Abstract
The naphthoquinone atovaquone is effective against Plasmodium and Pneumocystis carinii carinii. In Plasmodium, the primary mechanism of drug action is an irreversible binding to the mitochondrial cytochrome bc1 complex as an analog of ubiquinone. Blockage of the electron transport chain ultimately inhibits de novo pyrimidine biosynthesis since dihydroorotate dehydrogenase, a key enzyme in pyrimidine biosynthesis, is unable to transfer electrons to ubiquinone. In the present study, the effect of atovaquone was examined on Pneumocystis carinii carinii coenzyme Q biosynthesis (rather than electron transport and respiration) by measuring its effect on the incorporation of radiolabeled p-hydroxybenzoate into ubiquinone in vitro. A triphasic dose-response was observed, with inhibition at 10 nM and then stimulation up to 0.2 μM, followed by inhibition at 1 μM. Since other naphthoquinone drugs may also act as analogs of ubiquinone, diospyrin and two of its derivatives were also tested for their effects on ubiquinone biosynthesis in P. carinii carinii. In contrast to atovaquone, these drugs did not inhibit the incorporation of p-hydroxybenzoate into P. carinii carinii ubiquinone.
Ubiquinone (coenzyme Q [CoQ]) (Fig. 1A) plays a pivotal role in cellular respiration by participating in inner mitochondrial membrane electron transport by accepting electrons from a number of dehydrogenase enzymes and passing them to the cytochromes and eventually to molecular oxygen (6, 8, 30). Biosynthesis of ubiquinone (1, 22, 26, 29, 33) and the reactions that occur in different cellular compartments are probably best understood from studies with subcellular fractions of rat liver cells (1, 22, 29). Ubiquinone is composed of a benzoquinone ring and a lipophilic polyprenyl chain. In the rat liver, the precursor of the benzoquinone moiety, p-hydroxybenzoic acid (PHBA), is formed in the cytosol from the aromatic amino acids tyrosine or phenylalanine, whereas PHBA is formed directly from chorismic acid in organisms that have the shikimic acid pathway (i.e., plants, bacteria, and some fungi). The polyprenyl chain of CoQ is formed by a branch pathway in isoprenoid biosynthesis by the polymerization of five-carbon isopentenyl units; the number of the isopentenyl units in the chain distinguishes the CoQ homologs. The major CoQ homolog has been used as a taxonomic criterion for the verification of the phylogenetic relationships between organisms (32). There appears to be two independent sites where ubiquinone is synthesized in eukaryotic cells: one is the endoplasmic reticulum (ER)-Golgi system and the other is the mitochondrion (13, 33). After the transfer of a polyprenyl P-P chain to PHBA, followed by several additional reactions (1, 22, 26, 29, 33), the completed CoQ homolog is produced.
FIG. 1.
Structures of compounds relevant to the present study. (A) Ubiquinone (CoQ); n, number of isopentenyl units. (B) Atovaquone (556C80). (C) Diospyrin (R = H) and diospyrin dimethylether (R = CH3). (D) Diospyrin dimethylether hydroquinone (R = CH3).
Purified Pneumocystis carinii carinii (P. carinii) isolated from methylprednisolone-immunosuppressed rats was shown to contain CoQ10 as the major CoQ homolog (smaller amounts of CoQ9 were also detected) (10). Since CoQ10 was not detected in the lungs of healthy, untreated or in the lungs of immunosuppressed, uninoculated rat controls, this suggested that the pathogen was at least capable of synthesizing CoQ10. Recently, the incorporation in vitro of chorismate, PHBA, tyrosine, and mevalonate into P. carinii CoQ was demonstrated (10, 24, 28); thus, this organism can synthesize de novo both moieties of ubiquinone. The P. carinii pentafunctional gene for enzymes in the shikimic acid pathway has been cloned and characterized (2), suggesting that the pathway is functional in this organism. This gene is localized in the nucleus; hence, it is likely that ubiquinone biosynthesis in this organism occurs in the ER-Golgi system, although synthesis in the mitochondria cannot be ruled out.
Several hydroxynaphthoquinone drugs that are effective against protozoan infections (e.g., malaria, trypanosomiasis, and leishmaniasis) also have activity against P. carinii. Atovaquone (Fig. 1), first used as an antimalarial agent, was also found to have therapeutic activity against P. carinii pneumonia (PCP). This has been demonstrated both in animal models (19) and in humans (7, 14, 20). The mechanism of action of atovaquone against Plasmodium is believed to result from the irreversible binding of the drug to a 11.5-kDa protein of the mitochondrial cytochrome bc1 complex, thus inhibiting electron transport (12, 14–16). Since dihydroorotate dehydrogenase (DHOD), a key step in de novo pyrimidine synthesis, is coupled to the electron transport chain at complex III and because the parasite cannot salvage host pyrimidines, the mechanism of cidal drug action is thought to be the blockage of pyrimidine biosynthesis as a consequence of electron transport inhibition.
It was previously reported that the 50% inhibitory concentration (IC50) for P. carinii O2 consumption was 5 × 10−8 M atovaquone (14, 15). Thus, the respiratory chain was also implicated as the site of action in P. carinii. It was hypothesized, however, that unlike Plasmodium, P. carinii could salvage host pyrimidines, and the depletion of ATP (resulting from inhibition of respiration) was proposed as a mode by which P. carinii is killed by the drug (15). Moreover, unlike Plasmodium DHOD activity, which is inhibited by 1 nM atovaquone (12), the activity of this enzyme in P. carinii lysates was not inhibited by concentrations of ≤10 μM (21). Although atovaquone and other hydroxynaphthoquinone drugs are recognized as ubiquinone analogs, details on the mechanisms of their antimicrobial activities in different organisms remain unclear.
To test the hypothesis that oxidative phosphorylation is highly active in the respiratory chain of P. carinii and that the consequence of the drug's efficacy against PCP is the disruption of ATP synthesis, direct measurements of cellular ATP were performed. The effect of atovaquone on the ATP content of P. carinii organisms is described in a separate report (M. T. Cushion, et al., submitted for publication). In the present study, the effect of atovaquone on CoQ biosynthesis in P. carinii was examined by the incorporation in vitro of radiolabeled PHBA into CoQ. The results were compared with those obtained with another group of naphthoquinoid drugs which appear to be promising as antiparasitic agents (Fig. 1). Diospyrin, a natural product of Diospyros montana stem bark, and two of its derivatives (17, 18) exhibit activity in vitro against Plasmodium, Leishmania, and Trypanosoma at micromolar concentrations (18, 34). In an attempt to better understand the mechanism of antiparasitic activities of different quinoid drugs, these compounds were also examined for their effects on ubiquinone biosynthesis in P. carinii.
MATERIALS AND METHODS
Organisms.
P. carinii was isolated from infected rat lungs by using the corticosteroid-immunosuppressed rat model of Boylan and Current (4). Viral antibody-negative female Lewis rats (Harlan Sprague-Dawley, Indianapolis, Ind.) were immunosuppressed with methylprednisolone acetate (Depo-Medrol; Upjohn Co., Kalamazoo, Mich.) and were twice inoculated intratracheally with cryopreserved organisms containing 106 to 107 mixed-life-cycle stages by previously described methods (23). After 8 to 10 weeks of immunosuppression, moribund rats were killed and their lungs were perfused, excised, and cut into small pieces. The P. carinii organisms were isolated by homogenization (Stomacher; Tekmar, Cincinnati, Ohio) by the procedures described earlier; the mucolytic agent glutathione was included to detach organisms from host cells and other P. carinii organisms. Purification involved sieving and a series of centrifugation steps at different speeds, followed by membrane filtration. The purities of these preparations (>95 to 100%) were quantified by microscopic, biochemical, and immunochemical analyses (23). Routinely, these preparations contained 10 to 30% cystic forms and 80 to 95% viable organisms (23). Aliquots of the final organism preparations were used for total protein analysis (25).
Incorporation in vitro of PHBA into P. carinii CoQ.
Organisms (108 to 109) were centrifuged into a pellet at 925 × g for 10 min, and then the pellet was resuspended in 10 ml of serum-free RPMI 1640 medium supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and the radiolabeled precursor (24, 28). The organisms were incubated with radiolabeled [U-14C]PHBA for two days at 37°C in a 5% CO2 atmosphere. The organisms were washed with 0.85% NaCl and centrifuged into a packed pellet, and then the lipids were extracted. p-Hydroxybenzoate (specific activity, 33 mCi/mmol) was obtained from Tracer Lab (Boston, Mass.) or from Sigma (11.3 mCi/mmol); in each assay 5 or 10 μCi was added to the medium.
To test the effects of naphthoquinone drugs on P. carinii CoQ synthesis, atovaquone was dissolved in the primary solvent ethanol; diospyrin and its analogs were dissolved in dimethyl sulfoxide (DMSO). Two separate preparations of each diospyrin-based drug were tested in these CoQ biosynthesis studies. Various concentrations of each drug were added to radiolabeled PHBA before the organisms, suspended in the RPMI 1640 medium, were introduced into the reaction mixture. The ethanol concentration in the final incubation mixture was <0.2%, and the DMSO concentration was 0.1%.
Extraction of lipids and determination of P. carinii CoQ radioactivity.
The lipid extraction, purification, and fractionation methods used for studies on the incorporation in vitro of radiolabeled precursors into P. carinii ubiquinones were as described previously (28). Briefly, total lipids were extracted by a neutral solvent system as described by Bligh and Dyer (3) and were purified by biphasic partitioning as described by Folch et al. (11). The neutral lipid fraction was obtained by adsorption column chromatography (Unisil; Clarkson Co., Williamsport, Pa.) by elution with chloroform (CHCl3) and was then resolved by 1-dimensional thin-layer chromatography (TLC) on 0.25-mm Silica Gel H glass-backed plates (Analtech, Inc., Newark, Del.) prewashed with methanol. The TLC plates were developed with the solvent system petroleum ether-diethyl ether-acetic acid (80:20:1; vol/vol/vol) (9, 10). After visualization with I2 vapor, the ubiquinone band was scraped off the TLC plate and its radioactivity was determined by liquid scintillation spectrometry.
Incorporation of PHBA into the P. carinii total ubiquinone TLC fraction was expressed as picomoles of PHBA per milligram of protein from the original organism preparations. The effects of the drugs on CoQ biosynthesis were compared to those of vehicle controls.
RESULTS
Effects of atovaquone on the incorporation in vitro of PHBA into P. carinii ubiquinones.
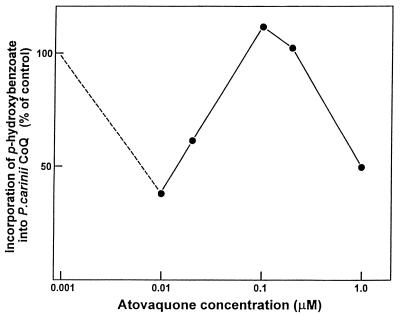
Incorporation of PHBA into P. carinii CoQ exhibited a triphasic response to increasing atovaquone concentrations (Table 1; Fig. 2). A dramatic inhibition of CoQ biosynthesis was detected at 10 nM, an effect that was reproducibly observed. At this concentration, incorporation was less than 50% of that for the untreated controls. At between 0.1 and 0.2 μM, incorporation occurred at levels comparable to or higher than those for the controls. The higher IC50 in the triphasic dose-response curve was observed at 1.0 μM.
TABLE 1.
Effects of naphthoquinone drugs on incorporation in vitro of radiolabeled p-hydroxybenzoate into P. carinii ubiquinonea
| Drug and concn (μM) | Radioactivity (dpm)b | Incorporation of PHBA into CoQ (pmol/mg of protein)c |
|---|---|---|
| Atovaquoned | ||
| 0 | 5,221–5,684 | 151.8 ± 2.4 |
| 0.01 | 2,037–2,516 | 59.3 ± 2.0 |
| 0.02 | 3,263–3,453 | 92.8 ± 1.0 |
| 0.1 | 5,815–6,668 | 170.5 ± 4.3 |
| 0.2 | 5,205–6,105 | 155.3 ± 5.4 |
| 1.0 | 2,458–2,905 | 74.3 ± 2.1 |
| Diospyrine | ||
| 0 | 525–1,037 | 66.0 ± 4.3 |
| 0.01 | 468–1,105 | 58.1 ± 6.5 |
| 0.05 | 495–832 | 56.2 ± 3.3 |
| 0.1 | 389–974 | 56.7 ± 5.1 |
| 0.5 | 411–1,042 | 56.5 ± 8.0 |
| 1 | 468–1,168 | 65.5 ± 6.3 |
| 5 | 474–1,200 | 63.2 ± 7.8 |
| 10 | 498–1,168 | 66.5 ± 6.2 |
| 25 | 403–1,037 | 59.9 ± 6.0 |
| 50 | 394–921 | 58.6 ± 4.6 |
| 100 | 442–1,105 | 60.6 ± 6.5 |
| Diospyrin dimethylethere | ||
| 0 | 252–397 | 48.2 ± 3.7 |
| 0.01 | 205–242 | 45.9 ± 1.9 |
| 0.05 | 237–435 | 53.9 ± 5.6 |
| 0.1 | 232–446 | 46.2 ± 2.5 |
| 0.5 | 137–382 | 46.7 ± 7.7 |
| 1 | 177–347 | 43.9 ± 5.2 |
| 5 | 226–279 | 51.5 ± 2.6 |
| 10 | 247–421 | 49.3 ± 4.8 |
| 25 | 253–381 | 46.6 ± 4.9 |
| 50 | 242–392 | 45.8 ± 3.4 |
| 100 | 221–432 | 46.4 ± 4.6 |
| Diospyrin dimethylether hydroquinonee | ||
| 0 | 274–689 | 58.2 ± 10.9 |
| 0.01 | 523–679 | 79.6 ± 5.0 |
| 0.05 | 295–453 | 63.9 ± 9.1 |
| 0.1 | 342–568 | 56.2 ± 6.3 |
| 0.5 | 418–621 | 63.0 ± 7.4 |
| 1 | 379–584 | 53.2 ± 6.4 |
| 5 | 442–668 | 77.2 ± 7.5 |
| 10 | 368–768 | 62.4 ± 10.6 |
| 25 | 354–820 | 61.1 ± 12.0 |
| 50 | 361–558 | 56.4 ± 7.2 |
| 100 | 279–515 | 48.5 ± 5.4 |
Purified organisms were incubated for 2 days in 10 ml of serum-free RPMI 1640 medium containing [U-14C]p-hydroxybenzoate and various concentrations of a drug. Values are means ± standard errors of the means.
Values are ranges of radioactivity in the ubiquinone band isolated by TLC.
Protein content of organisms prior to incubation.
Organisms were incubated with 10 μCi of the substrate (n = 4).
Organisms were incubated with 5 μCi of the substrate (n = 6 except in the assays with 0.01 and 5 μM, in which n = 3).
FIG. 2.
Effects of atovaquone on P. carinii CoQ biosynthesis. Purified organisms were incubated with [U-14C]PHBA for 2 days in the presence of different concentrations of the drug. There was a reproducible decrease in PHBA incorporation into CoQ at 10 nM, which then increased to control levels or higher at increased atovaquone concentrations. A decrease to half of the control value (IC50) was then observed with 1 μM atovaquone. Values represent means ± standard errors of the means for four separate experiments.
Effects of diospyrin and its derivatives on the incorporation of PHBA into P. carinii ubiquinones.
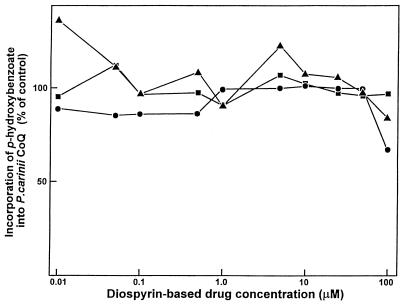
Diospyrin, diospyrin dimethylether, and diospyrin dimethylether hydroquinone did not inhibit the incorporation in vitro of PHBA into P. carinii ubiquinones when incubation was done for 48 h at concentrations up to 100 μM (Table 1; Fig. 3); dose-dependent reductions in CoQ biosynthesis were not observed.
FIG. 3.
Effects of diospyrin (circles), diospyrin dimethylether (squares), and diospyrin dimethylether hydroquinone (triangles). Purified organisms were incubated with [U-14C]PHBA for 2 days in the presence of different concentrations of a compound. Values represent means ± standard errors of the means for three or six separate experiments. Inhibition of ubiquinone biosynthesis was not observed at concentrations of these naphthoquinone drugs up to 100 μM.
DISCUSSION
Effects of atovaquone on P. carinii metabolism and respiration.
Atovaquone is effective in clearing organisms from patients with PCP with low to moderate numbers of organisms (7, 12, 20). It was previously reported that the drug inhibited P. carinii respiration (measured polarographically) at an IC50 of 50 nM (14, 15). Since it is known that atovaquone's activity in Plasmodium is the consequence of binding to the mitochondrial cytochrome bc1 complex (13–15) and that it apparently also binds to the P. carinii bc1 complex (14), it is not surprising that inhibition of P. carinii respiration was observed.
The present study addresses an effect of atovaquone other than electron transport and respiration. Atovaquone had a triphasic dose-effect on PHBA incorporation into P. carinii ubiquinone. Inhibition was observed at 10 nM atovaquone (the lowest concentration tested), which was lower than the concentration reported to be required for detectable inhibition of respiration (14), reduction of cellular ATP (Cushion et al., submitted), inhibition of DHOD activity, or inhibition of organism proliferation in primary cultures (21).
In a previous study, CoQ7 and CoQ8 were not detected by high-pressure liquid chromatography (HPLC) and/or gas-liquid chromatography–mass spectroscopy (10) methods, indicating that these homologs do not accumulate to readily detectable levels in the organism. By using more sensitive metabolic radiolabeling techniques, it was recently found that the HPLC fractions eluting with authentic CoQ7, CoQ8, CoQ9, and CoQ10 were all radioactive (D. Sul et al., unpublished data). The high specific activity of the shorter homologs and the conversion of radiolabeled CoQ8 to CoQ9 and CoQ10 suggest that the longer homologs can be formed by elongation of the polyprenyl chains of completed CoQ molecules. The biosynthesis of CoQ homologs by elongation of CoQ polyprenyl chains would represent a novel mechanism for CoQ10 biosynthesis.
Information on the regulatory mechanisms that control steps in CoQ biosynthesis in any cell type is severely lacking in the literature. On the basis of the available data on P. carinii and what is currently known about ubiquinone biosynthesis in general, we propose the following hypotheses or scheme as a working model to explain the observations on atovaquone's effect on P. carinii (Fig. 4). (i) At the low (10 nM) concentration, as an analog of CoQ, atovaquone may inhibit de novo CoQ biosynthesis by activating putative product feedback mechanisms that reduce the incorporation of PHBA into CoQ. In untreated cells, the enzyme would be regulated by the accumulation of free CoQ (CoQ10). This probably occurs at a cellular site closely associated with the mitochondrion or the ER-Golgi, where PHBA (which has also been transported into the organelle from the cytosol) condenses with heptaprenyl P-P or octaprenyl P-P. Following several reactions, including three S-adenosylmethionine (SAM)-dependent methyltransfer steps, the intermediate is converted to the completed CoQ7 molecule (a homolog radiolabeled with CoQ precursors in P. carinii). The completed CoQ product is translocated to the cytosolic side of the Golgi (or ER) membrane, where elongation of the polyprenyl chain occurs by the sequential addition of isopentenyl units, producing the major homolog CoQ10, which accumulates in the organism (10). Alternatively, completed polyprenyl chains could be formed prior to condensation with PHBA. In this scheme, it is proposed that feedback control involving inhibition by accumulation of CoQ10 in a free pool decreases the PHBA-hexaprenyltransferase activity in the Golgi-ER system. Translocation of CoQ10 to the inner mitochondrial membrane may require binding to a carrier protein which can target it to the mitochondrion. In the mitochondrion, CoQ participates in electron transport and interacts with the membrane, forming quinol and quinone pools in the membrane (6, 30). Thus, atovaquone is effective as a ubiquinone analog at triggering this feedback control of PHBA-polyprenyltransferase activity. (ii) At concentrations between 20 nM and 0.2 μM, atovaquone competes for sites on a carrier and/or binds to some (but not all) cytochrome bc1 complexes in the mitochondrial inner membrane, resulting in a reduction in the level of electron transport. At these concentrations, atovaquone may displace and prevent the binding of ubiquinone from some cytochrome bc1 complexes; i.e., the drug binds irreversibly to some of the bc1 complexes present in the membrane. This would result in detectable inhibition of respiration (decreased respiration in P. carinii carinii was detected with atovaquone at 50 nM [14, 15]). The inhibition of respiration may then trigger upregulation of the biosyntheses of components of CoQ intracellular transport (e.g., carrier) and/or the electron transport chain (e.g., ubiquinone) in response to the need to increase the cell's respiratory capacity. The upregulation of these biosynthetic activities might override the negative, end product feedback control(s) which atovaquone, at lower concentrations, could activate as a ubiquinone analog. Thus, with atovaquone at between 20 nM and 0.2 μM, CoQ biosynthesis (incorporation of PHBA into P. carinii CoQ) is increased to normal or higher levels. However, at these drug concentrations, ample electron transport could be maintained by CoQ molecules still in place within the mitochondrial inner membrane; thus, O2 consumption is only slightly affected, and the inhibition of oxidative phosphorylation, as measured by ATP levels in the cell, is not detectable (ATP pools may also be maintained by synthesis in the glycolytic pathway). (iii) At the higher drug concentrations (>1 μM), we hypothesize that sufficient amounts of atovaquone become irreversibly bound to most cytochrome bc1 complexes in the mitochondrion, resulting in a detectable reduction in cellular ATP levels. The reduction of ATP would result in decreased cellular metabolism, including those involved in de novo CoQ biosynthesis (reduction of PHBA incorporation into P. carinii CoQ). At these high atovaquone concentrations, the reduced level of synthesis of ATP probably also inhibits P. carinii folate biosynthesis. Inhibition of p-aminobenzoate incorporation into folates by atovaquone was observed at the IC50 of the drug (1.4 μM) for P. carinii (5). Detectable growth inhibition of P. carinii organisms would then become obvious at higher drug concentrations. It was shown that the proliferation of short-term primary cultures of P. carinii was inhibited with 3 μM but not with 0.3 μM atovaquone (21).
FIG. 4.
Proposed scheme for ubiquinone biosynthesis in P. carinii and the effects of atovaquone (Av). The precursors PHBA and geranyl P-P are formed in the cytosol. Elongation of the polyprenyl precursor by the addition of isopentenyl units may occur at the outer surface of the ER, and then heptaprenyl P-P is translocated to the ER and then to the Golgi apparatus lumen. At 10 nM atovaquone, the drug acts as an analog of CoQ10 and inhibits the PHBA-polyprenyltransferase activity (−), reducing the incorporation of PHBA into CoQ. In this model, it is proposed that decreased respiration stimulates the upregulation of biosyntheses of components involved in the intracellular translocation of CoQ (e.g., carrier) and/or electron transport (e.g., ubiquinone), which can override (++) the negative, end product feedback control(s). With atovaquone at concentrations of >1 μM, respiration is sufficiently inhibited and the reduction in ATP production by oxidative phosphorylation becomes measureable. The lack of ATP causes a decline in overall cellular metabolism, resulting in a decrease (−) in the rate of PHBA incorporation into CoQ. Ca, carrier.
Experiments on the effects of atovaquone on the PHBA-polyprenyltransferase activity in P. carinii were not directly tested in the present study, but it is now feasible to perform these studies. Procedures by which efficient incorporation of radiolabeled precursors into P. carinii molecules occurs have been developed with a cell-free system (28). The effects of the drug on PHBA incorporation into P. carinii CoQ will be examined as part of a separate study with this cell-free system. These experiments would represent among the few studies conducted on the regulation of PHBA-polyprenyltransferase in eukaryotic cells.
The present study demonstrated that atovaquone has potent effects on P. carinii ubiquinone biosynthesis, suggesting that there may be several possible mechanisms of action of the drug on this pathogen, and these mechanisms may also occur with other organisms, such as Toxoplasma and Plasmodium. Atovaquone-resistant strains have been identified among these organisms. Mutations in the cytochrome b gene appear to explain the development of some P. carinii-resistant strains (31). Since atovaquone was found to have a profound effect on other processes besides electron transport, it is possible that atovaquone resistance may also involve changes in components that function in the biosynthesis or intracellular translocation of ubiquinone.
Effects of diospyrin, diospyrin dimethylether, and diospyrin dimethylether hydroquinone on P. carinii ubiquinone biosynthesis.
It has been suggested that the mechanisms of action of quinoid drugs with antiparasite activity, as well as quinoid metabolites of some other drugs (e.g., primaquine), are by their action as analogs of ubiquinone (8, 15). Since diospyrin and its derivatives did not have an effect on P. carinii CoQ biosynthetic rates, this strongly suggests that the mechanism of action of atovaquone differed from those of the diospyrin-based quinoid drugs. Additional studies on the effects of the diospyrin-based drugs on the metabolic processes of the parasitic organisms shown to be sensitive to these compounds (18, 33) would aid in understanding the mechanism by which these drugs clear those infections. Recently, evidence for the inhibition of type I DNA topoisomerase activity by diospyrin was obtained in Leishmania donovani promastigotes (27). This observation is consistent with our results indicating that the drug reduces the cellular ATP content of P. carinii (Cushion et al., submitted), with no effect on the incorporation of PHBA into CoQ.
ACKNOWLEDGMENTS
We thank W. Gutteridge for kindly providing us with atovaquone.
This study was supported by NIH/NIAID grants RO1 AI38758 (to E.S.K.) and International Foundation for Science, Stockholm, Sweden, grant F/1836 (to B.H.).
REFERENCES
- 1.Appelkvist E L, Aberg F, Guan Z, Parmryd I, Dallner G. Regulation of coenzyme Q biosynthesis. Mol Aspects Med. 1994;15:S37–S46. doi: 10.1016/0098-2997(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Banerji S, Wakefield A E, Allen A G, Marshall D J, Peters S E, Hopkin J M. The cloning and characterization of the arom gene of Pneumocystis carinii. J Gen Microbiol. 1993;139:2901–2914. doi: 10.1099/00221287-139-12-2901. [DOI] [PubMed] [Google Scholar]
- 3.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 4.Boylan C J, Current W L. Improved model of Pneumocystis carinii pneumonia: induced laboratory infection in Pneumocystis-free animals. Infect Immun. 1992;60:1589–1597. doi: 10.1128/iai.60.4.1589-1597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comley J C, Mullin R J, Wolfe L A, Hanlon M H, Ferone R. Microculture screening assay for primary in vitro evaluation of drugs against Pneumocystis carinii. Antimicrob Agents Chemother. 1991;35:1965–1974. doi: 10.1128/aac.35.10.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane F L. Development of concepts for the role of ubiquinones in biological membranes. In: Lenaz G, Barnabei O, Rabbi A, Battino M, editors. Highlights in ubiquinone research. London, United Kingdom: Taylor & Francis; 1990. pp. 3–17. [Google Scholar]
- 7.Dohn M N, Frame P T, Baughman R P, Lafon S W, Smulian A G, Caldwell P, Rogers M D. Open-label efficacy and safety trial of 42 days of 566C80 for Pneumocystis carinii pneumonia in AIDS patients. J Eukaryot Microbiol. 1992;38:220S–221S. [PubMed] [Google Scholar]
- 8.Ellis J E. Coenzyme Q homologs in parasitic protozoa as targets for chemotherapeutic attack. Parasitol Today. 1994;10:296–301. doi: 10.1016/0169-4758(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 9.Ellis J E, Setchell K D, Kaneshiro E S. Detection of ubiquinone in parasitic and free-living protozoa, including species devoid of mitochondria. Mol Biochem Parasitol. 1994;65:213–224. doi: 10.1016/0166-6851(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 10.Ellis J E, Wyder M A, Zhou L, Gupta A, Rudney H, Kaneshiro E S. Composition of Pneumocystis carinii neutral lipids and identification of coenzyme Q10 as the major ubiquinone homolog. J Eukaryot Microbiol. 1996;43:154–170. doi: 10.1111/j.1550-7408.1996.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 11.Folch J, Lees M, Sloane-Stanley G H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 12.Fry M, Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80) Biochem Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Rudney H. 4-Hydroxybenzoate polyprenyltransferase from rat liver. Methods Enzymol. 1985;110:327–334. doi: 10.1016/s0076-6879(85)10090-x. [DOI] [PubMed] [Google Scholar]
- 14.Gutteridge W. 566C80, An antimalarial hydroxynaphthoquinone with broad spectrum: experimental activity against opportunistic parasitic infections of AIDS patients. J Eukaryot Microbiol. 1991;38:141S–143S. [PubMed] [Google Scholar]
- 15.Gutteridge W E. Pneumocystis carinii: potential targets for chemotherapeutic attack. In: Coombs G H, North M J, editors. Biochemical protozoology. London, United Kingdom: Taylor & Francis; 1991. pp. 35–51. [Google Scholar]
- 16.Haile L G, Flaherty J F. Atovaquone: a review. Ann Pharmacother. 1993;27:1488–1494. doi: 10.1177/106002809302701215. [DOI] [PubMed] [Google Scholar]
- 17.Hazra B, Pal S, Ghosh R, Banerjee A. Studies on changes in tumour-inhibitory activities through structural modification of a diospyrin derivative. Med Sci Res. 1994;22:621–623. [Google Scholar]
- 18.Hazra B, Ghosh R, Banerjee A, Kirby G C, Warhurst D C, Phillipson J D. In vitro antiplasmodial effects of diospyrin, a plant-derived naphthoquinoid, and a novel series of derivatives. Phytother Res. 1995;9:72–74. [Google Scholar]
- 19.Hughes W T, Gray V L, Gutteridge E W, Latter V S, Pudney M. Efficacy of a hydroxynaphthoquinone, 566C80, in experimental parasitic infections of AIDS pneumonitis. Antimicrob Agents Chemother. 1990;34:225–228. doi: 10.1128/aac.34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes W T. The role of atovaquone tablets in treating Pneumocystis carinii pneumonia. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:247–252. doi: 10.1097/00042560-199503010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ittarat I, Asawamahasakda W, Bartlett M S, Smith J W, Meshnick S R. Effects of atovaquone and other inhibitors on Pneumocystis carinii dihydroorotate dehydrogenase. Antimicrob Agents Chemother. 1995;39:325–328. doi: 10.1128/aac.39.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalén A, Norling B, Appelkvist E L, Dallner G. Ubiquinone biosynthesis by the microsomal fraction from rat liver. Biochim Biophys Acta. 1987;926:70–78. doi: 10.1016/0304-4165(87)90183-8. [DOI] [PubMed] [Google Scholar]
- 23.Kaneshiro E S, Wyder M A, Zhou L H, Ellis J E, Voelker D R, Langreth S G. Characterization of Pneumocystis carinii preparations developed for lipid analysis. J Eukaryot Microbiol. 1993;40:805–815. doi: 10.1111/j.1550-7408.1993.tb04479.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaneshiro E S, Ellis J E, Zhou L H, Rudney H, Gupta A, Jayasimhulu K, Setchell K D R, Beach D H. Isoprenoid metabolism in Pneumocystis carinii. J Eukaryot Microbiol. 1994;41:93S. [PubMed] [Google Scholar]
- 25.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Poon W W, Marbois B N, Faull K F, Clarke C F. 3-Hexaprenyl-4-hydroxybenzoic acid forms a predominant intermediate pool in ubiquinone biosynthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1995;320:305–314. doi: 10.1016/0003-9861(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 27.Ray S, Hazra B, Mittra B, Das A, Majumder H K. Diospyrin, a bisnaphthoquinone: a novel inhibitor of type I DNA topoisomerase of Leishmania donovani. Mol Pharmacol. 1998;54:994–999. doi: 10.1124/mol.54.6.994. [DOI] [PubMed] [Google Scholar]
- 28.Sul D, Kaneshiro E S. Ubiquinone synthesis by Pneumocystis carinii; incorporation of radiolabeled polyprenyl chain and benzoquinone ring precursors. J Eukaryot Microbiol. 1997;44:60S. doi: 10.1111/j.1550-7408.1997.tb05780.x. [DOI] [PubMed] [Google Scholar]
- 29.Teclebrhan T, Jakobsson-Borin A, Brunk U, Dallner G. Relationship between the endoplasmic reticulum-Golgi membrane system and ubiquinone biosynthesis. Biochim Biophys Acta. 1995;1256:157–165. doi: 10.1016/0005-2760(95)00016-6. [DOI] [PubMed] [Google Scholar]
- 30.Van den Bergen C W, Wagner A M, Krab K, Moore A L. The relationship between electron flux and the redox poise of the quinone pool in plant mitochondria. Interplay between quinol-oxidizing and quinone-reducing pathways. Eur J Biochem. 1994;226:1071–1078. doi: 10.1111/j.1432-1033.1994.01071.x. [DOI] [PubMed] [Google Scholar]
- 31.Walker D J, Wakefield A E, Dohn M N, Miller R F, Baughman R P, Hossler P A, Bartlett M S, Smith J W, Kazanjian P, Meshnik S R. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J Infect Dis. 1998;178:1767–1775. doi: 10.1086/314509. [DOI] [PubMed] [Google Scholar]
- 32.Yamada Y, Kondo K. Coenzyme Q system in the classification of the yeast genera Rhodotorula and Cryptococcus and the yeast-like genera Sporobolomyces and Rhodosporidium. J Gen Appl Microbiol. 1973;19:59–77. [Google Scholar]
- 33.Yamamoto T, Sugawara H, Shimizu S, Momose K. Possible existence of an intermediate pool of ubiquinone in rat heart mitochondria. Int J Biochem. 1990;22:89–91. doi: 10.1016/0020-711x(90)90082-e. [DOI] [PubMed] [Google Scholar]
- 34.Yardley V, Snowdon D, Croft S, Hazra B. In vitro activity of diospyrin and derivatives against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei brucei. Phytother Res. 1996;10:559–562. [Google Scholar]