Abstract
Background
The retina, as part of the central nervous system (CNS) with limited capacity for self-reparation and regeneration in mammals, is under cumulative environmental stress due to high-energy demands and rapid protein turnover. These stressors disrupt the cellular protein and metabolic homeostasis, which, if not alleviated, can lead to dysfunction and cell death of retinal neurons. One primary cellular stress response is the highly conserved unfolded protein response (UPR). The UPR acts through three main signaling pathways in an attempt to restore the protein homeostasis in the endoplasmic reticulum (ER) by various means, including but not limited to, reducing protein translation, increasing protein-folding capacity, and promoting misfolded protein degradation. Moreover, recent work has identified a novel function of the UPR in regulation of cellular metabolism and mitochondrial function, disturbance of which contributes to neuronal degeneration and dysfunction. The role of the UPR in retinal neurons during aging and under disease conditions in age-related macular degeneration (AMD), retinitis pigmentosa (RP), glaucoma, and diabetic retinopathy (DR) has been explored over the past two decades. Each of the disease conditions and their corresponding animal models provide distinct challenges and unique opportunities to gain a better understanding of the role of the UPR in the maintenance of retinal health and function.
Method
We performed an extensive literature search on PubMed and Google Scholar using the following keywords: unfolded protein response, metabolism, ER stress, retinal degeneration, aging, age-related macular degeneration, retinitis pigmentosa, glaucoma, diabetic retinopathy.
Results and conclusion
We summarize recent advances in understanding cellular stress response, in particular the UPR, in retinal diseases, highlighting the potential roles of UPR pathways in regulation of cellular metabolism and mitochondrial function in retinal neurons. Further, we provide perspective on the promise and challenges for targeting the UPR pathways as a new therapeutic approach in age- and disease-related retinal degeneration.
Keywords: Unfolded protein response, Metabolism, Endoplasmic reticulum stress, Retinal degeneration, Aging, Age related macular degeneration, Retinitis pigmentosa, Glaucoma, Diabetic retinopathy
Background
The retina is a thin layer of neural tissue that lies at the back of the eye and is responsible for sensing and processing the light input to generate visual signals and transmitting the information to the brain via the optic nerve. The vertebrate retina develops embryonically as an evagination from the developing neural tube and is thus part of the central nervous system (CNS) [1]. The structure of the retina is highly organized, consisting of multiple layers of photosensory neurons (photoreceptors), interneurons (bipolar cells, amacrine cells, and horizontal cells), projection neurons (retinal ganglion cells, RGCs), and their synapses. In addition, retinal blood vessels, which are enriched in the inner retina, and glial cells (astrocytes, Müller cells, and microglia) function as the supporting systems and form an integrated network with retinal neurons to maintain the metabolic and immune homeostasis in the retina. In mammals, retinal neurons are terminally differentiated at the early stage of life and do not regenerate [2]. Thus, severe injuries and loss of retinal neurons, such as light-sensing photoreceptors and projection neurons (RGCs), are often irreversible and subsequently lead to significant degeneration of the retina and catastrophic vision loss and blindness.
Relative to other CNS counterparts, retinal neurons are subjected to a greater level of environmental challenges and stresses [3, 4]. For example, retinal photoreceptors are constantly exposed to light, which can cause light toxicity and oxidative damage. In addition, photoreceptor cells have a high metabolic demand and a high protein turnover rate to maintain their physiological function and structural integrity [4]. The outer segments (OS) of photoreceptors, as the major site for visual phototransduction, are composed of highly specialized, disc-like structures enriched in lipids and proteins, which are prone to light-induced oxidative damage. To overcome the damage, the photoreceptor OS undergo daily shedding and renewal [5]. This process requires constant synthesis and proper folding of new proteins. In addition, major functions of photoreceptors, including phototransduction and neurotransmission, consume significant amounts of energy. These unique characteristics make photoreceptors highly susceptible to perturbations in the mitochondria and ER, which are the central hubs that govern metabolic and protein homeostasis.
To cope with the stress conditions, cells have developed a broad range of sophisticated stress response mechanisms to prevent and mitigate potential damages. These cellular signaling pathways, activated by distinct stressors, attempt to return the cell to homeostasis. However, if the stress conditions cannot be resolved, cells will activate programmed cell death signaling to eliminate damaged cells. Therefore, the stress response pathways are not only critical to maintaining long-term retinal integrity and function, but may also participate in disease pathophysiology by promoting cell death and degeneration. In addition to intrinsic stresses in retinal neurons, metabolic changes resulting from dysfunction and loss of retinal blood vessels, which reduces oxygen and nutrient supply to the retinal tissue, are also a frequent cause of neuronal death and degeneration. This can be seen in a number of ischemic retinal diseases such as diabetic retinopathy (DR) [6]. Imbalance of retinal microenvironment, governed by the blood-retinal barrier (BRB) consisting of tight junctions between neighboring vascular endothelial cells (inner BRB) or retinal pigment epithelium (RPE) (outer BRB), and glial cells, can activate cellular stress signaling in retinal neurons ultimately impacting their survival and function, resulting in vision impairment and blindness.
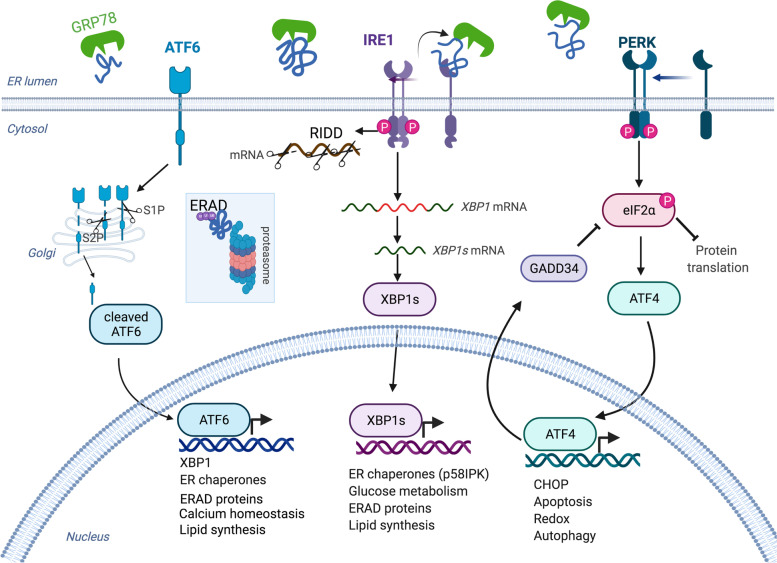
Among the various types of cellular stress responses, ER-associated signaling pathways, including the unfolded protein response (UPR), ER-associated degradation (ERAD), autophagy, and integrated stress response (ISR), play a central role in promoting and maintaining a balanced and functional proteome in a cell. The UPR is activated upon a stress condition, where excessive unfolded or misfolded proteins accumulate in the ER, referred to as ER stress. To alleviate ER stress, the ER resident chaperone protein glucose-regulated protein 78 (GRP78; also known as immunoglobulin binding protein, BiP), dissociates from trans-ER membrane proteins activating transcription factor 6 (ATF6), inositol requiring enzyme 1 (IRE1), and PKR-like endoplasmic reticulum kinase (PERK). Subsequently, GRP78 binds to unfolded and misfolded proteins to promote their folding or refolding and as well keep them in a soluble form to prevent protein aggregation [7]. The dissociation of GRP78 from ATF6, IRE1, and PERK activates each of these proteins, which serve as ER stress sensors, and their downstream signaling cascades (Fig. 1). These signaling pathways work synergistically to restore the ER homeostasis via a variety of processes including increasing protein degradation, decreasing protein translation, and increasing production of chaperones and foldases that facilitate protein folding [7]. Activation of the UPR is an important mechanism required for cells to maintain the protein and ER homeostasis, especially in neural tissues such as the retina [8]. In addition, the UPR has been linked to a wide array of physiological processes such as glucose and lipid metabolism, mitochondrial function, redox regulation, calcium homeostasis, autophagy, just to name a few [9]. Understanding the role and regulation of the UPR in retinal development, maintenance, and aging, and its implication in retinal dysfunction and degeneration, could provide novel insights into the pathogenesis of retinal disease and lead to new treatments.
Fig. 1.
Schematic diagram depicts the three branches of the unfolded protein response (UPR) and resulting downstream targets reviewed in the context of aging and retinal disease. Each branch is activated when the resident ER chaperone, GRP78, releases from IRE1, PERK, and ATF6 to bind accumulated unfolded or misfolded proteins. The resultant signaling cascades activate downstream effectors, such as XBP1 and ATF4, in an attempt to re-establish homeostasis through multiple means including repressing protein translation, promoting ERAD (endoplasmic reticulum associated protein degradation) and RIDD (regulated IRE1-dependent decay of mRNA), and upregulating the expression of ER chaperones and foldases. In addition, the UPR pathways regulates genes involved in a broad range of ER stress-dependent and independent cellular processes including autophagy, glucose metabolism, lipid synthesis, cytoskeletal reorganization, and calcium regulation
Here, we describe recent advances in understanding the mechanisms and signaling pathways of cellular stress response, with a major focus on the UPR, in retinal cells during aging and common retinal diseases, such as age-related macular degeneration (AMD), retinitis pigmentosa (RP), achromatopsia, glaucoma, and diabetic retinopathy (DR). We highlight a potential role of the UPR in regulation of cellular metabolism and mitochondrial function in retinal neurons and their therapeutic implications in protecting against age- and disease-related retinal degeneration and restoring neuronal and synaptic function.
Aging
Aging is a major risk factor for chronic human disease, including a broad range of neurodegenerative diseases in the eye. Epidemiologic research demonstrates that the frequency of visual impairment from all causes increases significantly past the age of 60 and the prevalence of common retinal diseases such as AMD, DR, and glaucoma, also increases with age [10, 11]. Aging is a multifaceted process in which accumulation of stress over time results in alterations in cellular signaling, metabolic control, and protein homeostasis, ultimately causing substantial changes in morphology, structure, and function in cells and tissues. Clinical studies have shown a continuous decline of retinal function with aging in normal human subjects aged 10 to 69 years and a reduction in central retinal thickness and retinal nerve fiber layer thickness in elderly population with age of 65 years or older [12, 13]. In experimental models, wild-type mice after 12 months of age demonstrate decreased retinal thickness, reduced retinal function, and a loss of retinal neurons including RGCs, bipolar cells, and peripheral photoreceptors [14–16]. In addition, the dendritic field size in subtypes of RGCs decreases with aging, suggesting that morphological changes other than cell loss of retinal neurons also contribute to age-related functional deficits [17]. Loss of synapses and increased synaptic remodeling in the neural retina is another characteristic of aged retina, which is evidenced by fewer photoreceptor synapses and displaced presynaptic photoreceptor ribbons from the outer plexiform layer (OPL) to the outer nuclear layer (ONL) along with aberrantly extended bipolar dendrites in mouse models of premature aging [18–21]. Interestingly, retraction of photoreceptor synapses has also been reported in human retinal degenerative diseases, such as AMD, retinitis pigmentosa, and retinal detachment [22]. These overlapping phenotypes suggest common underlying mechanisms for retinal degeneration during aging and disease conditions.
A functional UPR for maintaining the protein and ER homeostasis is critical for healthy aging [23]. As the organism ages, the expression levels of UPR proteins show changes and the ability of the cell to respond to cellular stress declines. In human lens, the baseline levels of GRP78, IRE1, and ATF6 increase progressively from ages 50 to 90 years [24]. Similarly, the levels of C/EBP homologous protein (CHOP) increase in aged mouse brain and retina [25]. Conversely, the baseline level of spliced XBP1 (XBP1s; the activated form of XBP1) decreases with age in the mouse retina [18]. In human, this variation extends to the individual with aged monozygotic twins showing differential expression of XBP1s correlated to cognitive function [26]. Furthermore, the changes in UPR components appear to be tissue-specific. For example, phosphorylated PERK levels are reduced in aged pancreas but increased in aged kidney [27, 28]. In aged rat retina, effectors in the PERK pathway, such as phosphorylated eukaryotic translation initiation factor-2α (eIF2α) and NF-E2-related factor 2 (Nrf2) are reduced, whereas other downstream effectors, such as ATF4 and CHOP, are elevated compared to younger controls [29]. These changes may suggest an increase in cellular stress in the ER coupled with disrupted protein homeostasis. In human retina, the presence of protein aggregates of nonphosphoylated tau and α-synuclein increases substantially with advanced age, further supporting the presence of protein misfolding and dyshomeostasis in aged retinas [30].
In addition to the changes in the basal levels of UPR proteins, the ability of aging cells to respond to cellular stress declines [31, 32]. Our recent study has shown that the ER stress stimulator, thapsigargin, was able to induce a robust activation of the UPR in the retina of young adult mice but failed to increase XBP1s expression in the retina of 13-month-old mice [18]. This finding suggests that declined function of the UPR pathways may contribute to neuronal dysfunction and degeneration in aging mice [18] and retinal diseases [33]. Further supporting this notion, conditional knockout (cKO) of XBP1 in retinal neurons results in accelerated retinal degeneration and retinal function decline with aging. At the age of 12–14 months, XBP1 cKO mice show significant structural and functional deficits that resemble wild-type mice twice that age, including reduced retinal thickness, loss of RGCs, and morphological defects of retinal synapses [18, 20]. Remarkably, a strikingly similar phenotype featuring age-related increase in ectopic photoreceptor-bipolar synapses is also observed in ER membrane protein complex 3 (Emc3) cKO mice [21], liver kinase B1 (Lkb1) cKO mice, and AMP activated protein kinase, alpha 1 and 2 subunits (AMPKα1/AMPKα2) double cKO mice [19]. Functionally, both light- and dark-adapted electroretinograms (ERG) show reduced amplitudes in all of these aging cKO models; the optokinetic response also deteriorates in mice with aging [15, 18, 20, 21]. AMPK functions as an energy sensor, whose activation increases glucose uptake and glycolysis, promotes fatty acid oxidation, and enhances mitochondrial biogenesis to restore energy supply and balance [34]. These findings suggest that maintaining the ER homeostasis and energy metabolism is critical for retinal neuronal survival and function during aging. Intriguingly, the retinas from aged XBP1 cKO mice have an overall decrease in baseline glycolysis and in maximum glycolytic response, compared to age-matched wild-type mice, and these changes may contribute to accelerated retinal neurodegeneration in these mice [12]. A progressive decline in metabolic control due to impaired function of nutrient-sensing pathways results in perturbations in energy metabolism in aged animals [35]. Together, these studies suggest restoring the UPR function may protect against metabolic defects, thus reducing the long-term stress associated with aging and tissue deterioration in age-related disease.
Age-related macular degeneration
Age-related macular degeneration (AMD) is a leading cause of severe, irreversible vision loss in elderly populations [36]. Approximately 10% of individuals over the age of 65 years and 25% of those over the age of 75 years in developed countries have been diagnosed with AMD. As life expectancy increases, so too does the prevalence of AMD. It is expected that by 2040, nearly 300 million people worldwide will be affected by the disease [37, 38]. The rapid increase in disease prevalence renders AMD a significant global health concern that negatively influences the well-being of the population. Clinically, AMD can be categorized into two stages, early and late AMD. Early stages of the disease are characterized by small extracellular deposits or drusen, depigmentation of the retinal pigment epithelium (RPE) layer, and impaired RPE functionality [39, 40]. Advanced stages of the disease can be subclassified into non-neovascular (or dry) and neovascular (or wet) AMD. Both forms of advanced-stage AMD are accompanied by loss of photoreceptors and geographic atrophy (GA), but neovascular AMD (nAMD) is distinguished by presence of pathological angiogenesis in the macula, or macular neovascularization (MNV) [41, 42]. According to the anatomic location and origination of the new vessels, MNV can be classified into three major types. Type 1 and Type 2 MNV originate from the choroid and proliferate under the RPE (Type 1) or breaks through the RPE to reach subretinal space (Type 2), while Type 3 MNV originates from the retina and grows toward the RPE [41]. Regardless of the type of the MNV, these malformed vessels lack appropriate pericyte coverage and tight junctions between endothelial cells and are therefore prone to leakage or rupture. Common lesions caused by MNV include exudation, hemorrhages, and edema in the macula, which is often associated with severe visual impairment [39, 43].
AMD is a multifactorial disease involving the interplay between advanced age, environmental risk factors, and genetic factors. Common variants found in the complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) genes have been shown to increase the risk of AMD [39]. Environmental factors that are responsible in part for disease onset and progression include modifiable risk factors like cigarette smoke and diet, but also hyperopia, hypertension, and sex (female) [44, 45]. The complex etiology poses significant challenges to the development of therapeutics for AMD. Current standard treatment options include intravitreal anti-vascular endothelial growth factor (VEGF) therapies for MNV in patients with wet AMD or nAMD, which significantly reduce vascular leakage in most cases, and inhibit vascular growth in some; however, its overall long-term effect on MNV regression or inhibition of MNV expansion remains suboptimal [43, 46]. In addition, no effective treatment is available for patients with early AMD and late stage AMD with GA [47]. Limitations on treatment options for AMD leave much to be discovered regarding the pathophysiology of the disease and the underlying molecular mechanisms, particularly initiation of the early-stage damage and dysfunction of the RPE.
The RPE is a monolayer of cuboidal epithelial cells located between choroidal vasculature and the outer segments of the photoreceptors. Basolaterally, RPE cells form the outer BRB by tight junctions and adhere to a highly organized basement membrane, known as Bruch’s membrane, which separates RPE cells from fenestrated endothelium of the choroidal capillaries [48, 49]. Apically, the RPE faces the light-sensitive photoreceptor outer segments (POS) and plays a crucial role in nourishing the outer retina, detoxifying and phagocytosing damaged POS, and regenerating visual pigment to maintain the process of phototransduction. In addition, the RPE serves as an essential component of a metabolic ecosystem in the eye [50–52]. In this system, glucose from the choroid is transported through the RPE to photoreceptors; photoreceptors then convert glucose to lactate, which is provided as a fuel to the RPE and neighboring retinal cells [53]. Lactate also suppresses glycolysis in the RPE that further preserves glucose for use by photoreceptors [54]. Without an intact RPE, critical processes such as photoreceptor morphogenesis and metabolic homeostasis are impaired and photoreceptor cells are likely to undergo degeneration [55, 56].
A prominent characteristic of early AMD is the accumulation of drusenoid deposits in the subretinal space and the thickening of the Bruch’s membrane [39]. Possible contributing factors to these pathological changes include malfunction of macrophages that fail to remove cell debris from subretinal space [57], dysregulation of lipid metabolism associated with aging [58], and accumulation of lipoproteins in Bruch’s membrane [59]. In addition, disturbed protein homeostasis plays a central role in this process. In human donor eyes, accumulation of amyloid β, a major component of amyloid plaques found in the brains of the patients with Alzheimer’s disease, was observed in drusen, correlating with complement activation and RPE/photoreceptor degeneration in AMD [60–63]. Ubiquitin-positive aggregates were also identified in soft and hard drusen in aged human retinas [30]. These findings suggest an implication of protein dyshomeostasis in the pathogenesis of AMD. In parallel with drusen formation, accumulation of lipids and protein modifications in the extracellular matrix leads to structural and compositional changes in Bruch’s membrane (reviewed in [64]). These changes impair the bidirectional nutrient transfer from the RPE to the choriocapillaris, further contributing to RPE and photoreceptor degeneration.
In response to nutrient shortage and disturbed metabolism, cells activate adaptive signaling pathways and molecules, among which is the AMPK/mammalian target of rapamycin (mTOR) pathway [65]. Activation of AMPK increases energy production and regulates a wide variety of metabolism-related stress responses, such as anti-oxidant defense, autophagy and mitophagy [66]. In the RPE from human donor eyes with AMD, AMPK activity was drastically reduced, suggesting that insufficient AMPK activation may be implicated in AMD [65]. Pharmacological activation of AMPK by metformin (1,1-dimethylbiguanide hydrochloride) protects photoreceptors and the RPE from light- and oxidative stress-induced damage [67]; conversely, retina-specific knockout of AMPK leads to retinal dysfunction and age-related neurodegeneration, suggesting an essential role of AMPK in retinal neuronal survival and function [68]. Interestingly, conditional deletion of AMPK in the neuroretina also induces a secondary degeneration of the RPE, which is perhaps not surprising given the close interdependence between the RPE and the retina as a metabolic ecosystem. In contrast, in the context of glaucoma (discussed below), hyperactivation of AMPK results in significant morphological changes and functional decline in RGCs, whereas depletion of AMPK rescues both structure and function in RGCs [69]. These discrepant results suggest that AMPK may activate distinct downstream pathways that exert varying or even opposite effects on cell metabolism and stress response in different cell types (i.e. RPE cells and RGCs). Therefore, understanding cell-specific signaling pathways in response to distinct stressors is critical to the formulation of effective interventions.
Closely related to dysregulation of cellular metabolism are increased oxidative stress and ER stress, which play a major role in RPE damage and AMD pathogenesis [39]. Recent work highlights a close interplay between these two types of stress [58, 70]. Cigarette smoke, a major environmental risk factor, activates oxidative stress and ER stress in RPE cells resulting in RPE apoptosis and cell death, disruption of the barrier function, and thickening and deposit accumulation on Bruch’s membrane [71–75]. Inhibition of ER stress or reduction of oxidative stress both protect RPE cells from cigarette smoke extract (CSE)-induced apoptosis and cell death [74, 76]. Moreover, alleviating ER stress significantly reduces mitochondrial fragmentation and decreases reactive oxygen species (ROS) generation in CSE-challenged RPE cells, further suggesting a close interplay between ER stress and oxidative stress [76]. Increased oxidative stress stimulates an upregulation of genes, such as transcription factor, Nrf2, to restore redox homeostasis [76]. In aging RPE, the Nrf2 signaling was found less functional in response to oxidative stress, which makes aging RPE vulnerable to oxidative damage [77]. Overexpression of Nrf2 significantly improves survival and barrier function of RPE cells challenged with oxidative stress and in animal models of retinal degeneration [78]. Genetic and/or pharmacological approaches to enhance Nrf2 function hold great promise for developing new treatments for AMD and other retinal degenerative diseases.
Like oxidative stress, ER stress has been implicated in the RPE pathologies associated with AMD [3, 74, 76, 79, 80]. In response to ER stress induced by CSE, all three UPR branches can be activated [76]. Among these branches, the IRE1/XBP1 pathway has been shown to be essential for RPE survival and function during stress conditions and for maintaining the RPE structural integrity by regulating calcium-dependent RhoA/Rho kinase signaling and actin cytoskeleton organization [74, 79, 80]. In human RPE cells, inhibition of XBP1 intensifies CSE-induced apoptosis; in contrast, suppression of the PERK/ATF4/CHOP pathway improves RPE cell survival, suggesting that the XBP1 pathway and the PERK/ATF4/CHOP pathway play differential roles in RPE survival during AMD [74]. Interestingly, despite the pro-apoptotic role of CHOP in mediating ER stress-related cell death in many cell types, silencing of CHOP gene in the RPE results in reduced Nrf2 activation and a marked increase in apoptosis [76]. Similarly, deficiency of CHOP advances rod photoreceptor cell death in degenerative retinal diseases such as Retinitis Pigmentosa [81]. These results suggest that maintaining a certain level of CHOP is necessary for Nrf2 activation and cell survival in the RPE and photoreceptors during stress conditions. However, excessive CHOP activation by ER stress can be detrimental to cell survival and function contributing to neurodegeneration [82].
Past studies have highlighted the importance of molecular chaperone proteins in protecting the RPE during AMD pathogenesis. Endoplasmic reticulum protein 29 (ERp29) is a multifunctional ER chaperone belonging to the protein disulfide isomerase family. As a putative ER chaperone, ERp29 facilitates the folding and trafficking of secretory and membrane proteins, such as connexin 43, which is an integral membrane protein that forms the gap junctions [83]. In addition, ERp29 functions as a regulator of cellular stress response by direct interacting with PERK and ATF6 in the UPR pathways and upregulating/enhancing the function of other ER chaperones (reviewed in [84]). While highly expressed in normal secretory epithelial cells, the levels of ERp29 were found significantly reduced in the RPE in both AMD patients and cells exposed in vitro to CSE. Overexpression of ERp29 protected RPE cells from CSE-induced ER stress, tight junction damage, and apoptosis. In contrast, ERp29 knockdown leads to decreased activation of the ATF6 pathway, reduced levels of p58IPK and Nrf2, and increased p-eIF2a and CHOP activation resulting in exacerbated CSE-triggered cell death [84–86]. Future studies are warranted to investigate the therapeutic potential of targeting specific protective UPR pathways, such as XBP1, or associated molecular chaperone proteins, such as Erp29, to restore the ER and protein homeostasis, for preventing RPE and photoreceptor damage in animal models of AMD.
Retinitis Pigmentosa
Retinitis Pigmentosa (RP) represents a group of rare genetic diseases where mostly rod-specific gene mutations cause slow and progressive rod, and subsequently secondary cone, degeneration leading to vision loss [87]. Genetic mutations in over 50 causal genes of RP have been identified [88]. In part due to the diversity and relative rarity of each mutated gene, currently there is only one Food and Drug Administration-approved treatment for RP, specific to the RPE65 mutation [89]. RPE65 encodes an all-trans retinyl ester isomerase in the RPE essential for production of the photopigment 11-cis-retinal. More common forms of RP are associated with misfolding of proteins caused by mutations of the rhodopsin gene (RHO). The resultant rhodopsin protein is a seven-transmembrane G-protein-coupled receptor responsible for initiating the phototransduction cascade in rod photoreceptor cells [88, 90, 91]. Over 200 mutations of the RHO gene have now been identified and may be inherited in an autosomal dominant or less frequently in an autosomal recessive manner [92, 93]. Autosomal recessive RP (arRP) is characterized by homozygous recessive inheritance of loss-of-function RHO mutations, such as those found in Receptor Expression Enhancer Protein 6 (REEP6). Mutant REEP6 proteins lead to retinal degeneration through defective formation and localization of guanyl cyclases and consequent alteration of the phototransduction pathway [94–96]. More commonly implicated, autosomal dominant RP (adRP) mutations such as P23H (proline substituted by histidine at position 23) and T17M (threonine substituted by methionine at position 17) are thought to be responsible for 20–30% of all adRP cases [91, 92]. These mutations have been shown to increase ER stress and activate the UPR and ERAD pathways in photoreceptors [97]. Selective activation of IRE1 decreases misfolded rhodopsin proteins in both the P23H and T17M models as well as a non-class II mutant rhodopsin, S334ter rhodopsin, in part through degradation by both ERAD and regulated IRE1-dependent mRNA decay (RIDD) [98]. Recent studies have shown that robust rhodopsin degradation precedes retinal degeneration and the IRE1 signal transduction pathway remains activated even after photoreceptor degeneration plateaus [33, 99]. Further evidence of the beneficial role of IRE1 points to the molecular chaperone, ER degradation-enhancing a-mannosidase-like 1 (EDEM1), which assists in regulation of protein degradation in the ER [100–102]. As a component of the IRE1 pathway, EDEM1 accelerates degradation and clearance of P23H rhodopsin proteins and in doing so may also promote the proper folding and transport of folding-competent mutant proteins [102]. This suggests that photoreceptor death may not be associated with insufficient activation of the IRE1 pathway and other pathways may contribute to the degeneration process. However, the cytoprotective features of the IRE1 pathway, such as EDEM1’s dual role of enhancing mutant rhodopsin degradation and promoting folding-competent protein, may prove useful in therapeutic interventions aiming to alleviate protein misfolding [102, 103].
In contrast to the IRE1 pathway that promotes protein folding and ERAD to alleviate ER stress, activation of PERK increases the phosphorylation of eIF2α, resulting in a decrease in global protein synthesis and an increase in ATF4 production [98]. The role of the PERK/ATF4 pathway in the pathogenesis of RP has been studied by several groups [104, 105]. Inhibition of PERK with GSK2606414A increases the production of both normal and mutant rhodopsin proteins resulting in increased protein aggregation, reduced photoreceptor survival, and decreased visual function. In contrast, enhancing eIF2α phosphorylation protects photoreceptors in P23H rats, suggesting that PERK activation to reduce global protein synthesis thus alleviating protein aggregation and ER stress is likely a protective response at the early stage of the disease [105]. Despite the early activation of PERK protecting photoreceptors again proteotoxicity and ER stress, long-term activation of PERK induces an increase in ATF translation and an upregulation of its downstream effector CHOP [106]. In T17M RP mouse model, elevated ATF4 levels accompanied by increased CHOP expression and reduced autophagy contribute to photoreceptor degeneration in RP [104]. Intriguingly, ablation of CHOP showed no effect on reducing photoreceptor death in two RP models [81, 107]. The mechanism behind these observations is not well understood, but earlier studies revealed that deletion of CHOP reduces protein expression of Nrf2, a key protective factor against oxidative damage, in the RPE [76]. Investigation of the downstream targets of CHOP in photoreceptors may provide new insights into the role of CHOP in RP. In addition, the protective effects of the PERK pathways are likely necessary for long-term photoreceptor survival and visual function in adRP by reducing mutant rhodopsin retention in the ER and diminishing rod photoreceptor degeneration [33]. This duality of the PERK signaling pathway may be specific to adRP models, wherein ER stress induced by protein misfolding can be alleviated by reduction of overall protein synthesis and upregulation of molecular chaperones [108–111].
In response to rhodopsin misfolding and ER stress in photoreceptor cells of adRP, a third UPR pathway, mediated by ATF6, is also activated [112]. Activation of ATF6 upregulates ER chaperones, such as GRP78, to promote protein folding and restore ER homeostasis [113] [99, 111]. In addition, selective activation of ATF6 provides a protective action that can be closely tied to processes ensuring proper ER folding, such as ERAD. Independent of IRE1 and PERK, selective activation of ATF6 upregulated HMG-CoA reductase degradation protein 1 (HRD1) – dependent ERAD of amyloid precursor protein [114]. In adRP models, activation of ATF6 decreased the levels of class II mutant rhodopsin, including P23H and T17M, while sparing monomeric WT rhodopsin production [98]. As with the IRE1 pathway, elucidating the role of molecular chaperones involved in specific UPR branches may improve targeted gene therapies for adRP. Recent findings demonstrated that intravitreal AAV injection of the GRP78 chaperone alleviates ER stress, suppresses apoptosis, and improves ERG responses in a rat P23H RHO model [114]. GRP78 alongside the co-chaperone and ER DNAJ protein 5 (ERdj5/DNAJC10) are also required for formation of the C110-C187 disulfide bond in WT rhodopsin. Knockdown of ERjd5 decreased expression of WT and mutant P23H rhodopsin, suggesting the importance of DNAJ proteins in maintaining the ER stress response [110, 115]. Viral-mediated overexpression of GRP78 and ERdj5 further supports these findings with results showing an overall reduction in ER stress and enhanced photoreceptor cell survival in the P23H RHO mouse model [110, 114, 116]. Although ATF6 signaling ensures degradation of mutant rhodopsin proteins present in RP, it cannot regulate proper folding of mutant rhodopsin [111]. This contrasts with ER chaperones downstream of IRE1, like EDEM1, which possess both improved mutant rhodopsin degradation and restoration of folding-competent P23H rhodopsin [102]. Altogether, these recent findings elucidating the proposed mechanism of each UPR pathway presents new opportunities for targeted therapies focusing on individual branches of the UPR and their co-chaperones [98, 111, 114].
Achromatopsia
Achromatopsia is a rare autosomal recessive disorder characterized by impaired cone photoreceptor function, leading to decreased visual acuity beginning at birth or early infancy, nystagmus, and reduced or absent color vision [117–119]. Six genes have been identified in close association with achromatopsia, including the gene encoding ATF6. Mutations of ATF6 result in autosomal recessive retinal cone dystrophy and convey increased susceptibility to ER stress from hypoxia, protein misfolding, and light damage [120–122]. In animal models, global ATF6 knockout mice show normal retinal morphology and function at a young age but develop photoreceptor dysfunction with increasing age [117]. Knockout of ATF6 in a P23H-KI model of RP impairs rhodopsin clearance and accelerates retinal degeneration and functional deficits [112]. A phenotypic correlation is seen in patients with ATF6 mutation-induced achromatopsia who present foveal hypoplasia, supporting a role of ATF6 in cone development [117, 121, 123]. Interestingly, using human stem cell-derived retinal organoids, a recent study shows that genetic variants that disrupt ATF6 function lead to impaired cone development and a loss of cone OS/IS [120]. The contradictory results from human and animal studies are believed to be associated with the intrinsic biologic differences and environmental factors that influence the role of ER stress and the UPR pathways in murine and human retinal development [117, 120, 123]. In addition, further insight into the presence of non-functioning peripheral cones may offer advances in pre-existing therapeutic interventions, such as gene therapy for achromatopsia associated with GNAT2, CNGA3, and CNGB3 mutations [117, 124, 125].
Recent investigations into the associations between ATF6, photoreceptor integrity, and achromatopsia reveal the diversity among the roles and potential mutations of ATF6. Current studies have begun to highlight these diverse molecular defects and the associated defects seen in specific steps of ATF6 activation. For example, Class 1 ATF6 mutants possess impaired trafficking from the ER to the Golgi apparatus whereas Class 3 mutations show an impaired basic leucine zipper (bZIP) domain [126]. Further exploration into the stepwise activation of ATF6 may prove of use for potential therapeutic strategies, including gene replacement therapy for defective transcriptional activators and gene editing for mononucleotide mutations. ATF6 small molecule agonists, such as ATF6-activating (AA) compounds AA147 and AA263, and antagonists, such as Ceapin-A7, have been shown to selectively modulate the ATF6 arm of the UPR pathway [108, 127, 128], Downstream targets of ATF6 may also serve as potential targets in achromatopsia. As seen in adRP models, overexpression of GRP78 and ERdj5 by AAV mediated delivery decreases aggregation of mutant proteins and may be possible regulators of ATF6 translocation to the nucleus [114, 116]. Although ATF6 is essential for regulating ER stress in retinal photoreceptors, the mechanisms behind ATF6-associated achromatopsia and its preference for central cone photoreceptor degeneration remains unclear. Future therapeutic interventions for achromatopsia, or any other AT6-associated disease conditions, must take into account that modulating ATF6 activation in cones may have catastrophic consequences for color vision. Thus, strategies targeting individual cell types (e.g. through specific viral variants) or specific regions (e.g. outer retina) should be considered over broad or systemic treatments.
Glaucoma
Glaucoma is a leading cause of irreversible blindness characterized by progressive degeneration of RGCs and their axons resulting in a loss of visual field and central vision, if left untreated. In 2013, approximately 64.3 million people aged 40–80 years worldwide were affected by primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG) and the numbers were estimated to increase to 76.0 million in 2020 and 111.8 million in 2040 [129]. Vision loss in glaucoma often starts from the periphery and progresses without noticeable symptoms in patients until late stages. Thus, RGCs undergo a prolonged course of degeneration after the disease onset, which provides a valuable window for intervention upon a timely diagnosis. Glaucoma is multifactorial disease. Among many identified risk factors, elevated intraocular pressure (IOP) is the most predominant, and the only modifiable factor causing RGC degeneration. An increase in the IOP occurs as a result of a buildup of aqueous humor due to reduced drainage of aqueous fluid caused by a stiff and less permeable trabecular meshwork (TM) and increased outflow resistance at the TM [130, 131]. In addition to increased stiffness of the TM, there is also morphological and biochemical changes including extracellular deposits within the cribriform layer of the TM [132]. Current clinical treatment for glaucoma focuses on pharmacological, laser, or surgical therapies to lower IOP, either by increasing aqueous humor drainage or decreasing its production [133]. Experimentally, multiple mouse models have been developed to recapitulate increased IOP using a variety of techniques including intracameral injection of microbeads, laser photocoagulation, episcleral vein cauterization, and injection of hypertonic saline and hyaluronic acid [134]. Increased IOP leads to loss of RGCs and their axons and optic-disc cupping, suggesting a causal role of high IOP in glaucomatous RGC damage and neuropathy [134].
Genetic factors play an important role in the pathogenesis of glaucoma. Recent studies have identified multiple genomic loci and genetic variants that contribute to glaucoma development [135–137]. Paired Box Gene 6 (PAX6) is a transcription factor that regulates development of the eye and its dysregulation or mutation can lead to aniridia (a complete or partial absence of the iris) and congenital glaucoma [136, 138]. CAV1/CAV2 are genes that encode caveolin-1 and caveolin-2 proteins, respectively, which can bind to cholesterol and are therefore important in maintaining membrane homeostasis and cholesterol metabolism, as well as regulating TM outflow [135, 139]. GAS7 encodes growth arrest-specific protein 7, which plays a pivotal role in cell division and neuronal development [135, 137, 140]. These findings not only provide insights into the molecular mechanisms of glaucoma but also present an opportunity for developing genetic screening for early diagnosis and potentially for gene therapy or overexpression of functional proteins in RGCs.
Cell stress signaling in TM cell damage and increased IOP
Multiple studies have shown that dysregulation of the UPR pathways in TM cells are involved in the development of glaucoma. It is important to understand the mechanisms that lead to ER stress in TM cells in order to prevent the subsequent damage. Mutations in the MYOC gene, which encodes myocilin protein, have been linked to increased IOP in juvenile open-angle glaucoma (JOAG) and adult-onset POAG [141]. Mechanistically, mutations of myocilin cause protein misfolding resulting in accumulation of misfolded myocilin proteins in the ER and increased ER stress in TM cells [142, 143]. Treatment with phenylbutyric acid (PBA), a chemical chaperone that promotes protein folding and alleviates protein aggregation thus reducing ER stress, successfully prevents TM cell death and lowers IOP in glaucoma models associated with MYOC mutations [142]. In addition, mutant myocilin proteins interact with components of the extracellular matrix (ECM), including fibronectin, elastin, and collagen type IV and I, resulting in aberrant accumulation of ECM proteins in the ER and dysregulation of the ECM, which contributes to reduced outflow of aqueous humor and increased IOP in some glaucoma cases [144]. Inhibition of PERK by GSK2606414 reduces cell survival, while activation of this pathway by salubrinal, which inhibits elF2α dephosphorylation, increases cell survival, suggesting a protective effect of PERK activation in stressed TM cells [145]. Interestingly, in another study, inhibition of PERK by LDN-0060609 was shown to reduce DNA damage, improve cell survival and restore cell function in human TM cells [146]. The paradoxical results from the two studies may be in part attributable to the specific pharmacological inhibitors or stress conditions; further investigation of these compounds and which downstream pathways they affect is essential for the development of therapies that incorporate them.
ATF4 is a major downstream effector in the PERK pathway and studying this component of the pathway can help to better understand the conflicting evidence previously discussed on PERK. In this mechanism, elF2α phosphorylation increases ATF4 protein production while reducing global protein translation. As a transcription factor, ATF4 binds to the promotor of the aquaporin 1 (AQP1) gene and negatively regulates its transcription in TM cells [146, 147]. Reduced expression of AQP1 is believed to be responsible for increased resistance to aqueous humor outflow that leads to elevated IOP in glaucoma associated with increased endothelin-1 (ET-1) level in aqueous humor [147]. In addition, activation of the elF2α/ATF4/CHOP pathway increases apoptosis and inflammation in human TM cells, in part through promoting ER stress-induced apoptosis, increasing ROS production, upregulating inflammatory genes such as endothelial-leukocyte adhesion molecule 1 and Interleukin 8 [148]. Activation of ATF4 also results in increased protein synthesis that increases the ER protein load, thereby exacerbating ER stress in TM cells [149]. Importantly, increased ATF4 and CHOP expression have been observed in TM from patients with POAG, suggesting that the activation of ATF4/CHOP pathway is implicated in TM cell injury and IOP increase in human glaucoma [148–150]. In addition to primary glaucoma, elevated ER stress in TM cells has been implicated in dexamethasone-induced ocular hypertension, which resembles glucocorticoid-induced glaucoma in human patients [151]. Relative to the ATF4/CHOP pathway, the implication of the IRE1/XBP1 and ATF6 UPR branches in ER stress-associated TM cell dysfunction and cell death are less well studied (Fig. 2). Future studies are warranted to investigate whether targeting these understudied UPR pathways may lead to new avenues for reducing TM injury and inflammation in glaucoma models.
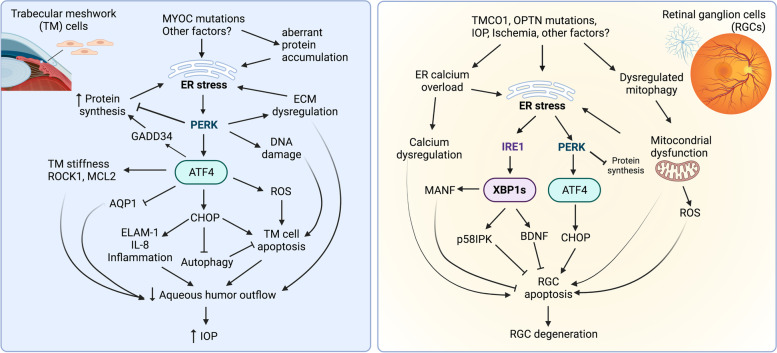
Fig. 2.
Implication of the UPR pathways in the pathogenesis of glaucoma. Left panel: Mutations of MYOC gene and other factors induces ER stress resulting in activation of the PERK/ATF4/CHOP pathway. Activation of this pathway leads to increased reactive oxygen species (ROS), inflammation, and DNA damage, promoting TM cell apoptosis. In addition, ATF4 increases TM stiffness contributing to reduced outflow of aqueous humor and increased IOP. Right panel: ER stress is induced by multiple factors, including mutations of genes such as TMCO1 and OPTN, increased IOP, ischemia, and others, in retinal ganglion cells (RGCs) during glaucoma. Activation of XBP1 increases expressions of ER chaperones and neurotrophic factors, such as brain derived neurotrophic factor (BDNF), p58IPK, and mesencephalic astrocyte-derived neurotrophic factor (MANF), reducing apoptosis of RGCs. Activation of ATF4/CHOP, mitochondrial dysfunction, and calcium dyshomeostasis, contribute to RGC cell death and degeneration
Cellular stress signaling in RGC damage
Like in TM cells, ER stress plays a pivotal role of in RGC cell death associated with glaucoma [152–155]. Some examples are RGC injuries caused by genetic variants of transmembrane and coiled-coil domain 1 (TMCO1) and optineurin (OPTN). TMCO1 encodes a transmembrane protein of the ER and functions as a calcium leak channel to prevent calcium overload and maintain calcium homeostasis in the ER [156]. TMCO1 is expressed ubiquitously in the body with high expression in RGCs and a genetic variant was recently identified as a risk factor for POAG [157, 158]. Deficiency or dysfunction of TMCO1 induces calcium overload in the ER, which in turn causes disturbance in protein synthesis and folding resulting in ER stress. Dysregulation of calcium signaling also increases ROS generation, over-activates mitophagy resulting in mitochondrial damage and impaired respiratory function, and promotes apoptosis [157, 159, 160]. OPTN encodes a protein that functions as a primary receptor of mitophagy and multiple mutations of OPTN protein have been identified associated with POAG [161]. Among these mutations, E50K is considered the most prevalent and is associated with normal-tension glaucoma, a subtype of POAG [162]. Overexpression of E50K mutant optineurin induces mitochondrial fission and enhanced mitochondrial degradation and mitophagy resulting in RGC degeneration [162]. Dysregulation of mitochondrial fission and mitophagy increases oxidative stress, which further intensifies mitochondrial dysfunction and damage resulting in a vicious cycle ultimately contributing to RGC cell death [163]. Another glaucoma-associated mutation of OPTN, 691_692insAG (or 2bpIns-OPTN), was shown to increase ER stress and upregulate CHOP expression resulting in cell death [164]. Future studies should investigate whether inhibition of ER stress prevents RGC degeneration induced by OPTN mutations in animal models of glaucoma.
AMPK is an energy sensor and a master regulator of cellular metabolism and mitochondrial dynamics [34]. However, the role of AMPK in regulation of energy homeostasis and mitochondrial function in RGCs and glaucoma appears to be less thoroughly investigated. A recent study demonstrates that AMPK is activated in RGCs in an ocular hypertension mouse model and in human glaucomatous retina tissue from patients with POAG [69]. Sustained activation of AMPK triggers RGC dysfunction and leads to RGC dendritic retraction and synapse elimination through inhibiting mammalian target of rapamycin complex 1 (mTORC1). Furthermore, when AMPK is depleted, RGC survival and retinal function is improved. These results suggest that chronic AMPK activation contributes to RGC cell death perhaps by inhibiting the energy consuming processes such as synaptic transmission and axon transport [69]. This finding is in apparent contrast to the protective role of AMPK in AMD (as described above) in which activation of AMPK mitigates photoreceptor and RPE degeneration. These discrepancies highlight the importance in understanding the signaling pathways in each specific type of neurons, which may possess unique mechanisms to combat different stresses and disease conditions.
In addition to metabolic disturbance, ER stress has been observed in RGCs in several animal models of glaucoma, including microbeads-induced ocular hypertension model, optic nerve crush model, and DBA/2 J (D2) mouse model [165–167]. Activation of the UPR pathways appears to play differential roles in glaucomatous RGC damage. Activation of the IRE1/XBP1 pathway protects RGCs from ER stress-induced damage in part through increasing expression of brain derived neurotrophic factor (BDNF); conversely, activation of the PERK-eIF2α-CHOP pathway can trigger RGC apoptosis [167, 168]. Combining the two approaches of over-expression of XBP1 and inhibition of eIF2α phosphorylation has been shown to not only protect RGC survival but also protect against axon degeneration and improve visual function in mouse models of traumatic optic nerve injury and microbeads-induced ocular hypertension [166]. However, in DBA/2 J mice deletion of CHOP results in modest protection to the RGC soma but does not protect against RGC axonal degeneration [165]. This could suggest that additional downstream effectors in the PERK/eIF2α pathway could be involved in RGC injury related to glaucoma.
Recent work demonstrates a potential role of an ER-resident chaperone p58IPK in RGC survival in glaucomatous conditions [169–171]. p58IPK is a multifunctional protein that acts as a co-chaperone of GRP78 in the process of protein folding and also plays a role in regulation of eIF2α phosphorylation, and thereby protein production, by inhibiting eIF2α kinases including double-stranded RNA-dependent protein kinase R [172–176], PERK [177, 178], and GCN2 (general control nonderepressible 2) [179]. p58IPK is highly expressed in the neural retina and its expression is upregulated under ER stress conditions [169]. Deletion of p58IPK results in fewer RGCs, accompanied by increased levels of CHOP and Bax (Bcl-2 Associated X-protein) in the retina of p58IPK knockout (KO) mice, and moreover, the p58IPK KOs are highly susceptible to ischemia-induced RGC loss compared to the wild-type animals. Conversely, overexpression of p58IPK attenuates oxidative stress and ER stress-induced apoptosis of cultured neural cells, suggesting a protective role of p58IPK in retinal neurons [169]. Overexpressing p58IPK using AAV protects against ER stress-induced cell death in cultured primary RGCs from both WT and p58IPK knockout mice [171]. In addition to p58IPK, recent studies identified mesencephalic astrocyte-derived neurotrophic factor (MANF) as an ER-localized neurotrophic factor, which inhibits ER stress-induced cell death of retinal neurons and improves RGC survival in a rat glaucoma model [171]. Therefore, enhancing the function of ER chaperones like p58IPK and MANF to restore protein homeostasis may offer exciting therapeutic potential for glaucomatous RGC degeneration (Fig. 2).
Diabetic retinopathy
Diabetic retinopathy (DR) is a major complication of diabetes characterized by progressive neurovascular injury and degeneration in the retina and is the most frequent cause of blindness in working-age adults. According to clinical manifestations, DR is classified into two large categories: non-proliferative DR (NPDR) and proliferative DR (PDR), representing the early and advanced stages of the disease, respectively. Major pathological characterization of NPDR includes retinal hemorrhages, microaneurysms, microvascular abnormalities, while PDR is distinguished by the development of retinal neovascularization (NV) due to aberrant blood vessel growth from the retina into the vitreous [6, 180, 181]. The fragile and malstructured blood vessels of retinal NV are prone to leakage and rupture, resulting in severe vitreous hemorrhage, fibrosis, tractional retinal detachment, and vision loss [180–182]. Leakage of injured retinal blood vessels and disruption of the BRB can also occur at early stages of DR, resulting in exudates and fluid accumulation in retinal tissue and thickening of the retina, known as diabetic macular edema (DME). DME is the most frequent cause of central vision loss in diabetic patients. In addition to vascular lesions, recent work recognizes the importance of diabetes-induced neural retina dysfunction and neurodegeneration in DR, although effective treatment for protection of retinal neurons and prevention of vision loss in DR is not yet available [183–187]. Currently, clinical managements for DR focus primarily on reducing vascular pathologies using a combination of anti-VEGF therapy, laser photocoagulation, and surgical treatment [188]. In many patients, in particular those with advanced DR, successful treatment in correcting vascular abnormalities and restoring the anatomical structure of the retina does not result in significant visual improvement [189]. New approaches to protect retinal cells and improve retinal function are urgently needed.
A number of molecular pathways and cellular processes, such as oxidative stress, ER stress, and inflammation, have been proposed in DR pathogenesis. Oxidative stress is considered a primary cause of retinal vascular damage in diabetes [190]. Major pathways contributing to ROS generation in diabetic retinal cells include activation of polyol and hexosamine biosynthetic pathways, advanced glycation end product (AGEs) production, protein kinase C (PKC) activation, mitochondrial dysfunction, and NADPH (nicotinamide adenine dinucleotide phosphate) oxidase activation [181, 191]. In addition, defects in the anti-oxidant defenses that scavenge free radicals and reduce oxidative stress also contribute to oxidative damage in the diabetic retina [192]. Studies have shown that during diabetes the DNA binding ability of Nrf2 is significantly reduced in retinal cells, and in contrast, the binding between Nrf2 and its inhibitor, Kelch like-ECH-associated protein 1 (Keap1) is increased resulting in enhanced Nrf2 degradation and decreased Nrf2 translocation to the nucleus [193, 194]. Inhibition of Keap1-Nrf2 interaction by small molecules to promote Nrf2 nuclear translocation and transcription activation of anti-oxidant defense genes alleviates oxidative stress, protects retinal cells from ischemic and inflammatory injury, and mitigates diabetic vascular damage [193, 195]. These findings suggest that targeting the anti-oxidant defense system and enhancing the cellular response to dampen oxidative stress and minimize oxidative damage of retinal cells could be a promising strategy for prevention and treatment of early-stage DR.
In addition to oxidative stress, ER stress has been shown to play a significant role in diabetes-associated retinal inflammation, endothelial cell injury, vascular leakage and vascular degeneration (Fig. 3) [196–202]. Recent studies also highlight the importance of the UPR signaling in maintaining retinal neuronal function and preventing neurodegeneration in diabetic conditions [203, 204]. Activation of the IRE1/XBP1 and PERK/ATF4/CHOP pathways differentially regulate retinal endothelial cell death, inflammation, and vascular permeability in animal models of diabetes [196, 199, 200, 205–207]. Preconditioning with mild ER stress activates XBP1-dependent UPR pathways, reducing retinal endothelial inflammation and vascular leakage [197]. Conversely, loss of XBP1 induces Müller glia activation and promotes retinal inflammation in DR [208]. Moreover, cells deficient of XBP1 are susceptible to oxidative stress-induced apoptosis and cell death and tight junction damage [74, 76, 79, 80]. These findings imply a vital role of XBP1 in maintaining cellular function and integrity in diabetic retinas. Conditional knockout of XBP1 in retinal neurons leads to early onset retinal function decline, neuronal loss, and enhanced Müller glia activation in diabetic mice [203], suggesting that the XBP1 pathway is critical for neuronal protection against diabetes induced retinal injury and dysfunction.
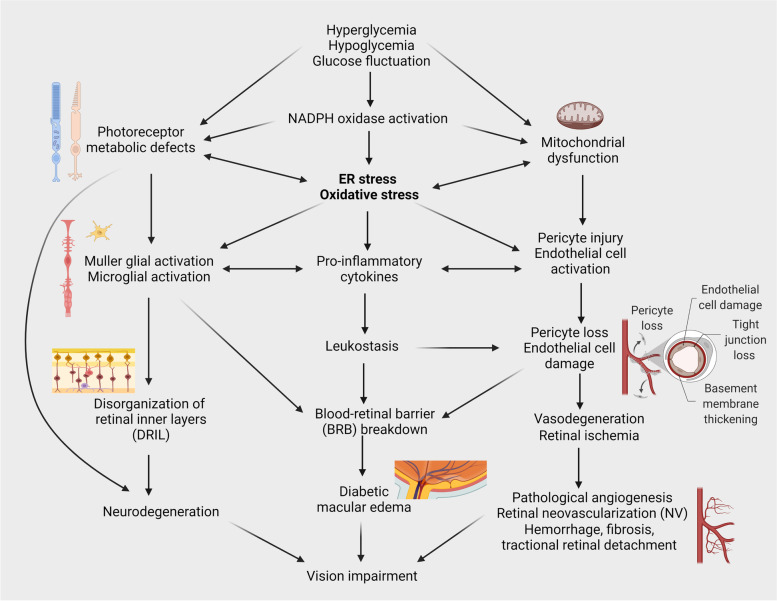
Fig. 3.
Role of ER stress and oxidative stress in retinal neurovascular damage in diabetic retinopathy (DR). Disturbance in glucose supply (hyperglycemia, hypoglycemia, glucose fluctuation, etc.) leads to metabolic defects, mitochondrial dysfunction, NADPH oxidase activation, resulting in increased ER stress and oxidative stress. Enhanced ER stress and oxidative stress play a central role in inducing vascular endothelial cell and pericyte damage, blood-retinal barrier (BRB) breakdown, glial activation, inflammation and diabetic macular edema. Cumulative loss of vascular cells and capillaries resulting in retinal ischemia, which ultimately leads to retinal neovascularization and neurodegeneration contributing to vision impairment in DR
The retina has high metabolic demands to support its function in generating and transmitting visual signals and maintain the normal structure of photoreceptors. In diabetes, retinal metabolism is disrupted due to elevated glucose levels, correlated with enhanced glycolysis and sorbitol oxidation, which has been implicated in the pathogenesis of DR [209–211]. Diverting upstream metabolites from glycolysis into other pathways, such as the hexosamine, diacylglycerol (DAG)/PKC, and AGE pathways, leads to endothelial injury in diabetes [212]. Systemic reduction of GLUT1 or deletion of GLUT1 in retinal neurons prevents polyol accumulation and improves retinal function in diabetic animals, suggesting a role of metabolic dysregulation in neurodegeneration in DR [209]. In addition, mitochondrial dysfunction and damage leads to reduced mitochondrial respiratory activity further contributing to the imbalance between glycolysis and oxidative phosphorylation in diabetic retinal cells [reviewed in [213]. As a major cellular stress response, the UPR has been shown to play an important role in regulation of glucose metabolism in retinal cells [18, 214]. The IRE1 branch functions as a nutrition sensor in cells under starvation and induces activation of XBP1 to restore energy homeostasis [215]. In glioma cells, silencing XBP1 suppresses hexokinase-2 (HK2) therefore inhibiting glycolysis and resulting in cell death [216]. Conditional knockout of XBP1 in retinal cells also leads to reduced glycolysis associated with retinal dysfunction and neurodegeneration [18], suggesting a role of XBP1 in regulation of retinal neuronal glycolysis. The exact function of XBP1 and other UPR pathways in regulation of retinal metabolism during diabetes remains to be elucidated.
Conclusions
The long-term and constant requirement for the retina to maintain protein and metabolic homeostasis is critical for preserving normal visual function and preventing retinal neurodegeneration throughout the lifetime. Studies over the past two decades have laid a groundwork for understanding how elements of the UPR respond to various stressors during aging and in common retinal disease conditions including AMD, RP, glaucoma, and DR in humans and in animal models. The findings reported so far clearly suggest that activation of the UPR signaling has a significant impact on retinal cell survival and function, not only through governing the homeostasis of protein production, modification, trafficking, and degradation, but also via regulation of cell metabolism, mitochondrial function, and calcium levels. Although the interactions between the UPR pathways, as well as their involvement in metabolic regulation, can vary in different cell types and are not necessarily consistent between disease conditions, the work described in this review provides hope that targeting the UPR pathways may lead to new therapeutic approaches for protecting retinal cells at the early stages of neurodegenerative disease.
As discussed earlier, aging is a significant risk factor for major neurodegenerative diseases in the retina, as it is for Alzheimer’s disease, Parkinson’s disease, and many others in the CNS. Aberrant protein aggregation and deposition, along with enhanced protein and lipid oxidation, correlate with chronic ER stress and oxidative stress in aging retinal tissue [18, 30, 217, 218]. While the disruption of proteostasis can be attributable to declined ability to activate the protective UPR pathways in aged cells [18], the mechanisms behind the dysfunction of the UPR during aging remain poorly understood. Several factors have been proposed to potentially mediate the failure of sensing ER stress and activation of the UPR, including disturbed redox balance in the ER, dysregulated calcium homeostasis, and increased nitrosylation of ER stress sensors and ER chaperones or foldases [219]. Whether targeting these factors could restore the function of the UPR in aging and diseased retinal cells warrants future investigation.
Another interesting question is how the UPR pathways interact and reciprocally regulate metabolic signaling pathways in retinal cells. The role of the UPR in metabolic diseases including obesity and diabetes has been extensively investigated. In addition to restoring the ER and protein homeostasis thereby improving cell survival and function, the UPR genes have also been shown to independently regulate pathways in glucose and lipid metabolism. Furthermore, multiple UPR molecules directly and indirectly regulate critical genes responsible for anti-oxidant defense and mitochondrial function. Yet the exact mechanisms by which the UPR signaling is implicated in metabolic regulation in response to stressors in each disease condition and in various retinal cell types are largely unknown. Understanding the interactions between these signaling pathways in coordinating cellular stress responses to maintain and improve the capacity for metabolic regulation and protein homeostasis could provide valuable insight for therapeutic intervention.
It is important to recognize that the retina is capable of dealing with significant cellular stress on a daily basis, often for decades, without significant functional decline or neurodegeneration even under disease conditions. The concept that an additional cause, such as compromised nutrient sensing due to advanced age or the breakdown of the BRB, is required for cellular stress response pathways to be overwhelmed thereby leading to functional decline and neurodegeneration is particularly intriguing. Recent development of new technologies, such as single cell multi-omics that enable multiple, and even simultaneous, genetic, transcriptomic, epigenetic, and proteomic analyses from individual cells using tissue sections [220], could generate precise information on the temporal and spatial changes of each signaling molecule in the UPR pathways in the retina during aging and under disease conditions. Furthermore, the emerging new experimental systems, including stem cell-derived human organoids and humanized animal models, demonstrate remarkable advantage in studying human retinal development and diseases [221]. Last but not least, the successful discovery of small molecules and pharmacological compounds targeting selective UPR signaling (reviewed in [108]) provides valuable tools for better understanding the implication of individual UPR pathways in disease progression and opens new avenues for developing drug treatments for retinal protection against neurodegeneration.
Acknowledgements
Not applicable.
Abbreviations
- AAV
Adeno-associated virus
- AGE
Advanced glycation end product
- AMD
Age-related macular degeneration
- AMPKα1
AMP activated protein kinase, alpha 1
- AMPKα2
AMP activated protein kinase, alpha 2
- APP
Amyloid precursor protein
- AQP1
Aquaporin 1
- ARMS2
Age-related maculopathy susceptibility 2
- ATF4
Activating transcription factor 4
- ATF6
Activating transcription factor 6
- Bax
Bcl-2 Associated X-protein
- BDNF
Brain derived neurotrophic factor
- BiP
Immunoglobulin binding protein
- BRB
Blood-retinal barrier
- CAV1
Caveolin-1
- CAV2
Caveolin-2
- CHOP
C/EBP homologous protein
- CFH
Complement factor H
- cKO
Conditional knockout
- CNS
Central nervous system
- CNV
Choroidal neovascularization
- DHA
di-docosahexaenoic acid
- DME
Diabetic macular edema
- DR
Diabetic retinopathy
- ECM
Extracellular matrix
- eIF2α
Eukaryotic translation initiation factor-2α
- Emc3
ER membrane protein complex 3
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- ERG
Electroretinogram
- ET-1
Endothelin-1
- GA
Geographic atrophy
- GAS7
Growth arrest-specific protein 7
- GCN2
General control nonderepressible 2
- GLUT1
Glucose transporter 1
- GRP78
Glucose-regulated protein 78
- HK2
Hexokinase-2
- HRD1
HMG-CoA reductase degradation protein 1
- IOP
Intraocular pressure
- IRE1
Inositol requiring enzyme 1
- ISR
Integrated stress response
- JOAG
Juvenile open-angle glaucoma
- Keap1
Kelch like-ECH-associated protein 1
- Lkb1
Liver kinase B1
- MANF
Mesencephalic astrocyte-derived neurotrophic factor
- MNV
Macular neovascularization
- mTOR
Mammalian target of rapamycin
- mTORC1
mTOR complex 1
- MYOC
Myocilin
- NADPH
Nicotinamide adenine dinucleotide phosphate
- nAMD
Neovascular AMD
- NPDR
Non-proliferative DR
- Nrf2
NF-E2-related factor 2
- NV
Neovascularization
- OCT
Optical coherence tomography
- ONL
Outer nuclear layer
- OPTN
Optineurin
- OS
Outer segments
- p58IPK
58 kDa inhibitor protein kinase
- PACG
Primary angle-closure glaucoma
- PAX6
Paired Box Gene 6
- PBA
Phenylbutyric acid
- PDR
Proliferative DR
- PERK
PKR-like endoplasmic reticulum kinase
- PKC
Protein kinase C
- POAG
Primary open-angle glaucoma
- POMC
Pro-opio-melanocortin
- POS
Photoreceptor outer segments
- RGCs
Retinal ganglion cells
- RHO
Rhodopsin gene
- RIDD
Regulated IRE1-dependent mRNA decay
- ROS
Reactive oxygen species
- RP
Retinitis pigmentosa
- RPE
Retinal pigment epithelium
- TM
Trabecular meshwork
- TMCO1
Transmembrane and coiled-coil domain 1
- UPR
Unfolded protein response
- VEGF
Vascular endothelial growth factor
- WT
Wild type
- XBP1
X-Box Binding Protein 1
- XBP1s
Spliced XBP1
Authors’ contributions
TMc, JJW, and SXZ conceived the review. TMc, AM, JP, MY, JJW, and SXZ performed literature search, wrote and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported, in part, by NIH/NEI Grants EY019949, EY025061, EY030970 (to SXZ), a research grant NGR G2019302 from the Brightfocus Foundation (to SXZ), and an Unrestricted Grant from Research to Prevent Blindness to the Department of Ophthalmology, the State University of New York at Buffalo.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Todd McLaughlin, Email: toddmcla@buffalo.edu.
Andy Medina, Email: andymedi@buffalo.edu.
Jacob Perkins, Email: jmp29@buffalo.edu.
Maria Yera, Email: mariayer@buffalo.edu.
Joshua J. Wang, Email: jianxinw@buffalo.edu
Sarah X. Zhang, Email: xzhang38@buffalo.edu
References
- 1.Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2:538–549. doi: 10.1016/j.stem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.VandenBosch LS, Reh TA. Epigenetics in neuronal regeneration. Semin Cell Dev Biol. 2020;97:63–73. doi: 10.1016/j.semcdb.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang SX, Sanders E, Fliesler SJ, Wang JJ. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp Eye Res. 2014;125C:30–40. doi: 10.1016/j.exer.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurley JB, Lindsay KJ, Du J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J Neurosci Res. 2015;93:1079–1092. doi: 10.1002/jnr.23583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2012;287:1642–1648. doi: 10.1074/jbc.R111.304428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2:e93751. [DOI] [PMC free article] [PubMed]
- 7.Zhang SX, Ma JH, Bhatta M, Fliesler SJ, Wang JJ. The unfolded protein response in retinal vascular diseases: implications and therapeutic potential beyond protein folding. Prog Retin Eye Res. 2015;45C:111–131. doi: 10.1016/j.preteyeres.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroeger H, Chiang WC, Felden J, Nguyen A, Lin JH. ER stress and unfolded protein response in ocular health and disease. FEBS J. 2019;286:399–412. doi: 10.1111/febs.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghemrawi R, Khair M. Endoplasmic reticulum stress and unfolded protein response in neurodegenerative diseases. Int J Mol Sci. 2020;21:6127. [DOI] [PMC free article] [PubMed]
- 10.Chou CF, Frances Cotch M, Vitale S, Zhang X, Klein R, Friedman DS, Klein BEK, Saaddine JB. Age-related eye diseases and visual impairment among U.S. adults. Am J Prev Med. 2013;45:29–35. doi: 10.1016/j.amepre.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luu J, Palczewski K. Human aging and disease: lessons from age-related macular degeneration. Proc Natl Acad Sci. 2018;115:2866–2872. doi: 10.1073/pnas.1721033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langrová H, Zrenner E, Kurtenbach A, Seeliger MW. Age-related changes in retinal functional topography. Invest Ophthalmol Vis Sci. 2008;49:5024–5032. doi: 10.1167/iovs.07-1309. [DOI] [PubMed] [Google Scholar]
- 13.Ryoo NK, Ahn SJ, Park KH, Ahn J, Seo J, Han JW, Kim KW, Woo SJ. Thickness of retina and choroid in the elderly population and its association with complement factor H polymorphism: KLoSHA eye study. Plos One. 2018;13:e0209276. doi: 10.1371/journal.pone.0209276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdinest N, London N, Lavy I, Morad Y, Levinger N. Vision through healthy aging eyes. Vision (Basel, Switzerland). 2021;5:46. [DOI] [PMC free article] [PubMed]
- 15.Ferdous S, Liao KL, Gefke ID, Summers VR, Wu W, Donaldson KJ, Kim Y-K, Sellers JT, Dixon JA, Shelton DA, et al. Age-related retinal changes in wild-type C57BL/6J mice between 2 and 32 months. Invest Ophthalmol Vis Sci. 2021;62:9–9. doi: 10.1167/iovs.62.7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Retinal photoreceptor density decreases with age. Ophthalmology. 1995;102:1853–1859. doi: 10.1016/s0161-6420(95)30784-1. [DOI] [PubMed] [Google Scholar]
- 17.Samuel MA, Zhang Y, Meister M, Sanes JR. Age-related alterations in neurons of the mouse retina. J Neurosci. 2011;31:16033–16044. doi: 10.1523/JNEUROSCI.3580-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin T, Falkowski M, Park JW, Keegan S, Elliott M, Wang JJ, Zhang SX. Loss of XBP1 accelerates age-related decline in retinal function and neurodegeneration. Mol Neurodegener. 2018;13:16. doi: 10.1186/s13024-018-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel MA, Voinescu PE, Lilley BN, de Cabo R, Foretz M, Viollet B, Pawlyk B, Sandberg MA, Vavvas DG, Sanes JR. LKB1 and AMPK regulate synaptic remodeling in old age. Nat Neurosci. 2014;17:1190–1197. doi: 10.1038/nn.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugita Y, Yamamoto H, Maeda Y, Furukawa T. Influence of aging on the retina and visual motion processing for optokinetic responses in mice. Front Neurosci. 2020;14:586013. doi: 10.3389/fnins.2020.586013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Qi X, Yang Y, Tian W, Liu W, Jiang Z, Li S, Zhu X. Loss of the ER membrane protein complex subunit Emc3 leads to retinal bipolar cell degeneration in aged mice. Plos One. 2020;15:e0238435. doi: 10.1371/journal.pone.0238435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan RKP, WoldeMussie E, Pow DV. Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Invest Ophthalmol Vis Sci. 2007;48:2782–2791. doi: 10.1167/iovs.06-1283. [DOI] [PubMed] [Google Scholar]
- 23.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang HZ, Yang LM. Activation of the unfolded protein response in aged human lenses. Mol Med Rep. 2015;12:389–393. doi: 10.3892/mmr.2015.3417. [DOI] [PubMed] [Google Scholar]
- 25.Naidoo N, Zhu J, Zhu Y, Fenik P, Lian J, Galante R, Veasey S. Endoplasmic reticulum stress in wake-active neurons progresses with aging. Aging Cell. 2011;10:640–649. doi: 10.1111/j.1474-9726.2011.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammadnejad A, Li W, Lund JB, Li S, Larsen MJ, Mengel-From J, et al. Global gene expression profiling and transcription factor network analysis of cognitive aging in monozygotic twins. Front Genet. 2021;12:675587. [DOI] [PMC free article] [PubMed]
- 27.Naidoo N, Davis JG, Zhu J, Yabumoto M, Singletary K, Brown M, Galante R, Agarwal B, Baur JA. Aging and sleep deprivation induce the unfolded protein response in the pancreas: implications for metabolism. Aging Cell. 2014;13:131–141. doi: 10.1111/acel.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda N, Kume S, Tanaka Y, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Haneda M, Koya D, et al. Altered unfolded protein response is implicated in the age-related exacerbation of proteinuria-induced proximal tubular cell damage. Am J Pathol. 2013;183:774–785. doi: 10.1016/j.ajpath.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Lenox AR, Bhootada Y, Gorbatyuk O, Fullard R, Gorbatyuk M. Unfolded protein response is activated in aged retinas. Neurosci Lett. 2015;609:30–35. doi: 10.1016/j.neulet.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leger F, Fernagut PO, Canron MH, Léoni S, Vital C, Tison F, Bezard E, Vital A. Protein aggregation in the aging retina. J Neuropathol Exp Neurol. 2011;70:63–68. doi: 10.1097/NEN.0b013e31820376cc. [DOI] [PubMed] [Google Scholar]
- 31.Martínez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell. 2017;16:615–623. doi: 10.1111/acel.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estébanez B, de Paz JA, Cuevas MJ, González-Gallego J. Endoplasmic reticulum unfolded protein response, aging and exercise: an update. Front Physiol. 2018;9:1744. doi: 10.3389/fphys.2018.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comitato A, Schiroli D, Montanari M, Marigo V. Calpain activation is the major cause of cell death in photoreceptors expressing a rhodopsin Misfolding mutation. Mol Neurobiol. 2020;57:589–599. doi: 10.1007/s12035-019-01723-5. [DOI] [PubMed] [Google Scholar]
- 34.Shirwany NA, Zou MH. AMPK: a cellular metabolic and redox sensor. A minireview. Front Biosci (Landmark edition) 2014;19:447–474. doi: 10.2741/4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 37.Age-Related Macular Degeneration (AMD) Data and Statistics. https://www.nei.nih.gov/learn-about-eye-health/outreach-campaigns-and-resources/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics.
- 38.Wei Q, Hu W, Lou Q, Yu J. NAD+ inhibits the metabolic reprogramming of RPE cells in early AMD by upregulating mitophagy. Discov Med. 2019;27:189–196. [PubMed] [Google Scholar]
- 39.Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7:31. doi: 10.1038/s41572-021-00265-2. [DOI] [PubMed] [Google Scholar]
- 40.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Li M, Messinger JD, Ferrara D, Curcio CA, Freund KB. Recognizing atrophy and mixed-type neovascularization in age-related macular degeneration via Clinicopathologic correlation. Transl Vis Sci Technol. 2020;9:8. doi: 10.1167/tvst.9.8.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, Waheed NK, Chakravarthy U, Rosenfeld PJ, Holz FG, et al. Consensus nomenclature for reporting Neovascular age-related macular degeneration data: consensus on Neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–636. doi: 10.1016/j.ophtha.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Hadziahmetovic M, Malek G. Age-related macular degeneration revisited: From pathology and cellular stress to potential therapies. Front Cell Dev Biol. 2020;8:612812. doi: 10.3389/fcell.2020.612812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Age-Related Eye Disease Study Research G The Age-Related Eye Disease Study: a clinical trial of zinc and antioxidants--Age-Related Eye Disease Study Report No. 2. J Nutr. 2000;130:1516S–1519S. doi: 10.1093/jn/130.5.1516S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armstrong RA, Mousavi M. Overview of risk factors for age-related macular degeneration (AMD) J Stem Cells. 2015;10:171–191. [PubMed] [Google Scholar]
- 46.Levine ES, Custo Greig E, Mendonca LSM, Gulati S, Despotovic IN, Alibhai AY, Moult E, Muakkassa N, Quaranta-El Maftouhi M, El Maftouhi A, et al. The long-term effects of anti-vascular endothelial growth factor therapy on the optical coherence tomography angiographic appearance of neovascularization in age-related macular degeneration. Int J Retina Vitreous. 2020;6:39. doi: 10.1186/s40942-020-00242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan Y, Jiang S, Gericke A. Age-related macular degeneration: role of oxidative stress and blood vessels. Int J Mol Sci. 2021;22:1296. [DOI] [PMC free article] [PubMed]
- 48.Fields MA, Del Priore LV, Adelman RA, Rizzolo LJ. Interactions of the choroid, Bruch's membrane, retinal pigment epithelium, and neurosensory retina collaborate to form the outer blood-retinal-barrier. Prog Retin Eye Res. 2020;76:100803. doi: 10.1016/j.preteyeres.2019.100803. [DOI] [PubMed] [Google Scholar]
- 49.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 50.Cheng SY, Cipi J, Ma S, Hafler BP, Kanadia RN, Brush RS, Agbaga MP, Punzo C. Altered photoreceptor metabolism in mouse causes late stage age-related macular degeneration-like pathologies. Proc Natl Acad Sci U S A. 2020;117:13094–13104. doi: 10.1073/pnas.2000339117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher CR, Ferrington DA. Perspective on AMD pathobiology: a bioenergetic crisis in the RPE. Invest Ophthalmol Vis Sci. 2018;59:AMD41–AMD47. doi: 10.1167/iovs.18-24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viegas FO, Neuhauss SCF. A metabolic landscape for maintaining retina integrity and function. Front Mol Neurosci. 2021;14:656000. doi: 10.3389/fnmol.2021.656000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swarup A, Samuels IS, Bell BA, Han JYS, Du J, Massenzio E, Abel ED, Boesze-Battaglia K, Peachey NS, Philp NJ. Modulating GLUT1 expression in retinal pigment epithelium decreases glucose levels in the retina: impact on photoreceptors and Müller glial cells. Am J Physiol Cell Physiol. 2019;316:C121–c133. doi: 10.1152/ajpcell.00410.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanow MA, Giarmarco MM, Jankowski CS, Tsantilas K, Engel AL, Du J, et al. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife. 2017;6:e28899. [DOI] [PMC free article] [PubMed]
- 55.Bird A. Role of retinal pigment epithelium in age-related macular disease: a systematic review. Br J Ophthalmol. 2021;105:1469-74. [DOI] [PubMed]
- 56.Bonilha VL, Rayborn ME, Bhattacharya SK, Gu X, Crabb JS, Crabb JW, Hollyfield JG. Retinal Degenerative Diseases. 2006. The retinal pigment epithelium apical microvilli and retinal function; pp. 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allingham MJ, Loksztejn A, Cousins SW, Mettu PS. Immunological aspects of age-related macular degeneration. Adv Exp Med Biol. 2021;1256:143–189. doi: 10.1007/978-3-030-66014-7_6. [DOI] [PubMed] [Google Scholar]
- 58.Abokyi S, To C-H, Lam TT, Tse DY. Central role of oxidative stress in age-related macular degeneration: evidence from a review of the molecular mechanisms and animal models. Oxidative Med Cell Longev. 2020;2020:7901270. [DOI] [PMC free article] [PubMed]
- 59.Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of β amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Isas JM, Luibl V, Johnson LV, Kayed R, Wetzel R, Glabe CG, Langen R, Chen J. Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci. 2010;51:1304–1310. doi: 10.1167/iovs.09-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer's a beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis. 2003;9:184–190. [PubMed] [Google Scholar]
- 64.Meng L-H, Chen Y-X. Lipid accumulation and protein modifications of Bruch's membrane in age-related macular degeneration. Int J Ophthalmol. 2021;14:766–773. doi: 10.18240/ijo.2021.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blasiak J, Pawlowska E, Sobczuk A, Szczepanska J, Kaarniranta K. The aging stress response and its implication for AMD pathogenesis. Int J Mol Sci. 2020;21:8840. [DOI] [PMC free article] [PubMed]
- 66.Jeon S-M. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L, Kong L, Wang J, Ash JD. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2018;115:10475–10480. doi: 10.1073/pnas.1802724115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu L, Brown EE, Keuthan CJ, Gubbi H, Grellier E-K, Roger J, et al. AMP-activated-protein kinase (AMPK) is an essential sensor and metabolic regulator of retinal neurons and their integrated metabolism with RPE. bioRxiv. 2020:2020.2005.2022.109165.
- 69.Belforte N, Agostinone J, Alarcon-Martinez L, Villafranca-Baughman D, Dotigny F, Cueva Vargas JL, Di Polo A. AMPK hyperactivation promotes dendrite retraction, synaptic loss, and neuronal dysfunction in glaucoma. Mol Neurodegener. 2021;16:43. doi: 10.1186/s13024-021-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen C, Zhong Y, Wang JJ, Yu Q, Plafker K, Plafker S, Zhang SX. Regulation of Nrf2 by X box-binding protein 1 in retinal pigment epithelium. Front Genet. 2018;9:658. doi: 10.3389/fgene.2018.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW. Cigarette smoke–related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006;47:729–737. doi: 10.1167/iovs.05-0719. [DOI] [PubMed] [Google Scholar]
- 72.Fujihara M, Nagai N, Sussan TE, Biswal S, Handa JT. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. Plos One. 2008;3:e3119. doi: 10.1371/journal.pone.0003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cano M, Wang L, Wan J, Barnett BP, Ebrahimi K, Qian J, Handa JT. Oxidative stress induces mitochondrial dysfunction and a protective unfolded protein response in RPE cells. Free Radic Biol Med. 2014;69:1–14. doi: 10.1016/j.freeradbiomed.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C, Cano M, Wang JJ, Li J, Huang C, Yu Q, Herbert TP, Handa JT, Zhang SX. Role of unfolded protein response dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid Redox Signal. 2014;20:2091–2106. doi: 10.1089/ars.2013.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kunchithapautham K, Atkinson C, Rohrer B. Smoke exposure causes endoplasmic reticulum stress and lipid accumulation in retinal pigment epithelium through oxidative stress and complement activation. J Biol Chem. 2014;289:14534–14546. doi: 10.1074/jbc.M114.564674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang C, Wang JJ, Ma JH, Jin C, Yu Q, Zhang SX. Activation of the UPR protects against cigarette smoke-induced RPE apoptosis through up-regulation of Nrf2. J Biol Chem. 2015;290:5367-80. [DOI] [PMC free article] [PubMed]
- 77.Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu DM, Ji X, Ivanchenko MV, Chung M, Piper M, Rana P, et al. Nrf2 overexpression rescues the RPE in mouse models of retinitis pigmentosa. JCI insight. 2021;6:e145029. [DOI] [PMC free article] [PubMed]
- 79.Zhong Y, Li J, Wang JJ, Chen C, Tran J-TA, Saadi A, Yu Q, Le Y-Z, MNA M, Anderson RE, Zhang SX. X-box binding protein 1 is essential for the anti-oxidant defense and cell survival in the retinal pigment epithelium. Plos One. 2012;7:e38616. doi: 10.1371/journal.pone.0038616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma JH, Wang JJ, Li J, Pfeffer BA, Zhong Y, Zhang SX. The role of IRE-XBP1 pathway in regulation of retinal pigment epithelium tight JunctionsXBP1 regulates the RPE tight junctions. Invest Ophthalmol Vis Sci. 2016;57:5244–5252. doi: 10.1167/iovs.16-19232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nashine S, Bhootada Y, Lewin AS, Gorbatyuk M. Ablation of C/EBP homologous protein does not protect T17M RHO mice from retinal degeneration. Plos One. 2013;8:e63205. doi: 10.1371/journal.pone.0063205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H, Liu B, Lian L, Zhou J, Xiang S, Zhai Y, Chen Y, Ma X, Wu W, Hou L. High dose expression of heme oxigenase-1 induces retinal degeneration through ER stress-related DDIT3. Mol Neurodegener. 2021;16:16. doi: 10.1186/s13024-021-00437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das S, Smith TD, Sarma JD, Ritzenthaler JD, Maza J, Kaplan BE, Cunningham LA, Suaud L, Hubbard MJ, Rubenstein RC, Koval M. ERp29 restricts Connexin43 oligomerization in the endoplasmic reticulum. Mol Biol Cell. 2009;20:2593–2604. doi: 10.1091/mbc.E08-07-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLaughlin T, Falkowski M, Wang JJ, Zhang SX. Molecular chaperone ERp29: a potential target for cellular protection in retinal and neurodegenerative diseases. Adv Exp Med Biol. 2018;1074:421–427. doi: 10.1007/978-3-319-75402-4_52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirsch I, Weiwad M, Prell E, Ferrari DM. ERp29 deficiency affects sensitivity to apoptosis via impairment of the ATF6-CHOP pathway of stress response. Apoptosis. 2014;19:801–815. doi: 10.1007/s10495-013-0961-0. [DOI] [PubMed] [Google Scholar]
- 86.Huang C, Wang JJ, Jing G, Li J, Jin C, Yu Q, Falkowski MW, Zhang SX. Erp29 attenuates cigarette smoke extract-induced endoplasmic reticulum stress and mitigates tight junction damage in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:6196–6207. doi: 10.1167/iovs.15-16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 88.Rivolta C, Sharon D, DeAngelis MM, Dryja TP. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet. 2002;11:1219–1227. doi: 10.1093/hmg/11.10.1219. [DOI] [PubMed] [Google Scholar]
- 89.Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, Wittes J, Pappas J, Elci O, McCague S, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suda K, Filipek S, Palczewski K, Engel A, Fotiadis D. The supramolecular structure of the GPCR rhodopsin in solution and native disc membranes. Mol Membr Biol. 2004;21:435–446. doi: 10.1080/09687860400020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dryja TP, McGee TL, Hahn LB, Cowley GS, Olsson JE, Reichel E, Sandberg MA, Berson EL. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990;323:1302–1307. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- 92.Daiger SP, Sullivan LS, Bowne SJ. Genes and mutations causing retinitis pigmentosa. Clin Genet. 2013;84:132–141. doi: 10.1111/cge.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Athanasiou D, Aguila M, Bellingham J, Li W, McCulley C, Reeves PJ, Cheetham ME. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog Retin Eye Res. 2018;62:1–23. doi: 10.1016/j.preteyeres.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Y, Xu CL, Velez G, Yang J, Tanaka AJ, Breazzano MP, Mahajan VB, Sparrow JR, Tsang SH. Novel REEP6 gene mutation associated with autosomal recessive retinitis pigmentosa. Doc Ophthalmol. 2020;140:67–75. doi: 10.1007/s10633-019-09719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arno G, Agrawal SA, Eblimit A, Bellingham J, Xu M, Wang F, Chakarova C, Parfitt DA, Lane A, Burgoyne T, et al. Mutations in REEP6 cause autosomal-recessive retinitis Pigmentosa. Am J Hum Genet. 2016;99:1305–1315. doi: 10.1016/j.ajhg.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agrawal SA, Burgoyne T, Eblimit A, Bellingham J, Parfitt DA, Lane A, Nichols R, Asomugha C, Hayes MJ, Munro PM, et al. REEP6 deficiency leads to retinal degeneration through disruption of ER homeostasis and protein trafficking. Hum Mol Genet. 2017;26:2667–2677. doi: 10.1093/hmg/ddx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiang WC, Kroeger H, Sakami S, Messah C, Yasumura D, Matthes MT, Coppinger JA, Palczewski K, LaVail MM, Lin JH. Robust endoplasmic reticulum-associated degradation of rhodopsin precedes retinal degeneration. Mol Neurobiol. 2015;52:679–695. doi: 10.1007/s12035-014-8881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiang WC, Hiramatsu N, Messah C, Kroeger H, Lin JH. Selective activation of ATF6 and PERK endoplasmic reticulum stress signaling pathways prevent mutant rhodopsin accumulation. Invest Ophthalmol Vis Sci. 2012;53:7159–7166. doi: 10.1167/iovs.12-10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hosokawa N, Wada I, Hasegawa K, Yorihuzi T, Tremblay LO, Herscovics A, Nagata K. A novel ER alpha-mannosidase-like protein accelerates ER-associated degradation. EMBO Rep. 2001;2:415–422. doi: 10.1093/embo-reports/kve084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hosokawa N, Tremblay LO, You Z, Herscovics A, Wada I, Nagata K. Enhancement of endoplasmic reticulum (ER) degradation of misfolded null Hong Kong alpha1-antitrypsin by human ER mannosidase I. J Biol Chem. 2003;278:26287–26294. doi: 10.1074/jbc.M303395200. [DOI] [PubMed] [Google Scholar]
- 102.Kosmaoglou M, Kanuga N, Aguila M, Garriga P, Cheetham ME. A dual role for EDEM1 in the processing of rod opsin. J Cell Sci. 2009;122:4465–4472. doi: 10.1242/jcs.055228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mendes HF, Cheetham ME. Pharmacological manipulation of gain-of-function and dominant-negative mechanisms in rhodopsin retinitis pigmentosa. Hum Mol Genet. 2008;17:3043–3054. doi: 10.1093/hmg/ddn202. [DOI] [PubMed] [Google Scholar]
- 104.Bhootada Y, Kotla P, Zolotukhin S, Gorbatyuk O, Bebok Z, Athar M, Gorbatyuk M. Limited ATF4 expression in degenerating retinas with ongoing ER stress promotes photoreceptor survival in a mouse model of autosomal dominant retinitis Pigmentosa. Plos One. 2016;11:e0154779. doi: 10.1371/journal.pone.0154779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Athanasiou D, Aguila M, Bellingham J, Kanuga N, Adamson P, Cheetham ME. The role of the ER stress-response protein PERK in rhodopsin retinitis pigmentosa. Hum Mol Genet. 2017;26:4896–4905. doi: 10.1093/hmg/ddx370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adekeye A, Haeri M, Solessio E, Knox BE. Ablation of the proapoptotic genes CHOP or Ask1 does not prevent or delay loss of visual function in a P23H transgenic mouse model of retinitis pigmentosa. Plos One. 2014;9:e83871. doi: 10.1371/journal.pone.0083871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grandjean JMD, Wiseman RL. Small molecule strategies to harness the unfolded protein response: where do we go from here? J Biol Chem. 2020;295:15692–15711. doi: 10.1074/jbc.REV120.010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Athanasiou D, Aguila M, Opefi CA, South K, Bellingham J, Bevilacqua D, Munro PM, Kanuga N, Mackenzie FE, Dubis AM, et al. Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration. Hum Mol Genet. 2017;26:305–319. doi: 10.1093/hmg/ddw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Athanasiou D, Bevilacqua D, Aguila M, McCulley C, Kanuga N, Iwawaki T, Chapple JP, Cheetham ME. The co-chaperone and reductase ERdj5 facilitates rod opsin biogenesis and quality control. Hum Mol Genet. 2014;23:6594–6606. doi: 10.1093/hmg/ddu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Athanasiou D, Kosmaoglou M, Kanuga N, Novoselov SS, Paton AW, Paton JC, Chapple JP, Cheetham ME. BiP prevents rod opsin aggregation. Mol Biol Cell. 2012;23:3522–3531. doi: 10.1091/mbc.E12-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee EJ, Chan P, Chea L, Kim K, Kaufman RJ, Lin JH. ATF6 is required for efficient rhodopsin clearance and retinal homeostasis in the P23H rho retinitis pigmentosa mouse model. Sci Rep. 2021;11:16356. doi: 10.1038/s41598-021-95895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, Lin JH, Muzyczka N, Lewin AS. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci U S A. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zarouchlioti C, Parfitt DA, Li W, Gittings LM, Cheetham ME. DNAJ proteins in neurodegeneration: essential and protective factors. Philos Trans R Soc Lond Ser B Biol Sci. 2018;373:20160534. [DOI] [PMC free article] [PubMed]
- 116.Aguila M, Bellingham J, Athanasiou D, Bevilacqua D, Duran Y, Maswood R, Parfitt DA, Iwawaki T, Spyrou G, Smith AJ, et al. AAV-mediated ERdj5 overexpression protects against P23H rhodopsin toxicity. Hum Mol Genet. 2020;29:1310–1318. doi: 10.1093/hmg/ddaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kohl S, Zobor D, Chiang WC, Weisschuh N, Staller J, Gonzalez Menendez I, Chang S, Beck SC, Garcia Garrido M, Sothilingam V, et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat Genet. 2015;47:757–765. doi: 10.1038/ng.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aboshiha J, Dubis AM, Carroll J, Hardcastle AJ, Michaelides M. The cone dysfunction syndromes. Br J Ophthalmol. 2016;100:115–121. doi: 10.1136/bjophthalmol-2014-306505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Michaelides M, Hunt DM, Moore AT. The cone dysfunction syndromes. Br J Ophthalmol. 2004;88:291–297. doi: 10.1136/bjo.2003.027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kroeger H, Grandjean JMD, Chiang WJ, Bindels DD, Mastey R, Okalova J, et al. ATF6 is essential for human cone photoreceptor development. Proc Natl Acad Sci U S A. 2021;118:e2103196118. [DOI] [PMC free article] [PubMed]
- 121.Mastey RR, Georgiou M, Langlo CS, Kalitzeos A, Patterson EJ, Kane T, Singh N, Vincent A, Moore AT, Tsang SH, et al. Characterization of retinal structure in ATF6-associated Achromatopsia. Invest Ophthalmol Vis Sci. 2019;60:2631–2640. doi: 10.1167/iovs.19-27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ansar M, Santos-Cortez RL, Saqib MA, Zulfiqar F, Lee K, Ashraf NM, Ullah E, Wang X, Sajid S, Khan FS, et al. Mutation of ATF6 causes autosomal recessive achromatopsia. Hum Genet. 2015;134:941–950. doi: 10.1007/s00439-015-1571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu M, Gelowani V, Eblimit A, Wang F, Young MP, Sawyer BL, Zhao L, Jenkins G, Creel DJ, Wang K, et al. ATF6 is mutated in early onset photoreceptor degeneration with macular involvement. Invest Ophthalmol Vis Sci. 2015;56:3889–3895. doi: 10.1167/iovs.15-16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sundaram V, Wilde C, Aboshiha J, Cowing J, Han C, Langlo CS, Chana R, Davidson AE, Sergouniotis PI, Bainbridge JW, et al. Retinal structure and function in achromatopsia: implications for gene therapy. Ophthalmology. 2014;121:234–245. doi: 10.1016/j.ophtha.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Michalakis S, Schon C, Becirovic E, Biel M. Gene therapy for achromatopsia. J Gene Med. 2017;19. 10.1002/jgm.2944. [DOI] [PubMed]
- 126.Chiang W-C, Chan P, Wissinger B, Vincent A, Skorczyk-Werner A, Krawczyński MR, Kaufman RJ, Tsang SH, Héon E, Kohl S, Lin JH. Achromatopsia mutations target sequential steps of ATF6 activation. Proc Natl Acad Sci. 2017;114:400–405. doi: 10.1073/pnas.1606387114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mendez AS, Alfaro J, Morales-Soto MA, Dar AC, McCullagh E, Gotthardt K, et al. Endoplasmic reticulum stress-independent activation of unfolded protein response kinases by a small molecule ATP-mimic. Elife. 2015;4:e05434. [DOI] [PMC free article] [PubMed]
- 128.Plate L, Cooley CB, Chen JJ, Paxman RJ, Gallagher CM, Madoux F, et al. Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. Elife. 2016;5:e15550. [DOI] [PMC free article] [PubMed]
- 129.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 130.Wang K, Li G, Read AT, Navarro I, Mitra AK, Stamer WD, Sulchek T, Ethier CR. The relationship between outflow resistance and trabecular meshwork stiffness in mice. Sci Rep. 2018;8:5848. doi: 10.1038/s41598-018-24165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stamer WD, Clark AF. The many faces of the trabecular meshwork cell. Exp Eye Res. 2017;158:112–123. doi: 10.1016/j.exer.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rohen JW. Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical considerations. Ophthalmology. 1983;90:758–765. doi: 10.1016/s0161-6420(83)34492-4. [DOI] [PubMed] [Google Scholar]
- 133.Schuster AK, Erb C, Hoffmann EM, Dietlein T, Pfeiffer N. The diagnosis and treatment of Glaucoma. Dtsch Arztebl Int. 2020;117:225–234. doi: 10.3238/arztebl.2020.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Biswas S, Wan KH. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019;97:e331–e340. doi: 10.1111/aos.13983. [DOI] [PubMed] [Google Scholar]
- 135.Wiggs JL, Pasquale LR. Genetics of glaucoma. Hum Mol Genet. 2017;26:R21–R27. doi: 10.1093/hmg/ddx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Y, Allingham RR, Qin X, Layfield D, Dellinger AE, Gibson J, Wheeler J, Ashley-Koch AE, Stamer WD, Hauser MA. Gene expression profile in human trabecular meshwork from patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2013;54:6382–6389. doi: 10.1167/iovs.13-12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rozpedek-Kaminska W, Wojtczak R, Szaflik JP, Szaflik J, Majsterek I. The genetic and endoplasmic reticulum-mediated molecular mechanisms of primary open-angle Glaucoma. Int J Mol Sci. 2020;21:4171. [DOI] [PMC free article] [PubMed]
- 138.Lima Cunha D, Arno G, Corton M, Moosajee M. The Spectrum of PAX6 mutations and genotype-phenotype correlations in the eye. Genes. 2019;10:1050. [DOI] [PMC free article] [PubMed]
- 139.Sohn J, Lin H, Fritch MR, Tuan RS. Influence of cholesterol/caveolin-1/caveolae homeostasis on membrane properties and substrate adhesion characteristics of adult human mesenchymal stem cells. Stem Cell Res Ther. 2018;9:86. doi: 10.1186/s13287-018-0830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zukerman R, Harris A, Vercellin AV, Siesky B, Pasquale LR, Ciulla TA. Molecular genetics of Glaucoma: subtype and ethnicity considerations. Genes. 2020;12:55. [DOI] [PMC free article] [PubMed]
- 141.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 144.Kasetti RB, Phan TN, Millar JC, Zode GS. Expression of mutant Myocilin induces abnormal intracellular accumulation of selected extracellular matrix proteins in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2016;57:6058–6069. doi: 10.1167/iovs.16-19610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Y, Osakue D, Yang E, Zhou Y, Gong H, Xia X, Du Y. Endoplasmic reticulum stress response of trabecular meshwork stem cells and trabecular meshwork cells and protective effects of activated PERK pathway. Invest Ophthalmol Vis Sci. 2019;60:265–273. doi: 10.1167/iovs.18-25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rozpedek-Kaminska W, Galita G, Siwecka N, Carroll SL, Diehl JA, Kucharska E, et al. The potential role of small-molecule PERK inhibitor LDN-0060609 in primary open-angle Glaucoma treatment. Int J Mol Sci. 2021;22:4494. [DOI] [PMC free article] [PubMed]
- 147.Zhao Y, Zhu H, Yang Y, Ye Y, Yao Y, Huang X, Zhang Y, Shu X, Chen X, Yang Y, et al. AQP1 suppression by ATF4 triggers trabecular meshwork tissue remodelling in ET-1-induced POAG. J Cell Mol Med. 2020;24:3469–3480. doi: 10.1111/jcmm.15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ying Y, Xue R, Yang Y, Zhang SX, Xiao H, Zhu H, Li J, Chen G, Ye Y, Yu M, et al. Activation of ATF4 triggers trabecular meshwork cell dysfunction and apoptosis in POAG. Aging. 2021;13:8628–8642. doi: 10.18632/aging.202677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kasetti RB, Patel PD, Maddineni P, Patil S, Kiehlbauch C, Millar JC, Searby CC, Raghunathan V, Sheffield VC, Zode GS. ATF4 leads to glaucoma by promoting protein synthesis and ER client protein load. Nat Commun. 2020;11:5594. doi: 10.1038/s41467-020-19352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peters JC, Bhattacharya S, Clark AF, Zode GS. Increased endoplasmic reticulum stress in human glaucomatous trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2015;56:3860–3868. doi: 10.1167/iovs.14-16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124:1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Inokuchi Y, Nakajima Y, Shimazawa M, Kurita T, Kubo M, Saito A, Sajiki H, Kudo T, Aihara M, Imaizumi K, et al. Effect of an inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Invest Ophthalmol Vis Sci. 2009;50:334–344. doi: 10.1167/iovs.08-2123. [DOI] [PubMed] [Google Scholar]
- 153.Ito Y, Shimazawa M, Akao Y, Nakajima Y, Seki N, Nozawa Y, Hara H. Lig-8, a bioactive lignophenol derivative from bamboo lignin, protects against neuronal damage in vitro and in vivo. J Pharmacol Sci. 2006;102:196–204. doi: 10.1254/jphs.fp0060711. [DOI] [PubMed] [Google Scholar]
- 154.Shimazawa M, Inokuchi Y, Ito Y, Murata H, Aihara M, Miura M, Araie M, Hara H. Involvement of ER stress in retinal cell death. Mol Vis. 2007;13:578–587. [PMC free article] [PubMed] [Google Scholar]
- 155.Tsuruma K, Shimazaki H, Nakashima K, Yamauchi M, Sugitani S, Shimazawa M, Iinuma M, Hara H. Annatto prevents retinal degeneration induced by endoplasmic reticulum stress in vitro and in vivo. Mol Nutr Food Res. 2012;56:713–724. doi: 10.1002/mnfr.201100607. [DOI] [PubMed] [Google Scholar]
- 156.Li J, Liu C, Li Y, Zheng Q, Xu Y, Liu B, Sun W, Li Y, Ji S, Liu M, et al. TMCO1-mediated Ca2+ leak underlies osteoblast functions via CaMKII signaling. Nat Commun. 2019;10:1589. doi: 10.1038/s41467-019-09653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Batchelor-Regan H, Xin B, Zhou A, Wang H. From disease description and gene discovery to functional cell pathway: a decade-long journey for TMCO1. Front Genet. 2021;12:652400. doi: 10.3389/fgene.2021.652400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, Danoy P, Casson R, Viswanathan AC, Liu JZ, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43:574–578. doi: 10.1038/ng.824. [DOI] [PubMed] [Google Scholar]
- 159.Sun Z, Zhang H, Wang X, Wang QC, Zhang C, Wang JQ, Wang YH, An CQ, Yang KY, Wang Y, et al. TMCO1 is essential for ovarian follicle development by regulating ER ca (2+) store of granulosa cells. Cell Death Differ. 2018;25:1686–1701. doi: 10.1038/s41418-018-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X, Wang QC, Sun Z, Li T, Yang K, An C, Guo C, Tang TS. ER stress mediated degradation of diacylglycerol acyltransferase impairs mitochondrial functions in TMCO1 deficient cells. Biochem Biophys Res Commun. 2019;512:914–920. doi: 10.1016/j.bbrc.2019.03.115. [DOI] [PubMed] [Google Scholar]
- 161.Chernyshova K, Inoue K, Yamashita S-I, Fukuchi T, Kanki T. Glaucoma-associated mutations in the Optineurin gene have limited impact on Parkin-dependent Mitophagy. Invest Ophthalmol Vis Sci. 2019;60:3625–3635. doi: 10.1167/iovs.19-27184. [DOI] [PubMed] [Google Scholar]
- 162.Shim MS, Takihara Y, Kim KY, Iwata T, Yue BY, Inatani M, Weinreb RN, Perkins GA, Ju WK. Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration. Sci Rep. 2016;6:33830. doi: 10.1038/srep33830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim KY, Perkins GA, Shim MS, Bushong E, Alcasid N, Ju S, Ellisman MH, Weinreb RN, Ju WK. DRP1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 2015;6:e1839. doi: 10.1038/cddis.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Medchalmi S, Tare P, Sayyad Z, Swarup G. A glaucoma- and ALS-associated mutant of OPTN induces neuronal cell death dependent on Tbk1 activity, autophagy and ER stress. FEBS J. 2021;288:4576–4595. doi: 10.1111/febs.15752. [DOI] [PubMed] [Google Scholar]
- 165.Marola OJ, Syc-Mazurek SB, Libby RT. DDIT3 (CHOP) contributes to retinal ganglion cell somal loss but not axonal degeneration in DBA/2J mice. Cell Death Discov. 2019;5:140. doi: 10.1038/s41420-019-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang L, Li S, Miao L, Huang H, Liang F, Teng X, Xu L, Wang Q, Xiao W, Ridder WH, 3rd, et al. Rescue of Glaucomatous Neurodegeneration by differentially modulating neuronal endoplasmic reticulum stress molecules. J Neurosci. 2016;36:5891–5903. doi: 10.1523/JNEUROSCI.3709-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hu Y, Park KK, Yang L, Wei X, Yang Q, Cho KS, Thielen P, Lee AH, Cartoni R, Glimcher LH, et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73:445–452. doi: 10.1016/j.neuron.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang H, Miao L, Liang F, Liu X, Xu L, Teng X, Wang Q, Ridder WH, 3rd, Shindler KS, Sun Y, Hu Y. Neuroprotection by eIF2alpha-CHOP inhibition and XBP-1 activation in EAE/optic neuritiss. Cell Death Dis. 2017;8:e2936. doi: 10.1038/cddis.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boriushkin E, Wang JJ, Li J, Jing G, Seigel GM, Zhang SX. Identification of p58IPK as a Novel Neuroprotective Factor for Retinal Neurons. Invest Ophthalmol Vis Sci. 2015;56:1374-86. [DOI] [PMC free article] [PubMed]
- 170.McLaughlin T, Zhang SX. The neuroprotective potential of endoplasmic reticulum chaperones. Neural Regen Res. 2015;10:1211–1213. doi: 10.4103/1673-5374.162696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McLaughlin T, Dhimal N, Li J, Wang JJ, Zhang SX. p58IPK is an endogenous Neuroprotectant for retinal ganglion cells. Front Aging Neurosci. 2018;10:267. [DOI] [PMC free article] [PubMed]
- 172.Lee TG, Tang N, Thompson S, Miller J, Katze MG. The 58,000-Dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol Cell Biol. 1994;14:2331–2342. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee TG, Tomita J, Hovanessian AG, Katze MG. Purification and partial characterization of a cellular inhibitor of the interferon-induced protein kinase of Mr 68,000 from influenza virus-infected cells. Proc Natl Acad Sci U S A. 1990;87:6208–6212. doi: 10.1073/pnas.87.16.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee TG, Tomita J, Hovanessian AG, Katze MG. Characterization and regulation of the 58,000-Dalton cellular inhibitor of the interferon-induced, dsRNA-activated protein kinase. J Biol Chem. 1992;267:14238–14243. [PubMed] [Google Scholar]
- 175.Melville MW, Hansen WJ, Freeman BC, Welch WJ, Katze MG. The molecular chaperone hsp40 regulates the activity of P58IPK, the cellular inhibitor of PKR. Proc Natl Acad Sci U S A. 1997;94:97–102. doi: 10.1073/pnas.94.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Polyak SJ, Tang N, Wambach M, Barber GN, Katze MG. The P58 cellular inhibitor complexes with the interferon-induced, double-stranded RNA-dependent protein kinase, PKR, to regulate its autophosphorylation and activity. J Biol Chem. 1996;271:1702–1707. doi: 10.1074/jbc.271.3.1702. [DOI] [PubMed] [Google Scholar]
- 177.van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J Biol Chem. 2003;278:15558–15564. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- 178.Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci U S A. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Roobol A, Roobol J, Bastide A, Knight JR, Willis AE, Smales CM. p58IPK is an inhibitor of the eIF2alpha kinase GCN2 and its localization and expression underpin protein synthesis and ER processing capacity. Biochem J. 2015;465:213–225. doi: 10.1042/BJ20140852. [DOI] [PubMed] [Google Scholar]
- 180.Antonetti DA, Silva PS, Stitt AW. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 2021;17:195–206. doi: 10.1038/s41574-020-00451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi: 10.1038/nrdp.2016.12. [DOI] [PubMed] [Google Scholar]
- 183.Reis A, Mateus C, Melo P, Figueira J, Cunha-Vaz J, Castelo-Branco M. Pure neuroretinal dysfunction in diabetic retinopathy occurring prior to endothelial and vascular damage. Acta Ophthalmol. 2013;91:0. [Google Scholar]
- 184.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Simo R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902–1912. doi: 10.1007/s00125-018-4692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee VK, Hosking BM, Holeniewska J, Kubala EC, Lundh von Leithner P, Gardner PJ, Foxton RH, Shima DT. BTBR Ob/Ob mouse model of type 2 diabetes exhibits early loss of retinal function and retinal inflammation followed by late vascular changes. Diabetologia. 2018;61:2422–2432. doi: 10.1007/s00125-018-4696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Joltikov KA, Sesi CA, de Castro VM, Davila JR, Anand R, Khan SM, Farbman N, Jackson GR, Johnson CA, Gardner TW. Disorganization of retinal inner layers (DRIL) and Neuroretinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:5481–5486. doi: 10.1167/iovs.18-24955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Diabetic retinopathy preferred practice pattern®. Ophthalmology. 2020;127:P66–p145. doi: 10.1016/j.ophtha.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 189.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1816. [DOI] [PMC free article] [PubMed]
- 190.Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for human health. Oxidative Med Cell Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kowluru RA. Mitochondrial stability in diabetic retinopathy: lessons learned from epigenetics. Diabetes. 2019;68:241–247. doi: 10.2337/dbi18-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kowluru RA, Mishra M. Epigenetic regulation of redox signaling in diabetic retinopathy: role of Nrf2. Free Radic Biol Med. 2017;103:155–164. doi: 10.1016/j.freeradbiomed.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Deliyanti D, Alrashdi SF, Tan SM, Meyer C, Ward KW, de Haan JB, Wilkinson-Berka JL. Nrf2 activation is a potential therapeutic approach to attenuate diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:815–825. doi: 10.1167/iovs.17-22920. [DOI] [PubMed] [Google Scholar]
- 194.Zhong Q, Mishra M, Kowluru RA. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:3941–3948. doi: 10.1167/iovs.13-11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hui Q, Karlstetter M, Xu Z, Yang J, Zhou L, Eilken HM, Terjung C, Cho H, Gong J, Lai MJ, et al. Inhibition of the Keap1-Nrf2 protein-protein interaction protects retinal cells and ameliorates retinal ischemia-reperfusion injury. Free Radic Biol Med. 2020;146:181–188. doi: 10.1016/j.freeradbiomed.2019.10.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583:1521–1527. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li J, Wang JJ, Zhang SX. Preconditioning with endoplasmic reticulum stress mitigates retinal endothelial inflammation via activation of X-box binding protein 1. J Biol Chem. 2011;286:4912–4921. doi: 10.1074/jbc.M110.199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li B, Wang HS, Li GG, Zhao MJ, Zhao MH. The role of endoplasmic reticulum stress in the early stage of diabetic retinopathy. Acta Diabetol. 2011;48:103–111. doi: 10.1007/s00592-009-0170-z. [DOI] [PubMed] [Google Scholar]
- 199.Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX. Activation of endoplasmic reticulum stress by hyperglycemia is essential for muller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012;61:492–504. doi: 10.2337/db11-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen Y, Wang JJ, Li J, Hosoya KI, Ratan R, Townes T, Zhang SX. Activating transcription factor 4 mediates hyperglycaemia-induced endothelial inflammation and retinal vascular leakage through activation of STAT3 in a mouse model of type 1 diabetes. Diabetologia. 2012;55:2533–2545. doi: 10.1007/s00125-012-2594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bhatta M, Ma J, Wang J, Sakowski J, Zhang S. Enhanced endoplasmic reticulum stress in bone marrow angiogenic progenitor cells in a mouse model of long-term experimental type 2 diabetes. Diabetologia. 2015;58:2181–2190. doi: 10.1007/s00125-015-3643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bhatta M, Chatpar K, Hu Z, Wang JJ, Zhang SX. Reduction of endoplasmic reticulum stress improves Angiogenic progenitor cell function in a mouse model of type 1 diabetes. Cell Death Dis. 2018;9:467. doi: 10.1038/s41419-018-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McLaughlin T, Siddiqi M, Wang JJ, Zhang SX. Loss of XBP1 leads to early-onset retinal neurodegeneration in a mouse model of type I diabetes. J Clin Med. 2019;8:906. [DOI] [PMC free article] [PubMed]
- 204.Sundstrom JM, Hernandez C, Weber SR, Zhao Y, Dunklebarger M, Tiberti N, Laremore T, Simo-Servat O, Garcia-Ramirez M, Barber AJ, et al. Proteomic analysis of early diabetic retinopathy reveals mediators of neurodegenerative brain diseases. Invest Ophthalmol Vis Sci. 2018;59:2264–2274. doi: 10.1167/iovs.17-23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ma JH, Wang JJ, Zhang SX. The unfolded protein response and diabetic retinopathy. J Diabetes Res. 2014;2014:14. doi: 10.1155/2014/160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhong Y, Wang JJ, Zhang SX. Intermittent but not constant high glucose induces ER stress and inflammation in human retinal pericytes. Adv Exp Med Biol. 2012;723:285–292. doi: 10.1007/978-1-4614-0631-0_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huang H, Jing G, Wang J, Sheibani N, Zhang S. ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J Inflamm. 2015;12:31. doi: 10.1186/s12950-015-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang J, Chen C, McLaughlin T, Wang Y, Le YZ, Wang JJ, Zhang SX. Loss of X-box binding protein 1 in Muller cells augments retinal inflammation in a mouse model of diabetes. Diabetologia. 2019;62:531–543. doi: 10.1007/s00125-018-4776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Holoman NC, Aiello JJ, Trobenter TD, Tarchick MJ, Kozlowski MR, Makowski ER, De Vivo DC, Singh C, Sears JE, Samuels IS. Reduction of Glut1 in the neural retina but not the RPE alleviates polyol accumulation and normalizes early characteristics of diabetic retinopathy. J Neurosci. 2021;41:3275–3299. doi: 10.1523/JNEUROSCI.2010-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Van den Enden MK, Nyengaard JR, Ostrow E, Burgan JH, Williamson JR. Elevated glucose levels increase retinal glycolysis and sorbitol pathway metabolism. Implications for diabetic retinopathy. Invest Ophthalmol Vis Sci. 1995;36:1675–1685. [PubMed] [Google Scholar]
- 211.Ido Y, Nyengaard JR, Chang K, Tilton RG, Kilo C, Mylari BL, Oates PJ, Williamson JR. Early neural and vascular dysfunctions in diabetic rats are largely sequelae of increased sorbitol oxidation. Antioxid Redox Signal. 2010;12:39–51. doi: 10.1089/ars.2009.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 213.Yumnamcha T, Guerra M, Singh LP, Ibrahim AS. Metabolic dysregulation and neurovascular dysfunction in diabetic retinopathy. Antioxidants (Basel). 2020;9:1244. [DOI] [PMC free article] [PubMed]
- 214.Kelly K, Wang JJ, Zhang SX. The unfolded protein response signaling and retinal Müller cell metabolism. Neural Regen Res. 2018;13:1861–1870. doi: 10.4103/1673-5374.239431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yao T, Deng Z, Gao Y, Sun J, Kong X, Huang Y, He Z, Xu Y, Chang Y, Yu KJ, et al. Ire1α in Pomc neurons is required for thermogenesis and Glycemia. Diabetes. 2017;66:663–673. doi: 10.2337/db16-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu Y, Hou X, Liu M, Yang Z, Bi Y, Zou H, Wu J, Che H, Li C, Wang X, et al. XBP1 silencing decreases glioma cell viability and glycolysis possibly by inhibiting HK2 expression. J Neuro-Oncol. 2016;126:455–462. doi: 10.1007/s11060-015-2003-y. [DOI] [PubMed] [Google Scholar]
- 217.Pinazo-Durán MD, Gallego-Pinazo R, García-Medina JJ, Zanón-Moreno V, Nucci C, Dolz-Marco R, Martínez-Castillo S, Galbis-Estrada C, Marco-Ramírez C, López-Gálvez MI, et al. Oxidative stress and its downstream signaling in aging eyes. Clin Interv Aging. 2014;9:637–652. doi: 10.2147/CIA.S52662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Long P, He M, Yan W, Chen W, Wei D, Wang S, Zhang Z, Ge W, Chen T. ALDH2 protects naturally aged mouse retina <i>via</i> inhibiting oxidative stress-related apoptosis and enhancing unfolded protein response in endoplasmic reticulum. Aging. 2021;13:2750–2767. doi: 10.18632/aging.202325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bhattarai KR, Chaudhary M, Kim HR, Chae HJ. Endoplasmic reticulum (ER) stress response failure in diseases. Trends Cell Biol. 2020;30:672–675. doi: 10.1016/j.tcb.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 220.Eisenstein M. The secret life of cells. Nat Methods. 2020;17:7–10. doi: 10.1038/s41592-019-0698-y. [DOI] [PubMed] [Google Scholar]
- 221.Kim J, Koo B-K, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.