Abstract
Background
Corynebacterium striatum is a gram-positive facultative anaerobe found in the environment and human flora that has historically been considered a contaminant. More recently, Corynebacterium striatum has been implicated in human infections, including respiratory infections, endocarditis, and bone and joint infections, particularly those involving hardware or implanted devices.
Case presentation
A 65-year-old man presented for washout of his left total knee arthroplasty following a revision 20 days prior. The patient underwent debridement of his left total knee and revision of the left total femur arthroplasty. Daptomycin was initiated empirically due to a previous rash from vancomycin. Operative tissue cultures grew Staphylococcus haemolyticus, Staphylococcus epidermidis and Corynebacterium striatum. Given concern for daptomycin resistance and the reliability of vancomycin susceptibility, daptomycin was discontinued and vancomycin initiated following a graded challenge. Within a few days, the patient developed a diffuse, blanching, erythematous, maculopapular rash and daptomycin was restarted. Over the next 72 h, his rash progressed and he met criteria for drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Daptomycin was stopped and oral linezolid initiated; rash improved. C. striatum returned with susceptibility to gentamicin, linezolid, vancomycin and daptomycin. Due to concern for adverse effects on long-term linezolid, daptomycin was restarted and was tolerated for 20 days, at which point purulent drainage from incision increased. The patient underwent another arthroplasty revision and washout. Operative cultures from this surgery were again positive for C. striatum. Repeat C. striatum susceptibilities revealed resistance to daptomycin but retained susceptibility to linezolid. Daptomycin was again changed to linezolid. He completed six weeks of linezolid followed by linezolid 600 mg daily for suppression and ultimately opted for disarticulation.
Conclusions
C. striatum has historically been regarded as a contaminant, particularly when grown in tissue culture in the setting of prosthetic joint infection. Based on the available literature and susceptibility patterns, the most appropriate first-line therapy is vancomycin or linezolid. Treatment with daptomycin should be avoided, even when isolates appear susceptible, due to the risk of development of high-level resistance (MIC > 256 µg/mL) and clinical failure.
Keywords: Antimicrobial resistance, Prosthetic joint infection, Corynebacterium striatum, Daptomycin
Background
C. striatum is a gram-positive facultative anaerobe found in the environment and in human nasopharynx and skin. Non-diphtheriae Corynebacterium species have historically been considered a contaminant. More recently, C. striatum has been increasingly implicated in a number of infections, including respiratory infections, endocarditis, and bone and joint infections, particularly those involving hardware or implanted devices [1]. While the specific virulence factors contributing to these infections are not well described, C. striatum has been noted to form biofilms on prosthetic material and has been implicated in nosocomial outbreaks. Adherence to a variety of prosthetic surfaces, including both hydrophobic and hydrophilic surfaces has been noted via binding of fibrinogen to the surface of the organism [2, 3]. Increasingly common reporting of these infections may be a result of evolving identification technologies in clinical microbiology laboratories, such as matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Although identification of C. striatum has not been clinically validated on the Vitek MS (bioMérieux) or the Bruker Biotyper, the organism is available in the current Knowledge Base. MALDI-TOF MS has been reported to more accurately identify Corynebacterium species compared to conventional biochemical methods [4–6]. In a comparison of the Bruker Biotyper and the Vitek MS, 85 C. striatum isolates were identified with 100% agreement, however, 4 isolates were misclassified by the RapID CB Plus (ThermoFisher) phenotypic identification system [7].
The presence of C. striatum in bone and joint infections, including those with prosthetic material, remains puzzling. A recent review of Corynebacterium species causing orthopedic infections included 13 cases of C. striatum. The study concluded that Corynebacterium species are most often a contaminant, as defined by Infectious Disease Society of America Prosthetic Joint Infection guidelines as being detected in only 1 of at least 2 samples cultured [8, 9]. However, another retrospective review of C. striatum reported that when isolated from hardware, the organism was considered pathogenic in 87% of cases, as determined by the treating infectious disease physician. These patients were treated with a significantly longer duration of intravenous (IV) antibiotics compared to patients with hardware infections caused by coagulase-negative staphylococci [10].
Case presentation
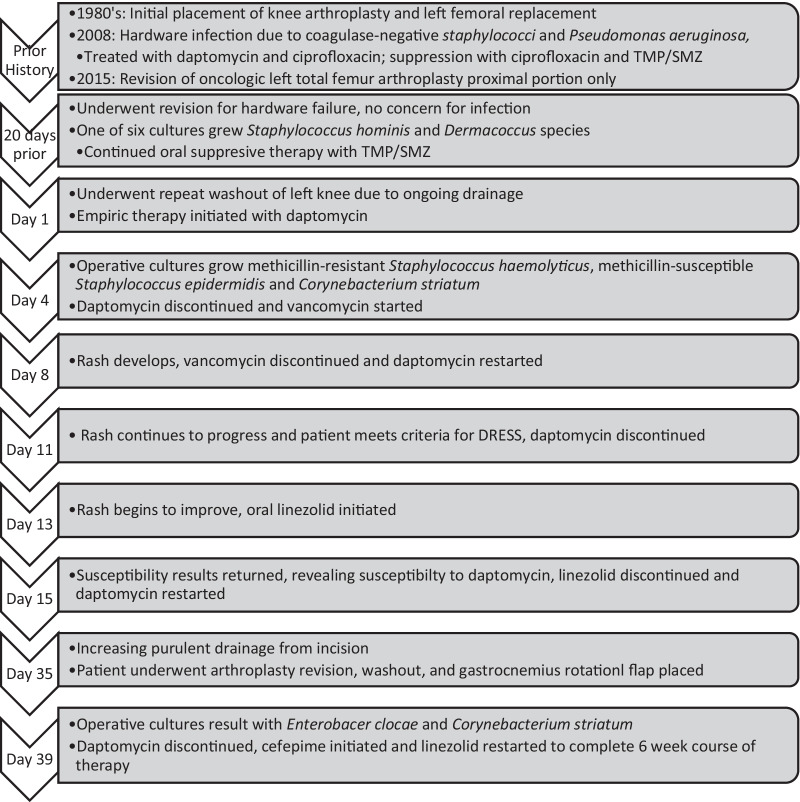
A 65-year-old man presented for repeat washout of his left total knee arthroplasty due to ongoing drainage following revision 20 days prior. The patient’s past medical history was significant for anxiety treated with sertraline. He had undergone multiple previous revisions of his knee arthroplasty and left femoral replacement, initially placed in the 1980s (Fig. 1). In 2008, he developed hardware infections with coagulase-negative staphylococci and Pseudomonas aeruginosa, treated with daptomycin and ciprofloxacin, followed by suppression with ciprofloxacin and trimethoprim-sulfamethoxazole. His labs were only notable for mild normocytic anemia.
Fig. 1.
Timeline of case events. TMP/SMZ sulfamethoxazole-trimethoprim, DRESS drug rash with eosinophilia and systemic symptoms
During his most recent revision of the tibial component and hinge mechanism for hardware failure 20 days prior to this admission, operative reports noted no concern for infection and pathology was negative for acute inflammation. One of six intra-operative cultures grew Staphylococcus hominis and Dermacoccus species; the patient continued oral trimethoprim-sulfamethoxazole suppressive therapy but discontinued oral ciprofloxacin based on cultures and concern for toxicity with long-term use.
During this admission, debridement of his left total knee wound and further revision of the left total femur arthroplasty revealed severe metallosis and a large hematoma. Operative tissue cultures were positive for methicillin-resistant Staphylococcus haemolyticus, methicillin-susceptible Staphylococcus epidermidis and Corynebacterium striatum via MALDI-TOF MS, in six of six cultures obtained. Daptomycin 6 mg/kg every 24 h was initiated empirically due to a previous rash from vancomycin. Linezolid was avoided at this time due to an anticipated treatment duration of more than 3 weeks and concern for subsequent adverse effects with prolonged linezolid therapy. Given concern for daptomycin resistance and the reliability of vancomycin susceptibility, daptomycin was discontinued and a vancomycin graded challenge was performed and tolerated on post-operative day four.
On day 4 of vancomycin, the patient developed a diffuse, blanching, erythematous, maculopapular rash and daptomycin was restarted. Over the next 72 h, his rash progressed, he became febrile to 38.9 , developed peripheral eosinophilia (1.45 K/cu mm, 13.8%; ref: 0–0.50 k/cu mm, 1.0–3.0%) and mildly elevated liver enzymes (AST 51 U/L, ALT 63 U/L), meeting criteria for drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Daptomycin was stopped. His rash began to improve and he was started on oral linezolid 600 mg every 12 h on post-operative day 13. At this time, sertraline was continued despite increased risk of serotonin syndrome, as linezolid was intended to be temporary while awaiting susceptibilities. C. striatum susceptibilities returned on post-operative day 15 and revealed susceptibility to gentamicin, linezolid, vancomycin and daptomycin (MIC 0.064 µg/mL via E-test, breakpoint < 1 µg/mL per CLSI m-45) [11] and resistance to ceftriaxone, clindamycin, doxycycline, meropenem, and trimethoprim-sulfamethoxazole; while no interpretation was available, levofloxacin had an MIC > 8 µg/mL. The patient was re-started on daptomycin.
The patient tolerated daptomycin for 20 days but then noted increasing purulent drainage from his incision. He underwent another arthroplasty revision, washout and gastrocnemius rotational flap. One operative tissue culture from this surgery was positive for Enterobacter cloacae complex, and five of six operative tissue cultures were again positive for rare C. striatum. Antibiotics were changed to linezolid and cefepime. Cefepime was selected due to concern for AmpC induction with the use of third generation cephalosporins over long courses of antibiotic therapy in Enterobacter cloacae. Due to the risk of serotonin syndrome when sertraline is combined with linezolid and the patient reporting little benefit from sertraline therapy, a taper was initiated. Repeat C. striatum susceptibilities revealed resistance to daptomycin (reported as MIC > 1 µg/mL), via E-test but retained susceptibility to linezolid (MIC 0.5 µg/mL). He completed six weeks of linezolid and cefepime followed by linezolid 600 mg daily for suppression, due to the presence of retained hardware. The linezolid suppression was continued for just under a year and a half (522 days) until the patient ultimately opted for disarticulation. At that time the patient noted symptoms consistent with optic neuritis and peripheral neuropathy, so linezolid was discontinued. The patient was treated post-operatively for 6 weeks with oral doxycycline as repeat cultures grew C. striatum susceptible to doxycycline and has not re-presented with infection after 12 months.
Discussion and conclusion
C. striatum exhibits limited susceptibility compared to other Corynebacterium species. Resistance to penicillins, cephalosporins, (including variable MICs reported for ceftaroline), carbapenems, clindamycin, and fluoroquinolones is common. Vancomycin, linezolid, and daptomycin often test susceptible [7]. Current CLSI guidelines provide interpretive criteria for susceptibility testing via broth microdilution. Daptomycin susceptibility is often determined via E-test. Disk diffusion has not been recommended since 2005, due to indeterminable differences in diffusion zone size as a result of slow diffusion because of the high molecular weight of daptomycin [11]. However, as with other organisms such as Staphylococcus aureus and Enterococcus spp, E-tests result in variability of susceptibility results depending on the calcium concentration of the Mueller–Hinton agar used [12], although calcium-supplemented E-tests are available and result in similarly accurate susceptibility testing [13]. A recently published report of 12 cases of monomicrobial C. striatum bone and joint infections reports treating 8 of those patients with an amoxicillin-rifampin combination. Amoxicillin MICs for 9 organisms in this series ranged from 0.38 to 3 mg/L (1 resistant organism, according to non-species related EUCAST criteria) and rifampin MICs ranged from < 0.002 to ≥ 32 mg/L (2 resistant organisms). Treatment with this combination resulted in clinical cure in 4 of 8 patients, although the majority of patients received glycopeptide therapy of unclear duration before receiving the amoxicillin-rifampin combination [14]. Application of these results in clinical practice is challenging because organisms included in this study had significantly higher β-lactam susceptibility rates than those reported elsewhere. The variability of antimicrobial agents tested and reported across the available literature makes drawing conclusions about susceptibility rates for C. striatum difficult. A recent systematic review of antimicrobial therapy for C. striatum infections reported 100% susceptibility for included isolates to vancomycin, linezolid, piperacillin/tazobactam, amoxicillin/clavulanate, and cefuroxime. However, the review included a total of 85 individual cases from the literature and reported susceptibilities for only 8 organisms for piperacillin/tazobactam, 3 organisms for amoxicillin/clavulanate, and 2 organisms for cefuroxime [15]. Reports on C. striatum not included in this review indicate that resistance to these agents may be higher, such as in the above study by Noussair and colleagues [14] that reported a resistance rate of 66% for piperacillin/tazobactam. These β-lactam/β-lactamase inhibitor combinations present a reasonable option for treatment if susceptibility to these agents has been confirmed.
The development of daptomycin resistance while on therapy is a significant concern, as illustrated in our case. Development of high level daptomycin resistance (MIC > 256 µg/mL) has been reported in vitro in isolates exposed to daptomycin for 24 h [12]. This has also been reported in prolonged treatment, one in a case of native valve endocarditis and in three cases of an infected left ventricular assist device [16–18]. While higher doses (> 6 mg/kg) of daptomycin have been studied for the treatment of S. aureus [19], the ideal dose for C. striatum PJI has not been established. Development of high level daptomycin resistance while on therapy has been reported in patients receiving doses ranging from 6 to 8 mg/kg [14–16, 20]. Two case reports of successful treatment of C. striatum endocarditis with daptomycin are available in the current literature, one that used 10 mg/kg of daptomycin and the other used daptomycin 6 mg/kg plus oral rifampin 300 mg twice daily [21, 22]. While these cases resulted in positive outcomes, more data are needed to recommend the use of high-dose daptomycin as monotherapy or with the addition of rifampin. The mechanism of daptomycin resistance in C. striatum is unique compared to other gram-positive organisms. In S. aureus, resistance to daptomycin develops via a variety of mechanisms attributed to a number of single nucleotide polymorphisms (SNPs). Acquisition of multiple SNPs results in resistance via membrane depolarization, cell wall thickening, and reduced affinity for daptomycin binding [23]. The mechanism of high-level daptomycin resistance in C. striatum differs in that a single mutation results in loss of function at phosphatidylglycerol synthase (pgsA2). Daptomycin activity is dependent on the bacterial cell membrane phosphatidylglycerol (PG) concentration. The pgsA2 mutation in C. striatum allows removal of PG from the cell membrane, altering membrane composition to maintain viability of the bacterial cell while conferring resistance to daptomycin [24]. This resistance phenotype has been shown to persist despite removal of daptomycin in culture media, unlike S. aureus, which often reverts once selective pressure is removed [17]. In general, routine susceptibility testing of daptomycin against C. striatum should not be recommended given the significant risk of development of high-level resistance on therapy.
Given our experience with this complicated case, we reviewed a series of prosthetic joint infections (PJI) with C. striatum isolated from operative cultures at our institution. We identified an additional 11 cases between July 1, 2015 and July 19, 2019 with a median follow-up of 1.4 years (range 0.4–4.4 years) following C. striatum isolation (Table 1).
Table 1.
Additional C. striatum hardware infection case details (n = 11)
| Age, median (range) | 70.4 years (54.3–91.6 years) |
|---|---|
| Gender, female | 6 (55%) |
| Involved joint | |
| Hip | 5 (45.5%) |
| Knee | 3 (27.3%) |
| Ankle | 1 (9.1%) |
| Elbow | 1 (9.1%) |
| Spine | 1 (9.1%) |
| Prosthetic joint age, median (range) | 4.5 years (0.07–38.2 years) |
| Prior surgical procedures, median (range) | 2 (1–18) |
| Presenting symptoms | |
| Drainage | 8 (72.7%) |
| Worsening pain | 8 (72.7%) |
| Sinus tract formation | 4 (36.4%) |
| Erythema | 3 (27.3%) |
| Dislocation | 2 (18.2%) |
| Exposed hardware | 1 (9.2%) |
| Additional organisms isolated with C. striatum in 10 patients with polymicrobial cultures | |
| Patient | |
| 1 | MRSA |
| 2 | MRSA; P. aeruginosa; E. faecalis; Bacillus species, not B. anthracis; P. mirabilis |
| 3 | CoNS |
| 4 | MRSA; P. aeruginosa |
| 5 | E. cloacae |
| 6 | E. faecalis |
| 7 | E. faecalis; C. acnes |
| 8 | MSSA; E. faecalis |
| 9 | MSSA; CoNS |
| 10 | MRSA; CoNS |
MRSA methicillin-resistant Staphylococcus aureus, CoNS coagulase-negative staphylococci, MSSA methicillin-susceptible Staphylococcus aureus
C. striatum susceptibilities were performed in seven (63.6%) patients, with all isolates susceptible to vancomycin. Susceptibilities at our institution are performed at an outside laboratory, and include ceftriaxone, clindamycin, doxycycline, erythromycin, gentamicin, levofloxacin, linezolid, meropenem, penicillin, and trimethoprim-sulfamethoxazole. Vancomycin and daptomycin susceptibilities are performed via E-test, if requested. However, only one patient had additional susceptibilities requested for their C. striatum isolate, which was susceptible to daptomycin, linezolid and resistant to fluoroquinolones. Ten (90.9%) patients were treated with vancomycin and one with daptomycin for a median of 42 days (range 17–105 days). Six (54.5%) required additional surgeries of the involved joint, including two amputations or disarticulations, a median of 44 days (range 5–614 days) following their isolation of C. striatum. Only one had C. striatum isolated from subsequent procedures, however this patient (#3) was initially treated with daptomycin, and was changed to vancomycin therapy following repeat C. striatum isolation. Daptomycin susceptibilities were not performed on either isolate from this patient. These cases highlight the appearance of C. striatum in complicated patients with PJI, although they likely under-represent the total cases at our institution, as the practice of identifying C. striatum to the species level is relatively new in our clinical microbiology laboratory.
C. striatum has historically been regarded as a contaminant, particularly when grown in tissue culture in the setting of PJIs, which are frequently polymicrobial. This case highlights the need to consider C. striatum a pathogen in certain clinical contexts. The most appropriate antimicrobial therapy for C. striatum PJI includes vancomycin or linezolid, but β-lactams may be considered if susceptible. Treatment of these infections with daptomycin should be avoided even when isolate appears susceptible due to the risk of developing high-level resistance leading to clinical failure.
Acknowledgements
Not applicable.
Abbreviations
- C. striatum
Corynebacterium striatum
- DRESS
Drug rash with eosinophilia and systemic symptoms
- MIC
Minimum inhibitory concentration
- MALDI-TOF MS
Matrix-assisted laser desorption ionization-time of flight mass spectrometry
- AST
Aspartate aminotransferase
- ALT
Alanine transaminase
- CLSI
Clinical and Laboratory Standards Institute
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- SNP
Single nucleotide polymorphisms
- pgsA2
Phosphatidylglycerol synthase
- PG
Phosphatidylglycerol
- S. aureus
Staphylococcus aureus
- PJI
Prosthetic joint infection
Authors' contributions
AS, CV, YH, MS, and JL contributed to the study conception and design. Material preparation, data collection and analysis were performed by CV, AS, and YH. The first draft of the manuscript was written by AS and CV, YH, MS, and JL commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funds, grants, or other support was received.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective chart review involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Oregon Health and Science University approved this study (STUDY00020228).
Consent for publication
The participant has consented to the submission of the case report to the journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee PP, Ferguson DA, Sarubbi FA. Corynebacterium striatum: an underappreciated community and nosocomial pathogen. J Infect. 2005;50:338–343. doi: 10.1016/j.jinf.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Ramos JN, Souza C, Faria YV, da Silva EC, Veras JFC, Baio PVP, Seabra SH, Moreira LO, Junior RH, Mattos-Guaraldi AL, Viera VV. Bloodstream and catheter-related infections due to different clones of multidrug-resistant and biofilm producer Corynebacterium striatum. BMC Infect Dis. 2019;19(672):1–11. doi: 10.1186/s12879-019-4294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Souza C, Faria YV, SantAnna LO, Viana VG, Seabra SH, De Souza MC, Vieira VV, Junior RH, Moreira LDO, de Mattos-Guaraldi AL. Biofilm production by multiresistant Corynebacterium striatum associated with nosocomial outbreaks. Mem Inst Oswaldo Cruz. 2015;110(2):242–248. doi: 10.1590/0074-02760140373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suwantarat N, Weik C, Romagnoli M, Ellis BC, Kwiatkowski N, Carroll KC. Practical utility and accuracy of matrix-assisted laser desporption ionization-time of flight mass spectrometry for identification of Corynebacterium species and other medically relevant coryneform-like bacteria. Am J Clin Pathol. 2016;145:22–28. doi: 10.1093/ajcp/aqv006. [DOI] [PubMed] [Google Scholar]
- 5.Esteban J, Nieto E, Calvo R, Fernandez-Roblas R, Valero-Guillen PL, Soriano F. Microbiological characterization and clinical significance of Corynebacterium amycolatum strains. Eur J Clin Microbiol Infect Dis. 1999;18:518–521. doi: 10.1007/s100960050336. [DOI] [PubMed] [Google Scholar]
- 6.Voisin S, Deruaz D, Freney J, Renaud FNR. Differentiation of Corynebacterium amycolatum, C. minutissimum, C striatum and related species by pyrolysis-gas-liquid chromatography with atomic emission detection. Res Microbiol. 2002;153:307–311. doi: 10.1016/S0923-2508(02)01324-4. [DOI] [PubMed] [Google Scholar]
- 7.McMullen AR, Anderson N, Wallace MA, Shupe A, Burnham CD. When good bugs go bad: epidemiology and antimicrobial resistance profiles of Corynebacterium striatum, an emerging multidrug-resistant, opportunistic pathogen. Antimicrob Agents Chemother. 2017;61(11):e01111–e1117. doi: 10.1128/AAC.01111-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalt F, Schulthess B, Sidler F, Herren S, Fucentese SF, Zingg PO, Berli M, Zinkernagel AS, Zbinden R, Achermann Y. Corynebacterium species rarely cause orthopedic infections. J Clin Microbiol. 2018;56(12):e01200–e1218. doi: 10.1128/JCM.01200-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckleberg JM, Rao N, Hanssen A, Wilson WR. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 10.Hahn WO, Werth BJ, Butler-Wu SM, Rakita RM. Multidrug-resistant Corynebacterium striatum associated with increased use of parenteral antimicrobial drugs. Emerg Infect Dis. 2016;22(11):1980–2014. doi: 10.1093/infdis/jiw480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. 3rd edn, CLSI guideline M45. Wayne, PA: Clinical and Laboratory Standards Institute; 2016
- 12.Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev. 2013;26(4):759–780. doi: 10.1128/CMR.00030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AP, Mushtaq S, Warner M, Livermore DM. Calcium-supplemented daptomycin Etest strips for susceptibility testing on Oso-Sensitest agar. J Antimicrob Chemother. 2004;53(5):860–862. doi: 10.1093/jac/dkh198. [DOI] [PubMed] [Google Scholar]
- 14.Noussair L, Salomon E, El Sayed F, Duran C, Bouchand F, Roux AL, Gaillard JL, Bauer T, Rottman M, Dinh A. Monomicrobial bone and joint infection due to Corynebacterium striatum: literature review and amoxicillin-rifampin combination as treatment perspective. Eur J Clin Microbiol Infect Dis. 2019;38:1269–1278. doi: 10.1007/s10096-019-03542-x. [DOI] [PubMed] [Google Scholar]
- 15.Milosavljevic MN, Milosavljevic JZ, Kocovic AG, et al. Antimicrobial treatment of Corynebacterium striatum invasive infections: a systematic review. Rev Inst Med Trop Sao Paulo. 2021;63:e49. doi: 10.1590/s1678-9946202163049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran TT, Jaijakul S, Lewis CT, Diaz L, Panesso D, Kaplan HB, Murray BE, Wagner A, Arias CA. Native valve endocarditis caused by Corynebacterium striatum with heterogeneous high-level daptomycin resistance: collateral damage from daptomycin therapy? Antimicrob Agents Chemother. 2012;56(6):3461–3464. doi: 10.1128/AAC.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.TeKippe EM, Thomas BS, Ewald GA, Lawrence SJ, Burnham CD. Rapid emergence of daptomycin resistance in clinical isolates of Corynebacterium striatum… a cautionary tale. Eur J Clin Microbiol Infect Dis. 2014;33(12):2199–2205. doi: 10.1007/s10096-014-2188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werth BJ, Hahn WO, Butler-Wu SM, Rakita RM. Emergence of high-level daptomycin resistance in Corynebacterium striatum in two patients with left ventricular assist device infections. Microb Drug Resist. 2016;22(3):233–237. doi: 10.1089/mdr.2015.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IDSA. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clinical Infectious Diseases. 2011; 52(3):e18–e55. [DOI] [PubMed]
- 20.Ajmal S, Saleh OA, Beam E. Development of high-grade daptomycin resistance in a patient being treated for Corynebacterium striatum infection. Antimicrob Agents Chemother. 2017;61(7):e00705–e717. doi: 10.1128/AAC.00705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez Guerrero ML, Molins A, Rey M, Romero J, Gadea I. Multi-drug resistant Corynebacterium striatum endocarditis successfully treated with daptomycin. Int J Antimicrob Agents. 2012;40:373–374. doi: 10.1016/j.ijantimicag.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Shah M, Murillo JL. Successful treatment of Corynebacterium striatum Endocarditis with Daptomycin plus Rifampin. Ann Pharmacother. 2005;39(10):1741–1744. doi: 10.1345/aph.1G242. [DOI] [PubMed] [Google Scholar]
- 23.Bayer AS, Schneider T, Sahl HG. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann NY Acad Sci. 2013;1277(1):139–158. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldner NK, Bulow C, Cho K, Wallace M, Hsu FF, Patti GJ, Burnham CA, Schlesinger P, Dantas G. Mechanism of high-level daptomycin resistance in Corynebacterium striatum. mSphere. 2018;3(4):e00371–e418. doi: 10.1128/mSphereDirect.00371-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.