Abstract
We examined the in vivo activity of human macrophage colony-stimulating factor (hM-CSF) against lethal Candida albicans infection in mice. In C. albicans-infected mice which had been immunosuppressed with cyclophosphamide, treatment with hM-CSF at a daily dose of 8 × 105 units/kg of body weight or greater slightly but significantly prolonged survival. Furthermore, the therapeutic efficacy of amphotericin B (AMPH-B) in infected mice was enhanced by its combined use with hM-CSF, while that of fluconazole (FLCZ) was not. The activities of peritoneal macrophages and neutrophils from mice administered hM-CSF plus AMPH-B in combination for inhibition of hyphal growth of C. albicans cells and intracellular phagocytosis and killing of the cells were greater than those of comparable phagocytic cells from control mice to which hM-CSF plus AMPH-B was not administered. These results suggest that intravenous administration of hM-CSF augments the efficacy of AMPH-B by enhancing the antifungal activities of macrophages and neutrophils. Therefore, it is expected that therapy with the combination AMPH-B and hM-CSF could improve the efficacy of AMPH-B and reduce the therapeutic dose of the antifungal drug that is required.
It is widely accepted that immunocompromised hosts, particularly those with impaired phagocytic cell function (mainly neutrophils and/or monocytes/macrophages) are at high risk of infections caused by Candida, Aspergillus, and several other opportunistic fungal pathogens. The survival, growth, and differentiation of progenitor cells of these phagocytes are known to be controlled by hemopoietic colony-stimulating factors (CSFs), of which four types of CSFs have been recognized: granulocyte CSF (G-CSF), granulocyte/macrophage CSF (GM-CSF), macrophage colony-stimulating factor (M-CSF), and multicolony-stimulating factor (interleukin-3 [IL-3]).
M-CSF is a hematopoietic glycoprotein that stimulates the proliferation and differentiation of mononuclear progenitors into mature cells (18, 30, 35) and promotes the production of other cytokines (9, 13, 20, 32) by monocytes/macrophages. These properties are indispensable to antimicrobial host defense, and there are an increasing number of reports describing the in vitro or in vivo antifungal effect of M-CSF (4, 14, 21, 22, 27, 28, 31). Although some reports of clinical trials with human M-CSF (hM-CSF) mentioned that it is useful as an adjunctive therapy in patients with invasive fungal infections treated with conventional antifungal agents (23, 24), there is limited backup information about the usefulness of M-CSF in combination with antifungal agents against fungal infections, and the clinical significance of M-CSF in combination with antifungal agents is still unclear.
The present studies were designed to evaluate the potential usefulness of hM-CSF in combination with two major antifungal drugs, amphotericin B (AMPH-B) and fluconazole (FLCZ), against Candida albicans infection in mice with immunosuppression induced by cyclophosphamide (CY). The results showed that the administration of hM-CSF in combination with AMPH-B markedly enhanced the preventive and therapeutic effects of the antifungal drug against Candida infection, while hM-CSF administered to mice in combination with FLCZ resulted in lower levels of enhancement of the effects of the antifungal drug. We further showed that the anti-Candida activities of hM-CSF-activated macrophages and neutrophils were enhanced by AMPH-B when it was administered together with M-CSF.
MATERIALS AND METHODS
Animals.
Male inbred C3H/HeN mice (specific pathogen free) were obtained from Charles River Japan, Kanagawa, Japan. The mice were 6 weeks of age at the time of the experiments.
Reagents.
hM-CSF was purified from human urine as described previously (10). The specific activity was 2.8 × 108 U/mg of protein, as determined by a mouse bone marrow colony formation assay (19), and the endotoxin content of the preparation containing 100 μg of hM-CSF was less than 0.03 endotoxin units as measured by the Limulus assay (Limulus HS-Single Test; Wako Pure Chemicals, Osaka, Japan). hM-CSF was dissolved to the required concentration in sterile saline before use. Commercially available intravenous (i.v.) preparations of AMPH-B (Fungizone) and FLCZ (Diflucan) were purchased from Bristol-Myers Squibb Co. (Tokyo, Japan) and Pfizer Inc. (Tokyo, Japan), respectively. The former was dissolved in 5% glucose immediately before use.
Microorganism.
C. albicans TIMM1768 (2), isolated from a patient with systemic candidiasis, was used in this study. The yeast was cultured on Sabouraud glucose agar. At the time of use, a small colony was taken from a subculture and inoculated into YPG broth (0.5% yeast extract, 1% polypeptone, 2% d-glucose) and was grown with shaking (200 rpm) for 16 h at 37°C. Yeast cells were harvested and washed three times with sterile saline by centrifugation at 500 × g for 5 min. The number of yeast cells in a suspension was measured with a hemocytometer, and the yeast cells were adjusted to a suitable concentration with sterile saline.
In vivo study.
Mice were injected intraperitoneally (i.p.) with 100 mg of CY (Shionogi Pharmaceuticals, Osaka, Japan) per kg of body weight. The immunosuppressed mice were infected i.v. with a lethal dose of C. albicans (5 × 104 cells per mouse) 5 days after CY injection. Beginning on the next day, the mice were administered the indicated i.v. doses of hM-CSF or vehicle sterile saline, alone and in combination with subcutaneous (s.c.) doses of AMPH-B (100 μg/kg) or FLCZ (500 μg/kg), once a day for 5 days. To detect the enhancing effect of hM-CSF, the minimal effective doses of AMPH-B and FLCZ were used. In some experiments, mice were administered an i.v. dose of M-CSF once a day for 4 days, beginning 1 day after CY injection. The animals were subsequently infected with C. albicans and were administered s.c. doses of antifungal drugs for 5 days, beginning 1 day after the infection. The number of animals surviving in each group was determined every day until the end of the 3-week experimental period. These experiments were repeated five or six times with groups of five mice each time. The total number of mice tested is indicated in the figure legends.
Preparation of neutrophils and macrophages.
hM-CSF and/or AMPH-B was injected into healthy mice in the same manner as described above for the in vivo study. To isolate murine neutrophils (1), the mice were injected i.p. with 2 ml of an 8% casein sodium (Tokyo Kasei, Tokyo, Japan) solution in saline. Six hours later, peritoneal cells were collected, washed with phosphate-buffered saline (PBS), and suspended in Tris-buffered ammonium chloride (1 mM Tris-acetate [pH 7.5], 0.833% ammonium chloride) to lyse the erythrocytes. The residual cells were resuspended in RPMI 1640 (Flow Laboratories, Inc., McLean, Va.) containing 7.5% heat-inactivated fetal calf serum and 100 μg of penicillin-streptomycin per ml, which was designated a complete medium, and then the cell suspension was layered onto 10 ml of 90% Ficoll Hypaque (Pharmacia Fine Chemicals, Piscataway, N.J.). After centrifugation at 300 × g for 30 min at room temperature, a neutrophil-rich pellet was obtained, washed with PBS, and resuspended in complete medium. The final cell suspension routinely consisted of more than 95% neutrophils, as confirmed by Diff-Quick staining (American Scientific Products, McGaw Park, Ill.).
To prepare murine macrophages, mice into which hM-CSF and/or AMPH-B was injected were i.p. administered 2 ml of 4% Brewer thioglycolate medium (Difco Laboratories, Detroit, Mich.). Four days later, the peritoneal cells were collected, and after lysing of the erythrocytes, the peritoneal cells were resuspended in complete medium. They were adjusted to 106 cells/ml with the same medium and were seeded in flat-bottom microplates (105 cells/well). After incubation for 1 h at 37°C in a 5% CO2 incubator, nonadherent cells were removed. Adherent cells consisting of over 95% macrophages were designated peritoneal macrophages (29).
Assay for Candida hyphal growth inhibition by phagocytes.
To determine neutrophil- or macrophage-mediated inhibition of C. albicans hyphal growth, we used a [3H]glucose incorporation assay technique (6). One hundred microliters of a neutrophil suspension or macrophage suspension (1 × 106 cells/ml) in complete medium was seeded into triplicate wells of flat-bottom microplates (100 μl/well), and then 100 μl of a C. albicans suspension (4 × 104 cells/ml) was added to these wells, yielding an effector cell:target cell (E/T) ratio of 25:1. After the cell mixtures were incubated for 16 h at 37°C in a humidified atmosphere of 5% CO2, the culture supernatants were removed and replaced by 50 μl of [3H]glucose (specific activity, 40 Ci/mmol [1,480 GBq/mmol]; ART 312, Glucose D-L5, 6-3H; American Radiolabeled Chemicals Inc., St. Louis, Mo.) diluted to 10 μCi/ml in sterile water. After an additional 1.5 h of incubation, 50 μl of 5.25% sodium hypochlorite was added to the incubation mixture. The growing Candida cells containing [3H]glucose in each well were collected with a MASH harvester, and [3H]glucose incorporation was measured with a scintillation counter. The percent growth inhibition of C. albicans was calculated as follows: 1 − (counts per minute of C. albicans incubated with effector cells/counts per minute of C. albicans incubated alone) × 100.
Assay of phagocytosis and C. albicans killing by macrophages.
For the phagocytosis assay, C. albicans cells were added to wells of flat-bottom microplates containing macrophages, yielding an E/T ratio of 2:1. The plates were centrifuged at 500 × g for 10 min. After a 30-min incubation, the wells were washed thoroughly and a 0.05% deoxycholic acid solution in sterile water was added to the wells to lyse the macrophages. To determine fungicidal activity, complete medium was added to the wells of the plate, which was then incubated for a further 5 h before the macrophages were lysed. After dilution with sterile water, the numbers of viable C. albicans cells in the wells were determined by the conventional plate count method on Sabouraud glucose agar as described above. The phagocytotic ability and fungicidal rate were calculated as follows: phagocytotic ability (in percent) = (number of remaining viable Candida cells in or on macrophages at 30 min of incubation/total number of Candida cells added) × 100, and fungicidal rate (in percent) = [1 − (number of viable Candida cells at 30 min − number of viable yeast cells at 5 h)/number of viable Candida cells at 30 min] × 100.
Statistical analysis.
Survival rates were evaluated by Kaplan-Meyer analysis and the Wilcoxon signed-rank test. The statistical significance of differences between groups of other data was determined by an analysis of variance by the Tukey-Kramer test. The difference was significant if P was <0.05.
RESULTS
Effect of hM-CSF against Candida infection.
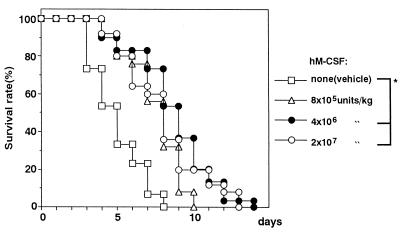
In the present study, mice with CY-induced leukopenia were infected with C. albicans. When mice were treated with CY at a single i.p. dose of 100 mg/kg, the total number of leukocytes in the peripheral blood decreased to approximately 60% of that for untreated control animals 4 days after the injection and then gradually returned to the normal level within 2 or 3 days (data not shown). Consistent with this, CY-treated animals were found to be highly susceptible to relatively small numbers of C. albicans cells (5 × 104 cells per mouse) 4 days after CY injection. All such mice infected with C. albicans died within 8 days, with a median survival time (MST) of 5.0 days (Fig. 1). CY-treated mice administered hM-CSF showed a slight but significant resistance to C. albicans. As seen in Fig. 1, mice receiving hM-CSF at a daily dosage of 8 × 105, 4 × 106, or 2 × 107 U per kg for 5 days from day 1 to day 5 postinfection had a slight but statistically significantly prolonged survival time compared with the survival times for the vehicle-treated controls. The greatest efficacy was obtained with 4 × 106 U of the cytokine per kg, with an MST of 9.0 days, although all the animals had died by 14 days.
FIG. 1.
Therapeutic effect of hM-CSF on C. albicans infection in CY-treated mice. Mice were inoculated i.v. with C. albicans (5 × 104 yeast cells per mouse) 5 days after CY injection. Vehicle or hM-CSF was given i.v. to the mice once daily for 5 days starting 1 day after infection. The total number of mice in each group was as follows: vehicle, n = 30; hM-CSF at 8 × 105 U/kg, n = 25; hM-CSF at 4 × 106 U/kg, n = 30; hM-CSF at 2 × 107 U/kg, n = 25. ∗, P < 0.05 (significant difference among the treatments).
Effect of hM-CSF in combination with antifungal drugs against Candida infection.
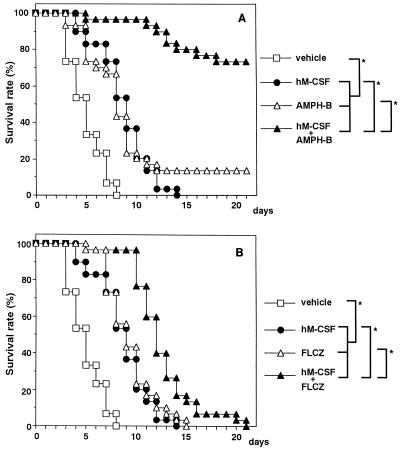
To study the potential usefulness of combination therapy of antifungal drugs with hM-CSF as an immunotherapeutic agent, experiments were conducted with the two major antifungal drugs, AMPH-B and FLCZ, which were administered to C. albicans-infected leukopenic mice, alone and in combination with hM-CSF, starting on day 1 postinfection. Low doses of the antifungal drugs which did not show a remarkable efficacy when they were used alone were chosen; daily doses of 100 μg of AMPH-B per kg and 500 μg of FLCZ per kg were given s.c. to mice once a day for 5 days starting 1 day after infection. Figure 2A shows the results of an experiment in which AMPH-B was administered alone and/or in combination with hM-CSF. That dose of AMPH-B was slightly efficacious in prolonging survival (MST, 9.0 days, versus 5.0 days for vehicle-treated controls). As indicated in Fig. 1, administration of the most effective dosage of hM-CSF (4 × 106 U/kg/day for 5 days) was begun 1 day after C. albicans infection. As shown in Fig. 2A, when hM-CSF treatment was combined with AMPH-B therapy, remarkable efficacy against Candida infection was evident. The MST was prolonged over 21 days (cf. MSTs of 9.0 days for AMPH-B alone and hM-CSF alone), and the survival rate over a 3-week period reached 73.3% (cf. survival rates of 13.3% for AMPH-B alone and 0% for hM-CSF alone). Figure 2B shows the results of FLCZ injection alone and/or in combination with hM-CSF. FLCZ alone was about as effective as AMPH-B alone in respect to MST (no significance was detected by the Tukey-Kramer test). The combined use of FLCZ with hM-CSF also significantly prolonged the MST to 12.0 days but did not increase the survival rate during the experimental period. These results demonstrate that therapy with AMPH-B in combination with hM-CSF is significantly more effective than AMPH-B monotherapy and that, in combination therapy with hM-CSF, the efficacy of AMPH-B treatment is significantly greater than that of FLCZ treatment.
FIG. 2.
Combined therapeutic effect of hM-CSF with AMPH-B (A) or FLCZ (B) administration on C. albicans infection in CY-treated mice. Mice were given hM-CSF (4 × 106 U/kg) as described in the legend to Fig. 1. AMPH-B (100 μg/kg) or FLCZ (500 μg/kg) was given s.c. to the mice once daily for 5 days concurrently with hM-CSF administration. The total number of mice in each group was 30. ∗, P < 0.05 (significant difference among the treatments).
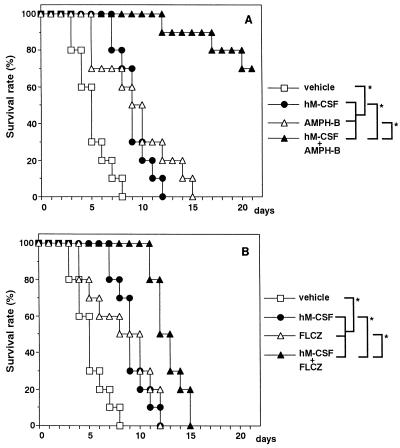
Figure 3 shows the results of experiments which were conducted under the same conditions as those for the experiments whose results are presented in Fig. 2, except that the administration of hM-CSF (4 × 106 U/kg/day for 4 days) was started 4 days before infection. As seen in Fig. 3, AMPH-B (Fig. 3A) and FLCZ (Fig. 3B) administered alone significantly prolonged the survival times (MSTs, 9.5 and 9.0 days, respectively) compared with that for the vehicle-treated control mice (MST, 5.0 days). The extent of survival was similar between groups of animals treated with AMPH-B and FLCZ alone (the difference was not significant). Pretreatment with hM-CSF rendered the mice more resistant to lethal Candida infection compared with the resistance of control mice treated with the vehicle (MSTs, 9.0 versus 5.0 days). In hM-CSF-pretreated mice, subsequent administration of AMPH-B and FLCZ significantly protected the animals from infection, as determined by prolongation of the survival time (MSTs, >21.0 days for AMPH-B-treated mice and 12.5 days for FLCZ-treated mice) and the survival rate (70% for AMPH-B-treated mice).
FIG. 3.
Combined prophylactic effect of hM-CSF with AMPH-B (A) or FLCZ (B) administration on C. albicans infection in CY-treated mice. Mice were inoculated i.v. with C. albicans (5 × 104 yeast cells per mouse) 5 days after CY injection. Vehicle or hM-CSF (4 × 106 U/kg) was given i.v. to mice once daily for 4 days starting 1 day after CY injection until 1 day before infection. AMPH-B (100 μg/kg) or FLCZ (500 μg/kg) was given s.c. to the mice once daily for 5 days after infection. The total number of mice in each group was 10. ∗, P < 0.05 (significant difference among the treatments).
Effect of hM-CSF, alone and in combination with AMPH-B, on macrophage function in ex vivo assays.
The possible immunopotentiating activity of hM-CSF, alone and in combination with AMPH-B, against macrophages was studied with ex vivo assay systems in which the growth-inhibitory activities, phagocytic activities, and fungicidal activities of macrophages from mice treated with 4 × 106 U of the cytokine per kg and/or 100 μg of AMPH-B per kg against C. albicans were tested. As shown in Table 1, macrophages harvested from mice that had been treated with hM-CSF in combination with AMPH-B significantly inhibited C. albicans hyphal growth compared with the inhibition caused by the corresponding cells from mice treated with vehicle or AMPH-B alone. Table 1 also shows that treatment with AMPH-B alone did not significantly enhance the fungal growth-inhibitory activities of macrophages but that the ability of hM-CSF to increase the antifungal activities of macrophages did appear, although to a slight extent, to be enhanced by the use of AMPH-B in combination. Furthermore, as shown in Table 2, macrophages from mice treated with hM-CSF in combination with AMPH-B were more active than the corresponding cells from vehicle-treated mice in the phagocytosis and intracellular killing of Candida cells. These abilities of macrophages from mice treated with hM-CSF and AMPH-B in combination were more prominent than those of macrophages from mice treated with the cytokine alone or AMPH-B alone (Table 2).
TABLE 1.
Effect of treatment of mice with hM-CSF and AMPH-B on in vitro C. albicans growth-inhibitory activities of macrophagesa
| Treatment | [3H]glucose incorporation (cpm [% inhibition]) |
|---|---|
| None (C. albicans only) | 11,107.0 ± 468.3 |
| Vehicle | 7,153.7 ± 2,258.9 (35.6) |
| hM-CSF (4 × 106 U/kg) | 4,225.7 ± 724.7 (62.0) |
| AMPH-B (100 μg/kg) | 5224.7 ± 900.0 (53.0) |
| hM-CSF + AMPH-B | 2843.0 ± 479.5 (74.4)b |
Macrophages were prepared from mice administered hM-CSF and/or AMPH-B. After incubating macrophages and C. albicans (E/T, 25:1) for 16 h, [3H]glucose incorporation into viable C. albicans was determined. Data are expressed as mean ± standard deviation counts per minute and represent the percent incorporation inhibition for triplicate samples.
P < 0.05 (significant difference among the treatments). The results for all treatment were significantly different from those of no treatment.
TABLE 2.
Effect of treatment of mice with hM-CSF and AMPH-B on in vitro phagocytic and fungicidal activities of macrophagesa
| Treatment | % Phagocytic activity | % Fungicidal activity |
|---|---|---|
| Vehicle | 17.1 ± 1.8 | 24.6 ± 12.6 |
| hM-CSF (4 × 106 U/kg) | 21.2 ± 1.4 | 40.2 ± 16.8 |
| AMPH-B (100 μg/kg) | 19.8 ± 3.1 | 29.8 ± 22.1 |
| hM-CSF + AMPH-B | 29.5 ± 2.7b | 53.0 ± 14.9b |
Macrophages were prepared as described in footnote a of Table 1. After incubating macrophages and C. albicans (E/T, 2:1) for 30 min, macrophages were washed and lysed or were cultured for a further 5 h before lysis. Phagocytosis of macrophages was determined after 30 min of incubation, and fungicidal activity was determined after 5 h. Data are expressed as means ± standard deviations for triplicate samples from four experiments.
P < 0.05 (significant difference among the treatments).
Effect of hM-CSF, alone and in combination with AMPH-B, on neutrophil function in ex vivo assays.
Since neutrophils are known to be more potent than macrophages as direct effector cells against C. albicans, the fungal growth-inhibitory activities of neutrophils harvested from mice that had been treated with hM-CSF, alone and in combination with AMPH-B, were investigated by using ex vivo assay systems which were essentially the same as those used to test macrophages. Table 3 shows that the hyphal growth-inhibitory activities of neutrophils, which appeared to be more potent than those of macrophages (Table 1), were significantly increased by in vivo treatment with hM-CSF in combination with AMPH-B compared with the activities after vehicle treatment, as was the case for macrophages.
TABLE 3.
Effect of treatment of mice with hM-CSF and AMPH-B on in vitro C. albicans growth-inhibitory activities of neutrophilsa
| Treatment | [3H]glucose incorporation (cpm [% inhibition]) |
|---|---|
| None (C. albicans only) | 34,111.7 ± 1,111.5 |
| Vehicle | 14,618.3 ± 858.9 (57.1) |
| hM-CSF (4 × 106 U/kg) | 10,529.0 ± 1,688.9 (69.1) |
| AMPH-B (100 μg/kg) | 12,605.3 ± 1,103.3 (63.0) |
| hM-CSF + AMPH-B | 8,897.7 ± 1,807.4 (73.9)b |
Neutrophils were prepared from mice administered hM-CSF and/or AMPH-B. [3H]glucose incorporation was assayed as described in footnote a of Table 1.
P < 0.05 (significant difference among the treatments). The results for all treatments were significantly different from those of no treatment.
DISCUSSION
Only a limited number of papers have reported that M-CSF-treated monocytes increase the host's capability to kill fungal cells intracellularly (14, 35) and that the administration of M-CSF to neutropenic mice infected with fungal microorganisms significantly improved the survival of the animals compared with the survivals obtained for placebo-treated controls (5). These data suggest, however, that M-CSF directly enhances host resistance to fungal infection by functionally activating monocytes or macrophages. One of the aims of the present study was to confirm these abilities of hM-CSF by using both in vivo and ex vivo assay systems.
The results of the in vivo study demonstrate that hM-CSF restores the reduced resistance of mice with CY-induced leukopenia to fungi. Our findings are similar to those presented in previous papers in which the effectiveness of recombinant hM-CSF against C. albicans infection in rats or mice was described (5, 31). In this connection, it has been demonstrated that hM-CSF enhances the fungicidal activities of macrophages by augmenting phagocytosis (12, 14) and superoxide production (27). The therapeutic efficacy of exogenous hM-CSF in the animal model of systemic candidiasis may also be associated with its capabilities to increase the number of monocytes/macrophages in the peripheral blood (31) and to enhance the number and activities of neutrophils by stimulating CSF (32) and IL-8 (9) production.
Our greater interest was on the potential of M-CSF to improve the clinical outcome of therapy when used in combination with certain antifungal drugs. Antifungal chemotherapy is generally required for patients with fungal infections, and at present the main treatment is AMPH-B or FLCZ. However, the clinical usefulness is hampered by the serious side effects of AMPH-B and the limited efficacy of FLCZ, particularly in immunocompromised patients. Therefore, some supportive therapy with immunotherapeutic agents is believed to be necessary for the immunological improvement of such patients.
Like G-CSF and GM-CSF, M-CSF is a promising candidate as an immunotherapeutic agent for antifungal therapy. Prophylactic administration of M-CSF to mice infected with C. albicans following chemotherapy resulted in longer survival times (5). When F344 rats with established C. albicans infection were treated with a combination of FLCZ and M-CSF, there was an increase in the survival time compared with that for rats receiving FLCZ alone (31).
Similarly, it would appear that combination therapy of AMPH-B, a “gold standard” antifungal drug, with M-CSF enhances the therapeutic efficacy of AMPH-B without increasing the dose and/or lowers the toxicity of AMPH-B by reducing the dose of the antifungal drug that is needed. However, there is little information about the usefulness of M-CSF in combination with AMPH-B against fungal infections. Thus, we were tempted to investigate the therapeutic efficacy of AMPH-B, as well as that of FLCZ, when the drugs were used in combination with hM-CSF against C. albicans infection in CY-induced neutropenic mice. The results of this study demonstrate that the therapeutic administration of hM-CSF improved survival and that when hM-CSF was combined with AMPH-B, survival was remarkably prolonged. When given with hM-CSF, FLCZ also increased the survival significantly, but to a lesser degree than AMPH-B did, while both agents applied alone exhibited a lower degree of efficacy than when they were applied with hM-CSF. Several investigators have shown the synergistic benefits of FLCZ combined with M-CSF (4, 22, 31), as well as with G-CSF (26, 37) or IL-1 (15), in fighting fungal infections. The mechanism of the synergistic action between FLCZ and M-CSF or some other cytokine by which fungal growth is inhibited remains to be answered. Since FLCZ does not have substantial immunomodulating activity (3, 7, 25), it looks likely that it and cytokines may not act synergistically on phagocytes to potentiate their antifungal activities but that FLCZ concentrated in phagocytes may directly inhibit the phagocytized fungi (33). In contrast, AMPH-B has a potent immunostimulatory effect on macrophages and/or polymorphonuclear leukocytes (8, 34, 36). Its stimulation of the antifungal activities of macrophages is believed to be due to activation of an oxidative burst upon phagocytosis (34), NO2− production (11), and tumor necrosis factor alpha production (17). Apart from this, as macrophages also function as a concentrated reservoir of AMPH-B, those into which the antibiotic is incorporated can be more active against Candida than macrophages into which AMPH-B is not incorporated (16). On the other hand, it is possible that some pharmacokinetic interactions between hM-CSF and FLCZ or AMPH-B could result in the potentiation of the activities of the antifungal drugs through an intervention of the macrophages on the level and/or retention time of the drugs in the blood and tissues. Pharmacokinetic studies on this issue are warranted.
The present ex vivo studies demonstrated that AMPH-B plus hM-CSF enhanced the growth-inhibitory activities of both of the two major types of effector cells, macrophages and neutrophils, against Candida. Clinical evaluation of the combination therapy with AMPH-B plus hM-CSF in the treatment of patients with disseminated candidiasis and other deep-seated mycoses is thus warranted.
REFERENCES
- 1.Abe S, Satoh T, Tokuda Y, Tansho S, Yamaguchi H. A rapid colorimetric assay for determination of leukocyte-mediated inhibition of mycelial growth of Candida albicans. Microbiol Immunol. 1994;38:385–388. doi: 10.1111/j.1348-0421.1994.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi K, Itoh N, Abe F, Abe S, Uchida K, Ishizuka M, Takeuchi T, Yamaguchi H. Enhancement by ubenimex (bestain) of host resistance to Candida albicans infection. J Antibiot. 1992;45:1778–1784. doi: 10.7164/antibiotics.45.1778. [DOI] [PubMed] [Google Scholar]
- 3.Bodey G P. Azole anti-fungal agents. Clin Infect Dis. 1992;14(Suppl. 1):5161–5169. [Google Scholar]
- 4.Brummer E, Nassar F, Stevens D A. Effect of macrophage colony-stimulating factor on anticryptococcal activity of bronchoalveolar macrophages: synergy with fluconazole for killing. Antimicrob Agents Chemother. 1994;38:2158–2161. doi: 10.1128/aac.38.9.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cenci E, Bartocci A, Puccetti P, Mocci S, Stanley E R, Bistoni F. Macrophage colony-stimulating factor in murine candidiasis: serum and tissue levels during infection and protective effect of exogenous administration. Infect Immun. 1991;59:868–872. doi: 10.1128/iai.59.3.868-872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djeu J Y, Parapanissios A, Halkias D, Friedman H. A rapid 3H-glucose incorporation assay for determination of lymphoid cell-mediated inhibition of Candida albicans growth. J Immunol Methods. 1986;92:73–77. doi: 10.1016/0022-1759(86)90505-3. [DOI] [PubMed] [Google Scholar]
- 7.Drummond D C, Wong C W, Whitman L M, McCormack J G. The effects of amphotericin B, fluconazole and miconazole on neutrophil and lymphocyte function in a guinea pig model. J Antimicrob Chemother. 1995;36:375–384. doi: 10.1093/jac/36.2.375. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand J A, Kimball K, Burke J F, Dinarello C A. Amphotericin B treatment of human mononuclear cells in vitro results in secretion of tumor necrosis factor and interleukin-1. Clin Res. 1988;36:456a. [Google Scholar]
- 9.Hashimoto S, Yoda M, Yamada M, Yanai N, Kawashima T, Motoyoshi K. Macrophage colony-stimulating factor induces interleukin-8 production in human monocytes. Exp Hematol. 1996;24:123–128. [PubMed] [Google Scholar]
- 10.Hatake K, Motoyoshi K, Ishizaka Y, Takaku F, Miura Y. Purification of human urinary colony-stimulating factor by higher performance liquid chromatography. J Chromatogr. 1985;344:339–344. doi: 10.1016/s0378-4347(00)82037-x. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann J L, Dubois N, Fourgeaud M, Basset D, Lagrange P H. Synergic inhibitory activity of amphotericin-B and γ interferon against intracellular Cryptococcus neoformans in murine macrophage. J Antimicrob Chemother. 1994;34:1051–1058. doi: 10.1093/jac/34.6.1051. [DOI] [PubMed] [Google Scholar]
- 12.Hume D A, Pavli P, Donahue R E, Fidler I J. The effect of human recombinant macrophage colony-stimulating factor (CSF-1) on the murine mononuclear phagocyte system in vivo. J Immunol. 1988;141:3405–3409. [PubMed] [Google Scholar]
- 13.Ishizaka Y, Motoyoshi K, Hatake K, Saito M, Takaku F, Miura Y. Mode of action of human urinary colony-stimulating factor. Exp Hematol. 1986;14:1–8. [PubMed] [Google Scholar]
- 14.Karbassi A, Becker J M, Foster J S, Moore R N. Enhanced killing of Candida albicans by murine macrophages treated with macrophage colony-stimulating factor: evidence for augmented expression of mannose receptors. J Immunol. 1987;139:417–421. [PubMed] [Google Scholar]
- 15.Kullberg B J, Van't Wout J W, Poell R J M, Van Furth R. Combined effect of fluconazole and recombinant human interleukin-1 on systemic candidiasis in neutropenic mice. Antimicrob Agents Chemother. 1992;36:1225–1229. doi: 10.1128/aac.36.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legrand P, Vertut-Doi A, Bolard J. Comparative internalization and recycling of different amphotericin B formulations by a macrophage-like cell line. J Antimicrob Chemother. 1996;37:519–533. doi: 10.1093/jac/37.3.519. [DOI] [PubMed] [Google Scholar]
- 17.Louie A, Baltch A L, Franke M A, Smith R P, Gordon M A. Comparative capacity of antifungal agents to stimulate murine macrophages to produce tumor necrosis factor alpha: an effect that is attenuated by pentoxifylline, liposomal vesicles, and dexamethasone. J Antimicrob Chemother. 1994;34:975–987. doi: 10.1093/jac/34.6.975. [DOI] [PubMed] [Google Scholar]
- 18.Metcalf D. The molecular biology and function of the granulocyte-macrophage colony-stimulating factor. Blood. 1986;67:257–267. [PubMed] [Google Scholar]
- 19.Motoyoshi K, Suda T, Kusumoto K, Takaku F, Miura Y. Granulocyte-macrophage colony-stimulating and binding activities of purified human urinary colony-stimulating factor to murine and human bone marrow cells. Blood. 1982;60:1378–1386. [PubMed] [Google Scholar]
- 20.Motoyoshi K, Yoshida K, Hatake K, Saito M, Miura Y, Yanai N, Yamada M, Kawashima T, Wong G G, Temple P A, Leary A C, Witek-Giannoti J S, Fujisawa M, Yuo A, Okabe T, Takaku F. Recombinant and native human urinary colony-stimulating factor directly augments granulocytic and granulocyte-macrophage colony-stimulating factor production of human peripheral blood monocytes. Exp Hematol. 1989;17:68–71. [PubMed] [Google Scholar]
- 21.Nassar F, Brummer E, Stevens D A. Effect of in vivo macrophage colony-stimulating factor on fungistasis of bronchoalveolar and peritoneal macrophages against Cryptococcus neoformans. Antimicrob Agents Chemother. 1994;38:2162–2164. doi: 10.1128/aac.38.9.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassar F, Brummer E, Stevens D A. Macrophage colony-stimulating factor (M-CSF) induction of enhanced anticryptococcal activity in human monocyte-derived macrophages: synergy with fluconazole for killing. Cell Immunol. 1995;164:113–118. doi: 10.1006/cimm.1995.1149. [DOI] [PubMed] [Google Scholar]
- 23.Nemunaitis J, Meyers J D, Buckner C D, Shannon-Dorcy K, Mori M, Shulman H, Bianco J A, Higano C S, Groves E, Storb R, Hansen J, Appelbaum F R, Singer J W. Phase I trial of recombinant human macrophage colony-stimulating factor in patients with invasive fungal infections. Blood. 1991;78:907–913. [PubMed] [Google Scholar]
- 24.Nemunaitis J, Shannon-Dorcy K, Appelbaum F R, Meyers J, Owens A, Day R, Ando D, O'Neill C, Buckner D, Singer J. Long-term follow up of patients with invasive fungal disease who received adjunctive therapy with recombinant human macrophage colony-stimulating factor. Blood. 1993;82:1422–1427. [PubMed] [Google Scholar]
- 25.Pawelec G, Ehninger G, Rehbein A, Schaudt K, Jaschonek K. Comparison of the immunosuppressive activities of the anti-mycotic agents itraconazole, fluconazole, ketoconazole and miconazole on human T-cells. Int J Immunopharmacol. 1991;13:299–304. doi: 10.1016/0192-0561(91)90111-j. [DOI] [PubMed] [Google Scholar]
- 26.Polak-Wyss A. Protective effect of human granulocyte colony stimulating factor (hG-CSF) on Candida infections in normal and immunosuppressed mice. Mycoses. 1991;34:109–118. doi: 10.1111/j.1439-0507.1991.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 27.Roilides E, Lyman C A, Mertins S D, Cole D J, Venzon D, Pizzo P A, Chanock S J, Walsh T J. Ex vivo effects of macrophage colony stimulating factor on human monocyte activity against fungal and bacterial pathogens. Cytokine. 1996;8:42–48. doi: 10.1006/cyto.1996.0006. [DOI] [PubMed] [Google Scholar]
- 28.Roilides E, Sein T, Holmes A, Chanock S, Blake C, Pizzo P A, Walsh T J. Effects of macrophage colony-stimulating factor on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Infect Dis. 1995;172:1028–1034. doi: 10.1093/infdis/172.4.1028. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai T, Yamada M, Shimamura S, Motoyoshi K. Recombinant human macrophage-colony stimulating factor suppresses the mouse mixed lymphocyte reaction. Cell Immunol. 1996;171:87–94. doi: 10.1006/cimm.1996.0177. [DOI] [PubMed] [Google Scholar]
- 30.Ulich T R, del Castillo J, Watson L R, Yin S, Garnich M B. In vivo hematologic effects of recombinant human macrophage colony-stimulating factor. Blood. 1990;75:846–850. [PubMed] [Google Scholar]
- 31.Vitt C R, Fidler J M, Ando D, Zimmerman R J, Aukerman S L. Antifungal activity of recombinant human macrophage colony-stimulating factor in models of acute and chronic candidiasis in the rat. J Infect Dis. 1994;169:369–374. doi: 10.1093/infdis/169.2.369. [DOI] [PubMed] [Google Scholar]
- 32.Warren M K, Ralph P. Macrophage growth factor CSF-1 stimulates human monocyte production of interferon, tumor necrosis factor, and colony stimulating activity. J Immunol. 1986;137:2281–2285. [PubMed] [Google Scholar]
- 33.Wildfeuer A, Laufen H, Haferkamp O. Interaction of fluconazole and human phagocytic cells: uptake of the antifungal agent and its effects on the survival of ingested fungi in phagocytes. Arzneim-Forsch/DrugRes. 1990;40:1044–1047. [PubMed] [Google Scholar]
- 34.Wolf J E, Masoff S E. In vivo activation of macrophages oxidative burst activity by cytokines and amphotericin B. Infect Immun. 1990;58:1296–1300. doi: 10.1128/iai.58.5.1296-1300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong G W, Temple P A, Leary A C, Witek-Giannotti J S, Yang Y C, Ciarletta A B, Chung M, Murtha P, Kiriz R, Kaufman R J, Ferenz C R, Sibley B S, Turner K J, Hewick R M, Clark S C, Yanai N, Yamada M, Saito M, Motoyoshi K, Takaku F. Human CSF-1: molecular cloning and expression of 4-kb cDNA encoding the human urinary protein. Science. 1987;235:1504–1508. doi: 10.1126/science.3493529. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi H, Abe S, Tokuda Y. Immunomodulating activity of antifungal drugs. Ann N Y Acad Sci. 1993;685:447–457. doi: 10.1111/j.1749-6632.1993.tb35905.x. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Uchida K, Klein T W, Friedman H, Yamaguchi H. Immunomodulators and fungal infections: use of antifungal drugs in combination with G-CSF. In: Friedman H, editor. Microbial infections. New York, N.Y: Plenum Press; 1992. pp. 231–241. [DOI] [PubMed] [Google Scholar]