Abstract
Background
Preterm infants experience a range of morbidity related to the immaturity of their organ systems and to concurrent disease states. There is concern that an unfavourable environment in the neonatal intensive care unit (NICU) may compound this morbidity. Modification of the environment could minimize the iatrogenic effects. Developmental care is a broad category of interventions designed to minimize the stress of the NICU environment. These interventions may include elements such as control of external stimuli (vestibular, auditory, visual, tactile), clustering of nursery care activities, and positioning or swaddling of the preterm infant. Individual strategies have also been combined to form programs, such as the 'Newborn Individualized Developmental Care and Assessment Program' (NIDCAP) (Als 1986).
Objectives
In preterm infants, do developmental care interventions reduce neurodevelopmental delay, poor weight gain, length of hospital stay, length of mechanical ventilation, physiological stress and other clinically relevant adverse outcomes?
Search methods
The Neonatal Review Group search strategy was utilized. Searches were made of MEDLINE from 1966 to June, 2005 and of CINAHL, The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2005), and conference and symposia proceedings in the English language from 1990 to June, 2005. A search of EMBASE was also made from 2003 to June 2005. A list of all relevant articles was sent to two experts in the field to identify any omissions or additional unpublished studies.
Selection criteria
Randomized trials in which elements of developmental care are compared to routine nursery care for infants < 37 weeks gestation and that measured clinically relevant outcomes. Reports were in English or a language for which a translator was available.
Computerized searches were conducted and all potentially relevant titles and abstracts were extracted. Retrieved articles were assessed for relevance independently by two reviewers, based on predetermined criteria. Articles that met all criteria for relevance were assessed for methodological quality based on predetermined criteria. Articles judged to have the appropriate quality by both reviewers were included in the analysis.
Data collection and analysis
Data were extracted independently by the two authors. Meta‐analyses were conducted for each intervention where the same outcome measures and/or instruments were used within comparable time points.
Main results
This review detected 36 eligible randomized controlled trials involving four major groups of developmental care interventions, 19 sub‐groups and multiple clinical outcomes. In addition, the long‐term outcomes of a previously included trial were added to the review.
The results of the review indicate that developmental care interventions demonstrate limited benefit to preterm infants with respect to: decreased moderate‐severe chronic lung disease, decreased incidence of necrotizing enterocolitis and improved family outcome. Conversely, an increase in mild lung disease and an increase in the length of stay were demonstrated in infants receiving developmental care compared to controls. There is also very limited evidence of the long‐term positive effect of NIDCAP on behavior and movement at 5 years corrected age but no effect on cognition. Other individualized developmental care interventions have also demonstrated some effect in enhancing neurodevelopmental outcome. Although a limited number of other benefits were demonstrated, those results were from single studies with small sample sizes. The lack of blinding of the assessors was a significant methodological flaw in half of the studies. The cost of the interventions and personnel was not considered in any of the studies.
Authors' conclusions
Because of the inclusion of multiple interventions in most studies, the determination of the effect of any single intervention is difficult. Although there is evidence of limited benefit of developmental care interventions overall, and no major harmful effects reported, there were a large number of outcomes for which no or conflicting effects were demonstrated. The single trials that did show a significant effect of an intervention on a major clinical outcome were based on small sample sizes, and the findings were often not supported in other small trials.
Before a clear direction for practice can be supported, evidence demonstrating more consistent effects of developmental care interventions on important short‐ and long‐term clinical outcomes is needed. The economic impact of the implementation and maintenance of developmental care practices should be considered by individual institutions.
Keywords: Humans; Infant, Newborn; Infant, Premature; Developmental Disabilities; Developmental Disabilities/prevention & control; Environment, Controlled; Intensive Care, Neonatal; Intensive Care, Neonatal/methods; Intensive Care, Neonatal/standards; Length of Stay; Randomized Controlled Trials as Topic; Respiration, Artificial; Stress, Physiological; Stress, Physiological/prevention & control; Treatment Outcome; Weight Gain
Plain language summary
Developmental care for promoting development and preventing morbidity in preterm infants
Developmental care interventions may help preterm infants cope better with the environment of the Neonatal Intensive Care Unit (NICU). Preterm infants (babies born before 37 weeks) can develop a range of problems because their organs are not mature. There is concern that an unfavourable environment in the NICU can add to these problems and negatively affect the infant's growth, with the brain being particularly vulnerable. Developmental care refers to a range of strategies designed to reduce the stresses of the NICU. These include reducing noise and light, minimal handling and giving longer rest periods. The review of trials suggests that these interventions may have some benefit to the outcomes of preterm infants; however, there continues to be conflicting evidence among the multiple studies. Before a clear direction for practice can be supported, evidence demonstrating more consistent effects of developmental care interventions on important short‐ and long‐term clinical outcomes is needed. The economic impact of the implementation and maintenance of developmental care practices should be considered by individual institutions.
Background
The striking difference between the intrauterine environment and the neonatal intensive care unit (NICU) is obvious. The sensory impact of the NICU has been postulated to adversely influence the neurodevelopmental outcome of preterm infants. However, it is unclear from the published literature whether environmental stimuli which are of high intensity (Cornell 1976), or low intensity (Rothchild 1966) or are simply inappropriate in their nature (Lawson 1977; Gottfried 1985) are more detrimental to the infant's development.
'Developmental care', introduced in the mid 1980's (Als 1986), provides a strategy to address the environmental concerns. Developmental care is an approach that was designed to modify the NICU environment so as to minimize the stress experienced by the preterm infant. Different strategies have been used to modify the extrauterine environment to decrease a variety of stresses including noise and light reduction, minimal handling and the provision of longer rest periods.
Preterm infants experience a range of morbidity related to the immaturity of their organ systems and to concurrent disease states. There is concern that an unfavourable environment in the NICU may compound this morbidity. Modification of the environment could minimize the iatrogenic effects. A negative impact of the NICU environment can be manifested in a number of ways by the preterm infant. Typical markers of stress are physiological parameters such as increased heart rate and decreased oxygen saturation. Growth of the infant is negatively affected by increased energy expenditure which may occur during routine nursery care. The adverse effects of the environment might also extend the infant's recovery from typical preterm illnesses. The preterm infant's rapidly developing brain is particularly vulnerable to a stressful environment. The detrimental effects of this stress could have short and long term implications for compromised neurobehavioural development.
Developmental care is a broad category of interventions that is designed to minimize the stress of the NICU environment. A number of elements are included under the umbrella of developmental care such as control of external stimuli (vestibular, auditory, visual, tactile), clustering of nursery care activities, and positioning or swaddling of the preterm infant so as to provide a sense of containment similar to the intrauterine experience. One or more of these elements may be included in developmental care interventions. Programs such as the 'Newborn Individualized Developmental Care and Assessment Program' (NIDCAP) (Als 1986), utilize a combination of these strategies depending upon the needs of each infant. Developmental care interventions that are individualized to the needs of the infant include a pre‐assessment using an instrument designed for this purpose. Behavioural observations are conducted on each baby, including respiratory status, colour, visceral responses (e.g. gagging, hiccoughing), motor state (e.g. tone, posture), facial expressions (e.g. grimace, smile), and attention (Als 1984). This individualized assessment is used as a measure of the infant's tolerance to the environment and caregiving activities. The findings then become the basis for the developmental interventions used to decrease the postulated detrimental effects of the neonatal intensive care environment.
Objectives
The overall objective of this review is to examine the evidence for the beneficial or adverse effects of developmental care in preterm infants. The specific categories of developmental interventions included in this review are: i) positioning ii) clustering of nursery care activities iii) modification of external stimuli iv) individualized developmental care interventions
The primary objective is to estimate the effect of developmental care interventions on neurobehavioural development, as well as other clinically important outcomes.
The effects of the specific interventions, listed by category of developmental care intervention, were assessed using the following comparisons: i) Intentional Positioning a) nesting vs no nesting b) prone vs supine c) swaddling vs no swaddling
ii) Clustering of Nursery Care Activities (no trials were found in this category)
iii) Modification of External Stimuli a) vestibular stimulation vs control b) auditory stimulation vs control c) visual stimulation vs control d) tactile stimulation vs control e) vestibular and auditory stimulation vs control f) vestibular and visual stimulation vs control g) vestibular and tactile stimulation vs control h) auditory and visual stimulation vs control i) auditory and tactile stimulation vs control j) visual and tactile stimulation vs control k) vestibular, auditory and visual stimulation vs control l) vestibular, auditory and tactile stimulation vs control m) auditory, visual and tactile stimulation vs control n) vestibular, auditory, visual, tactile vs control
iv) Individualized Developmental Care a) NIDCAP vs control b) other individualized interventions vs control
Methods
Criteria for considering studies for this review
Types of studies
All randomized trials, including cluster trials, in which elements of developmental care are compared to routine nursery care.
Types of participants
Infants < 37 weeks gestation.
Types of interventions
Developmental care is a broad term that is used to describe a variety of interventions that control the NICU environment. One or more interventions can be used for each infant according to the infant's needs. Interventions eligible for inclusion in this review are those that evaluate: a) positioning b) clustering of nursery care activities c) modification of external stimuli d) individualized developmental care interventions
Types of outcome measures
a) neurobehavioural development as measured by standardized instruments, including Bayley Scales of Infant Development, Neurobehavioral Assessment of the Preterm Infant, Assessment of Preterm Infant's Behavior b) weight gain c) length of hospital stay d) length of mechanical ventilation e) physiological parameters including heart rate, oxygen saturation f) other clinically relevant outcomes
Search methods for identification of studies
See: Neonatal Review Group search strategy. Searches were made of MEDLINE from 1966 to June, 2005. In addition the following were searched: CINAHL, The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2005), and conference and symposia proceedings in the English language including the National Association of Neonatal Nurses, and the American Pediatric Society/Society for Pediatric Research, from 1990 to June, 2005; and EMBASE from 2003 to June, 2005. Computerized searches were conducted using the following MeSH headings: prematurity, clinical trial, acoustic stimulation, noise, physical stimulation, touch, baths, nursing care, stress, child development, music or music therapy, environment, infant warmer, incubators, and kinesthesis. The following text words were also used: developmental care (tw), infant stimulation (tw), lighting in the NICU (tw), auditory stimulation in infants (tw), visual stimulation (tw), vestibular stimulation (tw), tactile stimulation (tw), minimal handling (tw), infant positioning (tw), infant over‐stimulation‐touch (tw), NICU light (tw), swaddling (tw), NIDCAP (tw), and behavioral state (tw). All potentially relevant titles and abstracts identified by the reviewers were retrieved. The reference lists/bibliographies of each article were reviewed independently for additional relevant titles and these were also retrieved. A list of all relevant articles was sent to two experts in the field to identify any omissions or additional unpublished studies.
Data collection and analysis
The systematic review followed the method described in the Cochrane Collaboration Handbook. All of the articles that were retrieved from the search were assessed for relevance independently by the two reviewers, based on a review of the entire article. Criteria for relevance included trials that utilized randomized experimental designs, tested elements of developmental care in preterm infants and measured clinically relevant outcomes. The articles that met all relevance criteria were assessed for methodological quality independently by the two reviewers. Disagreements between the reviewers were resolved through discussion. Those articles judged to have the appropriate quality by both the reviewers were included in the analysis. Missing data were obtained from the original authors, where contact was possible and a response was received.
Analysis of the broad intervention of 'developmental care' was not possible because of the diversity of elements within that category. As described in Objectives, we assessed the effect of specific interventions after classifying them into four broad groups of developmental care intervention. Clusters of two or more elements of developmental care were treated as a single intervention; the individual elements within such combinations were not analyzed in isolation.
Treatment effect on outcomes reported as dichotomous variables were analyzed using relative risk and risk difference and their 95% confidence intervals. Treatment effects on outcomes from single trials measured as continuous variables were analyzed using mean difference and its 95% confidence interval; for multiple trials, weighted mean difference was calculated. We conducted meta‐analyses, using a fixed effect model, in instances where more than one trial assessed treatment effect on the same outcome in similar populations, and used similar outcome measures and/or instruments. Heterogeneity was evaluated using the I2 statistic.
Results
Description of studies
All of the 36 studies that met the relevance criteria were included in the review. When comparing the four categories of developmental care interventions (positioning, clustering of nursery care activities, modification of external stimuli, and individualized developmental care), a number of similarities and differences are apparent. The number of trials within each category varied from 0 to 22. The total sample sizes in the individual studies ranged from 16 to 259, but the sample size in 22 of the studies was less than 50. The sample sizes among the trials was less variable in the positioning and individualized developmental care categories than in the modification of external stimuli category. The individualized developmental care trials were unique in the fact that those interventions required specially trained personnel to administer. In the other two categories with trials, the regular nursing staff provided the interventions. The infants in the individualized developmental care studies were generally more sick than infants in the other categories, who were non‐ventilated, stable preterm infants. The interventions in the modification of external stimuli trials were usually of shorter duration than in the positioning or individualized developmental care trials (days on intervention versus weeks). In keeping with this difference, the outcomes in the positioning studies were shorter term than in the modification of external stimuli or individualized developmental care studies, where both short and long term outcomes were included. A large number of outcomes was examined in the 36 studies, but only a few were common among them. Among the studies having common outcomes, the methods of measurement varied. Details of each included study are in the Table of Included Studies.
Fifty studies were excluded from the review. The reason for exclusion in all but four cases was because they did not meet one or more of the relevance criteria. In two cases, a trial was included in a previous review and in two cases, the population was restricted to a subgroup of preterm infants.
Risk of bias in included studies
The included studies were assessed using the following key criteria: blindness of randomization, blindness of intervention, completeness of follow‐up and blinding of outcome measurement. Completeness of follow‐up was considered adequate if less than 10% of the randomized subjects were lost to follow‐up. Additional criteria of study quality included: absence of co‐intervention bias, objective criteria for measuring outcomes and defined exclusion/inclusion criteria. The developmental care interventions cannot be blind to those providing care but should be blind to the assessors of the outcomes. Of the 36 studies, the allocation concealment was adequate in 10 studies, unclear in 23 studies, and inadequate in three studies. In 18 studies, blinding of the assessors was either partial, absent or unclear. Seventeen studies demonstrated clear evidence of complete follow‐up of study participants. It should be noted that none of the studies met all of the methodological quality criteria.
Effects of interventions
This review detected 36 eligible randomized controlled trials, randomizing a total of 2220 participants. The majority of these trials were of small sample size, however. There are two follow‐up studies originating from Westrup 2000: Kleberg 2002 (one year follow‐up) and Westrup 2004 (five year follow‐up). The results will be presented by category of developmental intervention.
I) POSITIONING
a) Nesting versus no nesting Length of hospital stay (two trials): Beckman 1997 found a significantly longer length of hospital stay in the nested infants (mean difference 8.1 days, 95% CI 0.1, 16.1). However, Aebi 1991 found no significant difference between groups in length of stay. A meta‐analysis could not be performed because standard deviations were not reported by Aebi 1991. However, there is no evidence from these two studies that nesting shortens duration of hospital stay.
Weight at discharge from study (one trial): Beckman 1997 found no difference between groups.
b) Swaddling versus no swaddling Neurobehavioural development (one trial): Short 1996 found a significantly higher score on the Morgan Neonatal Neurobehavioral Exam in the swaddled group (mean difference 6.2, 95% CI 2.6, 9.8). A higher score indicates higher behavioural maturation at 34 weeks corrected age.
c) Prone versus supine There were no randomized trials comparing prone versus supine positioning.
II) CLUSTERING OF NURSERY CARE ACTIVITIES
No randomized trials were found which tested the effect of clustering of nursery care activities.
III) MODIFICATION OF EXTERNAL STIMILUI
The comparison of modification of external stimuli versus control was divided into multiple sub‐groups to allow analysis of effects of individual elements (visual, auditory, tactile or vestibular stimulation), or a combination of some or all of these stimuli.
a) Vestibular stimulation versus control Physiologic parameters (two trials): Korner 1975 found no evidence of effect of vestibular stimulation on the infant's heart rate, respiratory rate, or body temperature. Keller 2003 found a significantly lower heart rate and respiratory rate in the experimental group. These two trials demonstrate conflicting evidence that vestibular stimulation improves physiologic outcomes.
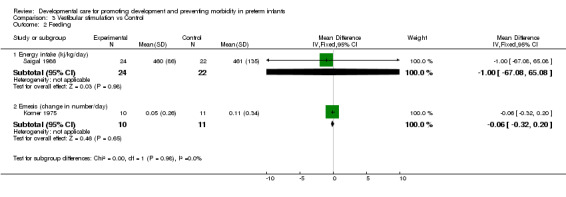
Feeding (two trials): Saigal 1986 found no evidence of effect of vestibular stimulation on energy intake. Korner 1975 found no evidence of effect of vestibular stimulation on frequency of emesis. There is no evidence from these two studies that vestibular stimulation improves feeding outcomes.
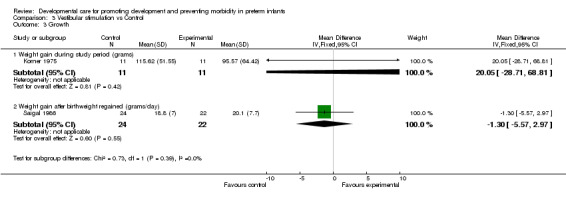
Growth (three trials): Korner 1975 and Keller 2003 reported no differences in weight gain during the study period. Saigal 1986, who reported weight gain after birth weight was regained, also found no significant effect of vestibular stimulation on weight gain. There is no evidence from these three studies that vestibular stimulation improves growth.
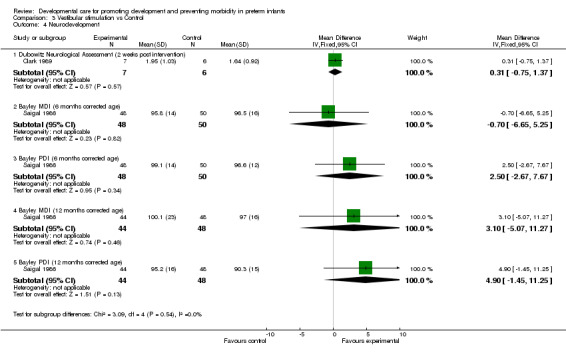
Neurodevelopment (five trials): Clark 1989 found no evidence of effect of vestibular stimulation on the Dubowitz Neurological Assessment administered two weeks post‐intervention. Saigal 1986 found no evidence of effect on the Bayley Scales of Infant Development, either MDI or PDI, administered at either 6 or 12 months corrected age. Darrah 1994 reported a number of neurodevelopmental outcomes at several time points to 18 months corrected age and found no differences between groups. Korner 1983 reported a significant effect in favor of the experimental group on some items of the neurobehavioral assessment at 34 ‐ 35 weeks post conceptional age as measured by the LAPPI (orientation to visual and auditory stimuli; spontaneous motor behavior; irritability and/or hypertonicity; time spent in visual alert, inactive state). Only summary scores were reported. Keller 2003 found a significantly higher neuromuscular maturity score in the experimental group. These five trials measuring neurodevelopment using different instruments, demonstrate conflicting evidence that vestibular stimulation improves neurodevelopmental outcomes.
Sleep/Wake states (two trials): Thoman 1991 found that vestibular stimulation resulted in significantly more frequent quiet sleep states and longer periods in quiet sleep post‐term. Cordero 1986 found that infants in the vestibular stimulation group showed an increase in the proportion of quiet sleep. No tables are available as these data could not be combined in a meta‐analysis because of variations in outcome measurement. However, there is evidence that vestibular stimulation facilitates quiet sleep.
Age at discharge (one trial): Thoman 1991 found no evidence of effect of vestibular stimulation on age at discharge. No table is available for this outcome as standard deviations were not reported by the author.
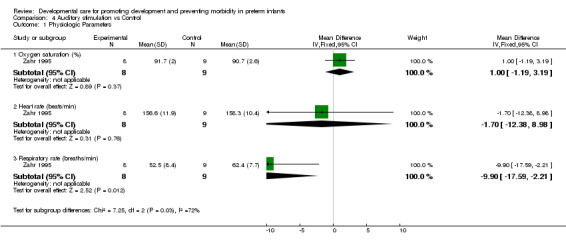
b) Auditory stimulation versus control Physiologic parameters (one trial): Zahr 1995 found no evidence of effect of auditory stimulation on heart rate, and oxygen saturation; but did find that auditory stimulation significantly lowered respiratory rate (mean difference ‐9.9 breaths/min, 95% CI ‐17.6, ‐2.2). Growth (one trial): Chapman 1984 found no evidence of effect of auditory stimulation on days to regain birth weight or on weight, length or head circumference at discharge.
c) Visual stimulation versus control No randomized trials were found which tested the effect of visual stimulation.
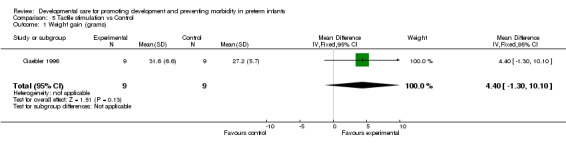
d) Tactile stimulation versus control Weight gain (three trials): Scott 1983 found that overall weight gain was significantly greater in the experimental group. Gaebler 1996 found a trend to greater weight gain in the experimental group, but it did not achieve statistical significance. A meta‐analysis of these two trials could not be performed on the weight gain outcome because Scott 1983 did not report standard deviations; but these trials appear to provide some evidence that tactile stimulation improves short‐term weight gain. Helders 1989 found a significant positive effect of tactile stimulation on weight‐for‐length at 12 months corrected age in girls.
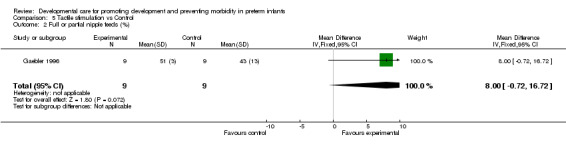
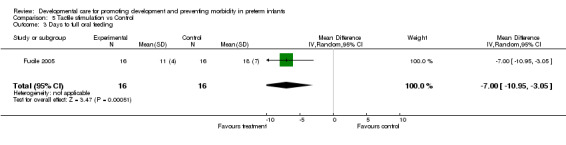
Full or partial nipple feeds (one trial): Gaebler 1996 found no evidence of effect of tactile stimulation on the ability to achieve full or partial nipple feeds. Days to full oral feeding (one trial): Fucile 2005 found a significantly shorter length of time to full oral feedings in the experimental group (seven days).
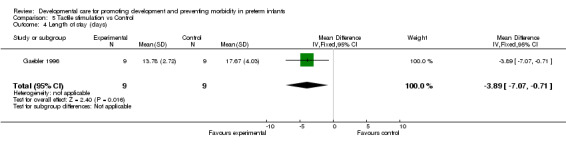
Length of hospital stay (one trial): Gaebler 1996 found a significantly shorter length of stay in infants receiving tactile stimulation (mean difference ‐3.9 days, 95% CI ‐7.1, ‐0.7).
e) Vestibular and auditory stimulation versus control Feeding (one trial): Gatts 1994 found no evidence of effect of vestibular and auditory stimulation on length of time tube feeding, or on energy intake. Growth (two trials): Gatts 1994 found no evidence of effect of vestibular and auditory stimulation on weight at discharge. Kramer 1976 found a significantly better weight gain (mean difference 46.0 grams, 95% CI 17.1, 74.9) and head circumference gain (mean difference 0.3 cm/week, 95% CI 0.1, 0.4) to 36 weeks corrected age in infants receiving vestibular and auditory stimulation. There are conflicting results with respect to the benefit of vestibular and auditory stimulation on growth outcomes.
Length of stay (one trial): Gatts 1994 found a shorter length of hospital stay in infants receiving vestibular and auditory stimulation which, after controlling for gestational age and weight upon entry to the study, reached statistical significance. Neurodevelopment (three trials): Gatts 1994 found significantly higher Brazelton Neonatal Behavioral Assessment Scale scores on Orientation and Range of State cluster scores in infants receiving vestibular and auditory stimulation; while Kramer 1976 did not demonstrate any differences. A meta‐analysis could not be performed on this outcome because values were not reported by Kramer 1976 and because it was not clear at what time point Gatts' assessment was made. Barnard 1983 found significantly higher Bayley MDI scores in the experimental group at 24 months corrected age. These three trials demonstrate conflicting results with respect to the benefit of vestibular and auditory stimulation on neurodevelopment outcomes.
f) Vestibular and visual stimulation vs control No randomized trials were found that compared vestibular and visual stimulation versus control, or on energy intake.
g) Vestibular and tactile stimulation vs control No randomized trials were found that compared vestibular and tactile stimulation versus control.
h) Auditory and visual stimulation vs control Feeding (one trial): Mann 1986 found time feeding was significantly shorter in infants receiving auditory and visual stimulation. No table is available for this outcome as the means and standard deviations were not reported by the author. Growth (one trial): Mann 1986 found weight gain was significantly better in infants receiving auditory and visual stimulation. No table is available for this outcome as the means and standard deviations were not reported by the author.
Sleep/Wake states (one trial): Mann 1986 found hours of sleep were significantly longer in infants receiving auditory and visual stimulation. No table is available for this outcome as the standard deviations were not reported by the author.
i) Auditory and tactile stimulation vs control No randomized trials were found that compared auditory and tactile stimulation versus control.
j) Visual and tactile stimulation vs control No randomized trials were found that compared visual and tactile stimulation versus control.
k) Vestibular, auditory and visual stimulation vs control No randomized trials were found that compared vestibular, auditory and visual stimulation versus control.
l) Vestibular, auditory and tactile stimulation vs control No randomized trials were found that compared vestibular, auditory and tactile stimulation versus control.
m) Auditory, visual and tactile stimulation vs control No randomized trials were found that compared auditory, visual and tactile stimulation versus control.
n) Vestibular, auditory, visual and tactile stimulation vs control Physiologic parameters (two trials): White‐Traut 1993 found no evidence of effect of vestibular, auditory, visual and tactile stimulation on pulse rate and oxygen saturation. In the 1997 trial, however, White‐Traut did demonstrate significantly lower pulse and respiratory rates in infants receiving vestibular, auditory, visual and tactile stimulation. No table is available as a meta‐analysis was not performed because means and standard deviations were given over multiple time periods. The trials demonstrate conflicting results with respect to the benefit of vestibular, auditory, visual and tactile stimulation on physiologic parameters.
Feeding (two trials): White‐Traut 1988 found that vestibular, auditory, visual and tactile stimulation resulted in improved Nursing Child Assessment Feeding Scale scores. White‐Traut 2002 found that vestibular, auditory, visual and tactile stimulation resulted in a faster transition to complete nipple feeding. No table is available for this outcome as the standard deviations were not reported by the author.
Neurodevelopment (three trials): In the 1993, 1997, and 2002 studies, White‐Traut found significantly better behavioural states in infants receiving vestibular, auditory, visual and tactile stimulation. A meta‐analysis could not be performed because no values were available from the author. In addition, the 2002 study differs from the other two studies in time of outcome assessments and in timing of observations. All of the reports suggest however, that vestibular, auditory, visual and tactile stimulation improve behavioural states. Length of hospital stay (one trial): White‐Traut 2002 found that infants receiving vestibular, auditory, visual and tactile stimulation were discharged significantly earlier than control infants. No table is available for this outcome as no standard deviations were reported by the author.
IV) INDIVIDUALIZED DEVELOPMENTAL CARE
a) NIDCAP vs control Respiratory support in infants surviving to discharge (five trials): Infants receiving NIDCAP had significantly fewer ventilation days based on the meta‐analysis (Als 1986; Als 1994; Westrup 2000) (WMD ‐8.3 days, 95% CI ‐15.82, ‐0.77). NIDCAP resulted in no differences in days in oxygen (Als 1986, Als 1994, Als 2004) (WMD ‐1.78 days, 95% CI ‐12.6, 9.04). There was, however, significant heterogeneity among the studies so that the results should be viewed with caution. Westrup 2000 demonstrated a lower age for oxygen withdrawal (weeks) for infants receiving NIDCAP (WMD ‐5.9, 95% CI ‐8.37, ‐3.43), as well as fewer days on CPAP (WMD ‐17.1 days, 95% CI ‐29.54, ‐4.66). Als 2003 found no group differences in respiratory support based on a three‐site trial. However, the data could not be included in the meta‐analysis because there were site differences. The evidence of the effect of NIDCAP on respiratory support from these five trials is conflicting.
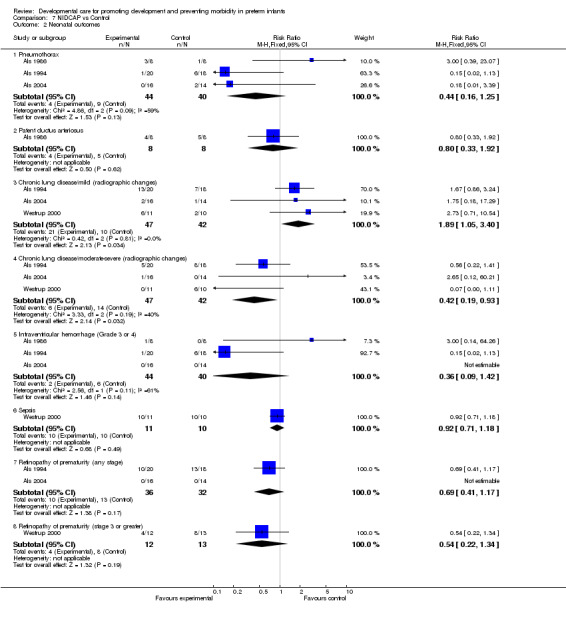
Neonatal outcomes in infants surviving to discharge (five trials): The meta‐analysis of Als 1986, Als 1994 and Als 2004 found no evidence of the effect of NIDCAP on incidence of pneumothorax (RR 0.44, 95% CI 0.16, 1.25). Als 1986 found no evidence of effect on incidence of PDA. The meta‐analysis of Als 1994, Als 2004 and Westrup 2000 which assessed the effect of NIDCAP on chronic lung disease (radiographic changes), found evidence of effect in favour of the control group in mild disease (RR 1.89, 95% CI 1.05, 3.40). Conversely, with moderate to severe chronic lung disease, a significant effect was found in favour of the NIDCAP group (RR 0.42, 95% CI 0.19, 0.93). The meta‐analysis of Als 1986, Als 1994 and Als 2004 found no evidence that NIDCAP affected the incidence of IVH, Grade 3 or 4 (RR 0.36, 95% CI 0.09, 1.42). Westrup 2000 found no evidence of effect of NIDCAP on incidence of either sepsis or ROP (stage 3 or greater). The meta‐analysis of Als 1994 and Als 2004 found no evidence of effect on incidence of ROP (any stage). Als 2003 found a benefit in favour of NIDCAP on the incidence of necrotizing enterocolitis. In summary, a statistically significant effect of NIDCAP was found on moderate‐severe chronic lung disease and incidence of necrotizing enterocolitis.
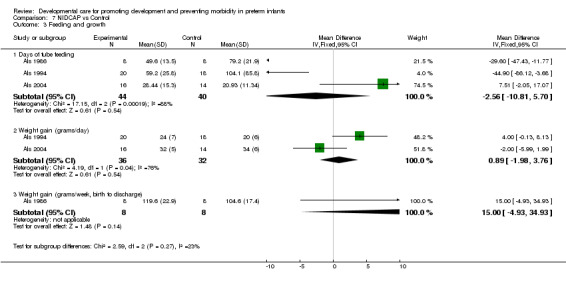
Feeding and growth in infants surviving to discharge (five trials): The meta‐analysis of Als 1986, Als 1994 and Als 2004 demonstrated no effect of NIDCAP in reducing tube feeding days (WMD ‐2.56 days, 95% CI ‐10.81, 5.7). The meta‐analysis of Als 1994, 2004 found no effect of NIDCAP on weight gain (gm/day) (WMD 0.89 grams/day, 95% CI ‐1.98, 3.76). There was however, significant heterogeneity among the studies so that the results should be viewed with caution. Westrup 2000 also found no differences between groups on weight gain (gm/day) (standard deviations not available). Als 1986 found no evidence of effect of NIDCAP on weekly weight gain (gms). Westrup 2000 found no evidence of effect of NIDCAP on head growth (cm/wk). Als 2003 found no group differences in days of parenteral feeding, transition to full enteral feeding, average daily weight gain and growth at two weeks after the EDC. Als 2004 also found no differences in growth at both 2 weeks and 9 months corrected age. In summary, NIDCAP did not effect feeding and growth in infants surviving to nine months corrected age.
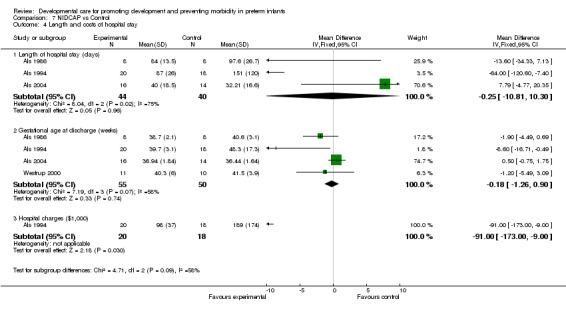
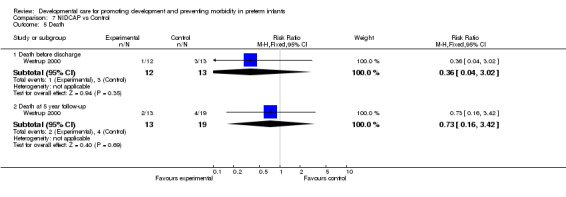
Length and cost of hospital stay in infants surviving to discharge (five trials): NIDCAP had no effect on gestational age at discharge (Als 1986; Als 1994; Als 2004 and Westrup 2000) (WMD ‐0.18 weeks, 95% CI ‐1.26, 0.90). The meta‐analysis of Als 1986, Als 1994 and Als 2004 found no differences between groups on hospital stay (WMD ‐0.25 weeks, 95% CI ‐10.81, 10.3). The infants included in the Als 2004 study were a more mature group of infants. Als 1994 found significantly lower hospital charges for infants receiving NIDCAP (mean difference ‐91 (US$1000), 95% CI ‐173, ‐9). Als 2003 found no group differences in length of stay and hospital charges based on a three‐site trial. However, the data could not be included in the meta‐analysis because there were site differences. In summary, only one of the five trials found a positive effect of NIDCAP on lower hospital charges. No studies evaluated the economic cost of NIDCAP, however. Death (one trial): Westrup 2000 found no evidence of effect of NIDCAP on death before discharge or at five year follow‐up.
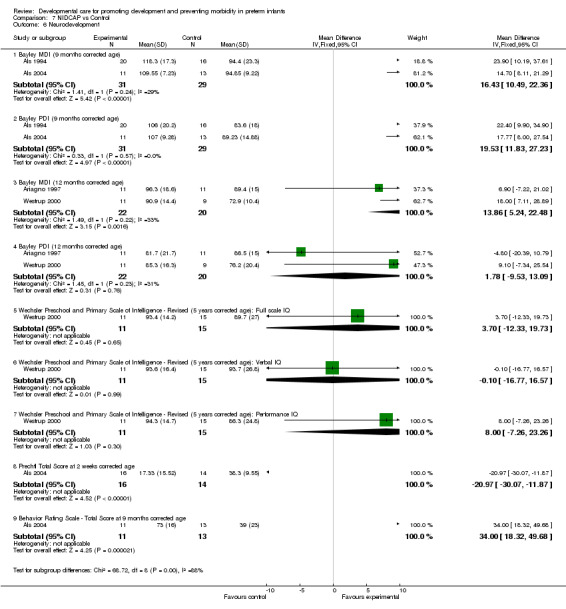
Neurodevelopment (four trials): The evidence of effect of NIDCAP on neurodevelopment from these four trials is conflicting. The meta‐analysis of Als 1994 and Als 2004 found a significant effect of NIDCAP on Bayley scores at 9 months corrected age in the 60 infants tested (MDI: mean difference 16.43, 95% CI 10.49, 22.36) (PDI: mean difference 19.53, 95% CI 11.83, 27.23). The infants included in the Als 2004 study were a more mature group of infants. The meta‐analysis of Ariagno 1997 and Westrup 2000 found a significant difference in Bayley MDI at 12 months corrected age (WMD 13.86, 95% CI 5.24, 22.48) but no difference in the Bayley PDI at 12 months corrected age. Based on the Westrup 2000 study subjects, Kleberg 2002 found significantly higher MDI scores at 1 year of age in the NIDCAP group but no difference in PDI scores. Als 2003 and Als 2004 found significant group differences in APIB scores in favour of the NIDCAP group. Als 2004 also found significant differences in the Prechtl scores at two weeks corrected age in favour of the NIDCAP group. Based on the Westrup 2000 study subjects, Westrup 2004 report a significant positive impact on behavior and movement at five years in favour of the NIDCAP group, however there were no differences in cognition. Family outcome (one trial): Als 2003 found a significant group difference in family stress and perception of the child in favour of the NIDCAP group.
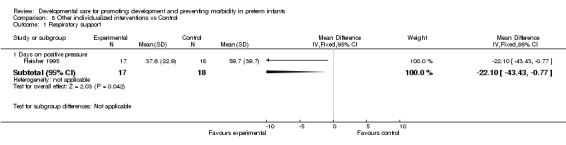
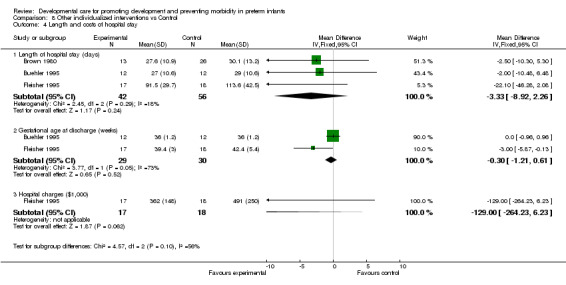
b) Other individualized interventions vs control Respiratory support (one trial): Fleisher 1995 found a significant effect of individualized developmental care interventions in reducing ventilation days (mean difference ‐22.1 days, 95% CI ‐43.4, ‐0.8).
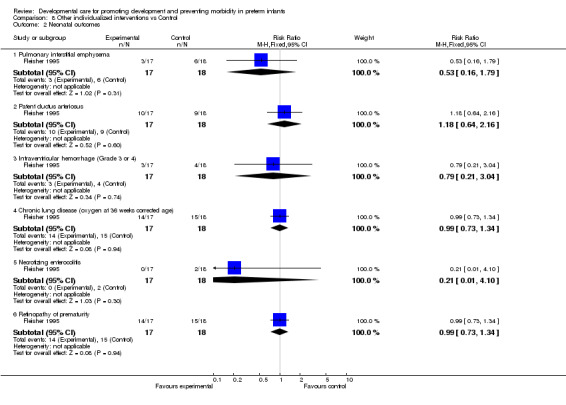
Neonatal outcomes (one trial): Fleisher 1995 found no evidence of effect of individualized developmental care interventions on incidence of PDA, chronic lung disease, IVH (Grade 3 or 4), NEC or ROP.
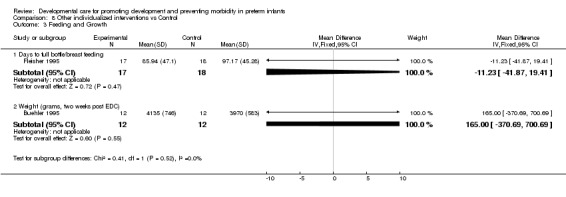
Feeding and growth (three trials): Neither Fleisher 1995, who measured days to full bottle/breast feeding, nor Buehler 1995, who measured weight at 2 weeks corrected age, found a significant of effect of individualized developmental care interventions on feeding or growth. A third trial, Brown 1980, found no effect on rate of weight gain and growth at 12 months (no data are reported).
Length and cost of hospital stay (three trials): Brown 1980 found no evidence of effect of individualized developmental care interventions on length of hospital stay. Buehler 1995 and Fleisher 1995 found no effect on gestational age at discharge or length of hospital stay. Fleisher 1995 found no effect on hospital charges.
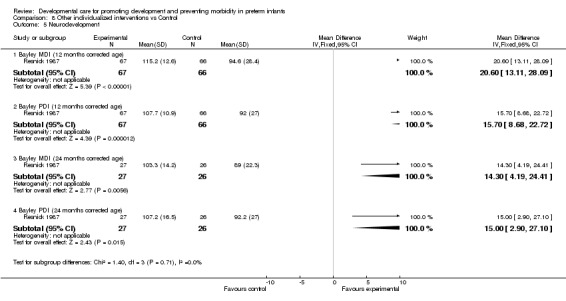
Neurodevelopment (three trials): Brown 1980 found no effect of individualized developmental care interventions on neurobehavioral assessment at discharge and at 12 months (no data are reported). Resnick 1987, Resnick 1988 found a significant effect on the Bayley Scales of Infant Development at 12 months corrected age. Values were only available for the 1987 study (PDI: mean difference 15.7, 95% CI 8.7, 22.7; MDI: mean difference 20.6, 95% CI 13.1, 28.1). Resnick 1987 also found a significant effect on MDI and PDI scores at 24 months corrected age (mean difference 15.0, 95% CI 2.9, 27.1). Evidence from these trials suggests that there is some effect of individualized developmental care interventions in enhancing neurodevelopmental outcomes.
Discussion
The developmental care interventions in this review included a wide range of outcomes. The interventions themselves were also very different from one another so the results could not be combined for an estimate of overall effectiveness. Although there is evidence of limited benefit of developmental care interventions and no major harmful effects reported, there were a large number of outcomes for which no or conflicting effects were demonstrated. The single trials that did show a significant effect of an intervention on a major clinical outcome were usually based on small sample sizes. A number of trials demonstrated a statistically significant effect of the intervention but the outcomes are of questionable clinical value. In addition, the cost of the interventions and personnel was not considered in any of the studies. Cost could be a significant factor in the overall evaluation of developmental care interventions. By comparison group, findings from this review indicate the following:
a) For modification of external stimuli, only two interventions demonstrated clinically important outcomes. Tactile stimulation demonstrated improved short‐term growth outcomes and shorter length of stay. Vestibular, auditory, visual and tactile stimulation demonstrated improved faster transition to full nipple feeding and shorter length of stay.
b) There is limited evidence of the effect of NIDCAP on moderate‐severe chronic lung disease, necrotizing enterocolitis and family outcome. There is also very limited evidence of the long‐term effect of NIDCAP on behavior and movement at five years corrected age but no effect on cognition. Other individualized developmental care interventions have also demonstrated some effect in enhancing neurodevelopmental outcomes. It should be noted, however, that the neurodevelopmental outcome results (up to 12 months corrected age) from the NIDCAP trials were conflicting with respect to benefit. The cost of the interventions in this category is considerable because of the need for specially trained personnel. An economic evaluation which takes account of both the increased costs of the intervention and cost savings resulting from reduced length and cost of hospital stay was not reported.
There were a number of overall design limitations of the presently available trials. Because of the nature of the intervention, blinding was not possible. However, blinding of the outcome assessors, although possible, was clearly evident in only 18 of the 36 trials. In several studies, there was contamination of the intervention by existing developmental care practices (control group received the experimental intervention "when necessary"). There were also differences in the maturity of the infants included.
Meta‐analysis was limited in this review due to the large variation in outcomes and limited number of randomized trials that were included in each outcome. Although many of the studies measured similar outcomes, the methods of measurement were too dissimilar to be included in a meta‐analysis. Alternatively, the authors reported the significance level but no specific data were provided. The strengths of the review include: a rigorous search strategy and review methodology, and inclusion of a broad range of developmental care interventions and outcomes.
Authors' conclusions
Implications for practice.
The results of the review indicate that developmental care interventions demonstrate limited benefits to preterm infants with respect to: decreased moderate‐severe chronic lung disease, decreased incidence of necrotizing enterocolitis and improved family outcome. There is also very limited evidence of the long‐term effect of NIDCAP on behavior and movement at five years corrected age but no effect on cognition. Other individualized developmental care interventions have also demonstrated some effect in enhancing neurodevelopmental outcomes. It should be noted, however, that the neurodevelopmental outcome results (up to 12 months corrected age) from the NIDCAP trials were conflicting with respect to benefit. The lack of blinding of the assessors was a significant methodological flaw in half of the studies. The cost of the interventions and personnel was not considered in any of the studies.
Before a clear direction for practice can be supported, evidence demonstrating more consistent effects of developmental care interventions on important short‐ and long‐term clinical outcomes is needed. The economic impact of the implementation and maintenance of developmental care practices should be considered by individual institutions.
Implications for research.
More high quality randomized trials, including cluster trials, undertaken by different investigators in different settings, are required to assess the effects of developmental care interventions on clinical outcomes. These trials should measure both short and long term effects, and should incorporate cost‐effectiveness analysis. Long term neurodevelopmental follow‐up data should include consistent timing of assessment and method of measurement. In order to facilitate meta‐analyses of these data, future research in this area should involve outcome measures consistent with those in previous studies. In addition, published reports should include all relevant data (means and standard deviations) to allow inclusion of the results in a meta‐analysis. Individualized developmental care interventions demonstrate some promising results and should be studied further in randomized trials. Future randomized trials could also include the study of the following untested developmental care interventions: prone versus supine positioning, clustering of nursery care activities, and visual stimulation.
What's new
| Date | Event | Description |
|---|---|---|
| 10 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 31 December 2005 | New search has been performed | This review updates the existing review of "Developmental care for promoting development and preventing morbidity in preterm infants" which was published in The Cochrane Library, Issue 4, 2003 (Symington 2003). Six new studies were included in this update. Two studies were added to the section on Modification of External Stimuli (Fucile 2005, Keller 2003). Two new studies were added to the NIDCAP section (Als 2003, Als 2004) and the long‐term outcomes from the Westrup 2000 study were also included in the NIDCAP section (Kleberg 2002, Westrup 2004). Conclusions of the review were altered. |
| 31 December 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the Hamilton Health Sciences Corporation Research Foundation for providing funding for this review and Patricia Austin for her assistance in literature search and retrieval. We also thank the following authors for providing us with additional or corrected data: Dr. C. Beckmann, J. Constantinou, Dr. B. Fleisher, Dr. B. Westrup.
Data and analyses
Comparison 1. Nesting vs No nesting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay (days) | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | 8.10 [0.06, 16.14] |
| 2 Weight at discharge from study (grams) | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | 17.5 [‐104.20, 139.20] |
1.1. Analysis.
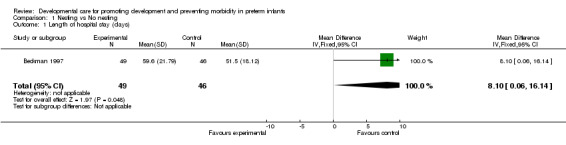
Comparison 1 Nesting vs No nesting, Outcome 1 Length of hospital stay (days).
1.2. Analysis.
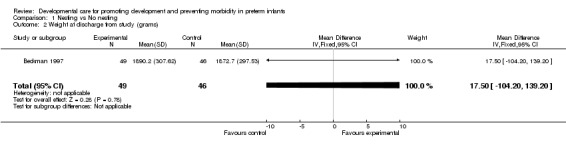
Comparison 1 Nesting vs No nesting, Outcome 2 Weight at discharge from study (grams).
Comparison 2. Swaddling vs No swaddling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morgan Neonatal Neurobehavioral Exam | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [2.62, 9.78] |
2.1. Analysis.
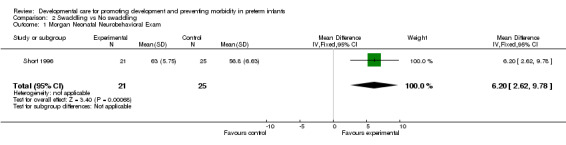
Comparison 2 Swaddling vs No swaddling, Outcome 1 Morgan Neonatal Neurobehavioral Exam.
Comparison 3. Vestibular stimulation vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physiologic Parameters | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Heart rate change (beats/min) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 2.22 [‐3.43, 7.87] |
| 1.2 Respiratory rate change (breaths/min) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐8.84, 7.34] |
| 1.3 Temperature change (degrees C) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.30, 0.22] |
| 2 Feeding | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Energy intake (kj/kg/day) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐67.08, 65.08] |
| 2.2 Emesis (change in number/day) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.32, 0.20] |
| 3 Growth | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Weight gain during study period (grams) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 20.05 [‐28.71, 68.81] |
| 3.2 Weight gain after birthweight regained (grams/day) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐5.57, 2.97] |
| 4 Neurodevelopment | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Dubowitz Neurological Assessment (2 weeks post intervention) | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.75, 1.37] |
| 4.2 Bayley MDI (6 months corrected age) | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐6.65, 5.25] |
| 4.3 Bayley PDI (6 months corrected age) | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐2.67, 7.67] |
| 4.4 Bayley MDI (12 months corrected age) | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐5.07, 11.27] |
| 4.5 Bayley PDI (12 months corrected age) | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [‐1.45, 11.25] |
3.1. Analysis.
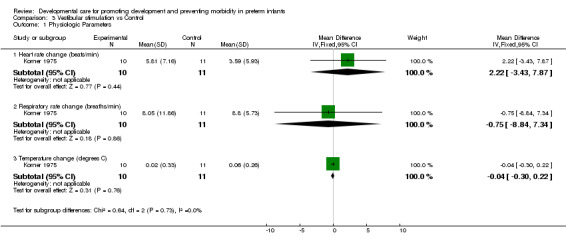
Comparison 3 Vestibular stimulation vs Control, Outcome 1 Physiologic Parameters.
3.2. Analysis.
Comparison 3 Vestibular stimulation vs Control, Outcome 2 Feeding.
3.3. Analysis.
Comparison 3 Vestibular stimulation vs Control, Outcome 3 Growth.
3.4. Analysis.
Comparison 3 Vestibular stimulation vs Control, Outcome 4 Neurodevelopment.
Comparison 4. Auditory stimulation vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physiologic Parameters | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oxygen saturation (%) | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.19, 3.19] |
| 1.2 Heart rate (beats/min) | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐12.38, 8.98] |
| 1.3 Respiratory rate (breaths/min) | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐9.90 [‐17.59, ‐2.21] |
| 2 Growth | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Days to regain birthweight | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.57, 3.17] |
| 2.2 Weight at discharge (grams) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐52.87, 64.87] |
| 2.3 Length at discharge (cms) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.99, 0.39] |
| 2.4 Head circumference at discharge (cms) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.28, 0.54] |
4.1. Analysis.
Comparison 4 Auditory stimulation vs Control, Outcome 1 Physiologic Parameters.
4.2. Analysis.

Comparison 4 Auditory stimulation vs Control, Outcome 2 Growth.
Comparison 5. Tactile stimulation vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain (grams) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 4.40 [‐1.30, 10.10] |
| 2 Full or partial nipple feeds (%) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐0.72, 16.72] |
| 3 Days to full oral feeding | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐10.95, ‐3.05] |
| 4 Length of stay (days) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐3.89 [‐7.07, ‐0.71] |
5.1. Analysis.
Comparison 5 Tactile stimulation vs Control, Outcome 1 Weight gain (grams).
5.2. Analysis.
Comparison 5 Tactile stimulation vs Control, Outcome 2 Full or partial nipple feeds (%).
5.3. Analysis.
Comparison 5 Tactile stimulation vs Control, Outcome 3 Days to full oral feeding.
5.4. Analysis.
Comparison 5 Tactile stimulation vs Control, Outcome 4 Length of stay (days).
Comparison 6. Vestibular and auditory stimulation vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Feeding | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Length of time tube feeding (days) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐15.59, 3.19] |
| 1.2 Energy intake (kcal/kg/day) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐1.32, 15.32] |
| 2 Growth | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Weight at discharge (grams) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐177.0 [‐382.18, 28.18] |
| 2.2 Weight gain to 36 weeks corrected age (grams) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 46.0 [17.07, 74.93] |
| 2.3 Head circumference gain to 36 weeks corrected age (cm/week) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.14, 0.38] |
| 3 Length of stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Length of stay (days) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐6.5 [‐16.74, 3.74] |
6.1. Analysis.
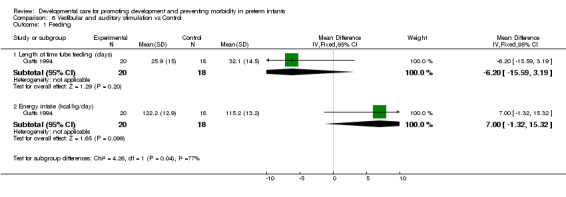
Comparison 6 Vestibular and auditory stimulation vs Control, Outcome 1 Feeding.
6.2. Analysis.
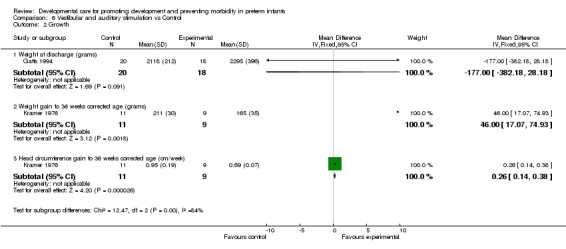
Comparison 6 Vestibular and auditory stimulation vs Control, Outcome 2 Growth.
6.3. Analysis.
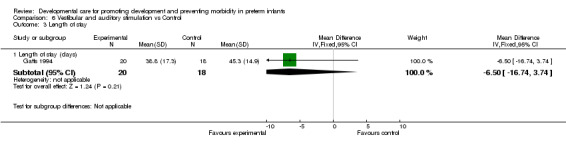
Comparison 6 Vestibular and auditory stimulation vs Control, Outcome 3 Length of stay.
Comparison 7. NIDCAP vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory support | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Days of ventilation | 3 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐8.30 [‐15.82, ‐0.77] |
| 1.2 Days on CPAP | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐17.1 [‐29.54, ‐4.66] |
| 1.3 Days in oxygen | 3 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐1.78 [‐12.60, 9.04] |
| 1.4 Age at oxygen withdrawal (post conceptional age in weeks) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐8.37, ‐3.43] |
| 2 Neonatal outcomes | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Pneumothorax | 3 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.16, 1.25] |
| 2.2 Patent ductus arteriosus | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.33, 1.92] |
| 2.3 Chronic lung disease/mild (radiographic changes) | 3 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.05, 3.40] |
| 2.4 Chronic lung disease/moderate‐severe (radiographic changes) | 3 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.93] |
| 2.5 Intraventricular hemorrhage (Grade 3 or 4) | 3 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.42] |
| 2.6 Sepsis | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| 2.7 Retinopathy of prematurity (any stage) | 2 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.17] |
| 2.8 Retinopathy of prematurity (stage 3 or greater) | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.34] |
| 3 Feeding and growth | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Days of tube feeding | 3 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐2.56 [‐10.81, 5.70] |
| 3.2 Weight gain (grams/day) | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.89 [‐1.98, 3.76] |
| 3.3 Weight gain (grams/week, birth to discharge) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 15.0 [‐4.93, 34.93] |
| 4 Length and costs of hospital stay | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Length of hospital stay (days) | 3 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐10.81, 10.30] |
| 4.2 Gestational age at discharge (weeks) | 4 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐1.26, 0.90] |
| 4.3 Hospital charges ($1,000) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐91.0 [‐173.00, ‐7.00] |
| 5 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Death before discharge | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.02] |
| 5.2 Death at 5 year follow‐up | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.16, 3.42] |
| 6 Neurodevelopment | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Bayley MDI (9 months corrected age) | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 16.43 [10.49, 22.36] |
| 6.2 Bayley PDI (9 months corrected age) | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 19.53 [11.83, 27.23] |
| 6.3 Bayley MDI (12 months corrected age) | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 13.86 [5.24, 22.48] |
| 6.4 Bayley PDI (12 months corrected age) | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.78 [‐9.53, 13.09] |
| 6.5 Wechsler Preschool and Primary Scale of Intelligence ‐ Revised (5 years corrected age): Full scale IQ | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐12.33, 19.73] |
| 6.6 Wechsler Preschool and Primary Scale of Intelligence ‐ Revised (5 years corrected age): Verbal IQ | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐16.77, 16.57] |
| 6.7 Wechsler Preschool and Primary Scale of Intelligence ‐ Revised (5 years corrected age): Performance IQ | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐7.26, 23.26] |
| 6.8 Prechtl Total Score at 2 weeks corrected age | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐20.97 [‐30.07, ‐11.87] |
| 6.9 Behavior Rating Scale ‐ Total Score at 9 months corrected age | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 34.0 [18.32, 49.68] |
7.1. Analysis.
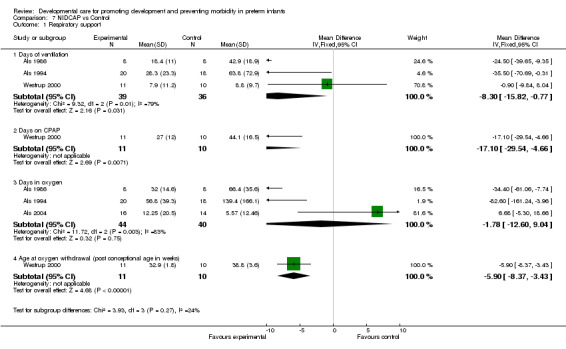
Comparison 7 NIDCAP vs Control, Outcome 1 Respiratory support.
7.2. Analysis.
Comparison 7 NIDCAP vs Control, Outcome 2 Neonatal outcomes.
7.3. Analysis.
Comparison 7 NIDCAP vs Control, Outcome 3 Feeding and growth.
7.4. Analysis.
Comparison 7 NIDCAP vs Control, Outcome 4 Length and costs of hospital stay.
7.5. Analysis.
Comparison 7 NIDCAP vs Control, Outcome 5 Death.
7.6. Analysis.
Comparison 7 NIDCAP vs Control, Outcome 6 Neurodevelopment.
Comparison 8. Other individualized interventions vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory support | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Days on positive pressure | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐22.1 [‐43.43, ‐0.77] |
| 2 Neonatal outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Pulmonary interstitial emphysema | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.16, 1.79] |
| 2.2 Patent ductus arteriosus | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.64, 2.16] |
| 2.3 Intraventricular hemorrhage (Grade 3 or 4) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.21, 3.04] |
| 2.4 Chronic lung disease (oxygen at 36 weeks corrected age) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |
| 2.5 Necrotizing enterocolitis | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.10] |
| 2.6 Retinopathy of prematurity | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |
| 3 Feeding and Growth | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Days to full bottle/breast feeding | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐11.23 [‐41.87, 19.41] |
| 3.2 Weight (grams, two weeks post EDC) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 165.0 [‐370.69, 700.69] |
| 4 Length and costs of hospital stay | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Length of hospital stay (days) | 3 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐3.33 [‐8.92, 2.26] |
| 4.2 Gestational age at discharge (weeks) | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.21, 0.61] |
| 4.3 Hospital charges ($1,000) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐129.0 [‐264.23, 6.23] |
| 5 Neurodevelopment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Bayley MDI (12 months corrected age) | 1 | 133 | Mean Difference (IV, Fixed, 95% CI) | 20.60 [13.11, 28.09] |
| 5.2 Bayley PDI (12 months corrected age) | 1 | 133 | Mean Difference (IV, Fixed, 95% CI) | 15.70 [8.68, 22.72] |
| 5.3 Bayley MDI (24 months corrected age) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 14.30 [4.19, 24.41] |
| 5.4 Bayley PDI (24 months corrected age) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 15.0 [2.90, 27.10] |
8.1. Analysis.
Comparison 8 Other individualized interventions vs Control, Outcome 1 Respiratory support.
8.2. Analysis.
Comparison 8 Other individualized interventions vs Control, Outcome 2 Neonatal outcomes.
8.3. Analysis.
Comparison 8 Other individualized interventions vs Control, Outcome 3 Feeding and Growth.
8.4. Analysis.
Comparison 8 Other individualized interventions vs Control, Outcome 4 Length and costs of hospital stay.
8.5. Analysis.
Comparison 8 Other individualized interventions vs Control, Outcome 5 Neurodevelopment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aebi 1991.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: 1.5kg or less, included ill infants Alive at 6 months Sample size ‐ 100 (Experimental: 54; Control: 46) | |
| Interventions | Experimental: positioned in a 'hemimetra' (plastic shell) before 7 days of age until discharge Control: routine positioning | |
| Outcomes | Length of stay Psychomotor development ("Illingsworth" and "Touwen") at 3 months corrected age | |
| Notes | Age at enrollment: before 7 days of life Duration of follow‐up: 2 years Country of study: Switzerland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Als 1986.
| Methods | Blinding of randomization ‐ Inadequate (phase lag design) Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Unclear | |
| Participants | Birth weight: <1.25kg IMV within 48 hours of birth IMV > 24 hours of first 48 hours of life At least 60% oxygen for at least 2 hours of first 48 hours of life No genetic abnormality, congenital infection, major maternal illness Sample size ‐ 16, 14 at 9 month follow‐up (Experimental: 8; Control: 8) | |
| Interventions | Experimental: NIDCAP, by specially trained personnel until discharge Control: routine care | |
| Outcomes | IVH PDA FiO2 (birth to Day 10) Number of days ventilated Number of days on oxygen Air leaks BPD Weight gain Corrected age at discharge Length of stay Number of days to full bottle/breast feeds (above outcomes measured at discharge) Assessment of Preterm Infants' Behavior at 1 month corrected age Bayley Scales of Infant Development at 3, 6, 9 months corrected age Kangaroo Box Paradigm at 9 months corrected age | |
| Notes | Age at enrollment: by Day 9 Duration of follow‐up: 9 months corrected age Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Als 1994.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: <1.25kg > 24 weeks and <30 weeks gestational age Alive at 48 hours IMV within first 3 hours IMV > 24 hours of first 48 hours of life Inborn, Singleton, English speaking parent Sample size ‐ 38, 36 at 9 months corrected age (Experimental: 20; Control: 18) (Experimental: 20 at 9 months follow‐up; Control: 16 at 9 months follow‐up) | |
| Interventions | Experimental: NIDCAP, by specially trained personnel until discharge Control: routine care (included Primary Care and "standard developmental protocol") | |
| Outcomes | IVH ROP Pneumothorax Days of ventilation Days in oxygen BPD Days of tube feeding Weight gain (birth to 2 weeks corrected age) Length of stay Age at discharge Hospital costs (above outcomes measured at discharge) Bayley Scales of Infant Development (9 months corrected age Assessment of Preterm Infants' Behavior (2 weeks corrected age) | |
| Notes | Possible contamination by overlap of standard and individualized developmental care protocols Age at enrollment: within 3 hours of life Duration of follow‐up: 9 months corrected age Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Als 2003.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: <1.25kg <28 weeks gestational age Alive at 48 hours IMV within first 3 hours IMV > 24 hours of first 48 hours of life Singleton, English speaking parent Sample size ‐ 110 . 92 at 42 weeks post EDC. (Experimental: 45; Control: 47) | |
| Interventions | Experimental: NIDCAP, by specially trained personnel until discharge Control: routine care and elements of developmental care | |
| Outcomes | IVH ROP Days of ventilation Days in oxygen BPD Days of tube feeding Weight gain (birth to 2 weeks corrected age) Length of stay Age at discharge Hospital costs (above outcomes measured at discharge) Steroid use Assessment of Preterm Infants' Behavior (2 weeks corrected age) Parenting Stress Index Mother's View of the Child | |
| Notes | Study period 1990‐1992 Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Als 2004.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Adequate | |
| Participants | 28 4/7‐33 3/7 weeks gestational age <72 hours of mechanical ventilation including CPAP Live in Greater Boston area Mother<=14 years old No major maternal medical or psychiatric illness Absence of chronic maternal medication treatment No history of maternal substance abuse at any time Telephone access Apgar at 5 min >=7 Appropriate for gestational age Normal initial head ultrasound, MRI, EEG <=72 hours of vasopressors No congenital or chromosomal abnormality No congenital or acquired infection No absence of prenatal care No known prenatal brain lesions No neonatal seizures English speaking parent Sample size ‐ 33. (Experimental: 16; Control: 14) | |
| Interventions | Experimental: NIDCAP, by specially trained personnel until discharge Control: routine care and elements of developmental care | |
| Outcomes | Medical outcomes at 2 weeks corrected age: Average daily weight gain; days on oxygen; days before bottle feeding; age at discharge; Pediatric Complication Scale; Weight; Length; Head Circumference; Pneumothorax; IVH; BPD; ROP Medica outcomes at 9 months corrected age: Weight; Length; Head circumference Developmental care experience Assessment of Preterm Infants' Behavior at 2 weeks corrected age Prechtl at 2 weeks corrected age Bayley Scales of Infant Development (9 months corrected age) Weight, height , head circumference at 9 months corrected age EEG Sleep EEG MRI (DTI and T2) | |
| Notes | Sample includes only infants at low risk for adverse medical outcomes 67% of eligible infants not enrolled including 50% refusal rate Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ariagno 1997.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ Unclear Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Inadequate | |
| Participants | Birth weight: 1.25kg or less 30 weeks gestational age or less IMV within first 3 hours of birth and continued for > 24 hours in first 48 hours of life Sample size ‐ 40; 35 at 42 weeks corrected age; 23 at 2 years corrected age (Experimental: 11 at 2 years; Control 12 at 2 years) | |
| Interventions | Experimental: NIDCAP, by specially trained personnel until discharge Control: Routine care | |
| Outcomes | Sleep‐Wake states for 2 days during intervention Neurobehavioral Assessment of the Preterm Infant at 36 weeks corrected age Assessment of Preterm Infants' Behavior at 42 weeks corrected age Bayley Scales of Infant Development at 4, 12 and 24 months corrected age | |
| Notes | Age at enrollment: > 48 hours of life Duration of follow‐up: 2 years corrected age Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Barnard 1983.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Inadequate | |
| Participants | <35 weeks gestational age, well infants Sample size ‐ 88; 82 at 4 months;77 at 8 months; 72 at 2 years (Experimental: Fixed interval ‐ 26; Self‐activating ‐ 23; Quasi self‐activating ‐ 10; Control ‐ 28 | |
| Interventions | Experimental: Fixed interval stimulation ‐ rocking 15 minutes /hour and recorded heartbeat. Self‐activating stimulation ‐ rocking for 15 minutes commenced when inactive for 90 seconds and recorded heartbeat. Quasi self‐activating stimulation* ‐ rocking for maximum 15 minutes/hour when inactive for 90 seconds and recorded heartbeat (duration of above interventions was about 3 weeks) Control: No rocking or recorded heartbeat | |
| Outcomes | Bayley Scales of Infant Development at 18 and 24 months corrected age Parent‐Infant Interaction (feeding scale) at discharge, 4 and 8 months corrected age Teaching‐ Interaction scale at 4, 8 and 24 months corrected age HOME at 24 months corrected age Brazelton at 34 weeks, prior to discharge and 1 month following discharge home Sleep‐Wake states on Day 1,4, 8, 12 and 34 weeks and after transfer to crib | |
| Notes | *Created post‐hoc following mechanical problem 109 variables tested Age at enrollment: 3 ‐ 15 days Duration of follow‐up: 24 months corrected age Country of Study: U.S.A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Beckman 1997.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: 0.6‐1.499kg 26‐32 weeks gestational age No IVH >Grade 2 Included ill infants Sample size ‐ 102; 95 at completion of study (Experimental: 52, 49 completed study; Control: 50, 46 completed study) | |
| Interventions | Experimental: Positioned in 'snuggle‐up' until 1.8kg Control: Positioned with blanket rolls until 1.8kg | |
| Outcomes | Weight gain Length of stay | |
| Notes | Age at enrollment: when moved to isolette Duration of follow‐up: hospital discharge SD for LOS and weight at discharge provided by author Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Brown 1980.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Inadequate | |
| Participants | Birth weight : 1‐1.75kg 37 weeks gestational age or less Black mother Mother at least 18 years old Bottle feeding No abnormalities Out of NICU by 24 hours of age Comparison group ‐ Preterm infants Sample size ‐ 67; 41 at discharge; 37 at 1 year (Experimental: Infant stimulation group ‐ 13, Mother training group ‐ 14, Infant stimulation group/Mother training group ‐ 14 Control: 26 | |
| Interventions | Experimental: *Infant stimulation group ‐ tactile, vestibular, auditory, visual stimulation for 30 minutes at feeding time twice each day 5 days/week; Mother training group ‐ teaching of infant stimulation program to mother; Infant stimulation group/Mother training group ‐ both interventions as previously described (duration of above interventions was about 6 days) Comparison group: no additional stimulation | |
| Outcomes | Length of stay Weight gain Brazelton Neonatal Behavioral Assessment prior to discharge Bayley Scales of Infant Development at 12 months corrected age HOME at 9 months corrected age | |
| Notes | Age at enrollment: when stable (Range 3‐9 days) Duration of follow‐up: 1 year Country of study: USA *Individualized program dependent on infant condition and method of feeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Buehler 1995.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Adequate | |
| Participants | Birth Weight: 2.5kg or less 30‐34 weeks gestational age No ventilatory support Sample size ‐ 24 (Experimental: 12; Control: 12) | |
| Interventions | Experimental: individualized developmental care interventions, by specially trained personnel until discharge Control: routine care, including a standard developmental care protocol and primary nursing | |
| Outcomes | Number of days on oxygen Mean daily pulmonary index (Day 3‐10) Number of days with apnea HMD CLD IVH Number of days on TPN and gavage feeding Weight gain Number of medical diagnoses at discharge Age at discharge Weight at 2 weeks corrected age EEG and EP's at 2 weeks corrected age Assessment of Preterm Infants' Behavior at 2 weeks corrected age | |
| Notes | Contamination of control group by existing developmental care practices Age at enrollment: < 48 hours Duration of follow‐up: 2 weeks corrected age Country of Study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chapman 1984.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Adequate | |
| Participants | Preterm, stable infants Sample size ‐ 259 (Experimental: Group A ‐ 83, Group B ‐ 80; Control: 82) | |
| Interventions | Experimental: Group A ‐ During feeding, lullaby during first half of hospitalization, parents voice during second half of hospitalization. Group B ‐ During each feeding, lullaby alternating with parents voice throughout hospitalization; (duration of above interventions was about 5 weeks) Control: routine care | |
| Outcomes | Anthropometric measures during study period | |
| Notes | No inclusion criteria stated Age at enrollment: Day 5 Duration of follow‐up: to hospital discharge Country of study: Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Clark 1989.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ partial Completeness of follow‐up ‐ Inadequate | |
| Participants | Preterm, non‐ventilated infants Sample size ‐ 26 (Experimental: 7; Control: 6 at follow‐up) | |
| Interventions | Experimental: rocking/ oscillating mattress, for 3 15‐minute periods/day for 2 weeks Control: regular mattress | |
| Outcomes | Dubowitz Neurological Assessment tested pre‐ and post‐intervention and 2 weeks post‐intervention | |
| Notes | No inclusion criteria stated Age at enrollment: unclear Duration of follow‐up: 2 weeks after treatment Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cordero 1986.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: 1‐1.53kg Appropriate for gestational age Non‐ventilated Sample size ‐ 17 (Experimental: 10; Control: 7) | |
| Interventions | Experimental: rocking isolette for 2 weeks Control: regular isolette | |
| Outcomes | Sleep‐Wake states on Day 1 and 14 of study period | |
| Notes | Age at enrollment: unclear Duration of follow‐up: study completion (2 weeks) Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Darrah 1994.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Inadequate | |
| Participants | <32 weeks gestational age, post‐acute illness Admitted to NICU Sample size ‐ 107 (Experimental: 23; Control: 29) | |
| Interventions | Experimental: waterbed until transferred to open cot (duration of intervention about 5 weeks) Control: no waterbed | |
| Outcomes | Movement Assessment of Infants at 4 and 8 months corrected age Dubowitz score at post‐intervention and at 40 weeks corrected age Infant Neurological International Battery at 4, 8 and 12 months corrected age Peabody Developmental Motor Scales at 4, 8, 12 and 18 months corrected age | |
| Notes | Age at enrollment: 2‐7 days Duration of follow‐up: 18 months corrected age Country of study: Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fleisher 1995.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Inadequate | |
| Participants | Birth weight:
1.25kg or less
30 weeks gestational age or less No IMV in first 3 hours of life or for >24 hours in first 48 hours of life Sample size ‐ 40; 35 completed study (Experimental: 17; Control: 18) |
|
| Interventions | Experimental: individualized developmental care interventions, by specially trained personnel until discharge Control: routine care | |
| Outcomes | Days on ventilation IVH PDA NEC ROP PIE CLD Number of days on tube feeds Length of stay Age at discharge Hospital costs (above outcomes measured at discharge) Assessment of Preterm Infants' Behavior at 42 weeks corrected age | |
| Notes | Age at enrollment: after 48 hours Duration of follow‐up: 42 weeks corrected age Country of study: USA Corrected data for CLD provided by author. SD for LOS, ventilation days, tube feeding days, GA at discharge and hospital charges provided by author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fucile 2005.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ Yes Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Adequate | |
| Participants | 26‐29 weeks gestation Appropriate for gestational age Gavage feeding No chronic medical complications including BPD, IVH, PVL, NEC, congenital anomalies Sample size ‐ 32 (Experimental: 16; Cortol: 16) | |
| Interventions | Experimental: Oral stimulation program: Stroking the perioral and intraoral structures for 15 minutes once daily for 10 days, 15‐30 minutes before gavage feeding. Control: Researchers hands in isolette, not touching infant, for 15 minutes | |
| Outcomes | Days to transition to full bottle feeds. | |
| Notes | Age at enrollment: 48 hours after discontinuing NP‐CPAP Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gaebler 1996.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Adequate | |
| Participants | 30‐34 weeks gestational age Stable sample size ‐18 (Experimental: 9; Control: 9) | |
| Interventions | Experimental: 5 minute stroking and perioral/ intraoral stimulation protocol before feeds, 3 times/day for 5 days/week Control: 5 minute stroking protocol before feeds | |
| Outcomes | Weight gain during hospitalization Length of stay Revised Neonatal Motor Assessment Scale at Day 3 and 5. Neurobehavioral assessment | |
| Notes | Age at enrollment: unclear Duration of follow‐up: 5 days Country of study: U.S.A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gatts 1994.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: 1‐2kg Stable at 15 days Sample size ‐ 38 (Experimental: 20; Control: 18) | |
| Interventions | Experimental: intermittent rocking bed and sound during hospitalization Control: regular bed | |
| Outcomes | Weight gain
Caloric intake
Tube feeding days Days from birth to first nipple feed Brazelton at discharge Length of stay |
|
| Notes | Age at enrollment: 5‐7 days Duration of follow‐up: to hospital discharge Country of study: U.S.A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Helders 1989.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Inadequate | |
| Participants | Birth weight: 1.5kg 32 weeks gestational age or less, stable Sample size ‐ 149; 51 at 12 months corrected age (Experimental: 67; Control: 82) | |
| Interventions | Experimental: nursed in hammock when in supine position, supported with flannelette blanket when in lateral position; during hospitalization Control: nursed on sheepskin alternating lateral positions | |
| Outcomes | Weight for length at 3,6,12 months corrected age | |
| Notes | Age at enrollment: admission to the intermediate care nursery Duration of follow‐up: 12 months corrected age Country of study: Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Keller 2003.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Inadequate | |
| Participants | <31 weeks gestation <1500 grams Stable Normal head ultrasound No oxygen or intravenous No congenital anomalies or metabolic disorders Sample size ‐ 20 (Experimental: 10; Control 10) | |
| Interventions | Experimental: Supine in hammock for 3 hour session for 10 consecutive days. Control: Nested in prone position for same period of time. | |
| Outcomes | Weight gain Heart rate Respiratory rate Ballard Assessment of Maturity Scale | |
| Notes | Age at enrollment: 4.5 weeks. Country of study: Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Korner 1975.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: <2kg, healthy <34 weeks gestational age Nursed in incubator by <120 hrs of age Sample size ‐ 21 (Experimental: 10; Control: 11) | |
| Interventions | Experimental: oscillating waterbed for 7 days Control: no waterbed | |
| Outcomes | Physiological parameters Weight gain Emesis Apnea (outcome data from first 9 days) | |
| Notes | Age at enrollment: 3‐5 days of age Duration of follow‐up: 9 days Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Korner 1983.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Inadequate | |
| Participants | Preterm infants with RDS Sample size ‐ 56 (Experimental: 12; Control: 8) | |
| Interventions | Experimental: waterbed for 7 days Control: no waterbed | |
| Outcomes | Neurobehavioral assessment at 34‐35 weeks corrected age | |
| Notes | Age at enrollment: <4 days of age Duration of follow‐up: 34‐35 weeks corrected age Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kramer 1976.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Unclear | |
| Participants | <34 weeks gestational age, healthy Sample size ‐ 20 (Experimental: 11; Control: 9) | |
| Interventions | Experimental: rocking waterbed, woman's voice and heartbeat during hospitalization Control: standard care | |
| Outcomes | Weight gain Dubowitz score Brazelton (above outcomes measured at discharge) | |
| Notes | Age at enrollment: Day 2 Duration of follow‐up: 36 weeks corrected age Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mann 1986.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ No Completeness of follow‐up ‐ Unclear | |
| Participants | <36 weeks gestational age No longer requires intensive care Sample size ‐ 41 (Experimental: 20; Control: 21) | |
| Interventions | Experimental: 12 hours at night light and noise reduced Control: Lighting always on during hospitalization | |
| Outcomes | Sleep‐Wake states assessed over 48 hours at: discharge home, term, 6 and 12 weeks corrected age Weight at discharge, EDC, 6 and 12 weeks post‐EDC Hours spent feeding at discharge, EDC, 6 and 12 weeks post‐EDC Hours awake at discharge, EDC, 6 and 12 weeks post‐EDC | |
| Notes | Age at enrollment: when intensive care not required (1‐63 days) Duration of follow‐up: 12 weeks corrected age. Country of study: UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Resnick 1987.
| Methods | Blinding of randomization ‐ Inadequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Inadequate | |
| Participants | Birth weight: .50‐1.8kg, included ill infants Sample size: 255; 228 at discharge; 133 at 1 year follow‐up (Experimental: 27 at 2 years; Control: 26 at 2 years) | |
| Interventions | Experimental: water mattress; pictures; exercise program during hospitalization Control: routine care | |
| Outcomes | Bayley Scales of Infant Development at 1 & 2 years corrected age | |
| Notes | Age at enrollment: after first 24 hours of age Duration of follow‐up: 2 years Country of study: USA Standard deviation of Bayley PDI at 24 months confirmed with author to be 16.5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Resnick 1988.
| Methods | Blinding of randomization ‐ Inadequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Unclear | |
| Participants | Birth weight: <1.8kg, included ill infants Admitted to NICU within 24 hours of birth Live within district Sample size: 41 (Experimental: 21; Control: 20) | |
| Interventions | Experimental: minimum of 2 developmental care interventions per day while in NICU; after hospital discharge, weekly home visits until due date; home visits 2 times per month for 12 months; by specially trained personnel Control: referral to support services as indicated | |
| Outcomes | Bayley Scales of Infant Development at 6 & 12 months corrected age Greenspan‐ Lieberman Observations System at 6 & 12 months corrected age | |
| Notes | Age at enrollment: <24 hours of age Duration of follow‐up: 12 months corrected age Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Saigal 1986.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Inadequate | |
| Participants | Birth weight: .75‐1.75kg Non‐ventilated for 12 hrs prior to enrollment Sample size ‐ 122 (Experimental: 59 ‐ 44 at 12 months; Control: 63 ‐ 48 at 12 months follow‐up) | |
| Interventions | Experimental: constantly oscillating air mattress Control: regular mattress Median duration of intervention in days, 18; range 7‐68 days. | |
| Outcomes | Weight gain Apnea; both during study period Einstein test at term corrected age Habituation scores at 3 months corrected age Bayley Scales of Infant Development at 6 & 12 months corrected age Sleep‐Wake state at end of study period | |
| Notes | Age at enrollment: < 5 days of age Duration of follow‐up: 12 months Country of study: Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Scott 1983.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ No Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: <1.4kg <31 days of age Gained weight for 2 consecutive days, stable Sample size ‐ 34 (Experimental: 17; Control:17) | |
| Interventions | Experimental: nursed on lambs wool Control: nursed on cotton Median duration of study in days, 15; range 4‐37 days | |
| Outcomes | Weight gain during study period | |
| Notes | Age at enrollment: 2‐31 days Duration of follow‐up: 4‐37 days Country of study: U.K. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Short 1996.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Adequate | |
| Participants | Birth weight: <1.25kg 7‐21 days of age, post‐acute illness Appropriate for gestational age Sample size ‐ 50 (Experimental: 24; Control: 26) | |
| Interventions | Experimental: Positioning with swaddling and hip roll, during hospitalization for a minimum of 15 hours/day Control: routine positioning including blanket rolls | |
| Outcomes | Morgan Neonatal Neurobehavioral Exam | |
| Notes | Subjects in control group could be swaddled when necessary (numbers not described) Age at enrollment: 7‐21 days Duration of follow‐up: 34 weeks corrected age Country of study: USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Thoman 1991.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Inadequate | |
| Participants | 29‐33 weeks gestational age Stable Sample size ‐ 58; 45 at discharge (Experimental: 16 at follow‐up; Control: 17 at follow‐up) | |
| Interventions | Experimental: breathing teddy bear in isolette; for 3 weeks Control: non‐breathing teddy bear in isolette | |
| Outcomes | Post‐natal age at discharge Conceptional age at discharge Behaviour Sleep‐Wake states during study period | |
| Notes | Age at enrollment: Unclear Duration of follow‐up: 5 weeks corrected age Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Westrup 2000.
| Methods | Blinding of randomization ‐ Adequate Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes* Completeness of follow‐up ‐ Inadequate | |
| Participants | <32 weeks gestational age Requiring ventilatory assistance by 24 hours of age Sample size ‐ 25 (Experimental: 12; Control: 13 Survivors‐ Experimental: 11; Control: 10) | |
| Interventions | Experimental: NIDCAP, by specially trained personnel until discharge Control: routine care | |
| Outcomes | Duration of mechanical ventilation Duration of CPAP Length of time on oxygen BPD Weight gain Head growth Corrected age at discharge Mortality IVH Antibiotic therapy Sepsis Apnea ROP (above outcomes measured at discharge) | |
| Notes | Sample size calculated but study concluded before completion of enrollment Age at enrollment: randomized at birth, entered study at Day 3 Duration of follow‐up: to discharge. *Confirmation of adequacy of blinding provided by investigator. Country of study: Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
White‐Traut 1988.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Unclear | |
| Participants | 28‐35 weeks gestational age Appropriate for gestational age English speaking mother Vaginal delivery Mother 16 years old or more Stable Sample size ‐ 33 mother ‐ infant pairs Number randomized to each group ‐ 11 | |
| Interventions | Experimental: Group: Talking or singing for 15 minutes at 24‐36 hr, 37‐48 hr, 49‐60 hr, 61‐72 hr intervals; Group: RISS (Rice Technique) tactile (massage), vestibular motion (rocking), auditory (talking), visual (eye‐to‐eye contact). Control: parents received a lecture on infant clothing. | |
| Outcomes | NCAFS (feeding assessment scale), includes infant and maternal behavioral scores; completed prior to discharge | |
| Notes | Age at enrollment: 24 hours of age Duration of follow‐up: to hospital discharge Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
White‐Traut 1993.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Unclear | |
| Participants | Birth weight: <1.8 kg 30‐32 weeks gestational age Appropriate for gestational age Stable Sample size ‐ 40 (Experimental: 20; Control: 20) | |
| Interventions | Experimental: ATVV ‐ auditory stimuli (female voice); tactile stimuli (light stroking); visual stimuli (eye‐to‐eye contact); vestibular stimuli (rocking). All interventions given for 4 days Control: no additional stimulations | |
| Outcomes | Physiological parameters Behavioral states; during study period | |
| Notes | Age at enrollment: 33 weeks postconceptional age Duration of follow‐up: 4 days Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
White‐Traut 1997.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ Unclear Completeness of follow‐up ‐ Unclear | |
| Participants | Preterm infant Stable Sample size ‐ 54 (Experimental: ATVV ‐ 10; ATV ‐11; A ‐ 9; T ‐ 10. Control: 14.) | |
| Interventions | Experimental: Group ATVV ‐ auditory stimuli (female voice); tactile stimuli (light stroking); visual stimuli (eye‐to‐eye contact); vestibular stimuli (rocking). Group ATV ‐ auditory stimuli (female voice); tactile stimuli (light stroking); visual stimuli (eye‐to‐eye contact). Group A ‐ auditory stimuli only. Group T‐ tactile stimuli (light stroking) only. All interventions given for 4 days. Control: routine care. | |
| Outcomes | Physiological parameters Sleep‐Wake states; during study period Postnatal complication scale | |
| Notes | Age at enrollment: 33 weeks postconceptional age Duration of follow‐up: 4 days Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
White‐Traut 2002.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ Yes Blinding of outcome assessors ‐ Yes Completeness of follow‐up ‐ Adequate | |
| Participants | 23‐31 weeks gestational age Stable Sample size ‐ 37 (Experimental: 21; Control: 16) | |
| Interventions | Experimental: Group ATVV ‐ auditory stimuli (female voice); tactile stimuli (10 minute massage); visual stimuli (eye‐to‐eye contact); vestibular stimuli (rocking). Control: routine care. | |
| Outcomes | Behavioural state during study period Days from gavage feeding only to complete nipple feeding Length of hospital stay | |
| Notes | All subjects participated in a stress reduction program Age at enrollment: 32 weeks postconceptional age Duration of follow‐up: 3 weeks Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Zahr 1995.
| Methods | Blinding of randomization ‐ Unclear Blinding of intervention ‐ No Blinding of outcome assessors ‐ No Completeness of follow‐up ‐ Adequate | |
| Participants | Preterm infants, post‐acute illness Sample size ‐ 30 (17 in non‐crossover situation. Experimental: 9; Control: 8) | |
| Interventions | Experimental: earmuffs for 2 days Control: no earmuffs | |
| Outcomes | Physiological parametres Anderson Behavioral State Scale; during study period | |
| Notes | No inclusion criteria stated Age at enrollment: about 3 weeks of age Duration of follow‐up: 2 days Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamson‐Macedo 1994 | Not a randomized trial |
| Als 1998 | Not a randomized trial |
| Berlin 1998 | Not a developmental care intervention |
| Bottos 1985 | Not a randomized trial |
| Bozynski 1988 | Not a randomized trial |
| Brown 1997 | Not a randomized trial |
| Cartlidge 1988 | Not a randomized trial |
| Deiriggi 1990 | Not a randomized trial |
| Dollberg 2004 | Not a randomized trial |
| Duxbury 1984 | Not a randomized trial Term infants Not a developmental care intervention |
| Fearon 1997 | Not a randomized trial |
| Field 1980 | Not a randomized trial No clinical outcomes Term infants Not a developmental care intervention |
| Field 1980/a | Not a randomized trial Not a developmental care intervention No clinical outcomes |
| Fox 1993 | Not a randomized trial |
| Garcia 1993 | Not a randomized trial |
| Glass 1985 | Not a randomized trial |
| Goto 1999 | Not a randomized trial |
| Grauer 1989 | Not a randomized trial Term infants |
| Gray 1998 | Not a randomized trial |
| Gray 2004 | Included in another Cochrane review |
| Harrison 1991 | Not a randomized trial |
| Harrison 1996 | Infant massage not included in this review |
| Jay 1982 | Not a randomized trial |
| Jenni 1997 | Not a randomized trial Not a developmental care intervention |
| Jirapaet 1993 | Not a developmental care intervention |
| Katz 1971 | Not a randomized trial |
| Kelly 1989 | Not a randomized trial |
| Kleberg 2000 | Not a randomized trial |
| Korner 1982 | Not a randomized trial |
| Kurlak 1994 | Not a randomized trial |
| Lekskulchai 2001 | Participants >37 weeks |
| Masterson 1987 | Not a randomized trial |
| Mathai 2001 | Not a randomized trial |
| Monterosso 1995 | Not a randomized trial Not a developmental care intervention |
| Montfort 1997 | Not a randomized trial |
| Nelson 1997 | Not a randomized trial Term infants Not a developmental care intervention |
| Neu 1997 | Not a randomized trial |
| Ogi 2001 | Not a randomized trial |
| Parker 1992 | Not a randomized trial Control group received developmental care |
| Pressler 2002 | Not a randomized trial |
| Resnick 1998 | Not a randomized trial Not a developmental care intervention |
| Saunders 1995 | Not a randomized trial |
| Schwirian 1986 | Not a randomized trial |
| Sell 1992 | Not a randomized trial Not a developmental care intervention |
| Standley 1995 | Not a randomized trial |
| Standley 1998 | Not a randomized trial |
| White‐Traut 1999 | Included only preterm infants with periventricular leukomalacia |
| White‐Traut 2004 | Included only preterm infants with periventricular leukomalacia |
| Wilcox 1995 | Not a randomized trial |
| Zahr 1995/a | Not a randomized trial Not a developmental care intervention |
Characteristics of ongoing studies [ordered by study ID]
Tyebkhan 2004.
| Trial name or title | Not known |
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Starting date | Not known |
| Contact information | Not known |
| Notes |
Sources of support
Internal sources
Hamilton Health Sciences Corporation Research Development Fund, Canada.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Aebi 1991 {published data only}
- Aebi U, Nielsen J, Sidiropoulos D, Stucki M. Outcome of 100 randomly positioned children of very low birthweight at 2 years. Child: Care, Health and Development 1991;17:1‐8. [DOI] [PubMed] [Google Scholar]
Als 1986 {published data only}
- Als H, Lawhon G, Brown E, Gibes R, Duffy FH, McAnulty G, Blickman JG. Individualized behavioral and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: Neonatal intensive care unit and developmental outcome. Pediatrics 1986;78:1123‐32. [PubMed] [Google Scholar]
Als 1994 {published data only}
- Als H, Lawhon G, Duffy F, McAnulty GB, Gibes‐Grossman R, Blickman JG. Individualized developmental care for the very low‐birth‐weight preterm infant: medical and neurofunctional effects. JAMA 1994;272:853‐8. [PubMed] [Google Scholar]
Als 2003 {published data only}
- Als H, Gilkerson L, Duffy FH, McAnulty GB, Buehler DM, Vandenberg K, Sweet N, Sell E, Parad RB, Ringer SA, Butler SC, Blickman JG, Jones KJ. A three‐center, randomized, controlled trial of individualized developmental care for very low birth weight preterm infants: Medical, neurodevelopmental parenting and caregiving effects. Journal of Developmental and Behavioural Pediatrics 2003;24:399‐408. [DOI] [PubMed] [Google Scholar]
Als 2004 {published data only}
- Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, Warfield SK, Huppi PS, Butler SC, Conneman N, Fischer C, Eichenwald EC. Early experience alters brain function and structure. Pediatrics 2004;113(4):846‐57. [DOI] [PubMed] [Google Scholar]
Ariagno 1997 {published data only}
- Ariagno RL, Thoman EB, Boeddiker MA, Kugener MA, Constantinou JC, Mirmiran M, Baldwin RB. Developmental care does not alter sleep and development of premature infants. Pediatrics 1997;100:1‐7. [DOI] [PubMed] [Google Scholar]
Barnard 1983 {published data only}
- Barnard KE, Bee HL. The impact of temporally patterned stimulation on the development of preterm infants. Child Development 1983;54:1156‐67. [PubMed] [Google Scholar]
Beckman 1997 {published data only}
- Beckmann CA. Use of neonatal boundaries to improve outcomes. Journal of Holistic Nursing 1997;15:54‐67. [DOI] [PubMed] [Google Scholar]
Brown 1980 {published data only}
- Brown JV, LaRossa MM, Aylward GP, Davis DJ, Rutherford PK, Bakeman R. Nursery‐based intervention with prematurely born babies and their mothers: Are there effects?. Journal of Pediatrics 1980;97:487‐91. [DOI] [PubMed] [Google Scholar]
Buehler 1995 {published data only}
- Buehler DM, Als H, Duffy FH, McAnulty GB, Liederman J. Effectiveness of individualized developmental care for low‐risk preterm infants: Behavioral and electrophysiologic evidence. Pediatrics 1995;96:923‐32. [PubMed] [Google Scholar]
Chapman 1984 {published data only}
- Chapman JS. Longitudinal follow‐up of prematurely born children: Predischarge outcomes of hospital stimulation programme. Nursing Papers 1984;16:30‐48. [PubMed] [Google Scholar]
Clark 1989 {published data only}
- Clark DL, Cordero L, Goss KC, Manos D. Effects of rocking on neuromuscular development in the premature. Biology of the Neonate 1989;56:306‐14. [DOI] [PubMed] [Google Scholar]
Cordero 1986 {published data only}
- Cordero L, Clark DL, Schott L. Effects of vestibular stimulation on sleep states in premature infants. American Journal of Perinatology 1986;3:319‐24. [DOI] [PubMed] [Google Scholar]
Darrah 1994 {published data only}
- Darrah J, Piper M, Byrne P, Watt MJ. The use of waterbeds for very low‐birthweight infants: Effects on neuromotor development. Developmental Medicine and Child Neurology 1994;36:989‐99. [DOI] [PubMed] [Google Scholar]
Fleisher 1995 {published data only}
- Fleisher BE, Vandenberg K, Constantinou J, Heller C, Benitz, WE, Johnson A, Rosenthal A, Stevenson DK. Individualized developmental care for very‐low‐birth‐weight premature infants. Clinical Pediatrics 1995;10:523‐9. [DOI] [PubMed] [Google Scholar]
Fucile 2005 {published data only}
- Fucile S, Gisel EG, Lau C. Effect of oral stimulation program on sucking skill maturation of preterm infants. Developmental Medicine and Child Neurology 2005;47:158‐62. [DOI] [PubMed] [Google Scholar]
Gaebler 1996 {published data only}
- Gaebler CP, Hanzlik, JR. The effects of a prefeeding stimulation program on preterm infants. American Journal of Occupational Therapy 1996;50:184‐92. [DOI] [PubMed] [Google Scholar]
Gatts 1994 {published data only}
- Gatts JD, Wallace DH, Glasscock GF, McKee E, Cohen RS. A modified newborn intensive care unit environment may shorten hospital stay. Journal of Perinatology 1994;14:422‐7. [PubMed] [Google Scholar]
Helders 1989 {published data only}
- Helders PJM, Cats BP, Debast S. Effects of a tactile stimulation/range‐finding programme on the development of VLBW‐neonates during the first year of life. Child: Care, Health and Development 1989;15:369‐79. [DOI] [PubMed] [Google Scholar]
Keller 2003 {published data only}
- Keller A, Arbel N, Merlob P, Davidson S. Neurobehavioral and autonomic effects of hammock positioning in infants with very low birth weight. Pediatric Physical Therapy 2003;15:3‐7. [DOI] [PubMed] [Google Scholar]
Korner 1975 {published data only}
- Korner AF, Kraemer HC, Haffner ME, Cosper LM. Effects of waterbed flotation on premature infants: A pilot study. Pediatrics 1975;56:361‐367. [PubMed] [Google Scholar]
Korner 1983 {published data only}
- Korner AF, Schneider P, Forrest T. Effects of vestibular‐proprioceptive stimulation on the neurobehavioral development of preterm infants: A pilot study. Neuropediatrics 1983;14:170‐5. [DOI] [PubMed] [Google Scholar]
Kramer 1976 {published data only}
- Kramer LI, Pierpont ME. Rocking waterbeds and auditory stimuli to enhance growth of preterm infants. Preliminary report. Journal of Pediatrics 1976;88:297‐9. [DOI] [PubMed] [Google Scholar]
Mann 1986 {published data only}
- Mann NP, Haddow R, Stokes L, Goodley S, Rutter N. Effect of night and day on preterm infants in a newborn nursery: randomised trial. BMJ 1986;293:1265‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Resnick 1987 {published data only}
- Resnick MB, Eyler FD, Nelson RM, Eitzman DV, Bucciarelli RL. Developmental intervention for low birth weight infants: improved early developmental outcome. Pediatrics 1987;80:68‐74. [PubMed] [Google Scholar]
Resnick 1988 {published data only}
- Resnick MB, Armstrong S, Carter, RL. Developmental intervention program for high‐risk premature infants: Effects on development and parent‐infant interactions. Journal of Developmental and Behavioral Pediatrics 1988;9:73‐8. [PubMed] [Google Scholar]
Saigal 1986 {published data only}
- Saigal S, Watts J, Campbell D. Randomized clinical trial of an oscillating air mattress in preterm infants: Effect on apnea, growth and development. Journal of Pediatrics 1986;109:857‐64. [DOI] [PubMed] [Google Scholar]
Scott 1983 {published data only}
- Scott S, Cole T, Lucas P, Richards M. Weight gain and movement patterns of very low birthweight babies nursed on lambswool. Lancet 1983;2:1014‐6. [DOI] [PubMed] [Google Scholar]
Short 1996 {published data only}
- Short MA, Brooks‐Brunn JA, Reeves DS, Yeager J, Thorpe JA. The effects of swaddling versus standard positioning on neuromuscular development in very low birth weight infants. Neonatal Network 1996;15:2‐31. [PubMed] [Google Scholar]
Thoman 1991 {published data only}
- Thoman EB, Ingersoll EW, Acebo C. Premature infants seek rhythmic stimulation, and the experience facilitates neurobehavioral development. Journal of Developmental and Behavioural Pediatrics 1991;12:11‐8. [PubMed] [Google Scholar]
Westrup 2000 {published data only}
- Kleberg A, Westrup B, Stjernqvist K, Lagercrantz H. Indications of improved cognitive development at one year of age among infants born very prematurely who received care based on the Newborn Individualized Developmental Care and Assessment Program (NIDCAP). Early Human Development 2002;68:83‐91. [DOI] [PubMed] [Google Scholar]
- Westrup B, Bohm B, Lagercrantz H, Stjernqvist K. Preschool outcome in children born very prematurely and cared for according to the Newborn Individualized Developmental Care and Assessment Program (NIDCAP). Acta Paediatrica 2004;93:498‐507. [DOI] [PubMed] [Google Scholar]
- Westrup B, Kleberg A, Eichwald K, Stjernqvist K, Lagercrantz H. A randomized controlled trial to evaluate the effects of the newborn individualized developmental care and assessment program in a Swedish setting. Pediatrics 2000;105:66‐72. [DOI] [PubMed] [Google Scholar]
White‐Traut 1988 {published data only}
- White‐Traut RC, Nelson MN. Maternally administered tactile, auditory, visual, and vestibular stimulation: relationship to later interactions between mothers and premature infants. Research in Nursing & Health 1988;11:31‐9. [DOI] [PubMed] [Google Scholar]
White‐Traut 1993 {published data only}
- White‐Traut RC, Nelson MN, Silvestri JM, Patel MK, Kilgallon D. Patterns of physiologic and behavioral response of intermediate care preterm infants to intervention. Pediatric Nursing 1993;19:625‐9. [PubMed] [Google Scholar]
White‐Traut 1997 {published data only}
- White‐Traut RC, Nelson MN, Silvestri JM, Cunningham N, Patel M. Responses of preterm infants to unimodal and multimodal sensory intervention. Pediatric Nursing 1997;23:169‐75. [PubMed] [Google Scholar]
White‐Traut 2002 {published data only}
- White‐Traut RC, Nelson MN, Silvestri JM, Vasan U, Littau S, Meleedy‐Rey P, Gu G, Patel M. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Developmental Medicine and Child Neurology 2002;44:91‐7. [DOI] [PubMed] [Google Scholar]
Zahr 1995 {published data only}
- Zahr LK, Traversay J. Premature infant responses to noise reduction by earmuffs: effects on behavioral and physiologic measures. Journal of Perinatology 1995;15:448‐55. [PubMed] [Google Scholar]
References to studies excluded from this review
Adamson‐Macedo 1994 {published data only}
- Adamson‐Macedo EN, Roiste A, Wilson A, De Carvalho, Dattani I. TAC‐TIC therapy with high‐risk, distressed, ventilated preterms. Journal of Reproductive and Infant Psychology 1994;12:249‐52. [Google Scholar]
Als 1998 {published data only}
- Als H. Developmental care in the newborn intensive care unit. Current Opinion in Pediatrics 1998;10:138‐42. [DOI] [PubMed] [Google Scholar]
Berlin 1998 {published data only}
- Berlin LJ, Brooks‐Gunn J, McCarton C, McCormick MC. The effectiveness of early intervention: Examining risk factors and pathways to enhanced development. Preventive Medicine 1998;27:238‐45. [DOI] [PubMed] [Google Scholar]
Bottos 1985 {published data only}
- Bottos M, Pettenazzo A, Giancola G, Stefani D, Pettenza G, Viscolani B, Rubaltelli FF. The effect of a 'containing' position in a hammock versus the supine position on the cutaneous oxygen level in premature and term babies. Early Human Development 1985;11:265‐73. [DOI] [PubMed] [Google Scholar]
Bozynski 1988 {published data only}
- Bozynski MEA, Naglie RA, Nicks JJ, Burpee B, Johnson RV. Lateral positioning of the stable ventilated very‐low‐birth‐weight infant. American Journal of Diseases of Children 1988;142:200‐2. [DOI] [PubMed] [Google Scholar]
Brown 1997 {published data only}
- Brown LD, Heerman JA. The effect of developmental care on preterm infant outcome. Applied Nursing Research 1997;10:190‐7. [DOI] [PubMed] [Google Scholar]
Cartlidge 1988 {published data only}
- Cartlidge PHT, Rutter N. Reduction of head flattening in preterm infants. Archives of Disease in Childhood 1988;63:755‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deiriggi 1990 {published data only}
- Deiriggi PM. Effects of waterbed flotation on indicators of energy expenditure in preterm infants. Nursing Research 1990;39:140‐7. [PubMed] [Google Scholar]
Dollberg 2004 {published data only}
- Dollberg S, Yacov G, Mimouni FB, Barak M. The effect of positioning on energy expenditure in preterm infants: A feasibility study. American Journal of Perinatology 2004;21(7):391‐4. [DOI] [PubMed] [Google Scholar]
Duxbury 1984 {published data only}
- Duxbury ML, Henly SJ, Broz LJ, Armstrong GD, Wachdorf CM. Caregiver disruptions and sleep of high‐risk infants. Heart Lung 1984;13:141‐7. [PubMed] [Google Scholar]
Fearon 1997 {published data only}
- Fearon I, Kisilevsky BS, Hains SM, Muir DW, Tranmer J. Swaddling after heel lance: Age specific effects on behavioral recovery in preterm infants. Journal of Developmental and Behavioural Pediatrics 1997;18:222‐32. [PubMed] [Google Scholar]
Field 1980 {published data only}
- Field T. Supplemental stimulation of preterm neonates. Early Human Development 1980;4:301‐14. [DOI] [PubMed] [Google Scholar]
Field 1980/a {published data only}
- Field T. Alleviating stress in intensive‐care unit neonates. Journal of the American Optometric Association 1987;87:646‐50. [PubMed] [Google Scholar]
Fox 1993 {published data only}
- Fox RE, Viscardi RM, Taciak VI, Niknafs H, Cinoman MI. Effect of position on pulmonary mechanics in healthy preterm newborn infants. Journal of Perinatology 1993;13:205‐11. [PubMed] [Google Scholar]
Garcia 1993 {published data only}
- Garcia AP, White‐Traut R. Preterm infants' responses to taste/smell and tactile stimulation during and apneic episode. Journal of Pediatric Nursing 1993;8:245‐52. [PubMed] [Google Scholar]
Glass 1985 {published data only}
- Glass P, Avery GB, Subramanian KNS, Keys MP, Sostek AM, Friendly DS. Effect of bright light in the hospital nursery on the incidence of retinopathy of prematurity. New England Journal of Medicine 1985;313:401‐4. [DOI] [PubMed] [Google Scholar]
Goto 1999 {published data only}
- Goto K, Mirmiran M, Adams MM, Longford RV, Baldwin RB, Boeddiker MA, Ariagno RL. More awakenings and heart rate variability during supine sleep in preterm infants. Pediatrics 1999;103:603‐9. [DOI] [PubMed] [Google Scholar]
Grauer 1989 {published data only}
- Grauer TT. Environmental lighting, behavioral state, and hormonal response in the newborn. Scholarly Inquiry for Nursing Practice 1989;3:53‐66. [PubMed] [Google Scholar]
Gray 1998 {published data only}
- Gray K, Dostal S, Ternullo‐Retta C, Armstrong MA. Developmentally supportive care in a neonatal intensive care unit: A research utilization project. Neonatal Network 1998;17:33‐8. [PubMed] [Google Scholar]
Gray 2004 {published data only}
- Gray PH, Paterson S, Finch G, Hayes M. Cot‐nursing using a heated, water‐filled mattress and incubator care: A randomized clinical trial. Acta Paediatrics 2004;93:350‐5. [PubMed] [Google Scholar]
Harrison 1991 {published data only}
- Harrison L, Olivet L, Cunningham K, Bodin MB, Hicks C. Effects of gentle human touch on preterm infants: Pilot study results. Neonatal Network 1996;15:35‐41. [PubMed] [Google Scholar]
Harrison 1996 {published data only}
- Harrison LL, Leeper J, Yoon M. Preterm infants' physiologic responses to early parent touch. Western Journal of Nursing research 1991;13:698‐713. [DOI] [PubMed] [Google Scholar]
Jay 1982 {published data only}
- Jay SS. The effects of gentle human touch on mechanically ventilated very‐short‐gestation infants. Maternal‐Child Nursing Journal 1982;11:199‐256. [PubMed] [Google Scholar]
Jenni 1997 {published data only}
- Jenni OG, Siebenthal K, Wolf M, Keel M, Duc G, Bucher HU. Effect of nursing in the head elevated tilt position (15 degrees) on the incidence of bradycardic and hypoxemic episodes in preterm infants. Pediatrics 1997;100:622‐5. [DOI] [PubMed] [Google Scholar]
Jirapaet 1993 {published data only}
- Jirapaet K. The effect of vertical pulsating stimulation on apnea of prematurity. Journal of the Medical Association of Thailand 1993;76:319‐26. [PubMed] [Google Scholar]
Katz 1971 {published data only}
- Katz V. Auditory stimulation and developmental behavior of the premature infant. Nursing Research 1971;20:196‐201. [PubMed] [Google Scholar]
Kelly 1989 {published data only}
- Kelly MK, Palisano RJ, Wolfson MR. Effects of developmental physical therapy program on oxygen saturation and heart rate in preterm infants. Physical Therapy 1989;69:467‐74. [DOI] [PubMed] [Google Scholar]
Kleberg 2000 {published data only}
- Kleberg A, Westrup B, Stjernqvist K. Developmental outcome, child behaviour and mother‐child interaction at 3 years of age following Newborn Individualized Developmental Care and Intervention Program (NIDCAP) intervention. Early Human Development 2000;60:123‐5. [DOI] [PubMed] [Google Scholar]
Korner 1982 {published data only}
- Korner AF, Ruppel EM, Rho JM. Effects of water beds on the sleep and motility of theophylline‐treated preterm infants. Pediatrics 1982;70:864‐9. [PubMed] [Google Scholar]
Kurlak 1994 {published data only}
- Kurlack LO, Ruggins NR, Stephenson TJ. Effect of nursing position on incidence, type, and duration of clinically significant apnoea in preterm infants. Archives of Disease in Childhood Fetal Neonatal Edition 1994;71:F16‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lekskulchai 2001 {published data only}
- Lekskulchai R, Cole J. Effect of a developmental program on motor performance in infants born preterm. Australian Journal of Physiotherapy 2001;47:169‐76. [DOI] [PubMed] [Google Scholar]
Masterson 1987 {published data only}
- Masterson J, Zucker C, Schulze K. Prone and supine positioning effects on energy expenditure and behavior of low birth weight neonates. Pediatrics 1987;80:689‐92. [PubMed] [Google Scholar]
Mathai 2001 {published data only}
- Mathai S, Fernandez A, Mondkar J, Kanbur W. Effects of tactile‐kinesthetic stimulation in preterms: A controlled trial. Indian Pediatrics 2001;38:1091‐8. [PubMed] [Google Scholar]
Monterosso 1995 {published data only}
- Monterosso L, Coenen A, Percival P, Evans S. Effect of postural support nappy on 'flattened posture' of the lower extremeties in very preterm infants. Journal of Paediatrics and Child Health 1995;31:350‐4. [DOI] [PubMed] [Google Scholar]
Montfort 1997 {published data only}
- Monfort K, Case‐Smith J. The effects of neonatal positioner on scapular rotation. American Journal of Occupational Therapy 1997;51:378‐84. [DOI] [PubMed] [Google Scholar]
Nelson 1997 {published data only}
- Nelson KG, Goldenberg RL, Hoffman HJ, Cliver SP. Growth and development during the first year in a cohort of low income term‐born American children. Acta Obstetricia et Gynecologica Scandinavica 1997;165:87‐92. [PubMed] [Google Scholar]
Neu 1997 {published data only}
- Neu M, Browne JV. Infant physiologic and behavioral organization during swaddled versus unswaddled weighing. Journal of Perinatology 1997;17:193‐8. [PubMed] [Google Scholar]
Ogi 2001 {published data only}
- Ogi S, Arisawa K, Takahashi T, Akiyama T, Goto Y, Fukuda M, Saito H. The developmental effects of an early intervention program for very low birthweight infants. No To Hattatsu 2001;33:31‐6. [PubMed] [Google Scholar]
Parker 1992 {published data only}
- Parker SJ, Zahr LK, Cole JG, Brecht ML. Outcome after developmental intervention in the neonatal intensive care unit for mothers of preterm infants with low socioeconomic status. Journal of Pediatrics 1992;120:780‐5. [DOI] [PubMed] [Google Scholar]
Pressler 2002 {published data only}
- Pressler JL, Hepworth JT. A quantitative use of the NIDCAP tool. Clinical Nursing Research 2002;11:89‐102. [DOI] [PubMed] [Google Scholar]
Resnick 1998 {published data only}
- Resnick MB, Gomatam SV, Carter RL, Ariet M, Roth J, Kilgore KL, Bucciarelli RL, Mahan CS, Curran JS, Eitzman DV. Educational disabilities of neonatal intensive care graduates. Pediatrics 1998;102:308‐14. [DOI] [PubMed] [Google Scholar]
Saunders 1995 {published data only}
- Saunders AN. Incubator noise: A method to decrease decibels. Pediatric Nursing 1995;21:265‐8. [PubMed] [Google Scholar]
Schwirian 1986 {published data only}
- Schwirian PM, Eesley T, Cuellar L. Use of water pillows in reducing head shape distortion in preterm infants. Research in Nursing & Health 1986;9:203‐7. [DOI] [PubMed] [Google Scholar]
Sell 1992 {published data only}
- Sell EJ, Hill‐Mangan S, Holberg CJ. Natural course of behavioral organization in premature infants. Infant Behavior and Development 1992;15:461‐78. [Google Scholar]
Standley 1995 {published data only}
- Standley JM, Moore RS. Therapeutic effects of music and mother's voice on premature infants. Pediatric Nursing 1995;21:509‐12. [PubMed] [Google Scholar]
Standley 1998 {published data only}
- Standley JM. The effect of music and mulitmodal stimulation on responses of premature infants in neonatal intensive care. Pediatric Nursing 1998;24:532‐8. [PubMed] [Google Scholar]
White‐Traut 1999 {published data only}
- White‐Traut RC, Nelson MN, Silvestri JM, Patel M, Vasan U, Han BK, Cunningham N, Burns K, Kopischke K, Bradford L. Developmental intervention for preterm infants diagnosed with periventricular leukomalacia. Research in Nursing & Health 1999;22:131‐43. [DOI] [PubMed] [Google Scholar]
White‐Traut 2004 {published data only}
- White‐Traut RC, Nelson MN, Silvestri JM, Patel M, Berbaum M, Gu G‐G, Rey PM. Developmental patterns of physiologic response to a multisensory intervention in extremely premature and high risk infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2004;33(2):266‐75. [DOI] [PubMed] [Google Scholar]
Wilcox 1995 {published data only}
- Wilcox S. Happy Babies. Nursing Times 1995;91:48‐51. [PubMed] [Google Scholar]
Zahr 1995/a {published data only}
- Zahr LK, Balian S. Responses of premature infants to routine nursing interventions and noise in the NICU. Nursing Research 1995;44:179‐85. [PubMed] [Google Scholar]
References to ongoing studies
Tyebkhan 2004 {published data only}
- Not known. Ongoing study Not known.
Additional references
Als 1984
- Als H. Manual for the naturalistic observation of newborn behavior (preterm and full term). Boston: The Children's Hospital, 1985:1‐19. [Google Scholar]
Als 1986
- Als H, Lawhon G, Brown E, Gibes R, Duffy FH, McAnulty G, Blickman JG. Individualized behavioural and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: neonatal intensive care unit and developmental outcome. Pediatrics 1986;78:1123‐32. [PubMed] [Google Scholar]
Cornell 1976
- Cornell EH, Gottfried AW. Intervention with premature human infants. Child Development 1976;47:32‐9. [PubMed] [Google Scholar]
Gottfried 1985
- Gottfried AW, Gaiter JL. Infant stress under intensive care. University Park Press, 1985. [Google Scholar]
Lawson 1977
- Lawson K, Daum C, Turkewitz G. Environmental characteristics of a neonatal intensive care unit. Child Development 1977;48:1633‐9. [PubMed] [Google Scholar]
Rothchild 1966
- Rothchild BT. Incubator isolation as a possible contributing factor to the high incidence of emotional disturbance among premature born persons. The Journal of Genetic Psychology 1966;110:287‐304. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Symington 2000
- Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001814] [DOI] [PubMed] [Google Scholar]
Symington 2001
- Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001814] [DOI] [PubMed] [Google Scholar]
Symington 2003
- Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD001814] [DOI] [PubMed] [Google Scholar]


