Abstract
Context
Recombinant human thyrotropin (rhTSH) is currently not Food and Drug Administration approved for the treatment of high-risk patients with differentiated thyroid cancer (DTC).
Objective
The goal of our study was to compare the outcomes in higher-risk patients with metastatic DTC prepared for radioiodine (RAI) therapy with rhTSH vs thyroid hormone withdrawal (THW).
Methods
A retrospective chart review was performed of patients with metastatic DTC in follow-up at MedStar Washington Hospital Center and MedStar Georgetown University Hospital from 2009 to 2017. Patients were divided according to their preparation for RAI therapy, with assessment of progression-free survival (PFS) and overall survival (OS).
Results
Fifty-five patients with distant metastases (16 men, 39 women) were prepared for RAI therapy exclusively either with rhTSH (n = 27) or with THW (n = 28). There were no statistically significant differences between the groups regarding clinicopathological features and history of RAI therapies. The median follow-up time for patients with rhTSH-aided therapies was 4.2 years (range, 3.3-5.5 years) and for patients with THW-aided therapies was 6.8 years (range, 4.2-11.6 years) (P = .002). Multivariate analysis showed that the method of thyrotropin stimulation was not associated with a difference in PFS or OS.
Conclusion
As has been shown previously for low-risk DTC, this study indicates that the mode of preparation for RAI therapy does not appear to influence the outcomes of patients with metastatic DTC. PFS and OS were similar for patients with THW-aided or rhTSH-aided RAI therapies.
Keywords: differentiated thyroid cancer, metastatic DTC, recombinant TSH, thyroid hormone withdrawal, radioiodine therapy
The use of recombinant human thyrotropin (rhTSH) to facilitate radioiodine (RAI) uptake as a diagnostic tool in the follow-up of differentiated thyroid cancer (DTC) dates to November 1998 in the United States. rhTSH (Thyrogen) was originally approved by the Food and Drug Administration (FDA) for diagnostic purposes to identify residual tumor via the stimulation of serum thyroglobulin secretion with or without a concomitant diagnostic RAI scan. In February 2005, rhTSH was approved by the European Medicines Agencies as an alternative to thyroid hormone withdrawal for pretherapy stimulation for remnant ablation of low-risk patients with DTC. Regulatory approval soon followed in Australia, selected Asian countries, and many South American countries [1]. In December 2007, the FDA issued its approval for the use of rhTSH in combination with RAI to perform postsurgical ablation of thyroid tissue in patients with DTC [2]. The FDA and the European Medicines Agencies do not currently approve the use of rhTSH (Thyrogen, Genzyme Corporation) for facilitation of RAI therapy in higher-risk patients with metastatic DTC.
In clinical practice, however, cumulative experience with the use of rhTSH for thyroid remnant ablation in the last decade has prompted many physicians to extend its use (without specific FDA approval) as an aid to RAI therapy of patients with metastatic disease. Data from prospective [3] and retrospective studies [4] have suggested that rhTSH can be as effective as thyroid hormone withdrawal (THW) in the preparation for RAI therapy in metastatic thyroid cancer. In 2012, we reported a retrospective study analyzing the relative efficacy and side effect profile of preparation by THW vs rhTSH in RAI treatment of metastatic thyroid cancer [5]. That study included patients treated and monitored at MedStar Washington Hospital Center and MedStar Georgetown University Hospital between 1996 and 2009, and similar efficacy was found as preparation for the RAI treatment of metastatic thyroid cancer whether THW or rhTSH was employed.
Nevertheless, the extant 2015 American Thyroid Association thyroid cancer management guidelines state there are currently insufficient outcome data to recommend rhTSH-mediated preparation for patients with distant metastatic disease being treated with 131I (Recommendation 74—No recommendation, Insufficient evidence) [6]. Besides the studies previously mentioned [3, 4], a recently published study has compared 2 groups of metastatic DTC treated with 131I and prepared either by THW or rhTSH at each treatment. The authors found no difference in the outcome of either group [7]. Therefore, the efficacy of rhTSH in assisting RAI therapy in the setting of metastatic disease remains a relevant topic of discussion, and our aim was to provide additional data that might help lead to resolution of the controversy.
The goal of this study was to compare the progression-free survival (PFS) and overall survival (OS) of patients with metastatic DTC prepared for RAI therapy with rhTSH vs THW.
Materials and Methods
The present report updates our experience since the earlier study [5]. We performed a retrospective analysis of medical charts of RAI-avid patients with metastatic thyroid cancer in follow-up at MedStar Washington Hospital Center and MedStar Georgetown University Hospital from 1996 to 2017. The study group was subdivided into those prepared for RAI therapy by THW alone or with rhTSH alone. The former group was off thyroid hormone for at least 4 weeks, while the latter was given rhTSH 0.9 mg intramuscularly for 2 consecutive days, followed by a therapeutic activity of 131I within 24 to 30 hours. The mode of preparation was chosen based on the attending physician’s preference. A posttherapy scan was performed after 7 to 10 days. TSH levels at the time of therapy were documented for both groups. All patients were instructed to follow a low-iodine diet for at least 2 weeks before RAI therapy, and urine iodine levels were checked for compliance prior to therapy. A subset of patients that had a prior RAI therapy prepared with THW and subsequently with rhTSH is described separately. The study was approved by the MedStar Health Research Institutional Review Board.
Response to therapy was assessed according to the revised RECIST 1.1 criteria [8]. Complete response (CR) is defined by the disappearance of all target lesions, while partial response (PR) corresponds to at least a 30% reduction in the sum of their diameters. Progressive disease (PD) represents at least a 20% increase in the sum of diameters of target lesions, taking the smallest sum documented as a reference. Stable disease (SD) represents neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD [8]. PFS was assessed from the date of last RAI therapy to the date of a computed tomography or magnetic resonance imaging scan confirming progression of target lesions. OS was considered the interval between the last follow-up and the first radioiodine therapy. The primary end point of this study was difference in PFS between the groups. The secondary end point was OS.
Statistical Analysis
All data extracted from electronic medical records were summarized using descriptive statistics such as mean with SDs or medians and range for continuous variables, and frequencies and percentages for categorical variables. Continuous variables were compared by 2-sample t test and Wilcoxon rank sum test as appropriate, while categorical variables were compared by chi-square and Fisher exact tests as appropriate. Kaplan-Meier curves were provided for OS and PFS, and log rank test was used to compare the curves. Multivariate Cox proportional hazards models were conducted controlling for the independent variables that were significant in the bivariate analysis to examine the risk of progression and risk of death. Statistical significance was defined as P less than or equal to .05. Analyses were performed with SAS 9.4 (SAS Institute Inc).
Results
From 1996 to 2017 we identified 124 patients with metastatic DTC that were treated with RAI. We excluded 21 patients for lack of relevant follow-up data, 25 patients because of non-RAI avid metastases, 6 patients younger than 18 years at first RAI therapy, and 4 patients for incomplete data from the beginning of their disease. In 13 patients, THW was used as a method of preparation in initial treatments and rhTSH in subsequent ones throughout the follow-up; this group is described separately.
Our final cohort consisted of 55 patients (39 women) who were prepared for RAI therapy exclusively either with rhTSH or with THW. The baseline characteristics of patients are summarized in Table 1. Twenty-seven patients (49%) were prepared exclusively with rhTSH, whereas 28 patients (51%) were prepared exclusively with THW. The median age was 59 years (range, 47.5-65.5 years) for the rhTSH group and 41 years (range, 30.0-63.5 years) for the THW group (P < .05). In the entire cohort, 42 patients (76%) had papillary thyroid cancer, either classic or follicular variant, 6 patients (22%) had follicular thyroid cancer, 1 patient (4%) had Hürthle cell thyroid cancer, and 1 patient (4%) had poorly differentiated thyroid cancer. There was no statistically significant difference in pathologic features between the groups, regarding tumor size, histologic type, and extrathyroidal extension. All patients had distant metastases. The groups were balanced as to metastatic sites, except for macronodular lung metastasis, for which there were 14 patients (52%) in the rhTSH group and 6 patients (21%) in the THW group (P < .05). Metastases to the liver, pleura, skin, or mediastinal lymph nodes were considered atypical.
Table 1.
Baseline characteristics
| rhTSH (n = 27) | THW (n = 28) | P | |
|---|---|---|---|
| Age, y | .02 | ||
| Median | 59 | 41 | |
| Range | 47.5-65.5 | 30-63.5 | |
| Sex | .09 | ||
| Male | 5 (19%) | 11 (39%) | |
| Female | 22 (81%) | 17 (61%) | |
| Tumor size, cm | 3.6 ± 2.7 | 3.9 ± 2.3 | .44 |
| Histology | .70 | ||
| PTC | 12 (44%) | 15 (54%) | |
| PTCFV | 7 (26%) | 6 (21%) | |
| FTC | 6 (22%) | 4 (14%) | |
| HTC | 1 (4%) | 3 (11%) | |
| PD | 1 (4%) | 0 | |
| Extrathyroidal extension | 12 (60%) | 10 (40%) | .18 |
| Distribution of metastasis | |||
| Lung—micronodular | 19 (70%) | 19 (68%) | .84 |
| Lung—macronodular | 14 (52%) | 6 (21%) | .02 |
| Bone | 13 (48%) | 9 (32%) | .22 |
| Brain | 2 (7%) | 1 (7%) | .53 |
| Atypical | 5 (19%) | 2 (7%) | .21 |
| sTg before first therapy, ng/mL | .96 | ||
| Median (range) | 602 (132.5-6962.5) | 900 (66.2-1944.0) | |
| TSH at time of RAI therapy | 112.4 ± 54.3 | 71.8 ± 32.6 | .01 |
| Activity at first therapy, mCi | .48 | ||
| Mean ± SD | 223.3 ± 113.8 | 196.2 ± 89.7 | |
| Median (range) | 197.7 (143.7-314) | 192.1 (148.8-255) | |
| Dosimetry-based therapy | 24 (89%) | 14 (50%) | .002 |
| Empiric-based therapy | 3 (11%) | 14 (50%) | .004 |
| Mean urine iodine, µg/L | 88.8 ± 44.7 | 114.2 ± 113.0 | .88 |
| No. of RAI therapies | .22 | ||
| Median (range) | 1 (1-2) | 2 (1-2) | |
| Mean total cumulative activity, mCi | 329 ± 197 | 418 ± 228 | .12 |
| Follow-up time, y | |||
| Median (range) | 4.2 (3.3-5.5) | 6.8 (4.2-11.6) | .002 |
Abbreviations: FTC, follicular thyroid cancer; HTC, Hürthle cell thyroid cancer; PD, poorly differentiated thyroid cancer; PTC, papillary thyroid cancer; PTCFV, papillary thyroid cancer follicular variant; RAI, radioiodine; sTg, stimulated thyroglobulin; TSH, thyrotropin.
Thyrotropin levels at the time of therapy were adequately elevated in both groups, with higher levels in the rhTSH group (median of 112.7 mIU/L the day after rhTSH stimulation vs 68.9 mIU/L after THW, P = .01). Mean urine iodine levels were similar between the groups. There was no statistical difference in RAI activity used in the first therapy in both groups, but in the rhTSH group 24 out of 27 patients (89%) received a dosimetry-based therapy, while in the THW group 14 out of 28 patients (50%) were dosimetrically treated (P = .002). There was no statistically significant difference in the mean number of RAI therapies and in the mean cumulative activity between the groups. The median follow-up time for patients prepared with rhTSH was 4.2 years (range, 3.3-5.5 years) and for patients prepared with THW was 6.8 years (range, 4.2-11.6 years) (P = .002).
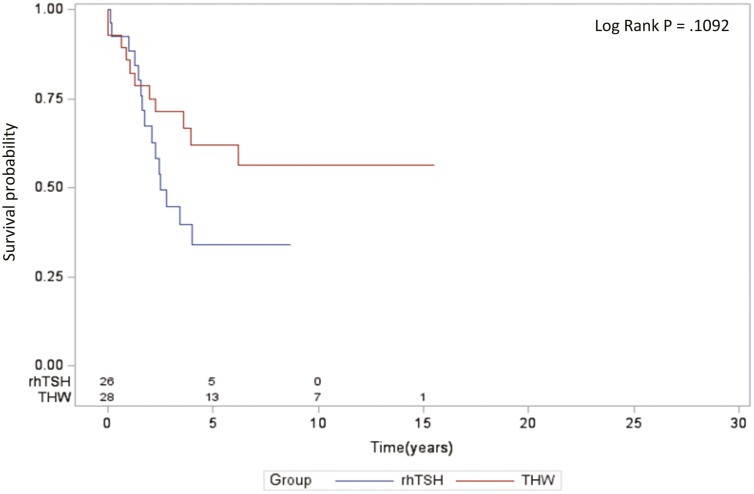
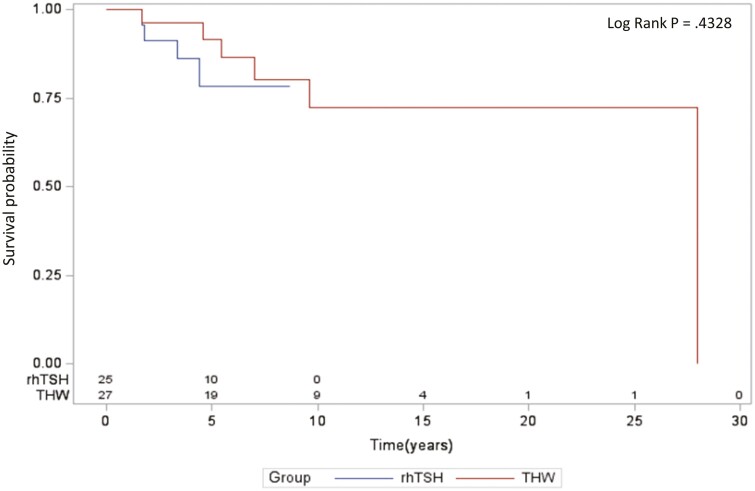
There was no statistically significant difference in the PFS between the groups of patients prepared with rhTSH or THW (Fig. 1). Multivariate analysis adjusting for age, sex, macronodular lung metastases, number of RAI therapies, and cumulative activity of RAI revealed that older age at the time of diagnosis was the only variable significantly associated with a greater risk of progression. The method of thyrotropin stimulation, either rhTSH or THW, was not associated with a difference in PFS (Table 2). Similarly, no statistically significant difference was observed in the OS between the groups (Fig. 2). Multivariate analysis adjusting for the same variables demonstrated that both older age at diagnosis and higher total cumulative activity of RAI were associated with a greater risk of death. The method of thyrotropin stimulation was not associated with a difference in the OS of these patients (Table 3).
Figure 1.
Progression-free survival in recombinant human thyrotropin (rhTSH) and thyroid hormone withdrawal (THW) groups.
Table 2.
Multivariate analysis for progression-free survival
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Arm THW | 0.481 | 0.184-1.258 | .13 |
| Age at diagnosis, y | 1.047 | 1.016-1.080 | .003 |
| Female sex | 1.017 | 0.395-2.616 | .97 |
| Total cumulative activity of 131I | 1.000 | 0.997-1.003 | .95 |
| Multiple macronodular lung metastases | 0.537 | 0.213-1.355 | .19 |
| No. of repeated therapies | 1.751 | 0.736-4.165 | .20 |
Abbreviation: THW, thyroid hormone withdrawal.
Figure 2.
Overall survival in recombinant human thyrotropin (rhTSH) and thyroid hormone withdrawal (THW) groups.
Table 3.
Multivariate analysis for overall survival
| Parameter | Odds ratio | 95% CI | P |
|---|---|---|---|
| Arm THW | 0.850 | 0.153-4.715 | .85 |
| Age at diagnosis, y | 1.087 | 1.017-1.162 | .01 |
| Female sex | 6.207 | 0.923-41.761 | .06 |
| Total cumulative activity of 131I | 1.007 | 1.001-1.013 | .03 |
| Multiple macronodular lung metastases | 0.362 | 0.063-2.072 | .25 |
| No. of repeated therapies | 0.226 | 0.032-1.596 | .13 |
Abbreviation: THW, thyroid hormone withdrawal.
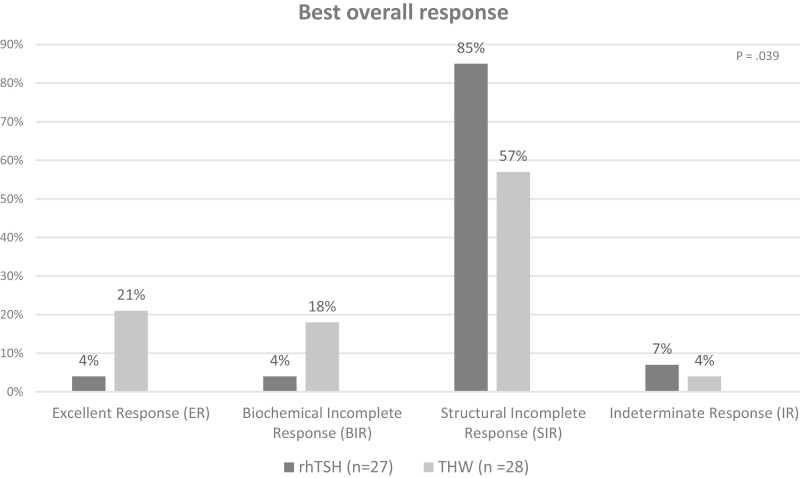
In the rhTSH group, structural incomplete response was observed in 23 patients (85%), followed by indeterminate response in 2 patients (7%), and biochemical incomplete response and excellent response (1 patient each—4% each). In the THW group, structural incomplete response was seen in 16 patients (57%), excellent response in 6 patients (21%), biochemical incomplete response in 5 patients (18%) and indeterminate response in 1 patient (4%) (Fig. 3). The difference between the types of response was statistically significant (P = .039).
Figure 3.
Best overall response.
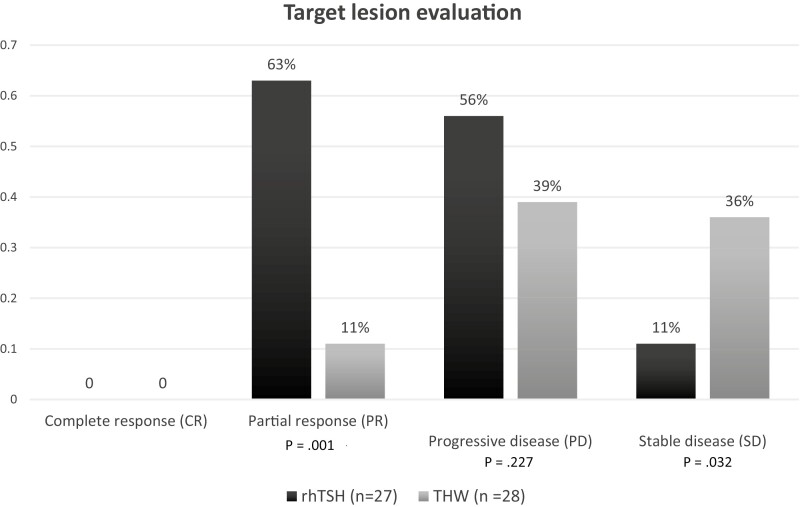
Regarding target lesion evaluation, no patient achieved CR. PR was observed in 17 out of 27 patients (63%) in the rhTSH group, compared to 3 out of 28 patients (11%) in the THW group (P < .001). PD was found in 15 patients (56%) in the rhTSH group, and in 11 patients (39%) in the THW group (P = .227). SD was more often observed in the THW group (36% vs 11%, P = .032) (Fig. 4).
Figure 4.
Target lesion evaluation according to RECIST 1.1.
A third group of patients was composed of patients who had initial RAI therapy with THW but had subsequent therapies prepared with rhTSH. The baseline characteristics of this group are described in Table 4. This group is characterized by more therapies (2.8 ± 1.0) and higher mean cumulative activity of radioiodine (584.6 ± 161.1 mCi). Responses to therapy in this group were biochemical incomplete response in 3 patients (23%) and structural incomplete response in 10 patients (77%).
Table 4.
Baseline characteristics of the mixed group (thyroid hormone withdrawal–aided followed by recombinant human thyrotropin–aided therapies)
| THW → rhTSH (n = 13) | |
|---|---|
| Age, y | |
| Median | 54 |
| Range | 23-73 |
| Sex | |
| Male | 6 |
| Female | 7 |
| Tumor size, cm | 2.6 ± 1.7 |
| Histology | |
| PTC | 4 |
| PTCFV | 2 |
| FTC | 6 |
| PTCTC | 1 |
| Extrathyroidal extension | 5 |
| Distribution of metastasis | |
| Lung—micronodular | 7 |
| Lung—macronodular | 7 |
| Bone | 6 |
| Brain | 2 |
| Atypical | 0 |
| sTg before first therapy, ng/mL | |
| Median (range) | 255 (31.6-22 997.5) |
| TSH at time of RAI therapy | 73.8 ± 70.5 |
| Activity at first therapy, mCi | |
| Mean ± SD | 185.1 ± 112 |
| Median (range) | 152.2 (26.4-370) |
| Dosimetry-based therapy | 8 (61.5%) |
| Median No. of RAI therapies (range) | 2 (2-5) |
| Mean total cumulative activity, mCi | 584.6 ± 161.1 |
| Follow-up time, y | |
| Median | 5.7 |
| Range | 1.7-41.5 |
| Best overall response | |
| BIR | 3 (23%) |
| SIR | 10 (77%) |
Abbreviations: BIR, biochemical incomplete response; FTC, follicular thyroid cancer; HTC, Hürthle cell thyroid cancer; PD, poorly differentiated thyroid cancer; PTC, papillary thyroid cancer; PTCFV, papillary thyroid cancer follicular variant; RAI, radioiodine; rhTSH, recombinant human thyrotropin; SIR, structural incomplete response; sTg, stimulated thyroglobulin; THW, thyroid hormone withdrawal.
Discussion
In this retrospective study, PFS and OS of patients with metastatic DTC were statistically comparable, regardless of the mode of preparation for RAI therapy, either with rhTSH or with THW.
Ideally, a prospective noninferiority study to compare patients with metastatic DTC prepared for RAI therapy with exogenous or endogenous thyrotropin would be based on the best-quality evidence. Yet in a systematic literature review that included both residual disease and metastatic DTC, no randomized controlled clinical trials were identified comparing rhTSH vs THW-mediated therapy for metastatic DTC [9].
The first evidence of the efficacy of rhTSH-aided RAI therapy came from several case reports [10-13]. Patients were selected for their inability to produce endogenous thyrotropin, for example, in patients with hypopituitarism, those with large tumor burden producing sufficient thyroid hormone to suppress thyrotropin, or because of a medical contraindication to hypothyroidism after THW, such as in patients with unstable angina. Despite the limitations of drawing definitive conclusions from case reports, the studies indicated that rhTSH reliably elevated thyrotropin to levels considerably higher than 30 mUI/L, that most metastatic lesions demonstrated RAI uptake on posttherapy scans, and that patients benefited from the avoidance of symptoms of hypothyroidism [14].
Larger studies have addressed the use of rhTSH preparation for RAI therapy in patients with DTC and distant metastases. Jarzab et al [3] prospectively evaluated 54 patients who received rhTSH to aid RAI therapy for advanced DTC including 49 patients with distant metastases, of whom 31 were RAI avid. There was no control group with THW-aided therapy, and patients served as their own controls comparing the outcome of rhTSH-aided treatment vs the outcome of previous THW-aided therapies. The authors concluded that rhTSH-aided RAI therapy could be equally effective as THW-aided RAI therapy [3]. Tala et al [4] published a retrospective study that included 175 patients with metastatic DTC of whom 35 patients were prepared with THW only, 58 patients with rhTSH only, and 82 patients had THW followed by rhTSH in subsequent therapies. No difference was noted in OS among the groups independent of how patients were prepared for RAI therapy [4]. In a retrospective study from our group, the outcomes in 15 patients prepared for RAI therapy with rhTSH were compared to that for 41 patients prepared after THW. Similar efficacy and safety profiles of either method of preparation for RAI treatment of RAI-avid metastases were observed [5]. In another retrospective study, Campopiano et al [7] compared 2 groups of patients with metastatic DTC treated with 131I and prepared either by THW (n = 34) or rhTSH (n = 43) at each treatment. After 4 years of follow-up, although 93% of the patients had structural disease, 91% of them obtained clinical benefit from RAI therapy in terms of SD, CR, or PR. The authors concluded that the preparation with either recombinant or endogenous thyrotropin had no effect on the efficacy of RAI therapy and on the outcome of the 2 groups [7]. The findings of the present study extend and corroborate earlier results indicating that the method of thyrotropin stimulation was not associated with a difference in OS, and we also demonstrated that PFS is similarly not affected. Consistent with the literature [15, 16], age at diagnosis was the only independent variable that affected both the risk of progression and the risk of death in our population. In fact, age is such an important prognostic factor in thyroid cancer that it is incorporated into relevant thyroid cancer staging systems [17, 18]. Another finding is that the total cumulative activity of RAI is associated with an increased risk of death. We hypothesize that this is related to metastatic lesions less responsive to RAI, leading to persistent disease, repeated therapies, and poorer outcomes, rather than to an adverse effect of higher RAI activities per se.
As observed by Campopiano et al [7], we also found a significant number of patients with structural disease in our cohort (Fig. 3). This finding may be related to factors intrinsic to the tumor, such as molecular abnormalities associated with more aggressiveness, or to the fact that our hospital is a referral center for patients with advanced cases, who are more likely to have persistent disease. Although we have not performed a subanalysis for different metastatic sites, we also found that our patients benefited from RAI therapy, as shown by the number of patients with PR and with SD (Fig. 4). For patients with PD, there was no statistical difference between the groups, suggesting that factors other than the mode of preparation for RAI therapy may play a role in disease progression.
Several studies have evaluated the negative effects of levothyroxine withdrawal on patients’ quality of life [19, 20]. On the other hand, the cumulative experience with rhTSH has shown that it is well tolerated, with mild adverse effects [21]. Moreover, the use of rhTSH is associated with better quality of life during the interval of RAI therapy, with minimal economic effect on the work productivity of patients, compared to levothyroxine withdrawal [21]. Previous studies have examined the cost-effectiveness of rhTSH in Europe and in the United States [22, 23]. Renal RAI clearance in rhTSH-aided therapies is higher than when THW-aided because of preserved renal function during euthyroidism. As a result, lower levels of radiation exposure in extrathyroidal tissues, such as blood and bone marrow, are expected. Yet the accelerated clearance of RAI could be considered a disadvantage for the use of rhTSH because it could result in reduced residence time of RAI in the metastatic lesions. In fact, iodine biokinetics are affected by the use of rhTSH for remnant ablation with significantly lower residence times of the RAI in the whole body and blood [24]. We recommend a dosimetric approach to therapy to determine the optimal safe RAI activity to be administered to each individual, especially for high-risk patients. In our study population, dosimetry was performed in 89% of patients in the rhTSH-aided RAI therapy group and in 50% of THW-aided RAI therapy group. Among the disadvantages of employing rhTSH for therapies is its high cost and lack of availability in some countries.
This study is limited by its retrospective design and possible selection bias. However, the groups were statistically comparable regarding the clinicopathological features between patients prepared with THW and rhTSH, as well as for the RAI therapies administered. Another possible limitation is the number of patients in the final analysis, with patients returning to their referring institutions and lost to long-term follow-up. However, a strength of this study lies in the inclusion of patients prepared exclusively with THW or with rhTSH throughout their management. An additional cohort of 13 patients was analyzed who had THW-aided RAI therapy for initial therapy and were subsequently prepared with rhTSH. A direct comparison of the OS and PFS of these patients to those with exclusive preparations is statistically challenging because of the confounding effect of previous therapies. The mean number of RAI therapies and the mean total cumulative activity is higher for this latter group of patients, who also had a longer follow-up time. We include a description of the outcomes in this group because patients with this history are frequently seen at other institutions.
We conclude that the mode of preparation for RAI therapy does not decisively affect the outcome of RAI therapy of patients with metastatic DTC. While PFS and the OS were similar for patients with THW-aided or rhTSH-aided RAI therapies, future controlled studies are warranted to further confirm these observations.
Glossary
Abbreviations
- CR
complete response
- DTC
differentiated thyroid cancer
- FDA
Food and Drug Administration
- OS
overall survival
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- RAI
radioiodine
- rhTSH
recombinant human thyrotropin
- StD
stable disease
- TSH
thyrotropin
- THW
thyroid hormone withdrawal
Financial Support
This work was supported by The Catherine Heron and Al Schneider Fellowship in Thyroid Cancer Research.
Disclosures
D.V.N. has been a speaker and consultant for Jubilant DraxImage. The other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Haugen BR, Cooper DS, Emerson CH, et al. Expanding indications for recombinant human TSH in thyroid cancer. Thyroid. 2008;18(7):687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. FDA. Accessed October 3, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020898s038lbl.pdf
- 3. Jarzab B, Handkiewicz-Junak D, Roskosz J, et al. Recombinant human TSH-aided radioiodine treatment of advanced differentiated thyroid carcinoma: a single-centre study of 54 patients. Eur J Nucl Med Mol Imaging. 2003;30(8):1077-1086. [DOI] [PubMed] [Google Scholar]
- 4. Tala H, Robbins R, Fagin JA, Larson SM, Tuttle RM. Five-year survival is similar in thyroid cancer patients with distant metastases prepared for radioactive iodine therapy with either thyroid hormone withdrawal or recombinant human TSH. J Clin Endocrinol Metab. 2011;96(7):2105-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, Mete M, Jonklaas J, Wartofsky L. Radioiodine treatment of metastatic thyroid cancer: relative efficacy and side effect profile of preparation by thyroid hormone withdrawal versus recombinant human thyrotropin. Thyroid. 2012;22(3):310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campopiano MC, Podestà D, Bianchi F, et al. No difference in the outcome of metastatic thyroid cancer patients when using recombinant or endogenous TSH. Eur J Endocrinol. 2020;183(4):411-417. [DOI] [PubMed] [Google Scholar]
- 8. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 9. Ma C, Xie J, Liu W, et al. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane Database Syst Rev. 2010;2010(11):CD008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rudavsky AZ, Freeman LM. Treatment of scan-negative, thyroglobulin-positive metastatic thyroid cancer using radioiodine 131I and recombinant human thyroid stimulating hormone. J Clin Endocrinol Metab. 1997;82(1):11-14. [DOI] [PubMed] [Google Scholar]
- 11. Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997;82(11):3637-3642. [DOI] [PubMed] [Google Scholar]
- 12. Adler ML, Macapinlac HA, Robbins RJ. Radioiodine treatment of thyroid cancer with the aid of recombinant human thyrotropin. Endocr Pract. 1998;4(5):282-286. [DOI] [PubMed] [Google Scholar]
- 13. Colleran KM, Burge MR. Isolated thyrotropin deficiency secondary to primary empty sella in a patient with differentiated thyroid carcinoma: an indication for recombinant thyrotropin. Thyroid. 1999;9(12):1249-1252. [DOI] [PubMed] [Google Scholar]
- 14. Robbins R, Folb L, Tuttle RM. Treatment of metastatic thyroid cancer with radioiodine following preparation by recombinant human thyrotropin. In: Wartofsky L, Van Nostrand D, eds. Thyroid Cancer: A Comprehensive Guide to Clinical Management. Springer; 2016. [Google Scholar]
- 15. Ganly I, Nixon IJ, Wang LY, et al. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid. 2015;25(10):1106-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid. 2015;25(1):125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1050-1057; discussion 1057-1058. [PubMed] [Google Scholar]
- 18. Tuttle RM, Morris LF, Haugen BR, et al. Thyroid—differentiated and anaplastic carcinoma. AJCC Cancer Staging Manual. 8th ed. Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 19. Duntas LH, Biondi B. Short-term hypothyroidism after levothyroxine-withdrawal in patients with differentiated thyroid cancer: clinical and quality of life consequences. Eur J Endocrinol. 2007;156(1):13-19. [DOI] [PubMed] [Google Scholar]
- 20. An JH, Song KH, Kim DL, Kim SK. Effects of thyroid hormone withdrawal on metabolic and cardiovascular parameters during radioactive iodine therapy in differentiated thyroid cancer. J Int Med Res. 2017;45(1):38-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reiners C, Lassmann M, Luster M. Recombinant human thyrotropin: safety and quality of life evaluation. J Endocrinol Invest. 2012;35(6 Suppl):30-35. [PubMed] [Google Scholar]
- 22. Borget I, Remy H, Chevalier J, et al. Length and cost of hospital stay of radioiodine ablation in thyroid cancer patients: comparison between preparation with thyroid hormone withdrawal and Thyrogen. Eur J Nucl Med Mol Imaging. 2008;35(8):1457-1463. [DOI] [PubMed] [Google Scholar]
- 23. Wang TS, Cheung K, Mehta P, Roman SA, Walker HD, Sosa JA. To stimulate or withdraw? a cost-utility analysis of recombinant human thyrotropin versus thyroxine withdrawal for radioiodine ablation in patients with low-risk differentiated thyroid cancer in the United States. J Clin Endocrinol Metab. 2010;95(4):1672-1680. [DOI] [PubMed] [Google Scholar]
- 24. Hanscheid H, Lassmann M, Luster M, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006;47(4):648-654. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.