Abstract
Aminoglycoside acetyltransferase was detected in Mycobacterium kansasii and M. fortuitum but not in M. avium-M. intracellulare when they were screened by a radioassay. Aminoglycoside phosphotransferase and nucleotidyltransferase activities were absent from all three species tested. Acetyltransferases from both M. kansasii and M. fortuitum displayed relatively high Kms, all at the millimolar level, for substrates including tobramycin, neomycin, and kanamycin A. The Km of each substrate was well above the corresponding maximum achievable level in serum. The low affinities of these enzymes for their substrates suggested that drug modification in vivo was very unlikely. Among the various substrates tested, no apparent positive correlation was found between substrate affinity and resistance level. The presence of aminoglycoside-modifying enzymes in these mycobacterial species was therefore not shown to confer resistance to aminoglycosides.
Production of aminoglycoside-modifying enzymes (AMEs) is by far the most common mechanism of aminoglycoside resistance in gram-positive and gram-negative bacteria (17, 24, 28); and three classes of AMEs, namely, aminoglycoside acetyltransferase (AAC), aminoglycoside phosphotransferase (APH), and aminoglycoside nucleotidyltransferase (ANT), have been identified (3, 12). Despite the long history of the study of aminoglycoside resistance mechanisms in gram-positive and gram-negative bacteria, little has been known about the details of the mechanisms of aminoglycoside resistance in mycobacteria. Presumably, the aminoglycoside resistance mechanisms resemble those of the gram-positive and gram-negative bacteria. In gram-positive and gram-negative bacteria, these mechanisms include production of AMEs that inactivate the drugs, the presence of an uptake barrier, and target site modification (6, 7). AMEs have been reported in some mycobacteria (16), the mycolic acid-rich cell wall envelope of mycobacteria has been found to render permeation of hydrophilic solutes slow (19), and mutations in the rpsL and rrs genes have also been identified in streptomycin-resistant strains of Mycobacterium tuberculosis (15).
Only a few studies of AMEs in mycobacteria have been reported, and these have mainly been confined to the studies of the Runyon group IV rapid growers (16, 29, 31, 33). These studies reported on the presence of acetyltransferase and phosphotransferase in rapidly growing mycobacteria but did not find them to be responsible for resistance, as no positive correlation had been found between the resistance levels (MICs) and the degree of inactivation of aminoglycosides (16). Further investigations suggested that these enzymes might have metabolic functions, in addition to the ability to inactivate aminoglycosides (30). Mitsuhashi and coworkers (22) also detected kanamycin acetyltransferase from a resistant strain of M. tuberculosis but gave no information about the sensitive strains which might also produce a certain amount of the enzyme. A strain of M. intracellulare studied by other investigators (23) was found to have no AME. Whether other mycobacterial species produce AMEs or not remains uncertain as studies are scarce. The present study was aimed at screening for AMEs in M. kansasii, M. avium-M. intracellulare, and M. fortuitum and to examine their role(s) in aminoglycoside resistance.
MATERIALS AND METHODS
Organisms.
Clinical isolates of M. kansasii (n = 19), M. avium-M. intracellulare complex (n = 24), and M. fortuitum (n = 30) were included in this study. Standard strains M. kansasii ATCC 12478, M. avium-M. intracellulare ATCC 35718, and M. fortuitum ATCC 6841 were also tested. Escherichia coli NCTC 11186 (E. coli R5/W677), which produces AAC(6′)-I (2, 5), Staphylococcus aureus STA 446 (a gift from R. Vanhoof, Pasteur Institute, Brussels, Belgium), which produces ANT(4′,4") (32), and Streptomyces ribosidificus ATCC 21294, which produces APH(3′) (11), were used as control organisms in AME screening assays.
Preparation of cell extracts.
All isolates of mycobacteria were grown in Middlebrook 7H9 broth (Difco, Detroit, Mich.) supplemented with glycerol (2.0 μl/ml) and oleic acid-dextrose-catalase (Difco) (100 μl/ml) until cultures reached the late logarithmic phase (1 week for rapid growers and 2 to 3 weeks for slow growers). The cells were then harvested by centrifugation, and the pellets were washed twice with cold phosphate-buffered saline (PBS). The washed cells were resuspended in PBS (4°C) in microcentrifuge tubes and were disrupted mechanically by ultrasonication (five 30-s bursts at 60-s intervals with an amplitude of 12 μm in an ice bath) by using an MSE 150-W ultrasonic disintegrator (Mass Scientific Equipments, Sussex, England). The disrupted materials were centrifuged for 1 min at 11,600 × g with a microcentrifuge (Mass Scientific Equipments), and the supernatant was collected. Cell extracts of the control organisms E. coli NCTC 11186, S. aureus STA 446, and S. ribosidificus ATCC 21294 were prepared in the same way, except that they were grown in Mueller-Hinton broth (Oxoid, Hampshire, England). The crude extracts were used either fresh after preparation or were aliquoted and kept at −70°C for not more than 1 month. The protein concentrations of the crude extracts were determined by the method of Lowry et al. (21) by using bovine serum albumin (Sigma, St. Louis, Mo.) as the standard.
Screening for AMEs.
AMEs were assayed by the phosphocellulose paper binding method (12). Radiolabeled [1-14C]acetyl coenzyme A ([1-14C]acetyl-CoA); (specific activity, 50 to 62 mCi/mmol), [1-14C]ATP (specific activity, 50 to 62 mCi/mmol), and [γ-32P]ATP (specific activity, ∼3,000 Ci/mmol) were purchased from Amersham (Buckinghamshire, England). Each class of AMEs was screened with four selected aminoglycosides (all from Sigma); these included gentamicin, tobramycin, kanamycin A, and amikacin for acetyltransferase; streptomycin, spectinomycin, kanamycin A, and amikacin for nucleotidyltransferase; and streptomycin, neomycin, kanamycin A, and amikacin for phosphotransferase. Reaction mixtures consisting of the crude extract, the antibiotic being studied (at a final concentration of 100 μg/ml), and the labeled cofactor in the appropriate buffer were incubated at 37°C for 60 min. The amount of radioactivity associated with each aminoglycoside tested was measured with the Wallac 1410 liquid scintillation counter (Pharmacia, Wallac Oy, Turku, Finland). Both blank controls, in which PBS replaced the enzyme, and positive controls, in which enzyme prepared from the control strains was used, were included.
The incorporation of radiolabeled cofactors into the substrate that resulted from enzymatic modification was determined by subtracting the radioactivity (in disintegrations per minute) in negative blank controls from that in the test samples. For crude screening, enzyme was regarded to be present when the radioactivity counts of the test samples were five times or more than those of the negative blank controls (33).
Kinetic parameters.
Kinetic studies were carried out for acetyltransferase in the crude extracts of M. kansasii ATCC 12478 and M. fortuitum ATCC 6841. A fixed-time radioassay was used for initial rate determination. The assay mixture contained 15 μl of drug (at various concentrations) as the substrate, 15 μl of 1 mM [1-14C]acetyl-CoA (specific activity, 9.3 mCi/mmol; Amersham) (8), 45 μl of Tris-maleate buffer (pH 7.8) (10), and 75 μl of crude extract in the same buffer. The substrates studied included tobramycin, neomycin, and kanamycin A. The drug, buffer, and crude extract were premixed and were preincubated at 37°C for 5 min. The reaction was started by the addition of [1-14C]acetyl-CoA. Samples of 20 μl each were withdrawn at 2, 4, 6, 8, 10, and 15 min and placed onto 10-mm2 Whatman P81 phosphocellulose paper (Whatman, Maidstone, England) square that had been heated at 80°C to stop the reaction. The squares were then immersed in hot (70 to 80°C) distilled water and washed five times with large volumes of distilled water and dried, and the radioactivity was counted. Trial runs were performed to select for the range of substrate concentrations for kinetic constant evaluation. The maximum drug concentrations tested were 30 mM for both tobramycin and neomycin and 20 mM for kanamycin A. The blank control rate at each drug concentration was determined similarly by stopped radioassay in which the buffer replaced the crude extract. This rate was then subtracted from the initial rate determined from the time course of the enzyme-catalyzed reaction to give the actual value. To ensure that enzyme activity was not limited by the cofactor concentration, the linearity of the enzyme activity with the amount of enzyme was verified by varying the volume of crude extract while keeping the drug in excess.
For the AAC from each selected strain, kinetic constants of each substrate, which included the Michaelis-Menten constant (Km), maximum initial velocity (Vmax), and the ratio of the two kinetic constants (Vmax/Km), were determined graphically with Hanes-Woolf plots. The mean values of three different experiments were considered.
RESULTS
Screening for AMEs.
At the substrate concentration selected for screening, acetyltransferases were detected in M. kansasii and M. fortuitum but not in M. avium-M. intracellulare, while none of the species produced nucleotidyltransferase or phosphotransferase. The presence of any acetyltransferase in the crude extract of each mycobacterial species being studied was screened with one substrate at a time by using gentamicin, tobramycin, kanamycin A, and amikacin as substrates. Of the 19 isolates of M. kansasii tested, high counts (at least five times that for the negative blank controls) were detected in 18 of them (94.7%) when they were screened with tobramycin and kanamycin A. With gentamicin, only 17 of them (89.5%) produced such counts, while with amikacin, none of the M. kansasii isolates tested demonstrated any acetylating activity. Similarly, all 30 M. fortuitum isolates tested demonstrated AAC activity for tobramycin, but when screened with gentamicin and kanamycin A, acetylating activities were detected in 16 (53.3%) and 12 (40.0%) of the isolates, respectively. Again, none of them was found to acetylate amikacin. Crude extracts from M. kansasii ATCC 12478 and M. fortuitum ATCC 6841 also displayed acetylating activities for gentamicin, tobramycin, and kanamycin A but not for amikacin.
Initial velocities.
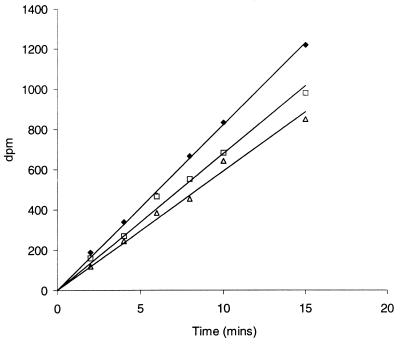
The time courses of the enzyme-catalyzed reactions for the acetyltransferase from M. kansasii ATCC 12478 for each substrate to be investigated at 10 mM are shown in Fig. 1. The substrates studied included tobramycin, neomycin, and kanamycin A. By least-squares regression analysis, the plots were all sufficiently linear (r2 ≥ 0.97) for the initial velocities to be determined directly from their slopes. Plots for all other concentrations of each substrate were also linear. Similar results were obtained for the acetyltransferase from M. fortuitum ATCC 6841. The initial velocities obtained from these plots were corrected for the blank control rates. The corrected values were subsequently used for kinetic constant determination with the Hanes-Woolf plot.
FIG. 1.
Acetylation of tobramycin (⧫), neomycin (□), and kanamycin A (▵) by a crude extract of M. kansasii ATCC 12478 at a drug concentration of 10 mM.
Kinetic analysis.
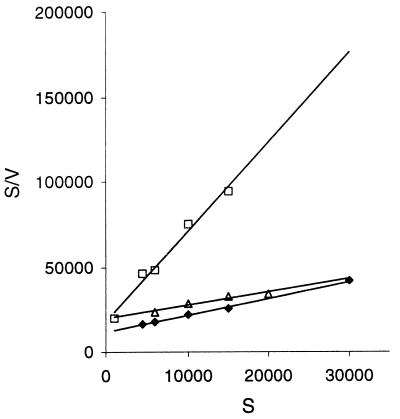
For the AACs from both M. kansasii ATCC 12478 and M. fortuitum ATCC 6841, plots of initial velocity versus substrate concentration for each substrate were hyperbolic, conforming to the Michaelis-Menten relationship. The acetyltransferase from M. kansasii was inhibited by neomycin at 30 mM, while that of M. fortuitum was inhibited by tobramycin, neomycin, and kanamycin A at concentrations above 15 mM. Acetylation of aminoglycosides by the enzyme acetyltransferase was actually a two-substrate reaction involving both the aminoglycoside and the cofactor acetyl-CoA. However, in this study, a pseudo-single-substrate reaction was generated for kinetic analysis by keeping the cofactor in excess. The cofactor concentration used was nonlimiting, as confirmed by the linear relationship between the initial velocity and the volume (or amount) of enzyme extract while keeping the aminoglycoside (neomycin, which had the lowest Km values, was selected) at a concentration in excess but with no substrate inhibition (i.e., 15 mM). Kinetic constants for the acetyltransferases from both M. kansasii ATCC 12478 and M. fortuitum ATCC 6841, as determined from the Hanes-Woolf plots, are summarized in Table 1. Such plots for M. kansasii ATCC 12478 are shown in Fig. 2, and similar results were obtained for M. fortuitum ATCC 6841.
TABLE 1.
Apparent kinetic parameters of AAC in crude extractsa of M. kansasii ATCC 12478 and M. fortuitum ATCC 6841 at 37°C and pH 7.8
| Strain | Substrate | Km (mM) | Vmax (nmol mg of protein−1 min−1) | Vmax/Km (10−4 min−1) |
|---|---|---|---|---|
| M. kansasii ATCC 12478 | Tobramycin | 13.04 ± 2.14 | 4.44 ± 0.83 | 0.74 ± 0.23 |
| Neomycin | 4.23 ± 0.87 | 1.68 ± 0.38 | 0.81 ± 0.10 | |
| Kanamycin A | 20.23 ± 4.94 | 4.26 ± 1.73 | 0.45 ± 0.13 | |
| M. fortuitum ATCC 6841 | Tobramycin | 2.56 ± 0.78 | 3.35 ± 0.10 | 3.23 ± 1.14 |
| Neomycin | 0.98 ± 0.09 | 1.35 ± 0.33 | 3.13 ± 1.05 | |
| Kanamycin A | 8.58 ± 1.28 | 10.7 ± 0.68 | 2.78 ± 0.29 |
The protein concentrations of the crude extracts in the reaction mixtures were 205 and 220 μg/ml for M. kansasii ATCC 12478 and M. fortuitum ATCC 6841, respectively.
FIG. 2.
Hanes-Woolf plot of acetyltransferase activity in a crude extract of M. kansasii ATCC 12478 for tobramycin (⧫), neomycin (□), and kanamycin A (▵). Abbreviations: S, substrate concentration (in micromolar); V, initial rate (in micromole liter−1 of reaction mixture minute−1).
DISCUSSION
Although the presence of AMEs, namely, acetyltransferase and phosphotransferase, had been demonstrated in M. fortuitum and M. chelonae (16, 29, 31, 33), it was unknown whether these enzymes were present in other mycobacterial species. Kinetic studies of the AMEs produced by mycobacteria were relatively scarce, even though the genetic determinants of AAC activity in M. fortuitum had been characterized (1). Knowledge of the kinetic constants, however, was crucial before the role of AMEs in aminoglycoside resistance in mycobacteria could be elucidated. In this study, we screened for the presence of AMEs in three clinically significant nontuberculous mycobacterial species and evaluated the kinetic constants of their AMEs, if any.
Preliminary screening for AMEs was carried out at the drug concentrations recommended in the literature (12), and the cutoff criteria described by Wallace et al. (33) were adopted. Owing to the lack of information about the Km of any substrate, multiple drugs including gentamicin, which is a broad-spectrum substrate for the various classes of AACs, were included to increase the sensitivity of detection. Such screening, however, provided very crude results, as the drug concentrations used for screening might exhibit substrate inhibition (4) for substrates with low Kms. Alternatively, little or no activity would be detected for substrates with high Kms. Knowledge of the Kms of various substrates was therefore essential before detailed screening was possible.
In this study, enzymatic activity on each selected substrate was screened at a concentration of 100 μg/ml (equivalent to 100 to 200 μM, depending on the molecular weight of the drug used). For those enzyme activities that had been evaluated over a range of substrate concentrations, no substrate inhibition was detected at those drug concentrations. As the drug concentration used for screening was much higher than the maximum achievable concentration (Cmax) of each drug in serum (25), a lack of any detectable in vitro activity at this drug level would imply the absence of such activity in vivo. Theoretically, those extracts that have counts below the cutoff level might still have some degree of activity when they are screened at more than one drug concentration, preferably below but at the same range as their Kms. Nevertheless, the AACs from both M. kansasii ATCC 12478 and M. fortuitum ATCC 6841 had Kms at the millimolar level for various substrates (Table 1), and the concentration was extremely high compared with the Cmax in vivo. Consequently, for those enzymes negative for activity in the preliminary screening, even if enzyme activity could be demonstrated with much higher substrate concentrations, such enzymes were unlikely to display any substantial activity on that substrate in vivo.
The screening for AMEs in the three selected nontuberculous mycobacterial species showed that acetyltransferase activity could be detected in both M. kansasii and M. fortuitum but not in M. avium-M. intracellulare. The broad-spectrum resistance of M. avium-M. intracellulare to the aminoglycosides shown previously (14) suggested that barrier effect played a role as the major mechanism of resistance in this species, and the absence of any modifying enzyme in these isolates further supported that suggestion. When screened for nucleotidyltransferase and phosphotransferase, the crude extracts of all strains studied gave counts comparable to those for the negative blank controls (data not shown); hence, all three mycobacterial species were unlikely to modify aminoglycosides with any of these two classes of AMEs in vivo.
The fixed-time radioassay provided an easy and convenient way for monitoring enzyme activity in crude extracts, and the time courses of the enzyme-catalyzed reactions were sufficiently linear (Fig. 1) for initial rate determinations. For all substrates tested, no substrate inhibition was observed up to a substrate concentration of 15 mM. Although it is a substrate, gentamicin was not included in the kinetic studies, as the proportions of the various components (gentamicins C1, C1a, and C2) and, hence, the molecular weight of the drug were unknown. Statistically, the Hanes-Woolf plot was preferred for graphical determination of the Km and Vmax (13), and in this study, kinetic constants from such plots (Fig. 2) were reported (Table 1). However, studies with both Lineweaver-Burk and Eadie-Hofstee plots were also performed, and the results were comparable (data not shown).
Unlike gram-positive and gram-negative bacteria, the purified enzymes of which displayed Km values at the low- to submicromolar level (9), the AACs in the crude extracts of M. kansasii ATCC 12478 and M. fortuitum ATCC 6841 had Km values at the millimolar level (Table 1). The affinities of these enzymes for the substrates were, hence, relatively low, and the enzymes alone were unlikely to be responsible for resistance. Furthermore, if the enzymes were responsible for resistance, then there should be a correlation between the resistance levels and the kinetic constants of the enzymes. In general, the lower Km of a particular substrate reflects a higher affinity of the enzyme for that substrate, resulting in a higher level of resistance against such a substrate in an organism. The in vitro activities of the various substrates against the selected mycobacterial strains had been determined and reported previously (14). In that study both M. kansasii ATCC 12478 and M. fortuitum ATCC 6841 were found to be more susceptible to neomycin than to tobramycin and kanamycin A. Kinetic studies, however, showed that the affinities of their AACs for neomycin were higher in comparison with those for tobramycin or kanamycin A, as reflected by their corresponding Kms (Table 1). Hence, no apparent positive correlation was found between the affinities of the enzymes for these substrates and their resistance levels.
For the AACs of both selected strains of M. kansasii and M. fortuitum, the values of the other two kinetic constants, Vmax and Vmax/Km, were low (Table 1) compared with those of the AACs of other bacteria (26). The Vmax/Km ratios, which reflected the rates of catalytic turnover at substrate concentrations below Km levels, were all on the order of 10−4 (Table 1), indicating that these modifying enzymes are ineffective in terms of their abilities to modify drugs. Similarly Vmaxs, which reflected the rates of catalytic turnover at saturating antibiotic concentrations, were also relatively low. The enzymes alone were therefore unlikely to bring about resistance even if an in vivo drug concentration as high as the corresponding Km was achieved. The diverse origins of the AMEs, however, suggested that they might have a metabolic role other than the conferral of drug resistance (27).
In mycobacteria, the rate of uptake across the mycolic acid-containing mycobacterial cell envelope appears to be the important limiting factor for drug translocation (19), and this putative barrier may differ substantially between different mycobacterial species. Moreover, the differences in the physiochemical properties (particularly the hydrophilicities) of the various aminoglycosides can markedly alter their permeation. Therefore, despite the high Km values of their AACs and the lack of a positive correlation between the affinities of these enzymes for those substrates studied and their corresponding resistance levels, aminoglycoside resistance in mycobacteria might still be conferred by an interplay between the cell wall barrier and the AAC activity. In fact, such an interplay had been demonstrated for beta-lactam resistance in mycobacteria (20), for which the permeability coefficients had been shown to be up to 1,000-fold lower than those for gram-negative bacilli (18).
In conclusion, the absence of AMEs in M. avium-M. intracellulare ruled out their possible role as a mechanism of resistance in this species. Although acetylating activities were found in the crude extracts of M. kansasii and M. fortuitum, the modifying enzymes could not be the sole mechanism responsible for resistance, as reflected by their low affinities for the substrates and their ineffectiveness at substrate modification. However, despite the poor activity and the relatively high Km, the enzyme could still play a role in conferring resistance if the rate of uptake of the aminoglycoside into the cell was relatively slow. Further studies on their uptake are, hence, worthwhile and would probably give us a clearer picture.
ACKNOWLEDGMENT
This study was supported by UGC Earmarked Grant (grant CUHK 4237/98M) from the Research Grants Council of Hong Kong.
REFERENCES
- 1.Anisa J A, Carlos M, Brigitte G, Gomez-Lus R. Characterization of the chromosomal aminoglycoside 2′-N-acetyltransferase gene from Mycobacterium fortuitum. Antimicrob Agents Chemother. 1996;40:2350–2355. doi: 10.1128/aac.40.10.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benveniste R, Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971;10:1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste R, Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- 4.Bongaerts G P A, Vliegenthart J S. Effect of aminoglycoside concentration on reaction rates of aminoglycoside-modifying enzymes. Antimicrob Agents Chemother. 1988;33:740–746. doi: 10.1128/aac.32.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broughall J M, Reeves D S. The acetyltransferase enzyme method for the assay of serum gentamicin concentration and a comparison with other methods. J Clin Pathol. 1975;28:140–145. doi: 10.1136/jcp.28.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan L E. Aminoglycoside resistance. In: Bryan L E, editor. Antimicrobial drug resistance. Orlando, Fla: Academic Press, Inc.; 1984. pp. 241–247. [Google Scholar]
- 7.Bryan L E. General mechanisms of resistance to antibiotics. J Antimicrob Chemother. 1988;22(Suppl. A):1–15. doi: 10.1093/jac/22.supplement_a.1. [DOI] [PubMed] [Google Scholar]
- 8.Culebras E, Perez-Dioz J C, Blazequez J, Alonso R, Baquero F. In vitro synergy between aminoglycoside deployed against staphylococcus species harbouring 6′-aminoglycoside acetyltransferase, 2"-aminoglycoside acetyltransferase enzyme. J Antimicrob Chemother. 1994;33:747–755. doi: 10.1093/jac/33.4.747. [DOI] [PubMed] [Google Scholar]
- 9.Davies J, Wright G D. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 1997;5:234–240. doi: 10.1016/S0966-842X(97)01033-0. [DOI] [PubMed] [Google Scholar]
- 10.Davies J E. Aminoglycoside-aminocyclitol antibiotics and their modifying enzymes. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 691–713. [Google Scholar]
- 11.Davies J E, Yagisawa M. The aminocyclitol glycosides (aminoglycosides) In: Vining L C, editor. Biochemistry and genetic regulation of commercially important antibiotics. Reading, Mass: Addison Wesley Publishing Company; 1983. pp. 329–354. [Google Scholar]
- 12.Haas M J, Dowding J E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- 13.Henderson P J F. Statistical analysis of enzyme kinetic data. In: Eisenthal R, Danson M J, editors. Enzyme assays—a practical approach. New York, N.Y: Oxford University Press; 1992. pp. 277–316. [Google Scholar]
- 14.Ho Y I I, Chan C Y, Cheng A F B. In-vitro activities of aminoglycoside-aminocyclitols against mycobacteria. J Antimicrob Chemother. 1997;40:27–32. doi: 10.1093/jac/40.1.27. [DOI] [PubMed] [Google Scholar]
- 15.Honoré N, Cole S T. Streptomycin resistance in mycobacteria. Antimicrob Agents Chemother. 1994;38:238–242. doi: 10.1128/aac.38.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hull S I, Wallace R J, Jr, Bobey D G, Price K E, Goodhines R A, Swenson J M, Silcox V A. Presence of aminoglycoside acetyltransferase and plasmids in Mycobacterium fortuitum. Am Rev Respir Dis. 1984;129:614–618. [PubMed] [Google Scholar]
- 17.Jacoby G A, Archer G L. New mechanisms of bacterial resistance to antimicrobial agents. N Engl J Med. 1991;324:601–612. doi: 10.1056/NEJM199102283240906. [DOI] [PubMed] [Google Scholar]
- 18.Jarlier V, Nikaido H. Permeability barrier to hydrophilic solutes in Mycobacterium chelonei. J Bacteriol. 1990;172:1418–1423. doi: 10.1128/jb.172.3.1418-1423.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarlier V, Nikaido H. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol Lett. 1994;123:11–18. doi: 10.1111/j.1574-6968.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- 20.Jarlier V, Gutmann L, Nikaido H. Interplay of cell wall barrier and β-lactamase activity determines high resistance to β-lactam antibiotics in Mycobacterium chelonae. Antimicrob Agents Chemother. 1991;35:1937–1939. doi: 10.1128/aac.35.9.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Mitsuhashi S, Tanaka T, Kawabe H, Umezawa H. Biochemical mechanism of kanamycin resistance in Mycobacterium tuberculosis. Microbiol Immunol. 1977;21:325–327. doi: 10.1111/j.1348-0421.1977.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 23.Mizuguchi Y, Udou T, Yamada T. Mechanism of antibiotic resistance in Mycobacterium intracellulare. Microbiol Immunol. 1983;27:425–431. doi: 10.1111/j.1348-0421.1983.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 24.Neu H C. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 25.Phillips I, Shannon K P. Aminoglycosides and aminocyclitols. In: O'Grady F, Lambert H P, Finch R G, Greenwood D, editors. Antibiotics and chemotherapy. New York, N.Y: Churchill Livingstone; 1997. pp. 164–201. [Google Scholar]
- 26.Radika K, Northrop D B. Correlation of antibiotic resistance with Vmax/Km ratio of enzymatic modification of aminoglycosides by kanamycin acetyltransferase. Antimicrob Agents Chemother. 1984;25:479–482. doi: 10.1128/aac.25.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rather P N. Origins of the aminoglycoside modifying enzymes. Drug Resist Updates. 1998;1:285–291. doi: 10.1016/s1368-7646(98)80044-7. [DOI] [PubMed] [Google Scholar]
- 28.Silver L L, Bostian K A. Discovery and development of new antibiotics: the problem of antibiotic resistance. Antimicrob Agents Chemother. 1993;37:377–383. doi: 10.1128/aac.37.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udou T, Mizuguchi Y, Wallace R J., Jr Patterns and distribution of aminoglycoside-acetylating enzymes in rapidly growing mycobacteria. Am Rev Respir Dis. 1987;136:338–343. doi: 10.1164/ajrccm/136.2.338. [DOI] [PubMed] [Google Scholar]
- 30.Udou T, Mizuguchi Y, Wallace R J., Jr Does aminoglycoside-acetyltransferase in rapidly growing mycobacteria have a metabolic function in addition to aminoglycoside inactivation? FEMS Microbiol Lett. 1989;48:227–230. doi: 10.1111/j.1574-6968.1989.tb03304.x. [DOI] [PubMed] [Google Scholar]
- 31.Udou T, Mizuguchi Y, Yamada T. Biochemical mechanisms of antibiotic resistance in a clinical isolate of Mycobacterium fortuitum. Am Rev Respir Dis. 1986;133:653–657. doi: 10.1164/arrd.1986.133.4.653. [DOI] [PubMed] [Google Scholar]
- 32.Vanhoof R, Godard C, Content J, Nyssen H J, Hannecart-Pokorni E. Detection by polymerase chain reaction of genes encoding aminoglycoside-modifying enzymes in methicillin-resistant Staphylococcus aureus isolates of epidemic phage types. J Med Microbiol. 1994;41:282–290. doi: 10.1099/00222615-41-4-282. [DOI] [PubMed] [Google Scholar]
- 33.Wallace R J, Jr, Hull S I, Bobey D G, Price K E, Swenson J M, Stele L C, Christensen L. Mutational resistance as the mechanism of acquired drug resistance to aminoglycosides and antibacterial agents in Mycobacterium fortuitum and Mycobacterium chelonei. Am Rev Respir Dis. 1985;132:409–416. doi: 10.1164/arrd.1985.132.2.409. [DOI] [PubMed] [Google Scholar]