Abstract
Background
Medications with an unfavorable risk–benefit profile in the elderly, and for which there are safer alternatives, are designated as potentially inappropriate medications (PIM). The RIME trial (Reduction of Potentially Inappropriate Medication in the Elderly) was based on PRISCUS, a list of PIM that was developed in 2010 for the German pharmaceuticals market. In this trial, it was studied whether special training and the PRISCUS card could lessen PIM and undesired drug–drug interactions (DDI) among elderly patients in primary care.
Methods
A three-armed, cluster-randomized, controlled trial was carried out in two regions of Germany. 137 primary care practices were randomized in equal numbers to one of two intervention groups—in which either the primary care physicians alone or the entire practice team received special training—or to a control group with general instructions about medication. The primary endpoint was the percentage of patients with at least one PIM or DDI (PIM/DDI) per practice. The primary hypothesis was that at 1 year this endpoint would be more effectively lowered in the intervention groups compared to the control group.
Results
Among 1138 patients regularly taking more than five drugs, 453 (39.8%) had at least one PIM/DDI at the beginning of the trial. The percentages of PIM/DDI at the beginning of the trial and 1 year later were 43.0% and 41.3% in the intervention groups and 37.0% and 37.6% in the control group. The estimated intervention effect of any intervention (69 practices) versus control (68 practices) was 2.3% (p = 0.36), while that of team training (35 practices) versus physician training (34 practices) was 4.3% (p = 0.22).
Conclusion
The interventions in the RIME trial did not significantly lower the percentage of patients with PIM or DDI.
Currently, 21.4% of the German population is aged 65 or over (1). Among this age group, polypharmacy is present in 45%: that is, 45% of people in this age group are taking at least five drugs, in some cases more (2, 3). An estimated 10%–50% of these patients are receiving potentially inappropriate medication (PIM) (4– 6), meaning drugs with an unfavorable risk–benefit ratio and for which safer alternatives exist (7). Prescriptions of PIM are associated with adverse events such as falls, cognitive impairment, or increased hospital admissions (8– 10).
German instruments for identifying PIM in older people include the PRISCUS list, which also identifies alternatives (11), and the FORTA criteria, which assess the appropriateness of drug substances by medical indication (12). Undesirable drug–drug interactions (DDIs) are another problem in older patients with multiple morbidities (13). An international panel of experts has identified numerous interactions that are to be avoided, such as the combination of oral anticoagulants (OACs) or antiplatelet drugs (APDs) with nonsteroidal anti-inflammatory drugs (NSAIDs), or the triple combination of renin–angiotensin–aldosterone system (RAAS) blockers, NSAIDs, and diuretics; the latter combination increases the risk of renal insufficiency (14).
A meta-analysis of randomized controlled trials (RCTs) on reducing potentially inappropriate prescribing showed a 21% reduction in DDIs (15). Another meta-analysis found insufficient evidence that the proportion of patients with PIM can be reduced by pharmaceutical intervention (16). The quality of the studies, which often included only inpatients, was found to be low. A systematic review in the outpatient setting (RCTs only) showed wide variation in the interventions performed and the primary endpoints (17).
The Reduction of Potentially Inappropriate Medication in the Elderly (RIME) study reported here was designed for German primary care practices as a randomized controlled trial to investigate the effect of a pragmatic intervention based on the PRISCUS list (18).
Methods
The primary research question was whether an intervention based on the PRISCUS list (11) and suitable for everyday use could reduce the percentage of older (≥ 70 years) primary care patients with PIM/DDIs (eTables 1a, 1b). Secondary questions were whether the intervention had an effect on quality of life, hospital admissions, or mortality. In this three-arm, cluster-randomized trial, primary care practices as clusters were randomized to an intervention group with intensive training given to the primary care physician alone, or to an intervention group with training given to the entire practice team, or else to a control group (18).
Study population
Primary care physicians from the practice networks of Witten/Herdecke University and Hannover Medical School recruited patients aged 70 years or older who in the previous 3 months had received six or more drug agents for regular, long-term use. Other inclusion criteria were ability and willingness to participate in data collection. Exclusion criteria were being cared for in a nursing home, legal incapacity, and being uncontactable on the telephone.
Primary and secondary endpoints
The primary endpoint was the difference in the percentages of patients at the practices with at least one PIM/DDI at baseline (T0) and 12 months later (T1). Secondary endpoints were overall mortality, the percentage of patients with at least one hospital admission, and mean quality of life as assessed using the EQ-5D health-related quality of life questionnaire (19).
Determination of case numbers
With a PIM/DDI prevalence of 25% (4, 20, 21), a 9% reduction to 16% (relative risk: 0.64) was taken to be clinically significant. In comparable studies, the intracluster correlation coefficient was 0.086 (22, 23). This resulted (t-test with power = 90% and type I error = 5%) in a sample size of 140 clusters of 12 patients each (1680 patients).
Ethics
The study adhered to the provisions of the Declaration of Helsinki (current version of 2013). It was approved by the ethics boards of the Hannover Medical School and the University of Witten/Herdecke.
Study design and survey instruments
In addition to the collection of sociodemographic data, comorbidities were assessed using a modified version of the Charlson Comorbidity Index (24). Data on function were collected by geriatric assessment by the primary care physician (Manageable Geriatric Assessment, MAGIC) (25). In telephone interviews, study participants were asked by trained interviewers about items that included the following:
Prescription and nonprescription medications (by pharmaceutical central number)
Visits to the doctor and admissions to hospital as an inpatient
Lifestyle and level of education
Pain
Depression (Geriatric Depression Scale, GDS-15 [26])
Health-related quality of life (European Quality of Life – 5 Dimensions [19], SF-12 [27]).
Utilization of health and social care services (FIMA questionnaire [28]).
Physical activity (PRISCUS-PAQ [29]), and
Vulnerability (Vulnerable Elders Survey, VES-13 [30]).
In telephone interviews after 6 and 12 months, additional questions were asked about unwanted drug reactions.
Randomization
Practices were randomized once they had 12 patients enrolled in the study, or when all the potential patients had been enrolled, or at the end of the recruitment phase. A patient was considered as enrolled when the baseline examination and initial telephone interview had been completed. Randomization was in blocks of variable length, stratified by region (Witten/Herdecke and Hannover). Patients and telephone interviewers were unaware of study group allocation.
Intervention
For the intervention, a short form of the PRISCUS list (PRISCUS card) was developed. In addition to PIM, it includes three clinically relevant interactions (APD + NSAID, OAC + NSAID, ACE inhibitor or AT1 antagonist [+/– diuretic] + NSAID), and general suggestions for managing polypharmacy in older people (eTables 1a, 1b). Interactions between OACs or APDs and NSAIDs were scored as undesirable DDI unless a proton pump inhibitor (PPI) was given. In addition, prescription of an APD and an OAC or of more than one APD were scored as adverse DDIs because, although these combinations may be within guidelines, they can lead to complications, especially as patients get older, and at least require close monitoring and intensive patient education (14). A manual was prepared containing detailed information about PIM and drug substances on the PRISCUS list as well as about alternative drugs. A telephone hotline was set up to allow consultation with a clinical pharmacologist about any particular problems.
The primary care physicians in the intervention group were invited to a training workshop where the PRISCUS card and manual were presented and handed out. Physicians who were unable to attend on the dates offered received training at their practice. For those practices randomized to team training, training was always held at the practice; medically trained team members at the practice were given a PRISCUS card specifically adapted for them. Primary care physicians in the control group were invited to a workshop on general aspects of geriatric pharmacotherapy.
Statistics
Continuous characteristics are reported as mean values with standard deviation (SD); categorical characteristics are reported as frequencies in percentages. Patient characteristics were pooled by practice and averaged per study arm. Statistical analyses for the primary research questions were carried out at practice level using the intention-to-treat (ITT) principle. To assess efficacy, differences between the percentages of patients with PIM/DDIs at baseline and after 1 year for each practice were compared using analysis of variance (ANOVA), with the study center as covariable. Missing values for patient PIM/DDI status were replaced by the baseline value. In a sensitivity analysis, patients with a completed 1-year telephone interview in the intervention groups and the control group were compared, stratified by study center and weighted according to the number of patients at each practice. Secondary endpoints were evaluated at practice level by ANOVA with the study center as covariable.
In a planned subgroup analysis, the two intervention groups (physician versus team training) were compared under the hypothesis that training the entire practice team would be more effective; the second subgroup analysis limited the evaluation to patients assessed as vulnerable.
All analyses were performed using the SAS software package, version 9.4 (2016, SAS Institute Inc., Cary, NC, USA).
Results
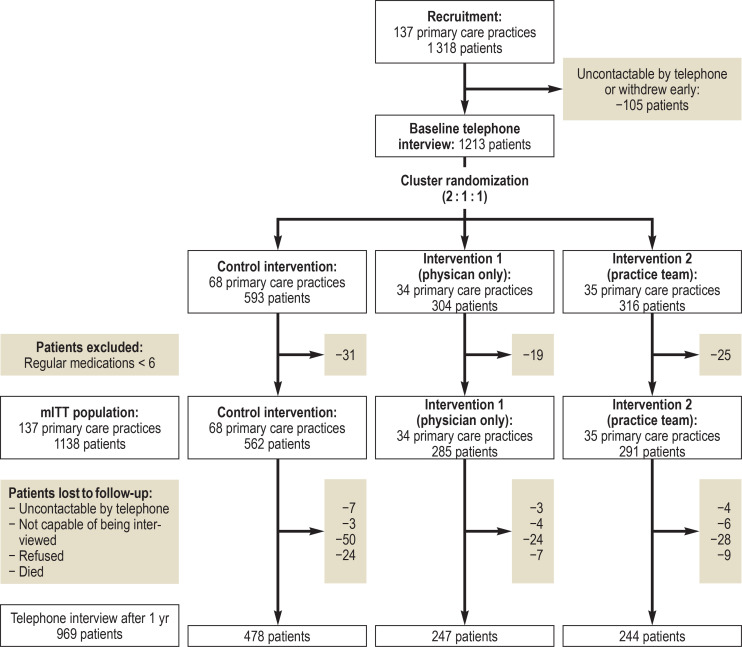
From September 2012 to June 2013, 137 primary care practices with a potential total of 1318 polypharmacy patients were enrolled. Of the 1318 patients, 105 could not be contacted for the baseline interview or withdrew from the study early. The 137 primary care practices with 1213 patients were randomized 2:1:1 to the control group (68 practices), the intervention group with physician training (34 practices), or the intervention group with practice team training (35 practices) (figure 1). After 75 patients with fewer than six regular prescriptions had been excluded, 1138 patients from 137 practices remained for the modified ITT evaluation.
Figure 1.
Study flow diagram
mITT, modified „intention to treat“
The training of the intervention groups was held mainly in the practices. More intensive group training was aimed for, but was only possible in 8 (21%) of 37 practices in Hannover and 10 (31%) of 32 practices in Witten/Herdecke due to the limited willingness of physicians to participate. Problems in the conduct of the study in the Witten/Herdecke study center meant that some of the training sessions did not take place as promptly after randomization as planned. At the end of 1 year, 969 patients (physician training group: 247 patients; team training group: 244 patients; control group: 478 patients) took part in the final telephone interview (figure 1). Sensitivity analysis was performed separately with 74 practices (37 intervention, 37 control) for Hannover and 63 practices (32 intervention, 31 control) for Witten/Herdecke.
Baseline characteristics
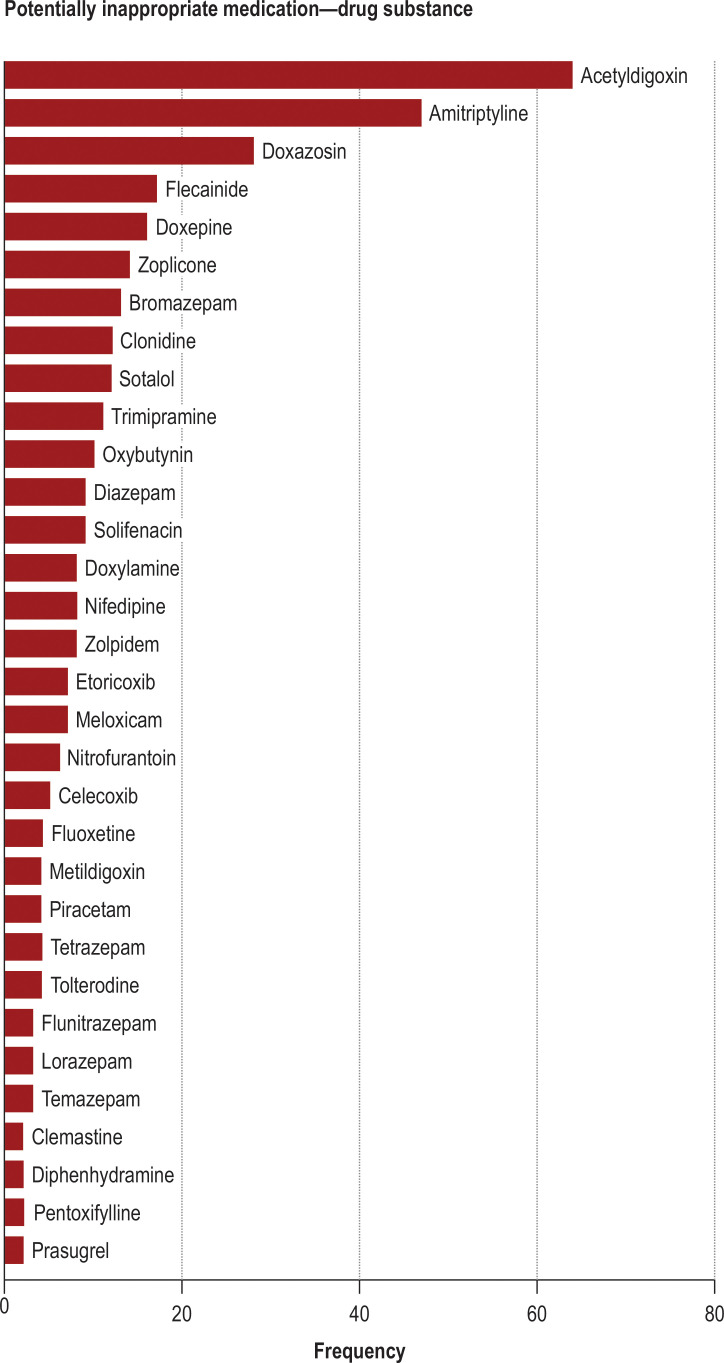
At the start of the study, characteristics were similar in the intervention and the control groups. On average, 8 to 9 patients were recruited per practice (mean age 78 years, half of the patients were men; Table 1, e Table 2). On average, patients were taking more than 9 different medications. The percentage of patients with at least one PIM ranged from 25% to 29%, and 18%–23% had an DDI. The overall percentage of patients with a PIM or DDI was 37%–43%. Among the total of 367 PIMs, acetyldigoxin (17%) and amitriptyline (13%) were the most common (figure 2). A total of 286 DDIs occurred in 225 patients. The combination of ACE inhibitors, AT1 antagonists, or renin inhibitors (A2) with NSAIDs was the most common, seen in 178 patients, 88 of whom were additionally taking a diuretic. The combination of APD or OAC with a NSAID without PPI was found in 70 cases (efigure).
Table 1. Patient characteristics at baseline.
| Number of patients | ||||
|
All N = 1138 |
Intervention 1 n = 285 |
Intervention 2 n = 291 |
Control n = 562 |
|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age in years | 77.5 (4.92) | 77.5 (5.11) | 77.6 (5.03) | 77.4 (4.77) |
| Men (%) | 50.0 | 49.5 | 46.7 | 52.0 |
| ISCED | 2.9 (0.71) | 2.9 (0.70) | 2.9 (0.70) | 2.9 (0.72) |
| Current smoker (%) | 5.9 | 4.9 | 6.2 | 6.2 |
| Diabetes mellitus (%) | 46.6 | 50.5 | 42.3 | 46.8 |
| Hypertension (%) | 88.1 | 91.2 | 86.6 | 87.4 |
| Coronary heart disease (%) | 34.7 | 31.9 | 34.0 | 36.5 |
| CCI (modified) | 3.1 (2.24) | 3.2 (2.28) | 3.0 (2.19) | 3.1 (2.26) |
| eGFR (mL/min) | 59.5 (17.95) | 59.6 (18.38) | 60.6 (18.29) | 58.8 (17.54) |
| VES-13 | 2.9 (2.59) | 2.8 (2.52) | 3.0 (2.57) | 2.9 (2.63) |
| VES-13 >2 (%) | 516 (45.3) | 124 (43.5) | 143 (49.1) | 249 (44.3) |
| EQ-5D | 67.6 (19.09) | 68.2 (18.73) | 68.1 (17.69) | 67.0 (19.97) |
| GDS-5 | 0.74 (0.95) | 0.66 (0.88) | 0.83 (0.96) | 0.74 (0.98) |
| More than one fall (%) | 12.2 | 14.7 | 11.0 | 11.6 |
| MAGIC sum score | 2.5 (1.46) | 2.4 (1.40) | 2.6 (1.39) | 2.5 (1.52) |
| Number of medications | 9.3 (2.69) | 9.5 (2.71) | 9.2 (2.59) | 9.2 (2.74) |
| PIM (%) | 26.5 | 28.1 | 28.9 | 24.6 |
| DDI (%) | 19.8 | 23.2 | 20.3 | 17.8 |
| Patients with PIM or DDI (%) | 39.8 | 42.8 | 43.0 | 36.7 |
CCI, Charlson Comorbidity Index; DDI, undesirable drug–drug interaction; eGFR (CG/DD), estimated glomerular filtration rate (Cockcroft–Gault with Dubois–Dubois correction); EQ-5D, European Quality of Life 5 Dimensions; GDS-5, Geriatric Depression Scale; ISCED, International Standard Classification of Education; MAGIC, Manageable Geriatic Assessment; PIM, potentially inappropriate medication; SD, standard deviation; VES-13, Vulnerable Elders Survey 13
Table 2. Results for the primary endpoint reduction in the proportion of patients with at least 1 PIM or DDI in the mITT analysis and the sensitivity analysis, and results for the secondary endpoints (all results shown at practice level).
| Analysis | Group | N | Mean (SD) at T0 | Mean (SD) at T1 | Effects estimates (SE) | P value |
| Primary endpoint | ||||||
| Patients with at least 1 PIM or DDI (mITT) |
Intervention 1 + 2 | 69 | 43.0 (20.8) | 41.3 (17.5) | 2.3 (2.4) | 0.36 |
| Control | 68 | 37.0 (19.0) | 37.6 (20.3) | Reference | ||
| Patients with at least 1 PIM or DDI (mITT) |
Intervention 1 | 34 | 43.4 (21.9) | 44.0 (15.0) | Reference | |
| Intervention 2 | 35 | 42.5 (19.9) | 38.7 (19.5) | 4.3 (3.5) | 0.22 | |
| Only vulnerable patients (mITT) | Intervention 1 + 2 | 69 | 44.8 (32.4) | 43.5 (29.5) | −1.6 (3.4) | 0.63 |
| Control | 67 | 43.5 (30.5) | 40.7 (32.7) | Reference | ||
| Sensitivity analysis for Hannover |
Intervention 1 + 2 | 37 | 43.2 (17.1) | 42.9 (15.8) | 3.7 (3.7) | 0.32 |
| Control | 37 | 37.1 (22.8) | 38.7 (25.3) | Reference | ||
| Sensitivity analysis for Witten/Herdecke |
Intervention 1 + 2 | 32 | 41.8 (25.6) | 39.5 (21.0) | 1.4 (4.0) | 0.73 |
| Control | 31 | 35.1 (21.1) | 35.1 (21.7) | Reference | ||
| Secondary endpoints | ||||||
| Deaths Proportion per practice |
Intervention 1 + 2 | 69 | 3.0 (5.7) | −1.5 (1.2) | 0.22 | |
| Control | 68 | 4.6 (8.4) | Reference | |||
| EQ-5D T0–T1 |
Intervention 1 + 2 | 69 | 67.6 (7.26) | 67.6 (6.26) | 0.31 (0.97) | 0.74 |
| Control | 68 | 67.0 (7.97) | 67.0 (8.55) | Reference | ||
| Hospital admissions Proportion per practice |
Intervention 1 + 2 | 69 | 39.6 (18.7) | 8.5 (3.2) | 0.01 | |
| Control | 68 | 31.0 (18.5) | Reference | |||
Modified intention to treat (mITT) analysis: all practices and the 1138 patients with at least 6 regular prescriptions were included in the analysis; missing values at T1 were replaced by the patient’s baseline value at T0. No replacement was made for secondary outcome parameters.
Sensitivity analysis: only patients with a standard interview at T1 were included in the calculation of percentages per practice. The analysis was stratified by study center with weighting according to the number of patients per practice at T1.
Vulnerable patients subgroup: only patients with a Vulnerable Elders Survey (VES)-13 score ≥ 3 at T0 were included in the analysis.
Group comparisons: effects estimate (SE, “standard error”) versus reference category from ANOVA with study center as covariable.
DDI, undesirable drug–drug interaction; EQ-5D, European Quality of Life – 5 Dimensions; mean (± SD), mean and standard deviation at the beginning (T0) and end (T1) of the study for percentages per practice without replacement values; PIM, potentially inappropriate medication
Figure 2.
Medication at baseline: most common potentially inappropriate medications (1138 patients)
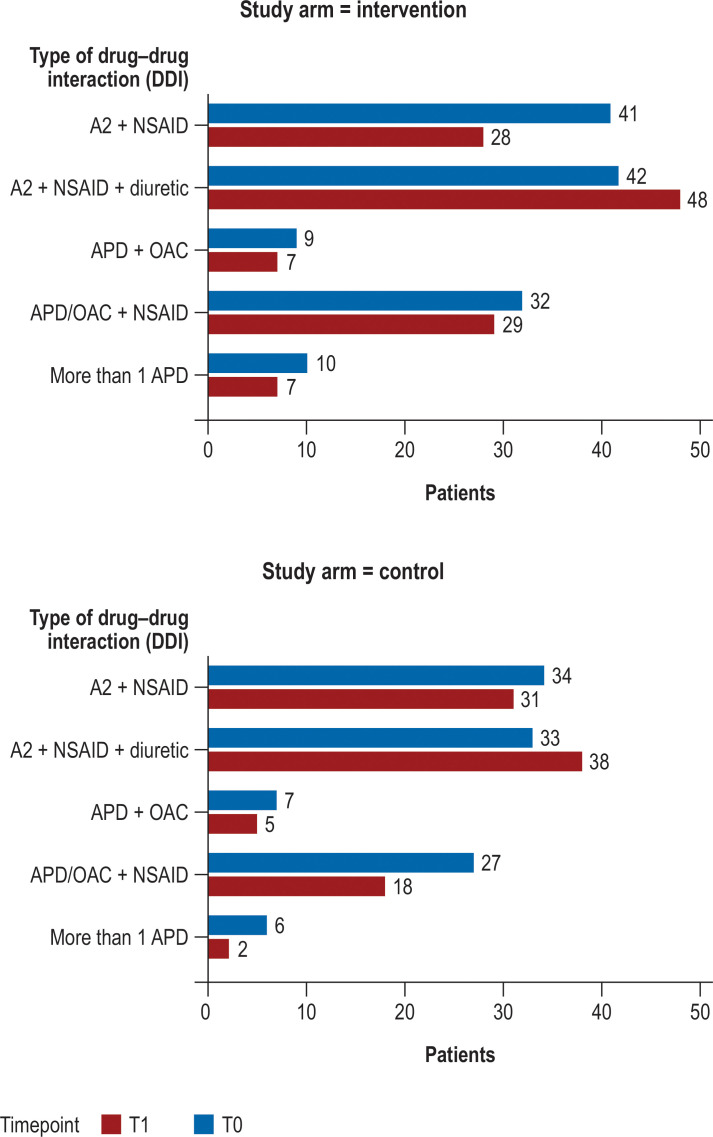
Figure 3.
Number of undesirable drug–drug interaction at timepoints T0 and T1 by type and study arm (in the 969 patients able to be interviewed at the end of 1 year)
A2, drug class that includes ACE inhibitors, AT1-antagonists, and renin inhibitors; NSAID, nonsteroidal anti-inflammatory or antiphlogistic drug; OAC, oral anticoagulant; APD, antiplatelet drug
eTable 1a-1 https://www.aerzteblatt.de/callback/image.asp?id=121242
eTable 1a-2 https://www.aerzteblatt.de/callback/image.asp?id=121243
eTable 1b-1 https://www.aerzteblatt.de/callback/image.asp?id=121244
eTable 1b-2 https://www.aerzteblatt.de/callback/image.asp?id=121245
Primary and secondary endpoints
At the end of 12 months, the mean percentage of patients with PIM/DDI had decreased slightly in the intervention group while remaining about the same in the control group. The intervention effect in the modified ITT population was 2.3% (P = 0.36, Table 2). The secondary comparison between the 2 intervention arms showed no significant advantage of training the practice team (4.3%, P = 0.22). In the sensitivity analysis, the estimated intervention effect was nonsignificantly greater for the Hannover study center (3.7%, P = 0.32, Table 2) than for Witten/Herdecke (1.4%, P = 0.73). For vulnerable patients, there was no difference between the intervention and the control group.
In the intervention group there were 16 deaths, in the control group there were 24 (difference 1.5%, P = 0.22). There were no differences as to quality of life. The percentage of hospital admissions was higher in the intervention group than in the control group (39.6% versus 31.0%, P = 0.01).
At patient level (n = 969), there was an increase in PIM in the control group (from 115 to 124 patients) versus almost no change in the intervention group (from 135 to 136 patients, eTable 3). The number of DDIs decreased slightly overall in both groups, but unevenly both as to DDI type (Figure 3) and as to study center (etable 4).
eTable 3. Number and percentage of patients with at least one PIM or DDI at T0 and T1, by study arm (969 patients).
|
PIM or DDI n (%) |
PIM n (%) |
DDI n (%) |
|||||
| Study arm | Time | ||||||
| Intervention 1 (physician) (n = 247) |
T0 | 102 | 41.3 | 66 | 26.7 | 55 | 22.3 |
| T1 | 104 | 42.1 | 70 | 28.3 | 52 | 21.1 | |
| Intervention 2 (practice team) (n = 244) |
T0 | 106 | 43.4 | 69 | 28.3 | 53 | 21.7 |
| T1 | 97 | 39.8 | 66 | 27.0 | 44 | 18.0 | |
| Control (n = 478) |
T0 | 170 | 35.6 | 115 | 24.1 | 83 | 17.4 |
| T1 | 176 | 36.8 | 124 | 25.9 | 76 | 15.9 | |
DDI, undesirable drug–drug interaction; PIM, potentially inappropriate medication; T0, start of study;
T1, end of study
eTable 4. Numbers of potentially inappropriate medications and undesirable drug–drug interactions at T0 und T1 by study arm and study center (969 patients).
| PIM: n (%) | DDI: n (%) | ||||||
| Both | Study center | Both | Study center | ||||
| Time | HMS | WHU | HMS | WHU | |||
| All | T0 | 302 | 173 (57.2) | 129 (42.7) | 241 | 138 (57.2) | 103 (42.7) |
| T1 | 305 | 189 (62.0) | 116 (38.0) | 213 | 110 (51.6) | 103 (48.4) | |
| Study arm | |||||||
| Intervention 1 (physician) |
T0 | 82 | 41 (50.0) | 41 (50.0) | 67 | 37 (55.2) | 30 (44.8) |
| T1 | 79 | 42 (53.2) | 37 (46.8) | 64 | 30 (46.9) | 34 (53.1) | |
| Intervention 2 (practice team) |
T0 | 83 | 53 (63.9) | 30 (36.1) | 67 | 40 (59.7) | 27 (40.3) |
| T1 | 81 | 57 (70.4) | 24 (29.6) | 55 | 27 (49.1) | 28 (50.9) | |
| Control | T0 | 137 | 79 (57.7) | 58 (42.3) | 107 | 61 (57.0) | 46 (43.0) |
| T1 | 145 | 90 (62.1) | 55 (37.9) | 94 | 53 (56.4) | 41 (43.6) | |
DDI, undesirable drug–drug interaction; HMS, Hannover Medical School; PIM, potentially inappropriate medication; T0, start of study; T1, end of study; WHU, Witten/Herdecke University
Discussion
This study put to the test an easily implemented intervention that could be realistically imported into the existing primary care system and aimed at preventing selected potentially inappropriate medications and selected relevant drug interactions. The main effect of the intervention, which comprised physician or practice team training, the provision of information and materials, and telephone consultation with clinical pharmacologists on request failed to reach statistical significance in either the ITT analysis (difference 2.3%) or the sensitivity analysis (maximum difference 3.7%), and given the originally assumed difference of 9% would not have been clinically relevant.
By comparison, studies that have been effective in improving medication showed differences particularly in terms of the intensity of the intervention. The DQIP trial (31) focused on the risk of concomitant prescription of NSAIDs and antiplatelet drugs. The intervention was multifaceted and included physician education, patient-specific warning signals about prescription through the practice’s electronic prescribing system, and monitoring of the frequency of concomitant prescription with feedback to the prescribing physician. This combination of measures reduced, for example, concomitant prescribing of NSAIDs and antiplatelet drugs without gastroprotection (from 1.5% to 0.6%) and also hospital admissions among high-risk patients (31).
Other studies have shown the significance of providing supporting information for prescribing through the practice software. In the PRIMA-eDS study, an electronic prescribing tool displayed recommendations directly at the time of prescribing (32). This intervention resulted in a clear reduction in the number of medications, although the combined endpoint of unplanned inpatient hospital admissions and mortality was statistically significantly reduced only in a per protocol analysis. Another element of the intervention in many studies is the involvement of pharmacologists and/or pharmacists. A recent systematic review (16) found reductions in PIMs and DDIs, and/or improvement in the quality of prescribing (33) for various care settings. Most studies, e.g., the WestGem study (34, 35), involved pharmaceutical care and less intervention targeting the prescriber (16). Studies conducted in primary care practices that involved pharmacists showed positive results (35– 37). In many countries pharmaceutical care is already routine, but in Germany it tends to be the exception.
In the PRIMUM study, medical practice staff other than the physician were included (38). The intervention consisted of a preliminary consultation between the staff member and the patient, a review of all the medication, computer-assisted optimization of medication, and a consultation between the patient and the physician. At the end of 6 months, no differences were shown between the intervention and the control group. The authors of the PRIMUM study argued that even at the beginning of the study, there was little scope for optimization of patients’ medication, and that baseline quality of life and functioning were good. According to data from the AgeCoDe cohort study (39) and prescription data (40), PIM prescriptions have been declining in Germany over the past 10 years. It is possible that while the RIME study was being carried out, there was an overlapping of effects from general awareness about polypharmacy and from the study intervention. Moreover, a discussion is needed about how many of the PRISCUS drugs and interactions are actually avoidable, i.e., to what extent PIM lists can flag up risks but cannot really, without complex intervention, contribute to their avoidance.
As to the secondary endpoints in our study, no important effects were found. Against expectations, in the intervention group the percentage of patients admitted to hospital at least once had gone up. Clarifying and changing medication is not a typical reason for admission in Germany. Unfortunately, the hospital admission diagnoses were not available, so it was not possible to make any assessment of causality. It is therefore unclear how far the rise in hospital admissions was a consequence of the intervention, although it seems unlikely given the small modifications made to the medication. The differences in how the two study centers implemented the training were not relevant in the separate analysis.
In the RIME study, the intervention was designed to be as pragmatic as possible for everyday use in primary care. Common, simple tools for detecting DDIs were integrated in a routine way into the practices’ various electronic prescribing systems. Given the complexity of drug safety in patients with polypharmacy, it may be that the intervention was not intensive enough. Furthermore, at patient level the originally planned sample size was not quite reached. However, in a randomized study this does not bias the main estimate, so we consider nevertheless that the study is valid.
Conclusion
A “pragmatic” approach such as that used in the RIME study is apparently not suited to achieving substantial effects. Future interventions will require a higher intensity of medication monitoring, which could be achieved by digital drug safety programs as well as by increased exchange of ideas between healthcare professionals.
eTable 2. Group characteristics at baseline in the 137 primary care practices.
| Number of practices |
All N = 137 |
Intervention 1 n = 34 |
Intervention 2 n = 35 |
Control n = 68 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Patients per practice | 8.3 (2.37) | 8.4 (2.53) | 8.3 (2.56) | 8.3 (2.22) |
| Age in years | 77.6 (2.04) | 77.6 (2.33) | 77.7 (1.96) | 77.5 (1.95) |
| Men (%) | 50.2 (20.54) | 49.9 (17.69) | 47.6 (20.86) | 51.7 (21.81) |
| ISCED | 2.9 (0.30) | 2.9 (0.27) | 2.9 (0.25) | 3.0 (0.34) |
| Current smoker (%) | 5.6 (8.27) | 5.1 (7.27) | 5.4 (7.98) | 5.9 (8.96) |
| Diabetes mellitus (%) | 46.5 (19.66) | 50.0 (21.60) | 43.8 (21.83) | 46.2 (17.41) |
| Hypertension (%) | 88.5 (11.25) | 91.9 (10.34) | 86.2 (12.78) | 88.1 (10.61) |
| CHD (%) | 35.8 (17.67) | 33.0 (15.79) | 36.0 (22.27) | 37.1 (15.93) |
| CCI (modified) | 3.1 (0.83) | 3.1 (0.93) | 3.0 (0.85) | 3.1 (0.78) |
| eGFR (mL per minute) | 59.5 (7.94) | 60.2 (8.14) | 59.9 (7.18) | 59.0 (8.27) |
| VES-13 | 2.9 (1.00) | 2.9 (1.08) | 3.0 (0.99) | 2.8 (0.98) |
| EQ-5D | 67.3 (7.60) | 67.4 (6.87) | 67.8 (7.72) | 67.0 (7.97) |
| GDS-5 | 0.7 (0.38) | 0.7 (0.35) | 0.8 (0.33) | 0.7 (0.41) |
| More than one fall (%) | 12.3 (11.37) | 15.3 (11.85) | 11.4 (12.42) | 11.2 (10.44) |
| MAGIC sum score | 2.5 (0.53) | 2.4 (0.57) | 2.7 (0.50) | 2.5 (0.53) |
| Number of medications | 9.3 (1.16) | 9.4 (1.20) | 9.1 (1.07) | 9.3 (1.20) |
| PIM | 26.8 (17.80) | 29.4 (18.01) | 28.5 (18.45) | 24.7 (17.35) |
| DDI | 19.6 (16.42) | 22.5 (16.34) | 19.4 (16.22) | 18.3 (16.62) |
| Patients with PIM and/or DDI | 40.0 (20.07) | 43.4 (21.90) | 42.5 (19.90) | 37.0 (19.02) |
CCI, Charlson Comorbidity Index; CHD, coronary heart disease; DDI, undesirable drug–drug interaction; eGFR (CG/DD), estimated glomerular filtration rate (Cockcroft–Gault with Dubois–Dubois correction); EQ-5D, European Quality of Life – 5 Dimensions; GDS-5, Geriatric Depression Scale; ISCED, International Standard Classification of Education; MAGIC, Manageable Geriatic Assessment; PIM, potentially inappropriate medication; SD, standard deviation; VES-13, Vulnerable Elders Survey 13
eFigure.
Medication at baseline: Occurrence of drug–drug interactions of concern (data from 1138 patients)
A2, drug class that includes ACE inhibitors, AT1-antagonists, and renin inhibitors;
NSAID, nonsteroidal anti-inflammatory or antiphlogistic drug; OAC, oral anticoagulant; APD, antiplatelet drug
Acknowledgments
Translated from the original German by Kersti Wagstaff
Footnotes
Conflict of interest statement Professor Thiem is a consultant in geriatrics and represents the German Geriatrics Society in the guideline group of the German College of General Practitioners responsible for updating the “Multimedication” guideline. He is a member and representative of the German Geriatrics Society in the Drug Therapy Safety Working Group of the German Society of Internal Medicine. He has received lecture fees from MediConsult and Novartis and reimbursement of travel expenses from MediConsult. The other authors declare no conflict of interest.
Study registration, funding
This study is registered with the German Clinical Trials Registry (DRKS00003610). The RIME study is funded by the German Federal Ministry of Education and Research as a subcomponent of the collaborative project “Development of a Model of Health Care for Older Patients with Multimorbidity” (PRISCUS II).
Data sharing
Upon request to the corresponding author, during the period from 3 to 36 months after publication of the article data will be shared with scientists who submit a methodologically sound analysis proposal.
References
- 1.Statistisches Bundesamt Deutschland - Genesis-Online. Ergebnis 12211-0002 (destatis.de) (last accessed on 9 October 2021) [Google Scholar]
- 2.Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–995. doi: 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Moßhammer D, Haumann H, Mörike K, Joos S. Polypharmacy—an upward trend with unpredictable effects. Dtsch Arztebl Int. 2016;113:627–633. doi: 10.3238/arztebl.2016.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tommelein E, Mehuys E, Petrovic M, Somers A, Colin P, Boussery K. Potentially inappropriate prescribing in community-dwelling older people across Europe: a systematic literature review. Eur J Clin Pharmacol. 2015;71:1415–1427. doi: 10.1007/s00228-015-1954-4. [DOI] [PubMed] [Google Scholar]
- 5.Nothelle SK, Sharma R, Oakes A, Jackson M, Segal JB. Factors associated with potentially inappropriate medication use in community-dwelling older adults in the United States: a systematic review. Int J Pharm Pract. 2019;27:408–423. doi: 10.1111/ijpp.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storms H, Marquet K, Aertgeerts B, Claes N. Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: a systematic review. Eur J Gen Practice. 2017;23:69–77. doi: 10.1080/13814788.2017.1288211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch Intern Med. 1991;151:1825–1832. [PubMed] [Google Scholar]
- 8.Endres HG, Kaufmann-Kolle P, Steeb V, Bauer E, Bottner C, Thuermann P. Association between potentially inappropriate medication (PIM) use and risk of hospitalization in older adults: an observational study based on routine data comparing PIM use with use of PIM alternatives. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146811. e0146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhlack DC, Hoppe LK, Weberpals J, Brenner H, Schöttker B. The association of potentially inappropriate medication at older age with cardiovascular events and overall mortality: a systematic review and meta-analysis of cohort studies. J Am Med Dir Assoc. 2017;18:211–220. doi: 10.1016/j.jamda.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Xing XX, Zhu C, Liang HY, et al. Associations between potentially inappropriate medications and adverse health outcomes in the elderly: a systematic review and meta-analysis. Ann Pharmacother. 2019;53:1005–1019. doi: 10.1177/1060028019853069. [DOI] [PubMed] [Google Scholar]
- 11.Holt S, Schmiedl S, Thuermann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107:543–551. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn-Thiel AM, Weiss C. Wehling: Consensus validation of the FORTA (Fit fOR The Aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging. 2014;31:131–140. doi: 10.1007/s40266-013-0146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmiedl S, Rottenkolber M, Szymanski J, et al. Preventable ADRs leading to hospitalization—results of a long-term prospective safety study with 6,427 ADR cases focusing on elderly patients. Expert Opin Drug Saf. 2018;17:125–137. doi: 10.1080/14740338.2018.1415322. [DOI] [PubMed] [Google Scholar]
- 14.Anrys P, Petit AE, Thevelin S, et al. An international consensus list of potentially clinically significant drug-drug interactions in older people. J Am Med Dir Assoc. 2021;22:2121–2133e24. doi: 10.1016/j.jamda.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Gray SL, Hart LA, Perera S, Semla TP, Schmader KE, Hanlon JT. Meta-analysis of interventions to reduce adverse drug reactions in older adults. J Am Geriatr Soc. 2018;66:282–288. doi: 10.1111/jgs.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rankin A, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2018;9 doi: 10.1002/14651858.CD008165.pub4. CD008165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clyne B, Fitzgerald C, Quinlan A, et al. Interventions to address potentially inappropriate prescribing in community-dwelling older adults: a systematic review of randomized controlled trials. J Am Geriatr Soc. 2016;64:1210–1222. doi: 10.1111/jgs.14133. [DOI] [PubMed] [Google Scholar]
- 18.Thiem U, Wilm S, Greiner W, et al. Reduction of potentially inappropriate medication in the elderly: design of a cluster-randomised controlled trial in German primary care practices (RIME) Ther Adv Drug Saf. 2020;12 doi: 10.1177/2042098620918459. 2042098620918459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amann U, Schmedt N, Garbe E. Prescribing of potentially inappropriate medications for the elderly: an analysis based on the PRISCUS list. Dtsch Arztebl Int. 2012;109:69–75. doi: 10.3238/arztebl.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert I, Kupper-Nybelen J, Ihle P, Thuermann P. Prescribing potentially inappropriate medication (PIM) in Germany‘s elderly as indicated by the PRISCUS list An analysis based on regional claims data. Pharmacoepidemiol Drug Saf. 2013;22:719–727. doi: 10.1002/pds.3429. [DOI] [PubMed] [Google Scholar]
- 22.Flottorp S, Oxman AD, Havelsrud K, Treweek S, Herrin J. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ. 2002;325 doi: 10.1136/bmj.325.7360.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fretheim A, Oxman AD, Havelsrud K, Treweek S, Kristoffersen DT, Bjorndal A. Rational prescribing in primary care (RaPP): a cluster randomized trial of a tailored intervention. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030134. e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Barkhausen T, Junius-Walker U, Hummers-Pradier E, Mueller CA, Theile G. „It‘s MAGIC“- development of a manageable geriatric assessment for general practice use. BMC Fam Pract. 2015;16 doi: 10.1186/s12875-014-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 27.Ware J Jr., Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Seidl H, Bowles D, Bock JO, et al. FIMA—questionnaire for health-related resource use in an elderly population: development and pilot study. Gesundheitswesen. 2015;77:46–52. doi: 10.1055/s-0034-1372618. [DOI] [PubMed] [Google Scholar]
- 29.Trampisch U, Platen P, Burghaus I, et al. Reliability of the PRISCUS-PAQ Questionnaire to assess physical activity of persons aged 70 years and older. Z Gerontol Geriatr. 2010;43:399–406. doi: 10.1007/s00391-010-0118-5. [DOI] [PubMed] [Google Scholar]
- 30.Saliba D, Elliott M, Rubenstein LZ, et al. The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 31.Dreischulte T, Donnan P, Grant A, Hapca A, McCowan C, Guthrie B. Safer prescribing—a trial of education, informatics, and financial incentives. N Engl J Med. 2016;374:1053–1064. doi: 10.1056/NEJMsa1508955. [DOI] [PubMed] [Google Scholar]
- 32.Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369 doi: 10.1136/bmj.m1822. m1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samsa GP, Hanlon JT, Schmader KE, et al. A summated score for the medication appropriateness index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891–896. doi: 10.1016/0895-4356(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 34.Köberlein-Neu J, Mennemann H, Hamacher S, et al. Interprofessional medication management in patients with multiple morbidities—a cluster-randomized trial (the WestGem study) Dtsch Arztebl Int. 2016;113:741–748. doi: 10.3238/arztebl.2016.0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose O, Mennemann H, John C, et al. Priority setting and influential factors on acceptance of pharmaceutical recommendations in collaborative medication reviews in an ambulatory care setting—analysis of a cluster randomized controlled trial (WestGem-Study) PLoS One. 2016;11 doi: 10.1371/journal.pone.0156304. e0156304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fried TR, Niehoff KM, Street RL, et al. Effect of the tool to reduce inappropriate medications on medication communication and deprescribing. J Am Geriatr Soc. 2017;65:2265–2271. doi: 10.1111/jgs.15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clyne B, Smith SM, Hughes CM, et al. Effectiveness of a multifaceted intervention for potentially inappropriate prescribing in older patients in primary care: a cluster-randomized controlled trial (OPTI-SCRIPT Study) Ann Fam Med. 2015;13:545–553. doi: 10.1370/afm.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muth C, Uhlmann L, Haefeli WE, et al. Effectiveness of a complex intervention on prioritising multimedication in multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-017740. e017740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmermann T, Kaduszkiewicz H, van den Bussche H, et al. (AgeCoDe-Study Group): Potentially inappropriate medication in elderly primary care patients: a retrospective, longitudinal analysis. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:941–949. doi: 10.1007/s00103-013-1767-5. [DOI] [PubMed] [Google Scholar]
- 40.Muhlack DC, Hoppe LK, Stock C, Haefeli WE, Brenner H, Schöttker B. The associations of geriatric syndromes and other patient characteristics with the current and future use of potentially inappropriate medications in a large cohort study. Eur J Clin Pharmacol. 2018;74:1633–1644. doi: 10.1007/s00228-018-2534-1. [DOI] [PubMed] [Google Scholar]