Abstract
Background
Circular RNA (circRNA) has been shown to play an important role in a variety of cardiovascular diseases, including myocardial infarction (MI). However, the role of circRbms1 in MI progression remains unclear.
Methods
An MI mouse model was constructed in vivo, and cardiomyocytes were cultured under hypoxia condition to induce a cardiomyocyte injury model in vitro. The expression levels of circRbms1, microRNA (miR)-742-3p, and forkhead box O1 (FOXO1) were determined by quantitative real-time PCR. Cell viability, migration, invasion, and apoptosis were measured using Cell Counting Kit-8 assay, transwell assay, and flow cytometry. Meanwhile, western blot analysis was used to examine the protein levels of apoptosis markers and FOXO1. Additionally, dual-luciferase reporter assay, RNA pull-down assay, and RIP assay were employed to verify the interactions between miR-742-3p and circRbms1 or FOXO1.
Results
CircRbms1 was upregulated in the heart tissues of MI mice and hypoxia-induced cardiomyocytes. Hypoxia induced cardiomyocyte injury by suppressing cell viability, migration, and invasion, and promoting apoptosis. Function experiments showed that circRbms1 overexpression aggravated hypoxia-induced cardiomyocyte injury, while its silencing relieved cardiomyocyte injury induced by hypoxia. Furthermore, circRbms1 sponged miR-742-3p. MiR-742-3p overexpression alleviated hypoxia-induced cardiomyocyte injury, and its inhibitor reversed the suppressive effect of circRbms1 silencing on hypoxia-induced cardiomyocyte injury. Further experiments showed that FOXO1 was a target of miR-742-3p, and its expression was positively regulated by circRbms1. The inhibitory effect of miR-742-3p on hypoxia-induced cardiomyocyte injury was reversed by FOXO1 overexpression.
Conclusion
CircRbms1 regulated the miR-742-3p/FOXO1 axis to mediate hypoxia-induced cardiomyocyte injury, suggesting that circRbms1 might be an effective target for MI treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s11658-022-00330-y.
Keywords: Myocardial infarction, Hypoxia, CircRbms1, MiR-742-3p, FOXO1
Introduction
Myocardial infarction (MI) refers to the phenomenon of severe and persistent ischemia and necrosis of the myocardium caused by coronary artery occlusion [1, 2]. Clinically, MI is often accompanied by arrhythmia, heart failure, shock, and other complications, so it can seriously endanger the life of the patient [3, 4]. Many studies have shown that MI is mainly due to cardiomyocyte apoptosis leading to cardiomyocyte injury [5, 6]. Therefore, revealing the targets and potential molecular mechanisms that affect cardiomyocyte injury is of great significance for the development of new and effective MI treatment options.
Circular RNA (circRNA) with a covalently closed structure is a special type of noncoding RNA that is insensitive to nucleases and more stable than ordinary linear RNA [7, 8]. Emerging research confirms that circRNAs are becoming powerful regulators of human diseases [9, 10]. CircRNA has been found to be closely associated with malignant progression of cancer and can be used as a biomarker for cancer treatment [11, 12]. In addition, circRNA is also abnormally expressed in neurodegenerative diseases such as Alzheimer’s disease, and it has been confirmed to have an important function in disease development [13]. Importantly, circRNA also plays a key role in a variety of cardiovascular diseases, including MI [14]. For example, circ_0060745 silencing was shown to relieve hypoxia-induced cardiomyocyte injury, alleviating MI [15].
In the GEO database (GSE133503), by analyzing the differentially expressed circRNA in the heart tissues of two controls and two MI mice, we found that mmu_circ_0001022 (from Rbms1 gene, also named circRbms1; the homologous circRNA in human is) was highly expressed in the heart tissues of MI mice. However, its role and function in MI had not been studied. Therefore, we chose circRbms1 as the object of this study to evaluate its role in MI by exploring its regulatory effect on hypoxia-induced cardiomyocyte injury. The proposed circRbms1/microRNA (miR)-742-3p/forkhead box O1 (FOXO1) axis improved the molecular mechanism of circRbms1 regulating cardiomyocyte injury and provides a new potential target for the treatment of MI.
Materials and methods
MI mouse models
Twelve male C57BL/6 mice were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China) and divided into two groups (n = 6 per group): MI group and sham group. All mice were housed with free access to food and water under standard conditions and subjected to a 12 h/12 h light/dark cycle. MI mouse models were constructed as previously described [16]. Briefly, C57BL/6 mice were anesthetized by intraperitoneal injection of 3% pentobarbital sodium (40 mg/kg; Sigma-Aldrich, St. Louis, MI, USA) and then the thoracic cavity was exposed. The left anterior descending coronary artery of the mice was ligated to construct an MI model. Sham group mice underwent the same surgery, but did not undergo ligation of the coronary arteries. After surgery, mice were kept separately and their health status was monitored daily. After 3 days, all mice were sacrificed to collect heart tissues. The infarct size of heart tissues was assessed by 2, 3, 5-trivinyltetrazolium chloride (TTC) staining (Solarbio, Beijing, China) and measured by Image J software. The animal study was approved by the institutional review board of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-JDYXY-2019-012) and was performed in compliance with the Basel Declaration. All animals received humane care according to the Guide for the Care and Use of Laboratory Animals.
Cell culture
Mouse cardiomyocytes (H9c2) (ATCC, Manassas, VA, USA) were cultured in DMEM medium (Gibco, Carlsbad, CA, USA) containing 10% FBS (Gibco) and 1% penicillin–streptomycin (10,000 U/mL, Gibco) at 37 ℃ in a humidified incubator under hypoxia (2% O2) or normal (21% O2) conditions.
Quantitative real-time PCR (qRT-PCR)
RNAsimple (Tiangen, Beijing, China) was used to isolate total RNA from heart tissues and H9c2 cells. Using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland), the RNA was reverse transcribed into cDNA. The RT procedure was 37 ℃ for 15 min and 85 ℃ for 5 s. After that, SYBR Green PCR Kit (Takara, Dalian, China) was used for qRT-PCR in a PCR system. The amplification process was as follows: denaturation at 95 ℃ for 5 min, followed by 40 cycles at 95 ℃ for 15 s, annealing at 55 ℃ for 30 s, and extension at 60 ℃ for 60 s. GAPDH or U6 was used as the internal control. Data were analyzed using the 2−ΔΔCt method. The primer sequences were as follows: circRbms1, F 5′-CTGAGCCTGGACTCCATTCG-3′, R 5′-ACCAGGAGTTTCTGGTTATGGT-3′; Rbms1, F 5′-CTGAGCAAGACAAACCTCTACAT-3′, R 5′-GGCCTTATCCAAAATCGCCTT-3′; miR-742-3p, F 5′-GCCGAGGAAAGCCACCATGCTGG-3′, R 5′-CAGTGCGTGTCGTGGAGT-3′; FOXO1, F 5′-CCCAGGCCGGAGTTTAACC-3′, R 5′-GTTGCTCATAAAGTCGGTGCT-3′; GAPDH, F 5′-GGTGAAGGTCGGTGTGAACG-3′, R 5′-CTCGCTCCTGGAAGATGGTG-3′; U6, F 5′-CTCGCTTCGGCAGCACATATACT-3′, R 5′-ACGCTTCACGAATTTGCGTGTC-3′.
Identification of circRNA circular characteristic
In RNase R assay, H9c2 cells were treated with RNAsimple to obtain RNA, and then the RNA was incubated with RNase R (Geneseed, Guangzhou, China) for 30 min. Nontreated RNA was used as mock. qRT-PCR was used to measure circRbms1 and linear Rbms1 expression. In Actinomycin D (ActD) assay, H9c2 cells were incubated with ActD solution (R&D, Minneapolis, MN, USA) for 1 h. After further culturing for indicated times (0, 4, 8, and 12 h), the expression of circRbms1 and linear Rbms1 was determined by qRT-PCR.
Cell transfection
For cell transfection, all oligonucleotides and vectors were synthesized from Ribobio (Guangzhou), including the circRbms1 small interference RNA (si-circRbms1) and its controls (si-NC), miR-742-3p mimic and inhibitor (miR-742-3p and anti-miR-742-3p) or their controls (miR-NC and anti-NC). The mimic and inhibitor of miR-742-3p were designed and synthesized by Sangon (Shanghai, China). The circRbms1 overexpression vector and FOXO1 overexpression vector was synthesized by subcloning a sequence of circRbms1 and FOXO1 into the pCD5-ciR vector and pcDNA3.1 vector, respectively. Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) was used to transfect them into cells. The concentration of oligonucleotides was 50 nM, and the concentration of vectors was 4.0 µg. After transfection for 24 h, the cells were cultured under hypoxia for 24 h.
Cell Counting Kit-8 (CCK8) assay
H9c2 cells were seeded into 96-well plates (5 × 103 cells per well). After incubating for 48 h, H9c2 cells were incubated with CCK8 solution (Dojindo, Kumamoto, Japan) for 4 h. Then, cell viability was evaluated at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Transwell assay
Transwell chambers (BD Biosciences, San Jose, CA, USA) precoated with Matrigel (BD Biosciences) were used for measuring cell invasion, and noncoated chambers were used for detecting cell migration. H9c2 cells were suspended with serum-free medium and then seeded on the upper chambers (2 × 105 cells per well for cell migration and 4 × 105 cells per well for cell invasion). The complete medium was added into lower chambers. Twenty-four hours later, the cells on the bottom of chambers were fixed with methanol (Beyotime, Shanghai, China) and stained with crystal violet (Beyotime). The migrated and invaded cell numbers were counted under a microscope (100×) (Leica, Wetzlar, Germany).
Flow cytometry
Annexin V-FITC Apoptosis Detection Kit was obtained from Dojindo. According to the kit instructions, the H9c2 cell suspensions (5 × 105 cells per mL) were suspended with binding buffer and incubated with Annexin V-FITC and propidium iodide. Cell apoptosis rate was analyzed by flow cytometry (Beckman Coulter, Miami, FL, USA). For detecting cell cycle process, the H9c2 cells were fixed with 70% alcohol and then treated with RNase A and propidium iodide. Cell cycle distribution was analyzed by flow cytometry.
Western blot (WB) analysis
RIPA Lysis Buffer (Beyotime) was used to extract total protein, and BCA Protein Assay Kit (Beyotime) was used to quantify the protein. Afterwards, protein samples (30 µg) were separated by 10% SDS-PAGE gel and transferred to PVDF membranes (Beyotime). Next, the membranes were blocked with skimmed milk for 2 h. After incubating with primary antibodies against Bcl-2 (26Kda, 1:2,000, BA0412, Boster, Wuhan, China), Bax (20Kda, 1:1,500, BA0315-2, Boster), Cleaved-caspase 3 (17Kda, 1:1,000, AC033, Beyotime), FOXO1 (82Kda, 1:2,000, AF603, Beyotime), or GAPDH (36Kda, 1:2,000, A00227, Boster), the membrane was then incubated with HRP Conjugated AffiniPure Goat Anti-rabbit/mouse IgG (H + L) (1:10,000, BA1056, Boster). The protein signals were visualized using BeyoECL Star (Beyotime). EasySee Western Marker (25-90Kda, DM201-01, Transgen Biotech, Beijing, China) was used as a molecular weight standard.
Dual-luciferase reporter assay
The sequences of circRbms1 or FOXO1 3′UTR containing the predicted miR-742-3p binding sites were inserted into pGL3 vector (Promega, Madison, WI, USA) to build the wild-type (WT) vectors. The mutant-type (MUT) vectors were built in the same way. HEK 293 T cells (ATCC) were transfected with the circRbms1-WT/MUT or FOXO1-3′UTR-WT/MUT vectors and miR-742-3p mimic or miR-NC for 48 h. Dual-Luciferase Reporter Assay System (Promega) was used to detect the Firefly and Renilla luciferase activities to evaluate relative luciferase activity.
RNA pull-down assay
H9c2 cells were transfected with biotin-labeled miR-742-3p probe (Bio-miR-742-3p) or negative control probe (Bio-miR-NC) (synthesized by Sangon). After 48 h, the cells were lysed and then the cell lysates were incubated with magnetic beads (Invitrogen) at 4 ℃ overnight. After purifying RNA, the enrichment of circRbms1 was analyzed by qRT-PCR.
RIP assay
According to the instructions of RNA Immunoprecipitation Kit (Sigma-Aldrich), H9c2 cells were lysed and then the cell lysates were incubated with magnetic beads conjugated with antibodies against IgG (anti-IgG) or Ago2 (anti-Ago2) overnight at 4 ℃. Then, qRT-PCR was used to determine the enrichment of circRbms1 and miR-742-3p.
Statistical analysis
Statistical analyses was conducted with GraphPad Prism 6.0 (GraphPad, La Jolla, CA, USA). The data are presented as mean ± standard deviation from three independent experiments. Significance of difference was determined using Student’s t-test or one-way analysis of variance followed by Tukey’s post-hoc test. P < 0.05 was considered statistically significant.
Results
CircRbms1 was highly expressed in MI mice and hypoxia-induced H9c2 cells
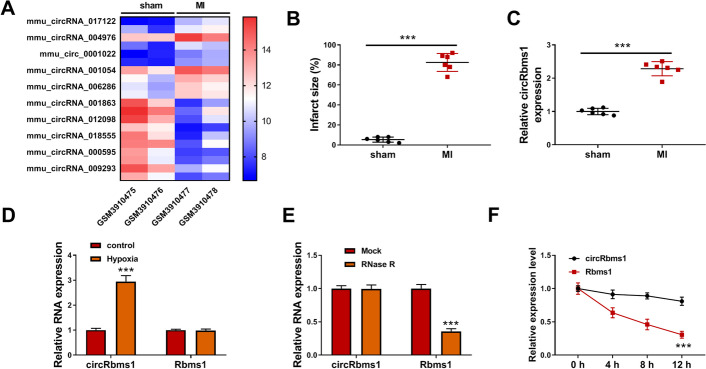
According to the cut-off criteria of values (P < 0.05 and |log2 fold change (log2FC)| > 1.0) in the GEO database (GSE133503), a total of 20 differentially expressed circRNAs were screened in the heart tissues of sham mice and MI mice, and mmu_circ_0001022 (circRbms1) was an upregulated circRNA in MI mice (Fig. 1A). In constructed MI mice, we calculated the infarct size and found that the infarct size was significantly increased in the MI group (Fig. 1B). Using qRT-PCR, we discovered that circRbms1 was indeed highly expressed in the heart tissues of MI mice compared with the sham group (Fig. 1C). In H9c2 cells induced by hypoxia, the expression of circRbms1 was significantly increased, but the expression of linear Rbms1 was not changed (Fig. 1D). To further confirm the circular characteristic of circRbms1, RNase R assay and ActD assay were performed, and the results showed that circRbms1 could resist the digestion of RNase R and its expression was more stable than linear Rbms1 (Fig. 1E, F). These data suggest that circRbms1 is a stability circRNA and might play an important role in the progression of MI.
Fig. 1.
The expression of circRbms1 in MI mice and hypoxia-induced H9c2 cells. A Heat map showing the differentially expressed circRNA in the heart tissues of two sham mice and two MI mice from the GEO database (GSE133503). B Infarct size in the sham mice and MI mice. C Expression of circRbms1 in the heart tissues of sham mice (n = 6) and MI mice (n = 6), assessed by qRT-PCR. D Expression of circRbms1 in hypoxia-treated and untreated H9c2 cells, assessed by qRT-PCR. E, F RNase R assay (E) and ActD assay (F) to confirm the circular characteristic of circRbms1. All experiments were repeated three times. ***P < 0.001
Knockdown of circRbms1 alleviated hypoxia-induced H9c2 cell injury
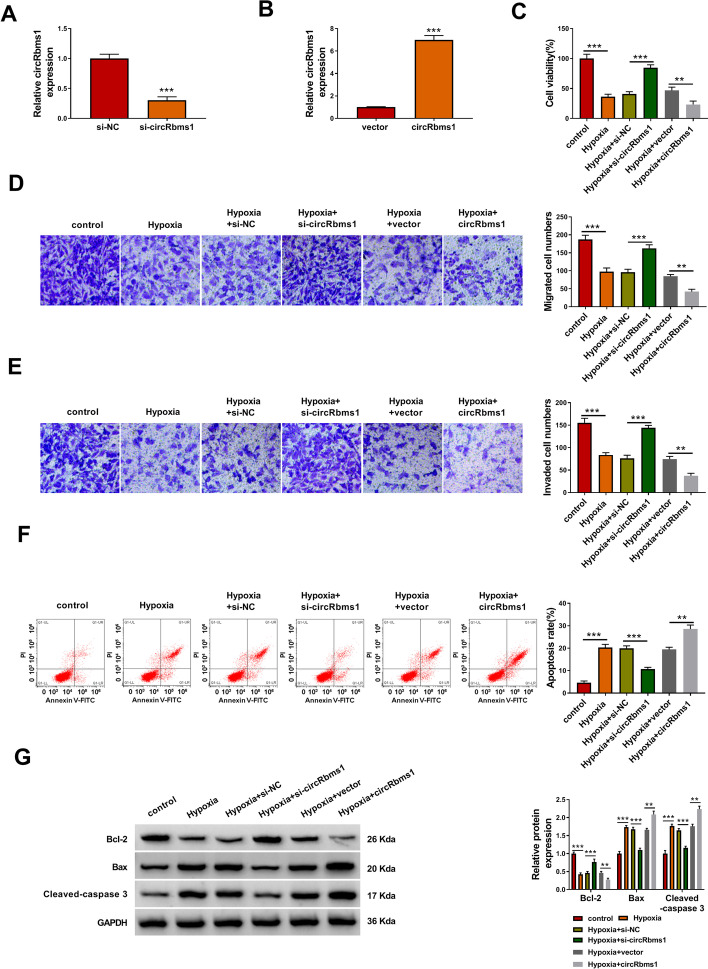
To explore the role of circRbms1 in MI progression, the siRNA and overexpression vector of circRbms1 were constructed. After transfecting H9c2 cells with si-circRbms1 and circRbms1 overexpression vector, the expression of circRbms1 was markedly decreased and increased, respectively (Fig. 2A, B). Then, H9c2 cells transfected with si-circRbms1 and circRbms1 overexpression vector were treated with hypoxia. By measuring cell viability, migrated cell numbers, and invaded cell numbers, we discovered that hypoxia could inhibit the viability, migration, and invasion of H9c2 cells (Fig. 2C–E). However, the inhibitory effect of hypoxia on H9c2 cell viability, migration, and invasion could be reversed by circRbms1 silencing and aggravated by circRbms1 overexpression (Fig. 2C–E). Besides, circRbms1 knockdown also hindered the apoptosis rate of H9c2 cells promoted by hypoxia, while its overexpression enhanced hypoxia-induced H9c2 cell apoptosis (Fig. 2F). In addition, silenced cicRbms1 also increased anti-apoptosis marker Bcl-2 protein expression and decreased apoptosis marker Bax and Cleaved-caspase 3 protein levels in hypoxia-induced H9c2 cells, while its overexpression had the opposite effect (Fig. 2G). We measured the cell cycle distribution in H9c2 cells in which circRbms1 was silenced or overexpressed. Hypoxia increased the cell number in the G0/G1 phase and reduced the cell number in the S phase, indicating that hypoxia induced cell cycle arrest. Knockdown of circRbms1 could promote the cell cycle in hypoxia-induced H9c2 cells, while its overexpression could aggravate cell cycle arrest (Additional file 1: Fig. S1A, B). All data reveal that circRbms1 promoted hypoxia-induced H9c2 cell injury, suggesting that it might accelerate the progression of MI.
Fig. 2.
Knockdown of circRbms1 alleviated hypoxia-induced H9c2 cell injury. A, B qRT-PCR was used to assess circRbms1 expression to evaluate the transfection efficiency of si-circRbms1 (50 nM) or circRbms1 overexpression vector (4.0 µg) in H9c2 cells. C–G H9c2 cells were transfected with or without si-NC (50 nM), si-circRbms1 (50 nM), vector (4.0 µg) or circRbms1 (4.0 µg), and then treated with hypoxia. Untreated H9c2 cells were used as control. CCK8 assay (C), transwell assay (D, E) and flow cytometry (F) were used to determine cell viability, migrated and invaded cell numbers, and cell apoptosis rate, respectively. G WB analysis was performed to test the protein levels of Bcl-2, Bax, and Cleaved-caspase 3. All experiments were repeated three times. **P < 0.01, ***P < 0.001
CircRbms1 directly sponged miR-742-3p
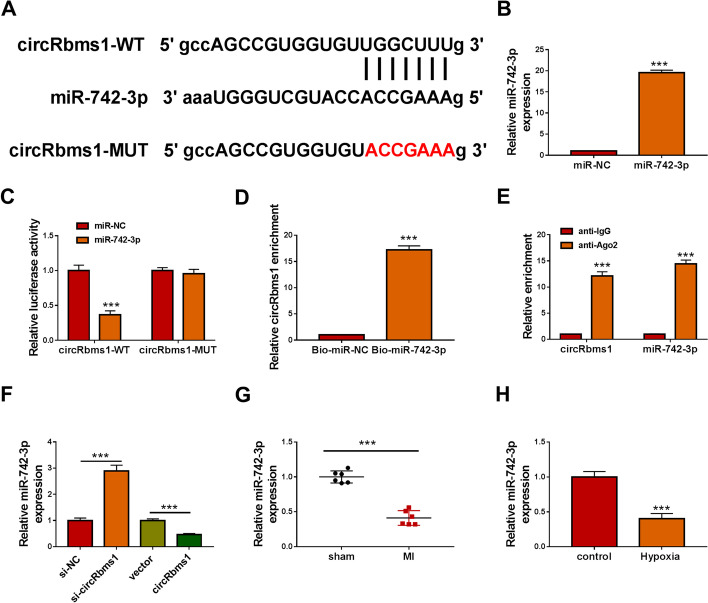
CircRNA has been shown to regulate cell biological functions as a competitive endogenous RNA (ceRNA) of microRNA (miRNA) [17, 18]. To investigate the mechanism of circRbms1, the Starbase tool (http://starbase.sysu.edu.cn/) was used to predict the targeted miRNA for circRbms1. Analysis revealed the presence of miR-742-3p complementary binding sites on circRbms1 (Fig. 3A). Subsequently, miR-742-3p mimic was built to perform function experiments. The transfection efficiency of miR-742-3p mimic was confirmed by detecting its expression after transfection (Fig. 3B). Then, the results of dual-luciferase reporter assay showed that the luciferase activity of circRbms1-WT vector could be inhibited by miR-742-3p overexpression, while that of the circRbms1-MUT vector had not changed (Fig. 3C). RNA pull-down assay indicated that the enrichment of circRbms1 was markedly increased in the Bio-miR-742-3p probe compared with the Bio-miR-NC probe (Fig. 3D). Also, RIP assay results suggested that the expression of circRbms1 and miR-742-3p could be enriched in anti-Ago2 (Fig. 3E). These data confirmed that there was an interaction between circRbms1 and miR-742-3p. Further experiments revealed that miR-742-3p expression was promoted by circRbms1 knockdown and repressed by circRbms1 overexpression (Fig. 3F). In the heart tissues of MI mice and hypoxia-induced H9c2 cells, we found that miR-742-3p expression was significantly lower compared with the corresponding controls (Fig. 3G, H).
Fig. 3.
CircRbms1 directly sponged miR-742-3p. A The fragments of circRbms1-WT and circRbms1-MUT. B The transfection efficiency of miR-742-3p mimic (50 nM) in H9c2 cells was confirmed by detecting miR-742-3p expression using qRT-PCR. Dual-luciferase reporter assay (C), RNA pull-down assay (D), and RIP assay (E) were used to verify the interactions between circRbms1 and miR-742-3p. F In H9c2 cells transfected with si-NC (50 nM), si-circRbms1 (50 nM), vector (4.0 µg), or circRbms1 (4.0 µg), the expression of miR-742-3p was examined by qRT-PCR. G MiR-742-3p expression in the heart tissues of sham mice (n = 6) and MI mice (n = 6) was assessed using qRT-PCR. H The expression of miR-742-3p in hypoxia-treated and untreated H9c2 cells was measured by qRT-PCR. All experiments were repeated three times. ***P < 0.001
MiR-742-3p could relieve hypoxia-induced H9c2 cell injury
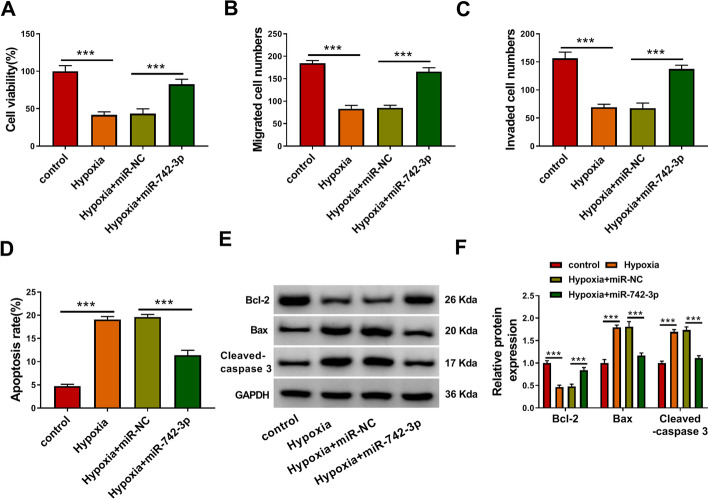
To confirm the function of miR-742-3p in MI, we evaluated its regulation on hypoxia-induced H9c2 cell injury. The results showed that miR-742-3p overexpression reversed the suppressive effect of hypoxia on the viability, migration, and invasion of H9c2 cells (Fig. 4A–C). Furthermore, miR-742-3p also inhibited the apoptosis rate of H9c2 cells induced by hypoxia (Fig. 4D). The increased Bcl-2 protein level and the decreased Bax and Cleaved-caspase 3 protein levels in the presence of miR-742-3p in hypoxia-induced H9c2 cells also indicated that miR-742-3p could recover the apoptosis-promoting effect of hypoxia on H9c2 cells (Fig. 4E, F). These results reveal that miR-742-3p could protect cardiomyocytes from hypoxia-induced injury.
Fig. 4.
MiR-742-3p relieved hypoxia-induced H9c2 cell injury. H9c2 cells were transfected with or without miR-NC (50 nM) or miR-742-3p mimic (50 nM), and then treated with hypoxia. Untreated H9c2 cells were used as control. Cell viability, migrated and invaded cell numbers, and cell apoptosis rate were measured using CCK8 assay (A), transwell assay (B, C) and flow cytometry (D). E, F The protein levels of Bcl-2, Bax and Cleaved-caspase 3 were determined using WB analysis. All experiments were repeated three times. ***P < 0.001
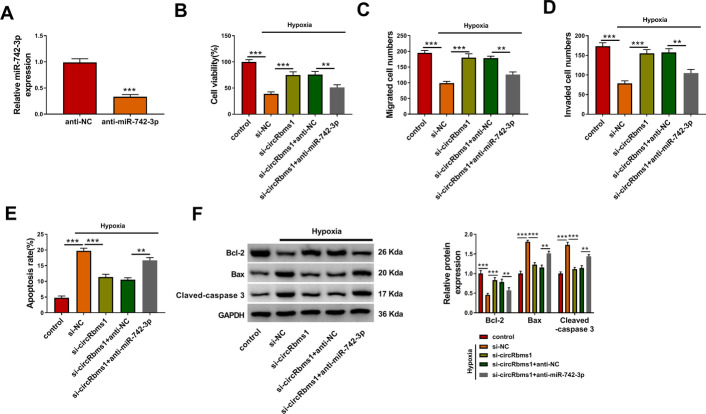
MiR-742-3p inhibitor reversed the inhibitory effect of circRbms1 silencing on hypoxia-induced H9c2 cell injury
To determine whether circRbms1 regulated hypoxia-induced cardiomyocyte injury by sponging miR-742-3p, rescue experiments were performed. After the H9c2 cells were transfected with anti-miR-742-3p, miR-742-3p expression was indeed reduced (Fig. 5A), confirming the transfection effectiveness of anti-miR-742-3p. Then, H9c2 cells transfected with si-circRbms1 and anti-miR-742-3p were treated with hypoxia. As shown in Fig. 5B–D, the promoting effects of circRbms1 silencing on the viability, the migrated cell numbers, and the invaded cell numbers of hypoxia-induced H9c2 cells were abolished by miR-742-3p inhibitor. Also, miR-742-3p inhibitor reversed the suppressive effect of circRbms1 knockdown on the apoptosis rate of hypoxia-induced H9c2 cells (Fig. 5E). By measuring the protein levels of Bcl-2, Bax, and Cleaved-caspase 3, we confirmed that the upregulatory effect of circRbms1 silencing on Bcl-2 expression and the downregulatory effect on Bax and Cleaved-caspase 3 expression could be reversed by miR-742-3p inhibitor (Fig. 5F).
Fig. 5.
Effects of circRbms1 silencing and miR-742-3p inhibitor on hypoxia-induced H9c2 cell injury. A After transfecting with anti-NC (50 nM) or anti-miR-742-3p (50 nM) into H9c2 cells, the expression of miR-742-3p was assessed by qRT-PCR. B–F H9c2 cells were transfected with si-NC (50 nM), si-circRbms1 (50 nM), si-circRbms1 (50 nM) + anti-NC (50 nM), or si-circRbms1 (50 nM) + anti-miR-742-3p (50 nM), and then treated with hypoxia. Untreated H9c2 cells were used as control. CCK8 assay (B), transwell assay (C, D), and flow cytometry (E) were employed to examine cell viability, migrated and invaded cell numbers, and cell apoptosis rate, respectively. F WB analysis was used to test the protein levels of Bcl-2, Bax, and Cleaved-caspase 3. All experiments were repeated three times. **P < 0.01, ***P < 0.001
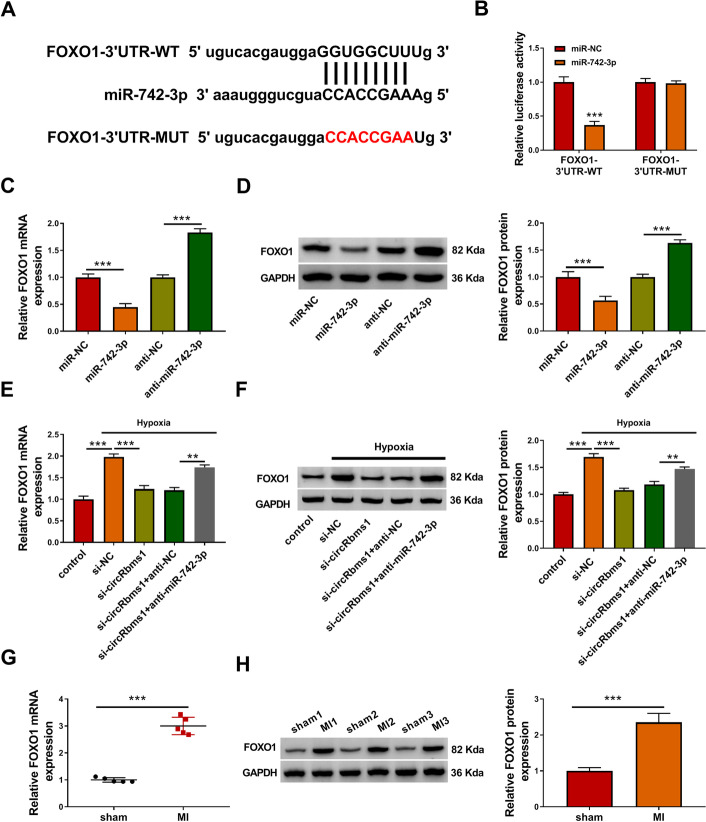
FOXO1 was targeted by miR-742-3p
Furthermore, the Starbase tool also predicted that miR-742-3p could target the 3′UTR of FOXO1 (Fig. 6A). Dual-luciferase reporter assay results revealed that the miR-742-3p overexpression could reduce the luciferase activity of FOXO1-3′UTR-WT vector, while not affecting that of the FOXO1-3′UTR-MUT vector (Fig. 6B). Through assessing the mRNA and protein expression levels of FOXO1, we discovered that FOXO1 expression was markedly inhibited by miR-742-3p overexpression and notably enhanced by miR-742-3p inhibition (Fig. 6C, D). Meanwhile, circRbms1 knockdown inhibited the mRNA and protein expression of FOXO1 promoted by hypoxia, while this effect was also reversed by miR-742-3p inhibitor (Fig. 6E, F). Additionally, we measured FOXO1 expression in the heart tissues of MI mice, and found that it was remarkably upregulated compared with the sham group (Fig. 6G, H).
Fig. 6.
FOXO1 was targeted by miR-742-3p. A The sequences of FOXO1-3′UTR-WT and FOXO1-3′UTR-MUT. B Dual-luciferase reporter assay was used to confirm the interaction between miR-742-3p and FOXO1. C, D H9c2 cells were transfected with miR-NC (50 nM), miR-742-3p (50 nM), anti-NC (50 nM), or anti-miR-742-3p (50 nM). The mRNA and protein expression levels of FOXO1 were measured using qRT-PCR and WB analysis. E, F H9c2 cells were transfected with si-NC (50 nM), si-circRbms1 (50 nM), si-circRbms1 (50 nM) + anti-NC (50 nM), or si-circRbms1 (50 nM) + anti-miR-742-3p (50 nM), and then treated with hypoxia. Untreated H9c2 cells were used as control. qRT-PCR and WB analysis were used to determine the mRNA and protein expression levels of FOXO1. G–H The mRNA and protein expression levels of FOXO1 in the heart tissues of sham mice and MI mice were examined using qRT-PCR (n = 6 per group) and WB analysis (n = 3 per group). All experiments were repeated three times. **P < 0.01, ***P < 0.001
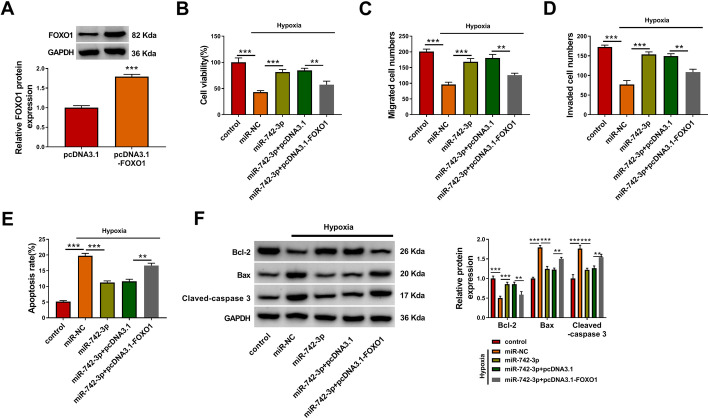
Overexpressed FOXO1 partially reversed the regulation of miR-742-3p on hypoxia-induced H9c2 cell injury
To confirm that miR-742-3p regulated hypoxia-induced H9c2 cell injury via targeting FOXO1, we constructed the pcDNA3.1 FOXO1 overexpression vector to carry out the rescue experiments. The increased FOXO1 expression confirmed that the transfection efficiency of pcDNA3.1-FOXO1 was good (Fig. 7A). In hypoxia-induced H9c2 cells co-transfected with miR-742-3p mimic and pcDNA3.1-FOXO1, we discovered that the enhancing effect of miR-742-3p on the viability, the migrated cell numbers, and the invaded cell numbers was abolished by FOXO1 overexpression (Fig. 7B–D). In addition, the inhibitory effect of miR-742-3p on the apoptosis rate, the Bax and Cleaved-caspase 3 protein levels, and the promoting effect on Bcl-2 protein level was also reversed by FOXO1 overexpression (Fig. 7E, F). All results indicate that miR-742-3p alleviated hypoxia-induced H9c2 cell injury by regulating FOXO1. Above all, our data show that circRbms1 sponged miR-742-3p to upregulate FOXO1, thereby inhibiting proliferation, migration, and invasion and promoting apoptosis in hypoxia-induced cardiomyocyte cells (Fig. 8).
Fig. 7.
Effects of miR-742-3p and FOXO1 on hypoxia-induced H9c2 cell injury. A H9c2 cells were transfected with pcDNA3.1 and pcDNA3.1-FOXO1, and the protein expression of FOXO1 was detected by WB analysis. B–F H9c2 cells were transfected with miR-NC (50 nM), miR-742-3p (50 nM), miR-742-3p (50 nM) + pcDNA3.1 (4.0 µg), or miR-742-3p (50 nM) + pcDNA3.1-FOXO1 (4.0 µg), and then treated with hypoxia. Untreated H9c2 cells were used as control. Cell viability, migrated and invaded cell numbers, and cell apoptosis rate were determined by CCK8 assay (B), transwell assay (C, D), and flow cytometry (E). F WB analysis was employed to examine the protein levels of Bcl-2, Bax and Cleaved-caspase 3. All experiments were repeated three times. **P < 0.01, ***P < 0.001
Fig. 8.
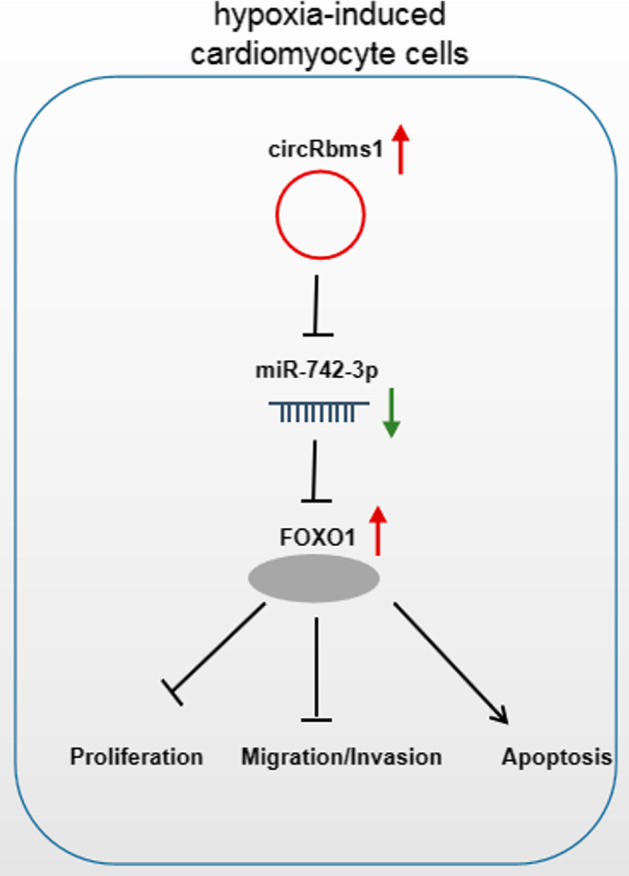
Mechanism diagram for this study. In hypoxia-induced cardiomyocyte cells, circRbms1 inhibited proliferation, migration, and invasion, while promoting apoptosis by regulating miR-742-3p/FOXO1
Discussion
Cardiovascular disease is one of the major diseases that seriously affect public health. Therefore, exploring the pathogenesis of MI is of great significance for disease diagnosis and treatment. In this study, we explored the role of a newly discovered circRNA, circRbms1, which was screened in the GEO database to be differentially expressed in the heart tissues of MI mice. In MI mice and hypoxia-induced H9c2 cells, we confirmed that circRbms1 had a significant high expression. Further experiments showed that overexpressed circRbms1 aggravated hypoxia-induced cell injury in simulated cardiomyocyte, while its knockdown protected cardiomyocytes from hypoxia-induced injury. These findings confirm the key function of circRbms1 in regulating cardiomyocyte injury and indicate that circRbms1 might have clinical significance in the treatment of MI.
The ceRNA mechanism of circRNA has been confirmed in many studies [19, 20]. For example, circ_28313 could act as a ceRNA for miR-195a to regulate osteoclast differentiation [21]. Zhang et al. reported that circNRIP1 could facilitate gastric cancer proliferation and metastasis by sponging miR-149-5p [22]. Circ_010567 was found to increase myocardial fibrosis through targeting miR-141 [23]. In MI, circCDYL had been discovered to serve as a miR-4793-5p sponge to enhance cardiomyocyte proliferation [24]. Using bioinformatics analysis and experimental verification, we confirmed that circRbms1 contained miR-742-3p binding sites. Past studies had shown that miR-742-3p was significantly underexpressed in the liver of obese mice, and might be associated with the progression of nonalcoholic fatty liver disease [25]. In our research, miR-742-3p was discovered to be lowly expressed in the heart tissues of MI mice and hypoxia-induced H9c2 cells. Gain-of-function experiments showed that miR-742-3p overexpression could relieve hypoxia-induced cardiomyocyte injury, suggesting that miR-742-3p might inhibit MI progression. Furthermore, miR-742-3p inhibitor reversed the effect of circRbms1 knockdown on hypoxia-induced cardiomyocyte injury, indicating that circRbms1 might participate in the regulation of MI progression via sponging miR-742-3p.
In addition, FOXO1 was confirmed to be a target of miR-742-3p. FOXO1 is a member of the O subgroup of the FOX family and is involved in regulating various biological processes, including oxidative stress, proliferation, and apoptosis [26, 27]. In addition, FOXO1 has been shown to play a vital role in embryonic development, fat formation, and tumor formation [28–30]. Ma et al. showed that FOXO1 could increase hypoxia–reoxygenation cardiomyocyte injury [31], and Qiu et al. proposed that knockdown of FOXO1 could inhibit hydrogen-peroxide-induced cardiomyocyte oxidative stress and apoptosis [32]. Here, we confirmed that FOXO1 was upregulated in the heart tissues of MI mice and hypoxia-induced H9c2 cells, and found that circRbms1 sponged miR-742-3p to positively regulate FOXO1. Further analysis verified that miR-742-3p targeted FOXO1 to relieve hypoxia-induced cardiomyocyte injury. The pro-cardiomyocyte injury effect of FOXO1 was also demonstrated in our study.
Of course, our study has some limitations. Although mmu_circ_0001022 is the homologous circRNA of hsa_circ_0056866 in mice, we have not verified the function and mechanism of hsa_circ_0056866 in human cardiomyocytes. Therefore, more research is needed to explore the function of circRbms1 in human cardiomyocytes to further determine its feasibility as a therapeutic target for MI.
In summary, our study revealed the role of a new circRNA in MI progression. This research suggests that silenced circRbms1 could alleviate cardiomyocyte injury after hypoxia by regulating the miR-742-3p/FOXO1 axis. Our findings provide a potential target for MI treatment and a reference for the study of circRbms1.
Supplementary Information
Additional file 1: Fig. S1. Effects of circRbms1 knockdown on cell cycle process in hypoxia-induced H9c2 cells. H9c2 cells were transfected with or without si-NC (50 nM), si-circRbms1 (50 nM), vector (4.0 µg), or circRbms1 (4.0 µg), and then treated with hypoxia. Untreated H9c2 cells were used as control. A, B Flow cytometry was used to assess the cell cycle distribution. All experiments were repeated three times. ***P < 0.001
Acknowledgements
None.
Abbreviations
- circRNA
circular RNA
- MI
myocardial infarction
- miR
microRNA
- FOXO1
forkhead box O1
- qRT-PCR
quantitative real-time PCR
- CCK8
Cell Counting Kit-8
- WB
western blot
- ActD
actinomycin D
- WT
wild type
- MUT
mutant type
Authors’ contributions
Bo Liu was responsible for drafting the manuscript. Bo Liu and Kai Guo contributed to the analysis and interpretation of data. Bo Liu and Kai Guo contributed to the data collection. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Please contact the corresponding author for data requests.
Declarations
Ethics approval and consent to participate
Our study was approved by the institutional review board of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-JDYXY-2019-012) and was performed in compliance with the Basel Declaration. All animals received humane care according to the Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reference
- 1.Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 2015;72(3):865–7. doi: 10.1007/s12013-015-0553-4. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, White HD, Joint, ESCAAHAWHFTFftRoMI Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Simms-Thomas F. Myocardial infarction. Clin J Oncol Nurs. 2000;4(3):141–4. [PubMed] [Google Scholar]
- 4.Lisowska A, Makarewicz-Wujec M, Filipiak KJ. Risk factors, prognosis, and secondary prevention of myocardial infarction in young adults in Poland. Kardiol Pol. 2016;74(10):1148–53. doi: 10.5603/KP.a2016.0098. [DOI] [PubMed] [Google Scholar]
- 5.Han F, Chen Q, Su J, Zheng A, Chen K, Sun S, et al. MicroRNA-124 regulates cardiomyocyte apoptosis and myocardial infarction through targeting Dhcr24. J Mol Cell Cardiol. 2019;132:178–88. doi: 10.1016/j.yjmcc.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM. Myocardial ischemia–reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428–42. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Patop IL, Wust S, Kadener S. Past, present, and future of circRNAs. EMBO J. 2019;38(16):e100836. doi: 10.15252/embj.2018100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foruzandeh Z, Zeinali-Sehrig F, Nejati K, Rahmanpour D, Pashazadeh F, Seif F, et al. CircRNAs as potent biomarkers in ovarian cancer: a systematic scoping review. Cell Mol Biol Lett. 2021;26(1):41. doi: 10.1186/s11658-021-00284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su H, Zou D, Sun Y, Dai Y. Hypoxia-associated circDENND2A promotes glioma aggressiveness by sponging miR-625-5p. Cell Mol Biol Lett. 2019;24:24. doi: 10.1186/s11658-019-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang HD, Jiang LH, Sun DW, Hou JC, Ji ZL. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25(1):1–7. doi: 10.1007/s12282-017-0793-9. [DOI] [PubMed] [Google Scholar]
- 12.Qu S, Liu Z, Yang X, Zhou J, Yu H, Zhang R, et al. The emerging functions and roles of circular RNAs in cancer. Cancer Lett. 2018;414:301–9. doi: 10.1016/j.canlet.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Akhter R. Circular RNA and Alzheimer's disease. Adv Exp Med Biol. 2018;1087:239–43. doi: 10.1007/978-981-13-1426-1_19. [DOI] [PubMed] [Google Scholar]
- 14.Altesha MA, Ni T, Khan A, Liu K, Zheng X. Circular RNA in cardiovascular disease. J Cell Physiol. 2019;234(5):5588–600. doi: 10.1002/jcp.27384. [DOI] [PubMed] [Google Scholar]
- 15.Zhai C, Qian G, Wu H, Pan H, Xie S, Sun Z, et al. Knockdown of circ_0060745 alleviates acute myocardial infarction by suppressing NF-kappaB activation. J Cell Mol Med. 2020;24(21):12401–10. doi: 10.1111/jcmm.15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Li G, Peng J, Ren L, Lei L, Ye H, et al. CircMACF1 attenuates acute myocardial infarction through miR-500b-5p-EMP1 axis. J Cardiovasc Transl Res. 2021;14(1):161–72. doi: 10.1007/s12265-020-09976-5. [DOI] [PubMed] [Google Scholar]
- 17.Cao M, Zhang L, Wang JH, Zeng H, Peng Y, Zou J, et al. Identifying circRNA-associated-ceRNA networks in retinal neovascularization in mice. Int J Med Sci. 2019;16(10):1356–65. doi: 10.7150/ijms.35149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong DD, Dang YW, Lin P, Wen DY, He RQ, Luo DZ, et al. A circRNA–miRNA–mRNA network identification for exploring underlying pathogenesis and therapy strategy of hepatocellular carcinoma. J Transl Med. 2018;16(1):220. doi: 10.1186/s12967-018-1593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou XY, Yang H, Bai YQ, Li XL, Han SY, Zhou BX. hsa_circ_0006916 promotes hepatocellular carcinoma progression by activating the miR-337-3p/STAT3 axis. Cell Mol Biol Lett. 2020;25(1):47. doi: 10.1186/s11658-020-00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du J, Zhang G, Qiu H, Yu H, Yuan W. The novel circular RNA circ-CAMK2A enhances lung adenocarcinoma metastasis by regulating the miR-615-5p/fibronectin 1 pathway. Cell Mol Biol Lett. 2019;24:72. doi: 10.1186/s11658-019-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Chen X, Ouyang Z, Shen Y, Liu B, Zhang Q, Wan L, et al. CircRNA_28313/miR-195a/CSF1 axis modulates osteoclast differentiation to affect OVX-induced bone absorption in mice. RNA Biol. 2019;16(9):1249–62. doi: 10.1080/15476286.2019.1624470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Wang S, Wang H, Cao J, Huang X, Chen Z, et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18(1):20. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem Biophys Res Commun. 2017;487(4):769–75. doi: 10.1016/j.bbrc.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Wang Z, Cheng Q, Wang Z, Lv X, Wang Z, et al. Circular RNA (circRNA) CDYL induces myocardial regeneration by ceRNA after myocardial infarction. Med Sci Monit. 2020;26:e923188. doi: 10.12659/MSM.923188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma M, Duan R, Shen L, Liu M, Ji Y, Zhou H, et al. The lncRNA Gm15622 stimulates SREBP-1c expression and hepatic lipid accumulation by sponging the miR-742-3p in mice. J Lipid Res. 2020;61(7):1052–64. doi: 10.1194/jlr.RA120000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing YQ, Li A, Yang Y, Li XX, Zhang LN, Guo HC. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018;193:124–31. doi: 10.1016/j.lfs.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Shi G, Liao PY, Cai XL, Pi XX, Zhang MF, Li SJ, et al. FoxO1 enhances differentiation and apoptosis in human primary keratinocytes. Exp Dermatol. 2018;27(11):1254–60. doi: 10.1111/exd.13775. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Lu Y, Tian M, Huang Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J Mol Endocrinol. 2019;62(3):R239-R53. doi: 10.1530/JME-18-0178. [DOI] [PubMed] [Google Scholar]
- 29.Ferdous A, Hill JA. FoxO1 in embryonic development. Transcription. 2012;3(5):221–5. doi: 10.4161/trns.21051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Ren YA, Pangas SA, Adams J, Zhou W, Castrillon DH, et al. FOXO1/3 and PTEN depletion in granulosa cells promotes ovarian granulosa cell tumor development. Mol Endocrinol. 2015;29(7):1006–24. doi: 10.1210/me.2015-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma M, Hui J, Zhang QY, Zhu Y, He Y, Liu XJ. Long non-coding RNA nuclear-enriched abundant transcript 1 inhibition blunts myocardial ischemia reperfusion injury via autophagic flux arrest and apoptosis in streptozotocin-induced diabetic rats. Atherosclerosis. 2018;277:113–22. doi: 10.1016/j.atherosclerosis.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Qiu Z, Wang L, Mao H, Xu F, Sun B, Lian X, et al. miR-370 inhibits the oxidative stress and apoptosis of cardiac myocytes induced by hydrogen peroxide by targeting FOXO1. Exp Ther Med. 2019;18(4):3025–31. doi: 10.3892/etm.2019.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Effects of circRbms1 knockdown on cell cycle process in hypoxia-induced H9c2 cells. H9c2 cells were transfected with or without si-NC (50 nM), si-circRbms1 (50 nM), vector (4.0 µg), or circRbms1 (4.0 µg), and then treated with hypoxia. Untreated H9c2 cells were used as control. A, B Flow cytometry was used to assess the cell cycle distribution. All experiments were repeated three times. ***P < 0.001
Data Availability Statement
Please contact the corresponding author for data requests.