Abstract
As the most phenotypically diverse mammalian species that shares human environments and access to sophisticated healthcare, domestic dogs have unique potential to inform our understanding of the determinants of aging. Here we outline key concepts in the study of aging and illustrate the value of research with dogs, which can improve dog health and support translational discoveries. We consider similarities and differences in aging and age-related diseases in dogs and humans and summarize key advances in our understanding of genetic and environmental risk factors for morbidity and mortality in dogs. We address health outcomes ranging from cancer to cognitive function and highlight emerging research opportunities from large-scale cohort studies in companion dogs. We conclude that studying aging in dogs could overcome many limitations of laboratory models, most notably, the ability to assess how aging-associated pathways influence aging in real-world environments similar to those experienced by humans.
Keywords: dogs, aging, multimorbidity, lifespan, cognition, translational research
1. INTRODUCTION
The domestic dog, Canis familiaris, is the most phenotypically variable mammal in the world. A trip to the local dog park quickly reveals extraordinary variation in size, shape, color, and behavior (1). Moreover, breeds vary in the risk of specific diseases and in expected lifespan (1, 2). The largest breeds, like Great Danes and Bouviers, typically live just 6 or 7 years, whereas Chihuahuas and Toy Poodles can easily reach 15 years of age. As in humans, with increased age, the risk for dogs of being diagnosed with many different diseases, and of dying from those diseases, increases exponentially (3). The variation that we see among dog breeds in risk of mortality, and in the causes of mortality, underscores dogs’ value as a powerful model to identify the genetic and environmental factors that influence aging, as well as the underlying molecular mechanisms of these factors. These insights are likely to inform our understanding of aging not only in dogs but in all mammals, including humans.
Most of what we know about the biology of aging comes from decades of research on laboratory organisms—yeast, nematodes, fruit flies, and rodents (4). We have learned a great deal about mechanisms of aging in these species, but it is not clear to what extent these lessons apply to longer-lived species like humans, or more to the point of this review, to the domestic dog—a genetically variable species living outside of the lab in an environmentally variable world, and a species that, like us, receives individualized care for diseases (5).
We are motivated to write this review not only because of an interest in improving healthy aging in dogs but also because what we learn from dogs could inform our understanding of human aging. Moreover, researchers working with dogs and their owners can ask questions that can prove difficult in human populations. For example, healthcare decisions for individual dogs vary based on the goals of each dog’s owner, meaning that dogs with similar diseases may be treated quite differently and may be euthanized at a time of the owner’s choosing. This creates the opportunity not only to compare outcomes from different treatment choices but also to explore the reasons different choices were made, including finances, logistics, and perceived quality of life. By contrast, the standard of care for human medical decision making is applied more consistently, the role of insurance often minimizes the impact of finance-based healthcare choices, and the option for palliative care is generally reserved only for very late stages of terminal disease.
A review on aging in dogs is timely for two important reasons. First, two large-scale studies on aging in dogs have launched recently. The Golden Retriever Lifetime Study (GRLS) is following more than 3,000 Golden Retrievers (GRs) over their lifetimes with a focus on cancer, and the Dog Aging Project (DAP) is tracking tens of thousands of dogs of all breeds for their entire lives, looking more generally at the biological and environmental determinants of aging (6, 7). Second, although veterinary medicine benefits from 22 distinct clinical specialties, from internal medicine to radiology to dermatology and more, veterinary gerontology is notably absent from this list. Our hope is that this review will inspire readers to pursue novel research questions with ongoing cohort studies, and that this work might also lay the groundwork for the creation of a formal veterinary gerontology field.
2. THE BASICS OF AGING
Aging, or senescence, describes the age-related decline that affects almost all molecular, cellular, and physiological processes and, in turn, disease risk and the key fitness traits of survival and reproduction. For centuries, people have wondered why we age, and why some organisms live so much longer than others. Experimental studies of aging began in the early twentieth century, starting with foundational work on the fruit fly, Drosophila melanogaster (8), and later investigating aging in single-celled yeast, nematode worms, mice, and rats.
Experimental studies of aging have progressed along two different paths. The first, a quantitative genetic approach, dates back to the earliest studies and has sought to measure the relative contributions of genetic and environmental factors to variation in lifespan and aging-related traits. Importantly, this approach has established that in experimental organisms like fruit flies, and also in humans, both genes and environment play an important role in explaining why some individuals live much longer than others (9). This work is important for thinking about variation in lifespan in dogs, as it suggests that there is not likely to be a single magic bullet that explains why, for example, Great Danes are so much shorter lived than Chihuahuas. Rather, a very large number of factors likely influence these differences, most of which make relatively small contributions.
The second research path has sought to understand the underlying molecular mechanisms of aging. Contrary to the quantitative genetic argument that aging will be shaped by a very large number of factors, each of small effect, molecular biologists and geneticists have identified individual genes and environmental interventions that can dramatically increase lifespan in relatively short-lived lab organisms. Interestingly, studies across diverse model organisms suggest that some of these genetic pathways associated with aging are highly evolutionarily conserved. For example, the relationship between lifespan and genes associated with the insulin/insulin-like growth factor (IGF) signaling pathway is found in common among organisms that diverged almost a billion years ago. In dogs, a single mutation in IGF1, a gene in this pathway, has a major effect on size in dogs (10). Whether this gene also explains why large-breed dogs are short-lived remains to be seen.
Lab-based studies have also established several hallmarks of aging that appear to be highly evolutionarily conserved, including telomere shortening, loss of mitochondrial function, failure to repair or eliminate damaged proteins, etc. (11). In most cases, although we observe these and other changes with age, many questions remain. Do these hallmarks account for natural variation in aging? Are they a cause or consequence of aging-related increases in disease risk and mortality? And if so, which hallmarks are most important? And perhaps most importantly, to what extent do the age-associated pathways discovered in the lab account for variation in aging in the real world?
3. GENETICS OF AGING
It is difficult to determine whether lab-based discoveries apply to a highly variable species like humans, in part because they are so long-lived. The companion dog provides us with an opportunity to integrate the two major approaches in aging research: identifying the genetic and environmental factors that shape aging and age-related disease and discovering the underlying mechanisms responsible for this variation, in a manageable time frame (that is, less than the 50+ years required to observe young adult life through old age in humans). Working with companion dogs, we have the potential to discover biomarkers that could transform the diagnosis, prognosis, treatment, and ultimately prevention of age-related morbidity and disease.
As with so many other traits, breed history accounts for much of the variation in aging among dogs (2), which is unsurprising, given their history of artificial selection. In traits that have been selected for directly, such as appearance, variation in one or only a handful of genes can often strongly predict phenotypic variation. For example, variation in coat type, length, and pattern is due in large part to mutations in just three genes (12). Some deleterious, Mendelian traits have been discovered in dogs, with these genes and their traits coming along for the ride during the strong artificial selection from a limited number of founders (e.g., leukocyte adhesion deficiency) (13). Other disease conditions are the direct anatomic or physiologic consequences of artificially selected morphological traits (e.g., the respiratory problems in bulldogs linked to selection for brachycephaly). From a veterinary medicine perspective, the identification of large-effect genetic diseases has sometimes allowed us to implement breeding programs that remove these deleterious alleles from future generations; in other instances, the morphology that produces the disease remains popular despite its consequences. In either case, the presence of these alleles in the dog genome, combined with the fact that dogs share more of their genomic sequence with humans than do rodents, allows us to use dogs with these diseases as models of human aging and age-related disease (14).
In human populations, we expect that variation in aging will be due primarily to common loci with very small effects. Most disease-associated, recessive Mendelian traits will be kept at bay by natural selection. Although this is generally true in dogs as well, small effective population sizes and a history of inbreeding within many breeds have made it difficult for selection to eliminate these highly deleterious alleles. In dogs, the IGF1 gene might be one such example of a single variant exerting a large effect on expected lifespan. Variation in IGF1 accounts for up to 50% of variation in size across dog breeds (10, 15). Interestingly, from flies to mice to humans, functional mutations in the IGF signaling pathway have been associated not only with body size but also with longevity (16-18). At least one study has suggested that variation in IGF1 and the IGF1 receptor gene is associated with longevity in dogs (19). Moreover, increased IGF1 signaling in large breeds not only plays a critical role in growth and development but likely also results in increased protein synthesis by mTOR, an important mechanism in the biology of aging (20). So one might hypothesize that large-breed dogs are short-lived due to selection for an IGF1 allele that favors large size, which would represent an example of antagonistic pleiotropy (21). However, researchers have yet to demonstrate that the association with IGF1 and lifespan in dogs is causal and not simply due to the close statistical association between size and lifespan.
In general, we expect the genetic architecture of diseases to look quite different from that of traits that have been actively selected for. Aside from specific cases of genes of large effect, most breed-level variation for disease traits likely results from several sources. For instance, rare variants have become prevalent in some breeds owing to population bottlenecks, and related to this, variation among breeds in the degree of inbreeding, as well as variation in pathways that influence growth, development, and the physiological pace of aging, have emerged (22). A quantitative and population genetics perspective on disease can thus shed light on the kinds of disease-associated genes we expect to find. Population genetic models point to a negative correlation between the effect size of an allele on disease and the frequency of that allele in the population (23). In particular, in searching for genes associated with variation in disease risk, we expect to find two categories: (a) large-effect loci that are usually quite rare, associated with Mendelian traits (e.g., muscular dystrophy), and (b) small-effect loci distributed across the genome that, in aggregate, increase the risk of disease (24).
With the advent of relatively inexpensive whole-genome sequencing, researchers have begun to identify the many genes that underlie complex traits, including those associated with age-related diseases, using dog populations. One of the most powerful aspects of studying dogs to understand the genetic architecture of aging-related traits is their high linkage disequilibrium (LD), which is a direct consequence of strong artificial selection (14). High-LD regions can be extensive in dogs, often reaching several megabases long within breeds, making it easier to identify the causal genetic variants underlying phenotypic traits. These variants can then be validated across breeds, where LD breaks down by two orders of magnitude (from megabases to tens of kilobases) (14). In many cases, the relatively simple genetic architecture of dogs has permitted effective disease trait mapping using a limited set of genetic markers in a few hundred dogs (25).
3.1. Breed-Associated Diseases
Because much of the genetic variation in dogs is related to breed history, we can assume that genes account for variation among breeds not only in size, shape, and behavior but also in rates of occurrence of specific age-associated diseases (2). This among-breed variation, coupled with the relative lack of genetic variation found between two dogs of the same breed, makes it relatively easy to identify heritable components of breed-specific diseases (26). Thanks to this genetic structure, we have been able to take advantage of breeds at high risk of developing specific disease outcomes to identify genetic variants that have been difficult to identify in human populations (27). For instance, Bernese Mountain Dogs are more than 10 times as likely as other dog breeds to die of cancer, even when adjusting for age and sex (28). Further study in this breed identified variants in the CDKN2A/B gene region that were associated with histiocytic sarcoma, the most common tumor type identified in Bernese Mountain Dogs (29). The analogous gene region in humans encodes for p16, a tumor suppressor, and abnormalities in this region have been associated with several cancer outcomes in humans, including histiocytic sarcoma (30, 31).
Similarly, Dalmatians show increased risk of urolithiasis (stones formed in the urinary tract) owing to increased excretion of uric acid (32). Studies of Dalmatian pedigrees confirm that the trait is heritable (33), and linkage mapping localized the hyperuricemia trait to the SLC2A9 gene in Dalmatians (34). Subsequent work showed that this same gene is responsible for urate transport capacity in humans (35).
Thus, we can often use dog breed information as a surrogate for specific genetic information. Importantly, however, not all breed predispositions are due to specific genetic mutations. Sometimes the inherited physical factors associated with breed phenotypes can predispose a dog to certain conditions. For instance, all deep-chested, large, and giant-sized dog breeds are at increased risk for developing gastric dilatation volvulus, a condition in which the stomach fills with gas and then twists upon itself, blocking both the entrance and exit of the stomach. Though this can happen to any breed of dog, all large-breed dogs are up to 10 times more likely to develop the condition than are small-breed dogs (36).
3.2. Epigenetic Changes with Age
Although DNA sequence is subject to the forces of natural selection across many generations, other aspects of the genome can change over shorter periods of time and even within the course of an individual’s lifespan. One such change includes chemical modifications of DNA sequence itself, including patterns of DNA methylation, which are considered a hallmark of aging (11). In so-called epigenetic clocks, DNA methylation patterns can predict chronological age with high accuracy and capture aspects of biological age that predict mortality above and beyond chronological age (37). In model organisms, these epigenetic clocks are also responsive to age-extending interventions, like caloric restriction, making them a useful biomarker for quantifying how interventions, genotypes, and environmental exposures alter the pace of aging (38, 39). At least three epigenetic clocks have been successfully developed in dogs (40-42), with the goal of employing them to understand mechanisms of breed differences in aging and to evaluate the efficacy of age-altering interventions. However, their utility might be limited, as these three clocks have different underlying loci, and much work is needed to fully understand the biological import of these epigenetic changes. Indeed, as epigenetic clocks become increasingly precise (i.e., can predict chronological age more accurately), there is less biological variation in the difference between epigenetic age and chronological age (age acceleration), limiting our ability (and power) to detect interindividual differences in the pace of aging and the efficacy of interventions (43). Nevertheless, epigenomic studies of aging in dogs (and other species) are still relatively new, and more data from larger cohorts of dogs may help to uncover novel, potentially modifiable, molecular mechanisms of aging.
4. PHYSICAL ENVIRONMENTAL IMPACTS ON AGING AND AGE-RELATED DISEASES
Any environmental factor that damages cellular macromolecules or interferes with repair processes can affect aging and age-related disease outcomes. Importantly, environmental toxicants have been shown to target stem cells, the main source of tissue regeneration in the adult body, accelerating the aging process and increasing the incidence of age-related disease outcomes (44). The aging process in humans is highly responsive to diverse environmental influences, such as chemical exposures, ultraviolet radiation, chronic inflammation, infectious diseases, and nutrition (45). This is important to note when considering the use of dogs as a model for aging in humans because dogs share human physical and chemical environments to a much greater extent than other animal species. In fact, dogs live in more than 63 million households in the United States. They have been used to assess human exposures to chemical contaminants, heavy metals, environmental carcinogens, and pollutants in air and water, as dogs have been shown to be good predictors of environmental exposures in people (46, 47).
Much of the work using companion animals as sentinels to identify environmental hazards for people has focused on spontaneous tumor development in dogs. This is because dogs naturally develop the same cancers as humans, but do so much more quickly (48). A classic example of this is a study of mesothelioma, a malignant lung cancer, in which the owner’s exposure to asbestos was found to be significantly associated with this cancer outcome in their dog (49). Thus, the diagnosis of mesothelioma in the companion dog served as an early warning of the environmental exposure to this hazardous material for the humans within the household.
Tobacco smoking and exposure to environmental tobacco smoke (ETS) have been associated with several age-related diseases in humans, including respiratory and cardiovascular diseases and several types of cancers (50). In addition, smoking has been associated with age acceleration as measured using a human epigenetic clock (51). ETS exposure has been evaluated in dogs, and measurements of biomarkers detected in dog hair and urine have been validated for use in quantifying ETS exposure (52, 53). ETS has been associated with health outcomes in dogs that include upper and lower airway disease, lymphoma, sinonasal cancers, and lung cancers (54-56). Associations between ETS exposure and longevity have not yet been examined in a large-scale study involving dog populations.
Pesticides are a heterogeneous group of chemicals that dogs and humans are exposed to both in and around the home and yard/garden. Several pesticides have carcinogenic effects in both humans and dogs, but the most consistent evidence of risk of exposure to pesticides and carcinogenic effects comes from phenoxy acid–containing herbicides (57). One such chemical, 2,4-dichlorophenoxyacetic acid, has been associated with development of lymphoma and testicular cancer in both dogs and humans, as well as bladder cancer in dogs (58-60).
Other chemical mixtures, including compounds like organic solvents, polychlorinated biphenyls, and heavy metals, have been identified at waste landfills and have been causally linked to adverse health effects in humans (61). Humans and their companion dogs can become exposed to these chemicals through air, water, and/or soil (62). Residential proximity to environmental hazard-containing sites has been used to estimate chemical exposure and cancer risk in dogs in several observational studies (54, 60, 63). Mortality due to cancer is also higher among human populations living near the same waste sites compared with the general population (64).
5. CLINICAL CONSEQUENCES OF AGING AND AGE-RELATED DISEASE
Aging is inevitable, but the experience of aging, including physical, medical, social, and emotional dimensions, varies widely among individuals within a species. A nuanced ability to define a more desirable aging experience creates the possibility to measure “successful aging” and to identify pathways that might lead in that direction (65). Healthy aging implies continued normal daily activities, mobility, cognitive function, social engagement, and general well-being, which may persist despite the presence of one or more diagnosed diseases (66, 67). In people, beyond awareness of specific diseases that are frequent in the aging population, several syndromes of aging are defined that might impact general well-being. These include the coexistence of more than one chronic condition, termed multimorbidity (68-70), and the chronic activation of the inflammatory response, defined as “inflammaging” (71). Limited attempts have been made to study these age-related syndromes in animal models (but see 72-74). But it is clear to dog owners and veterinarians that aging dogs not only acquire specific diagnoses of age-related disease but also experience changes to normal daily routine, mobility, cognitive function, and social engagement. Owners can document and describe the changes in their aging dogs’ behaviors and activities, and veterinarians can document and describe disease states and physiologic changes. Healthspan describes the period of life free of disease or disability (75, 76). The related concept of quality of life is an exquisitely important factor to aging dogs, because elective euthanasia is available to owners when prognosis or perceived quality of life is poor (77, 78). Lifespan itself is truncated in companion dogs when euthanasia is elected because good quality of life—or healthspan—has ended; in this way, lifespan and healthspan may be more closely tied in companion dogs than in any other species (76, 79, 80).
5.1. Effect of Age on Disease Risk in Dogs
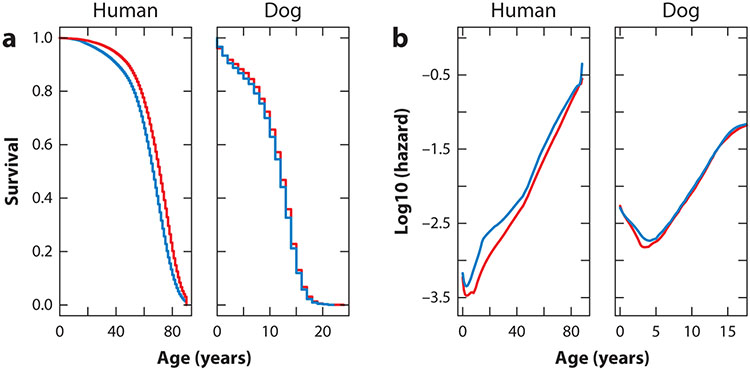
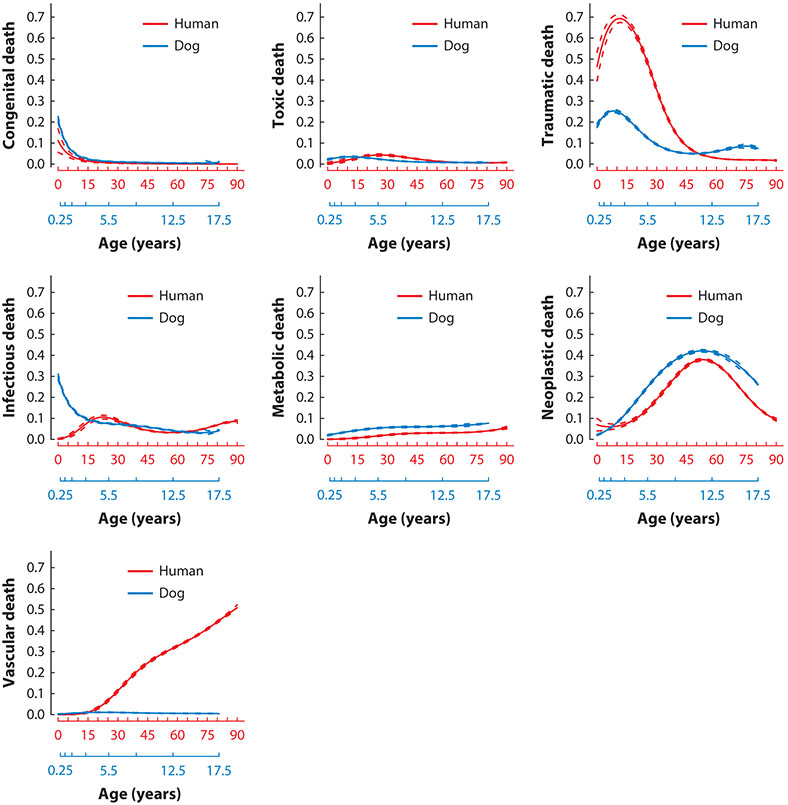
Veterinary medicine includes nearly all of the specialties seen in human medicine. Currently, 22 veterinary specialty organizations are recognized, several of which govern recognition of more than one specialty. To earn board-certified specialty status, veterinarians complete rigorous postgraduate training (internships and residencies), education, and examination requirements. Although specialties as diverse as cardiology and sports medicine exist, gerontology is glaringly absent from the list of recognized veterinary medical specialties. Given owner commitment and access to specialty care, the first clinical consequence of aging that has been described and quantified in dogs is age-related disease diagnosis, which varies among dogs of different sizes and breed backgrounds (81). For example, the likelihood of a diagnosis of chronic kidney disease or osteoarthritis rises with age in dogs, whereas the likelihood of a diagnosis of idiopathic epilepsy declines after middle age (82-84). Similarly, causes of death vary among size and breed categories of dogs, between reproductively intact and sterilized dogs (see the sidebar titled Effect of Endogenous Hormone Exposure on Aging), and by age at the time of death (2, 28, 85, 86). The overall pattern of mortality is similar between dogs and humans, though the strong effect of size in dogs is not mirrored in humans (22) (Figure 1). Moreover, some causes of mortality show similar age trajectories in both species, whereas for other causes there are marked differences. The likelihood of trauma as a cause of death peaks in the first quarter of a dog’s projected lifespan, similar to humans, and the likelihood of cancer as a cause of death generally increases over the lifespan in both dogs and people, peaking in late middle age and then tapering off again over the last quarter of life. By contrast, the likelihood of vascular disease as a cause of death increases steadily across the projected human lifespan but is an unlikely cause of death in dogs at any age (87) (Figure 2).
EFFECT OF ENDOGENOUS HORMONE EXPOSURE ON AGING.
In both human and dog populations, females are longer-lived than are males, though the effect size of sex on longevity is much stronger in humans (87). Hormones can act as either growth factors or inhibitors, depending on the sex of the dog and the affected tissue (150, 151). In dogs, castration/spay status and age at alteration are commonly used as proxy measures for total endogenous hormone exposure. For some cancers, such as mammary tumors, less exposure to sex hormones has been shown to be protective, whereas for others, such as osteosarcoma, lymphoma, and prostate cancer, less exposure to sex hormones is associated with an increased risk of disease (85, 150, 152-155). In addition, there is some limited evidence that the age of the dog at the time of castration/spay can impact the risk of developing cancer in some breeds (155).
Figure 1.
Age-specific (a) survival and (b) mortality for humans and dogs. Females are shown in red and males in blue. Human data are from the US Census Bureau, and canine data are from the VetCompass database. Depending on the breed, dogs are at least 5–10-times shorter-lived than humans. However, as these survivorship and mortality curves illustrate, the effect of age on mortality rate is similar in the two species. Mortality declines after birth and then increases exponentially (a straight line on a log-linear plot, as shown in panel b). Life expectancy is higher in females than in males for both species, though the difference is greater in humans than in dogs. Figure reproduced from Hoffman et al. (87) according to the terms of a Creative Commons CC-BY license.
Figure 2.
Proportion of deaths at each age due to disease in particular pathophysiological processes in humans (red) and dogs (blue). Note that dog age has been rescaled to roughly approximate human age. Figure reproduced from Hoffman et al. (87) according to the terms of a Creative Commons CC-BY license.
5.2. Multimorbidity
Multimorbidity, the co-occurrence of two or more chronic diseases within an individual, can be viewed as a marker of the deteriorating health of an aging individual, or as a syndrome that differentially affects certain members of the population and contributes to deteriorating health (88). Improved understanding of this hallmark of aging is essential. Multimorbidity is not successfully modeled in laboratory species, in which many strains are genetically modified to amplify the likelihood of developing specific diseases, and for which chronic disease management is seldom a goal. Because canine patients are managed as valued individuals, aging dogs frequently experience sequential diagnosis and subsequent concurrent management of multiple conditions, or multimorbidity, which can be studied through individual canine patient veterinary medical records (74, 87). Additionally, the health outcomes of dogs likely are modulated by the therapies recommended by their veterinarians and administered by their owners. Beyond a simple list of the diseases acquired, a key target of multimorbidity research is to understand the proportion to which any individual diagnosis in a list of morbidities contributes to risk of death (or euthanasia) and the extent to which any given therapy decreases that risk (68, 89, 90).
5.3. Frailty
Frailty is a well-described phenomenon in aging people that is associated with increased disability and risk of death (91-93). Several scales exist for measuring frailty in humans, and most include axes such as isolated muscle strength, gait speed, balance, unintentional or excessive weight loss (which is often associated with sarcopenia), cognitive decline, and social isolation (70, 94). Disability on assessments such as the Activities of Daily Living scale also closely parallels frailty in aging people (92). Although the superior frailty instrument remains a matter of debate, meta-analysis confirms that even gait speed alone is a powerful predictor of human mortality (95).
It is clear to dog owners and their veterinarians that aging dogs experience a phenomenon of frailty, including declining energy levels, limitations in mobility, and changes in cognition (96-98). Although owners of aging dogs may compensate for their dogs’ deteriorating function by purchasing ramps to get into cars or beds, or altogether avoiding stairs or slick floors, owners may not consider these changes to be evidence of illness or injury, and both owners and veterinarians may describe this deterioration as normal aging. Defining healthy aging in older dogs and differentiating healthy aging from disease are essential. At this time, such observations have not been quantified on any scale, and so the description of what counts as normal aging cannot be defined (99). Lacking a history of the measurement of these dimensions in practice, frailty is currently the most difficult consequence of aging and age-related disease to describe in dogs. Whereas some components of human frailty and Activities of Daily Living instruments are poorly suited to translation to dogs, common physical assessments and tasks that many dogs perform in their daily environments can be used as comparable metrics (70, 73). For example, nutritional decline is determined in humans through routine weight, body mass index, and appetite measurements. Although body mass index is not used in veterinary practice owing to the morphological diversity of dogs, the use of an analogous measure, body condition score, is common, and weight and appetite are documented routinely (100). Other factors relating to frailty include measures of physical activity, mobility, muscle loss, cognition, mood, and social relations—many of which have already been assessed in dogs via low-tech, widely applicable approaches (101-106). Incorporating these factors into a description of canine frailty will capture a wide range of physiologic parameters that vary over a lifetime and at different rates among individuals.
6. COGNITIVE AGING
Given our coevolutionary history and possible evolutionary convergences between dogs and humans, dogs may provide a more powerful model for many aspects of human cognition than our closest primate relatives (107). Numerous studies have focused on dog cognitive aging in both laboratory and companion dog populations, drawing on diverse techniques ranging from neuropsychological tests and measures of neuropathology to behavioral questionnaires assessing symptoms of dementia. Each of these approaches presents unique strengths and weaknesses, but together they provide valuable insights into how dog cognition changes with age, the extent to which cognitive aging varies among individuals and breeds, the neuropathological hallmarks of cognitive aging, and lifestyle factors that may influence these processes.
6.1. Studies of Cognitive Aging in Laboratory Dogs
Over the last three decades, and until recently, most research on dogs as a model of cognitive aging has focused on laboratory Beagles. Similarly to humans, aging Beagles exhibit age-related declines in those cognitive processes that rely on prefrontal functions, such as working memory and reversal learning (108). Performance in Beagles declines with age in tasks requiring discrimination based on size, space, or body orientation but does not decline for procedural learning or simple visual discrimination, suggesting that, as in humans, not all cognitive abilities are affected by age in dogs (109). Two key findings from laboratory studies are that (a) even within relatively homogeneous populations of Beagles, the extent to which individual dogs become impaired varies substantially, and this variability increases with age, and (b) on average, initial cognitive impairments often develop in middle age (6–7 years), suggesting that many dogs may experience some degree of cognitive impairment for approximately half of their lives (110, 111).
One of the primary motivations for laboratory work with Beagles has been the development of a dog model of Alzheimer’s disease. Alzheimer’s disease in humans is characterized by progressive dementia and neuropathological features, including β-amyloid (Aβ) plaques and neurofibrillary tangles made of hyperphosphorylated tau protein. Although evidence for neurofibrillary tangles in dogs is limited, aged Beagles spontaneously develop diffuse Aβ plaques that resemble those in Alzheimer’s disease (112). As is observed in humans, in Beagles these plaques appear first in the frontal cortex and later in the temporal and occipital regions (113). Importantly, the extent of this pathology is linked to the severity of cognitive impairment, suggesting similar underlying mechanisms in canine and human dementia (114, 115).
6.2. Studies of Cognitive Aging in Companion Dogs
Studies with laboratory populations have provided foundational knowledge about canine cognitive aging but have been limited by relatively small sample sizes, and also by the predominant focus on a single breed, Beagles. In contrast, studies with companion dogs have benefited from access to larger and more heterogeneous populations living in environments shared with humans. These studies also incorporate diverse methods, ranging from behavioral questionnaires to neuropsychological tests and spontaneous problem-solving tasks implemented by citizen scientists.
Questionnaire measures have focused largely on developing scales to detect canine cognitive dysfunction (CCD). CCD is characterized by age-related cognitive and behavioral impairments as well as neuropathological features and is typically diagnosed by veterinarians based on behavioral indicators rather than neuropathology or neuropsychological testing (116). Several scales are currently available to assess symptoms of CCD, which include signs of disorientation, decreased social interaction, changes in activity, loss of housetraining, and increased anxiety (117). Although less objective than neuropsychological tests, CCD questionnaires can be administered quickly and capture a broad range of behavioral changes associated with cognitive impairment, showing good predictive validity for clinical diagnoses and associations with Alzheimer’s disease–like neuropathology (97, 118, 119). When deployed at scale in large epidemiological studies, CCD scales estimate a disease prevalence of ~14%, which is much higher than the ~2% prevalence based on clinical diagnoses, suggesting that CCD is underdiagnosed in companion dogs (120).
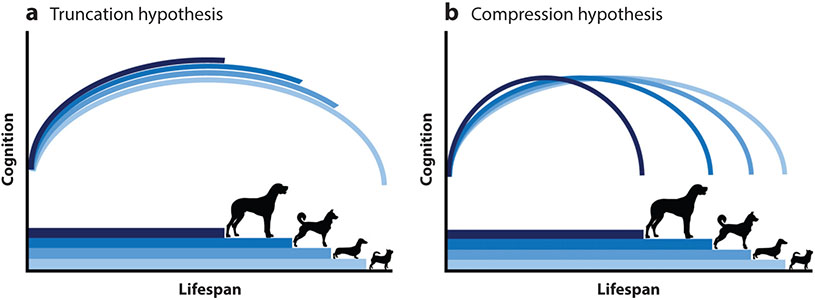
A second major line of research with companion dogs involves neuropsychological testing implemented at universities, working dog training centers, animal shelters, and dog day care facilities. Recently, experimental studies of companion dog cognition have also been implemented using citizen science, with experiments performed by dog owners in the home environment (101). Although these studies occasionally use methods similar to those employed in the lab (e.g., 121), more often they rely on spontaneous nonverbal problem-solving tasks—an approach modeled after methods used by developmental psychologists working with preverbal children. This approach allows testing of larger and more diverse samples and is beginning to yield insights about the pattern and pace of cognitive aging in companion dog populations (122-124). One emerging trend from this work is that although many cognitive traits exhibit an inverted-U-shaped trajectory, with improvements in early life and declines in late life, the extent of decline varies across breeds and cognitive processes. Nonetheless, a cross-sectional study of more than 4,000 dogs revealed similar cognitive aging trajectories across breeds, regardless of breed lifespan (125) (Figure 3). Whereas physiological aging scales in a way that short-lived and long-lived breeds alike experience decline, the delay in cognitive changes is such that smaller breeds are more likely to live to the advanced ages associated with major cognitive impairment. This finding is consistent with epidemiological studies of CCD, which have found no age × longevity interaction in disease prevalence and greater odds of impairment in smaller dogs (120, 126).
Figure 3.
Alternative models of aging in dogs. (a) A schematic of the truncation hypothesis, in which larger and smaller dog breeds have similar aging trajectories of a given phenotype (e.g., cognition). Under this hypothesis, large dog breeds experience limited age-associated declines because they typically die before the more precipitous decline experienced by longer-lived, smaller breeds. (b) The alternative, compression hypothesis, in which aging trajectories scale with lifespan, such that larger breeds have an accelerated aging trajectory. Figure reused with permission from Watowich et al. (125).
6.3. Lifestyle Factors Influencing Dog Cognitive Aging
Even within breeds, there is variation in whether and, if so, when dogs exhibit cognitive decline. It is therefore crucial to identify factors that might delay, mitigate, or prevent cognitive changes that decrease quality of life. One potential environmental mediator is diet. In the lab, aging Beagles fed an antioxidant-enriched diet showed fewer age-related impairments on cognitive tasks involving spatial learning and memory, oddity discrimination, and reversal learning when compared to a control group (127, 128). This dietary intervention also affected neuropathology; dogs fed the antioxidant-enriched diet exhibited reduced Aβ pathology (129). Other dietary interventions, including supplementation with medium-chain triglycerides and antioxidant capsules, have also shown some promise in laboratory populations (130, 131). However, we still know little about the effects of diet on cognitive aging in companion dogs, and initial studies in companion dogs have found no benefits associated with antioxidant-enriched foods (132).
In addition to diet, both cognitive stimulation and exercise are positively associated with brain health and successful cognitive aging in dogs. Aging Beagles that were housed with kennel mates, provided opportunities for regular exercise, and routinely exposed to novel stimuli showed improved performance on multiple cognitive measures in comparison to a control group without these forms of enrichment (128). The combination of diet and behavioral enrichment yielded additive benefits for cognitive function, associated with maintenance of high levels of brain-derived neurotrophic factor into old age (133). In companion dogs, lifelong training has also been associated with preserved attentional processes in older age, suggesting similar protective effects of lifelong cognitive stimulation in humans (134).
Although age-related cognitive decline occurs spontaneously in dogs, the pace and extent of impairment are likely to have several modifiable risk factors, including diet, exercise, and cognitive stimulation. Perhaps surprisingly, few studies have considered the role of lifestyle factors on cognitive aging in companion dogs. Although many of these dogs have relatively enriched lives compared to laboratory populations, their diet and exercise patterns and the amount of cognitive stimulation they receive vary widely, creating rich opportunities for future large-scale studies of lifestyle factors affecting cognitive aging.
7. DATA SOURCES FOR AGE-RELATED RESEARCH
Complete mortality data, including all causes of mortality and the age at which death occurred, are rarely obtainable in companion dog populations owing to the scarcity of population-based registries and lack of census data pertaining to dogs. However, some data sources can be used to investigate mortality and disease incidence and/or prevalence in dog populations. As we show here, these databases offer excellent opportunities to ask questions about aging in dogs, but each also has inherent biases that must be kept in mind.
7.1. Existing Databases
The Veterinary Medical Database is one of the largest and oldest clinic-based data sets that includes information on all causes of mortality in domestic animal populations, primarily dogs and cats, seen at participating clinics (http://vmdb.org). This data set was begun as an initiative of the National Cancer Institute in 1964 and includes veterinary patient data, covering the full range of possible medical diagnoses, collected from 26 university-affiliated veterinary teaching hospitals in the United States and Canada. Given that veterinary teaching hospitals typically act as tertiary care facilities for veterinary care, the cases and disease diagnoses contained in the Veterinary Medical Database are unlikely to be representative of those typically seen in the general dog population owing to referral bias, a type of systematic error that occurs as a result of selecting only dogs that have been referred to tertiary care centers and therefore do not represent the total dog population (135).
The Veterinary Companion Animal Surveillance System (VetCompass) collects clinical data from private veterinary practices in the United Kingdom and is therefore less likely to be affected by referral bias. However, this data set may be influenced by misclassification bias, a type of systematic information bias that may result in inaccurate identification of exposures or outcomes (136). This data set contains medical information about more than 15 million animals contributed from more than 1,800 participating veterinary practices located throughout the United Kingdom (137). VetCompass Australia began collecting clinical data from primary care facilities in 2013, and pilot projects are underway in Spain, Germany, and New Zealand (138, 139). Thus far, this data set has been used to investigate both specific causes of mortality in dogs and all causes of mortality within specific breeds of dogs, but it has not been used to document all causes of mortality within all breeds of dogs (140).
Two well-established veterinary insurance databases from the United Kingdom and Sweden have been used to determine the incidence and prevalence of specific conditions in dogs (86, 141). A limitation with both data sets is that not all diagnoses are confirmed histologically, and the Swedish data contain mortality rates primarily in dogs younger than 10 years of age, after which dogs are no longer insured. However, comparison of mortality rates across breeds within the Swedish data is informative given that the limitations occur equally across all breeds of dogs. For instance, when comparing overall mortality rates for breeds within the Swedish data set, German Shepherd Dogs had a total mortality rate of 634 deaths per 10,000 dog years at risk compared to the overall total mortality for all dog breeds, which was 283 deaths per 10,000 dog years at risk, showing that German Shepherd Dogs do have a higher mortality rate when compared to other dog breeds (86).
7.2. Emerging Sources of Data
Recent large-scale efforts have been made to collect data about companion dogs and their environments in systematic ways over long periods of time to better understand the factors associated with disease outcomes and healthy aging. One such study, the GRLS, enrolled a cohort of more than 3,000 purebred GRs under the age of two years in order to collect information about them throughout their lifetimes (6). Owners and veterinarians supply information about the dogs and their environment through questionnaires, and the dogs receive routine healthcare, including annual examination and biospecimen collection. Therefore, this data set includes information about each dog’s environment, diet, and physical activity, coupled with annual biological sample sets and detailed health outcomes. This data set will be considered complete when mortality of all enrolled dogs has reached 100%, predicted to occur by 2030.
An even larger data set is being compiled by the DAP, a multiyear longitudinal study that is tracking tens of thousands of dogs over the course of their lives (7). Dogs of any breed, size, and age are eligible for participation in the DAP, though enrollment is currently limited to the 50 US states. It is anticipated that the total number of dogs enrolled in the DAP will eventually exceed 100,000, as enrollment is ongoing and there is no limit to the total number of participants that will be included in the project. Thus, this data set represents the largest primary, publicly available data source of health information in dogs collected explicitly for use in translational medical research.
This longitudinal study includes detailed owner-reported comprehensive information about enrolled dogs’ lifestyle, exercise, diet, behavior, and health, as well as data extracted from veterinary medical records submitted by participants. Targeted surveys address age-related topics, such as owner-reported cognitive decline and veterinarian-reported multimorbidity. Dogs participate in periodic tasks, such as gait speed assessments and exercises designed to measure cognition. A subset of dogs (n = 10,000) have whole-genome sequencing performed from cheek swabs, and a subset of those dogs (n = 1000) have a comprehensive biospecimen sample kit collected annually. Because the DAP is an Open Science study, these diverse types of raw data for each dog will be deidentified and made available to the research community.
8. CONCLUDING REMARKS
In this review, we have focused on the diverse causes and consequences of aging in companion dogs. Although dogs offer a powerful translational model for human health, we note that the reverse is also true. For example, human medicine has inspired veterinarians to develop canine equivalents of limb-sparing surgery in osteosarcoma (142, 143), safer and more effective drugs for epilepsy (144, 145), and management of congestive heart failure (146, 147).
Similarly, canine research has also been inspired by the rich history of studies designed to discover the biological and environmental determinants of health in human populations (148, 149). Researchers are now bringing this powerful approach to the study of aging in companion dogs, efforts that represent a new paradigm in translational aging research. Large-scale longitudinal studies in dogs will help us to identify dog breeds at increased risk of developing heritable diseases and thus identify the genetic mutations associated with disease outcomes. Furthermore, given that companion dogs share our environment, these longitudinal studies are likely to help us identify risk factors that threaten health and longevity in both dog and human populations. These studies also offer the opportunity to test interventions designed to delay or decrease the impact of aging on morbidity and mortality (7). Finally, by tracking not only disease incidence but also detailed information about cognition, frailty, and multimorbidity, we will better understand the aging process in dogs and, through these discoveries, gain further insight into the aging process in humans.
Aging:
age-specific increase in morbidity and mortality risk, owing to decline in intrinsic molecular and physiological processes (senescence)
Golden Retriever Lifetime Study (GRLS):
cohort study of more than 3,000 Golden Retriever dogs to identify factors associated with health and disease
Dog Aging Project (DAP):
ongoing longitudinal study of companion dogs, with the goal of discovering the biological, environmental, and lifestyle determinants of healthspan
Evolutionarily conserved:
biological processes that function similarly across species owing to inheritance from a common ancestor and limited independent evolution
Mendelian traits:
phenotypes controlled by a single genetic locus
Brachycephaly:
condition of having a shortened skull as compared to what is typical within a species
Complex traits:
phenotypes influenced by multiple genetic loci and environmental factors
Linkage disequilibrium (LD):
nonrandom association of alleles at two or more loci in the genome
Epigenetic clock:
loci (CpG sites) in the genome where methylation levels can predict individual chronological age with high precision
Multimorbidity:
co-occurrence of two or more chronic diseases within an individual, associated with poorer health outcomes
Inflammaging:
result of chronic low-grade inflammation, believed to accelerate the biological aging process
Healthspan:
period of lifespan during which an individual is free from illness, disability, or chronic disease
Frailty:
declining functional activity and wellness, assessed by scales that include muscle strength, gait speed, balance, weight loss, and/or cognitive decline
ACKNOWLEDGMENTS
All of the authors are supported in part by a National Institute on Aging grant in support of the Dog Aging Project, U19 AG057377. We thank Dr. Jessica Hoffman for help with access to original figures.
Footnotes
DISCLOSURE STATEMENT
D.P. is paid to review intramural grant proposals for Waltham Petcare Science Institute.
RELATED RESOURCES
Darwin’s Ark website: https://www.darwinsark.org
Dog Aging Project website: https://www.dogagingproject.org
Golden Retriever Lifetime Study website: https://www.morrisanimalfoundation.org/golden-retriever-lifetime-study
VetCompass website: https://www.rvc.ac.uk/vetcompass
Using imputation to map complex traits in dogs with low-pass whole-genome sequencing: Buckley RM, Harris AC, Wang G-D, Whitaker DT, Zhang Y-P, Ostrander EA. 2021. Best practices for analyzing imputed genotypes from low-pass sequencing in dogs. bioRxiv. https://www.biorxiv.org/content/10.1101/2021.04.29.441990v1?ct=ct
LITERATURE CITED
- 1.Wayne RK, Ostrander EA. 2007. Lessons learned from the dog genome. Trends Genet. 23(11):557–67 [DOI] [PubMed] [Google Scholar]
- 2. Fleming JM, Creevy KE, Promislow DEL. 2011. Mortality in North American dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J. Vet. Intern. Med 25(2):187–98 Large-scale analysis of effects of size and age on diverse causes of mortality by breed.
- 3.Kraus C, Pavard S, Promislow DEL. 2013. The size-life span trade-off decomposed: why large dogs die young. Am. Nat 181(4):492–505 [DOI] [PubMed] [Google Scholar]
- 4.Fontana L, Partridge L, Longo VD. 2010. Extending healthy life span—from yeast to humans. Science 328(5976):321–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. 2014. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLOS ONE 9(3):e90501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy MK, Page RL, Jensen WA, Olson PN, Haworth JD, et al. 2015. The Golden Retriever Lifetime Study: establishing an observational cohort study with translational relevance for human health. Philos. Trans. R. Soc. Lond. B 370(1673):20140230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaeberlein M, Creevy KE, Promislow DEL. 2016. The dog aging project: translational geroscience in companion animals. Mamm. Genome 27(7–8):279–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearl R, Parker SL. 1921. Experimental studies on the duration of life. I. Introductory discussion of the duration of life in Drosophila. Am. Nat 55(641):481–509 [Google Scholar]
- 9.Melzer D, Pilling LC, Ferrucci L. 2020. The genetics of human ageing. Nat. Rev. Genet 21(2):88–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, et al. 2007. A single IGF1 allele is a major determinant of small size in dogs. Science 316(5821):112–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153(6):1194–217 In-depth analysis of traits that decline with age in diverse organisms.
- 12.Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, et al. 2009. Coat variation in the domestic dog is governed by variants in three genes. Science 326(5949):150–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kijas JMH, Bauer TR Jr., Gäfvert S, Marklund S, Trowald-Wigh G, et al. 1999. A missense mutation in the β-2 integrin gene (1TGB2) causes canine leukocyte adhesion deficiency. Genomics 61(1):101–7 [DOI] [PubMed] [Google Scholar]
- 14. Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, et al. 2005. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438(7069):803–19 First high-quality draft sequence of the dog genome and outline of genetic differences between breeds.
- 15.Boyko AR, Quignon P, Li L, Schoenebeck JJ, Degenhardt JD, et al. 2010. A simple genetic architecture underlies morphological variation in dogs. PLOS Biol. 8(8):e1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. 1996. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N. Engl. J. Med 335(18):1363–67 [DOI] [PubMed] [Google Scholar]
- 17.Edgar BA. 2006. How flies get their size: Genetics meets physiology. Nat. Rev. Genet 7(12):907–16 [DOI] [PubMed] [Google Scholar]
- 18.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, et al. 2011. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med 3(70):70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones P, Chase K, Martin A, Davern P, Ostrander EA, Lark KG. 2008. Single-nucleotide-polymorphism-based association mapping of dog stereotypes. Genetics 179(2):1033–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sándor S, Kubinyi E. 2019. Genetic pathways of aging and their relevance in the dog as a natural model of human aging. Front. Genet 10:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams GC. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11(4):398–411 [Google Scholar]
- 22.Yordy J, Kraus C, Hayward JJ, White ME, Shannon LM, et al. 2020. Body size, inbreeding, and lifespan in domestic dogs. Conserv. Genet 21(1):137–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, et al. 2008. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet 9(5):356–69 [DOI] [PubMed] [Google Scholar]
- 24.Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK, et al. 2003. The nature and identification of quantitative trait loci: a community’s view. Nat. Rev. Genet 4(11):911–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrander EA, Dreger DL, Evans JM. 2019. Canine cancer genomics: lessons for canine and human health. Annu. Rev. Anim. Biosci 7:449–72 [DOI] [PubMed] [Google Scholar]
- 26.Vaysse A, Ratnakumar A, Derrien T, Axelsson E, Rosengren Pielberg G, et al. 2011. Identification of genomic regions associated with phenotypic variation between dog breeds using selection mapping. PLOS Genet. 7(10):e1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cadieu E, Ostrander EA. 2007. Canine genetics offers new mechanisms for the study of human cancer. Cancer Epidemiol. Biomark. Prev 16(11):2181–83 [DOI] [PubMed] [Google Scholar]
- 28.Egenvall A, Bonnett BN, Hedhammar Å, Olson P. 2005. Mortality in over 350,000 insured Swedish dogs from 1995–2000: II. Breed-specific age and survival patterns and relative risk for causes of death. Acta Vet. Scand 46:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abadie J, Hédan B, Cadieu E, De Brito C, Devauchelle P, et al. 2009. Epidemiology, pathology, and genetics of histiocytic sarcoma in the Bernese mountain dog breed. J. Hered 100(Suppl. 1):S19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar R, Khan SP, Joshi D-D, Shaw GR, Ketterling RP, Feldman AL. 2011. Pediatric histiocytic sarcoma clonally related to precursor B-cell acute lymphoblastic leukemia with homozygous deletion of CDKN2A encoding p16INK4A. Pediatr. Blood Cancer 56(2):307–10 [DOI] [PubMed] [Google Scholar]
- 31.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, et al. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413(6851):86–91 [DOI] [PubMed] [Google Scholar]
- 32.Schaible RH. 1986. Genetic predisposition to purine uroliths in Dalmatian dogs. Vet. Clin. N. Am. Small Anim. Pract 16(1):127–31 [DOI] [PubMed] [Google Scholar]
- 33.Bannasch DL, Ling GV, Bea J, Famula TR. 2004. Inheritance of urinary calculi in the Dalmatian. J. Vet. Intern. Med 18(4):483–87 [DOI] [PubMed] [Google Scholar]
- 34.Bannasch D, Safra N, Young A, Karmi N, Schaible RS, Ling GV. 2008. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLOS Genet. 4(11):e1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz A, Gautschi I, Schild L, Bonny O. 2018. Human mutations in SLC2A9 (Glut9) affect transport capacity for urate. Front. Physiol 9:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glickman LT, Glickman NW, Pérez CM, Schellenberg DB, Lantz GC. 1994. Analysis of risk factors for gastric dilatation and dilatation-volvulus in dogs. J. Am. Vet. Med. Assoc 204(9):1465–71 [PubMed] [Google Scholar]
- 37.Horvath S, Raj K. 2018. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet 19(6):371–84 [DOI] [PubMed] [Google Scholar]
- 38.Maegawa S, Lu Y, Tahara T, Lee JT, Madzo J, et al. 2017. Caloric restriction delays age-related methylation drift. Nat. Commun 8:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn O, Gronke S, Stubbs TM, Ficz G, Hendrich O, et al. 2017. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol. 18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Ma J, Hogan AN, Fong S, Licon K, et al. 2020. Quantitative translation of dog-to-human aging by conserved remodeling of the DNA methylome. Cell Syst. 11(2):176–85.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson MJ, vonHoldt B, Horvath S, Pellegrini M. 2017. An epigenetic aging clock for dogs and wolves. Aging 9(3):1055–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvath S, Lu AT, Haghani A, Zoller JA, Brooke RT, et al. 2021. Epigenetic clock and methylation studies in dogs. bioRxiv. 10.1101/2021.03.30.43760443 [DOI] [Google Scholar]
- 43.Zhang Q, Vallerga CL, Walker RM, Lin T, Henders AK, et al. 2019. Improved precision of epigenetic clock estimates across tissues and its implication for biological ageing. Genome Med. 11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, et al. 2005. Cell intrinsic alterations underlie hematopoietic stem cell aging. PNAS 102(26):9194–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karol MH. 2009. How environmental agents influence the aging process. Biomol. Ther 17(2):113–24 [Google Scholar]
- 46.APPA. 2020. APPA National Pet Owners Survey Statistics: Pet Ownership and Annual Expenses. Stamford, CT: Am. Pet Prod. Assoc. [Google Scholar]
- 47.Backer LC, Grindem CB, Corbett WT, Cullins L, Hunter JL. 2001. Pet dogs as sentinels for environmental contamination. Sci. Total Environ 274(1–3):161–69 [DOI] [PubMed] [Google Scholar]
- 48.Rowell JL, McCarthy DO, Alvarez CE. 2011. Dog models of naturally occurring cancer. Trends Mol. Med 17(7):380–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glickman LT, Domanski LM, Maguire TG, Dubielzig RR, Churg A. 1983. Mesothelioma in pet dogs associated with exposure of their owners to asbestos. Environ. Res 32(2):305–13 [DOI] [PubMed] [Google Scholar]
- 50.Lee KWK, Pausova Z. 2013. Cigarette smoking and DNA methylation. Front. Genet 4:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao X, Zhang Y, Breitling LP, Brenner H. 2016. Relationship of tobacco smoking and smoking-related DNA methylation with epigenetic age acceleration. Oncotarget 7(30):46878–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knottenbelt CM, Bawazeer S, Hammond J, Mellor D, Watson DG. 2012. Nicotine hair concentrations in dogs exposed to environmental tobacco smoke: a pilot study. J. Small Anim. Pract 53(11):623–26 [DOI] [PubMed] [Google Scholar]
- 53.Bertone-Johnson ER, Procter-Gray E, Gollenberg AL, Ryan MB, Barber LG. 2008. Environmental tobacco smoke and canine urinary cotinine level. Environ. Res 106(3):361–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marconato L, Leo C, Girelli R, Salvi S, Abramo F, et al. 2009. Association between waste management and cancer in companion animals. J. Vet. Intern. Med 23(3):564–69 [DOI] [PubMed] [Google Scholar]
- 55.Reif JS, Bruns C, Lower KS. 1998. Cancer of the nasal cavity and paranasal sinuses and exposure to environmental tobacco smoke in pet dogs. Am. J. Epidemiol 147(5):488–92 [DOI] [PubMed] [Google Scholar]
- 56.Reif JS, Dunn K, Ogilvie GK, Harris CK. 1992. Passive smoking and canine lung cancer risk. Am. J. Epidemiol 135(3):234–39 [DOI] [PubMed] [Google Scholar]
- 57.Dich J, Zahm SH, Hanberg A, Adami HO. 1997. Pesticides and cancer. Cancer Causes Control 8(3):420–43 [DOI] [PubMed] [Google Scholar]
- 58.Hayes HM, Tarone RE, Cantor KP, Jessen CR, McCurnin DM, Richardson RC. 1991. Case-control study of canine malignant lymphoma: positive association with dog owner’s use of 2,4-dichlorophenoxyacetic acid herbicides. J. Natl. Cancer Inst 83(17):1226–31 [DOI] [PubMed] [Google Scholar]
- 59.Hayes HM, Tarone RE, Casey HW. 1995. A cohort study of the effects of Vietnam service on testicular pathology of U.S. military working dogs. Mil. Med 160(5):248–55 [PubMed] [Google Scholar]
- 60.Glickman LT, Schofer FS, McKee LJ, Reif JS, Goldschmidt MH. 1989. Epidemiologic study of insecticide exposures, obesity, and risk of bladder cancer in household dogs. J. Toxicol. Environ. Health 28(4):407–14 [DOI] [PubMed] [Google Scholar]
- 61.Vrijheid M 2000. Health effects of residence near hazardous waste landfill sites: a review of epidemiologic literature. Environ. Health Perspect 108(Suppl. 1):101–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Upton AC, Kneip T, Toniolo P. 1989. Public health aspects of toxic chemical disposal sites. Annu. Rev. Public Health 10:1–25 [DOI] [PubMed] [Google Scholar]
- 63.Gavazza A, Presciuttini S, Barale R, Lubas G, Gugliucci B. 2001. Association between canine malignant lymphoma, living in industrial areas, and use of chemicals by dog owners. J. Vet. Intern. Med 15(3):190–95 [PubMed] [Google Scholar]
- 64.Comba P, Bianchi F, Fazzo L, Martina L, Menegozzo M, et al. 2006. Cancer mortality in an area of Campania (Italy) characterized by multiple toxic dumping sites. Ann. N.Y. Acad. Sci 1076:449–61 [DOI] [PubMed] [Google Scholar]
- 65.Rowe JW, Kahn RL. 1987. Human aging: usual and successful. Science 237(4811):143–49 [DOI] [PubMed] [Google Scholar]
- 66.Rowe JW, Kahn RL. 1997. Successful aging. Gerontologist 37(4):433–40 [DOI] [PubMed] [Google Scholar]
- 67.World Health organ. 2020. Ageing: healthy ageing and functional ability. http://www.who.int/ageing/active_ageing/en
- 68.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. 2012. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380(9836):37–43 [DOI] [PubMed] [Google Scholar]
- 69. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. 2004. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J. Gerontol. A 59(3):255–63 Translating these concepts from human gerontology is essential to the study of aging in dogs.
- 70.Malmstrom TK, Miller DK, Morley JE. 2014. A comparison of four frailty models. J. Am. Geriatr. Soc 62(4):721–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fülöp T, Dupuis G, Witkowski JM, Larbi A. 2016. The role of immunosenescence in the development of age-related diseases. Rev. Investig. Clin 68(2):84–91 [PubMed] [Google Scholar]
- 72.Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, Garcia-Garcia FJ, Olaso-Gonzalez G, et al. 2017. A new frailty score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J. Gerontol. A 72(7):885–91 [DOI] [PubMed] [Google Scholar]
- 73.Hua J, Hoummady S, Muller C, Pouchelon J-L, Blondot M, et al. 2016. Assessment of frailty in aged dogs. Am. J. Vet. Res 77(12):1357–65 [DOI] [PubMed] [Google Scholar]
- 74.Jin K, Hoffman JM, Creevy KE, O’Neill DG, Promislow DEL. 2016. Multiple morbidities in companion dogs: a novel model for investigating age-related disease. Pathobiol. Aging Age-Relat. Dis 6:33276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, et al. 2014. Geroscience: linking aging to chronic disease. Cell 159(4):709–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adams VJ, Watson P, Carmichael S, Gerry S, Penell J, Morgan DM. 2016. Exceptional longevity and potential determinants of successful ageing in a cohort of 39 Labrador retrievers: results of a prospective longitudinal study. Acta Vet. Scand 58:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belshaw Z, Asher L, Harvey ND, Dean RS. 2015. Quality of life assessment in domestic dogs: an evidence-based rapid review. Vet. J 206(2):203–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edney AT. 1998. Reasons for the euthanasia of dogs and cats. Vet. Rec 143(4):114. [DOI] [PubMed] [Google Scholar]
- 79.Mallery KF, Freeman LM, Harpster NK, Rush JE. 1999. Factors contributing to the decision for euthanasia of dogs with congestive heart failure. J. Am. Vet. Med. Assoc 214(8):1201–4 [PubMed] [Google Scholar]
- 80.Moore GE, Burkman KD, Carter MN, Peterson MR. 2001. Causes of death or reasons for euthanasia in military working dogs: 927 cases (1993-1996). J. Am. Vet. Med. Assoc 219(2):209–14 [DOI] [PubMed] [Google Scholar]
- 81.Bonnett BN, Egenvall A. 2010. Age patterns of disease and death in insured Swedish dogs, cats and horses. J. Comp. Pathol 142(Suppl. 1):S33–38 [DOI] [PubMed] [Google Scholar]
- 82.O’Neill DG, Elliott J, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. 2013. Chronic kidney disease in dogs in UK veterinary practices: prevalence, risk factors, and survival. J. Vet. Intern. Med 27(4):814–21 [DOI] [PubMed] [Google Scholar]
- 83.Anderson KL, O’Neill DG, Brodbelt DC, Church DB, Meeson RL, et al. 2018. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci. Rep 8(1):5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kearsley-Fleet L, O’Neill DG, Volk HA, Church DB, Brodbelt DC. 2013. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet. Rec 172(13):338. [DOI] [PubMed] [Google Scholar]
- 85. Hoffman JM, Creevy KE, Promislow DEL. 2013. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLOS ONE 8(4):e61082. Sterilization is associated with increased lifespan and with risk of certain categorical causes of death
- 86.Bonnett BN, Egenvall A, Hedhammar Å, Olson P. 2005. Mortality in over 350,000 insured Swedish dogs from 1995–2000: I. Breed-, gender-, age- and cause-specific rates. Acta Vet. Scand 46(3):105–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoffman JM, Creevy KE, Franks A, O’Neill DG, Promislow DEL. 2018. The companion dog as a model for human aging and mortality. Aging Cell 17(3):e12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. 2015. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J. Am. Med. Dir. Assoc 16(8):640–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. 2008. A physiologic index of comorbidity: relationship to mortality and disability. J. Gerontol. A 63(6):603–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Charlson M, Szatrowski TP, Peterson J, Gold J. 1994. Validation of a combined comorbidity index. J. Clin. Epidemiol 47(11):1245–51 [DOI] [PubMed] [Google Scholar]
- 91.Graham JE, Snih SA, Berges IM, Ray LA, Markides KS, Ottenbacher KJ. 2009. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology 55(6):644–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vermeulen J, Neyens JCL, van Rossum E, Spreeuwenberg MD, de Witte LP. 2011. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong CH, Weiss D, Sourial N, Karunananthan S, Quail JM, et al. 2010. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin. Exp. Res 22(1):54–62 [DOI] [PubMed] [Google Scholar]
- 94.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, et al. 2001. Frailty in older adults: evidence for a phenotype. J. Gerontol. A 56(3):M146–56 [DOI] [PubMed] [Google Scholar]
- 95.Liu B, Hu X, Zhang Q, Fan Y, Li J, et al. 2016. Usual walking speed and all-cause mortality risk in older people: a systematic review and meta-analysis. Gait Posture 44:172–77 [DOI] [PubMed] [Google Scholar]
- 96.Davies M 2012. Geriatric screening in first opinion practice—results from 45 dogs. J. Small Anim. Pract 53(9):507–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ. 2011. The canine cognitive dysfunction rating scale (CCDR): a data-driven and ecologically relevant assessment tool. Vet. J 188(3):331–36 [DOI] [PubMed] [Google Scholar]
- 98.Lund EM, Armstrong PJ, Kirk CA, Kolar LM, Klausner JS. 1999. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J. Am. Vet. Med. Assoc 214(9):1336–41 [PubMed] [Google Scholar]
- 99.Willems A, Paepe D, Marynissen S, Smets P, Van de Maele I, et al. 2017. Results of screening of apparently healthy senior and geriatric dogs. J. Vet. Intern. Med 31(1):81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laflamme D 1997. Development and validation of a body condition score system for dogs. Canine Pract. 22(4):10–15 [Google Scholar]
- 101.Stewart L, MacLean EL, Ivy D, Woods V, Cohen E, et al. 2015. Citizen science as a new tool in dog cognition research. PLOS ONE 10(9):e0135176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hesbach A 2003. A proposed canine movement performance test: the canine timed up and go test (CTUG). Orthoped. Phys. Ther. Pract 15:26 [Google Scholar]
- 103.Morgan EM, Heseltine JC, Levine GJ, Promislow DEL, Creevy KE. 2019. Evaluation of a low-technology system to obtain morphological and mobility trial measurements in dogs and investigation of potential predictors of canine mobility. Am. J. Vet. Res 80(7):670–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gonçalves LCVB, Simões ADGA, Millis DL, de Matos AJF. 2016. Development of a scale to evaluate mobility in dogs. Cienc. Rural 46(12):2210–15 [Google Scholar]
- 105.Reid J, Wiseman-Orr ML, Scott EM, Nolan AM. 2013. Development, validation and reliability of a web-based questionnaire to measure health-related quality of life in dogs. J. Small Anim. Pract 54(5):227–33 [DOI] [PubMed] [Google Scholar]
- 106.Bray EE, MacLean EL, Hare BA. 2014. Context specificity of inhibitory control in dogs. Anim. Cogn 17(1):15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.MacLean EL, Herrmann E, Suchindran S, Hare B. 2017. Individual differences in cooperative communicative skills are more similar between dogs and humans than chimpanzees. Anim. Behav 126:41–51 [Google Scholar]
- 108.Head E 2013. A canine model of human aging and Alzheimer’s disease. Biochim. Biophys. Acta 1832(9):1384–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Milgram NW, Head E, Weiner E, Thomas E. 1994. Cognitive functions and aging in the dog: acquisition of nonspatial visual tasks. Behav. Neurosci 108(1):57–68 [DOI] [PubMed] [Google Scholar]
- 110.Head E, Milgram NW, Cotman CW. 2001. Neurobiological models of aging in the dog and other vertebrate species. Funct. Neurobiol. Aging 2001:457–68 [Google Scholar]
- 111.Studzinski C, Christie L, Araujo J, Burnham W, Head E, et al. 2006. Visuospatial function in the beagle dog: an early marker of cognitive decline in a model of human aging and dementia. Neurobiol. Learn. Mem 86(2):197–204 [DOI] [PubMed] [Google Scholar]
- 112.Wisniewski HM, Wegiel J, Morys J, Bancher C, Soltysiak Z, Kim KS. 1990. Aged dogs: an animal model to study beta-protein amyloidogenesis. In Alzheimer’s Disease. Epidemiology, Neuropathology, Neurochemistry, and Clinics, ed. Maurer K, Riederer P, Beckmann H, pp. 151–68. Key Topics Brain Res. Vienna: Springer [Google Scholar]
- 113.Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. 2000. Region-specific age at onset of β-amyloid in dogs. 21(1):89–96 [DOI] [PubMed] [Google Scholar]
- 114.Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. 1996. β-Amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol. Learn. Mem 66(1):11–23 [DOI] [PubMed] [Google Scholar]
- 115.Urfer S, Darvas M, Keene D, Czeibert K, Kubinyi E, et al. 2020. Amyloid beta-42 levels in companion dog brains correlate with age and cognitive function. Innov. Aging 4(S1):887 [Google Scholar]
- 116.Dewey CW, Davies ES, Xie H, Wakshlag JJ. 2019. Canine cognitive dysfunction: pathophysiology, diagnosis, and treatment. Vet. Clin. N. Am. Small Anim. Pract 49(3):477–99 [DOI] [PubMed] [Google Scholar]
- 117.Chapagain D, Range F, Huber L, Virányi Z. 2018. Cognitive aging in dogs. Gerontology 64(2):165–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rofina JE, van Ederen AM, Toussaint MJM, Secrève M, van der Spek A, et al. 2006. Cognitive disturbances in old dogs suffering from the canine counterpart of Alzheimer’s disease. Brain Res. 1069(1):216–26 [DOI] [PubMed] [Google Scholar]
- 119.Madari A, Farbakova J, Katina S, Smolek T, Novak P, et al. 2015. Assessment of severity and progression of canine cognitive dysfunction syndrome using the CAnine DEmentia Scale (CADES). Appl. Anim. Behav. Sci 171:138–45 [Google Scholar]
- 120.Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ. 2010. Under diagnosis of canine cognitive dysfunction: a cross-sectional survey of older companion dogs. Vet. J 184(3):277–81 [DOI] [PubMed] [Google Scholar]
- 121.Wallis LJ, Virányi Z, Müller CA, Serisier S, Huber L, Range F. 2016. Aging effects on discrimination learning, logical reasoning and memory in pet dogs. Age 38(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Piotti P, Szabó D, Wallis L, Bognár Z, Stiegmann BS, et al. 2017. The effect of age on visuo-spatial short-term memory in family dogs. Pet Behav. Sci 4(4):17–19 [Google Scholar]
- 123.Szabó D, Gee NR, Miklósi A. 2016. Natural or pathologic? Discrepancies in the study of behavioral and cognitive signs in aging family dogs. J. Vet. Behav 11:86–98 [Google Scholar]
- 124.Wallis LJ, Range F, Müller CA, Serisier S, Huber L, Zsó V. 2014. Lifespan development of attentiveness in domestic dogs: drawing parallels with humans. Front. Psychol 5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Watowich MM, MacLean EL, Hare B, Call J, Kaminski J, et al. 2020. Age influences domestic dog cognitive performance independent of average breed lifespan. Anim. Cogn 23(4):795–805 Despite major differences in breed-specific lifespan, dog breeds share a common trajectory of cognitive decline.
- 126.Azkona G, García-Belenguer S, Chacón G, Rosado B, León M, Palacio J. 2009. Prevalence and risk factors of behavioural changes associated with age-related cognitive impairment in geriatric dogs. J. Small Anim. Pract 50(2):87–91 [DOI] [PubMed] [Google Scholar]
- 127.Cotman C, Head E, Muggenburg B, Zicker S, Milgram N. 2002. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol. Aging 23(5):809–18 [DOI] [PubMed] [Google Scholar]
- 128.Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, et al. 2005. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol. Aging 26(1):77–90 [DOI] [PubMed] [Google Scholar]
- 129.Pop V, Head E, Hill M-A, Gillen D, Berchtold NC, et al. 2010. Synergistic effects of long-term antioxidant diet and behavioral enrichment on β-amyloid load and non-amyloidogenic processing in aged canines. J. Neurosci 30(29):9831–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Head E, Murphey HL, Dowling ALS, McCarty KL, Bethel SR, et al. 2012. A combination cocktail improves spatial attention in a canine model of human aging and Alzheimer’s disease. J. Alzheimers Dis 32(4):1029–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pan Y, Larson B, Araujo JA, Lau W, de Rivera C, et al. 2010. Dietary supplementation with mediumchain TAG has long-lasting cognition-enhancing effects in aged dogs. Br. J. Nutr 103(12):1746–54 [DOI] [PubMed] [Google Scholar]
- 132.Chapagain D, Virányi Z, Huber L, Serra J, Schoesswender J, Range F. 2018. Effect of age and dietary intervention on discrimination learning in pet dogs. Front. Psychol 9:2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fahnestock M, Marchese M, Head E, Pop V, Michalski B, et al. 2010. BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol. Aging 33(3):546–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chapagain D, Virányi Z, Wallis LJ, Huber L, Serra J, Range F. 2017. Aging of attentiveness in border collies and other pet dog breeds: the protective benefits of lifelong training. Front. Aging Neurosci 9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bartlett PC, Van Buren JW, Neterer M, Zhou C. 2010. Disease surveillance and referral bias in the veterinary medical database. Prev. Vet. Med 94(3–4):264–71 [DOI] [PubMed] [Google Scholar]
- 136.Small Anim. Vet. Surveill. Netw. 2013. SAVSNET the next stage. BSAVA Companion 2013(2):4–5 [Google Scholar]
- 137.R. Vet. Coll. 2021. About VetCompass. https://www.rvc.ac.uk/vetcompass/about
- 138.Latter M 2016. VetCompass Australia—a leap forward for companion animals. Aust. Vet. J 94:N14 [Google Scholar]
- 139.O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. 2014. Approaches to canine health surveillance. Canine Genet. Epidemiol 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.R. Vet. Coll. 2021. Projects: research projects and opportunities, http://www.rvc.ac.uk/vetcompass/projects
- 141.Dobson JM, Samuel S, Milstein H, Rogers K, Wood JLN. 2002. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract 43(6):240–46 [DOI] [PubMed] [Google Scholar]
- 142.Wittig JC, Bickels J, Kellar-Graney KL, Kim FH, Malawer MM. 2002. Osteosarcoma of the proximal humerus: long-term results with limb-sparing surgery. Clin. Orthop. Relat. Res 2002:156–76 [DOI] [PubMed] [Google Scholar]
- 143.Mitchell KE, Boston SE, Kung M, Dry S, Straw RC, et al. 2016. Outcomes of limb-sparing surgery using two generations of metal endoprosthesis in 45 dogs with distal radial osteosarcoma. A Veterinary Society of Surgical Oncology retrospective study. Vet. Surg 45:36–43 [DOI] [PubMed] [Google Scholar]
- 144.Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, et al. 2005. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol. Assess 9:1–157 [DOI] [PubMed] [Google Scholar]
- 145.Muñana KR. 2013. Management of refractory epilepsy. Top. Companion Anim. Med 28:67–71 [DOI] [PubMed] [Google Scholar]
- 146.Chen HH, Redfield MM, Nordstrom LJ, Cataliotti A, Burnett JC Jr. 2003. Angiotensin II AT1 receptor antagonism prevents detrimental renal actions of acute diuretic therapy in human heart failure. Am. J. Physiol. Renal Physiol 284:F1115–9 [DOI] [PubMed] [Google Scholar]
- 147.Larouche-Lebel É, Loughran KA, Huh T, Oyama MA. 2020. Effect of angiotensin receptor blockers and angiotensin converting enzyme 2 on plasma equilibrium angiotensin peptide concentrations in dogs with heart disease. J. Vet. Intern. Med 35:22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stanziano DC, Whitehurst M, Graham P, Roos BA. 2010. A review of selected longitudinal studies on aging: past findings and future directions. J. Am. Geriatr. Soc 58(Suppl. 2):S292–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pearson H 2016. The Life Project: The Extraordinary Story of Our Ordinary Lives. London: Penguin Books Ltd. 416 pp. [Google Scholar]
- 150.Cooley DM, Beranek BC, Schlittler DL, Glickman NW, Glickman LT, Waters DJ. 2002. Endogenous gonadal hormone exposure and bone sarcoma risk. Cancer Epidemiol. Biomarkers Prev 11(11):1434–40 [PubMed] [Google Scholar]
- 151.Rhodes L, Ding VD, Kemp RK, Khan MS, Nakhla AM, et al. 2000. Estradiol causes a dose-dependent stimulation of prostate growth in castrated beagle dogs. Prostate 44(1):8–18 [DOI] [PubMed] [Google Scholar]
- 152.Sonnenschein EG, Glickman LT, Goldschmidt MH, McKee LJ. 1991. Body conformation, diet, and risk of breast cancer in pet dogs: a case-control study. Am. J. Epidemiol 133(7):694–703 [DOI] [PubMed] [Google Scholar]
- 153.Ru G, Terracini B, Glickman LT. 1998. Host related risk factors for canine osteosarcoma. Vet. J 156(1):31–39 [DOI] [PubMed] [Google Scholar]
- 154.Bryan JN, Keeler MR, Henry CJ, Bryan ME, Hahn AW, Caldwell CW. 2007. A population study of neutering status as a risk factor for canine prostate cancer. Prostate 67(11):1174–81 [DOI] [PubMed] [Google Scholar]
- 155.Hart BL, Hart LA, Thigpen AP, Willits NH. 2014. Long-term health effects of neutering dogs: comparison of Labrador Retrievers with Golden Retrievers. PLOS ONE 9(7):e102241. [DOI] [PMC free article] [PubMed] [Google Scholar]