Abstract
Background
F-18 fluorodeoxyglucose positron emission tomography computed tomography (PET/CT) is used to assess response of non-Hodgkin lymphoma (NHL) to chimeric antigen receptor T cell (CAR-T) therapy. We sought to describe metabolic and volumetric PET prognostic factors at one month post-CAR-T and identify which patients with partial response (PR) or stable disease (SD) are most likely to subsequently achieve complete response (CR), and which will develop progressive disease (PD) and death.
Methods
Sixty-nine patients with NHL received axicabtagene ciloleucel CAR-T therapy. One-month post-CAR-T infusion and PET/CT scans were segmented with a fixed absolute SUV maximum (SUVMax) threshold of 2.5 using a semiautomated workflow with manual modification to exclude physiologic uptake as needed. Metabolic tumor volume (MTV), total lesion glycolysis (TLG), SUVMax, and other lesion characteristics were calculated and associated with risk of PD and death.
Results
Patients with total MTV > 180 cc, presence of bone or parenchymal disease, SUVMax > 10, single lesion TLG > 245 g, or > 2 total lesions had increased risk of death. Patients with total MTV > 55 cc, total TLG > 250 cc, SUV Max > 10, or > 2 total lesions had increased risk of PD. For the subset of 28 patients with PR/SD, higher SUVMax was associated with increased risk of subsequent PD and death. While 86% of patients who had SUVMax ≥ 10 eventually had PD (HR 3.63, 1.13–11.66, p = 0.03), only 36% of those with SUVMax < 10 had PD.
Conclusions
Higher SUVMax at one month post-CAR-T is associated with higher risk of PD and death. SUVMax ≥ 10 may be useful in guiding early salvage treatment decisions in patients with SD/PR at one month.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01256-w.
Keywords: CAR-T, PET/CT, Non-Hodgkin lymphoma
Chimeric antigen receptor T cell (CAR-T) therapy provides an opportunity for long-term remission in patients with aggressive relapsed or refractory B cell non-Hodgkin lymphoma (NHL) [1–4]. However, the rate of disease progression after CAR-T therapy is still significant, and advancements in risk stratification are needed to further tailor therapy and monitoring.
In clinical practice, F-18 fluorodeoxyglucose (FDG) positron emission tomography computed tomography (PET/CT) is routinely obtained at baseline and then serially for response assessment and surveillance. Despite advancements in PET/CT imaging and analysis, current standards divide treatment response into only four categories: complete response (CR), partial response (PR), stable disease (SD), or relapsed/progressive disease (PD) [5]. Patients who have a CR to therapy have excellent prognosis with a fraction potentially cured, while those with PR or SD have a more mixed prognosis, with approximately half of patients eventually achieving CR [1, 2]. However, it is unknown which of these patients with PR/SD are more likely to convert to CR and can be safely monitored for continued response, and which should be considered for immediate salvage therapy to prevent PD that would limit further treatment options.
Metabolic PET/CT characteristics including metabolic tumor volume (MTV) and total lesion glycolysis (TLG) represent opportunities to provide more accurate prognosis and guide therapy, particularly for patients with SD or PR who have uncertain prognoses [6–9]. We sought to analyze metabolic and volumetric PET/CT prognostic factors at one month post-CAR-T and identify which patients with PR/SD are more likely to experience subsequent PD and death (complete methods in supplement).
Sixty-eight patients with NHL were treated with axi-cel CAR-T therapy between January 2018 and July 2020 (Additional file 1: Table S1). Of these, 27 (39%) achieved CR by Lugano criteria, 24 (35%) achieved PR, 4 (6%) had SD, and 13 (19%) experienced PD. With a median follow-up of 13.3 months (interquartile range 4.7–18.0 months), the OS at 6, 12, and 18 months was 75%, 65%, and 42%, respectively. EFS at 6, 12, and 18 months was 43%, 39%, and 37%, respectively. At last follow-up, 46 patients (67%) had experienced PD and 30 (43%) had died.
When analyzing one-month post-CAR-T infusion PET/CT for all 69 patients regardless of Lugano classification, multiple characteristics representing increased disease burden (MTV) and bulk (TLG of the largest lesion) were associated with increased risk of death and PD (Additional file 1: Tables S2 and S3).
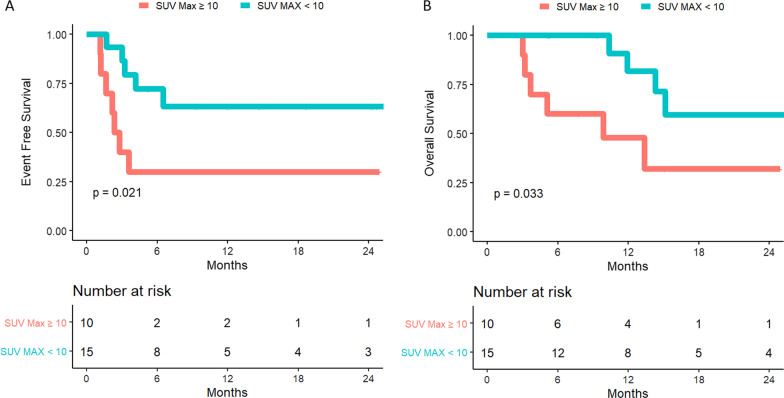
Twenty-eight patients (41%) had either PR or SD at one-month post-CAR-T infusion and were analyzed separately for predictors of PD and death. Of these, 12 (43%) had experienced progression at last follow-up. SUVMax was significantly associated with risk of PD as a continuous variable and when using a cutoff point of 10 (HR 3.63, 95% CI 1.13–11.66, p = 0.03) (Fig. 1A). While 86% of patients with PR/SD who had SUVMax > 10 at one month post-CAR-T eventually had PD, only 36% of those with SUVMax ≤ 10 had PD (Fig. 1A, B). No other PET characteristic was significantly associated with risk of subsequent PD in patients with PR/SD one-month post-CAR-T infusion. For patients with PR/SD at one month, those with SUVMax ≥ 10 had high subsequent PD rates and should be monitored closely and considered for early salvage.
Fig. 1.
Risk of progression (A) and death (B) stratified by SUVMax > 10 for patients with PR/SD at one month post-CAR-T. Patients with SUVMax > 10 at one-month post-CAR-T infusion had higher risk of death and progression than patients with SUVMax < 10, as demonstrated by the Kaplan–Meier curves above
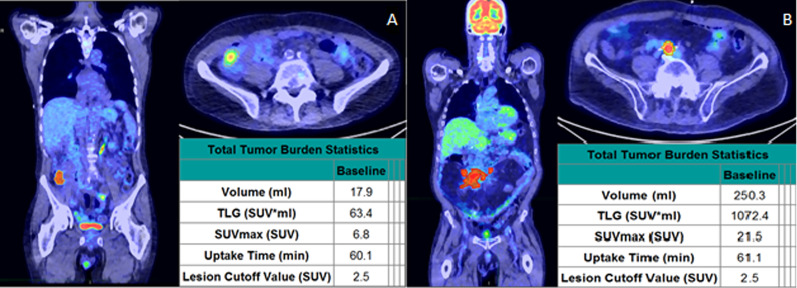
Currently, the Lugano criteria are still used for PET/CT assessment at one-month post-CAR-T infusion, dividing patients into only four broad categories, with significant clinical and prognostic heterogeneity between patients in the same category (Fig. 2A, B) [10]. Extracting and utilizing metabolic and volumetric data present in routinely obtained PET/CT scans may provide better prognostic discrimination and guidance of future treatments [7, 11, 12]. While several metabolic characteristics were analyzed, this study identified SUVMax ≥ 10 as a simple and effective prognostic predictor for patients with PR/SD at one-month post-CAR-T infusion. Using SUVMax > 10 to guide management one month after CAR-T infusion is a simple step beyond the Lugano system that may improve management decisions for this patient population. This is consistent with the recently published work from MD Anderson; combined, these two single institution studies support SUVMax > 10 as a useful biomarker one-month post-CAR-T infusion [6].
Fig. 2.
One-month post-CAR-T PET imaging and analysis. Two patients both assessed as having partial response one-month post-CAR-T therapy. The patient on the left pane (A SUVMax < 10) subsequently converted to complete response and is without progression at last follow-up, while the patient on the right (B SUVMax > 10) had subsequent progression and death
This analysis is limited by its retrospective nature, size, and the lack of widespread assessment of MTV and TLG in clinical practice. Fortunately, SUVMax ≥ 10 was identified as the key metric, which is readily evaluable on all PET/CT scans. Prospective validation of these findings is needed.
Supplementary Information
Additional file 1. Supplemental Tables. Supplemental Table 1: Patient and Treatment Characteristics. Supplemental Table 2: Risk of Progressive Disease According to One Month Post-CAR-T Infusion PET/CT Characteristics for All Patients. Supplemental Table 3: One-Month Post-CAR-T Infusion PET/CT Characteristics and Risk of Death. Supplemental Table 4: Association between PET/CT Characteristics and Death in Patients with PR or SD One-Month after CAR-T.
Authors' contributions
WB, SL, and YL designed the research. PJ, SA, UW, JCVB, JP, YL, and NB managed the clinical workflow reported. WB and JRY performed the imaging analysis. RB curated the clinical dataset. MH analyzed the data. WB wrote the paper. All authors aided in writing and revising the paper and guiding the analyses. All authors read and approved the final manuscript.
Funding
JP received research funding from Karyopharm unrelated to this work. YW received research funding from Incyte, InnoCare, LOXO Oncology, Novartis, Genentech, and MorphoSys, and is on advisory committees for Eli Lilly and TG Therapeutics, all unrelated this work. SMA received research funding from Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium, and Takeda, all unrelated to this work. YL provided consultancy and received grant support from Kite/Gilead, Celgene/BMS, Bluebird Bio, Janssen, Legend BioTech, and Takeda. YL also provided consultancy for Juno/BMS, Gamida Cells, Iovance, Fosun Kite, and Pfizer. YL also received grant support from Merck and Boston Scientific. YL also provided data review for Sorrento and Pfizer, and served on the scientific advisory committee for NexImmune. All funds were paid to the institution with no personal compensation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study and waiver of consent were approved by the Mayo Clinic Institutional Review Board (ID: 20-006748).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Scott C. Lester and Yi Lin are co-senior authors
References
- 1.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 3.Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490–3496. doi: 10.1200/JCO.2013.53.9593. [DOI] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 6.Al Zaki A, Feng L, Watson G, et al. Day 30 SUVmax predicts progression in lymphoma patients achieving PR/SD after CAR T-cell therapy. Blood Adv. 2022 doi: 10.1182/bloodadvances.2021006715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268–3276. doi: 10.1182/bloodadvances.2020001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malek E, Sendilnathan A, Yellu M, Petersen A, Fernandez-Ulloa M, Driscoll JJ. Metabolic tumor volume on interim PET is a better predictor of outcome in diffuse large B-cell lymphoma than semiquantitative methods. Blood Cancer J. 2015;5:e326. doi: 10.1038/bcj.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song MK, Yang DH, Lee GW, et al. High total metabolic tumor volume in PET/CT predicts worse prognosis in diffuse large B cell lymphoma patients with bone marrow involvement in rituximab era. Leuk Res. 2016;42:1–6. doi: 10.1016/j.leukres.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NN, Nagle SJ, Torigian DA, et al. Early positron emission tomography/computed tomography as a predictor of response after CTL019 chimeric antigen receptor -T-cell therapy in B-cell non-Hodgkin lymphomas. Cytotherapy. 2018;20(12):1415–1418. doi: 10.1016/j.jcyt.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Hu Y, Yang S, et al. Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25(6):1092–1098. doi: 10.1016/j.bbmt.2019.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental Tables. Supplemental Table 1: Patient and Treatment Characteristics. Supplemental Table 2: Risk of Progressive Disease According to One Month Post-CAR-T Infusion PET/CT Characteristics for All Patients. Supplemental Table 3: One-Month Post-CAR-T Infusion PET/CT Characteristics and Risk of Death. Supplemental Table 4: Association between PET/CT Characteristics and Death in Patients with PR or SD One-Month after CAR-T.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.