Abstract
Gastrointestinal (GI) problems are common in individuals with eating disorders (EDs) and associated with distress, impairment, and increased healthcare utilization. GI symptoms may be exacerbated by meals and other interventions central to ED recovery thereby contributing to negative clinical outcomes. Informed by models emphasizing the role of the brain-gut axis in the expression of GI symptoms, this article describes a program of research to adapt “brain-gut psychotherapies” for EDs. First, the role of the brain-gut axis in GI symptoms is described, and evidence-based brain-gut psychotherapies are reviewed, with an emphasis on cognitive behavioral therapy for GI disorders and gut-directed hypnotherapy. Next, future directions for research in EDs to (a) understand the impact of GI symptoms on illness course and outcome; (b) clarify target engagement; (c) evaluate brain-gut psychotherapies; and (d) optimize intervention reach and delivery are described. We present a conceptual model that emphasizes GI-specific anxiety and altered gut physiology as targets of brain-gut psychotherapies in EDs, and discuss several issues that need to be addressed in designing clinical trials to test these interventions. We also describe how engagement with multidisciplinary stakeholders and use of digital tools could speed translation from the laboratory to clinical settings.
Keywords: brain-gut psychotherapy, cognitive behavior therapy, digital intervention, disorder of gut-brain interaction, eating disorder, functional gastrointestinal disorder, GI-specific anxiety, gut-directed hypnotherapy
1 |. INTRODUCTION
Gastrointestinal (GI) problems are common in both “shape/weight-motivated eating disorders” (EDs; i.e., anorexia nervosa, bulimia nervosa, binge-eating disorder, other specified feeding or eating disorder; Murray et al., 2020, p. 2) and avoidant restrictive food intake disorder (ARFID) and are associated with distress, impairment, and increased healthcare utilization (Dooley-Hash, Lipson, Walton, & Cunningham, 2013; Riedlinger et al., 2020). Among the most frequent GI complaints in EDs are symptoms consistent with disorders of gut-brain interaction (DGBI; e.g., functional dyspepsia, irritable bowel syndrome [IBS]; Murray et al., 2020). DGBI (previously termed functional gastrointestinal disorders) are chronic GI disorders characterized by altered brain-gut interaction in the absence of detectable pathology or structural abnormalities explaining the symptoms (Black, Drossman, Talley, Ruddy, & Ford, 2020). Separating DGBI and EDs is challenging, as their somatic features overlap, and ED behaviors can have an adverse impact on GI function (Riedlinger et al., 2020), likely contributing to altered brain-gut interaction. However, GI symptoms also can precede ED symptoms and have been hypothesized to influence their development (Zucker & Bulik, 2020).
Regardless of origin, GI symptoms pose a significant challenge in ED treatment (Riedlinger et al., 2020). Common ED-related GI problems, like constipation, nausea, and abdominal fullness, can making eating uncomfortable physically, exacerbate fears of fatness and weight/shape concerns, and contribute to food avoidance and aversions. Consequently, patients may be less willing to engage in standard behavioral interventions central to the management of EDs (e.g., regular eating, food exposures). Moreover, although many patients are told that GI problems will resolve following restoration of a healthy body weight or discontinuation of ED behaviors, longitudinal data indicate that GI concerns persist after improvement in ED symptoms (Boyd, Abraham, & Kellow, 2010; Mack et al., 2016; Zucker & Bulik, 2020), perhaps contributing to relapse.
Novel approaches to the management of GI symptoms in patients with EDs are needed. To this end, we propose a program of research to investigate and adapt evidence-based psychotherapeutic interventions that target brain-gut interaction—that is, brain-gut psychotherapies (Palsson & Ballou, 2020)—for EDs. We focus on brain-gut psychotherapies rather than other evidence-based approaches for GI disorders (e.g., medications, dietary interventions) because: (a) psychotherapy is the first-line treatment for EDs in outpatient settings; (b) medication acceptance is low in some ED diagnostic groups (Halmi, 2008); and (c) certain dietary changes recommended in GI disorder management are contraindicated for patients with EDs (e.g., elimination diets). Nevertheless, optimal treatment of GI disorders involves a multidisciplinary approach that typically includes gastroenterologists addressing symptoms from within the GI tract (e.g., antispasmodics, fiber, or laxatives) or prescribing neuro-modulating medications aimed at regulating GI function (e.g., tricyclic antidepressants), dietitians assisting with dietary modification and eating behaviors, and other allied health professionals such as pelvic floor physical therapists to address musculoskeletal abnormalities, in addition to experts in psychogastroenterology. Thus, investigation of brain-gut psychotherapies for EDs will require a multidisciplinary approach.
2 |. PSYCHOTHERAPIES THAT TARGET BRAIN-GUT INTERACTION
Key to understanding the role of psychological factors in GI symptoms is the brain-gut axis—a complex, bidirectional communication pathway involving neural, immune, and endocrine systems. This pathway allows the GI tract (“the gut”) to send the central nervous system, including the cognitive and emotional centers of the brain, ongoing information about its activity. The brain receives these signals and sends information back to the gut, which chooses either to maintain homeostasis or modulate gut function based on shifting demands. During increased psychological stress, illness, or injury, the brain raises GI sensations to conscious awareness and alters GI function, resulting in GI symptoms. If this perceived threat is extreme or ongoing, brain-gut communication may become altered more permanently, a phenomenon known as brain-gut dysregulation. Brain-gut psychotherapies offer a logical pathway to address brain-gut dysregulation in a range of GI disorders by targeting and modifying cognitive-affective factors that drive GI symptoms.
2.1 |. Evidence-based brain-gut psychotherapies
Evidence-based brain-gut psychotherapies include relaxation training, mindfulness training, psychodynamic therapy, cognitive behavioral therapy for GI disorders (GI-CBT) and gut-directed hypnotherapy (GDH). Of these, GI-CBT and GDH are the most commonly used and empirically supported (Palsson & Ballou, 2020). Table 1 provides an overview of common interventions used in brain-gut psychotherapies, with specific examples. Many of the interventions overlap with strategies used in psychotherapies for EDs (e.g., self-monitoring, relaxation training, cognitive restructuring, behavioral exposures); however, the conceptualization of GI symptoms as resulting from dysregulation of the brain-gut axis offers a method of engaging patients in these treatments that is not employed systematically in the ED field. For instance, whereas CBT for EDs might address a GI symptom such as abdominal bloating by challenging associated thoughts about eating, shape, or weight, GI-CBT aims to reduce the abdominal bloating itself through modulation of the stress response, using cognitive strategies to challenge GI-specific worries (e.g., something must be wrong for my belly to feel like this) or through instruction of behavioral strategies such as diaphragmatic breathing. Additionally, GDH involves components not typically used in ED treatment including hypnotic induction and provision of GI-symptom focused suggestions.
TABLE 1.
Common interventions used in brain-gut psychotherapies
| Associated with: | ||||
|---|---|---|---|---|
| Many brain-gut psychotherapies | GI-CBT | GDH | Description and examples | |
| Psychoeducation | ✕ | Provide information about the relationship between the brain and the gut Examples: Description of the brain-gut axis, role of stress in GI functioning |
||
| Relaxation training | ✕ | Muscle relaxation and modification of autonomic arousal Examples: Diaphragmatic breathing, passive or progressive muscle relaxation |
||
| Self-monitoring | ✕ | Identify and monitor GI symptom triggers and responses Examples: Foods, substances, activities, environments, emotional or physical states or the GI symptoms themselves, which precipitate responses and outcomes |
||
| Therapeutic suggestions | ✕ | Use of therapeutic suggestion related to symptom reduction is a key feature and proposed mechanism of GDH. Typical components of a GDH session include hypnotic induction (i.e., narrowing of attention), physical relaxation, and use of metaphors and imagery. Facilitation of hypnotic trance, a state of deep relaxation and focused attention, is thought to increase receptivity and openness to changes in symptoms. Example: “Over time, you will notice less and less discomfort in your gut until only comfortable sensations remain.” |
||
| Cognitive restructuring | ✕ | Challenge common cognitive errors (e.g., symptom catastrophizing, negative future prediction) to address GI-specific anxiety Example: “My abdominal pain is a sign that something really serious is wrong, like having cancer” → “this pain is a sign of my sensitive GI tract. It is unpleasant, but it does not mean that I am unsafe” |
||
| Flexible coping | ✕ | Emphasizes the use of emotion-focused (passive) coping strategies for uncontrollable stressors and problem-focused (active) coping strategies for controllable stressors Examples: Often, decreasing problem-focused coping strategies such as internet searching, avoiding foods/activities, and increasing emotion-focused coping strategies such as practicing acceptance, relaxation, engagement in pleasant activities that turn attention away from symptoms |
||
| Behavioral exposures | ✕ | Engage in feared behaviors to challenge negative thoughts and beliefs and reduce GI-specific anxiety Examples: Eating foods that have been associated with symptoms in the past, going for a long walk without access to a toilet |
||
Abbreviations: GDH, gut-directed hypnotherapy; GI-CBT, cognitive behavioral therapy for gastrointestinal disorders.
3 |. DIRECTIONS FOR FUTURE RESEARCH ON BRAIN-GUT PSYCHOTHERAPIES FOR EDS
3.1 |. Understanding the impact of GI symptoms on ED course and outcome
Although clinical experience suggests that GI symptoms interfere with ED treatment, no studies have examined the impact of GI problems on illness course or outcome. This is an important step in demonstrating the need for systematic integration of brain-gut psychotherapies and other GI interventions into ED treatment. Longitudinal designs could be used to gather quantitative data regarding the effects of GI symptoms on treatment-related outcomes (e.g., dropout, ED symptom remission) and course of illness. Additionally, qualitative methods would be helpful in understanding patients’ perspectives about the impact of GI symptoms on ED recovery and the utility—or lack thereof—of current approaches to addressing GI complaints in EDs.
3.2 |. Clarifying target engagement
Adapting brain-gut psychotherapies for EDs requires elucidating the mechanisms by which GI symptoms perpetuate disordered eating (and vice versa). This approach aligns with the emphasis by the U.S. National Institute of Mental Health and other funding agencies on targeting disease mechanisms in clinical trials (Insel, 2015). Several candidate mechanisms of brain-gut dysregulation are worthy of investigation including visceral hypersensitivity (abnormally low threshold for perceiving and interpreting visceral sensations as painful or uncomfortable; Drossman, 2016), visceral interoception (“the perception and integration in the brain of afferent signals pertaining to the homeostatic state of the body”; Kerr et al., 2016, p. 521), and GI-specific anxiety (“the cognitive, affective, and behavioral response stemming from fear of GI sensations, symptoms, and the context in which these visceral sensations and symptoms occur”; Labus, Mayer, Chang, Bolus, & Naliboff, 2007, p. 89).
Figure 1 presents a theoretical model adapted from Drossman (2016) that emphasizes GI-specific anxiety and altered gut physiology as mechanisms underlying the association between EDs and GI symptoms. This transdiagnostic model of symptom maintenance extends ideas presented by Zucker and Bulik (2020) regarding the role of GI discomfort in the development of anorexia nervosa. Though research supports some of the model’s tenets (e.g., associations between GI symptoms and healthcare utilization), future studies are needed to test whether ED symptoms predict GI-specific anxiety and/or altered gut physiology (and vice versa), and whether these mechanisms mediate associations between EDs and GI problems. Longitudinal designs and experimental studies in which the proposed mechanisms are manipulated in patients with EDs would be useful in this regard. Particular attention should be paid to mechanisms that can be modified by psychotherapeutic interventions (e.g., GI-specific anxiety; Hesser, Hedman-Lagerlöf, Andersson, Lindfors, & Ljótsson, 2018). Research also is needed to enhance the measurement of GI-related treatment targets. For example, GI-specific anxiety currently is measured using a self-report questionnaire, the Visceral Sensitivity Index (Labus et al., 2004), and there is a need for the development of more objective indices. Such work lends itself well to an approach informed by the Research Domain Criteria framework (Cuthbert & Insel, 2013), in which GI-specific anxiety might be studied across multiple levels of analysis (e.g., circuits, physiology, behavior, self-report) using validated measures of potential threat (“anxiety”) adapted for GI-related stimuli.
FIGURE 1.
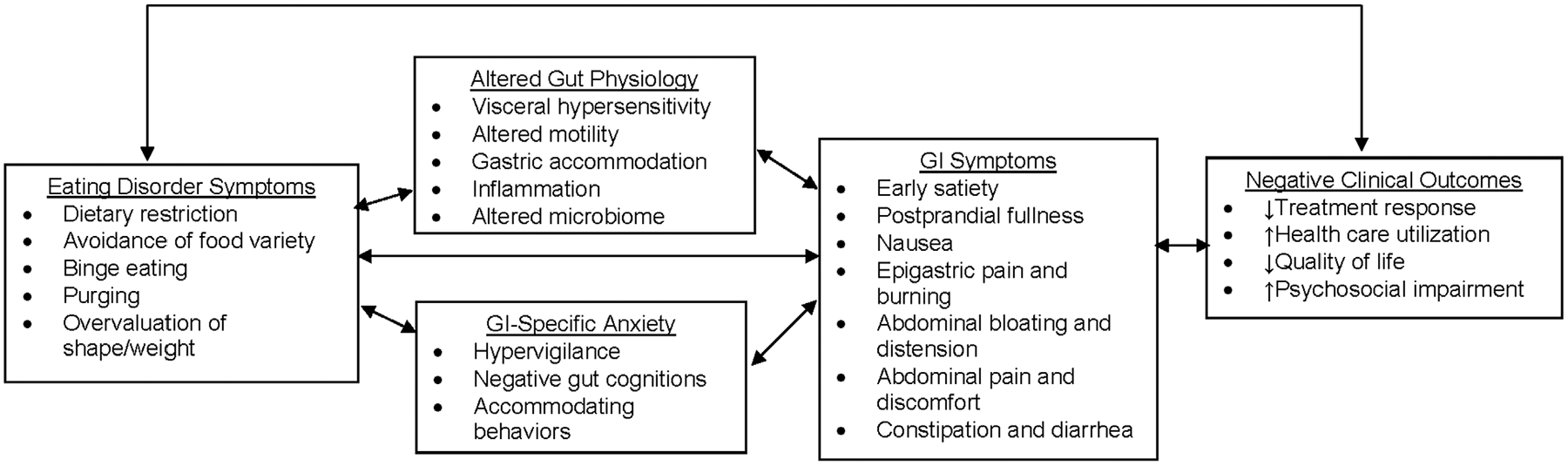
Theoretical model of mechanisms underlying associations between eating disorder symptoms and gastrointestinal problems
3.3 |. Evaluating brain-gut psychotherapies in patients with EDs
Brain-gut psychotherapies are not designed to address ED symptoms directly; consequently, these interventions likely will be of greatest use as adjuncts to existing treatments. In outpatient settings, studies might test whether adding brain-gut psychotherapy components to CBT or family based treatment results in improved retention and higher rates of symptom remission relative to standard versions of these interventions. Alternatively, brain-gut psychotherapies could be tested as strategies to enhance outcomes in higher levels of care, with an emphasis on supporting transition to less restrictive treatment settings and preventing readmission.
Future studies also should focus on identifying the patients for whom brain-gut psychotherapies are needed most. GI complaints are prevalent across the ED spectrum (Hetterich, Mack, Giel, Zipfel, & Stengel, 2019; Murray et al., 2020), suggesting that a transdiagnostic approach to treatment-development might be indicated. Nevertheless, some ED interventions (e.g., weight restoration, exposure to avoided foods) may be more likely to exacerbate, or be affected by, GI problems than others (e.g., abstinence from binge-eating), suggesting the need for a more nuanced approach to matching patients with EDs to brain-gut psychotherapies. Additionally, although some patients may find it validating to address their GI symptoms directly, acceptability of and engagement with brain-gut psychotherapies among individuals with EDs is yet to be determined.
Finally, research is needed to determine the optimal type of brain-gut psychotherapy to deliver in patients with EDs. GI-CBT and GDH are good places to start because they have the strongest evidence-base (Palsson & Ballou, 2020); however, their intervention components (Table 1) and treatment targets vary. GI-CBT would be especially useful for targeting GI-specific anxiety, as among individuals with IBS, changes in GI-specific anxiety mediate the effects of GI-CBT on improvements in IBS symptom severity (Hesser et al., 2018). Alternatively, one study found that IBS patients treated with GDH exhibited decreased neural activation during rectal distension (an index of visceral hypersensitivity; Lowén et al., 2013). Thus, if visceral hypersensitivity is found to underlie GI symptoms in patients with EDs, GDH could be an important focus of treatment-development efforts.
3.4 |. Optimizing intervention reach and delivery
Adapting brain-gut psychotherapies for EDs warrants studying the best settings and modalities through which to deliver these interventions. In general, reach of ED treatments is poor, and access to providers with expertise in EDs and psychogastroenterology is limited to a few academic medical centers. Moreover, because components of ED treatment that occur outside of therapy sessions or after discharge from higher levels of care may exacerbate GI symptoms (e.g., bloating in response to larger meals/snacks), intervention approaches are needed that extend treatment into daily life to support ongoing skill practice and maximize the potency of face-to-face therapy sessions.
Digital interventions offer an exciting modality through which to extend the reach of brain-gut psychotherapies for EDs and support skills practice between sessions and beyond. Digital adaptations of GI-CBT have been studied in patients with IBS (Palsson & Ballou, 2020), but have not been applied or adapted to patients with EDs. Further, the design of brain-gut psychotherapies for EDs would be strengthened through enhanced collaboration between the GI and ED fields to inform different settings and approaches through which these interventions could be delivered. Engaging all relevant stakeholders (e.g., gastroenterologists, dietitians, primary care physicians, patients, caregivers) in the design of psychological interventions has potential to increase their effectiveness when implemented in real-world settings (Lyon & Koerner, 2016). Studying ways to optimize reach and scale from the start of intervention development, rather than waiting until after initial intervention development and testing has concluded, will help to speed the translation of the intervention from laboratory-based development to widespread clinical delivery.
4 |. CONCLUSIONS
Brain-gut psychotherapies are promising tools to enhance engagement in and the efficacy of ED treatments. By targeting psychosocial influences on gut function, brain-gut psychotherapies offer a novel approach to ameliorating GI symptoms, which are common in patients with EDs and complicate treatment. We encourage future research to adapt brain-gut psychotherapies for EDs, with an emphasis on target engagement, multidisciplinary collaboration, and the use of digital tools to speed translation.
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: K01DK116925; National Institute of Mental Health, Grant/Award Number: T32MH082671
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed.
REFERENCES
- Black CJ, Drossman DA, Talley NJ, Ruddy J, & Ford AC (2020). Functional gastrointestinal disorders: Advances in understanding and management. Lancet, 396(10263), 1664–1674. 10.1016/S0140-6736(20)32115-2 [DOI] [PubMed] [Google Scholar]
- Boyd C, Abraham S, & Kellow J (2010). Appearance and disappearance of functional gastrointestinal disorders in patients with eating disorders. Neurogastroenterology & Motility, 22, 1279–1283. 10.1111//j.1365.2982.2010.01576.x [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine, 11, 126. 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley-Hash S, Lipson SK, Walton MA, & Cunningham RM (2013). Increased emergency department use by adolescents and young adults with eating disorders. International Journal of Eating Disorders, 46(4), 308–315. 10.1002/eat.22070 [DOI] [PubMed] [Google Scholar]
- Drossman DA (2016). Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology, 150 (6), 1262–1279. 10.1053/j.gastro.2016.02.032 [DOI] [PubMed] [Google Scholar]
- Halmi KA (2008). The perplexities of conducting randomized, double-blind, placebo- controlled treatment trials in anorexia nervosa patients. The American Journal of Psychiatry, 165(10), 1227–1228. 10.1176/appi.ajp.2008.08060957 [DOI] [PubMed] [Google Scholar]
- Hesser H, Hedman-Lagerlöf E, Andersson E, Lindfors P, & Ljótsson B (2018). How does exposure therapy work? A comparison between generic and gastrointestinal anxiety-specific mediators in a dismantling study of exposure therapy for irritable bowel syndrome. Journal of Consulting and Clinical Psychology, 86(3), 254–267. 10.1037/ccp0000273 [DOI] [PubMed] [Google Scholar]
- Hetterich L, Mack I, Giel KE, Zipfel S, & Stengel A (2019). An update on gastrointestinal disturbances in eating disorders. Molecular and Cellular Endocrinology, 497, 110318. 10.1016/j.mce.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Insel TR (2015). The NIMH experimental medicine initiative. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 14 (2), 151–153. 10.1002/wps.20227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, & Simmons WK (2016). Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(2), 521–528. 10.1038/npp.2015.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Chang L, Bolus R, & Naliboff BD (2007). The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: Further validation of the visceral sensitivity index. Psychosomatic Medicine, 69(1), 89–98. 10.1097/PSY.0b013e31802e2f24 [DOI] [PubMed] [Google Scholar]
- Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, & Naliboff BD (2004). The visceral sensitivity index: Development and validation of a gastrointestinal symptom-specific anxiety scale. Alimentary Pharmacology & Therapeutics, 20(1), 89–97. 10.1111/j.1365-2036.2004.02007.x [DOI] [PubMed] [Google Scholar]
- Lowén MB, Mayer EA, Sjöberg M, Tillisch K, Naliboff B, Labus J, …Walter SA (2013). Effect of hypnotherapy and educational intervention on brain response to visceral stimulus in the irritable bowel syndrome. Alimentary Pharmacology & Therapeutics, 37(12), 1184–1197. 10.1111/apt.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, & Koerner K (2016). User-centered design for psychosocial intervention development and implementation. Clinical Psychology: A Publication of the Division of Clinical Psychology of the American Psychological Association, 23(2), 180–200. 10.1111/cpsp.12154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack I, Cuntz U, Gramer C, Niedermaier S, Pohl C, Schwiertz A, …Penders J (2016). Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Scientific Reports, 6, 2672. 10.1038/srep26752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HB, Kuo B, Eddy KT, Breithaupt L, Becker KR, Dreier MJ, … Staller K (2020). Disorders of gut-brain interaction common among outpatients with eating disorders including avoidant/restrictive food intake disorder. International Journal of Eating Disorders. 10.1002/eat.23414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson OS, & Ballou S (2020). Hypnosis and cognitive behavioral therapies for the management of gastrointestinal disorders. Current Gastroenterology Reports, 22(7), 31. 10.1007/s11894-020-00769-z [DOI] [PubMed] [Google Scholar]
- Riedlinger C, Schmidt G, Weiland A, Stengel A, Giel KE, Zipfel S, …Mack I (2020). Which symptoms, complaints and complications of the gastrointestinal tract occur in patients with eating disorders? A systematic review and quantitative analysis. Frontiers in Psychiatry, 11, 195. 10.3389/fpsyt.2020.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker NL, & Bulik CM (2020). On bells, saliva, and abdominal pain or discomfort: Early aversive visceral conditioning and vulnerability for anorexia nervosa. International Journal of Eating Disorders, 53(4), 508–512. 10.1002/eat.23255 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed.


