Abstract
Serotonin 4 (5-HT4) receptor signaling does not only have the physiological function of improving cognition, but might also be helpful in the therapy of Alzheimer’s disease (AD) through regulation of the production of soluble amyloid-β protein precursor alpha (sAβPPα). To analyze the relationship between 5-HT4 receptor signaling and sAβPPα production, we stably transfected H4 cells with AβPP and 5-HT4 receptor (H4/AβPP/5-HT4 cells). We found that 24-h incubation with the 5-HT4 receptor agonist RS-67333 upregulates matrix metalloproteinase-9 (MMP-9). Furthermore, MMP-9 overexpression enhanced sAβPPα levels, whereas knockdown with MMP-9 siRNA decreased sAβPPα levels. When RS-67333 was injected for 10 days in Tg2576 mice, a model of amyloid-β peptide (Aβ) deposition, there was an increase in hippocampal levels of sAβPPα, C-terminal fragment α, and MMP-9, as well as a decrease in hippocampal senile plaque number and levels of the 40 amino acid peptide, Aβ40. Taken all together, these experiments demonstrate that 5-HT4 receptor stimulation induces expression of MMP-9 which cleaves AβPP through α-secretase-like activity, leading to an increase of sAβPPα levels and a reduction of Aβ load.
Keywords: α-secretase, amyloid-β protein precursor, matrix metalloproteinase 9, serotonin 4 receptor
INTRODUCTION
The serotonin 4 (5-HT4) G-protein coupled receptor belongs to a family of proteins consisting of at least thirteen G-protein coupled receptors and a ligandgated ion channel [1]. The 5-HT4 receptor has been shown to be involved in cognition and depression [2-4]. Recently, it has been suggested that the 5-HT4 receptor signaling might help cure Alzheimer’s disease (AD). Injection of receptor agonists not only increases hippocampal acetylcholine level in a dose-dependent manner [5], but also improves cognitive function in rodents [6]. Moreover, the receptor agonist RS-67333 affects the cleavage of the amyloid-β protein precursor (AβPP) with increase of soluble AβPPα (sAβPPα) and reduction of amyloid-β peptide (Aβ) in AβPP-overexpressing cells [7]. All these data point at the potential therapeutic relevance of understanding the mechanisms by which 5-HT4 receptors regulate AβPP processing. However, the chain of molecular events upregulating α-secretase has not yet been identified.
Cleavage of AβPP at the Lys687-Leu688 site through α-secretase leads to production of sAβPPα. Cleavage by β- and γ-secretases, in turn, leads to production of Aβ [8]. Because α- and β-secretases compete with each other for the production of sAβPPα and Aβ, respectively, upregulation of α-secretase activity is likely to counteract Aβ accumulation in the brain. A disintegrin and metalloproteinase (ADAM) 9, 10, and 17 are well known α-secretases, as they have been shown to play a major role in sAβPPα production [9-11]. Recently, it has been reported that also metalloproteinase 9 (MMP-9) has α-secretase activity, producing sAβPPα following induction by Aβ40 [12]. MMP-9 is a gelatinase which is elevated in the brain of AD patients [13]. It belongs to the MMPs, a family of structurally and functionally related zinc endopeptidases consisting of 23 different members in humans [14] with a variety of pathophysiological functions not only in development, but also in diseases such as cancer and arthritis because of the MMP proteolytic activities during angiogenic invasion and tissue disruption [14-16]. MMP-9 has also been shown to degrade Aβ fibrils [17]. In the present study, we have investigated the effects of the stimulation of the 5-HT4 receptor signaling through the receptor agonist RS-67333 onto the levels of MMP-9 using both stably transfected AβPP overexpressing cells and Tg2576, a model of Aβ deposition. We have demonstrated that 5-HT4 receptor stimulation induces MMP-9 to enhance AβPP cleavage and increase sAβPPα levels.
MATERIALS AND METHODS
Cells
H4 cells stably transfected with wild type AβPP (H4/AβPP) were kindly provided by Dr. Todd Golde (University of Florida, Gainesville, FL). These cells were used to stably express the 5-HT4 receptor and produce H4/AβPP/5-HT4 cells. Full-length human 5-HT4 receptor cDNA (Origene Technologies) was subcloned into pcDNA3 vector (Invitrogen). The 5-HT4 plasmid was transfected into H4/AβPP cells using FuGENE HD Transfection Reagent (Roche Diagnostics) and selecting a single clone by 100 μg/ml Zeocin and 600 μg/ml Geneticin in Dulbecco’s modified Eagle’s medium (DMEM) including 10% fetal bovine serum (FBS; Invitrogen).
Animals
All experiments were performed with the approval of the Columbia University Animal Care and Use Committee in accordance with the guidelines for the humane treatment of animals. AβPP-transgenic mice (Tg2576 mice) were obtained from a colony bred in our animal facility using mice initially provided by Dr. Karen Hsiao-Ashe (University of Minnesota). Mice were genotyped from tail samples as previously described [18]. Female Tg2576 mice ranging from 12 to 14-months of age were intraperitoneally injected once a day for 10 days with 3 mg/kg RS-67333 (Tocris Bioscience) or saline. After administration, mice were sacrificed and hippocampi from both hemispheres were stored at −80°C until use.
Immunodetection of sAβPPα and MMP-9
H4/AβPP/5-HT4 cells (2 × 105 cells) pre-cultured in DMEM with 10% FBS for 2 days were incubated in serum-free DMEM for 2 h prior to treatment. Cells were then treated with 5-HT (1 μM) (Sigma-Aldrich), RS-67333 (3 μM), RS-67333 plus GR-113808 (3 μM) (Sigma-Aldrich), or medium as a control for 1, 2, 4, 8, 24, or 48 h in serum-free DMEM. To analyze the sAβPPα protein secreted from H4/AβPP/5-HT4 cells, the harvested medium was concentrated 5 times using Microcon with YM-10 filter (Millipore) prior to immunoblotting using anti-sAβPPα (1 μg/ml) (2B3, IBL-America). For analysis of MMP-9 protein secreted from the cells, the medium (500 μl) was immunoprecipitated using the anti-MMP-9 antibody H-129 (1 μg/ml) (Santa Cruz Biotechnology) and Protein G-Agarose (20 μl/ml medium) (Roche Diagnostics). Hippocampi from the brain right hemisphere of Tg2576 mice were homogenized using T-PER (Thermo Scientific) and immunoblotting samples were prepared as previously described [19]. The reduced proteins (40 μg) were analyzed by immunoblotting with the anti-sAβPPα antibody 2B3 (1 μg/ml) (IBL America) or anti-C-terminal AβPP antibody (0.25 ng/ml) (Invitrogen). For normalization, anti-βIII tubulin antibodies (0.5 μg/ml) or anti-GAPDH antibodies (1 μg/ml) (Millipore) were used.
Gelatin zymography
Gelatin zymopraphy for gelatinases was performed according to the method of Okada et al. [20]. Briefly, concentrated medium treated with 5-HT, RS-67333, or medium as a control was mixed with a sampling buffer followed by incubation for 30 min at 37°C. For electrophoresis, 10% zymogram gelatin gel (Invitrogen) was used.
Knockdown of MMP-9
MMP-9 siRNA (5′-CAUCACCUAUUGGAUCCA Att-3′ and 5′-UUGGAUCCAAUAGGUGAUGtt-3′) or control siRNA (2 pmol) (Applied Biosystems) were transfected into H4/AβPP/5-HT4 cells using Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. Transfected cells precultured for 2 days in DMEM with 10% FBS were treated with RS-67333 (3 μM) or medium for another 24 h in serum-free DMEM. The medium was concentrated and subjected to immunoblotting using anti-sAβPPα and MMP-9 antibodies and gelatin zymography as described above.
Quantitative RT-PCR
Total RNAs were extracted from hippocampi taken from Tg2576 mice and used for reverse transcription with SuperScriptIII (Invitrogen) and PCR with MX3000 (Stratagene) using the following synthetic oligonucleotides: MMP-9 forward primer (5′-AGCGTCATTCGCGTGGATA-3′), and MMP-9 reverse primer (5′-CGTGTGAGTTCCAGGGCAC-3′). Each mRNA value was normalized to that of the housekeeping gene β-actin.
Histology
Tissue sections were deparaffinized in xylene and hydrated. Then using Biocare’s Diva pretreatment solution, sections were steamed for 45 min, followed by cooling for 20 min, and treatment with 0.3% hydrogen peroxide to block endogenous peroxidase. Tissue sections were then incubated in protein-free block (Biocare’s background sniper) for 15 min to inhibit the nonspecific binding of primary. Primary antibody (6E10 at 1: 400 Biocare Medical) was incubated for 60 min at room temperature. Detection was performed with horseradish peroxidase-conjugated respective secondary antibody (Dako) incubated for 30 min at room temperature. Color was developed with 3′,3′-diaminobenzidine (DAB substrate Kit, Vector Laboratories) and counterstaining with the Gill hematoxylin solution. A board-certified neuropathologist, who was blinded to the treatment versus the control group, analyzed a coronal section from each mouse and counted the total number of well-formed Aβ plaques in the hippocampus bilaterally.
Enzyme-linked immunosorbent assay (ELISA)
The homogenates were prepared from hippocampi of the left hemispheres, as previously described [21]. Human Aβ40 and Aβ42 levels from diluted samples (1: 2000) were measured using human amyloid-β 1-40/1-42 Kit (Invitrogen). Aβ amounts were normalized with the protein concentration calculated using BCA Protein Assay Reagent (Thermo Scientific).
Statistics
The intensity of sAβPPα and MMP-9 bands were quantified using Image J Program (NIH), and normalized with respect to tubulin. Values were reported as the mean ± S.E.M. Statistical analysis was performed with either Bonferroni/Dunn test or Student’s t-test. p values of less than 0.05 were considered significant.
RESULTS
Enhancement of sAβPPα production by stimulation of the 5-HT4 receptor
To determine whether 5-HT4 receptor stimulation leads to sAβPPα production, we used western blotting techniques following addition of the 5-HT4 agonist RS-67333 or vehicle control medium to H4/AβPP/5-HT4 cells for 1, 2, 4, 8, 24, or 48 h. In the presence of RS-67333, a sAβPPα band was increased from 8 h to 48 h (Fig. 1A). Levels of sAβPPα were significantly higher in RS-67333-treated cultures (102 ± 20.4% of control, 265 ± 46.6%, or 343 ± 56.3% at 8, 24, or 48 h, respectively, n = 4 per each group), as well as 5-HT-treated cultures (125 ± 16.8%, 216 ± 41.9%, or 261 ± 34.2% at 8, 24, or 48 h, respectively, n = 4 per each group) compared with vehicle-treated cultures (n = 4 per each group). The effect of RS-67333 was blocked by addition of the 5-HT4 receptor antagonist, GR-113808 (95.5 ± 2.40%, 64.4 ± 8.29%, and 64.9 ± 13.6% at 8, 24, or 48 h, respectively, n = 4 per each group; Fig. 1A and B). On the other hand, no Aβ40 and Aβ42 were detected in H4/AβPP/5-HT4 cells either by immunoblotting or ELISA both in basal conditions and after RS-67333 treatment (data not shown). These results are consistent with previous studies showing a RS-67333 induced increase in sAβPPα levels in AβPP overexpressing cells [7], and suggest that stimulation of 5-HT4 receptor signaling enhances sAβPPα production.
Fig. 1.
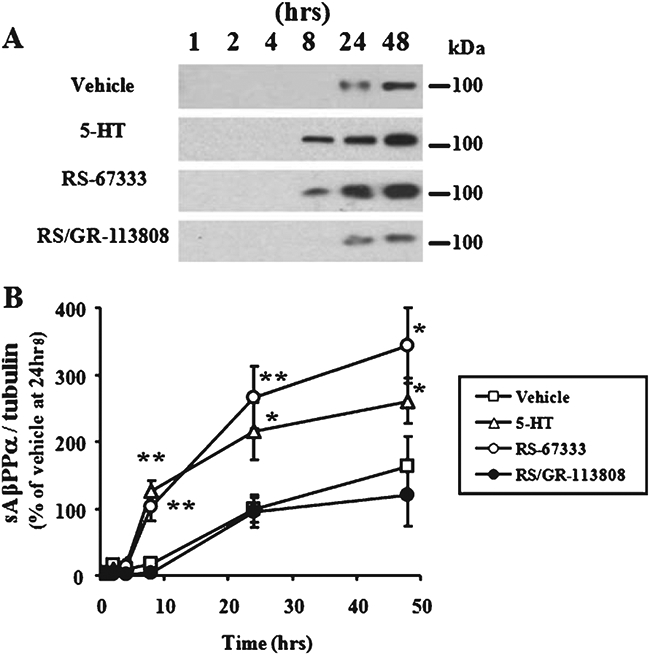
sAβPPα time-course following 5-HT4 receptor stimulation in H4/AβPP/5-HT4 cells. A) H4/AβPP/5-HT4 cells were treated with 5-HT (1 μM), the 5-HT4 agonist RS-67333 (3 μM), or RS-67333 (3 μM) plus the 5-HT4 antagonist GR-113808 (3 μM) for 1, 2, 4, 8, 24, or 48 h in a serum free medium. The concentrated medium was subjected to immunoblotting using anti-sAβPPα antibody. B) The sAβPPα band intensity was measured and normalized for tubulin intensity, and plotted based on normalized band intensities of 24 h vehicle-treated cells. Error bars show S.E.M. (n = 4). **p<0.01; *p<0.05.
Enhancement of MMP-9 expression by stimulation of the 5-HT4 receptor
Our next goal was to determine how 5-HT4 receptor stimulation leads to production of sAβPPα. To analyze proteolytic activity of MMP-9, the medium of H4/AβPP/5-HT4 cells was subjected to gelatin zymography following treatment with 1 μM 5-HT or 3 μM RS-67333 for 1, 2, 4, 8, or 24 h. Two bands were detected at 92 and 82 kDa at 24 h corresponding to the pro- and active form of MMP-9, respectively (Fig. 2A). Vehicle-treated cultures, in turn, showed no bands. Both bands were also detected using immunoblotting at 24 h following treatment with the 5-HT4 receptor agonist (Fig. 2B). These results suggest that 5-HT4 receptor signaling stimulation leads to an increase in MMP-9 expression.
Fig. 2.
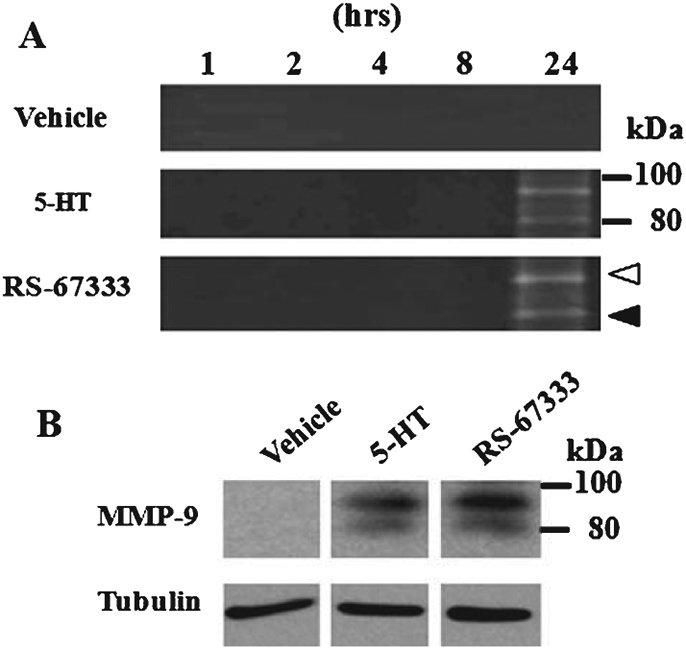
MMP-9 induction by 5-HT4 receptor stimulation in H4/AβPP/5-HT4 cells. A) H4/AβPP/5-HT4 cells were treated with serum-free medium, 5-HT (1 μM), or RS-67333 (3 μM) for 1, 2, 4, 8, or 24 h. Concentrated medium was analyzed in a gelatin zymography. Two bands were detected in RS-67333 treated cultures at 92 and 82 kDa corresponding to the pro and active form, respectively. B) Immunoprecipitated protein from the medium or RS-67333 (3 μM) treated for 24 h was subjected to immunoblotting using anti-MMP-9 antibody. Tubulin bands from cell lysates are also shown as an internal control.
Regulation of sAβPPα production by MMP-9
Next, we investigated whether MMP-9 regulates sAβPPα production. MMP-9 was transfected into H4/AβPP cells and the proteolytic activity of MMP-9 was analyzed (Fig. 3A). We found a remarkable increase in sAβPPα levels compared with mock transfected cultures using western blotting (Fig. 3A). These data are consistent with the observation that MMP-9 has α-secretase activity against AβPP [12].
Fig. 3.
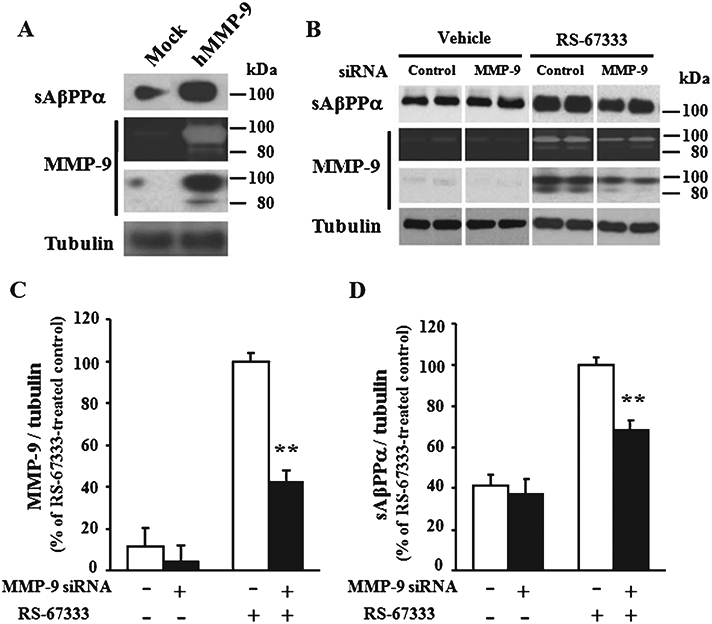
sAβPPα production by MMP-9 induction. A) H4/AβPP cells transfected with MMP-9 or mock plasmid were maintained for 48 h in a serum-free medium. The concentrated medium was analyzed by gelatin zymography and immunoblotting using anti-MMP-9 or sAβPPα antibodies. B) H4/AβPP/5-HT4 cells transfected with MMP-9 or control siRNA were cultured for 2 days and then treated with serum-free medium or RS-67333 (3 μM) for 24 h. The concentrated and immunoprecipitated medium were analyzed by the gelatin zymography and immunoblotting using anti-MMP-9 or sAβPPα antibody, respectively. The tubulin band from the cell lysate was used as an internal control. Band intensities of immunoreactive MMP-9 (C) and sAβPPα (D) were quantified, and the percent intensity normalized with tubulin was indicated. Error bars show S.E.M. (n = 4). **p < 0.01.
To add additional evidence in favor of the induction of sAβPPα production by MMP-9, we examined whether reduction in MMP-9 expression down-regulates levels of sAβPPα. MMP-9 siRNA was transfected into H4/AβPP/5-HT4 cells. sAβPPα protein or proteolytic activity was detected by immunoblotting or gelatin zymography. MMP-9 siRNA did not affect levels of sAβPPα protein in the absence of the 5-HT4 agonist RS-67333 (Fig. 3B-D). MMP-9 siRNA, in turn, caused a significant decrease of MMP-9 protein occurring after stimulation with the agonist compared to control siRNA transfected cultures (41.9 ± 5.95%, n = 4 for each group) (Fig. 3B and C). The gelatinase activity of MMP-9 was also decreased (Fig. 3B). Most importantly, a significant decrease of sAβPPα protein was observed in the medium of H4/AβPP/5-HT4 cells (68.6 ± 4.30%, n = 4 for each group) following stimulation with 3 μM RS-67333 (Fig. 3D), confirming that MMP-9 plays a key role in sAβPPα production.
Enhancement of sAβPPα, CTFα, and MMP-9 levels by stimulation of the 5-HT4 receptor in vivo
To validate findings on cell lines using an in vivo system, hippocampal levels of sAβPPα, C-terminal fragment α (CTFα), and MMP-9 were measured following intraperitoneal administration of 3 mg/kg RS-67333 in 10–12 month old Tg2576 mice for 10 days. We found an increase in sAβPPα (155 ± 16.3% of control vehicle, n = 10 for RS-67333 versus n = 10 for vehicle; Fig. 4A and B) and CTFα (243 ± 23.4% of control vehicle, n = 5; Fig. 4D). This was associated with an increase in precursor MMP-9 (365 ± 93.2% of vehicle-injected control mice, n = 10 for RS-67333 versus n = 10 for vehicle; Fig. 4A and C), even if no mature enzyme was detected in both groups (Fig. 4A). Interestingly, the increase in levels of MMP-9 protein was not accompanied by a change in its mRNA levels (Fig. 4E). Taken together, these findings validate results obtained in cell lines onto an in vivo system.
Fig. 4.
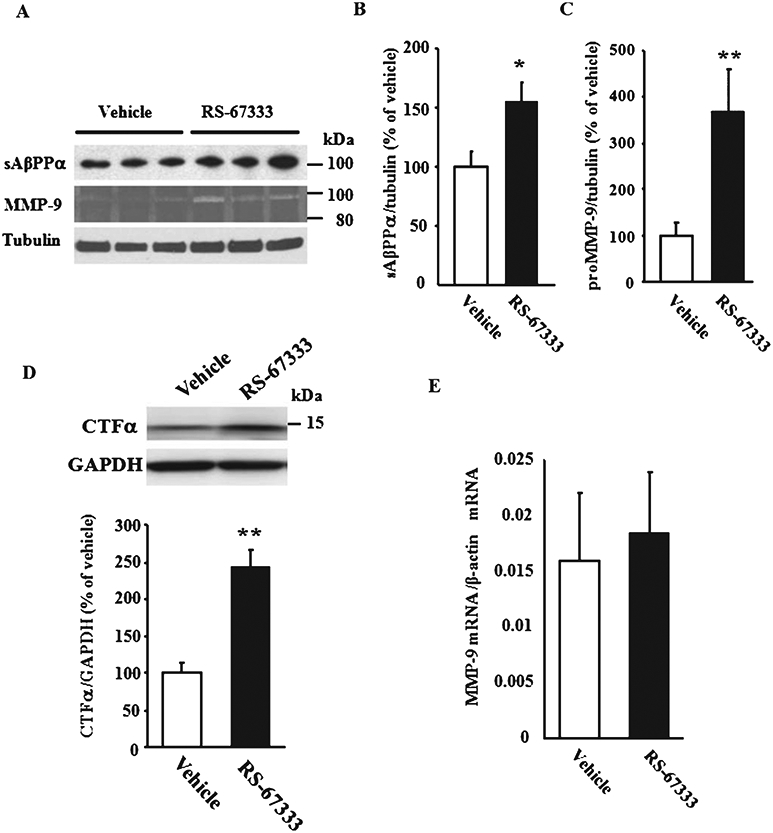
Effect of 5-HT4 receptor stimulation onto levels of MMP-9, sAβPPα, and CTFα in Tg2576 mice. A-D) RS67333 (3 mg/kg) or saline was intraperitoneally injected into female Tg2576 mice for 10 days. Mice were sacrificed and hippocampi were homogenized for immunoblotting using anti-sAβPPα, C-terminal AβPP, tubulin, or GAPDH antibody and for gelatin zymography. Band intensities of immunoreactive sAβPPα (n = 10 per each group) (B), proMMP-9 (n = 10 per each group) (C), and CTFα (n = 5 per each group) (D) were quantified and normalized for tubulin or GAPDH intensity. E) Quantitative RT-PCR analysis showed no difference in the expression levels of MMP-9 mRNA purified from the hippocampi of Tg2576 mice injected with RS67333 (3 mg/kg) or vehicle for 10 days. Data were normalized against β-actin (n = 5 each group). Error bars show S.E.M. **p < 0.01; *p < 0.05.
Aβ load reduction by stimulation of 5HT4 receptor in vivo
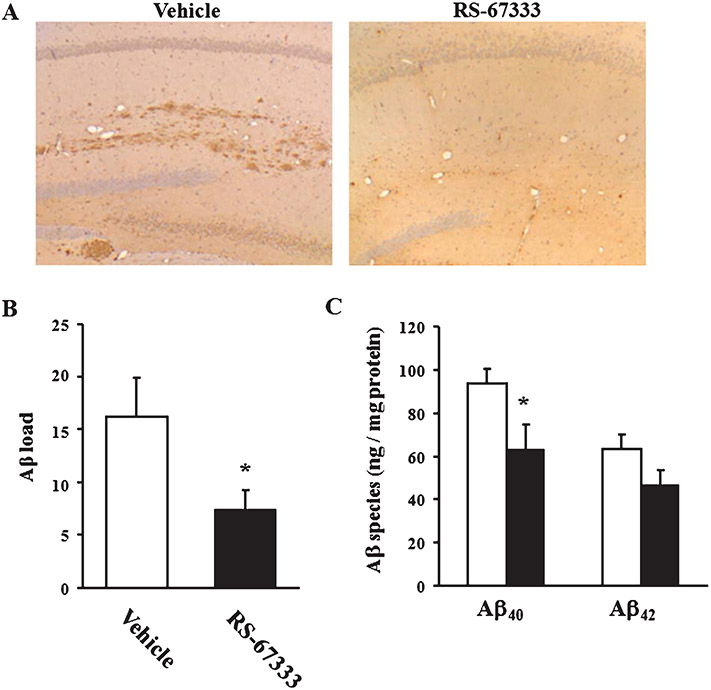
To further analyze the effect by MMP-9 upregulation, hippocampal amyloid plaques were immunostained and Aβ species were measured in 10–12 month old Tg2576 mice. The number of senile plaques in hippocampus was significantly decreased by injection of RS-67333 (45.7 ± 11.8% of control vehicle, n = 5 per each group; p < 0.05; Fig. 5A and B). Hippocampal levels of Aβ40 in RS-67333-treated mice were significantly decreased (63.5 ± 11.2 ng/mg protein) compared with those of vehicle-treated animals (93.5 ± 6.97 ng/mg protein; p < 0.05; Fig. 5C). We also observed a trend for a decrease in hippocampal levels of Aβ42 (47.1 ± 6.46 versus 63.5 ± 6.59ng/mg protein in controls, n = 10 for RS-67333-treated mice versus n = 9 in controls, p = 0.0970; Fig. 5C). Taken all together, these findings extend to the in vivo preparation results from cell lines, suggesting that stimulation of the 5-HT4 receptor signaling inhibits the amyloidogenic processing of AβPP through induction of the MMP-9 α-secretase activity.
Fig. 5.
Effect of 5-HT4 receptor stimulation on Aβ deposition in Tg2576 mice. A) Sections of hippocampus from vehicle (left) and treatment group (right) stained with 6E10 antibody (4 × objective; 40 × magnification). There is a reduction in amyloid plaques in the hippocampus after treatment with RS-67333 (3 mg/kg). B) A board-certified neuropathologist, who was blinded to the treatment versus the control group, analyzed a coronal section from each mouse and counted the total number of well-formed Aβ plaques in the hippocampus bilaterally. There are significantly fewer plaques in the treatment group versus the control group. Error bars show S.E.M. (n = 5 for each group). C) Concentrations of hippocampal Aβ40 or Aβ42 were measured by ELISA. ELISA signals are reported in nanograms per milligram protein. The blank or filled bar indicates the mean value for vehicle or RS67333 treatment group, respectively. Error bars show S.E.M. (n = 9 for vehicle and n = 10 for RS67333 group). *p < 0.05.
DISCUSSION
In this manuscript we have demonstrated that 5-HT4 receptor stimulation upregulates sAβPPα levels via a novel mechanism involving MMP-9. The finding that sAβPPα is upregulated by the treatment with a 5-HT4 receptor agonist is consistent with previous reports also showing the presence of a band for sAβPPα following stimulation of the 5-HT4 receptor signaling [22, 23]. It should be noted, however, that in these studies the band appeared at much shorter intervals (15–30 min versus 8h in our experiments) [22, 23]. A possible reason for this discrepancy is linked to the different type of preparation used in our studies compared with earlier reports, which might involve different signaling mechanisms. Different than our studies which were performed on neuroglioma-derived H4 cells, CHO cells stably transfected with 5-HT4 receptor demonstrated that 5-HT4 receptor signaling enhances sAβPPα production through Epac → Rap1 → Rac via a protein kinase A phosphorylation independent mechanism after a short interval [23]. By contrast, consistent with our findings showing an effect with a longer interval (6 h), serotonin treatment of rat smooth muscle cells upregulated MMP-13 via ERK1/2 [24]. Nevertheless, even if we do not find a short interval for the regulation of sAβPPα levels, the main finding that 5-HT4 receptor stimulation leads to sAβPPα elevation is still valid.
The MMP-9 knockdown study using siRNA showed that sAβPPα levels were 70% of control. MMP-9, in turn, was 40% of control. This discrepancy suggests that other proteinases besides MMP-9 are likely to be involved in the 5-HT4-receptor mediated upregulation of sAβPPα. For instance, ADAM9, 10, and 17 have been shown to act as α-secretases [9-11]. Additionally, down-regulation of β-secretase, another proteinase which has been shown to compete with α-secretase to favor Aβ production, might also be involved in the regulation of sAβPPα levels by 5-HT4-receptor stimulation. Notwithstanding, regardless of whether other proteinases in addition to MMP-9 are also involved in the increase of sAβPPα levels, our findings demonstrating that MMP-9 induction is a primary mechanism for sAβPPα upregulation by 5-HT4 receptor stimulation, are still valid.
Another interesting aspect of our studies is related to the reduction in plaque number and Aβ levels following 5-HT4 receptor stimulation. Based on our findings, this is likely to be at least in part due to enhancement of the MMP-9 α-secretase activity. However, other mechanisms can also be involved. For instance, MMP-9 has been found to degrade Aβ fibrils as well as monomeric Aβ peptide, whereas other Aβ-degrading proteinases such as neprilysin, endothelin-converting enzyme, and insulin-degrading enzyme are not capable of clearing Aβ42 fibrils [17]. Thus, one cannot exclude an effect of the 5-HT4 receptor stimulation not only on AβPP processing, but also degradation. Furthermore, Aβ40 and Aβ25-35 are known to induce MMP-9 expression both in vitro and in vivo [12, 25, 26], suggesting that MMP-9 can be upregulated by multiple mechanisms including Aβ and 5-HT4 receptors. Overall, these mechanisms lead to an improvement of Aβ load.
The increase in MMP-9 protein levels following treatment with RS-67333 was not accompanied by an increase in mRNA levels. There are several possible explanations for this finding. For instance, a different timing between change in mRNA and protein levels, such that collecting hippocampi at 10 days when protein levels are increased might not be appropriate to detect changes in mRNA levels. Additionally, contamination of glial cells might mask the increase in mRNA. Changes in the rate of mRNA translation might also explain the increase in protein levels with no changes in mRNA levels [27]. Finally, changes in mechanisms of protein degradation might be responsible for it. Investigating these possibilities goes beyond the goal of this manuscript. Nevertheless, our results are still significant as they support the possibility that 5-HT4-receptor agonists might be beneficial against AD.
In agreement with our results, long-term potentiation, a type of synaptic plasticity that is likely to be related to learning and memory, can be either reduced or enhanced through block or upregulation of MMP-9 activity, respectively [28, 29]. Recently, however, Mizoguchi et al. reported that disruption or inhibition of MMP-9 improves Aβ-mediated cognitive dysfunction and neurotoxicity. They have also found that Aβ40 enhances proteolytic activity of MMP-9 [26]. On the other hand, the concentrations of Aβ peptide used for the in vitro or in vivo experiments was much higher (10 μM or 900 pmol, respectively), compared with that of previous data [30, 31]. A possible scenario that might reconcile the apparently different results includes a positive effect on cognition by moderate amounts of Aβ and a negative effect with higher amounts of Aβ [30, 31].
Another mechanism through which 5-HT4 receptor signaling can improve cognition includes facilitation of neurotransmitter release. Using microdialysis, it has been shown that treatment with 5-HT4 receptor agonists such as RS67333 induces acetylcholine efflux [5]. The transmitter is known to play a key role in enhancement of cognition, suggesting another avenue through which stimulation of the 5-HT4 receptor signaling might improve cognitive dysfunction in disease state.
MMP-9 belongs to the family of the MMPs which includes various enzymes with different proteolytic activities such as collagenases, stromelysins, or gelatinases [14]. Following identification of the catalytic mechanisms of collagen type I-IV, pharmaceutical industries have focused during the last few decades onto developing MMP inhibitors to counteract arthritis and various cancers. In spite of the fact that MMP-9 and ADAM 9, 10, and 17 have been found to present α-secretase activity [8, 12, 17], direct proteinase activators for these enzymes have not been developed, probably due to the fact that activators of these enzymes are much more difficult to synthesize than inhibitors. Thus, proteinase activators acting indirectly through stimulation of receptors or kinases might be an excellent strategy to counteract neurodegenerative diseases. In the present study, we have demonstrated that stimulation of the 5-HT4 receptor signaling through a 5-HT4 receptor agonist can enhance MMP-9 activity, leading to sAβPPα production and Aβ reduction. Thus, 5-HT4 receptors are likely to constitute a promising drug target for the therapy of AD or other dementias.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH-NS049442). We thank Drs. Todd Golde (University of Florida) and Karen Hsiao-Ashe (University of Minnesota) for providing us with H4/AβPP cells and Tg2576 mice, respectively. We also thank Geping Zhang for her assistance with preparing the immunohistochemical sections for histologic assessment of plaque count.
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=1409).
REFERENCES
- [1].Hannon J, Hoyer D (2008) Molecular biology of 5-HT receptors. Behav Brain Res 195, 198–213. [DOI] [PubMed] [Google Scholar]
- [2].Lezoualc’h F (2007) 5-HT4 receptor and Alzheimer’s disease: The amyloid connection. Exp Neurol 205, 325–329. [DOI] [PubMed] [Google Scholar]
- [3].Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O, Haddjeri N, Piñeyro G, Sadikot AF, Debonnel G (2007) Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 55, 712–725. [DOI] [PubMed] [Google Scholar]
- [4].Tamburella A, Micale V, Navarria A, Drago F (2009) Antidepressant properties of the 5-HT4 receptor partial agonist, SL65.0155: Behavioral and neurochemical studies in rats. Prog Neuropsychopharmacol Biol Psychiatry 33, 1205–1210. [DOI] [PubMed] [Google Scholar]
- [5].Mohler EG, Shacham S, Noiman S, Lezoualc’h F, Robert S, Gastineau M, Rutkowski J, Marantz Y, Dumuis A, Bockaert J, Gold PE, Ragozzino ME (2007) VRX-03011, a novel 5-HT4 agonist, enhances memory and hippocampal acetylcholine efflux. Neuropharmacology 53, 563–573. [DOI] [PubMed] [Google Scholar]
- [6].Moser PC, Bergis OE, Jegham S, Lochead A, Duconseille E, Terranova JP, Caille D, Berque-Bestel I, Lezoualc’h F, Fischmeister R, Dumuis A, Bockaert J, George P, Soubrié P, Scatton B (2002) SL65.0155, a novel 5-hydroxytryptamine(4) receptor partial agonist with potent cognition-enhancing properties. J Pharmacol Exp Ther 302, 731–741. [DOI] [PubMed] [Google Scholar]
- [7].Cho S, Hu Y (2007) Activation of 5-HT4 receptors inhibits secretion of beta-amyloid peptides and increases neuronal survival. Exp Neurol 203, 274–278. [DOI] [PubMed] [Google Scholar]
- [8].Thinakaran G, Koo EH (2008) Amyloid precursor protein trafficking, processing, and function. JBiol Chem 283,29615–29619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA (1998) Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem 273, 27765–27767. [DOI] [PubMed] [Google Scholar]
- [10].Koike H, Tomioka S, Sorimachi H, Saido TC, Maruyama K, Okuyama A, Fujisawa-Sehara A, Ohno S, Suzuki K, Ishiura S (1999) Membrane-anchored metalloprotease MDC9 has an alpha-secretase activity responsible for processing the amyloid precursor protein. Biochem J 343, 371–375. [PMC free article] [PubMed] [Google Scholar]
- [11].Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F (1999) Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A 96, 3922–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Talamagas AA, Efthimiopoulos S, Tsilibary EC, Figueiredo-Pereira ME, Tzinia AK (2007) Abeta(1-40)-induced secretion of matrix metalloproteinase-9 results in sAPPalpha release by association with cell surface APP. Neurobiol Dis 28, 304–315. [DOI] [PubMed] [Google Scholar]
- [13].Bruno MA, Mufson EJ, Wuu J, Cuello AC (2009) Increased matrix metalloproteinase 9 activity in mild cognitive impairment. J Neuropathol Exp Neurol 68, 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y (2000) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 59, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE (1996) Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest 97, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, Lee JM (2006) Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem 281, 24566–24574. [DOI] [PubMed] [Google Scholar]
- [18].Trinchese F, Liu S, Battaglia F, Walter S, Mathews P, Arancio O (2004) Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol 55, 801–814. [DOI] [PubMed] [Google Scholar]
- [19].Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O (2006) Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell 126, 775–788. [DOI] [PubMed] [Google Scholar]
- [20].Okada Y, Gonoji Y, Naka K, Tomita K, Nakanishi I, Iwata K, Yamashita K, Hayakawa T (1992) Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem 267, 21712–21719. [PubMed] [Google Scholar]
- [21].Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ (1997) Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med 3, 67–72. [DOI] [PubMed] [Google Scholar]
- [22].Robert SJ, Zugaza JL, Fischmeister R, Gardier AM, Lezoualc’h F (2001) The human serotonin 5-HT4 receptor regulates secretion of non-amyloidogenic precursor protein. J Biol Chem 276, 44881–44888. [DOI] [PubMed] [Google Scholar]
- [23].Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, Fischmeister R, Lezoualc’h F (2003) Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol 5, 633–639. [DOI] [PubMed] [Google Scholar]
- [24].Shum JK, Melendez JA, Jeffrey JJ (2002) Serotonin-induced MMP-13 production is mediated via phospholipase C, protein kinase C, and ERK1/2 in rat uterine smooth muscle cells. J Biol Chem 277, 42830–42840. [DOI] [PubMed] [Google Scholar]
- [25].Hernández-Guillamon M, Delgado P, Ortega L, Pares M, Rosell A, García-Bonilla L, Fernández-Cadenas I, Borrell-Pagès M, Boada M, Montaner J (2009) Neuronal TIMP-1 release accompanies astrocytic MMP-9 secretion and enhances astrocyte proliferation induced by beta-amyloid 25-35 fragment. J Neurosci Res 87, 2115–2125. [DOI] [PubMed] [Google Scholar]
- [26].Mizoguchi H, Takuma K, Fukuzaki E, Ibi D, Someya E, Akazawa KH, Alkam T, Tsunekawa H, Mouri A, Noda Y, Nabeshima T, Yamada K (2009) Matrix metalloprotease-9 inhibition improves amyloid beta-mediated cognitive impairment and neurotoxicity in mice. J Pharmacol Exp Ther 331, 14–22. [DOI] [PubMed] [Google Scholar]
- [27].Huang T, Wan S, Xu Z, Zheng Y, Feng KY, Li HP, Kong X, Cai YD (2011) Analysis and prediction of translation rate based on sequence and functional features of the mRNA. PLoS One 6, e16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW (2008) Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA 105, 19520–19525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bozdagi O, Nagy V, Kwei KT, Huntley GW (2007) In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J Neurophysiol 98, 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Puzzo D, Privitera L, Fa’ M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM, Troy CM, Mercken M, Jung SS, Palmeri A, Arancio O (2010) Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol 69, 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A, Arancio O (2008) Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci 28, 14537–14545. [DOI] [PMC free article] [PubMed] [Google Scholar]