Abstract
Infection by human immunodeficiency virus type 1 (HIV-1) has been associated with increased cell death by apoptosis in infected and uninfected cells. The envelope glycoprotein complex ([gp120/gp41]n) of X4 HIV-1 isolates is involved in both infected and uninfected cell death via its interaction with cellular receptors CD4 and CXCR4. We studied the effect of the blockade of CXCR4 receptors by the agonist stromal derived factor (SDF-1α) and the antagonist bicyclam AMD3100 on apoptotic cell death of CD4+ cells in different models of HIV infection. In HIV-infected CEM or SUP-T1 cultures, AMD3100 showed antiapoptotic activity even when added 24 h after infection. In contrast, other antiviral agents, such as zidovudine, failed to block apoptosis under these conditions. The antiapoptotic activity of AMD3100 was also studied in coculture of peripheral blood mononuclear cells or CD4+ cell lines with chronically infected H9/IIIB cells. AMD3100 was found to inhibit both syncytium formation and apoptosis induction with 50% inhibitory concentrations ranging from 0.009 to 0.24 μg/ml, depending on the cell type. When compared to SDF-1α, AMD3100 showed higher inhibitory potency in all cell lines tested. Our data indicate that the bicyclam AMD3100 not only inhibits HIV replication but also efficiently blocks cell-surface-expressed HIV-1 envelope-induced apoptosis in uninfected cells.
The human immunodeficiency virus type 1 (HIV-1) viral envelope complex ([gp120/gp41]n) interacts with the CD4 molecule and several chemokine receptors, mainly CXCR4 and CCR5 (6, 15, 38) to drive the entry of viral particles into target cells. Consequently, chemokines such as the stromal derived factor (SDF-1α), the natural ligand of CXCR4 (5, 34), and RANTES, MIP-1α, and MIP-1β, the ligands of CCR5 (8), are able to suppress the entry of X4 (CXCR4 using) and R5 (CCR5 using) HIV-1 isolates, respectively. Recently, low-molecular-weight molecules, such as the bicyclam AMD3100, have been reported to act as potent and specific antagonists of CXCR4, showing strong inhibitory activity against X4 isolates of HIV (13, 14, 16, 30, 40, 41). Both the chemokines and the low-molecular-weight antagonists block virus-to-cell fusion without affecting HIV-1 attachment to the cell surface (12, 35).
X4 and R5 isolates of HIV differ in their tropisms and in their cytopathicities (1). Although R5 isolates can be highly pathogenic (22), X4 isolates are more cytopathic in vitro and have been postulated as a causal factor of AIDS (21). In fact, among HIV-infected people, R5 isolates are predominant in asymptomatic individuals, whereas the emergence of X4 isolates is usually associated to a faster decline of CD4+ T cell counts and the onset of AIDS (17).
The mechanisms leading to the cytopathicity of HIV-1 have been related to nonexclusive apoptotic and nonapoptotic events (26, 27, 44). Increased apoptosis in both infected and uninfected cells has been reported in different experimental models of HIV-1 infection (19, 20, 28, 43, 45). The envelope glycoprotein complex ([gp120/gp41]n) seems to be a major determinant of apoptotic events (9, 28). Soluble gp120 from both X4 and R5 isolates of HIV share CD4-dependent effects (37), and the infection by both types can induce CD4+ T-cell killing (46). However, other effects of gp120, such as the induction of apoptosis in CD8+ T-cells and neurons, appear to specifically involve CXCR4 signaling and are restricted to X4 isolates (23, 24). Similarly, the cell-surface-expressed HIV-1 envelope from X4 isolates is a potent inducer of apoptosis in CD4+ cell lines and primary CD4+ T cells (25) by a process that is independent of FAS/CD95 and involves the recruitment of the CD4 and CXCR4 receptors (4).
Apoptosis of uninfected cells may play a key role in AIDS pathogenesis (18, 23, 24), while the death of infected cells might serve to impair viral replication. A general blockade of apoptosis in vitro led to the survival of infected cells, thus enhancing viral production (7). Consequently, the inhibition of apoptosis during HIV disease should be focused on the specific protection of uninfected cells. The role of CXCR4 on the death of uninfected cells led us to characterize the activity of the CXCR4 antagonist AMD3100 as that of an inhibitor of one of the pathogenic mechanisms of HIV-1, the cell-surface-expressed HIV-1 envelope-induced apoptosis. Our data showed that AMD3100 is able to specifically inhibit this mechanism of apoptosis in cultures of infected and uninfected cells, thus suggesting a broader anti-HIV effect of AMD3100 in vitro when compared to other antiretrovirals that have no effect on cell-to-cell fusion and apoptosis induction, such as zidovudine (AZT).
MATERIALS AND METHODS
Cells.
CEM cells (clone 13) selected by high-level expression of CD4 were obtained from A. G. Hovanessian, Institut Pasteur, France. SUP-T1 and MT-4 cells were obtained from the American Type Culture Collection, Rockville, Md. Chronically HIV-1-infected H9/IIIB cells were obtained from R. C. Gallo at the National Institutes of Health, Bethesda, Md. All these cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated (56°C for 30 min) fetal calf serum and 2 mM glutamine (supplemented RPMI). Peripheral blood mononuclear cells (PBMC) were purified from healthy donors by Ficoll-Hypaque sedimentation and were cultured in supplemented RPMI containing 3 μg of phytohemagglutinin (PHA) per ml (Sigma) and 15 UI of interleukin-2 (IL-2) (Boehringer Inghelheim).
Infections and coculture.
HIV-1 infections were carried out by incubating 5 × 106 cells with a highly infectious HIV-1NL4-3 stock (material equivalent to 10,000 × the 50% tissue culture infective dose) at 37°C for 4 h in the absence or presence of antiviral agents. Unbound virus was removed by centrifugation, and cells were cultured in fresh supplemented RPMI containing the same concentration of antiviral agents. In some experiments, antiviral agents were added to the culture 24 h after infection. The virus production was monitored by measuring the concentration of viral core protein p24 in the culture supernatants by enzyme-linked immunosorbent assay (Coulter).
Coculturing of chronically infected H9/IIIB cells with target cells was performed as described (25). Briefly, 5 × 106 target cells (CEM, MT-4, SUP-T1, or PBMC stimulated for 2 days) were cultured in the presence of 5 × 105 H9/IIIB cells in 5 ml of supplemented RPMI (containing 25 UI of IL-2 per ml in PBMC cocultures). Before addition of chronically infected cells, target cells were incubated for 30 min at 37°C with the indicated concentrations of the CXCR4 agonist SDF-1α (Peprotech, London, United Kingdom) or the CXCR4 antagonist AMD3100. Cocultures were allowed to stand, usually for 24 h at 37°C, and were monitored for the development of cytopathicity as manifested by syncytium formation and ballooning of cells.
Detection of apoptosis.
At different times of infection or coculture, the level of apoptosis was determined by different methods. The standard method to determine apoptosis was propidium iodide staining. Briefly, cells were washed once in phosphate-buffered saline (PBS) and were incubated in labeling solution containing 0.1 mg of propidium iodide per ml and 0.1% Triton X-100 in PBS for 90 min and were analyzed. In some cases, apoptosis was quantified in intact nuclei by incubating cells in hypotonic labeling solution containing 3.4 mM sodium citrate, 0.05 mg of propidium iodide per ml, 0.1 mM EDTA, 1 mM Tris (pH 8), and 0.1% Triton X-100 as described (33). Analysis was carried out in a FACSCalibur (Becton Dickinson) by gating single cells or intact nuclei, respectively. When intact nuclei were analyzed, cell debris was eliminated by increasing the threshold of forward side scatter. In the fluorescence-based terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assays (In-Situ Cell Death Detection Kit; Boehringer Mannheim Biochemicals) cells were fixed in 1% paraformaldehyde (PFA), were washed twice in PBS, and were permeabilized in 70% ethanol for 30 min at −20°C. Labeling reactions using fluorescein-labeled dUTP were performed as indicated by the manufacturer. In some experiments, apoptosis was also monitored by annexin-V binding (Annexin-V FLUOS; Boehringer Mannheim Biochemicals). In these experiments, cells were simultaneously stained with the anti-CD4 antibody Leu3a (PerCP-coupled; Becton-Dickinson) to identify apoptotic cells as CD4+ cells. In both TUNEL and Annexin-V-binding experiments analysis was performed by gating single cells.
The measure of apoptosis in infected cultures by flow cytometry is handicapped by the presence of multinucleated giant cells. Propidium iodide (PI) labeling in hypotonic buffer gives the total number of apoptotic nuclei in the cultures; whereas fluorescence-based TUNEL, annexin-V-binding assays, and cell cycle analysis after PI staining measure single apoptotic cells, syncytium cells are not measured by the latter techniques.
As a positive control for apoptosis, cells (106/ml) were incubated for 24 h with 100 ng of the agonist anti-CD95/FAS monoclonal antibody (MAb) CH11 per ml (IgM; Immunotech).
Analysis of expression of cell surface antigens.
Expression of cell surface antigens was studied by fluorescence-activated cell sorter (FACS) analysis using the following MAbs: the anti-human CD4 MAb Leu-3a (PerCP-labeled IgG1; Becton Dickinson), the fluorescein isothiocyanate-labeled anti-human CD26 MAb Ta1 (Coulter), and the phycoerythrin-labeled anti-human CXCR4 12G5 MAb (IgG2a; R & D Systems, Minneapolis, Minn.). Before incubation with antibodies, cells were washed in ice-cold PBS. Incubations were performed at 4°C for 30 min. Cells were then washed again and fixed in PBS (containing 1% formaldehyde) for analysis in a FACSCalibur Flow Cytometer (Becton Dickinson) using the CellQuest software (Becton Dickinson).
Data analysis.
The 50% inhibitory concentration (IC50) of CXCR4 ligands was determined by coculturing different target cells with H9/IIIB cells in a ratio of 10 target cells for each infected cell in the presence of increasing concentrations of the test compound. Apoptosis data were obtained by PI staining, and IC50s were calculated by nonlinear regression of data as described (3).
RESULTS
AMD3100 blocks the apoptosis induced by HIV infection of CD4+ cell lines.
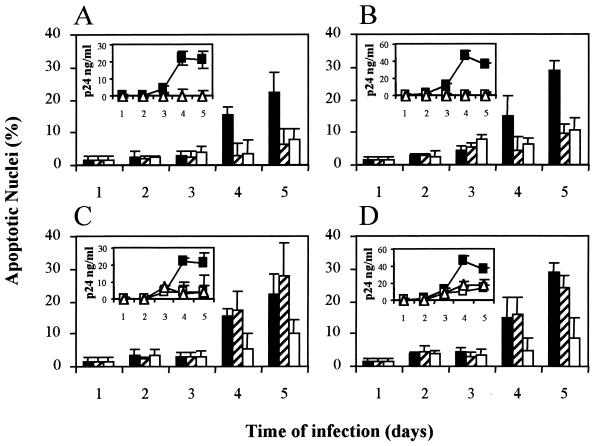
To assess the role of CXCR4 in the apoptosis following HIV infection, we studied the effect of the addition of the CXCR4 antagonist AMD3100 to HIV-1-infected cultures of CEM and SUP-T1 cells. This effect was compared to that of AZT, an antiretroviral agent known to be inefficient in blocking cell-surface-expressed envelope-induced apoptosis (4, 25). We hypothesized that the effect of AMD3100 on cell-to-cell fusion would be able to improve cell survival. As shown in Fig. 1, both compounds AZT and AMD3100, when added prior to infection, efficiently block HIV replication and, consequently, all the mechanisms of HIV-induced cell death (Fig. 1A and B), thus no apoptosis was observed in treated cultures. Conversely, strong differences were observed when the compounds were added to the culture 24 h after virus infection. In this case, the culture is a mixture of infected and uninfected cells, in which contacts between infected and uninfected cells play a major role in HIV-1 transmission (9). Both AZT and AMD3100 impaired viral growth as assessed by p24 production (Fig. 1). However, AZT was unable to block the total apoptosis in the infected culture, because of the lack of effect of this drug on the viral envelope glycoprotein-driven cell-to-cell fusion. Indeed, the AZT-treated and the untreated infected cultures showed similar syncytia at 4 and 5 days postinfection. In contrast, the CXCR4 antagonist AMD3100 efficiently blocked cell-to-cell fusion, as assessed by the lack of syncytia in the AMD3100-treated culture, and impaired the induction of apoptosis (Fig. 1C and 1D).
FIG. 1.
AMD3100 blocks the apoptosis induced by HIV-1 infection of CEM and SUP-T1 cells. Cultures of CEM (A) and SUP-T1 (B) cells were infected with HIV-1 NL4-3 (4 h, 37°C) in the absence (black columns) or the presence (striped columns) of 0.1 μg of the reverse transcriptase inhibitor AZT per ml or in the presence of 1 μg of the CXCR4 antagonist AMD3100 per ml (white columns). In a parallel experiment, CEM (C) and SUP-T1 cells (D) were infected in the absence of any compound. In this case, 24 h after infection, the cultures were left untreated (black columns) or the above indicated concentrations of AZT (striped columns) or AMD3100 (white columns) were added. At the indicated times, cultures were monitored for apoptosis by hypotonic propidium iodide staining as described in Materials and Methods. In each panel, inserts show the HIV-1 production measured by the content of viral core protein p24 in cell culture supernatants corresponding to a control infected cultures (solid squares), AZT-treated cultures (open squares), and AMD3100-treated cultures (open triangles). For each panel and insert, points represent the means ± the standard deviations of three different experiments.
AMD3100 inhibits HIV-1 envelope-induced apoptosis in PBMC.
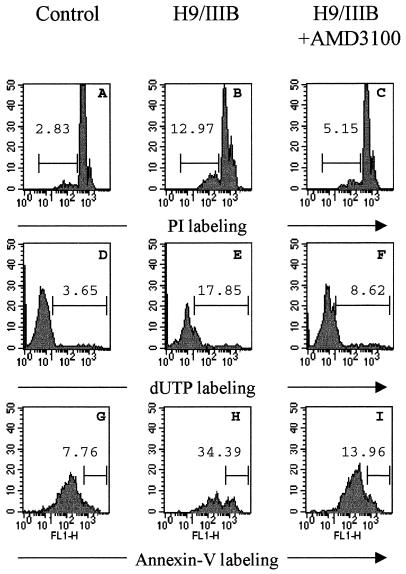
We have recently reported the role of CXCR4 in HIV-1 envelope-induced apoptosis (4). Here, we studied the effect of the CXCR4 antagonist AMD3100 on the apoptosis occurring in cocultures of chronically infected H9/IIIB cells with PBMC PHA activated for 2 days. In this model, apoptosis is strongly dependent on the interactions of the HIV-1 envelope complex glycoproteins expressed by H9/IIIB cells with cellular receptors on the surface of target cells (4). Apoptosis was measured by several methods (PI staining, TUNEL, and Annexin-V-binding assays) at the single cell level. Figure 2 shows increased apoptosis in cocultures of PBMC with H9/IIIB (Fig. 2B, E, and H) cells compared to control cultures (Fig. 2A, D, and G). This increased apoptosis is associated with a loss of CD4+ T cells (usually more than 50%), since cells fused into syncytia are not quantified by FACS analysis. The underrepresentation of single CD4+ T cells after cocultivation of PBMC with H9/IIIB becomes more evident when CD4+ T cells are gated by the simultaneous labeling with Annexin-V and anti-CD4 antibodies, (Fig. 2G). In addition, the percentage of apoptotic cells in this CD4+ T cell subset is higher than the values observed in CD4− cells (not shown), thus confirming that this mechanism of cell death is targeted mainly to CD4+ T cells (Fig. 2G and H). Both apoptosis induction and CD4+ T-cell loss due to syncytium formation were clearly blocked (more than 80%) by the addition of AMD3100 to the cocultures, as assessed by the three different methods used (Fig. 2C, F, and I).
FIG. 2.
HIV-1 envelope-induced apoptosis in PBMC is inhibited by AMD3100. PBMC stimulated for 2 days with PHA were cultured alone (A, D, and G) or were cocultured with H9/IIIB cells (in a ratio 10:1) in the absence (B, E, and H) or the presence (C, F, and I) of 10 μg of AMD3100 per ml. The apoptosis occurring in these cultures was monitored after 24 h of culture by PI staining (A, B, and C), fluorescent TUNEL assay using dUTP (D, E, and F), or Annexin-V binding (G, H, and I). The figure shows PI and dUTP/TUNEL labeling (A to F) obtained by gating total lymphocyte population as assessed by forward and side scatter, whereas Annexin-V labeling (G, H, and I) was obtained by gating the CD4+ T-cell population, after simultaneous staining of cells with Annexin-V and Per-CP-labeled MAb Leu3a. The percentage of apoptotic cells is indicated in each histogram. Note the higher apoptosis and cell loss when CD4+ T cells are gated (G, H, and I). The figure shows a single representative of the three experiments performed.
The specificity of the antiapoptotic activity of AMD3100 has been evaluated in PBMC incubated with the anti-FAS MAb CH11. AMD3100 at concentrations that completely blocked the cell-surface-expressed envelope-induced apoptosis did not modify the apoptosis induced by engaging the FAS receptor (data not shown). Thus, HIV envelope-induced apoptosis is efficiently and specifically inhibited by the CXCR4 antagonist AMD3100.
The antiapoptotic activity of AMD3100 is cell dependent.
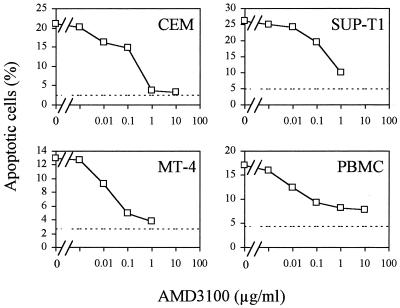
To evaluate the potency of CXCR4 ligands as inhibitors of HIV-1 envelope-induced apoptosis, we quantified the apoptosis occurring in cocultures of H9/IIIB with different target cells in the presence of increasing concentrations of the CXCR4 agonist SDF-1α or the CXCR4 antagonist AMD3100. In all cell lines tested, MT-4, CEM, SUP-T1, and PBMC, the bicyclam AMD3100 blocked both syncytium formation and single-cell killing by apoptosis in a dose-dependent manner. However, the IC50s were strongly dependent on cell type, ranging from 0.009 ± 0.004 and 0.013 ± 0.009 μg/ml (PBMC and MT-4 cells, respectively) to 0.24 ± 0.02 and 0.22 ± 0.03 μg/ml (SUP-T1 and CEM cells, respectively) (Fig. 3 and Table 1). Conversely, SDF-1α showed clear inhibitory effect only in MT-4 cells and PBMC but failed to block apoptosis in SUP-T1 and CEM cells (Table 1). Moreover, the IC50s found for SDF-1α were at least fivefold higher than those observed for AMD3100 (Table 1).
FIG. 3.
The potency of AMD3100 in inhibiting HIV-1 envelope-induced apoptosis is cell dependent. CEM, SUP-T1, MT-4 cells, and PBMC stimulated for 2 days were cocultured with H9/IIIB cells at a ratio of 10 target cells for each infected cell, in the absence or the presence of increasing concentrations of AMD3100, ranging from 1 ng/ml to 10 μg/ml. At 24 h of coculture, apoptosis was evaluated by PI staining. Dotted lines represent apoptosis levels of untreated cultures. The figure shows a single representative of the three experiments performed.
TABLE 1.
The inhibition of HIV-envelope-induced apoptosis by CXCR4 ligands
| Cell line | IC50 (μg/ml)a
|
|
|---|---|---|
| SDF-1α | AMD3100 | |
| CEM | >10 | 0.22 ± 0.03 |
| SUP-T1 | >1 | 0.24 ± 0.02 |
| MT-4 | 0.06 ± 0.03 | 0.013 ± 0.009 |
| PBMC | 0.12 ± 0.7 | 0.009 ± 0.004 |
Values were calculated from PI staining data by nonlinear regression as described in Materials and Methods. Experiments were performed in triplicate. Values are the means ± the standard deviations given by the fitting program.
We have previously noticed the correlation of SDF-1α antiapoptotic activity to the expression of CXCR4 (4). However, other authors have correlated the anti HIV-1 activity of SDF-1α to the expression of CD26, whose dipeptidyl peptidase IV activity regulates the affinity of SDF-1α for its receptor (42). We have evaluated the expression of these cell surface proteins and that of CD4 in the cell lines studied (CEM, SUP-T1, and MT-4) and in CD4+ T cells activated for 2 days with PHA/IL-2 (Table 2). The antiapoptotic activity of AMD3100 seems to inversely correlate to the expression of CXCR4 on the surface of target cells, which was low in MT-4 cells, moderate in stimulated CD4+ T-cells, and high in CEM or SUP-T1 cells. The potency of both AMD3100 and SDF-1α do not seem to be related to the expression of CD26, which was only clearly measurable in stimulated CD4+ T cells, or related to the expression of CD4, which was high in all the cells studied (Table 2).
TABLE 2.
The expression of CD4, CD26, and CXCR4 by different cell lines
| Cell line | Cell surface expressiona
|
||
|---|---|---|---|
| CD4 | CXCR4 | CD26 | |
| CEM | 99.6 ± 0.8 (164 ± 22) | 99.1 ± 0.8 (225 ± 65) | 1.1 ± 0.2 (5.2 ± 0.2) |
| SUP-T1 | 99 ± 1 (228 ± 15) | 99.8 ± 0.1 (425 ± 88) | 2.8 ± 0.4 (4.9 ± 0.5) |
| MT-4 | 98.6 ± 0.6 (138 ± 22) | 32 ± 12 (18 ± 6) | 1.5 ± 0.2 (3.7 ± 0.1) |
| PBMC (CD4+ T-cells)b | 100 (133 ± 42)b | 63 ± 14 (62 ± 22) | 52 ± 22 (19 ± 6) |
The expression of CD4, CD26, and CXCR4 was evaluated by FACS analysis as described in Materials and Methods. Results show the percentage of positive cells followed by the mean fluorescence intensity of the cell surface staining. Data represent the means ± the standard deviations of three independent experiments.
CD4+ T cells were gated for analysis of expression of CXCR4 and CD26.
DISCUSSION
The cytopathic effect of HIV-1 is the consequence of multiple levels of virus-to-cell interactions. Indeed, at least four HIV-1 gene products (Env, Tat, Nef, and Vpr) can contribute to CD4+ T-cell death (19, 28, 43, 45). However, the strong gp120-CD4 interaction suggests a specific action of HIV-1 envelope glycoprotein complex on the induction of apoptosis in CD4+ T cells (9, 28). In the infection model, two complementary mechanisms involving the HIV-1 envelope may explain the CD4+ cell death. According to the first scenario, infected cells die as a consequence of Env-mediated viral infection. In this case, cell death is not directly caused by the gp120/gp41 complex, but is caused by viral production, either by apoptosis or other mechanisms (20, 26). Such a dependence on infection would allow all HIV replication inhibitors to block HIV-1-induced apoptosis when added prior to infection (Fig. 1). In a second scenario, once the envelope glycoprotein complex expressed on the surface of infected cells it would be able to induce apoptosis of neighboring CD4+ or CD4− cells (23, 24, 28). In this case, productively infected cells would become apoptosis inducers, and no inhibition may be expected from all HIV replication inhibitors. However, inhibition may be achieved by those agents that block envelope-mediated contacts with target cells. Consistent with this hypothesis, it has been reported that the addition of the neutralizing anti-gp120 V3 loop MAb 110.4 to a culture of infected MT2 or CEM cells leads to improved cell viability (28, 44). Conversely, the addition of saquinavir, a protease inhibitor blocking a late step in the HIV-1 replicative cycle (9), or the treatment of an HIV-1-infected culture with the RT inhibitor AZT does not block cell-surface-expressed envelope-induced apoptosis (Fig. 1).
In the infection and coculture model presented here, the cell surface envelope glycoprotein plays a major role in apoptosis induction. It has been reported that the virus-associated envelope glycoprotein complex and recombinant envelope glycoproteins are not able to induce apoptosis in CD4+ T cells (4, 23, 45). In fact, cell-surface-expressed envelope glycoprotein complex and recombinant gp120 behave very differently. While the oligomeric complex, cell surface expressed or cross linked, is highly cytopathic for CD4+ T cells (2, 4), recombinant gp120 has been shown to induce cell death of different CD4− cell types, such as neurons and CD8+ T cells (23, 24), by its ability to interact with chemokine receptors. However, no apoptosis is observed in CD4+ T cells after short time cultures (reference 45 and unpublished data).
Apoptosis may play a key role in HIV-1 infection and pathogenesis in vivo. Lymph nodes and other lymphoid organs show continuous HIV-1 replication that may favor strong and persistent cell-to-cell contacts between infected and uninfected cells that lead to cell death by apoptosis of bystander cells (18). Accordingly, the blockade of CXCR4 by AMD3100, even without affecting the binding of gp120 to CD4 (12), could prevent uninfected cells from entering the irreversible apoptotic cascade. It should be noted that the IC50 of AMD3100 for the blockade of apoptosis is slightly higher than the values reported for its antiviral activity, which is in the 0.001-to-0.01-μg/ml range (16, 40, 41). Moreover, in cocultures of chronically infected and uninfected cells, AMD3100 inhibited apoptosis induction, syncytium formation, and p24 production (data not shown). Taken together, these data point out that in conditions where apoptosis is inhibited, cell-to-cell viral transmission and direct viral infection are also completely abolished. Therefore, AMD3100 seems to block CD4+ T-cell depletion in vitro by a dual mechanism. On the one hand, AMD3100 acts as a potent inhibitor of HIV-1 replication, thus blocking infection and further death of cells (Fig. 1). On the other hand, AMD3100 also blocks the death of uninfected cells by blocking cell-to-cell fusion and the induction of apoptosis by productively infected cells (Fig. 1 and 3).
The differences observed between the IC50s for antiviral (16, 40, 41) and antiapoptotic activities of AMD3100 (Table 1) in the cell lines tested are intriguing. Apoptosis induction requires cell-to-cell contacts, whereas antiviral activity is measured in cell-free virus preparations. The reported differences between cell-to-cell and virus-to-cell fusion probably account for the discrepancies found in these effects of AMD3100 (36). We have found a rough correlation between the level of expression of CXCR4 and the antiapoptotic activity of AMD3100. Although the exact relevance of this correlation is unclear, it seems logical to assume that higher concentrations of AMD3100 are necessary to block CXCR4 when this receptor is expressed at high density, because very low levels of coreceptor expression seem to be necessary to support apoptotic events (Table 2). Further investigations will be required to determine the stoichiometric requirements of CXCR4 in cell-to-cell fusion and apoptosis induction.
The therapeutic potential of targeting apoptosis as an approach towards the treatment of AIDS has been evaluated in vitro. It is not clear whether inhibition of apoptosis per se would be beneficial to HIV-1-infected patients. Inhibition of apoptosis by the use of caspase inhibitors leads to enhanced HIV replication in vitro (7), and a similar effect has been observed in experimental models of Bcl-2 overexpression (39). The specific inhibition of HIV-1-induced apoptosis by AMD3100 might represent a benefit in the treatment of HIV infection, since the strong antiviral activity of this compound should prevent the increased HIV replication observed in other models of apoptosis inhibition, as in the case of caspase inhibitors or Bcl-2 overexpression (7, 39).
The chemokine receptor CXCR4 is a seven-transmembrane G-protein-coupled receptor (29) that, in addition to its role in HIV infection, modulates trafficking of immune cells. As with many G-protein-coupled receptors, the interaction of the naturally occurring CXCR4 agonist SDF-1α activates different signaling pathways that result in increased intracellular calcium levels (5, 34) and phosphorylation of cellular substrates from the ERK pathway (11, 37). These signaling events lead to a chemotactic response in T- and B-lymphocytes (5) or the stimulation of pre-B lymphocytes (31). The importance of these SDF-1-dependent responses has been demonstrated by the fact that SDF-1-knockout mice die perinatally due to defects in B-cell lymphopoiesis and bone marrow myelopoiesis (32). Although this might raise concern as to the use of CXCR4 antagonists as therapeutic agents, the bicyclam AMD3100 did not show toxic effects in SCID-hu Thy-Liv mice at plasma concentrations which inhibit viral replication and potentially inhibit envelope-induced apoptosis in human PBMC (10). The results presented here add to the potential value of AMD3100 in the treatment of HIV-1 infected individuals bearing X4 isolates. However, further studies and current clinical trials (C. Hendrix, C. Flexner, R. Macfarland, C. Giandomenico, A. Schweiter, and G. Henson, Abstr. 6th Conf. Retrovir. Opportun. Infect., abstr. 610, 1999) of AMD3100 will be necessary to evaluate the role of CXCR4 in adult individuals and the therapeutic potential of AMD3100.
ACKNOWLEDGMENTS
We thank Ana María García and Arantxa Gutiérrez for excellent technical assistance.
This work was supported in part by the Fundació IRSICaixa and the Spanish “Fondo de Investigaciones Sanitarias” (FIS) project 98/0868. J. Blanco is a researcher of the “Fundació per a la Recerca Biomèdica Germans Trias i Pujol” FIS project 98/3047.
REFERENCES
- 1.Berkowitz R D, Alexander S, Bare C, Linquist-Stepps V, Bogan M, Moreno M E, Gibson L, Wieder E D, Kosek J, Stoddart C A, McCune J M. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J Virol. 1998;72:10108–10117. doi: 10.1128/jvi.72.12.10108-10117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berndt C, Mopps B, Angermuller S, Gierschik P, Krammer P H. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T-cells. Proc Natl Acad Sci USA. 1998;95:12556–12561. doi: 10.1073/pnas.95.21.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco J, Canela E I, Mallol J, Lluís C, Franco R. Characterization of adenosine receptors in brush border membranes. Br J Pharmacol. 1992;107:671–678. doi: 10.1111/j.1476-5381.1992.tb14505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco J, Jacotot E, Cabrera C, Cardona A, Clotet B, De Clercq E, Esté J A. The implication of the chemokine receptor CXCR4 in HIV-1 envelope-induced apoptosis is independent of the G protein-mediated signaling. AIDS. 1999;13:909–917. doi: 10.1097/00002030-199905280-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 6.Bour S, Geleziunas R, Wainberg M A. The human immunodeficiency virus type 1 (HIV-1) CD4 receptor and its central role in promotion of HIV-1 infection. Microbiol Rev. 1995;59:63–93. doi: 10.1128/mr.59.1.63-93.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinnaiyan A M, Woffedin C, Dixit V M, Nabel G J. The inhibition of apoptotic ICE-like proteases enhances HIV replication. Nat Med. 1997;3:333–338. doi: 10.1038/nm0397-333. [DOI] [PubMed] [Google Scholar]
- 8.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α and MIP-1β as the major suppressive factor produced by CD8+ T-cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 9.Corbeil J, Richman D D. Productive infection and subsequent interaction of CD4-gp120 at the cellular membrane is required for HIV-induced apoptosis of CD4+ T-cells. J Gen Virol. 1995;76:681–690. doi: 10.1099/0022-1317-76-3-681. [DOI] [PubMed] [Google Scholar]
- 10.Datema R, Rabin L, Hincenbergs M, Moreno M B, Warren S, Linquist V, Rosenwirth B, Seifert J, McCune J M. Antiviral efficacy in vivo of the anti-human immunodeficiency virus bicyclam SDZ SID 791 (JM 3100), an inhibitor of infectious cell entry. Antimicrob Agents Chemother. 1996;40:750–754. doi: 10.1128/aac.40.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Clercq E, Yamamoto N, Pauwels R, Balzarini J, Witvrouw M, De Vreese K, Debyser Z, Rosenwirth B, Peichl P, Datema R. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother. 1994;38:668–674. doi: 10.1128/aac.38.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donzella G A, Schols D, Lin S W, Esté J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 14.Doranz B J, Grovit-Ferbas K, Sharron M P, Mao S H, Goetz M B, Daar E S, Doms R W, O'Brien W A. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J Exp Med. 1997;186:1395–1400. doi: 10.1084/jem.186.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Souza M P, Harden V A. Chemokines and HIV-1 second receptors. Nat Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 16.Esté J A, Cabrera C, De Clercq E, Struyf S, Van Damme J, Bridger G, Skerlj R T, Abrams M J, Henson G, Gutierrez A, Clotet B, Schols D. Activity of different bicyclam derivatives against human immunodeficiency virus depends on their interaction with the CXCR4 chemokine receptor. Mol Pharmacol. 1999;55:67–73. doi: 10.1124/mol.55.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 18.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 19.Fujii Y, Otake K, Tashiro M, Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 1996;393:93–96. doi: 10.1016/0014-5793(96)00859-9. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi R T, Chen B K, Straus S E, Dale J K, Lenardo M J, Baltimore D. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glushakova S, Grivel J C, Fitzgerald W, Silwester A, Zimmerberg J, Margolis L B. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat Med. 1998;4:346–349. doi: 10.1038/nm0398-346. [DOI] [PubMed] [Google Scholar]
- 22.Gulizia R J, Levy J A, Mosier D E. The envelope gp120 gene of human immunodeficiency virus type 1 determines the rate of CD4-positive T-cell depletion in SCID mice engrafted with human peripheral blood leukocytes. J Virol. 1996;70:4184–4187. doi: 10.1128/jvi.70.6.4184-4187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brien W A, Verdin E. Apoptosis of CD8+ T-cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 24.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 25.Jacotot E, Krust B, Callebaut C, Laurent-Crawford A G, Blanco J, Hovanessian A G. HIV-1 envelope glycoproteins-mediated apoptosis is regulated by CD4 dependent and independent mechanisms. Apoptosis. 1997;2:47–60. doi: 10.1023/a:1026435625144. [DOI] [PubMed] [Google Scholar]
- 26.Kolesnitchenko V, King L, Riva A, Tani Y, Korsmeyer S J, Cohen D L. A major human immunodeficiency virus type 1-initiated killing pathway distinct from apoptosis. J Virol. 1997;71:9753–9763. doi: 10.1128/jvi.71.12.9753-9763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurent-Crawford A G, Krust B, Muller S, Riviere Y, Rey-Cuille M A, Bechet J M, Montagnier L, Hovanessian A G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;185:829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 28.Laurent-Crawford A G, Krust B, Riviere Y, Desgranges C, Muller S, Kieny M P, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retrovir. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 29.Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 30.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, Kishimoto T, Yamamoto N, Nagasawa T. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 34.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 35.Oravecz T, Pall M, Norcross M A. β-Chemokine inhibition of monocytotropic HIV-1 infection. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 36.Pantaleo G, Butini L, Graziosi C, Poli G, Schnittman S M, Greenhouse J J, Gallin J I, Fauci A S. Human immunodeficiency virus (HIV) infection in CD4+ T lymphocytes genetically deficient in LFA-1: LFA-1 is required for HIV-1-mediated cell fusion but not for viral transmission. J Exp Med. 1991;173:511–514. doi: 10.1084/jem.173.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popik W, Hesselgesser J E, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J Virol. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Premack B A, Schall T J. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 39.Sandstrom P A, Pardi D, Goldsmith C S, Chengying D, Diamond A M, Folks T M. Bcl-2 expression facilitates human immunodeficiency virus type-1 mediated cytopathic effects during acute spreading infections. J Virol. 1996;70:4617–4622. doi: 10.1128/jvi.70.7.4617-4622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schols D, Esté J A, Henson G, De Clercq E. Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antiviral Res. 1997;35:147–156. doi: 10.1016/s0166-3542(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 41.Schols D, Struyf S, Van Damme J, Esté J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shioda T, Hiroyuki K, Ohnishi Y, Tashiro K, Ikegawa M, Nakayama E E, Lu H, Kato A, Sakai Y, Liu H, Honjo T, Nomoto A, Iwamoto A, Morimoto C, Nagai Y. Anti-HIV-1 and chemotactic activities of human stromal cell derived factor 1α (SDF-1α) and SDF-1β are abolished by CD26/dipeptidyl peptidase IV-mediated cleavage. Proc Natl Acad Sci USA. 1998;95:6331–6336. doi: 10.1073/pnas.95.11.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart S A, Poon B, Jowett J B, Chen I S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terai C, Kornbluth R S, Pauza C D, Richman D D, Carson D A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Investig. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dheins J, Walczack H, Debatin K M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, McLane M F, Ratner L, O'brien W, Collman R, Essex M, Lee T H. Killing of primary CD4+ T-cells by non-syncytium inducing macrophage tropic human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1994;91:10237–10241. doi: 10.1073/pnas.91.21.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]