Abstract
Background
The outcomes of hematogenous periprosthetic joint infection (PJI) and reasons for failure are largely unknown.
Methods
The outcomes of consecutive patients with hematogenous PJI treated at our institution between 2010 and 2019 were evaluated. Failure was classified as persistence or relapse of infection or new infection. Failure-free survival was assessed using Kaplan-Meier analysis. Proportions between groups were compared with the Fisher exact test.
Results
One hundred thirty-two hematogenous PJI episodes involving knee (n = 76), hip (n = 54), shoulder (n = 1), or elbow (n = 1) prostheses experienced by 110 patients were included. The median follow-up (range) was 20.7 (0.2–89.9) months. Hematogenous PJIs were caused by Staphylococcus aureus (n = 49), Streptococcus spp. (n = 36), Enterococcus faecalis (n = 17), Enterobacterales (n = 16), coagulase-negative staphylococci (n = 9), and other (n = 6). Debridement and implant retention were performed in 50 (38%), prosthesis exchange or removal in 79 (60%), and no surgery in 3 episodes (2%). Treatment failed in 42 episodes (32%), including 6 infection-related deaths. Among 36 nonfatal failures, 21 were caused by a new pathogen and 8 by the same pathogen, in 7 episodes no pathogen was isolated. Of all nonfatal failures, 19 (53%) PJIs were of hematogenous origin. Identification of the primary focus, causative pathogen, and CRIME80 Score did not influence treatment outcome, but the failure rate was higher following prosthesis retention compared with multistage exchange.
Conclusions
Persistence-/relapse-free survival after treatment of hematogenous PJI was high (84%). New hematogenous PJI due to the same or a new pathogen occurred frequently, reducing treatment success to 62% after 4 years of follow-up, suggesting an individual predisposition to hematogenous PJI. The outcome was similar for different pathogens but worse in episodes treated with prosthesis retention compared with multistage exchange.
Keywords: hematogenous, outcome, periprosthetic joint infection, treatment failure
Hematogenous periprosthetic joint infection (PJI) is a well-described, distinct clinical entity [1, 2]. In contrast to postoperative and contiguous (per continuitatem) infection, the pathogen in hematogenous PJI spreads from a distant focus of infection or port of entry via the bloodstream to the prosthesis. Most hematogenous PJIs occur months or years after arthroplasty, and typically present with (sub)acute symptoms [3]. Therefore, some authors categorize all PJI episodes manifesting >3 months after implantation with acute symptoms as “late acute PJI” [4], misleadingly implying a hematogenous route of infection. The estimated incidence ranges from 10% to 35% [5–7]. However, as uniform definition criteria have not been established [8] and the route of infection is not reported in most articles, the real incidence of hematogenous PJI remains unknown.
Another controversy is the optimal surgical and antimicrobial treatment strategy for hematogenous PJI. In early postoperative PJI (within 3–4 weeks after implantation), the combination of debridement, antibiotics, and implant retention (DAIR) is recommended by most authors [9–12]. The background of this recommendation is that a “young” (immature) biofilm can be eradicated with biofilm-active antibiotics—in conjunction with thorough surgical debridement and exchange of mobile implant components. Compared with the exchange of the complete prosthesis, the DAIR strategy bears the advantages of lower surgical invasiveness including less blood loss, reduced bone and soft tissue damage, lower risk of superinfection, and faster recovery [13].
In acute hematogenous PJI, however, some authors suggest explantation of the prosthesis rather than the DAIR strategy, at least in a subgroup of patients with risk factors for treatment failure of the DAIR procedure. Conflicting data regarding the outcome of acute late (presumably hematogenous) PJI exist; some authors have reported failure rates of 21%–24% [6, 13, 14], while others demonstrated failure rates of 45%–59% [4, 15–17]. Several factors may explain this wide variability, including heterogenous definition criteria for failure, inaccurate diagnosis of hematogenous PJI, and nonuniform treatment strategies.
In the present study, we analyzed the outcome of hematogenous PJIs, which were diagnosed by comprehensive definition criteria, in particular excluding acute exacerbations of chronic postoperative PJI. In addition, the reasons for treatment failure were evaluated and classified as persisting or relapsing vs new infection. A systematic analysis of uniformly defined hematogenous PJI and risk factors for treatment failure is needed to improve the outcome of hematogenous PJI.
METHODS
Study Design
This retrospective cohort study was conducted in a university tertiary health care center, providing advanced specialty care to a population of about 4 million inhabitants. The orthopedic department has an interdisciplinary septic surgery unit, treating about 320 patients with PJI annually.
Patient Consent
The study protocol was approved by the institutional ethical committee of the Charité – University Medicine Berlin (EA04/040/14), and the need for informed consent was waived as data utilized in this retrospective study have been de-identified. The study was performed in accordance with the most recent iteration of the Declaration of Helsinki.
Study Population
All consecutive episodes of hematogenous PJI treated at our institution from January 2010 through November 2019 were screened for inclusion. If multiple prosthetic joints were infected simultaneously, each joint was counted as a separate episode. Another hematogenous PJI in the same patient was counted as a new episode if (i) another joint was affected or (ii) the same joint was affected with a different pathogen or the same pathogen more than one year after the previous episode. Episodes for which no follow-up was available after discharge or that were treated with a noncurative treatment approach were excluded. Episodes were identified through the institutional PJI database. Part of this cohort was referred to in a study evaluating characteristics of hematogenous PJI [3].
Definitions
PJI was diagnosed if at least 1 of the following criteria was fulfilled, as applied in previous studies [3, 18]: (i) macroscopic purulence or presence of a sinus tract, (ii) increased synovial fluid leukocyte count (>2 × 109/L) or percentage of granulocytes (>70%), (iii) isolation of a high-virulent organism from ≥1 sample/s OR isolation of an identical organism in ≥2 samples in case of low-virulent organisms, (iv) positive histopathology defined as >23 granulocytes in 10 high-power fields (ie, periprosthetic membrane type II or III according to Krenn & Morawietz) [19].
As in a previous study [3], hematogenous PJI was defined if the onset of symptoms was ≥1 month after joint surgery, the onset of symptoms was acute following a pain-free period, and at least 1 of the following 4 criteria were fulfilled: (i) positive blood cultures and evidence of a distant infectious focus consistent with the pathogen, (ii) positive prosthetic site culture and evidence of a distant infectious focus consistent with the pathogen, (iii) the onset of symptoms was ≥2 years after last arthroplasty surgery and evidence of a distant infectious focus or positive blood cultures, or (iv) judged as hematogenous PJI by the infectious diseases physician based on clinical and chronological features and microbiological findings.
Acute PJI was defined by duration of signs of infection at the prosthesis site of less than 4 weeks, with or without fever. PJI was classified as chronic if symptom duration exceeded 4 weeks.
Failure was defined as (i) persistent or relapsing PJI with the same or no isolated pathogen, (ii) a new PJI caused by a pathogen different from the index episode, or (iii) infection-related death within 3 months of PJI diagnosis and without an alternative explanation for death, for example, pulmonary embolism or other nonseptic complications. Any subsequent PJI episode after inclusion was considered a failure, irrespective of the time of occurrence and pathogenesis. However, these 2 characteristics were considered for inclusion of the episode as index hematogenous PJI in the cohort.
Failure due to hematogenous PJI was defined according to the above-mentioned criteria. If definition criteria for hematogenous infection were not fulfilled and no contiguous focus was present, failure was judged as early or delayed postoperative PJI. Failure that occurred during treatment of index hematogenous PJI was classified as early failure, and failure after completion of index multimodal treatment was considered late failure. The need for repeated debridement or modification of surgical strategy with secondary removal of the prosthesis in the immediate postoperative course after initial DAIR strategy, that is, during the same hospital stay, was not considered a failure.
Surgical and Antimicrobial Treatment
The institutional algorithmic approach and standardized antibiotic scheme were applied [20]. According to this standardized approach, acute infections were treated with DAIR in patients with well-fixed prostheses, uncompromised soft tissue conditions, and if the pathogen and its susceptibility were known and biofilm-active antibiotic treatment available. One-stage exchange was performed in cases of chronic infections or loose prostheses without history of previous revisions. Multistage exchange was applied in cases of compromised soft tissues, sinus tract, and/or in patients with a history of multiple previous revisions. Removal without reimplantation of the prosthesis (ie, resection arthroplasty in hips, permanent arthrodesis in knees) was performed only exceptionally in patients with severe comorbidities or if no functional benefit could be expected (eg, bedridden patients, compromised musculature).
If an implant was in situ after completion of surgical treatment, biofilm-active antibiotics (ie, rifampin for staphylococcal infection, ciprofloxacin for gram-negative rods) were administered whenever possible [2, 21].
Follow-up Evaluation
Patients were followed up in the outpatient clinic 3, 6, and 12 months after revision surgery, and thereafter by annual visits. Clinical, laboratory, and radiological evaluations were interpreted by the interdisciplinary team, consisting of an orthopedic surgeon and an infectious diseases physician. If patients did not appear for the scheduled follow-up visit, they were contacted by mail and/or phone using a standardized case report form. The CRIME80 Score recently proposed to predict failure risk after DAIR in late acute PJI was evaluated [4].
Statistical Analyses
Categorical variables were compared using the Fisher exact test or chi-square test, continuous variables using the Student t test or Mann-Whitney U test, as appropriate. A 2-sided P value <.05 was considered statistically significant. Probability of failure-free survival and 95% confidence interval were estimated using the Kaplan-Meier survival method, and subgroups were compared using the log-rank test. An alpha level of .05 was considered significant. For statistical analyses and graphics, Prism (version 9; GraphPad, San Diego, CA, USA) and SPSS (IBM SPSS Statistics, version 27, Ehningen, Germany) were used.
RESULTS
Demographics
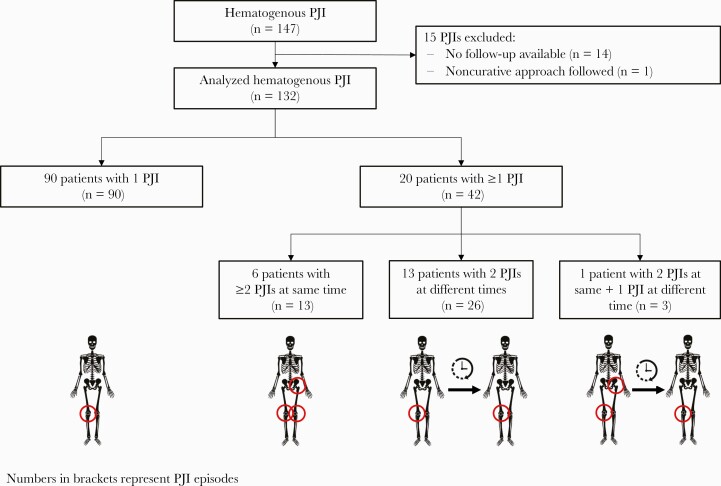
Of 147 identified hematogenous PJI episodes treated during the study period, 15 episodes were excluded due to loss to follow-up (n = 14) or noncurative approach (n = 1, establishment of permanent sinus tract) (Figure 1). One hundred thirty-two episodes in 110 patients were included in this study; respective baseline data are summarized in Table 1. Ninety patients with a single PJI episode were included once in the analysis, 20 patients suffered from multiple episodes either simultaneously or at different times and hence contributed more than 1 episode to the cohort. Patients with single vs multiple episodes were similar regarding sex, previous surgeries, implant fixation, and comorbidities, but patients with more than 1 episode were younger (median age, 66 vs 76 years; P = .014) and more often had intravascular devices (eg, cardiac pacemaker, long-term intravenous catheter, prosthetic heart valve, vascular prosthesis) in situ than patients with a single episode (40% vs 18%; P = .030).
Figure 1.
Flowchart of study patients. Of 147 identified hematogenous PJI episodes treated during the study period, 15 episodes were excluded. Abbreviation: PJI, periprosthetic joint infection.
Table 1.
Demographic Data and Comorbidities of 110 Patients With Hematogenous PJI, Stratified Into Those With Single and Multiple Episodes
| Characteristic | All Patients (n = 110) |
Patients With Single Episode (n = 90) |
Patients With Multiple Episodes (n = 20) |
P Value |
|---|---|---|---|---|
| Age,a median (range), y | 74 (36–92) | 76 (36–92) | 66 (49–87) | .014 |
| Sex, female | 54 (49) | 45 (50) | 9 (45) | .686 |
| Patients with ≥1 previous revision | 65/109 (60) | 55/89 (62) | 10 (50) | .331 |
| Patients with ≥1 previous septic revision | 47/108 (44) | 40/88 (46) | 7 (35) | .395 |
| No. of previous surgeries, median (range) | 1 (0–10) | 1 (0–10) | 0.5 (0–7) | .322 |
| Cemented prosthesis | 84 (76) | 70 (78) | 14 (70) | .459 |
| Fever/rigors | 35/105 (33) | 32/87 (37) | 3/18 (17) | .099 |
| Comorbidities | ||||
| Chronic renal disease | 44/106 (42) | 36/86 (42) | 8 (40) | .879 |
| Diabetes mellitus | 30 (27) | 26 (29) | 4 (20) | .581 |
| Rheumatoid arthritis | 9 (8) | 6 (7) | 3 (15) | .360 |
| Active or past malignancy | 26 (24) | 22 (24) | 4 (20) | .778 |
| Liver cirrhosis | 1 (1) | 0 (0) | 1 (5) | .182 |
| Chronic obstructive pulmonary disease | 6 (6) | 4 (4) | 2 (10) | .299 |
| Presence of implantable intravascular device(s) | 24 (22) | 16 (18) | 8 (40) | .030 |
Data are No. (%) of episodes if not indicated otherwise.
Bold represent P values indicating statistical significance.
Abbreviation: PJI, periprosthetic joint infection.
If a patient was included more than once, the age at the time of the first episode was considered.
Characteristics of Infection
Most episodes affected knee prostheses (n = 76), followed by hip prostheses (n = 54) (Table 2). Most presented with acute symptoms (76%).
Table 2.
Characteristics of 132 Hematogenous PJI Episodes
| Characteristic | Episodes (n = 132) |
|---|---|
| Affected prosthesis | |
| Knee | 76 (58) |
| Hip | 54 (41) |
| Shoulder | 1 (1) |
| Elbow | 1 (1) |
| Time from last surgery to infection, median (range), y | 2.5 (0.1–28.8) |
| Time from primary implantation to infection, median (range), y | 9.9 (0.1–34.6) |
| Acute manifestation (<4 wk symptom duration) | 98/129 (76) |
| Occurrence of infection after last surgery | |
| Early (<3 mo) | 17/130 (13) |
| Delayed (3–24 mo) | 43/130 (33) |
| Late (>24 mo) | 70/130 (54) |
| Clinical findings at admission | |
| New onset of joint pain after an uneventful course | 124/128 (97) |
| Local signs of inflammationa | 89/129 (69) |
| Fever (>38°C) | 46/126 (37) |
| Radiological findings at admission | |
| Implant loosening | 21 (16) |
| Laboratory findings at admission | |
| Serum C-reactive protein concentration, median (range), mg/L | 148 (3–579) |
| Synovial fluid leukocyte count, median (range), 109/L | 67.9 (0.8–2215.5) |
| Microbiologyb | |
| Staphylococcus aureusc | 49 (37) |
| Streptococcid | 36 (27) |
| Enterococcie | 17 (13) |
| Gram-negative rodsf | 16 (12) |
| S. epidermidis | 9 (7) |
| Clostridium spp.g | 2 (2) |
| Candia albicans | 2 (2) |
| Culture negative | 2 (2) |
| Portal of pathogen entry/primary infection focus | |
| Unknown | 39 (30) |
| Cardiovascular | 32 (24) |
| Urogenital tract | 18 (14) |
| Skin | 17 (13) |
| Oral cavity | 15 (11) |
| Gastrointestinal tract | 8 (6) |
| Otherh | 3 (2) |
Data are No. (%) of episodes, if not indicated otherwise.
Abbreviation: PJI, periprosthetic joint infection.
Including swelling, erythema, warmth at the index joint.
One mixed infection (S. agalactiae and E. coli); therefore, the sum exceeds 100%.
Including 7 methicillin-resistant S. aureus strains.
Including Streptococcus agalactiae (n = 13), S. dysgalactiae (n = 10), S. oralis/mitis (n = 5), S. gallolyticus (n = 3), S. gordonii (n = 2), S. anginosus (n = 2) S. canis, S. constellatus, S. pyogenes, S. sanguinis, and S. parasanguinis (n = 1 each). One patient had mixed streptococcal infection.
Including E. faecalis (n = 15), E. faecium (n = 2).
Including Escherichia coli (n = 9), Proteus mirabilis (n = 3), Campylobacter coli (n = 1), Haemophilus parainfluenzae (n = 1), Enterobacter cloacae (n = 1), Pseudomonas aeruginosa (n = 1).
Clostridium innocuum (n = 1), C. perfringens (n = 1).
Epidural abscess and meningitis (n = 1), contralateral PJI (n = 1), pneumonia (n = 1).
Microbiological Findings
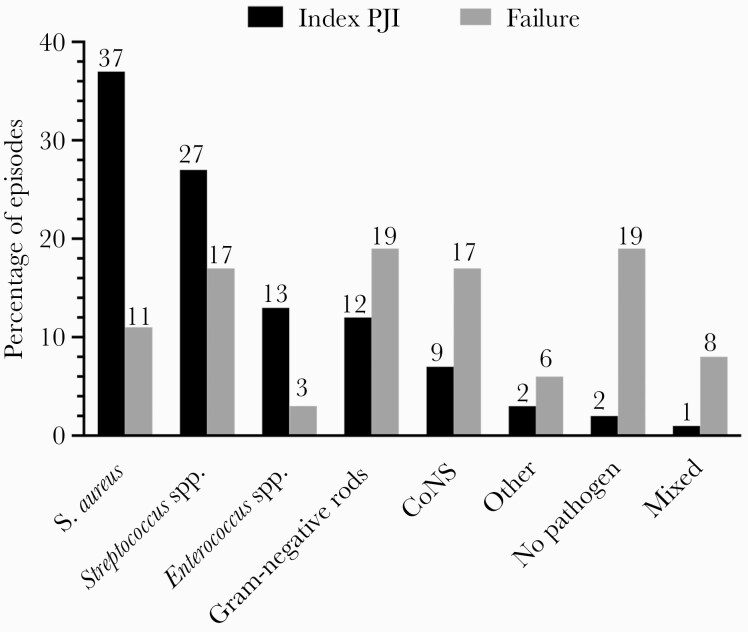
Isolated pathogens and primary infection foci are shown in Table 2 and Figure 2. Staphylococcus aureus and streptococci account for more than half of index episodes, followed by enterococci and gram-negative rods. The most common primary infection foci were cardiovascular infections. Bacteremia was documented in 61 of 99 episodes (62%), in which blood cultures were sampled.
Figure 2.
Causative pathogens and their frequency of index hematogenous PJI (black columns, %) and failures (gray columns, %). Abbreviations: CoNS, coagulase-negative staphylococci; PJI, periprosthetic joint infection.
Surgical and Antimicrobial Treatment
Therapeutic details are shown in Table 3. All but 3 PJI episodes were treated with revision surgery, and antimicrobial treatment was administered in all episodes. In 3 episodes, the known primary infectious focus was not addressed, including 1 patient with periodontitis and 2 patients with Streptococcus gallolyticus bacteremia who refused further diagnostics and/or treatment.
Table 3.
Treatment of 132 Hematogenous PJI Episodes
| Characteristic | Episodes (n = 132) |
|---|---|
| Surgical procedure for PJI, No. (%) | |
| No surgerya | 3 (2) |
| Retention of prosthesisb | 50 (38) |
| Removal of prosthesis | 79 (60) |
| Two-stage/multistage exchangec | 69 (87) |
| 1-stage exchange | 4 (5) |
| No reimplantation | 6 (8) |
| Median No. of surgeries performed (range) | 2 (1–6) |
| Treatment of primary focus/portal of pathogen entry | |
| No specific interventiond | 63 (48) |
| Antimicrobial treatment only | 42 (32) |
| Surgery | 27 (20) |
| Antimicrobial treatment | |
| Duration of treatment, median (range), wk | 15 (3–243) |
| Duration of intravenous treatment, median (range), wke | 4 (1–16) |
| Duration of oral treatment, median (range), wkf | 12 (3–24) |
| Episodes treated with biofilm-active antibiotics, No. (%)g | 72/93 (77) |
| Episodes treated with antimicrobial suppression, No. (%)h | 29/130 (22) |
Abbreviation: PJI, periprosthetic joint infection.
Due to hemodynamic instability.
In 46 episodes, mobile implant components were exchanged.
Median interval between ex- and reimplantation (range) was 74 (18–273) days.
Including 39 episodes with unknown focus, 11 episodes after a (para)medical intervention, and 10 episodes that originated from a noninfectious skin lesion. Refusal of further diagnostics/treatment in 3 episodes.
In 129 of 130 episodes with known antimicrobial treatment, intravenous therapy was administered initially.
In 116 of 127 episodes, oral therapy was administered.
That is, rifampin for Staphylococcus sp. or quinolones for gram-negative rods. Thirty-nine episodes were caused by enterococci, streptococci, or Candida spp., for which no biofilm-active treatment is available.
That is, prolonged oral treatment for 30–240 weeks.
Follow-up Evaluation
The median time from diagnosis to most recent contact or failure (range) was 20.7 (0.2–89.9) months. For episodes with a successful outcome, the median follow-up (range) was 23.2 (0.4–89.9) months.
Outcome Analysis
Of 132 hematogenous PJI episodes, infection-free status was reported in 90 episodes (68%) at the time of last contact. Of 42 failures, early failure occurred in 17 episodes (40%) before the surgical procedures and/or antimicrobial treatment were completed, and late failure occurred in 25 episodes (60%). Death was documented in 15 episodes (11%), in 6 (5%) of which mortality was attributed to hematogenous PJI, concomitant bacteremia, or primary infection.
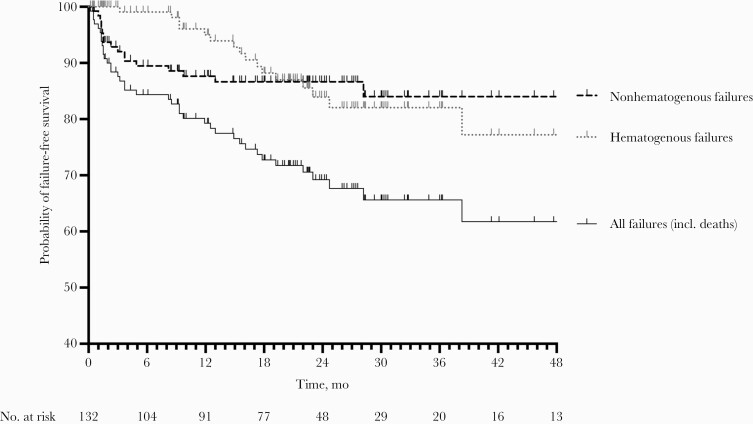
Most common pathogens isolated in failure episodes were gram-negative rods (n = 7), followed by coagulase-negative staphylococci (n = 6) and streptococci (n = 6) (Figure 2). Infection-free status subsisted in 79% after 12 months, 69% after 24 months, and 62% after 48 months (Figure 3). The success rate remained similar when only episodes with a minimum follow-up of 12 months were considered, that is, 84 infection-free episodes of 125 episodes (67%).
Figure 3.
Failure-free survival curve. Failures were stratified according to hematogenous and nonhematogenous route of infection and total failures.
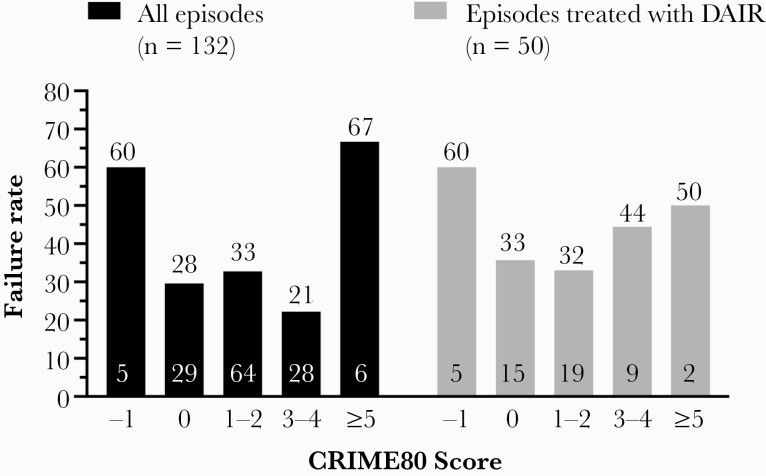
The failure rate was similar in subgroups stratified by pathogen, that is, S. aureus (14/49 episodes, 29%), coagulase-negative staphylococci (2/9 episodes, 22%), streptococci (12/35 episodes, 34%), enterococci (6/17 episodes, 35%), and gram-negative rods (6/15 episodes, 40%). Furthermore, the failure rate was similar whether the primary focus was identified or not (30/93 episodes, 32%, vs 12/39 episodes, 31%). In contrast, the outcome was worse in patients treated with DAIR/retention of the prosthesis (failure in 49%) vs implant removal/multistage exchange (failure in 32%; P = .033) (Figure 4). No correlation between CRIME80 Score and failure rate was observed, either in the subgroup treated with DAIR, as originally described, or in those treated with any surgical option (Figure 5).
Figure 4.
Failure-free survival curve of episodes treated with multistage exchange and those with prosthesis retention (DAIR). Abbreviation: DAIR, debridement, antibiotics, and implant retention.
Figure 5.
Failure rate according to CRIME80 Score for all episodes (black columns) and those treated with DAIR (gray columns). The numbers above the bar represent the failure rate (%), and the numbers at the bottom of the bar represent the absolute number of episodes with the respective CRIME 80 Score. Abbreviation: DAIR, debridement, antibiotics, and implant retention.
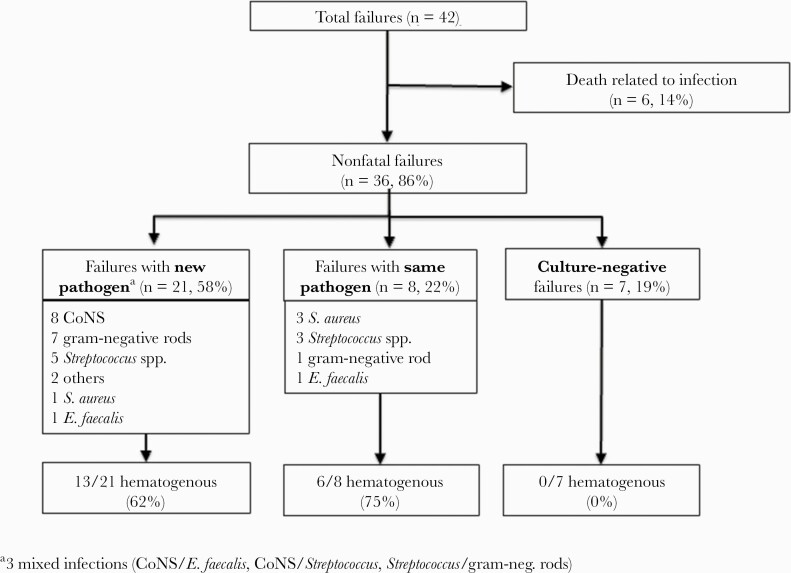
Among 126 episodes in surviving patients, reoccurrence of infection was documented in 36 episodes (29%), including infection by a new pathogen (in 21 episodes), by the same pathogen (in 8 episodes), and without pathogen isolation (in 7 episodes) (Figure 6). Of note, 19 episodes (53% of nonfatal failures) were caused by another episode of hematogenous spread onto the prosthesis, 13 by a new pathogen and 6 by the same pathogen as during index PJI. Most failures (34/42, 81%) occurred within 2 years after surgery (Figure 3), the majority of failures of nonhematogenous pathogenesis in the first year and of hematogenous pathogenesis in the second year after index PJI. In 5 out of 16 failures after DAIR (31%), the hematogenous route of infection led to reinfection, whereas after removal of the prosthesis 14 of 20 failures (70%) were again caused by hematogenous spread.
Figure 6.
Chart of failures according to microbiological findings and route of infection. Abbreviation: CoNS, coagulase-negative staphylococci.
DISCUSSION
In our cohort, treatment failure occurred in 32% of 132 hematogenous PJI episodes. In other studies, failure rates ranged from 21% to 55% [5, 6, 13, 14, 17, 22]. The wide span may reflect inaccurate diagnosis of the hematogenous route of infection. Considering all late acute PJI episodes as hematogenous might have led to misclassification of acute exacerbations of chronic postoperative PJIs that had remained clinically “silent” for a prolonged period.
Typically, late hematogenous PJIs present acutely after an asymptomatic period, whereas patients with acute exacerbation of a chronic postoperative low-grade infection tend to report at least some degree of perpetual discomfort, pain, and/or functional deficit. Wrongly classifying chronic infections as acute hematogenous PJI may lead to application of prosthesis retention (DAIR) instead of the indicated prosthesis exchange, which in turn may explain the worse outcome with DAIR. Furthermore, if surgery for hematogenous PJI is delayed for more than 3–4 weeks, acute infections “chronify” and, considering biofilm maturity, should be treated with prosthesis exchange. Thorough history-taking and sound clinical examination are crucial to define the origin of late acute PJI. In this study, therefore, we added additional criteria to confirm the diagnosis of hematogenous PJI, namely (i) positive blood or prosthetic site culture and evidence of a distant infectious focus consistent with the pathogen or (ii) judgment by the infectious diseases physician.
The management of hematogenous PJI remains controversial. Whereas guidelines recommend DAIR for acute hematogenous PJI, some authors advocate a 2-stage or multistage prosthesis exchange in specific populations due to poorer outcome with DAIR [17, 23]. In 1 study [5], prosthesis retention failed in 55% of 33 analyzed episodes. However, in a quarter of included episodes the “surgical” approach was limited to joint aspiration, likely explaining this poor outcome [5]. The importance of a meticulous debridement with exchange of all mobile parts of the prosthesis has recently been substantiated by a multicenter observational analysis of 340 “late acute” PJIs, in which the omission of mobile component replacement was found to be a significant risk factor for failure [4]. The authors reported treatment success of 55% in episodes of late acute PJI treated with DAIR [4], but “late acute” PJI probably included acute exacerbation of a chronic postoperative low-grade infection and contiguous infection from adjacent anatomic sights. Nevertheless, our data confirm worse outcomes if prosthesis retention is applied (Figure 4). However, due to the retrospective design of the study, a selection bias is likely.
During the first year, most failures in our study were caused by perioperative introduction of new pathogens or persistence of infection, whereas new hematogenous infection was the predominant cause of failure thereafter. Interestingly, a higher proportion of hematogenous failures was seen in patients who had undergone a 2-stage or multistage exchange, compared with less invasive treatment for index hematogenous PJI (ie, debridement and prosthesis retention). This observation squares with data that identified previous index joint revision arthroplasty as a risk factor for hematogenous PJI in patients with S. aureus bacteremia [24]. The larger prosthetic surfaces of revision vs primary arthroplasty implants may predilect for adherence of bacteria during bacteremia [24]. Among acute PJI, lower success rates were reported for hematogenous compared with nonhematogenous PJI [9, 13, 15]. In addition, among hematogenous PJI, worse outcomes have been observed in staphylococcal infections compared with PJI caused by other pathogens [5, 13, 14].
The above-mentioned retrospective multicenter cohort study analyzing 340 patients with “late acute” PJIs led to the proposal of the CRIME80 score as a tool to identify patients at risk for failure of treatment of such PJIs [4]. This score includes patient-specific (age >80 years, rheumatoid arthritis, male sex), prosthesis-specific (fracture as an indication for primary implantation), and infection-specific (CRP >150 mg/L, S. aureus as causative pathogen) factors. In our cohort, CRIME80 Score was not predictive for failure after DAIR in hematogenous PJI. Indeed, this score probably reflects the general risk for worse outcome of any PJI and any surgical procedure, like age, comorbidities, and high-virulent pathogens do. Therefore, it may be a mistake to decide against DAIR based upon this score, as the 2-/multistage prosthesis exchange may have even worse infection and functional outcomes with higher morbidity and mortality.
Interestingly, in our cohort, 58% of failures were caused by a new pathogen and 53% were due to another episode of hematogenous spread. Few patients experienced recurrent hematogenous PJI, attributed to a lack of source control (ie, identification and treatment of primary infection foci). These findings suggest a host-mediated predisposition to hematogenous PJI, a topic that urgently requires further research. However, a recent retrospective study including 47 hematogenous PJIs after investigating 542 bacteremia episodes found no patient-related risk factors for PJI during bacteremia, for example, chronic comorbidities [8]. The risk for development of PJI during bacteremia in this study depended on the pathogen and the timing of bacteremia following joint replacement surgery. Hematogenous PJIs occurred most commonly after bacteremia with S. aureus (21%) and beta-hemolytic streptococci (21%), whereas they were rare with gram-negative bacteria (1.3%). Interestingly, the risk of developing PJI was highest for bacteremia occurring within the first year after arthroplasty.
The identification of a primary focus is not necessary for the diagnosis of hematogenous PJI, as primary bacteremia (ie, bacteremia without primary focus) may occur in 10% to 49%, depending on the pathogen [25–28]. In our cohort, the outcome of hematogenous PJI was similar with or without an identified focus. This may possibly be explained by cure of undetected primary foci with prolonged antimicrobial treatment, which was administered for PJI treatment.
The overall mortality in our cohort was 11%, and the attributable mortality to infection was 5%. Other authors described mortalities ranging from 13% to 25% in patients with hematogenous PJIs [4, 5, 14, 15]. These high rates likely reflect severe comorbidities of the population suffering from, and possibly inherently prone to, hematogenous infection. Especially in frail and elderly patients, DAIR should be considered the first-line surgical treatment of acute hematogenous PJI, and highly invasive surgical strategies must be scrutinized. Stability of the prosthesis, soft tissue condition, and antimicrobial treatment options, rather than the CRIME80 or other general risk scores, should steer the surgical course. If the DAIR strategy fails, removal of the prosthesis can be subsequently performed, with a noninferior outcome vs primary 2-stage implant exchange [29].
Generally, patients with a joint prosthesis who present with fever and/or bacteremia should be immediately treated with antibiotics and closely monitored for symptoms of PJI. With respect to primary infection foci found to be common in our cohort, patients with prosthetic joints should be regularly assessed by their family doctor and should be advised to maintain good skin care and to undergo regular professional dental cleaning and professional pedicure to prevent (re)occurrence of hematogenous PJI.
This study has several limitations. The definition criteria for hematogenous PJI used in this study had not been previously validated, although they are based on rational consideration. These were adapted from the definition of acute PJI, excluding early postoperative and acute exacerbations of chronic PJI, as recently published [3]. Second, the outcome of hematogenous PJI treated with DAIR vs prosthesis exchange cannot be compared in this retrospective study. Higher perioperative risks of more invasive prosthesis exchanges may have led to a preference for the DAIR strategy. Third, the number of PJI episodes included in this study is smaller than the one used in the study that led to proposal of the CRIME80 Score and may therefore not suffice to disqualify the score. However, a general risk score should be universally applicable. Fourth, although we found similar failure rates in subgroups stratified by pathogen, we cannot exclude an influence of the pathogen on the outcome, as the sample size was not sufficient to detect significant differences, if present. Other authors with larger numbers assessing the outcome of late acute infections suggest that S. aureus is associated with poorer outcomes than other pathogens [4, 5].
In conclusion, treatment failure of hematogenous PJI in our cohort was high, but most failures were due to new hematogenous infection rather than persistence or relapse of the initial PJI episode. In 18% of affected patients, more than one episode of hematogenous PJI occurred. The outcome of hematogenous PJI was similar for different pathogens, but failure occurred significantly more often in patients treated with prosthesis retention vs multistage exchange.
Acknowledgments
Financial support. This work was supported by the PRO-IMPLANT Foundation, Berlin, Germany (www.pro-implant.org), which provided an unrestricted research grant. The funding body had no influence on the design of the study, the collection, analysis, and interpretation of data, or the writing of the manuscript. We acknowledge support for the Article Processing Charge from the DFG (German Research Foundation, 393148499) and the Open Access Publication Fund of the University of Greifswald.
Potential conflicts of interest. Within the past 36 months, C.P. received consulting fees from LINK, Zimmer, DePuy/Synthes, and Smith & Nephew. A.T. received research funding from Heraeus Medical, Curetis, InfectoPharm, and Osartis. N.R. and A.R. declare that they have no competing interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Ethics approval. This research study was conducted retrospectively from data obtained for clinical purposes. Approval was granted by the institutional Ethics Committee (EA04/040/14).
Author contributions. N.R. and A.R. collected, analyzed, and interpreted the patient data and were the major contributors in writing the manuscript draft. A.T. and C.P. participated in the study design and revised the manuscript draft. All authors read and approved the final manuscript.
Prior presentation. Parts of the results were presented at the German Congress of Orthopaedics and Traumatology (DKOU, virtual meeting, October 20–23, 2020) and at the 3rd World Arthroplasty Congress (virtual meeting, April 22–24, 2021).
Availability of data and material. The data sets used and/or analyzed during the current study are available from the authors on reasonable request.
References
- 1. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 2. Izakovicova P, Borens O, Trampuz A.. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev 2019; 4:482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rakow A, Perka C, Trampuz A, Renz N.. Origin and characteristics of haematogenous periprosthetic joint infection. Clin Microbiol Infect 2019; 25:845–50. [DOI] [PubMed] [Google Scholar]
- 4. Wouthuyzen-Bakker M, Sebillotte M, Lomas J, et al. Clinical outcome and risk factors for failure in late acute prosthetic joint infections treated with debridement and implant retention. J Infect 2019; 78:40–7. [DOI] [PubMed] [Google Scholar]
- 5. Rodriguez D, Pigrau C, Euba G, et al. Acute haematogenous prosthetic joint infection: prospective evaluation of medical and surgical management. Clin Microbiol Infect 2010; 16:1789–95. [DOI] [PubMed] [Google Scholar]
- 6. Chen W, Klemt C, Smith EJ, Tirumala V, Xiong L, Kwon Y-M.. Outcomes and risk factors associated with failures of debridement, antibiotics, and implant retention in patients with acute hematogenous periprosthetic joint infection. J Am Acad Orthop Surg. 2021; 29(23):1024–30. doi: 10.5435/JAAOS-D-20-00939. PMID: 33620172. [DOI] [PubMed] [Google Scholar]
- 7. Zeller V, Kerroumi Y, Meyssonnier V, et al. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J Infect 2018; 76:328–34. [DOI] [PubMed] [Google Scholar]
- 8. Honkanen M, Jamsen E, Karppelin M, et al. Periprosthetic joint infections as a consequence of bacteremia. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wouthuyzen-Bakker M, Sebillotte M, Huotari K, et al. Lower success rate of debridement and implant retention in late acute versus early acute periprosthetic joint infection caused by Staphylococcus spp. results from a matched cohort study. Clin Orthop Relat Res 2020; 478:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang CF, He L, Fang XY, et al. Debridement, antibiotics, and implant retention for acute periprosthetic joint infection. Orthop Surg 2020; 12:463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ottesen CS, Troelsen A, Sandholdt H, et al. Acceptable success rate in patients with periprosthetic knee joint infection treated with debridement, antibiotics, and implant retention. J Arthroplasty 2019; 34:365–8. [DOI] [PubMed] [Google Scholar]
- 12. Boyer B, Cazorla C.. Methods and probability of success after early revision of prosthetic joint infections with debridement, antibiotics and implant retention. Orthop Traumatol Surg Res 2021; 107:102774. [DOI] [PubMed] [Google Scholar]
- 13. Sendi P, Lötscher PO, Kessler B, et al. Debridement and implant retention in the management of hip periprosthetic joint infection: outcomes following guided and rapid treatment at a single centre. Bone Joint J 2017; 99-b:330–6. [DOI] [PubMed] [Google Scholar]
- 14. Konigsberg BS, Valle CJD, Ting NT, Qiu F, Sporer SM.. Acute hematogenous infection following total hip and knee arthroplasty. J Arthroplasty 2014; 29:469–72. [DOI] [PubMed] [Google Scholar]
- 15. Vilchez F, Martinez-Pastor JC, Garcia-Ramiro S, et al. Efficacy of debridement in hematogenous and early post-surgical prosthetic joint infections. Int J Artif Organs 2011; 34:863–9. [DOI] [PubMed] [Google Scholar]
- 16. Shohat N, Goswami K, Tan TL, Fillingham Y, Parvizi J.. Increased failure after irrigation and debridement for acute hematogenous periprosthetic joint infection. J Bone Joint Surg Am 2019; 101:696–703. [DOI] [PubMed] [Google Scholar]
- 17. Pansu N, Hamoui M, Manna F, et al. Implant retention and high rate of treatment failure in hematogenous acute knee and hip prosthetic joint infections. Med Mal Infect 2020; 50:702–8. [DOI] [PubMed] [Google Scholar]
- 18. Renz N, Yermak K, Perka C, Trampuz A.. Alpha defensin lateral flow test for diagnosis of periprosthetic joint infection: not a screening but a confirmatory test. J Bone Joint Surg Am 2018; 100:742–50. [DOI] [PubMed] [Google Scholar]
- 19. Krenn V, Morawietz L, Perino G, et al. Revised histopathological consensus classification of joint implant related pathology. Pathol Res Pract 2014; 210:779–86. [DOI] [PubMed] [Google Scholar]
- 20. Gellert M, Hardt S, Koder K, Renz N, Perka C, Trampuz A.. Biofilm-active antibiotic treatment improved the outcome of knee periprosthetic joint infection: results from a 6-year prospective cohort. Int J Antimicrob Agents 2020; 55:105904. [DOI] [PubMed] [Google Scholar]
- 21. Li C, Renz N, Trampuz A.. Management of periprosthetic joint infection. Hip Pelvis 2018; 30:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koh IJ, Han S-B, In Y, Oh K-J, Lee D-H, Kim TK.. Open debridement and prosthesis retention is a viable treatment option for acute periprosthetic joint infection after total knee arthroplasty. Arch Orthop Trauma Surg 2015; 135:847–55. [DOI] [PubMed] [Google Scholar]
- 23. Wouthuyzen-Bakker M, Sebillotte M, Lomas J, et al. Timing of implant-removal in late acute periprosthetic joint infection: a multicenter observational study. J Infect 2019; 79:199–205. [DOI] [PubMed] [Google Scholar]
- 24. Tande AJ, Palraj BR, Osmon DR, et al. Clinical presentation, risk factors, and outcomes of hematogenous prosthetic joint infection in patients with Staphylococcus aureus bacteremia. Am J Med 2016; 129:221.e11–20. [DOI] [PubMed] [Google Scholar]
- 25. Dahl A, Lauridsen TK, Arpi M, et al. Risk factors of endocarditis in patients with Enterococcus faecalis bacteremia: external validation of the NOVA Score. Clin Infect Dis 2016; 63:771–5. [DOI] [PubMed] [Google Scholar]
- 26. Ceci M, Delpech G, Sparo M, Mezzina V, Sánchez Bruni S, Baldaccini B.. Clinical and microbiological features of bacteremia caused by Enterococcus faecalis. J Infect Dev Ctries 2015; 9:1195–203. [DOI] [PubMed] [Google Scholar]
- 27. Kaasch AJ, Barlow G, Edgeworth JD, et al. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 2014; 68:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart AG, Paterson DL, Young B, et al. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections caused by ampc β-lactamase-producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: a pilot multicenter randomized controlled trial (MERINO-2) . Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim K, Zhu M, Cavadino A, et al. Failed debridement and implant retention does not compromise the success of subsequent staged revision in infected total knee arthroplasty. J Arthroplasty 2019; 34:1214–20.e1. [DOI] [PubMed] [Google Scholar]