Abstract
Hyperandrogenemia and obesity are common in women with polycystic ovary syndrome, but it is currently unclear how each alone or in combination contribute to reproductive dysfunction and female infertility. To distinguish the individual and combined effects of hyperandrogenemia and an obesogenic diet on ovarian function, prepubertal female rhesus macaques received a standard control (C) diet, testosterone (T) implants, an obesogenic Western-style diet (WSD), or both (T + WSD). After 5 to 6 years of treatment, the females underwent metabolic assessments and controlled ovarian stimulations. Follicular fluid (FF) was collected for steroid and cytokine analysis and the oocytes fertilized in vitro. Although the T + WSD females exhibited higher insulin resistance compared to the controls, there were no significant differences in metabolic parameters between treatments. Significantly higher concentrations of CXCL-10 were detected in the FF from the T group, but no significant differences in intrafollicular steroid levels were observed. Immunostaining of cleavage-stage embryos revealed multiple nuclear abnormalities in the T, WSD, and T + WSD groups. Single-cell DNA sequencing showed that while C embryos contained primarily euploid blastomeres, most cells in the other treatment groups were aneuploid. Despite yielding a higher number of mature oocytes, T + WSD treatment resulted in significantly reduced blastocyst formation rates compared to the T group. RNA sequencing analysis of individual blastocysts showed differential expression of genes involved in critical implantation processes between the C group and other treatments. Collectively, we show that long-term WSD consumption reduces the capacity of fertilized oocytes to develop into blastocysts and that the addition of T further impacts gene expression and embryogenesis.
Keywords: aneuploidy, diet, hyperandrogenemia, obesogenic, PCOS, preimplantation
Elevated levels of circulating testosterone (T; hyperandrogenism), as well as obesity/diet-induced metabolic dysfunction in women are associated with an increased frequency of female infertility (1, 2) and polycystic ovary syndrome (PCOS) (3-5). PCOS affects approximately 5% to 10% of reproductive age females (6), with patients exhibiting a wide range of clinical symptoms that typically includes metabolic issues such as insulin resistance and obesity (5, 7, 8). A PCOS diagnosis comprises at least 2 out of 3 features, including hyperandrogenism, infrequent or irregular ovulation (oligoovulation), and polycystic ovaries (9). Compromised or altered ovarian function contributes to the noted oligoovulation or anovulation (7, 8, 10). Qualitative and quantitative changes in intrafollicular steroid (11-17) and growth factor/cytokine (18, 19) levels have been frequently noted in both PCOS animal models and women with PCOS. Thus, alteration of the ovarian intrafollicular milieu (15, 19-21) is likely associated with the reduced oocyte competency observed in PCOS.
Although metabolic and endocrine dysfunction, as well as genetic factors, are known to contribute to the etiology of PCOS (7, 8, 22, 23), it is unclear how each effects ovarian physiology. Elevated T levels in PCOS are thought to directly promote small antral follicle development and survival, giving rise to the polycystic ovary phenotype (10, 24, 25). However, the obesity and metabolic defects that tend to accompany PCOS symptoms have also been implicated in contributing to ovarian dysfunction. In particular, reduced oocyte quality, chromosomal abnormalities, and the diminished capacity of oocytes to fertilize and develop into blastocysts were observed after consumption of an obesogenic high-fat diet (26-28). Of the different metabolic parameters measured in PCOS animal models and patients, insulin resistance is hypothesized to be the major contributor to reduced oocyte competency observed in these studies (22, 23, 29). It is currently unknown, however, how the diet itself or diet-induced changes in metabolic function and hyperandrogenemia each contribute to aberrant ovarian physiology and, thus, female infertility.
To define the individual contributions of diet and hyperandrogenemia alone or in combination on altered ovarian function, studies were initiated whereby rhesus macaque females received a standard low-fat control diet (C; 15% of calories from fat) or a high-fat Western-style diet (WSD; 36% of calories from fat) in the absence or presence of T implants (30). All treatments were initiated just prior to the onset of puberty to coincide with the stage at which PCOS symptoms typically emerge in women (31, 32). T implants increased circulating T levels to an average of 1.4 ng/mL, which is 3- to 5-fold above normal non-PCOS levels, but still well below the concentration detected in males (33). After 2 to 3 years of treatment, body weight and fat mass gain significantly increased, as well as insulin resistance, in the T + WSD group (34). By 3 years posttreatment, animals in both the T and the T + WSD groups exhibited a PCOS-like morphology (30, 35, 36), with numerous small antral follicles present in the periphery of the ovary (30). In the T + WSD females, significantly reduced circulating progesterone levels were also detected during the luteal phase. Relative to the controls, these animals had reduced blood flow and volume in the corpus luteum, indicating that T and WSD alone or in combination diminish luteal vascular function (30). The uterine milieu was also impacted by the treatments based on findings of impaired endometrial blood vessel formation in the T and WSD groups and reduced uterine decidualization in T + WSD females (30). Fertility trials conducted during this time revealed that WSD treatment caused a delay in the time to pregnancy, while the T + WSD treatment was associated with reduced fertility and abnormal fetal development (35).
After ~3.5 years of treatment, the females underwent a controlled ovulation (COv) cycle to aspirate the naturally selected follicle, from which a significantly increased number of degenerated oocytes were collected from the T, WSD, and T + WSD groups (37). Treatment specific changes in follicular fluid (FF) levels of cortisol, CC chemokine ligand-2 and -11, and fibroblast growth factor 2 were also observed. Moreover, mature metaphase II (MII) oocytes obtained from the WSD group often underwent abnormal multipolar divisions following in vitro fertilization (IVF) (37). Although these findings indicate that there are direct effects of T and/or WSD on the selected ovulatory follicle and the competency of its resident oocyte, COv cycles preclude the collection of sufficient oocyte numbers to assess the effects on maturation, fertilization, and preimplantation development. In this study, controlled ovarian stimulation (COS) cycles were conducted on the females to generate multiple follicles from which the impact of long-term (5-6 years) hyperandrogenemia, WSD consumption, or the combination of the 2 on the oocyte pre- and post-IVF could be assessed. Our COS protocol is analogous to that used in women undergoing infertility treatment (38), and because of shared female reproductive physiology between rhesus macaques and humans (39, 40), the findings of this work provide insight into how hyperandrogenemia and WSD consumption, both alone and in combination, impact oocyte/embryo competency and IVF outcomes.
Materials and Methods
Experimental Groups, Treatment Regimen, and Metabolic Measurements
All protocols involving animals were approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The housing and general care of rhesus macaques (Macaca mulatta) has been previously described (41). At the onset of the study, a cohort of 40 female rhesus macaques were equally divided into 4 treatment groups (n = 10/group) at ~2.5 years of age just prior to puberty, including C, T, WSD, and T + WSD, as described previously (37). Control animals consumed a standard chow diet (15% fat, 59% carbohydrate, 26% protein) and received cholesterol implants. Females in the T group received T implants to maintain their circulating blood T levels up to 3 to 5 times the normal circulating T concentration and fed a standard chow diet. The WSD group was fed an obesogenic high-fat diet (36% fat, 46% carbohydrate, 18% protein) and received a cholesterol implant. The T + WSD group received a T implant as well as the obesogenic diet. Each animal received an implant to control for their placement by surgical manipulation. All 10 C animals underwent COS protocols, while 7 T, 6 WSD, and 3 T + WSD females were able to undergo a COS protocol after 5 to 6 years of treatment. The fewer numbers of animals in the treatment groups relative to the control group was due to health issues, primarily endometriosis (42), which prevented further reproductive testing. Thus, only those females not diagnosed with endometriosis underwent COS cycles.
Weight, body mass index (BMI), body fat percentage, and homeostatic model assessment of insulin resistance (HOMA-IR) were measured after 5 years of treatment as previously described (34). In brief, body fat percentage for each animal was measured using dual-energy X-ray absorptiometry (Hologic QDR Discovery A; Hologic, Inc.). Fasting insulin and glucose levels were obtained to determine HOMA-IR values (fasting insulin*fasting glucose/405) as previously described (34). Low HOMA-IR values indicated high insulin sensitivity, whereas high HOMA-IR values correlated with low insulin sensitivity (insulin resistance). Animal weight and crown-rump length (CR) was measured and used to calculate BMI (BMI = weight/CR*CR).
Controlled Ovarian Stimulations and Collection of Oocytes
COS protocols were performed as previously published (38). Briefly, recombinant human gonadotropins, follicle-stimulating hormone and luteinizing hormone, were administered to promote the development of multiple ovarian follicles. Female rhesus macaques were anesthetized for laparoscopic follicular aspirations 36 hours after a bolus of human chorionic gonadotropin was administered to induce events necessary for the reinitiation of meiosis. Two individual follicles per ovary (n = 4 each animal) were chosen for aspiration based on their larger size and the increased likelihood that they could yield sufficient follicular fluid for further analyses. These aspirates were collected manually using a low dead space 3 mL syringe with a 22-gauge × 1.5inch needle (Ulticare, UltaMed Inc., Excelsior, MN, USA). Individual follicle aspirates were centrifuged to separate the FF from the cumulus oocyte complexes (COCs) and the granulosa cells. FF was stored at −80°C until analysis, and the oocytes from the individual aspirates were denuded from the surrounding cumulus cells of the COCs by gentle micropipetting. The remaining follicles were aspirated in bulk using vacuum suction. Bulk aspirated COCs were collected in Tyrode’s albumin lactate in pyruvate (TALP)-N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid (HEPES) medium with 0.3% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) and 1% heparin sodium salt solution at 37°C. Oocytes from the bulk aspirates were denuded by gentle micropipeting in TALP-HEPES medium containing 0.3% BSA and 3% hyaluronidase (Sigma-Aldrich). The developmental stage of each oocyte was recorded and categorized as either immature germinal vesicle, maturing metaphase I (MI), MII, or degenerated. Individual oocytes were placed in pre-equilibrated 100 μL TALP complete drops in a 10-well IVF dish (LifeGlobal, Guildford, CT, USA) in 5% CO2 at 37°C until IVF.
Analysis of Cytokine and Steroid Levels in FF
FF samples obtained from the individual follicular aspirates of each female were pooled and used for the analysis of 29 cytokines and 7 steroids by the Endocrine Technologies Core at ONPRC. The number of samples analyzed for steroid analysis included 10 C, 5 T, 6 WSD, and 3 T + WSD. For cytokine analysis, there were 9 C, 4 T, 4 WSD, and 1 T + WSD (only 1 female from the T + WSD group had sufficient FF volume for testing). Cytokine levels were determined by the Endocrine Technologies Core using a monkey 29-plex cytokine panel (Thermo Fisher, Waltham, MA, USA, catalog #LPC0005M) following the manufacturer’s instructions. Concentrations of each cytokine were calculated from a standard control curve. Samples were analyzed on a Milliplex Analyzer (EMD Millipore, Billerica, MA, USA) with XPonent Software version 3.1 (Luminex, Austin, TX, USA). The data were calculated using Milliplex Analyst software version 5.1 (EMD Millipore). All samples were analyzed in a single run and the intra-assay coefficients of variation (CVs) for all analytes were <20%. Steroid hormone analysis was performed by liquid chromatography-tandem mass spectrometry on a Shimadzu Nexera-LCMS-8050 using a previously described method (37). Accuracies for the steroid hormone assays ranged from 87.3% to 108.5%, and the intra-assay CV was < 11%. The list of the steroids and cytokines analyzed with their CVs and accuracies are included in Supplementary Tables 1 and 2, respectively (43).
IVF and Monitoring of Preimplantation Development
Fresh semen from 4 adult male rhesus monkeys (aged 10-13 years old) with proven fertility, obtained through the ONPRC Assisted Reproductive Technologies Core, was used for IVF throughout this study. The semen was obtained on the same day as IVF, and the sperm prepared as previously described (44) for use at a final concentration of 2 × 10 (6) sperm/mL in TALP-complete medium (6). IVF was performed the evening of the oocyte collection as previously described (44). In brief, sperm samples were treated with cyclic adenosine monophosphate (5 mg/mL) and caffeine (2 mg/mL) 15 minutes before fertilization to induce hyperactivation. Activated sperm (1 μL) were added to each well containing an oocyte. The IVF dishes were incubated in 5% CO2 at 37°C for 14 to 16 hours. Any remaining sperm were removed from fertilized oocytes by gentle micropipetting. Zygotes (n = 12) identified by the presence of 2 pronuclei and/or 2 polar bodies were randomly selected and transferred to custom EevaTM 12-well polystyrene petri dishes (Progyny, Inc., San Francisco, CA, USA) containing 100 μL of pre-equilibrated culture medium (IVF Bioscience, UK, BO-IVC) under mineral oil (SageTM, Trumbull, CT, USA) for time-lapse monitoring (TLM) at 37°C with 6% CO2, 5% O2, and 89% N2. The remaining zygotes were transferred to a 10-well IVF dish (LifeGlobal) and cultured in the same media as the TLM dish. Media was changed at day 3 post-IVF, and the embryos allowed to develop up to day 8 or until they reached the blastocyst stage. The maturation, fertilization, cleavage, and blastocyst formation rate were calculated as follows: maturation rate = (number of mature MII oocytes/total number of oocytes)*100; fertilization rate = (number of zygotes formed post-IVF/number of mature MII oocytes)*100; cleavage rate = (number of zygotes that cleaved/total number of zygotes)*100; and blastocyst formation rate = (number of blastocysts formed/number of cleaved embryos) *100.
Assessment of Initial Mitotic Divisions by Time-lapse Imaging
The division kinetics of zygotes were monitored using the EevaTM darkfield 2.2.1 time-lapse microscope system as previously described (45). Embryos were imaged every 5 minutes with a 0.6 second exposure time up to 8 days or until they developed into blastocysts. Each image was time stamped with a frame number, and all images compiled into an AVI movie using FIJI software version 2.0.0 (National Institutes of Health, Bethesda, MD, USA). A total of 309 zygotes from each of the 4 treatments were examined by TLM, of which 166 successfully divided (73 C, 39 T, 30 WSD, and 24 T + WSD). Given that the time intervals of the first 3 mitotic divisions are predictive of blastocyst formation in human embryos (46, 47), the timing between the appearance of the first cleavage furrow to the end of the first cytokinesis, the beginning of the second mitotic division, and the start of the third mitotic division were manually measured by 4 independent observers and represented as an average. Morphological features such as cellular fragmentation or asymmetrical/multipolar divisions were also recorded for each embryo based on their association with aneuploidy (48, 49).
Immunofluorescence-based Detection of Nuclear Integrity
Cleavage-stage embryos that underwent cellular fragmentation and/or multipolar divisions indicative of chromosomal abnormalities during TLM (48, 49) were collected at the 6- to 11-cell stage (2 C, 3 T, 4 WSD, 4 T + WSD) on day 2 to 3 of preimplantation development for immunostaining since they would likely arrest prior to the blastocyst stage. Removal of the zona pellucida (ZP) was accomplished by incubating the embryos in EmbryoMax Acidic Tyrode’s Solution (EMD Millipore) for ~30 seconds. The embryos were washed in 0.1% BSA plus 0.1% Tween 20 [phosphate-buffered saline (PBS)-T; Sigma-Aldrich] and fixed by incubation in 4% paraformaldehyde (Fisher Scientific) in PBS for 20 minutes at room temperature (RT). Embryos were washed with PBS-T for 30 minutes at RT to remove any fixative and permeabilized in 1% Triton-X (Calbiochem; Burlington, MA, USA). Nonspecific binding was blocked by incubation in 4% donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 30 minutes at RT. Embryos were incubated with a primary antibody (RRID AB_443298, https://antibodyregistry.org/search.php?q=AB_443298; Abcam, Cambridge, MA, USA, catalog #ab16048, rabbit monoclonal, 1:100) that recognized Lamin-B1 (LMNB1) as previously reported (48). LMNB1 antibody binding was detected by incubating samples with a donkey antirabbit secondary antibody conjugated with Alexa Fluor 488 (RRID AB_2535792, https://antibodyregistry.org/search.php?q=AB_2535792; Thermo Fisher; catalog #A-21206, 1:250) for 2 hours at RT. All antibodies were diluted in PBS-T + 1% donkey serum. DNA was stained with 1 μg/mL 4′,6-diamidino-2-phenylindole (Thermo Fisher, D1306, 1:1000) for 10 minutes. Between each step, the embryos were washed with PBS-T 3 times for 5 minutes each. Embryos were transferred to glass-bottom petri dishes (Mattek, Ashland, MA, USA), and LMNB1 immunolocalization was visualized on a Leica SP5 AOBS spectral confocal system located in the Imaging and Morphology Core at ONPRC. Z-stacks 1 to 5 μM apart were imaged sequentially to avoid spectral overlap between channels using the 10× and 20× objectives.
Single-cell DNA Sequencing and Copy Number Variation Analyses
Another cohort of cleavage-stage embryos that underwent TLM were collected at the 3- to 9-cell stage on day 2 to 3 of preimplantation development for single-cell DNA sequencing and copy number variation (CNV) analysis. The ZP was removed as described above, and the embryos were disassembled into single blastomeres and cellular fragments, if present, by incubating in Quinn’s advantage Ca2 + and Mg2+-free medium with HEPES plus 10% human albumin (CooperSurgical Inc., Trumbull, CT, USA) and 0.05% trypsin-EDTA (Thermo Fisher Scientific) as previously reported (48). Each sample was washed with Ca2 + and Mg2+-free PBS and collected individually for transfer to a sterile UltrafluxTM polymerase chain reaction (PCR) tube (VWR, Radnor, PA, USA). A total of 88 single blastomeres or fragments belonging to 16 cleavage-stage embryos from the C (40 blastomeres, 7 embryos), T (21 blastomeres, 3 embryos), WSD (19 blastomeres, 4 embryos), T + WSD (8 blastomeres, 2 embryos) groups were processed for DNA isolation, amplification, and library construction per the manufacturer’s instructions (Takara Bio-SMARTer® PicoPLEX® DNA-seq Kit, Shiga, Japan). Libraries were quantified using the Qubit high sensitivity DNA assay (Life Technologies, Carlsbad, CA, USA) and validated by PCR amplification of the adapter sequence. Only libraries with DNA quantities greater than the no-template controls were included for sequencing. DNA (50 ng) was prepared from each blastomere or euploid fibroblast, which served as a positive control for CNV analysis, and 25 ng from cellular fragments as previously described (48). DNA from pooled libraries was purified using a MagBead kit (Zymo Research, Irvine, CA, USA) and requantified by the Qubit high sensitivity DNA kit. Pooled multiplexed libraries were loaded at 1.6 pM and sequenced on the NextSeq 500 platform using a 75-cycle single-end protocol (Illumina, San Diego, CA, USA).
The quality of sequencing reads was assessed with FastqC (v 0.11.8), and low-quality bases and adapters were trimmed with Trimmomatic (v 0.39). Trimmed reads were deduplicated with fastqx collapser from FASTX Toolkit (v 0.0.14). Mapping to the most recent rhesus macaque reference genome (Mmul_10) from Ensembl was performed with burrows-wheeler-aligner (bwa mem v 0.7.17) with the “-M” parameter specified to mark shorter split hits as secondary. CNV was determined by integrating previously published bioinformatics pipelines called VNOWC and CHI (48, 50). The VNOWC pipeline generates variable-sized windows with a constant number of expected reads per window and uses circular binary segmentation to identify putative copy number changes between windows across each chromosome (51). To correct for GC bias across the genome, we also implemented the CHI pipeline, which uses the Hidden Markov Model (52) based on parameters determined previously (53). Following integration, 4000 reads per window size were used for chromosome plots as this was previously shown to yield accurate CNV calling (48).
RNA Sequencing of Blastocysts and Differential Gene Expression Analysis
Blastocysts were collected from all 4 treatment groups, the ZP was removed as described above, washed with Ca2 + and Mg2+-free PBS, and frozen at −80°C until RNA isolation. To analyze the effect of the treatments rather than embryo morphology, 38 blastocysts (12 C, 12 T, 10 WSD, and 4 T + WSD) that did not exhibit cellular fragmentation or asymmetrical/multipolar divisions were chosen for RNA sequencing (RNA-seq) analysis. RNA was extracted from the blastocysts using the ARCTURUS PicoPure RNA Isolation Kit (ThermoFisher Scientific, KIT0204), complimentary DNA (cDNA) was prepared using the SMART-Seq v4 Ultra Low Input RNA Kit for sequencing (TakaraBio, Shiga, Japan), and the amplified cDNA was purified using the Agencourt AMPure XP Kit (Beckman Coulter, Brea, CA, USA), all according to the manufacturers’ instructions. cDNA was then sheared to approximately 250 base pairs in length using a Covaris M220 sonicator. Sheared cDNA was resuspended in Tru-Seq Resuspension Buffer and libraries prepared using a Tru-Seq Nano kit (Illumina) according to the manufacturer’s instructions, except that 16 cycles of amplification were performed to account for low input samples. Fragment size was measured using a Fragment Analyzer 5200 and samples quantified with quantitative PCR and pooled at equimolar concentration. Multiplexed samples were sequenced across 7 lanes of a single-read, 75-cycle run on an Illumina HiSeq 4000 sequencer.
The sequencing data was demultiplexed using Illumina’s bcl2fastq software and sample quality assessed with FastQC (v 0.11.8), followed by trimming of low-quality bases and adapter sequences with Trimmomatic (v 0.39). Trimmed sequences were aligned via STAR (version 2.7.0) to the Mmul_10 rhesus macaque reference genome, and gene counts were obtained by specifying the “quantMode GeneCounts” parameter of STAR, along with the Mmul_10.99 Ensembl annotation gtf file. From the total number of blastocysts that underwent RNA-seq, only those samples that had more than 10 million total gene counts (n = 25) were retained for differential gene expression analysis (5 C, 10 T, 8 WSD, 2 T + WSD). Differential expression between embryo groups was performed with edgeR (version 0.28.0) using the “QLFTest” option. The Enrichr and G-profiler online tools were used for molecular pathway and gene ontology (GO) assessment.
Statistical Analysis
The analysis of variance (ANOVA) type III test was performed to assess the significance of the metabolic parameters between treatment groups. In addition, 1-way ANOVA and a post hoc t-test comparison with Bonferroni adjustment was performed to test for significance of the HOMA-IR results. Significant differences in FF levels of steroids and cytokines were assessed by 1-way ANOVA and a post hoc t-test comparison with Bonferroni adjustment. Statistical analyses of oocyte number, maturation rate, fertilization rate, and cleavage rate among the treatment groups was conducted using a chi-square test followed by post hoc pair-wise comparisons. Poisson regression was used to model the number of blastocysts formed between treatments. More specifically, a generalization of Poisson regression, negative binomial regression was used to accommodate overdispersion, followed by the 3 degree-of-freedom chi-square test for blastocyst numbers and formation rates. For the TLM analysis, a linear mixed effects model was used, followed by the 3 degree-of-freedom chi-square test, to determine statistical differences in initial mitotic timing between the embryo groups.
Results
Chronic Exposure to T + WSD Produces a Trend in Higher Insulin Resistance
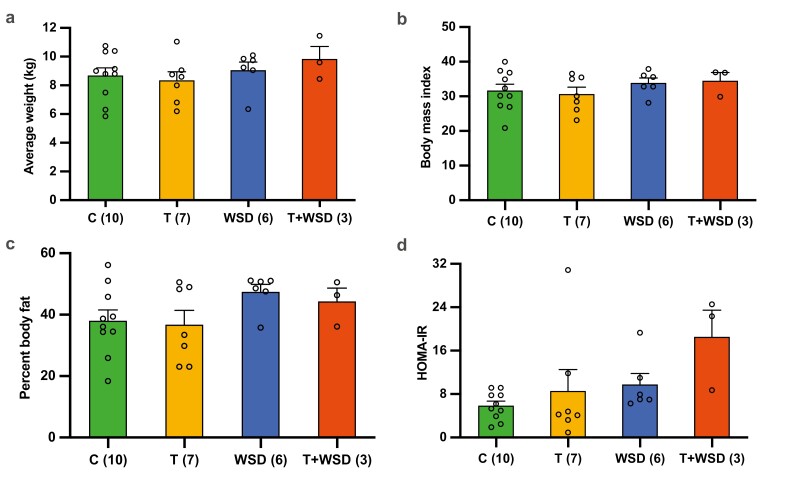
After 5 to 6 years of the treatment, the metabolic profile of the female rhesus macaques in each of the 4 groups was determined prior to undergoing a COS protocol. This included an assessment of weight (Fig. 1A), BMI (Fig. 1B), total percent body fat (Fig. 1C), and HOMA-IR (Fig. 1D). Despite previously reported gains in body weight and fat mass in the T + WSD group at 2 to 3 years of treatment (34), no significant differences in body condition or the metabolic parameters were observed between the treatment groups. A trend in increased insulin resistance was detected in the T + WSD group when compared to the other treatments, but this was not statistically significant (P = 0.06).
Figure 1.
Comparison of body condition and metabolic parameter measurements between treatment groups. Weight, body mass index (BMI), percent body fat, and insulin resistance in female rhesus macaques receiving a standard chow diet (control, C; n = 10), testosterone (T; n = 7), a Western-style diet (WSD; n = 6), or a combination of T + WSD (n = 3) for 5 to 6 years. The physical and metabolic parameters (mean ± SE of the mean) that were analyzed included (A) weight, (B) BMI, (C) percent body fat, and (D) insulin resistance [homeostatic model assessment of insulin resistance (HOMA-IR)] as determined by HOMA. There were no significant differences in body condition or metabolic parameters among the treatment groups, although HOMA-IR trended higher in the T + WSD females compared to the C group females (P = 0.06).
C-X-C Motif Ligand 10 Chemokine Levels in FF Is Altered by T Treatment
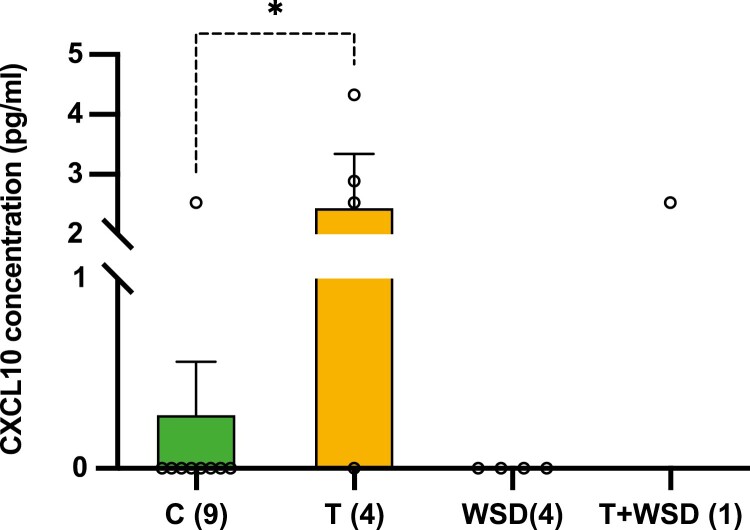
To determine whether the treatments affected the ovarian follicular milieu, FF samples obtained at the time of oocyte retrieval were examined for chemokine/cytokine/growth factor and steroid levels. Out of the 7 steroids (estradiol, estrone, androstenedione, T, progesterone, cortisol, and cortisone) analyzed, none significantly differed between the 4 treatment groups [Supplementary Table 1 (43)]. However, of the 29 chemokines/cytokines/growth factors assessed, a nearly 10-fold increase (P < 0.05) in the level of the chemokine, C-X-C motif ligand 10 (CXCL10), was observed in FF from the T group relative to C and WSD groups [Fig. 2; Supplementary Table 2 (43)]. While all FF samples from the T group had measurable levels of CXCL10, the CXCL10 concentration was below detectable limits in all WSD samples and in all but 1 sample in the C group. Because of limitations in the minimal volume required for analysis, cytokine levels were measured in only 1 T + WSD sample due to insufficient FF quantity. This prevented the inclusion of the T + WSD group in our statistical analysis. The level of CXCL10 in the 1 sample from the T + WSD animal (2.53 pg/mL) was equal to the mean CXCL10 level observed in the FF samples obtained from T treated animals.
Figure 2.
Effects of testosterone (T) or Western-style diet (WSD) treatment on the follicular fluid (FF) cytokine milieu during controlled ovarian stimulation cycles. Follicular fluid samples were collected from all the treatment groups and analyzed for 29 cytokines. The cytokine C-X-C motif chemokine 10 (CXCL10) was not detectable in any of the FF samples from the WSD group and all but 1 FF sample in the control (C) group. In contrast, the mean concentration (±SE of the mean) of CCXL10 was significantly higher in FF from T-treated animals when compared to FF from the C group. The number of FF samples that were analyzed from each group are shown in parentheses on the x-axis. Only 1 female from the T + WSD group had sufficient FF volume for the cytokine analysis and, therefore, was not included in the statistical analysis. Statistical significance was calculated by 1-way analysis of variance followed by a post hoc comparison with Bonferroni adjustment (*P < 0.05).
WSD Intake Reduces the Capacity of Fertilized Oocytes to Develop Into Blastocysts
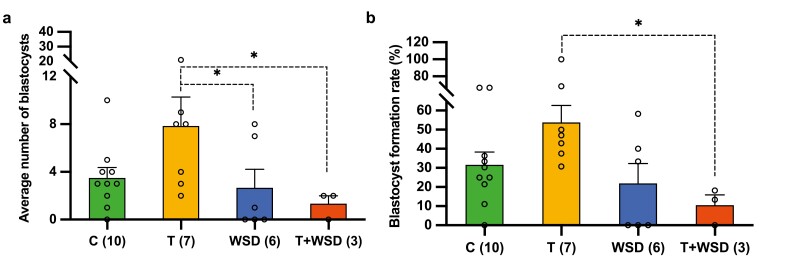
We next assessed the effects of hyperandrogenemia alone or in combination with consumption of a WSD on oocyte maturation and embryo development. Although not statistically significant, the average number of mature MII oocytes obtained per female varied among the treatments, with the highest being in the T and T + WSD animals (26 C, 32 T, 21 WSD, 33 T + WSD). Despite the larger number of mature oocytes collected, embryos from the WSD and T + WSD groups yielded the fewest blastocysts per female following IVF (P < 0.05) (Fig. 3A). Moreover, the blastocyst formation rate was lowest in the T + WSD group relative to both the control and WSD animals and significantly lower than the T group (P < 0.05) (Fig. 3B). Interestingly, the T group produced the highest number of blastocysts per female, but this was not significantly different between treatments. While WSD (16.1%; n = 5/31) and T + WSD (15%; n = 3/20) treatments produced a greater percentage of embryos with multipolar divisions than the C (9.7%; n = 6/62) and T (10.9%; n = 6/55) embryos, which may explain why fewer blastocysts were obtained from these groups, it also did not reach statistical significance. Lastly, the time intervals of the first 3 mitotic divisions predictive of blastocyst formation (46, 47), as well as the incidence of morphological characteristics such as cellular fragmentation, were not significant. These findings are consistent with previous human studies showing similar, albeit delayed, mitotic timing and overall morphology in embryos from PCOS women as well as those who are obese or overweight (54-57).
Figure 3.
Preimplantation developmental outcomes after long-term Western-style diet (WSD) consumption and/or hyperandrogenemia exposure. (A) The average number of blastocysts (±SE of the mean) formed per female were significantly reduced in the WSD and testosterone (T) + WSD groups (*P < 0.05). There was also a trend for the T group to yield a greater number of blastocysts per animal relative to the T + WSD (P = 0.065) or the WSD group (P = 0.12). (B) The mean blastocyst formation rate (±SE of the mean) in the T + WSD group was significantly lower relative to the blastocyst formation rate in the T group (*P < 0.05). The numbers in parentheses along the x-axes in (A) and (B) indicate the number of females that underwent the controlled ovarian stimulation protocol in each treatment group. Poisson regression, followed by the 3 degrees-of-freedom chi-square test, was used for calculating the statistical significance of the blastocyst numbers and blastocyst formation rate among the treatment groups.
Nuclear Integrity and Chromosome Segregation Are Compromised in T, WSD, and T + WSD Embryos
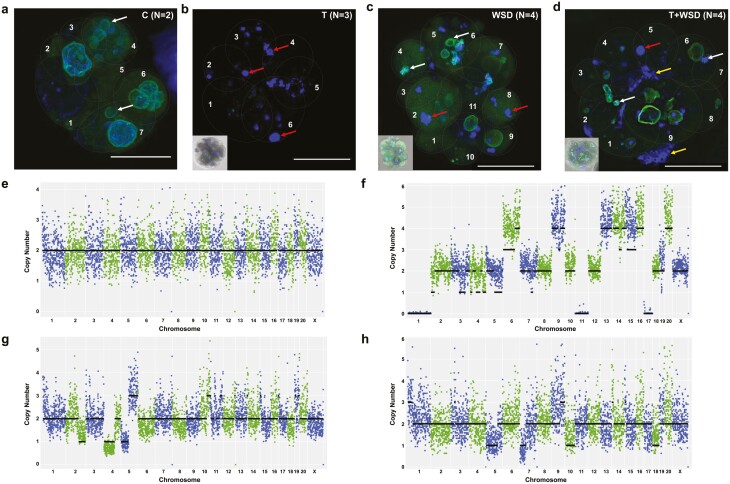
Cleavage-stage embryos from each of the 4 groups were immunolabelled with the nuclear envelope marker, LMNB1, and stained with 4′,6-diamidino-2-phenylindole to visualize DNA. While some micronuclei were noted in the control embryos, which is typical of rhesus preimplantation development (48), only primary nuclei with intact nuclear envelope were observed in each blastomere (Fig. 4A). In contrast, blastomeres in cleavage-stage embryos from the T, WSD, and the T + WSD groups lacked well-defined primary nuclei and possessed not only micronuclei, but also DNA without nuclear envelope, chromosome-containing cellular fragments, and nuclear fragmentation (Fig. 4B-4D), indicative of chromosomal abnormalities (48, 49). Thus, the remaining 16 cleavage-stage embryos were disassembled into single blastomeres, from which 88 individual cells (40 C, 21 T, 19 WSD, 8 T + WSD) were isolated for determining the absence or presence of aneuploidy. Karyotypic reconstruction of each embryo revealed predominantly euploid blastomeres with segmental errors only from the controls (Fig. 4E), while with the exception of 1 blastomere from the T + WSD group, almost all cells in the T, WSD, and T + WSD embryos were aneuploid (Fig. 4F-4H). In particular, these embryos primarily exhibited chaotic aneuploidy, with >4 losses and/or gains of different chromosomes, which we have shown is associated with multipolar divisions (48). This data, in conjunction with the numerous nuclear abnormalities detected by immunostaining, suggest that T and WSD alone or the combination of the 2 induces chromosomal instability in embryos.
Figure 4.
Assessment of nuclear integrity and copy number variation (CNV) in cleavage stage embryos from each treatment group. Cleavage stage embryos (6-11 cells) from the 4 treatment groups were collected and immunolabeled for the nuclear envelope marker LMNB1 (green) and stained with 4′,6-diamidino-2-phenylindole for DNA detection (blue). (A) Although a few micronuclei (white arrows) were present in cleavage stage embryos from the control group (n = 2), the main nucleus of each blastomere was enclosed by nuclear envelope. (B) In contrast, cleavage stage embryos in the testosterone (T) group (n = 3) lacked well-defined primary nuclei and instead possessed highly fragmented DNA without any evidence of nuclear envelope (red arrows). Besides micronuclei and nuclear fragmentation, the (C) Western-style diet (WSD; n = 4) and (D) T + WSD (n = 4) embryos also contained chromosome-containing cellular fragments (yellow arrows), which was more prominent in the T + WSD group. To aid in the visualization of each cell, blastomeres are numbered and outlined with a gray dashed line. Scale bar = 50 µm. CNV analysis was performed on individual blastomeres from other cleavage stage embryos in each treatment group and representative chromosome plots are shown. (E) While euploid blastomeres were primarily detected in the control group (n = 40 blastomeres from 7 embryos), (F-H) almost all cells in the T (n = 21 blastomeres from 3 embryos), WSD (n = 19 blastomeres from 4 embryos), and T + WSD (n = 8 blastomeres from 2 embryos) groups were aneuploid, with chaotic aneuploidy (>4 chromosomal losses and/or gains) being the predominant source. The chromosome number is listed in order on the x-axis, and copy number is represented on the y-axis.
Blastocyst RNA-seq Reveals Unique and Shared Effects of Each Treatment on Gene Expression
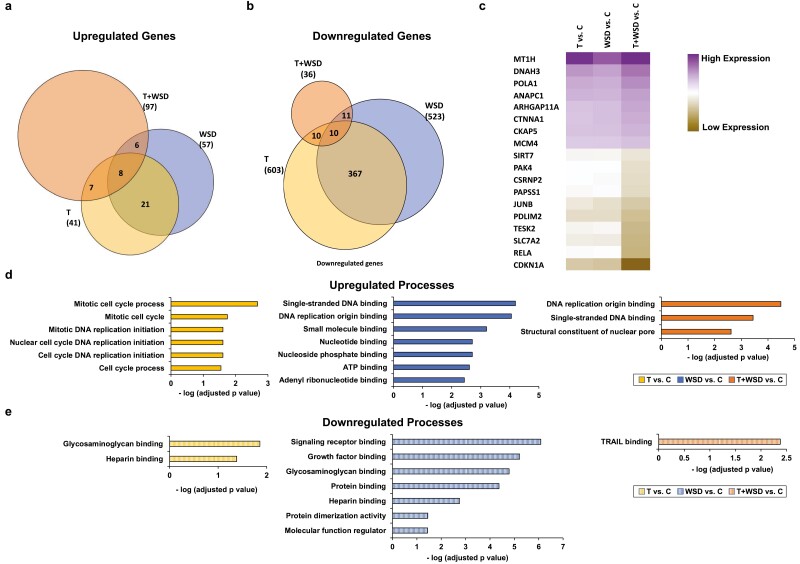
Because blastocysts that had undergone multipolar divisions during the first 3 mitoses or exhibited morphological characteristics indicative of poor embryo quality might have confounding effects on gene expression, we selected 38 blastocysts that only underwent normal bipolar divisions and lacked evidence of cellular fragmentation from the treatment groups for RNA-seq. Of the 38 blastocysts sequenced, we further restricted our analysis to 25 samples (5 C, 10 T, 8 WSD, 2 T + WSD) that contained a sufficient number of total gene counts for differential gene expression analysis [Supplementary Figure 1 (43)]. Differentially expressed genes (DEGs) that were statistically significant (P < 0.05) were identified in each embryo group compared to the controls (644 T, 580 WSD, 133 T + WSD). Both unique ([Supplementary Figure 3 (43)] and common DEGs were identified in the T, WSD and T + WSD treatment groups (Fig. 5A-5C). Among the common set of DEGs, some were significantly upregulated (n = 8) or downregulated (n = 10) in comparison to the control embryos, and the combined T + WSD treatment had the maximum impact on DEG regulation [Supplementary Figure 4 (43)]. GO and pathway analysis (Fig. 5D and 5E) of all DEGs revealed enrichment of mitosis, cell cycle, and DNA replication in blastocysts obtained from T-treated females, while heparin and glycosaminoglycan binding were downregulated in this group. In contrast, blastocysts from the WSD treatment group showed an upregulation of single-stranded DNA and DNA replication origin, as well as nucleotide/nucleoside phosphate, adenosine 5′-triphosphates, and ribonucleotide binding and a downregulation of signaling receptor, growth factor, glycosaminoglycan, protein, heparin binding, and protein dimerization. A similar analysis of the T + WSD treatment group demonstrated substantial overlap in upregulated functions with the WSD blastocysts, in addition to a noted downregulation of tumor necrosis factor-related apoptosis-inducing ligand binding. Of the genes upregulated in T + WSD blastocysts, the highest expressed include those involved in apoptosis (58) and microtubule function (59). Notably, several of these genes have also been correlated to repeated implantation failure (60) and epigenetic changes impacting placental development (61, 62), which may help explain why PCOS women are more likely to experience early pregnancy loss, preterm birth, and other pregnancy complications than women without PCOS (63, 64).
Figure 5.
RNA sequencing and differentially expressed gene (DEG) analysis identifies both distinct and shared effects of treatment on blastocyst gene expression. A total of 25 blastocysts (C = 5, T = 10, WSD = 8, T + WSD = 2) that contained sufficient total gene counts were included in the differential gene expression analysis. Venn diagrams showing the number of genes (in parentheses) that were uniquely (A) upregulated or (B) downregulated in each group, as well as those that were common between the different treatments. (C) A heat map of the adjusted P-values (P < 0.05) from the 18 shared genes [n = 8 upregulated (purple), n = 10 downregulated [brown]) among the treatment groups is depicted. Note that the maximum effect on gene expression was observed in the T + WSD blastocysts when each treatment was compared to the controls. The top gene ontology terms for all (D) upregulated and (E) downregulated DEGs in the blastocysts from the T, WSD, and T + WSD groups compared to the C blastocysts demonstrating that numerous biological processes important for peri-implantation development are impacted by T and/or WSD treatment. The x-axis is represented as the negative log of the P-value.
Discussion
PCOS is a complex disease with a wide range of clinical symptoms that is difficult to study in women due, in large part, to the unclear relationship between metabolic and/or endocrine dysfunction and their impact on ovarian physiology and female fertility. Using our established nonhuman primate model to distinguish the individual and combined effects of hyperandrogenemia and an obesogenic diet on oocyte/embryo competency, we show here that long-term WSD consumption with or without T induces treatment specific changes in female reproductive function. Because insulin resistance is thought to be a major contributor to reduced oocyte quality (22, 23, 29), we measured fasting insulin and glucose levels, but only a trend for greater insulin resistance was observed in the T + WSD females compared to controls. Unlike previous studies with this cohort of animals (34), no significant differences in body weight or fat mass were detected between treatment groups. The difference between the 2- to 3-year measurements and those conducted here may be due to the age of the females (7-8 years old) and the continued ad libitum consumption of a low-fat chow diet that allows for increased weight gain and adiposity without promoting outright insulin resistance (65-67). Additionally, the loss of animals in the WSD and T + WSD groups due to health issues between 3 and 5 years of treatment included those with the greatest BMI, which would skew the results of the remaining animals toward a healthier metabolic phenotype.
Despite the lack of metabolic differences, T treatment for >5 years did have an overt effect on ovarian follicles, as evidenced by a higher concentration of CXCL10 in FF. CXCL10 is a proinflammatory cytokine with antiangiogenic properties (68, 69), both of which could contribute to poor oocyte quality (70, 71). A proinflammatory environment activates natural killer cells, which in turn, leads to the production of CXCL10 (72). Natural killer cells were shown to be elevated in the FF of women who underwent unsuccessful IVF cycles (73), and there is evidence of an androgen receptor agonist directly inducing CXCL10 expression in primary prostate epithelial cells (74). Additionally, increased serum CXCL10 levels were also reported in lean women with PCOS and positively correlated to insulin resistance, but not with BMI (75). The chronic inflammation observed in women with PCOS (75-77) further supports the possibility that dysregulated cytokine synthesis and secretion may lead to altered ovarian follicle function.
In our previous study, we reported separate diet and androgen effects on the cytokine and steroid milieu in the dominant follicle during COv cycles (37). More specifically, the collection of mature MI/MII oocytes was associated with reduced FF levels of the chemokine C-C motif ligand 11 from the T + WSD treatment, while reduced chemokine C-C motif ligand 2 and fibroblast growth factor 2 levels were detected in the FF of T ± WSD group. A higher FF cortisol concentration was also observed in the T group compared to the C group, which we recently demonstrated correlates with a greater propensity for the resident oocyte to form a blastocyst following fertilization (78). We suspect that the differences detected between these 2 studies could be the use of COS cycles here since they induce the growth of multiple follicles that are heterogeneous in terms of follicle size and cellular content (38, 79, 80). Additionally, 3 T, 4 WSD, and 7 T + WSD animals could not undergo further reproductive testing due to health problems, thereby reducing statistical power. We also note that the WSD and T + WSD females who were excluded from the present study had the greatest metabolic dysfunction, including insulin resistance, after 5 years of treatment that may have further skewed our findings (81).
Since only the dominant follicle was sampled previously (37), COS cycles were performed here to obtain a sufficient number of oocytes to investigate the impact of chronic T, WSD, or T + WSD treatment on the oocyte-to-embryo transition. The T embryos produced the greatest number of blastocysts per female and the highest overall blastocyst formation rate. These results are similar to observations in previous human studies that reported increased oocyte and embryo yields in women receiving moderate amounts of T or T substrates for infertility treatment (82, 83). In contrast to the T embryos, WSD consumption was associated with a reduced blastocyst formation rate and the T + WSD group produced the least number of blastocysts in spite of having the highest number of mature oocytes. Analogous findings in women with PCOS were reported, wherein an equal or greater number of oocytes was obtained from the COS cycles, but postfertilization embryo development rates were lower in comparison to the normal control group (20, 84, 85). It should be noted, however, that these studies did not consider the effect of hyperandrogenemia and diet separately. From our analysis, we observed a significant reduction in blastocyst yield from the WSD group that was worsened by T addition. Thus, this suggests that the treatments alone or through their interactions with one another differentially alter ovarian function to exert the greatest negative effect on oocyte competency.
Assessment of cleavage stage embryos from each group revealed the presence of micronuclei in all treatments, but the incidence of this was lower within the control group, which is in accordance with our previous study showing baseline micronuclei levels in untreated rhesus embryos (48). Unlike the control group, however, the T, WSD, and T + WSD embryos also exhibited DNA without nuclear envelope, chromosome-containing cellular fragments, and nuclear fragmentation indicative of chromosomal abnormalities (48, 49). Indeed, single-cell DNA sequencing and CNV analysis of disassembled cleavage-stage embryos demonstrated that while euploid blastomeres were predominantly obtained from control embryos, almost all cells in the T, WSD, and T + WSD embryos were aneuploid. These data reinforce previous observations of abnormal chromosome rearrangement in mature oocytes from a high-fat diet rodent model, which upon fertilization, resulted in delayed embryo developmental progression (86). Additional studies suggest that weakened sister chromatid cohesion and/or telomere dysfunction in oocytes from high-fat diet fed mice are responsible for the effects of maternal obesity on oocyte quality and subsequent preimplantation embryogenesis (87, 88).
RNA-seq and differential expression analysis of blastocysts from each group revealed further consequences of the chronic T, WSD, and T + WSD treatments on genes and pathways important for implantation and other biological processes. Moreover, the GO term analyses of the most significantly expressed genes indicated that there were both individual and combined effects of each treatment on embryogenesis. One of the confounding factors with the T + WSD group was that only 2 blastocysts could be included in the DEG analysis due to reduced total read counts in the other samples. Despite this caveat, however, the T + WSD treatment had the maximum effect both in terms of fold change in gene expression and the significance of pathways identified as critical for peri-implantation development. This included the downregulation of genes associated with chromatin binding and DNA methylation, which are essential for the extensive epigenetic reprogramming that occurs during the oocyte-to-embryo transition (89, 90). It is important to note that the blastocysts chosen from each group for gene expression analysis were those that did not undergo multipolar divisions, demonstrating that T, WSD, and T + WSD can impact gene expression even in the absence of major morphological or cell division abnormalities. Future studies are required to understand the precise molecular effects of WSD and/or T treatment on preimplantation development, especially how diet and obesity can alter gene expression and the epigenetic state of the primate oocyte/embryo.
Acknowledgments
We thank the Division of Comparative Medicine and NHP Core for their animal care and research support, the Surgical Services Unit for their follicular aspiration expertise, Assisted Reproductive Technologies Core for obtaining and preparing the sperm sample for IVF, Endocrine Technologies Core for performing the cytokine and steroid analysis assays, Bioinformatics and Biostatistics Core for providing help with statistical analysis, and the Imaging and Morphology Core for confocal microscopy, all under the auspices of the NIH/OD ONPRC core grant (P51 OD011092). We also thank Dr. Jimi L. Rosenkrantz for her assistance with the DNA sequencing.
Financial Support
This work was supported by the National Centers for Translational Research in Reproduction grant P50 HD071836 (Project II; to J.D.H and S.L.C.) from the National Institutes of Health/National Institute of Child Health and Human Development and the Oregon National Primate Research Center grant P51 OD011092 from the National Institutes of Health/Office of the Director.
Author Contributions
S.R., S.L.C., and J.D.H. designed the study, performed experiments, analyzed data, and wrote the manuscript. M.J.M. and N.R-T were 2 independent observers for the TLM videos. B.D. performed the initial DNA sequencing and RNA-seq data analysis. M.J.M. and F.L. coordinated the metabolic tests and scheduled COS cycles. D.T. collected and helped analyze all of the metabolic data. All authors were involved in editing the manuscript.
Disclosures
Authors have nothing to disclose.
Data Availability
All RNA-seq data generated and analyzed during this study are included in the data repository, Gene Expression Omnibus (GSE186969), https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE186969.
References
- 1. Cardozo ER, Karmon AE, Gold J, Petrozza JC, Styer AK. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Hum Reprod. 2016;31(2):385-392. [DOI] [PubMed] [Google Scholar]
- 2. Provost MP, et al. Pregnancy outcomes decline with increasing body mass index: analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil Steril. 2016;105:663-669. [DOI] [PubMed] [Google Scholar]
- 3. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219-231. [DOI] [PubMed] [Google Scholar]
- 4. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270-284. [DOI] [PubMed] [Google Scholar]
- 5. Rothenberg SS, Beverley R, Barnard E, Baradaran-Shoraka M, Sanfilippo JS. Polycystic ovary syndrome in adolescents. Best Pract Res Clin Obstet Gynaecol. 2018;48:103-114. [DOI] [PubMed] [Google Scholar]
- 6. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28-38.e25. [DOI] [PubMed] [Google Scholar]
- 7. Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003;14(8):365-370. [DOI] [PubMed] [Google Scholar]
- 8. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25. [DOI] [PubMed] [Google Scholar]
- 10. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jakimiuk AJ, Weitsman SR, Magoffin DA. 5alpha-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84(7):2414-2418. [DOI] [PubMed] [Google Scholar]
- 12. Agarwal SK, Judd HL, Magoffin DA. A mechanism for the suppression of estrogen production in polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81(10):3686-3691. [DOI] [PubMed] [Google Scholar]
- 13. Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83(11):3984-3991. [DOI] [PubMed] [Google Scholar]
- 14. Hu Y, Cortvrindt R, Smitz J. Effects of aromatase inhibition on in vitro follicle and oocyte development analyzed by early preantral mouse follicle culture. Mol Reprod Dev. 2002;61(4):549-559. [DOI] [PubMed] [Google Scholar]
- 15. Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17(1):17-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brzyski RG, Grow DR, Sims JA, Seltman HJ. Increase in androgen:estrogen ratio specifically during low-dose follicle-stimulating hormone therapy for polycystic ovary syndrome. Fertil Steril. 1995;64(4):693-697. [DOI] [PubMed] [Google Scholar]
- 17. Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15(12):2471-2477. [DOI] [PubMed] [Google Scholar]
- 18. Teixeira Filho FL, Baracat EC, Lee TH, et al. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1337-1344. [DOI] [PubMed] [Google Scholar]
- 19. Franks S, Roberts R, Hardy K. Gonadotrophin regimens and oocyte quality in women with polycystic ovaries. Reprod Biomed Online. 2003;6(2):181-184. [DOI] [PubMed] [Google Scholar]
- 20. Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(1):13-21. [DOI] [PubMed] [Google Scholar]
- 21. Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cano F, García-Velasco JA, Millet A, Remohí J, Simón C, Pellicer A. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet. 1997;14(5):254-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87(3):1111-1119. [DOI] [PubMed] [Google Scholar]
- 24. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101(12):2622-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78(3):380-389. [DOI] [PubMed] [Google Scholar]
- 26. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. 2017;107(4):840-847. [DOI] [PubMed] [Google Scholar]
- 27. Boudoures AL, Saben J, Drury A, et al. Obesity-exposed oocytes accumulate and transmit damaged mitochondria due to an inability to activate mitophagy. Dev Biol. 2017;426(1):126-138. [DOI] [PubMed] [Google Scholar]
- 28. Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018;16(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59(6):1445-1453. [DOI] [PubMed] [Google Scholar]
- 30. Bishop CV, Mishler EC, Takahashi DL, et al. Chronic hyperandrogenemia in the presence and absence of a western-style diet impairs ovarian and uterine structure/function in young adult rhesus monkeys. Hum Reprod. 2018;33(1):128-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2008;93(5):1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nobels F, Dewailly D. Puberty and polycystic ovarian syndrome: the insulin/insulin-like growth factor I hypothesis. Fertil Steril. 1992;58(4):655-666. [DOI] [PubMed] [Google Scholar]
- 33. McGee WK, Bishop CV, Bahar A, et al. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. True CA, Takahashi DL, Burns SE, et al. Chronic combined hyperandrogenemia and western-style diet in young female rhesus macaques causes greater metabolic impairments compared to either treatment alone. Hum Reprod. 2017;32(9):1880-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bishop CV, Stouffer RL, Takahashi DL, et al. Chronic hyperandrogenemia and western-style diet beginning at puberty reduces fertility and increases metabolic dysfunction during pregnancy in young adult, female macaques. Hum Reprod. 2018;33(4):694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bishop CV, Xu F, Xu J, et al. Western-style diet, with and without chronic androgen treatment, alters the number, structure, and function of small antral follicles in ovaries of young adult monkeys. Fertil Steril. 2016;105(4):1023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bishop CV, Reiter TE, Erikson DW, et al. Chronically elevated androgen and/or consumption of a Western-style diet impairs oocyte quality and granulosa cell function in the nonhuman primate periovulatory follicle. J Assist Reprod Genet. 2019;36(7):1497-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stouffer RL, Zelinski-Wooten MB. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol. 2004;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phillips KA, Bales KL, Capitanio JP, et al. Why primate models matter. Am J Primatol. 2014;76(9):801-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stouffer RL, Woodruff TK. Nonhuman primates: a vital model for basic and applied research on female reproduction, prenatal development, and women’s health. Ilar J. 2017;58(2):281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy MJ, Halow NG, Royer PA, Hennebold JD. Leukemia inhibitory factor is necessary for ovulation in female rhesus macaques. Endocrinology. 2016;157(11):4378-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bishop CV, Luo F, Gao L, Fei SS, Slayden OD. Mild hyperandrogenemia in presence/absence of a high-fat, Western-style diet alters secretory phase endometrial transcriptome in nonhuman primates. F S Sci. 2020;1(2):172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ravisankar S, Murphy MJ, Redmayne-Titley et al. Supplementary data for: Long-term hyperandrogenemia and/or Western-style diet treatment impairs rhesus macaque preimplantation embryo development. Figshare. 2021. Doi: 10.6084/m9.figshare.16910482 [DOI] [Google Scholar]
- 44. Lanzendorf SE, Gliessman PM, Archibong AE, Alexander M, Wolf DP. Collection and quality of rhesus monkey semen. Mol Reprod Dev. 1990;25(1):61-66. [DOI] [PubMed] [Google Scholar]
- 45. Vera-Rodriguez M, Chavez SL, Rubio C, Reijo Pera RA, Simon C. Prediction model for aneuploidy in early human embryo development revealed by single-cell analysis. Nat Commun. 2015;6:7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong CC, Loewke KE, Bossert NL, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115-1121. [DOI] [PubMed] [Google Scholar]
- 47. Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99(4):1035-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daughtry BL, Rosenkrantz JL, Lazar NH, et al. Single-cell sequencing of primate preimplantation embryos reveals chromosome elimination via cellular fragmentation and blastomere exclusion. Genome Res. 2019;29(3):367-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chavez SL, Loewke KE, Han J, et al. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat Commun. 2012;3:1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vitak SA, Torkenczy KA, Rosenkrantz JL, et al. Sequencing thousands of single-cell genomes with combinatorial indexing. Nat Methods. 2017;14(3):302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Olshen AB, Venkatraman ES, Lucito R, Wigler M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 2004;5(4):557-572. [DOI] [PubMed] [Google Scholar]
- 52. Ha G, Roth A, Lai D, et al. Integrative analysis of genome-wide loss of heterozygosity and monoallelic expression at nucleotide resolution reveals disrupted pathways in triple-negative breast cancer. Genome Res. 2012;22(10):1995-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Knouse KA, Wu J, Amon A. Assessment of megabase-scale somatic copy number variation using single-cell sequencing. Genome Res. 2016;26(3):376-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bellver J, Mifsud A, Grau N, Privitera L, Meseguer M. Similar morphokinetic patterns in embryos derived from obese and normoweight infertile women: a time-lapse study. Hum Reprod. 2013;28(3):794-800. [DOI] [PubMed] [Google Scholar]
- 55. Wissing ML, Bjerge MR, Olesen AI, Hoest T, Mikkelsen AL. Impact of PCOS on early embryo cleavage kinetics. Reprod Biomed Online. 2014;28(4):508-514. [DOI] [PubMed] [Google Scholar]
- 56. Sundvall L, Kirkegaard K, Ingerslev HJ, Knudsen UB. Unaltered timing of embryo development in women with polycystic ovarian syndrome (PCOS): a time-lapse study. J Assist Reprod Genet. 2015;32(7):1031-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bartolacci A, Buratini J, Moutier C, et al. Maternal body mass index affects embryo morphokinetics: a time-lapse study. J Assist Reprod Genet. 2019;36(6):1109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Papouli E, Defais M, Larminat F. Overexpression of metallothionein-II sensitizes rodent cells to apoptosis induced by DNA cross-linking agent through inhibition of NF-kappa B activation. J Biol Chem. 2002;277(7):4764-4769. [DOI] [PubMed] [Google Scholar]
- 59. Tanaka M, Jin G, Yamazaki Y, Takahara T, Takuwa M, Nakamura T. Identification of candidate cooperative genes of the Apc mutation in transformation of the colon epithelial cell by retroviral insertional mutagenesis. Cancer Sci. 2008;99(5):979-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou X, Xu B, Zhang D, et al. Loss of CDYL results in suppression of CTNNB1 and decreased endometrial receptivity. Front Cell Dev Biol. 2020;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gonzalez TL, Sun T, Koeppel AF, et al. Sex differences in the late first trimester human placenta transcriptome. Biol Sex Differ. 2018;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roifman M, Choufani S, Turinsky AL, et al. Genome-wide placental DNA methylation analysis of severely growth-discordant monochorionic twins reveals novel epigenetic targets for intrauterine growth restriction. Clin Epigenetics. 2016;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boomsma CM, Fauser BC, Macklon NS. Pregnancy complications in women with polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):72-84. [DOI] [PubMed] [Google Scholar]
- 64. Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575-592. [DOI] [PubMed] [Google Scholar]
- 65. Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281(4):E757-E765. [DOI] [PubMed] [Google Scholar]
- 66. Hansen BC, Bodkin NL, Ortmeyer HK. Calorie restriction in nonhuman primates: mechanisms of reduced morbidity and mortality. Toxicol Sci. 1999;52(2 suppl):56-60. [DOI] [PubMed] [Google Scholar]
- 67. Wagner JD, Cann JA, Zhang L, Harwood HJ.Diabetes and obesity research using nonhuman primates. In: Abee CR, Mansfield K, Tardif S, Morris T, eds. Nonhuman Primates in Biomedical Research. 2nd ed. Elsevier; 2012:699-732. [Google Scholar]
- 68. Angiolillo AL, Sgadari C, Taub DD, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182(1):155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8(3):207-219. [DOI] [PubMed] [Google Scholar]
- 70. Tatone C, Amicarelli F, Carbone MC, et al. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14(2):131-142. [DOI] [PubMed] [Google Scholar]
- 71. Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. 2019;158(3):R79-R90. [DOI] [PubMed] [Google Scholar]
- 72. Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251-276. [DOI] [PubMed] [Google Scholar]
- 73. Krízan J, Cuchalová L, Síma P, Králícková M, Madar J, Vĕtvicka V. Altered distribution of NK and NKT cells in follicular fluid is associated with IVF outcome. J Reprod Immunol. 2009;82(1):84-88. [DOI] [PubMed] [Google Scholar]
- 74. Asirvatham AJ, Schmidt M, Gao B, Chaudhary J. Androgens regulate the immune/inflammatory response and cell survival pathways in rat ventral prostate epithelial cells. Endocrinology. 2006;147(1):257-271. [DOI] [PubMed] [Google Scholar]
- 75. Deng H, Li Z, Liu G, et al. Elevated serum interferon γ-inducible protein-10 in women with polycystic ovary syndrome. Gynecol Endocrinol. 2017;33(5):363-367. [DOI] [PubMed] [Google Scholar]
- 76. Tarkun I, Arslan BC, Cantürk Z, Türemen E, Sahin T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89(11):5592-5596. [DOI] [PubMed] [Google Scholar]
- 77. Shorakae S, Ranasinha S, Abell S, et al. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin Endocrinol (Oxf). 2018;89(5):628-633. [DOI] [PubMed] [Google Scholar]
- 78. Ravisankar S, Hanna CB, Brooks KE, et al. Metabolomics analysis of follicular fluid coupled with oocyte aspiration reveals importance of glucocorticoids in primate periovulatory follicle competency. Sci Rep. 2021;11(1):6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baart EB, Macklon NS, Fauser BJ. Ovarian stimulation and embryo quality. Reprod Biomed Online. 2009;18(suppl 2):45-50. [DOI] [PubMed] [Google Scholar]
- 80. Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170-207. [DOI] [PubMed] [Google Scholar]
- 81. Bishop CV, Takahashi D, Mishler E, et al. Individual and combined effects of 5-year exposure to hyperandrogenemia and Western-style diet on metabolism and reproduction in female rhesus macaques. Hum Reprod. 2021;36(2):444-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saharkhiz N, Zademodares S, Salehpour S, Hosseini S, Nazari L, Tehrani HG. The effect of testosterone gel on fertility outcomes in women with a poor response in in vitro fertilization cycles: A pilot randomized clinical trial. J Res Med Sci. 2018;23:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Haydardedeoğlu B, Işık AZ, Bulgan Kılıçdağ E. The combination of dehydroepiandrosterone, transdermal testosterone, and growth hormone as an adjuvant therapy in assisted reproductive technology cycles in patients aged below 40 years with diminished ovarian reserve. Turk J Obstet Gynecol. 2015;12(2):60-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ludwig M, Finas DF, al-Hasani S, Diedrich K, Ortmann O. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod. 1999;14(2):354-358. [DOI] [PubMed] [Google Scholar]
- 85. Jabara S, Coutifaris C. In vitro fertilization in the PCOS patient: clinical considerations. Semin Reprod Med. 2003; 21(3):317-324. [DOI] [PubMed] [Google Scholar]
- 86. Luzzo KM, Wang Q, Purcell SH, et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PloS One. 2012;7(11):e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yun Y, Wei Z, Hunter N. Maternal obesity enhances oocyte chromosome abnormalities associated with aging. Chromosoma. 2019;128(3):413-421. [DOI] [PubMed] [Google Scholar]
- 88. Ge J, et al. Telomere dysfunction in oocytes and embryos from obese mice. Front Cell Dev Biol. 2021;9:617225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu J, Xu J, Liu B, et al. Chromatin analysis in human early development reveals epigenetic transition during ZGA. Nature. 2018;557(7704):256-260. [DOI] [PubMed] [Google Scholar]
- 90. Eckersley-Maslin MA, Alda-Catalinas C, Reik W. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat Rev Mol Cell Biol. 2018;19(7):436-450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All RNA-seq data generated and analyzed during this study are included in the data repository, Gene Expression Omnibus (GSE186969), https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE186969.