Abstract
Introduction
During the COVID-19 pandemic, many tobacco users increased their tobacco use, and calls to quitlines decreased. Among inpatients, the pandemic also necessitated a rapid transition of intensive tobacco use counseling to telehealth counseling. No data exist comparing the outcomes of telehealth inpatient counseling with in-person (pre-telehealth) counseling.
Aims and Methods
We examined inpatient data from a large tobacco treatment program (TTP) during two comparable time periods 04/01/2019–09/30/2019 (pre-telehealth) and 04/01/2020–09/30/2020 (telehealth). The pre-telehealth and telehealth populations were compared using Pearson’s chi-square test for homogeneity on each populations’ patient, visit, and medication acceptance characteristics. Reach to “current tobacco users” was analyzed using TTP flowsheet and electronic health record (EHR) data in relation to aggregate EHR data in the data warehouse.
Results
Mean monthly tobacco treatment inpatient counseling and outreach visits increased 38.9% in the telehealth period (M = 376, SD = 36.7) compared with the pre-telehealth period (M = 271, SD = 50.0) (t(10) = 3.8, p = .004). Reach significantly increased from 32.8% to 65.9% among all “current tobacco users” admitted, including 31.8% to 66.6% in races at higher risk for COVID-19 severe disease. Pearson’s chi-square tests for homogeneity showed significant differences in the pre-telehealth and telehealth population distributions for age, visit type, ethnicity, and medication acceptance.
Conclusions
This study offers the first understanding of characteristics of patients, visits, and medication acceptances in pre-telehealth and telehealth tobacco use treatment for inpatient populations. Larger reach and counseling were identified in the telehealth population. This study’s findings on inpatient tobacco use treatment can inform future reach and engagement of large numbers of patients who use tobacco products.
Implications
This study provides the first analysis of inpatient tobacco use treatment transition to telehealth delivery of care during the COVID-19 pandemic. The transition resulted in increases in reach and cessation counseling. These findings can inform efforts to improve reach, engagement, and research on telehealth delivery of inpatient tobacco use treatment.
Introduction
With 34 million active cigarette smokers U.S., smoking remains the leading cause of preventable death, accounting for more than 480 000 deaths annually.1 In 2018, 55% of adults who smoke attempted to quit, but only 7.5% were successful.2 More than 16 million Americans are living with chronic diseases related to smoking, resulting in $170 billion in direct medical costs per year.1
The Joint Commission, U.S. Department of Health and Human Services, and the U.S. Preventive Services Task Force recommend that all nonpregnant adults be screened for tobacco use, advised to stop using, and provided behavioral and pharmacotherapy interventions (unless medically contraindicated).3–5 Such guidelines apply not only to outpatient care but also to inpatient settings.6 Studies indicate inpatient tobacco use treatment programs decrease hospital readmissions and reach patients who may otherwise have limited access to primary care and prevention services, including outpatient tobacco treatment.7,8
The pandemic disproportionately affected individuals from communities of color with increased risks of COVID-19 infection, hospitalization, and death.9,10 Academic medical centers that care for vulnerable and underserved populations provide unique opportunities to address tobacco use during hospitalization with patients who may otherwise not have access to tobacco treatment services. During the COVID-19 pandemic, many individuals with tobacco use disorder increased their smoking,11 and smoking emerged as a clear modifiable risk factor for severe outcomes in COVID-19, along with preexisting conditions related to smoking, such as cardiovascular disease, respiratory disease, and malignancy.12
The COVID-19 pandemic prompted inpatient tobacco use treatment programs to rapidly adapt from in-person counseling to telehealth visits (Supplement 1). As little previous research has examined such transitions, we sought to examine reach, outcomes, and associations with demographics and visit variables of pre-telehealth (ie, in-person) and telehealth time periods in tobacco use treatment from a large inpatient tobacco treatment program (TTP).
Methods
Institutional Settings
UNC Health is the academic health system at the University of North Carolina—Chapel Hill. The UNC TTP began in 2008 and cessation services were offered to inpatients at the 950 bed UNC Medical Center in Chapel Hill since 2010 and at the 83 bed UNC Hillsborough Hospital since 2019.
Data
Inclusion criteria: patients treated by the UNC TTP team during the time periods 04/01/2019–09/30/2019 (pre-telehealth) and 04/01/2020–09/30/2020 (telehealth). Tobacco use treatment telehealth services were fully operational by 04/01/2020. Pre-telehealth and telehealth populations were chosen from the same months in consecutive years to account for seasonal variability in inpatient populations. Flowsheet data obtained at the point-of-care included patient, visit, and treatment variables, including medication use. This study also used an i2b2 web application, linked to an institutional data warehouse, to obtain deidentified counts of inpatients on units served by the TTP team (see Supplement 1) from the two time periods above. i2b2 is a tool that allows the user to query the data warehouse to identify patient cohorts based on user-specified criteria (eg, diagnosis, date of admission, etc.). Results in i2b2 with aggregate counts >0 and <10 are reported as <10 to protect patient protected health information (PHI). Simple imputation of the mean (5) was performed to include these data with existing but unreported counts ranging from >0 and <10. This study was reviewed and approved by the UNC Human Subjects Institutional Review Board.
Analyses
Descriptive statistics, chi-square tests, and t tests were performed using R, V4.14, and SPSS V27 software. The pre-telehealth and telehealth populations were compared using Pearson’s chi-square test for homogeneity (without correction) on each population’s patient, visit, and medication acceptance characteristics (see Table 1 for full list). p values of <.05 were considered significant. Race categories were consolidated to reflect risk of higher versus not higher COVID-19 infection severity (ie, Black or African American, American Indian or Alaska Native, Native Hawaiian or Pacific Islander were identified as “higher risk for COVID-19 infection”; Asian, White or Caucasian, “other race,” and “unknown” were consolidated as “not higher risk for COVID-19 infection”).13 Although variability in staffing occurred in both time periods, the full-time-employee (FTE) hours for tobacco treatment specialists were averaged over the respective pre-telehealth and telehealth periods. In addition to visit count analysis with t test comparison of monthly visit means for the two periods, an FTE-adjusted calculation of telehealth visit counts is provided. Reach (numbers of patients served by the TTP) were analyzed using counts of patients with interactions (point-of-care data) in relation to counts of “current tobacco users” identified in i2b2 for each period and stratified by race category. Point-of-care missing data for medication acceptance was imputed as “declined.” Missing race data in i2b2 was imputed as “unknown.”
Table 1.
Patient and Visit Characteristics
| Characteristic | Pre-telehealth Characteristica (study population %) |
Telehealth Characteristica (study population %) |
|---|---|---|
| Number of unique patients | 1426 | 1810 |
| Mean age (SD) | 50 (15.5) | 48 (15.0) |
| Age 0 < 65 | 1147 (80.4) | 1548 (85.5) |
| Age ≥65 | 279 (19.6) | 262 (14.5) |
| Genderb | ||
| Female | 607 (42.6) | 804 (44.4) |
| Male | 819 (57.4) | 1006 (55.6) |
| Racec | ||
| Higher risk for COVID-19 | 469 (32.9) | 597 (33.0) |
| Not higher risk for COVID-19 | 957 (67.1) | 1213 (67.0) |
| Ethnicityd | ||
| Hispanic or Latinx | 21 (1.5) | 45 (2.5) |
| Not Hispanic or Latinx | 1405 (98.5) | 1765 (97.5) |
| Insurancee | ||
| General health insurance | 1051 (73.7) | 1290 (71.3) |
| No general health insurance | 375 (26.3) | 520 (28.7) |
| Visit types—all | 1624 (100) | 2255 (100) |
| Visit type—outreach only | 396 (24.4) | 733 (32.5) |
| Visit type—counseled | 1228 (75.6) | 1522 (67.5) |
| Medication outcome: for “counseled” visits | 1228 (100) | 1522 (100) |
| Medications accepted | 707 (57.6) | 781 (51.3) |
| Medications declined | 521 (42.4) | 741 (48.7) |
| Characteristics of patients accepting medication recommendationf | 707 visits | 781 visits |
| Age <65 yo | 586 (83% of age) | 666 (85% of age) |
| Age ≥65 yo | 121 (17% of age) | 115 (15% of age) |
| Gender: female | 328 (46% of gender) | 367 (47% of gender) |
| Gender: male | 379 (54% of gender) | 414 (53% of gender) |
| Race: higher risk for COVID-19 | 222 (31% of race) | 237 (30% of race) |
| Race: not higher risk for COVID-19 | 485 (69% of race) | 544 (70% of race) |
| Insurance: general health | 521 (74% of insurance) | 550 (70% of insurance) |
| Insurance: no general health | 186 (26% of insurance) | 231 (30% of insurance) |
aPatient characteristic count unless indicated otherwise.
bNo greater gender granularity is available in this dataset.
cRace evaluated as a binary variable with (1) “higher risk for COVID-19” comprised of dataset values of Black or African American, American Indian or Alaska Native, Native Hawaiian or Pacific Islander and (2) “not higher risk for COVID-19” comprised of Asian, Other Race, Unknown, White or Caucasian.
dEthnicity evaluated as a binary variable with (1) Hispanic or Latinx and (2) not Hispanic or Latinx including not Hispanic or Latinx, patient refused, and unknown.
eInsurance status: general health insurance = BCBS, Tricare, State Health Plan, Medicaid, Medicare, Medicare Advantage, commercial, agency; no general health insurance = Medicaid pending, Worker’s Compensation, liability; self-pay.
fAll characteristics that may have contributed to patient medication acceptance are not available in this dataset.
Results
Characteristics of patients treated and visits conducted by the inpatient TTP team, along with counts of medication recommendations accepted by patients are detailed (Table1). Missing medication acceptance values imputed as “declined”: pre-telehealth 2.2% and telehealth 1.7%.
The pre-telehealth population contained 1426 patients who were provided counseling or outreach only (ie, contact for offer of cessation counseling but patient declined or was unavailable for counseling). In comparison, the telehealth population showed 1810 patients received counseling or outreach only (26.9% increase).
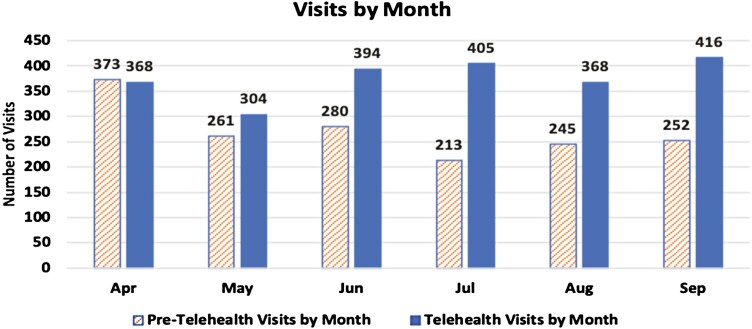
The pre-telehealth population had 1624 visits (total count of counseling or outreach only visits; 1228 counseling, 396 outreach only). The telehealth population had 2255 total visits (1522 counseling, 733 outreach only). This was a 38.9% increase in total visits in the telehealth period with a 23.9% increase in telehealth counseling visits and an 85.1% increase in telehealth outreach only visits. The mean telehealth monthly visit counts (M = 376, SD = 36.7) compared with the pre-telehealth monthly visit counts (M = 271, SD = 50.0) were significantly higher, t(10) = 3.8, p = .004 (Figure 1). The pre-telehealth time period averaged 2.1 TTP FTEs and the telehealth time period averaged 1.9 TTP FTEs, reflecting a 9.5% FTE decrease in the telehealth time period. If telehealth tobacco treatment specialist FTE is adjusted to 2.1 FTE, to reflect the pre-telehealth FTE allocation, telehealth visit counts would have risen to 2492 visits, a 53.4% increase over the pre-telehealth visit counts.
Figure 1.
Pre-telehealth and telehealth visits by month.
Full results of Pearson’s chi-square tests for homogeneity showed significant differences in the pre-telehealth and telehealth population distributions for medication acceptance, age, ethnicity, and visit type. The pre-telehealth population had proportionally more medication acceptances than the telehealth population (χ 2 = 10.7 (df = 1, N = 2750), p = .001). The telehealth population had proportionally more: patients with age <65 years (χ 2 = 14.8 (df = 1, N = 3236), p < .001), Hispanic patients (χ 2 = 4.1 (df = 1, N = 3236), p < .04), and visit type = outreach only (χ 2 = 30.2 (df = 1, N = 3879), p < .001) than the pre-telehealth population. Full chi-square analyses results are provided (Supplement 2).
A total of 20 758 inpatients were on TTP units in the pre-telehealth period, with 5540 (26.7%) classified as “higher risk for COVID-19 race category” (0.1% missing race values). 4352 (21.0%) of all inpatients on the TTP units were classified as “current tobacco users” with 1474 (33.9%) of the “higher risk for COVID-19” race category. The telehealth population queries identified a total of 17 970 inpatients, with 4718 (26.3%) of the “higher risk for COVID-19” race category (1.1% missing race values). 2746 (15.3%) of all telehealth inpatients on TTP units were classified as “current tobacco users” with 896 (32.6%) of the “higher risk for COVID-19” race category. Inpatient proportions of current tobacco users at higher risk for COVID-19 (33.9% vs. 32.6%) were not significantly different in the pre-telehealth compared with the telehealth population (χ 2 = 1.2 (df = 1, N = 7098), p = .28). In the telehealth period, of the 1312 patients with a COVID-19 diagnosis in i2b2, 369 (28.1%) were in the “higher risk for COVID-19” race category.
Using TTP patient counts in relation to i2b2 patient counts, reach of the TTP team to all current tobacco users was 32.8% (1426/4352) in the pre-telehealth period and 65.9% (1810/2746) in the telehealth period. Reach to “current tobacco users” of “higher risk of COVID-19” race category was 31.8% (469/1474) in the pre-telehealth period and 66.6% (597/896) in the telehealth period.
Discussion
The COVID-19 pandemic presented unique challenges and opportunities to inpatient tobacco use treatment. With staff, system, and patient cooperation, many barriers were overcome and new methods of care delivery successfully instituted. Despite a 9.5% reduction in TTP FTE allocation, the UNC TTP team significantly increased counts of patients served and visits conducted in the telehealth period. Reach increased for all “current tobacco users” and for those classified as belonging to the “higher risk of COVID-19” race category. Our study is the first to demonstrate the impact of transition to telehealth treatment of tobacco use for inpatients in response to the COVID-19 pandemic. Our TTP team reported improved workflows and efficiency with telehealth implementation, providing evidence for its continued use postpandemic. These improvements resulted from quicker transitions between visits, less travel to patient rooms, and fewer delays due to prolonged patient visits with other providers. The findings of increased visit numbers are similar to analyses by Kotsen et al.,14 but our study examined inpatient telehealth tobacco treatment delivery rather than outpatient delivery of such services. We also included expanded analyses of demographics, including insurance status, medication acceptance, and comparison with aggregate i2b2 data for analyses of reach.
A statistically significant difference in the proportion of tobacco cessation medication acceptance in the pre-telehealth and telehealth populations was identified with greater medication acceptance in the pre-telehealth population. Possible explanations for this difference include: (1) less effective communication or engagement with tobacco treatment specialists via remote (telehealth) interactions, resulting in poorer uptake of medication recommendations, and (2) less motivation to quit smoking with increased pandemic stress. The latter explanation is consistent with reports of decreased tobacco cessation engagement with national quit lines and increased tobacco sales during COVID-19.15
According to i2b2 data, inpatient counts and tobacco prevalence had decreased in the telehealth population compared with the pre-telehealth population. This likely reflected the health care system’s early response to the pandemic with attempts to reserve space for COVID-19 patients and limit nonurgent admissions. Lower inpatient counts also likely reflected health care hesitancy overall, perhaps disproportionately in current smokers during the pandemic.16 In the early phase of the pandemic, with growing evidence of smoking as a risk factor for severe COVID-19 infection, patients who smoke may have voluntarily limited their hospital admissions in an effort to protect themselves.
Despite decreases in absolute numbers of inpatients and “current tobacco users,” i2b2 analyses confirmed that the proportions of inpatients of the “higher risk for COVID-19” race category were not significantly different in the two populations. TTP point-of-care data showed a similarly stable race distribution, confirming consistent reach. We had hypothesized that inpatients of the race category “higher risk for COVID-19” would have increased substantially in the 2020 population. Using i2b2 aggregate data, we found that patients of the “higher-risk for COVID-19” category comprised a small increased proportion (28.1%) of patients with a COVID-19 diagnosis compared with their overall proportion of all inpatients in either the pre-telehealth (26.7%) or telehealth (26.3%) periods. This relative stability may reflect early pandemic health care hesitancy of these patients.
While multiple telehealth guidelines have been proposed, the discussion on telehealth reimbursement and best practices continues.17–19 Research is needed to determine optimally efficient and effective communication and telehealth practices including: (1) delivery method (eg, phone, video, and/or interactive voice recordings), (2) delivery content, and (3) delivery precision (ie, the right message via the right mode at the right time, based on specific patient characteristics). Also needed are further studies of patient and visit characteristics in tobacco cessation medication acceptance in telehealth populations. Video-based telehealth has been shown to increase the use of cessation pharmacotherapy compared with telephone-based interventions, with higher user satisfaction, but it is significantly more expensive than telephone counseling.20 Economic and outcome analyses (eg, quit rates, patient satisfaction, future use of medical services) can inform telehealth practice and reimbursement. Equally pressing is identification of optimal telehealth practices to address the digital divide and its potential to exacerbate care disparities.
Several limitations exist. Analyses reflect experiences and data from one academic medical center with resultant limitations in generalizability. This is a retrospective analysis of existing clinical data, with associated limits on data collected for nonresearch purposes with potential for missing, incomplete, or imprecise data although percentages of missing data were very low. Staff changes impeded an exact comparison of care delivered, and results likely underestimate improvement in numbers of patients served and visits conducted in the telehealth period.
This study offers an improved understanding of characteristics of patients, visits, and medication acceptances in the pre-telehealth and telehealth tobacco use treatment for inpatient populations. These findings can inform future reach and engagement of general populations participating in tobacco use treatment, especially those using a telehealth modality that may begin in the inpatient setting and continue as outpatient treatment.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We are grateful to the North Carolina Translational and Clinical Sciences Institute and their support and use of the i2b2 platform.
Funding
This work was funded in part by the National Cancer Institute grant number 3P30CA016086-43S1.
Declaration of Interests
None declared.
Data Availability
Data are not available for sharing due to privacy standards for protected health information contained in these data. Researchers may contact the corresponding author with any questions.
References
- 1. Centers for Disease Control and Prevention (CDC). Current Cigarette Smoking Among Adults in the United States.https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking. Accessed November 16, 2020.
- 2. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR. 2019;68(45):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiore MC, Goplerud E, Schroeder SA. The Joint Commission’s new tobacco-cessation measures—will hospitals do the right thing? NEJM. 2012;366(13):1172–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. U.S. Department of Health and Human Services. Smoking Cessation: A Report of the Surgeon General—Key Findings.2020. https://www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/2020-cessation-sgr-factsheet-key-findings/index.html. Accessed July 1, 2020.
- 5. USPSTF, Krist AH, Davidson KW, et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(3):265–279. [DOI] [PubMed] [Google Scholar]
- 6. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence 2008 Update. Quick Reference Guide for Clinicians . 2009. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/references/quickref/tobaqrg.pdf. Accessed January 31, 2021.
- 7. Cartmell KB, Dooley M, Mueller M, et al. Effect of an evidence-based inpatient tobacco dependence treatment service on 30-, 90-, and 180-day hospital readmission rates. Med Care. 2018;56(4):358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evison M, Pearse C, Howle F, et al. Feasibility, uptake and impact of a hospital-wide tobacco addiction treatment pathway: results from the CURE project pilot. Clin Med (Lond). 2020;20(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahajan UV, Larkins-Pettigrew M. Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties. J Public Health (Oxf). 2020;42(3):445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Snowden LR, Graaf G. COVID-19, social determinants past, present, and future, and African Americans’ health. J Racial Ethn Health Disparities. 2021;8(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kowitt SD, Cornacchione Ross J, Jarman KL, et al. Tobacco quit intentions and behaviors among cigar smokers in the United States in response to COVID-19. Int J Environ Res Public Health. 2020;17(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Risk for COVID-19 Infection, Hospitalization, and Death by Race/Ethnicity. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html. Accessed March 22, 2021.
- 14. Kotsen C, Dilip D, Carter-Harris L, et al. Rapid scaling up of telehealth treatment for tobacco-dependent cancer patients during the COVID-19 outbreak in New York City. Telemed J E Health. 2021;27(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929–1930. [DOI] [PubMed] [Google Scholar]
- 16. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Academy of Family Physicians (AAFP). Tobacco Cessation Telehealth Guide.https://www.aafp.org/dam/AAFP/documents/patient_care/tobacco/tobacco-cessation-telehealth-guide.pdf. Accessed February 5, 2021.
- 18. American Lung Association. Tobacco Cessation, Telehealth and a Pandemic: A Changing Landscape.https://www.lung.org/getmedia/83628722-434c-432b-84f4-754b25ea0cce/tobacco-cessation-and-telehealth_final.pdf. Accessed February 5, 2021.
- 19. Medicare Payment Advisory Committee. Report to the Congress: Medicare Payment Policy.2021. http://medpac.gov/docs/default-source/reports/mar21_medpac_report_to_the_congress_sec.pdf?sfvrsn=0. Accessed March 22, 2021.
- 20. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. JMIR. 2015;17(5):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not available for sharing due to privacy standards for protected health information contained in these data. Researchers may contact the corresponding author with any questions.