Abstract
Background
Hepatosplenic candidiasis (HSC) used to be reported in patients with acute myeloid leukemia (AML) without antifungal prophylaxis. The aim was to describe the clinical features and outcomes of HSC over the last 13 years in a single French hematology center.
Methods
All patients diagnosed with HSC between 2008 and 2020 were included in a single-center retrospective cohort study. Data were collected from patient charts, and HSC was classified according to the 2020 European Organisation for Research and Treatment of Cancer/Mycoses Study Group definitions.
Results
Sixty patients were included, with 18.3% proven, 3.3% probable, and 78.3% possible HSC according to the 2020 European Organization for Research and Treatment of Cancer Mycoses Study Group classification. Among them, 19 patients were treated for acute myeloid leukemia (AML), 21 for lymphomas, and 14 for acute lymphoblastic leukemia. HSC occurred in 13 patients after autologous stem cell transplantation for lymphoma. At HSC diagnosis, 13 patients were receiving antifungal prophylaxis. Candida colonization was present in 84.2%, with prior candidemia in 36.7% of cases. β-D-glucans was positive in 55.8%, and 45.8% of tissue biopsies were contributive. First-line antifungal therapy was azoles in 61.7%, and steroids were associated in 45% of cases. At 3 months of follow-up, partial response to antifungal therapy was 94.2%. At last follow-up (mean, 22.6 months), 41 patients (68.3%) presented a complete hematological remission and 22 patients were deceased, none because of HSC.
Conclusions
The epidemiology of HSC has changed in the last decade, with fewer cases occurring in the AML setting. A better identification of patients at risk could lead to specific prophylaxis and improved diagnosis.
Keywords: diagnosis, hepatosplenic candidiasis, treatment
Hepatosplenic candidiasis (HSC) is a rare complication occurring after a prolonged period of neutropenia, mainly in the context of hematological malignancies [1, 2]. The incidence is unknown and probably underestimated. In patients with acute myeloid leukemia (AML), the incidence rate has been reported in varying proportions, from 3% up to 29%, and, historically, HSC has rarely been diagnosed in patients with lymphoma [3–5].
HSC diagnosis may be challenging and relies on clinical, biological and radiological considerations. The revised European Organisation for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria require examination or recovery of yeasts by culture or amplification of fungal DNA from a tissue biopsy or a specimen from a sterile body site for a proven invasive fungal disease, and for probable disease, the positivity of (1,3)-β-D-glucans (BDG) in at least 2 consecutive serum samples in a patient with host factors and clinical features [6]. Regarding HSC, histological examination is rarely contributive. Classically, HSC is suspected in the presence of hepatic or splenic target-like lesions on the computed tomography (CT) scan [1, 7]. Although magnetic resonance imaging (MRI) remains the most sensitive radiological tool for HSC diagnosis [8], enhanced ultrasonography (US) with contrast [9] and positron emission tomography (PET) scan with 18-flurorodesoxyglucose showed encouraging results regarding both the diagnosis and follow-up of patients with HSC [10]. Furthermore, while blood cultures are often negative, dosage of biomarkers in serum such as BDG [11] or mannan antigens and antibodies could allow earlier diagnosis of HSC [12] and be useful to monitor outcome [13]. However, the usefulness of these tools for diagnosis purposes has not been investigated in large contemporary cohorts of HSC patients.
HSC treatment relies on antifungal therapy for 3–6 months [14]. In the 2010 Infectious Disease Society of America (IDSA) guidelines [15] and the 2012 European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines [16], fluconazole was recommended as first-line therapy, whereas liposomal amphotericin B (LAmB) was preferred in critically ill patients. Currently, the IDSA and ESCMID recommend use of echinocandins or LAmB as first-line therapy [15, 17]. Furthermore, case series with small sample sizes have suggested that corticosteroids were associated with improvement in symptoms and inflammatory response in HSC patients [14, 18]. Hence, the optimal treatment strategy for HSC remains unknown.
Finally, it is likely that the profile of patients with HSC has evolved in the past decade given that several major changes recently occurred in the field of HSC, such as the availability of new diagnostic tools (biomarkers, PET scan), the widespread use of antifungal prophylaxis in patients with AML since 2007 [19], and extended empirical antifungal treatment in neutropenic patients [2, 20]. However, no large contemporary cohort study of HSC patients has been reported to date.
The aim of this study was to describe a large cohort of HSC patients over a 13-year period, encompassing epidemiology, diagnosis workup, treatments, and outcomes in this rare disease.
METHODS
Study Design
This was a retrospective cohort study. All patients diagnosed with HSC between 2008 and 2020 and followed at Saint Louis Hospital (Paris, France) were included. Considering the retrospective study design, data collection from preexisting medical records, and respect for the anonymity of the patients included (referred to as studies “Hors Loi Jardé” in France), no ethical approval or administrative approval was necessary for this study. This study was submitted to the local Data Protection Officer (DPO) and, upon approval, was identified in the hospital study registry. The research was conducted in accordance with the Declaration of Helsinki. Patients were informed that their clinical data could be used, after anonymization, for research purposes.
Diagnostic of HSC
Patients were included from insurance database codes (CIM-10 codes B377, B378, and B379), and medical charts were reviewed to validate HSC diagnoses. We used the 2020 EORTC/MSG definitions [6] to define proven HSC as the demonstration of yeasts in a normally sterile site; probable HSC, in a patient with host factors, requires clinical criteria, such as imaging detection of lesions with typical “bull’s-eye aspect” in the liver or spleen, as well as mycological findings, following an episode of candidemia within the previous 2 weeks; and possible HSC is defined by the presence of host factors and radiological findings of small, target-like abscesses (bull’s-eye lesions) in the liver or spleen.
Before 2016, dosage of biomarkers (ie, BDG, Candida antigen, and serology) was not routinely performed. During this period, if a serum at HSC diagnosis was still available, we retrospectively performed these dosages. BDG testing was performed using the Fungitell assay (Cape Cod Diagnostics, Cape Cod, MA, USA) according to the manufacturer’s instructions. BDG was considered positive when >80 pg/mL. Samples were analyzed using PLATELIA Candida Ag Plus for antigen testing and PLATELIA Candida Ab PLUS (Biorad) for Candida serology. For antigen testing, we used the cutoff of 125 pg/mL to retain positivity, and for Candida serology, samples were considered positive when they were >10 UA/mL.
Moreover, in order to evaluate HSC prevalence over the study period, we obtained from insurance databases the number of patients who were hospitalized between 2013 and 2019 at Saint Louis Hospital for the treatment of hematological malignancies (CIM-10 codes C81–C96). We also evaluated HSC prevalence in selected subpopulations (ie, AML, lymphoma, ALL).
Variables Definitions
Data on patient characteristics at baseline, hematologic disease, Candida colonization, and type of treatment were collected from medical charts. Neutropenia was defined as a neutrophil count <500/mm3 and date of first signs of HSC by prolonged fever (≥3 days) despite broad-spectrum antibiotics and no alternative diagnosis. Furthermore, Candida colonization was defined as the presence of Candida at direct examination or culture of samples from the throat, lung, vagina, urine, skin, or stools before or at HSC diagnosis.
Follow-up
Partial response at 3 months was defined by a resolution of all clinical and biological signs, with imaging studies showing stable size or reduction of size or number of lesions. Complete response was defined as resolution of all clinical and biological signs with imaging studies showing resolved or calcified nodules. Vital status was recorded. Follow-up ended in May 2020.
Statistical Analyses
Continuous variables are expressed as mean (SD), and categorical variables as number (%). Survival curves considering overall survival were generated using the Kaplan-Meier method. Then, a proportional hazard Cox regression modeling was used to estimate hazard ratios (HRs) and 95% CIs, considering overall survival as the outcome. The model was adjusted for azole administration, use of corticosteroids, age at HSC diagnosis, length of aplasia >1 month (yes vs no), and hematological remission at HSC diagnosis (yes vs no). Proportional hazard assumption was graphically checked. Analyses were 2-sided and performed using R software (version 3.6.2).
RESULTS
Patients’ Characteristics
Sixty patients diagnosed with HSC between 2008 and 2020 and followed at Saint Louis Hospital were included. Patient characteristics are reported in Table 1. The mean age at diagnosis (SD) was 44.5 (17.7) years, and 35 (58.3%) were men. Patients had the following hematologic diseases: AML in 19 patients (31.7%), lymphoma in 21 (35%), ALL in 14 (23.3%), and other in 6 patients (bi-phenotypic leukemia in 2, chronic lymphocytic leukemia in 1, myelodysplastic syndrome [MDS] in 2, and aplastic anemia in 1). Overall, 36 patients (60%) had a disease of lymphoid lineage, and for lymphoma patients diffuse large B-cell lymphoma accounted for the majority (10 patients, 47.6%). Other types of lymphoma were T-cell lymphoma (4 patients, 19%), Burkitt lymphoma (3 patients, 14.2%), Hogdkin lymphoma, and mantle cell lymphoma (2 patients for each). Among these 36 patients, before the diagnosis of HSC, 13 lymphoma patients received autologous stem cell transplantation (auto-SCT). Two patients had received allo-SCT before HSC diagnosis.
Table 1.
Characteristics of the HSC Population
| Overall Population (n = 60), No. (%) or Mean ± SD |
|
|---|---|
| Baseline characteristics | |
| Male gender | 35 (58.3) |
| Age, y | 44.5 ± 17.7 |
| Hematologic disease | - |
| Acute myeloid leukemia | 19 (31.7) |
| Acute lymphoid leukemia | 14 (23.3) |
| Lymphoma | 21 (35.0) |
| Others | 6 (10.0) |
| Stem cell transplantation before HSC diagnosis | - |
| None | 32 (68.0) |
| Autologous | 13 (27.7) |
| Allogenic | 2 (4.3) |
| Characteristics at HSC diagnosis | |
| Candida colonization | 48 (84.2) |
| Neutropenia duration, d | 30.1 ± 32.6 |
| Hematologic disease control | 35 (58.3) |
| Prior candidemia | 22 (36.7) |
| EORTC/MSG classification | - |
| Possible | 47 (78.3) |
| Probable | 2 (3.3) |
| Proven | 11 (18.3) |
| Treatments | |
| Antifungal prophylaxis | 13 (22.0) |
| First-line antifungal therapy | - |
| None | 0 (0) |
| Azoles | 37 (61.7) |
| Caspofungine | 22 (36.7) |
| Liposomal amphotericin B | 1 (1.6) |
| Antifungal therapy combination | 3 (5.0) |
| Duration of curative treatment, mo | 7.4 ± 6.0 |
| Use of corticosteroids | 27 (45.0) |
| Delayed or modified chemotherapy | 8 (13.8) |
| Follow-up data | |
| Follow-up, mo | 22.6 ± 19.3 |
| Partial response at 3 mo | 49 (94.2) |
| Complete response at last follow-up | 53 (88.3) |
| Hematologic remission at last follow-up | 41 (68.3) |
| Death at last follow-up | 22 (36.7) |
Abbreviations: EORTC/MSG, European Organization for Research and Treatment of Cancer/Mycoses Study Group; HSC, hepatosplenic candidiasis.
The prevalence of HSC among all hematological patients ranged from 0.4% to 4% depending on the considered year. Detailed results are presented in Supplementary Table 1.
At HSC diagnosis, 13 patients were receiving antifungal prophylaxis, including 9 AML patients (posaconazole for 7 and voriconazole for the other 2), 3 MDS (posaconazole), and 1 ALL (caspofungin).
The mean duration of neutropenia before HSC diagnosis (SD) was 30.1 (32.6) days. At HSC diagnosis, 14 patients had neutropenia (24.6%). HSC was diagnosed after a first-line treatment for 35 (58.4%) patients, after a conditioning regimen for 14 patients (23.3%), and after a rescue treatment for 11 (18.3%) patients.
HSC Diagnosis
Candida colonization was found in 48 patients (84.2%), with 2 or more Candida species in 50% of cases, either at or before HSC diagnosis. Candida albicans was the most prevalent (79.2%), followed by C. glabrata (10.4%), C. krusei (6.3%), C. parapsilosis (2.1%), and C. tropicalis (2.1%).
According to the 2020 EORTC criteria, HSC was proven in 11 cases (18.3%), probable in 2 (3.3%), and possible in 47 (78.3%).
Twenty-two patients presented candidemia before HSC diagnosis (36.7%). Candidemia occurred in 8 of 13 patients previously treated with auto-SCT. Mycological findings are presented in Table 2.
Table 2.
Contribution of Diagnostic Tests Used for HSC
| No. Positive/No. Tested | |
|---|---|
| Mycological findings | |
| Prior candidemia | 22/60 |
| Positive ß-D-glucan | 29/52 |
| Positive Candida serology | 18/50 |
| Positive Candida antigen | 22/55 |
| Radiologic exams | |
| Contrast ultrasonography | 11/13 |
| CT scan | 43/43 |
| PET scan | 29/36 |
| Contributive biopsya | 11/24 |
Abbreviations: CT, computed tomography; HSC, hepatosplenic candidiasis; PCR, polymerase chain reaction; PET, positron emission tomography.
Examination or recovery of yeasts by culture or amplification of fungal DNA by PCR obtained by biopsy from a sterile body site.
HSC radiological diagnosis was made by abdominal CT scan in 48 cases (81.4%), ultrasound in 7 (11.7%), and PET scan in 4 (6.8%).
New tools for HSC diagnosis are presented in Table 2. Contrast US was performed for 13 patients (21.7%) with a suspicion of HSC and was positive in 11. A PET scan was performed at diagnosis for 36 patients and showed hypermetabolism of the liver and/or spleen in 29 cases (80.6%). For mycological criteria, dosage of BDG, Candida serology, and Candida antigen were available for 52, 50, and 55 patients, respectively. Dosage of BDG was positive for 29 patients (55.8%). Candida serology and antigen were positive in 18 (36%) and 22 (40%) cases, respectively. Overall, 24 patients (40%) had a tissue biopsy (19 liver, 4 skin, and 1 spleen). In 11 (45.8%), direct examination (10/11) or molecular analysis (1/11) by multiplex polymerase chain reaction (PCR) was positive for yeasts. All cultures remained negative. Of note, 9 of the 11 contributive biopsies originated from liver biopsies.
Treatment of HSC
As presented in Table 1, first-line antifungal therapy after HSC diagnosis relied on azoles in 37 patients (61.7%), among whom 30 received fluconazole, 5 voriconazole, and 2 posaconazole. Caspofungin was used in 22 (36.7%) and LAmB in 1 patient. Among patients, 35 (58.3%) were treated with the same agent for the whole duration of antifungal therapy. For 17 patients (28.3%), caspofungin was switched to oral treatment depending on microbiological findings. For the remaining patients, treatment was changed due to interaction with treatment, microbiological findings, or impossibility of oral administration (3, 3, and 2 patients respectively). An antifungal combination was used for 3 patients (5%). Corticosteroids were used in 27 patients (45%). The mean duration of curative treatment (SD) was 7.4 (6.0) months.
Follow-up
The mean follow-up duration (SD) was 22.6 (19.3) months. At 3 months, response status was available for 52 patients, of whom 49 were in partial response (94.2%). At last follow-up, complete response was observed in 53 patients (88.3%) (Table 1). In 25 patients, a PET scan was performed to evaluate radiological response, and a resolution of hepato-splenic hypermetabolism was observed in 21 patients. Furthermore, hematologic treatment was delayed for only 8 patients (13.8%) due to active infection or deterioration of general or biological status.
At last follow-up, 41 patients (68.3%) presented a complete hematological remission. Eleven patients (18.3%) received an allogeneic SCT, performed a mean time (SD) of 123.6 (64.3) days after HSC diagnosis. Post-allo-SCT HSC recurrence was diagnosed in 1 out of these 11 patients.
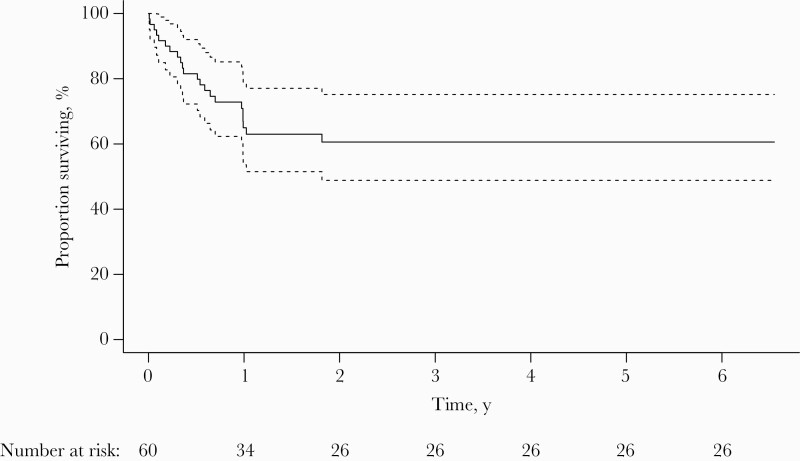
The overall survival of patients with HSC is represented in Figure 1. Overall, 22 patients died during the study period, mainly following disease progression (for 12 of them), but none from HSC. Other causes of death were infectious complications for 7 patients (3 complicated bacteriemia, 2 pneumonitis, 1 arthritis, and 1 infection due to Fusarium), metabolic complications, cardiogenic shock, and severe graft-vs-host disease (1 patient each). Overall survival at 3 and 6 months was 88.3% and 81.5%, respectively. As presented in Table 3, the absence of hematological remission at HSC diagnosis was associated with an increased risk of overall mortality (HR, 3.53; 95% CI, 1.13–11.03), while there was a borderline association for older age (HR, 1.02; 95% CI, 1.00–1.05).
Figure 1.
Overall survival. Dotted lines represent 95% CI.
Table 3.
Results of Cox Regression Modeling for Overall Survival
| HR | 95% CI | |
|---|---|---|
| Azole administration | 0.70 | 0.28–1.71 |
| Corticosteroid administration | 1.24 | 0.48–3.21 |
| Age at HSC diagnosis (per 1-y increase) | 1.02 | 1.00–1.05 |
| Neutropenia >1 mo | 0.97 | 0.36–2.58 |
| No hematological remission at HSC diagnosis | 3.53 | 1.13–11.03 |
Abbreviations: HR, hazard ratio; HSC, hepatosplenic candidiasis.
DISCUSSION
This study provides an updated overview of the epidemiology and outcome of HSC over 13 years in a single-site retrospective study. Little is known about the incidence and recent epidemiologic features of HSC. HSC has mainly been described in patients with AML after neutrophil recovery [21]. Since the first HSC descriptions, antifungal prophylaxis has been widely used for patients with AML, with a decreasing incidence of invasive aspergillosis in that setting [19]. However, to our knowledge, the impact of antifungal prophylaxis on the incidence of HSC has not been studied. As compared with a previously published cohort of HSC patients in the early 21st century [22], fewer patients with AML were diagnosed in our study (31.7% vs 66.7%), and more patients with malignancies of lymphoid lineage (35.0% vs 12.5% of lymphoma patients for instance). Antifungal prophylaxis in patients with AML probably partly explains the shift in HSC diagnoses in our study toward patients with “lymphoid diseases,” with only a third of HSC cases diagnosed among patients with AML. In our study, of the 19 patients diagnosed with AML only 9 had received antifungal prophylaxis before HSC diagnosis. The generalization of antifungal prophylaxis could reduce further the incidence of HSC in AML patients. Another plausible explanation for this shift is the evolution of chemotherapy for patients with ALL and lymphomas in the past decade. Novel treatments such as immunomodulating and immunosuppressive agents in addition to cytotoxic treatments are associated with an increased risk of invasive fungal infections among these patients [23, 24]. Furthermore, in France, L-asparaginase has been used since 2005 to treat adult ALL patients and is well known to disrupt the gastrointestinal tract, which might contribute to the development of HSC [25].
Interestingly, 13 patients were diagnosed with HSC after autologous transplantation for lymphomas. Among those patients, 8 had a previous candidemia (61.5%), which is a higher rate than usually observed. Moreover, a direct exam was positive for yeast in only 45.8% of biopsies (11/24). Also, patients presented a rapidly favorable evolution under antifungal treatment. In most previously published studies [2, 3, 22], reporting a majority of HSC cases occurring in patients with AML, the rate of previous candidemia was around 20%–30%, liver biopsies were positive in <40%, and the need for adjunctive corticosteroids was around 50%.
Regarding HSC diagnosis, although new diagnostic tools are being used, definite diagnosis remains challenging. BDG is now included as a mycologic criterion, in at least 2 consecutive serum samples, for the diagnosis of HSC by the EORTC [6], with a sensitivity of 77% for yeast infections [11]. Combined mannan-antimannan is also included as a mycologic criterion by the European Conference on Infections in Leukemia [26], with better sensitivity than BDG to detect disseminated candidiasis [27–29], and is useful as an early marker of HSC [12]. We retrospectively performed, for all patients with available serum, dosages of biomarkers (ie, BDG, mannan antigen, and antibodies). Dosage of BDG was positive for 29 patients (55.8%). Candida serology and antigen were positive in 18 (36%) and 22 (40%) cases, respectively, rates that are lower than observed in other studies [28].
Given the study period and considering the fact that dosages of biomarkers were not routinely performed before 2016, we rarely had 2 successive serum samples to fit the criteria for a probable diagnosis of HSC according to the 2020 EORTC/MSG classification. Of note, using only 1 positive sample for BDG at the time of HSC diagnosis, as recommended in the previous 2008 EORTC/MSG classification, we observed 14 probable cases (23.3%) and 35 possible (58.4%). As previously reported, candidemia was observed in only 36.7% of cases in our study, but having candidemia in the 2 weeks before the appearance of hepatosplenic lesions was still included as a mandatory clinical feature for a probable diagnosis of HSC in the 2020 EORTC/MSG definition that we used in our study. Regarding radiological diagnosis, CT scans were mostly performed in our study, but we also used PET scans at diagnosis for 36 patients and for follow-up. New diagnostic tools with better specificity and sensitivity are needed to improve HSC diagnosis.
In our study, the first-line treatment of HSC mainly relied on azoles or caspofungin. When compared with a former published study considering patients treated between 2000 and 2007, we report less frequent antifungal combination therapy and less delayed or modified chemotherapy, as recommended by several guidelines and authors [16, 30, 31]. The optimal first-line treatment remains debated. Both the IDSA and ESCMID recommend the use of echinocandins or LAmB as first-line therapy [15, 17]. This recommendation is based on the fact that patients are currently receiving more antifungal prophylaxis and thus might have an increased risk of developing infection with an azole-resistant organism. In particular, the use of echinocandins is supported by the results of a recent meta-analysis showing the superiority of echinocandins over amphotericin B and azoles [32]. However, fluconazole as a first-line treatment or following LAmB induction has been shown to be effective in some studies [7, 33, 34], although these data have not been readdressed for 30 years. In our study, first-line treatment relied mainly on azoles (61.7% of patients). Considering HSC, at last follow-up, complete response was observed in 88.3% of patients, but, given the size of the cohort, analyzing risk factors associated with response was not contributive.
From our study results, neither azole nor corticosteroid administration improved overall survival. However, due to the small sample size of our study and its nonrandomized design, drawing definite conclusions is unwise.
The physiopathology of HSC remains unclear. One main hypothesis is the deregulation of Th1⁄Th17 and Th2⁄Treg response after neutrophil recovery leading to an immune reconstitution syndrome as observed for cryptococcosis in AIDS [3, 35]. This could explain the efficacy of adjunctive corticosteroids for persisting fever despite antifungal treatment. The physiopathology might be different for cases occurring after lymphoid diseases. The CANHPARI study (Hepatosplenic Candidiasis: PET Scan and Immune Response Analysis), which is an ongoing multicenter prospective pilot study investigating the pathogenesis, diagnosis, and therapeutic strategies of hepatosplenic candidiasis (https://research.pasteur.fr/fr/project/canhpari/), should soon deliver interesting results and might give new insights on the pathogenesis of HSC [36].
This study has several strengths, such as the extended recruitment period and the large sample size, in contrast to the paucity of data regarding HSC in the literature. However, our study has some limitations. First, serum and PET scan were not available at diagnosis and follow-up for all patients in order to evaluate new diagnostic tools. Second, we may lack power to detect differences given the sample size and monocentric nature of the study, and our results may lack generalizability. Third, the retrospective observational design of this study precludes any causality assumption or conclusions regarding HSC treatment. Finally, results regarding HSC prevalence should be taken with caution as (i) the number of patients seen at the hospital (all hematological patients and for each subpopulation) refers to the number of hospitalizations over the considered year and is not strictly the number of diagnoses (ie, a patient with lymphoma can be hospitalized in 2013 for any reason although the diagnosis was made before) and (ii) given the very small number of HSC cases each year, prevalence rate estimates are prone to high uncertainty.
In conclusion, our study gives an updated overview of the epidemiology of HSC over a 13-year period. More patients with lymphoid diseases were noted when compared with previous reports, in which AML patients were predominant, possibly explained by the widespread use of antifungal prophylaxis in AML patients and new therapeutic regimens in lymphoid diseases. In the setting of lymphoid diseases, HSC could represent an emerging invasive fungal infection. This study also revealed less delayed chemotherapy treatment after HSC diagnosis than previously published, and hematological control was the strongest factor associated with survival in HSC. Nevertheless, this rare disease remains a challenge in hematological malignancies, and studies exploring the physiopathology of HSC, potentially leading to improvement of diagnostic tools, are urgently needed, as are studies on treatment optimization and prophylaxis.
Supplementary Material
Acknowledgments
We thank Dr. Picat and E. Segol from the biostatistics department for their helpful support.
Financial support. No funding was received for this review.
Potential conflicts of interest. The authors have declared no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Considering the retrospective study design, data collection from preexisting medical records, and respect for the anonymity of the patients included (referred to as studies “Hors Loi Jardé” in France), no ethical approval or administrative approval was necessary for this study. This study was submitted to the local Data Protection Officer (DPO) and, upon approval, was identified in the hospital study registry. The research was conducted in accordance with the Declaration of Helsinki. Patients were informed that their clinical data could be used, after anonymization, for research purposes.
References
- 1. Thaler M, Pastakia B, Shawker TH, et al. Hepatic candidiasis in cancer patients: the evolving picture of the syndrome. Ann Intern Med 1988; 108:88–100. [DOI] [PubMed] [Google Scholar]
- 2. Kontoyiannis DP, Luna MA, Samuels BI, Bodey GP.. Hepatosplenic candidiasis. A manifestation of chronic disseminated candidiasis. Infect Dis Clin North Am 2000; 14:721–39. [DOI] [PubMed] [Google Scholar]
- 3. Rammaert B, Desjardins A, Lortholary O.. New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses 2012; 55:e74–84. [DOI] [PubMed] [Google Scholar]
- 4. Sallah S, Semelka RC, Wehbie R, et al. Hepatosplenic candidiasis in patients with acute leukaemia. Br J Haematol 1999; 106:697–701. [DOI] [PubMed] [Google Scholar]
- 5. Anttila VJ, Elonen E, Nordling S, et al. Hepatosplenic candidiasis in patients with acute leukemia: incidence and prognostic implications. Clin Infect Dis 1997; 24:375–80. [DOI] [PubMed] [Google Scholar]
- 6. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kauffman CA, Bradley SF, Ross SC, Weber DR.. Hepatosplenic candidiasis: successful treatment with fluconazole. Am J Med 1991; 91:137–41. [DOI] [PubMed] [Google Scholar]
- 8. Anttila VJ, Lamminen AE, Bondestam S, et al. Magnetic resonance imaging is superior to computed tomography and ultrasonography in imaging infectious liver foci in acute leukaemia. Eur J Haematol 1996; 56:82–7. [DOI] [PubMed] [Google Scholar]
- 9. Görg C, Bert T, Klassen E, et al. Contrast enhanced sonographic patterns of hepatic candidiasis. Z Gastroenterol 2010; 48:678–82. [DOI] [PubMed] [Google Scholar]
- 10. Hot A, Maunoury C, Poiree S, et al. Diagnostic contribution of positron emission tomography with [18F]fluorodeoxyglucose for invasive fungal infections. Clin Microbiol Infect 2011; 17:409–17. [DOI] [PubMed] [Google Scholar]
- 11. Lamoth F, Cruciani M, Mengoli C, et al. ; Third European Conference on Infections in Leukemia (ECIL-3). β-glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis 2012; 54:633–43. [DOI] [PubMed] [Google Scholar]
- 12. Prella M, Bille J, Pugnale M, et al. Early diagnosis of invasive candidiasis with mannan antigenemia and antimannan antibodies. Diagn Microbiol Infect Dis 2005; 51:95–101. [DOI] [PubMed] [Google Scholar]
- 13. Guitard J, Isnard F, Tabone MD, et al. Usefulness of ß-D-glucan for diagnosis and follow-up of invasive candidiasis in onco-haematological patients. J Infect 2018; 76:483–8. [DOI] [PubMed] [Google Scholar]
- 14. Legrand F, Lecuit M, Dupont B, et al. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis 2008; 46:696–702. [DOI] [PubMed] [Google Scholar]
- 15. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ullmann AJ, Akova M, Herbrecht R, et al. ; ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 2012; 18(Suppl 7):53–67. [DOI] [PubMed] [Google Scholar]
- 17. Martin-Loeches I, Antonelli M, Cuenca-Estrella M, et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med 2019; 45:789–805. [DOI] [PubMed] [Google Scholar]
- 18. Chaussade H, Bastides F, Lissandre S, et al. Usefulness of corticosteroid therapy during chronic disseminated candidiasis: case reports and literature review. J Antimicrob Chemother 2012; 67:1493–5. [DOI] [PubMed] [Google Scholar]
- 19. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348–59. [DOI] [PubMed] [Google Scholar]
- 20. van Burik JH, Leisenring W, Myerson D, et al. The effect of prophylactic fluconazole on the clinical spectrum of fungal diseases in bone marrow transplant recipients with special attention to hepatic candidiasis. An autopsy study of 355 patients. Medicine (Baltim) 1998; 77:246–54. [DOI] [PubMed] [Google Scholar]
- 21. Pagano L, Mele L, Fianchi L, et al. Chronic disseminated candidiasis in patients with hematologic malignancies. clinical features and outcome of 29 episodes. Haematologica 2002; 87:535–41. [PubMed] [Google Scholar]
- 22. De Castro N, Mazoyer E, Porcher R, et al. Hepatosplenic candidiasis in the era of new antifungal drugs: a study in Paris 2000-2007. Clin Microbiol Infect 2012; 18:E185–7. [DOI] [PubMed] [Google Scholar]
- 23. Chan TS, Gill H, Hwang YY, et al. Breakthrough invasive fungal diseases during echinocandin treatment in high-risk hospitalized hematologic patients. Ann Hematol 2014; 93:493–8. [DOI] [PubMed] [Google Scholar]
- 24. Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006; 91:1068–75. [PubMed] [Google Scholar]
- 25. Schein PS, Rakieten N, Gordon BM, et al. The toxicity of Escherichia coli L-asparaginase. Cancer Res 1969; 29:426–34. [PubMed] [Google Scholar]
- 26. Mikulska M, Calandra T, Sanguinetti M, et al. ; Third European Conference on Infections in Leukemia Group. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care 2010; 14:R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sendid B, Caillot D, Baccouch-Humbert B, et al. Contribution of the Platelia Candida-specific antibody and antigen tests to early diagnosis of systemic Candida tropicalis infection in neutropenic adults. J Clin Microbiol 2003; 41:4551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sendid B, Poirot JL, Tabouret M, et al. Combined detection of mannanaemia and antimannan antibodies as a strategy for the diagnosis of systemic infection caused by pathogenic Candida species. J Med Microbiol 2002; 51:433–42. [DOI] [PubMed] [Google Scholar]
- 29. Ellis M, Al-Ramadi B, Bernsen R, et al. Prospective evaluation of mannan and anti-mannan antibodies for diagnosis of invasive Candida infections in patients with neutropenic fever. J Med Microbiol 2009; 58:606–15. [DOI] [PubMed] [Google Scholar]
- 30. Masood A, Sallah S.. Chronic disseminated candidiasis in patients with acute leukemia: emphasis on diagnostic definition and treatment. Leuk Res 2005; 29:493–501. [DOI] [PubMed] [Google Scholar]
- 31. Ford ES, Duke ER, Cheng GS, et al. Outcomes of hematopoietic cell transplantation in patients with mixed response to pretransplantation treatment of confirmed or suspected invasive fungal infection. Transplant Cell Ther 2021; 27:684.e1–9. [DOI] [PubMed] [Google Scholar]
- 32. Demir KK, Butler-Laporte G, Del Corpo O, et al. Comparative effectiveness of amphotericin B, azoles and echinocandins in the treatment of candidemia and invasive candidiasis: a systematic review and network meta-analysis. Mycoses 2021; 64:1098–110. [DOI] [PubMed] [Google Scholar]
- 33. Anaissie E, Bodey GP, Kantarjian H, et al. Fluconazole therapy for chronic disseminated candidiasis in patients with leukemia and prior amphotericin B therapy. Am J Med 1991; 91:142–50. [DOI] [PubMed] [Google Scholar]
- 34. de Pauw BE, Raemaekers JM, Donnelly JP, et al. An open study on the safety and efficacy of fluconazole in the treatment of disseminated Candida infections in patients treated for hematological malignancy. Ann Hematol 1995; 70:83–7. [DOI] [PubMed] [Google Scholar]
- 35. Dellière S, Guery R, Candon S, et al. Understanding pathogenesis and care challenges of immune reconstitution inflammatory syndrome in fungal infections. J Fungi (Basel) 2018; 4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Candon S, Rammaert B, Foray AP, et al. Chronic disseminated candidiasis during hematological malignancies: an immune reconstitution inflammatory syndrome with expansion of pathogen-specific t helper type 1 cells. J Infect Dis 2020; 221:1907–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.