Abstract
Age is a key factor affecting sexual selection, as many physical and social traits are age-related. Although studies of primate mate choice often consider particular age-related traits, few consider the collective effects of male age. We tested the hypothesis that female golden snub-nosed monkeys Rhinopithecus roxellana prefer prime aged males (10–15 years) over younger and older males. We examined a habituated, provisioned troop during a 3-year study in the Qinling Mountains, China. Prime age males were more likely to be resident males of 1-male units (OMUs) than males of other ages. Since females are free to transfer between OMUs, the number of females per OMU can be indicative of female preferences. We examined the number of females per OMU, and found that it increased with resident male age up to 7–8 years, and declined after 12 years, such that prime age resident males had more females than other resident males. Females also initiated extra-unit copulations with high-ranking prime age males at significantly higher rates than with other males. Nevertheless, females tended to transfer from OMUs with high-ranking, older resident males to those with low-ranking, younger resident males. Thus, females appear to use different strategies when choosing social mates and extra-unit mates (i.e., different social contexts). We speculate that females may perceive early signs of aging in males and trade off the benefits and costs of high rank versus male senescence. This study lays the groundwork for future studies that examine possible direct and indirect benefits of such strategies.
Keywords: extra-unit copulation, female transfer, male age, mate choice, 1-male unit, Rhinopithecus roxellana
In sexual selection theory, proposed by Darwin (1871), an individual’s fitness is affected both by adaptations that increase its chances of survival and by adaptations that increase its ability to obtain high quality and/or high quantities of mates with whom to reproduce. Darwin recognized 2 forms of sexual selection. One involves intrasexual selection in which same sex individuals compete with one another for access to mates. The other is intersexual selection in which members of 1 sex display a preference for particular characteristics in the opposite sex that are associated with enhanced reproductive performance and enhanced quantities or qualities in offspring. Sexual conflict, which may take the form of coercion, in which 1 sex gains access to mates by force or intimidation, has more recently been classified as a third form of sexual selection (Smuts and Smuts 1993; Muller and Wrangham 2009). Sex differences in mating biases stems from tendencies for 1 sex (usually females) of most species to invest more resources into each offspring than the other (usually males). These higher costs for females lead to a stronger emphasis on intrasexual selection, that is, male–male competition, among males for access to females and male coercion of females (Lawler 2009; Reddy and Mitani 2020). They also lead to a stronger emphasis on intersexual selection, that is, female choice, among females for the highest quality males. Females’ choices may also be influenced by competition with other females for mates or resources, particularly resources associated with particular males (Baniel et al. 2018; Garcia-Berro et al. 2019).
Most research on female choice has focused on the effects of genetic compatibility and/or single physical and behavioral characteristics of males (Coltman et al. 2002; Kruuk et al. 2002; MacDougall-Shackleton 2020; Stervander et al. 2020). Many such characteristics have been identified that influence a male’s ability to gain access to fertile females, including body size, rank, ornamentation, weapons, social skills, and experience (Andersson 1994). Direct relationships between such male traits and mating success are generally interpreted as evidence for sexual selection on male traits (Kervinen et al. 2016). Many of these traits are related to male age (Gasparini et al. 2019); thus, male age per se is likely to reflect the collective associations between 1 or more such traits and mating success to some extent. If so, cues of male age, including those not directly due to declines in male’s physiological state or physical abilities, for example, age-related changes in pelage color, may nevertheless provide useful information to females about aspects of male quality. However, the extent to which potential female mates use age-related cues is often unclear. As an initial step to fill this gap for primate species, we ask whether wild female golden snub-nosed monkeys Rhinopithecus roxellana bias their choices of mates toward males of particular ages.
Recent studies have found that some female mate preferences may indeed be associated with male age. However, different species may show different age-related preferences. Some studies report a positive relationship between females choice and male age: for example, yellowthroat birds Geothlypis trichas and black grouse Lyrurus tetrix (Freeman-Gallant et al. 2010; Martin and Festa-Bianchet 2011), others a negative relationship: soay sheep Ovis aries (Hayward et al. 2015), others a nonlinear (inverted U) relationship: lekking sandfly Lutzomyia longipalpis (Jones et al. 2000), and others the absence of a relationship: bighorn sheep Ovis canadensis (Martin and Festa-Bianchet 2011). Choices related to male age may also differ depending on the social context. For example, some female song birds choose middle-aged males with territories as social mates, but may also engage in extra-unit copulations with younger males (Griffith et al. 2002; Gao et al. 2019).
Individual male mating success and rank in most vertebrates typically increase in early life, reach a maximum, and then decrease at later ages (Jones et al. 2008; Nussey et al. 2013). This pattern also exists in some other nonhuman primates (e.g., baboons: Silk et al. 2020), but it is unclear how much such prime aged primate males, that is, fully mature middle-aged males that show no signs of senescence, may be preferred over sub-adult, young adult, and aging males by female conspecifics. Only a few primate studies have considered the effect of male age per se on female mate choice. For example, in rhesus macaques Macaca mulatta, male reproductive success appears to be associated with several factors including male age, weight, and dominance rank as well as individual female preferences (Bercovitch and Nurnberg 1996; Berard 1999). In hamadryas baboons Papio hamadryas hamadryas, tenure length in a 1-male unit (OMU) is related to male age and is likely a primary factor determining variation in a male’s lifetime reproductive success (Pines et al. 2015). Finally, adult female gorillas often leave breeding groups with old males to join groups led by younger males, because younger males are likely to be of higher reproductive and protective value (Baudouin et al. 2019). No study has examined this relationship in golden snub-nosed monkeys.
Golden snub-nosed monkeys live in multilevel societies in which the basic unit is the OMU (Qi et al. 2014). Each OMU contains a single resident male, 1 or more adult and sub-adult females, and immature offspring. Several associated OMUs form a cohesive breeding band and several bands comprise a troop. In addition, all-male units (AMUs), comprised of juvenile and bachelor males, follow the breeding band (Grueter et al. 2017) and interact with males and females. The species is a useful model for studying female preferences because females have an unusual degree of autonomy in mate choice. Within the multilevel structure, female membership in particular OMUs changes constantly as females freely leave their resident male to join a new OMU. Takeovers of OMUs by other males also occur frequently. Nevertheless, female preferences appear to strongly influence whether the resident male is replaced by another male (Fang et al. 2018) or whether the group becomes unstable and fissions (Kirkpatrick and Grueter 2010). Thus, OMU membership appears to be the outcome of a complicated process involving male–male competition, female preferences (Zhang et al. 2006; Qi et al. 2009), and possibly female–female competition. Females typically initiate matings both within the OMU and with other males outside the OMU. Their resident males attempt to block AMU males from access to the females in their unit, but often fail to prevent extra-unit copulations. As such, females appear to be able to express their preferences for both social mates and extra-unit mates by directly choosing to mate with resident males, seeking extra-unit copulations, joining other OMUs, or by deserting new resident males that have recently taken over their OMU (Fang et al. 2018). However, it is unclear how much male age is associated with their preferences.
Rhinopithecus roxellana are seasonal breeders. Although they mate all year round, matings reach a peak in autumn, and infants are born in the spring. Females generally give birth every second year (Zhao et al. 2008). Paternity analysis has shown that more than half of the offspring (57–75%) examined are sired by extra-unit males, that is, AMU members or resident males of OMUs other than the one the female belongs to (Guo et al. 2010; unpublished data). Both males and females display linear hierarchies (He et al. 2013) and disperse from their natal OMUs. Males and females have been observed to disperse between bands (Zhang et al. 2008; Li et al. 2021). However, only males have been observed dispersing to other troops. Related females are found in the same OMU more than expected by chance, and both related and unrelated females in different OMUs maintain affiliative relationships with one another during periodic visits to each other’s OMUs (Zhang et al. 2006; Guo et al. 2015; Ren et al. 2018). Males disperse from their natal OMUs at about 4 years old and initially join an AMU. They are able to sire offspring from the age of 5 or 6 years (our unpublished data) and attempt to form their own OMU from 7 or 8 years by attracting females or taking over the position of a resident male (Fang et al. 2018).
Here, we ask whether wild female golden snub-nosed monkeys display preferences for males of a particular age. We test the hypothesis that females display preferences for prime age males, given 1) that individual male mating success among some nonhuman primates peaks in prime aged males and 2) the likelihood that age-related changes in males provide useful cues to females about male quality. We predict that 1) prime aged males are more likely to be members of an OMU than an AMU. Although gaining and maintaining an OMU is likely to involve male–male competition, evidence from Fang et al. (2018) suggests that female preferences are the main factor deciding whether a takeover attempt is successful. Given the females are free to transfer from one OMU to another, we predict that 2) prime aged resident males have more females in their OMUs than resident males of other ages. We also predict that 3) prime age males engage in more extra-unit copulations than males of other ages, and 4) females that transfer between OMUs tend to transfer to units with prime age resident males from units with resident males of other ages.
Materials and Methods
Study site and subjects
Our research site was in the Dapingyu (DPY) region of the Guanyinshan National Nature Reserve (GNNR) in the Qinling Mountains, China (107°52′ –108°02′E, 33°20′ –33°44′N). The reserve has a semi-humid montane climate with an altitude from 1,150 to 2,574 m. Vegetation structure varies with altitude; it is dominated by deciduous broadleaf forest under 1,500 m, coniferous and deciduous broadleaf mixed forest between 1,500 and 2,200 m, and coniferous forest above 2,300 m (Wang et al. 2020).
The study troop, DPY, has been habituated and observed since 2009. During our study, there was a single AMU and a variable number (6–9) of OMUs within 2 bands (see details in the “Results” section).
Over the course of the study, the troop consisted of 70–95 individuals with 9–17 adult males (7+ years), 19–34 adult females (5+ years), 10–14 sub-adults (3–4 females: 4–5 years, 6–11 males: 4–6 years), 19–21 juveniles (1–3 years), and 11–13 infants (<1 year). All individuals except infants were identifiable from individual facial characteristics, scars, or evidence of previous injuries, etc. All adult females (n = 34) and all sub-adult and adult males (n = 28) were subjects of this study.
Data collection
This study was initiated in March 2018 and continued through December 2020. We tracked the troop at least 7 months every year from March to June (birth season), July to August, and September to December (mating season). Because the terrain in the troop’s home range consisted largely of rocky cliffs, we scattered apples and corn daily in a valley to attract the monkeys and allow observation. There were 2 periods of provisioning per day (started at 09:30 am and 15:00 pm, respectively). Each provisioning period lasted about 30–50 min, for a total of 376 days (620 h). Evidence suggests that provisioning does not affect their intake of natural food (Guo et al. 2010). The monkeys moved freely in the provisioning area: females and resident males generally remained in the provisioning area all day, whereas some AMU males spent only part of the day in the area (time was recorded). The monkeys were well habituated to observation, displaying tolerance as we moved through the troop collecting data. Provisioning and the presence of observers no doubt influenced some aspects of their social behavior. Staying in the provisioning area probably increased opportunities for females to express their preferences, because it increased the number of males they could access, but we have no reason to think it influenced which males were preferred.
We used all-occurrence sampling to record all mating behaviors involving males and agonistic interactions between resident males (used to determine ranks between resident males) beginning at 09:00 h and ending between 17:00 and 18:00 h on checksheets. We also used all occurrences sampling to keep track of individuals come and went from the provisioning area. We recorded behavioral data before, during, and after provisioning until the monkeys left the provisioning area usually between 17:00 and 18:00 h (3008 h).
Copulations were defined as episodes of mounting with intromission and pelvic thrusting, all of which were initiated by female solicitation. Individuals engaging in extra-unit copulation could be identified accurately and quickly. Agonistic interaction between males occurred mainly during periods of provisioning. Definitions of each type of aggression and submission followed Li et al. (2006). Aggressive interactions included contact aggression (biting, kicking, or hitting), chasing, and threatening, or counter-aggression. Submissive behavior included being displaced or retreating without contest. We determined a clear winner only if 1 male (the loser) fled along with the members of his unit from the other male (the winner). Otherwise, the outcome was considered a tie (Zhao and Tan 2011).
Male age estimation
Because it is difficult to determine the age of wild snub-nosed monkeys based on skull growth and healing, we used males (juveniles-adults) of known ages (36% or 69.2%) in the troop as a guide to estimate several unknown age males. In addition, we relied on criteria developed for captive golden snub-nosed monkeys (Liang et al. 2001). Like researchers of other wild primates, we concentrated on changes in dentition, skin, and pelage (e.g., Hill et al. 2001; Muller et al. 2006). We used the following criteria: canines and granulomas on both sides of their upper lip appear at about 5 years of age and align after 6.5 years of age. At the same time, long guard hairs begin to grow on the back, and males display substantial tooth growth. After 7.5 years, canines are thick, granulomas are full, and molting begins. The length of aciculum (long golden hair) on the dorsal side of the waist and the body size increases until 9. The incisors gradually wear down after the age of 11 years. Faces of young males are clear and light in color, but wrinkles develop about 12 years old. Wrinkles and blue skin around the eyes intensify with age. Teeth darken and wear more severely with time, and cavities become more numerous. The canine teeth began to wear down and fall out after the age of 16 years. The color of the coat darkens with age; older males have more reddish-brown on their crowns, more golden hair on their dorsum, and looser skin. The granulomas of older males shrink, and the ages of their offspring within the unit tend to be older, which can indirectly indicate a male’s age. The age distribution of unknown age males was not significantly different from known age males (Fisher’s exact test, P = 0.740). Prime age males were defined as 10–15 years. They were distinguished from old males based on clear signs of senescence beginning at about 16 years and from young adults based on body size increases until 9. Figure 1 shows examples of males of known ages.
Figure 1.
Males of known ages. Panels A–F show males 2, 4, 7, 11, 14, and 18 years of age.
Relatedness analyses
We collected hair samples from subjects by stepping gently on hair from their tails and waiting until they moved away. DNA was extracted from hair follicles following methods described by Allen et al. (1998). We used the genotypes at 20 highly polymorphic microsatellite loci to estimate the pairwise relatedness coefficient. Because the null alleles are pervasive in microsatellites (e.g., Kokita et al. 2013) , we will use the corrections given by Huang et al.’s (2016) to obtain reliable estimates. We first used Kalinowski and Taper’s (2006) estimator to estimate the null allele frequency (results were shown in Supplementary Table S1). We then evaluated the statistical performance of various estimators for our dataset with 100,000 Monte-Carlo simulations, and the estimator with the least root mean squared error (RMSE) in parent–offspring, full-sibs, half-sibs, and non-relatives would be chosen (results were shown in Supplementary Table S2). Although the RMSE of maximum-likelihood estimators is generally smaller than the method-of-moment estimators, they give a large proportion of zero estimates, which can yield many ties in the subsequent comparisons. Therefore, we would use the optimal method-of-moment estimator in the following analyses and the novel estimator B developed by Huang et al.’s (2016) was chosen. The null allele frequency estimation, Monte-Carlo simulation to obtain the RMSE for various relatedness estimators, and the pairwise relatedness estimations were performed in POLYRELATEDNESS V1.11 (Huang et al. 2016).
Data analysis
To examine the age distributions of males in OMUs and the AMU, and specifically to compare prime aged males to other age groups, we determined whether each male subject was a member of an OMU or the AMU at the end of the observation season each year. We grouped males into 5 age groups of 3 years each (sub-adults: 4–6 years, young adults: 7–9 years, middle or prime aged adults: 10–12 and 13–15 years, and old males: 16+ years), and calculated the percentage of males in each age group that belonged to OMUs versus the AMU. These groupings separate natural developmental stages: males from 4–6 years are adolescents that can mate and sometimes sire offspring, 7–9 year olds are considered young adults but may not be full size, 10–12 year olds have reached full size and strength, 13–15 year olds generally still appear powerful and healthy, but 16+ year olds show signs of senescence (see above). We used Fisher’s exact tests to compare the age distributions of males in OMUs and AMUs separately during each year of the study.
To examine whether the number of females in an OMU was related to the age of the resident male, we used a generalized linear mixed model (GLMM, lmer package) in R 3.5.3 in which we entered the number of females in each OMU in each year as the response variable, resident male age (as a continuous variable) and age squared (to test for the predicted curvilinear relationship) as fixed effects, and resident male identity (ID) as a random factor. To control for possible effects of male rank, female relatedness, female–male relatedness, and the recent reproductive performance of females in the OMU, we added each male’s ordinal rank (see below), the mean relatedness of females within the OMU, and the number of infants and yearlings present within the OMU/per female, respectively, as fixed effects. All variance inflation factor (VIF) values between fixed effects in this and subsequent GLMM analyses were less than 5, suggesting an acceptable level of multicollinearity (Akinwande et al. 2015). Exceptions were for age and age squared which were closely related (VIF > 10) but both effects needed to be retained in the model in order to test for the predicted curvilinear relationship between number of females and male age.
To construct male ranks, we used Elo-rating (Neumann et al. 2011), specifically the “EloRating” package in R, to calculate the ranks of resident males. Elo-rating is a method to measure an individual’s competitive successes. It is based on sequential agonistic interactions between individuals with a clear winners and losers (Albers and de Vries 2001; Neumann et al. 2011). After each aggressive (see above) interaction, the Elo-ratings of the 2 participants are updated based on the results of interaction (winning, losing, or a tie) (see details Albers and de Vries 2001). Thus, Elo-ratings can be extracted at any point in time during a study. In this research, all the males began with a rating of 1,000, and we allowed a burn in time of 1 month after which the Elo-rating scores were converted to ordinal ranks at various points in time. The rank of the original resident male when a female transferred out of his OMU and the rank of the new resident male when the female joined a new OMU were used in our analysis of transfer patterns. Ranks of all resident males at the end of each observation season were used to examine the relationship between male rank and age. A GLMM, in which rank was the response variable, male age was the fixed effects, and resident male ID was a random factor revealed a strong, positive relationship between rank and age (Age: estimate = −0.19, z = −5.1, P < 0.001, Figure 2).
Figure 2.
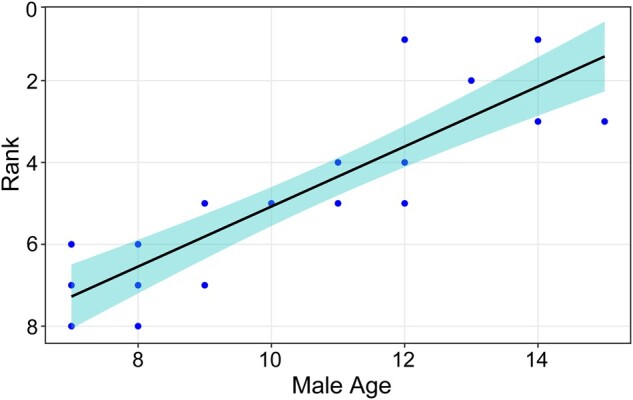
Correlation of resident male age and rank. Linear model: y = −0.755x + 12.658. Rank 1 represents the highest ranking male, rank 2 is the second highest ranking male, etc. The figure is for illustrative purposes only because some males are represented in more than 1 year.
To examine whether females were more likely to engage in extra-unit copulations with prime age males than males of other ages, we first established that there was no significant difference in the male age distribution of extra-unit copulations during the mating season versus other months (Fisher’s exact test: P = 1). Thus, we combined data on extra-unit copulations from the mating season and from other months in a GLMM. Frequencies of extra-unit copulations each year were entered for each male–female pair as the response variable, offset by the amount of time the pair spent together in the provisioning area, thus creating rates. Male age and male age squared (to test for a predicted curvilinear relationship) were fixed effects. We controlled for rank and female–male relatedness and availability of extra-unit males by entering male rank and female–male relatedness as additional fixed effects. The identities of the males and females were random factors. Bachelor males in the AMU were assigned the lowest rank.
Our sample of female transfers (n = 18) was too small for multivariate analysis; hence, we used Sign tests to analyze female transfer patterns between OMUs by resident male age, male rank, and female relatedness. We first examined whether there were significant tendencies to transfer to OMUs with prime aged males from those with males of other ages or vice-versa. We also asked whether there were significant tendencies for them to transfer from units with younger to older males or vice versa, or from lower ranking males to units with higher ranking males or vice-versa. For this analysis, we looked at ranks of the original resident male when the female transferred out and the ranks of the new resident male when female joined. We also asked whether females tended to transfer from OMUs with more females to those with fewer females or vice-versa. We asked whether there were significant tendencies for females to transfer to OMUs with more closely related females than in their original OMU by comparing each female’s mean degree relatedness to the females in her original OMU to that in her new OMU. Finally, we asked whether females tended to transfer to OMUs with less closely related males than their original resident male.
Results
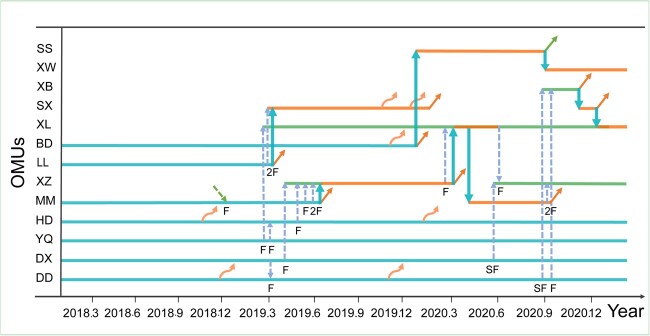
During the course of the study, we identified 16 OMUs. Seven of the 16 OMUs were present at the beginning of the study in March 2018. Thirteen adult subjects were resident males of the 16 OMUs for varying amounts of time over the course of the study (see Figure 3 for details). Six of the 13 males also spent varying amounts of time in the AMU. Four of the original 7 resident males retained their residency throughout the study period, 4 new OMUs formed, and 8 OMUs experienced takeovers. Three of the 8 takeovers occurred in spring, 1 in summer, 3 in autumn, and 1 in winter. The remaining male subjects were members of the AMU (11) or sub-adults (4) that had not yet left their natal OMU. Fourteen females (12 adults and 2 sub-adults) transferred from one OMU to another (Figure 3), 7 old females died, 5 adult males disappeared, and 2 adult males joined the troop.
Figure 3.
Dynamics of OMUs and female transfer from 2018 to 2020. Capital letters on the left list the ID of the resident male at the beginning of the study or of a newly formed OMU.  , OMs existing at the beginning of the study;
, OMs existing at the beginning of the study;  , newly formed OMUs;
, newly formed OMUs;  , takeovers;
, takeovers;  , the direction of takeover;
, the direction of takeover;  , female immigrated to DPY;
, female immigrated to DPY;  , female transfer between OMU;
, female transfer between OMU;  , male emigrated from the troop;
, male emigrated from the troop;  , male returning to the AMU from an OMU; and
, male returning to the AMU from an OMU; and  in the pdf file of this paper, the size of this symbol, along with the other sympbols, is too large, please reduce size of these symbols female died.
in the pdf file of this paper, the size of this symbol, along with the other sympbols, is too large, please reduce size of these symbols female died.
Male age distribution
The age distributions of males in OMUs and the AMU differed significantly in each year of the study (Fisher’s exact test: 2018: P < 0.01; 2019: P < 0.01; 2020: P < 0.05, Figure 4). In each year, the majority of 10–15 year old males (considered prime aged) were in OMUs (2018: 5 out of 5 or 100%, 2019: 6 out of 7 or 85.7%, 2020: 5 out of 7 or 71.4%). The majority of males older than 15 or younger than 10 were in the AMU (2018: 8 out of 9 or 88.9%, 2019: 9 out of 12 or 75%, 2020: 9 out of 13 or 69.2%).
Figure 4.
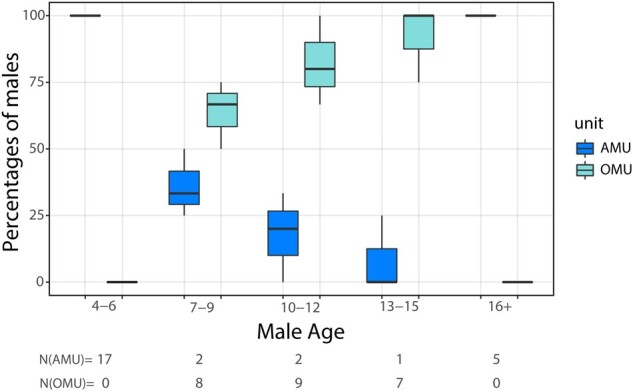
Percentages of male subjects in each age class that belong to the AMU or are resident males in an OMU. Box plots show medians, IQRs, and ranges over the 3 years of the study. N(AMU) and N(OMU) are sample sizes of males in the AMU and OMUs, respectively. The figure is for illustrative purposes only, since some males were represented in more than 1 year.
Number of females in OMUs
There was a significant nonlinear association between the age of resident male and the number of females in his OMU (Figure 5, age: estimate = 1.072, z = 2.074, P < 0.05; age2: estimate = −0.050, z = −2.205, P < 0.05). There were no significant associations between number of females and male rank (estimate = −0.084, z = −1.055, P = 0.291), mean relatedness of females (estimate = −0.682, z = −0.858, P = 0.391), female–male relatedness (estimate = −0.534, z = −0. 671, P = 0.602), or recent female reproductive performance (estimate = −0.002, z = −0.055, P = 0.996). Numbers of females increased with male age at first and then decreased. In general, 7-year-old males had OMUs containing only 1 or 2 females. Numbers of females reached a peak by the age about 12 years and then declined. After about 15 years old, males lost females and re-entered the AMU or lived alone. The results suggest that the age of 15 years is a turning point in male life history with regard to retaining females in an OMU.
Figure 5.
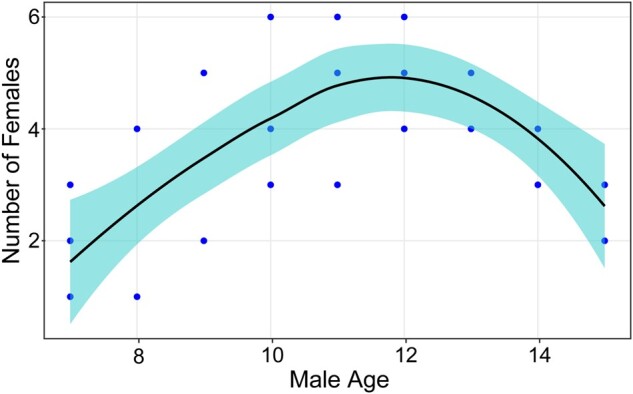
Number of females per OMU as a function of the age of the male. Local polynomial regression: y = −16.716 + 3.704x − 0.159x2. The figure is for illustrative purposes only since some males were represented in more than 1 year.
Extra-unit copulation
We observed a total of 79 instances in which females solicited copulation with extra-unit males (5 in 2018, 35 in 2019, and 39 in 2020). Our GLMM revealed significant associations between rates of extra-unit copulations (frequencies per day the pair was together in the provisioning area) and both male age (estimate = 2.015, z = 1.919, P < 0.05) and age squared (estimate = −0.421, z = −2.763, P < 0.01), suggesting a curvilinear relationship between extra-unit copulations and male age (Figure 6). There was also a positive association between rates of extra-unit copulations and male rank (estimate = −0.301, z = −3.950, P < 0.001). However, there was no significant relationship between rates and female–male relatedness (estimate = −0.673, z = −0.768, P = 0.428). Most extra-unit copulations were with resident males from other units (n = 64% or 81.0%) rather than with bachelors from the AMU (n = 15% or 19.0%). There was no significant difference in the age distributions among these 2 kinds of males (Fisher’s exact test: P = 1).
Figure 6.
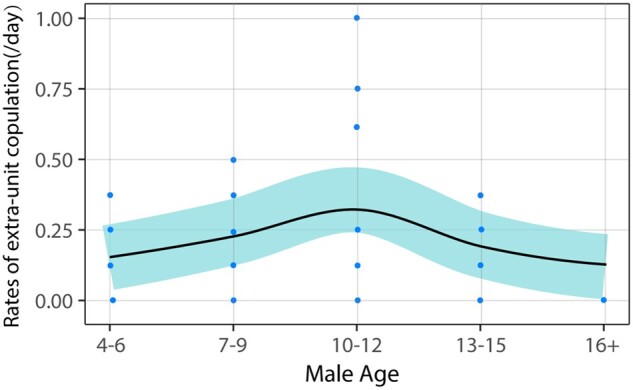
Rates of female extra-unit copulation as a function of the age of the male. The figure is for illustrative purposes only since some males were represented in more than 1 year.
Female transfer
There were 18 cases (involving 14 females) in which females transferred from one OMU to another (Figure 3). In 12 cases, adult females left their original OMU and formed an OMU with a bachelor male that had no other females (n = 5) or joined an established OMU (n = 7). In 2 of the 12 cases, sub-adult females left their natal unit to join a new unit. In all 12 of these cases, the resident male retained the OMU after the female transferred. In the remaining 6 cases, adult females transferred to an established OMU after which the original resident male was left with no females and returned to the AMU. In no case, did we analyze transfers that occurred immediately following a takeover. Thirteen (72%) transfers took place during the birth season and 5 (28%) took place during the mating season. In 15 of 17 cases in which females transferred from one known resident male to another, they went from older to younger resident males (Sign test: n = 17, x = 2, P < 0.001). In 13 cases, they transferred from prime aged resident males to young resident adults (7–9 years) (Sign test: n = 17, x = 4, P < 0.05). Notably, in 9 of the 13 cases, females transferred from resident males that were 13–15 years old, that is, resident males that were in the top half of the age range that we designated as prime. In all 17 cases, they transferred from higher ranking resident males to lower ranking resident males (Sign test: n = 17, x = 0, P < 0.001). This was not surprising given that resident male rank was positively and linearly correlated with male age; younger resident males were typically lower in rank than older resident males (Figure 1). There was no significant association tendency for females to transfer to OMUs with fewer or more females than their original OMU (Sign test: n = 13, x = 7, P = 1). Nor were there significant association between the females’ mean degree of relatedness to other females in her original OMU versus her new OMU (Sign test: n = 15, x = 6, ties = 4, P = 0.791) or between her relatedness to the male of her original OMU versus her new OMU (Sign test: n = 15, x = 9, ties = 2, P = 0.607). Females were in a variety of reproductive states when they transferred: 1 was pregnant, and 6 were with infants or unweaned yearlings (i.e., lactating). An additional 6 females were with weaned offspring older than 2 years, and 5 had no offspring. Whether females with weaned offspring or no offspring were cycling or anestrous is not clear.
Discussion
The results of our study suggest that prime age males (10–15 years) are more likely to be resident males in OMUs than older and younger males, and that prime age males have more females in their OMUs than younger and older males. Male rank, female relatedness, and recent female reproductive performance do not apparently affect the number of females in each OMU. Although it is sometimes difficult to tease apart the effects of male–male competition and female mate choice if females prefer males with the highest competitive ability, evidence suggests that females strongly influence the outcomes of attempted takeovers of OMUs and often favor the lower ranking of the 2 males (Fang et al. 2018). Moreover, females are free to transfer between OMUs. Thus, these findings are consistent with female preferences for prime age males as social partners over those of other ages and ranks. In addition, females tend to initiate extra-unit copulations disproportionately with high ranking prime age males, most of which are resident males of different OMUs. Given that females initiate extra-unit copulations, these results suggest that females display also preferences for high ranking prime aged extra-unit males. Since paternity analysis suggests that males in OMUs sire a disproportionate number of offspring (Qi et al. 2020), such preferences could potentially lead to increased mating success, and perhaps increased reproductive success, for high ranking prime aged males compared with males of other ages (Sutherland 1989; Clutton-Brock and Isvaran 2007; McCleery et al. 2008). Although direct evidence is not yet available, the possibility that reproductive success may be higher in prime aged males than in other males of this species is consistent with a general trend among vertebrates for male mating and reproductive success to increase with age until senescence sets in at which point it decreases (Coltman et al. 2002; Hammers et al. 2012). On the other hand, females appear to transfer primarily from high ranking prime aged resident males to lower ranking younger resident males, suggesting that different factors may influence female decisions to change OMUs, particularly when their resident male reaches 13–15 years of age. Below we discuss in more detail these apparently different preferences that females display in different social contexts (i.e., when choosing new social mates versus extra-unit mates).
Our results suggest that male golden snub-nosed monkeys begin adulthood with few females, if any, but acquire females over time, reaching a maximum number of females at about 12 years of age, after which males tend to lose females (Figure 5). This pattern is similar to other primate species with OMUs. For example, in Thomas’ langurs and hamadryas baboons (Steenbeek 1999; Pines et al. 2015), resident males acquire nearly all their females before they are halfway into their tenure. It has been hypothesized that prime aged males in species with OMUs are better at defending themselves from competition from other males, because prime aged males are both higher ranking and physically stronger than other males (Mainguy and Côté 2007; Raveh et al. 2010). As such, it is thought that prime aged males are also better able to protect females and infants in their OMUs from outside males and other dangers. However, once resident males reach about 13 years, males may lose females and be less likely to acquire new females. This may due to the fact that the physical condition of males gradually begins to decrease and is accompanied by a decline in their ability to protect females from harassment from other males. Such a decline in physical condition and ability of defend mates with age is likely to be exacerbated and accelerated by the typically high rates of physical injury that they incur from frequent male–male aggressive interaction, which is typical for highly polygamous species (Kirkwood and Rose 1991; Ricklefs 1998; Kappeler and Schaik 2004). In addition, given that 13–15-year-old resident males are very likely to be replaced within a year or 2, females could conceivably benefit by transferring from them to younger males if doing so lowers the risk of infanticide, a common risk associated with takeovers in some species with OMUs (Lukas and Huchard 2014). However, the risk of infanticide appears to be very low in this population; so far no evidence of infanticidal behavior has been found. Our findings that most female transfers from prime aged males to younger males involved males in the upper range (13–15 years) that we considered prime age (10–15 years) suggest that, in spite of their high rank, females may have perceived or anticipated cues of senescence earlier than we suspected. In the case of golden snub-nosed monkeys, females in OMUs led by males that are in upper range of what we considered prime age may be in a position in which they must trade off the benefits of rank versus the costs of age, and females may benefit by leaving high ranking males that show signs of aging.
The results for extra-unit copulation suggest that females prefer to mate with high ranking prime aged males when mating outside of their own OMU. Their preference for prime aged males in this social context is likely to be an important factor contributing to the mating success of males of this age, since these extra-unit copulations appear to be responsible for the majority of offspring born (57–75%) (Guo et al. 2010; our unpublished data). Why females appear to prefer prime aged males for extra-unit copulation is not yet clear. Many studies have shown that male age is closely related to indicators of quality that may affect female fitness. It could be that prime aged males are likely to be more reproductively competent than other males, and that females are more likely to conceive when mating with them (Alberts et al. 2006). In addition, prime aged males have survived a long time; therefore, they may have genes related to longevity to pass to offspring. Prime aged males that belong to an OMU also tend to be high-ranking (Figure 2); hence, their offspring may acquire heritable physical or psychological traits that facilitate the acquisition of high rank. Indeed, male rank and paternity are correlated with one another in many mammalian species and with male age, forming inverted U-shaped functions with male age (Setchell et al. 2006; Pines et al. 2015; Perlman et al. 2016). Finally, to the extent that infanticide is a risk in this population, extra-unit copulation could lower that risk, should the resident male be replaced (Qi et al. 2020). Alternatively, biases favoring extra-unit mating with prime age males could be related to mate guarding rather than female preferences, if resident males find it difficult to prevent matings between their females and prime age extra-unit males. However, this is unlikely because in most cases females approach and solicit extra-unit males after moving some distance away from their resident male and OMU. It may also be that prime age males may be more receptive to solicitations by extra-unit females, but currently there are no data to test this possibility.
On the other hand, female transfers suggest that females tend to transfer from older, high-ranking resident males to younger, low-ranking resident males (see also Zhao et al. 2008), although the specific factors driving this tendency are not clear. First, when young females mature within their natal OMUs, their fathers are likely to have reached prime age or older. Given the high frequency of OMU takeovers, a young female’s father may be an older resident male in a different OMU. Thus, some transfers to younger males may function to avoid inbreeding. In our study, females WX and DH left their natal OMUs and its resident males (DX and DD) to build a new OMU with younger males. Although we didn’t find evidence that females tend to transfer to new males that are less closely related than their original males. Mothers (or sisters) may also transfer to the same OMU as had their daughters (or sisters), that is, an OMU with a younger male, given that females tend to be in OMUs with female kin (Ren et al. 2018). For example, DH’s mother transferred into the same OMU as had her daughter, and sisters YB and HH both transferred to XZ’s OMU. However, female–female relatedness is not likely to be a major factor in transfer patterns since we found no significant tendency for females to transfer to OMUs that contained more closely related females than their original OMUs. Given that young adult resident males have fewer females than prime aged resident males, it could be that females that transfer to new OMUs with young adult males to avoid female–female competition for resources, including attention and sperm from the male. Indeed, several studies provide evidence that females may discriminate against sperm-exhausted males who had consorted with several females or were very old males (Harris and Moore 2005; Briefer et al. 2013; Vuarin et al. 2019). However, we found no tendencies for females to transfer to OMU with fewer females. In some primate species, females are more likely to be attracted by novel mates (Wikberg et al. 2017; Baudouin et al. 2019); for example, males XW and XB came from other troops. Transferring females also appear to be particularly attracted to younger resident males who only recently formed an OMU (SS and XZ). Such males and their novel genomes may provide opportunities to females to diversify the genetic make ups of their offspring. Younger resident males tend to be lower ranking than older resident males (Figure 2), perhaps presenting a disadvantage to females, given that higher ranking males are better able to provide a stable OMU in which to raise offspring (Silk et al. 2020). It is unknown yet whether the advantages of young resident male outweigh the potential disadvantages of having a lower ranking resident male, for example, priority of access to food for the unit, protection from harassment from other males (Moller and Jennions 2001). Finally, although females transferred when they were in a variety of reproductive states, the fact that most of the transfers occurred during the birth season, rather than the mating season may reflect a broader reproductive strategy for females rather simply a mating strategy.
Overall, our findings suggest that female golden snub-nosed monkey preferences may differ depending on whether they are choosing social mates in the form of resident males or mates for extra-unit copulation. There appear to be a number of possible benefits to choosing high ranking prime age males for extra-unit copulation and for transferring from OMUs with older prime age males to those with lower ranking younger males.
It is also unclear whether females focus on particular cues that are functionally linked, as do females in many species, including the size and quality of secondary sexual characteristics, for example, antler size, tail feathers, and plumage coloration (Nussey et al. 2009; Evans et al. 2011; Kervinen et al. 2016) or more general cues related to age. Do females attend to more than one age-related cue, such that age can be used to capture the cumulative effects of multiple cues more effectively than single cues? The results of this study suggest that future studies that compare the effects of male age per se with the effects of particular age-related cues would be helpful in answering this question in particular and in further understanding the dynamics of female mating strategies.
Ethical Note
All research protocols reported here adhere to the regulatory requirements of China and were approved, and that the research complied with the protocols approved by the animal care committee of the Wildlife Protection Society of China (SL-2012-42).
Data Availability
The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
The idea for this study originally came from B.L., P.Z., and X.Y. Behavioral data were collected by X.Y. and H.H. Help by others is accordingly acknowledged. The statistical analyses were performed by X.Y., C.M.B., R.H., and K.H. The manuscript was prepared by X.Y. and C.M.B. with edits and additional suggestions for data analysis and interpretation provided by C.M.B. The project and all guidance were coordinated by X.W., H.Z., and C.W. All authors contributed to the article and the submitted version was approved by B.L. and P.Z.
Acknowledgments
We gratefully acknowledge Guanyinshan National Nature Reserve for permission to carry out this research. We thank He Zhang for assistance with statistical analysis. We also thank all who helped us during the field work and all members of the Golden Monkey Research Team in the College of Life Sciences, Northwest University. All research protocols reported in this manuscript were reviewed and approved by the Chinese Academy of Science.
Funding
This study was funded by the National Natural Science Foundation of China [31730104, 31770425, 32071495, and 31770411], the National Key Program of Research and Development, Ministry of Science and Technology [2016YFC0503200], the Strategic Priority Research Program of the Chinese Academy of Sciences [XDB31000000], and the Natural Science Basic Research Plan in Shaanxi Province of China [2019JM-258].
Conflict of Interest
All co-authors declare that they have no conflict of interests.
Contributor Information
Xi Yang, Shaanxi Key Laboratory for Animal Conservation, College of Life Sciences, Northwest University, Xi’an 710069, China.
Carol M Berman, Department of Anthropology and Graduate Program in Evolution, Ecology and Behavior, State University of New York at Buffalo, NY 14261, USA.
Hanyu Hu, Shaanxi Key Laboratory for Animal Conservation, College of Life Sciences, Northwest University, Xi’an 710069, China.
Rong Hou, Shaanxi Key Laboratory for Animal Conservation, College of Life Sciences, Northwest University, Xi’an 710069, China.
Kang Huang, Shaanxi Key Laboratory for Animal Conservation, College of Life Sciences, Northwest University, Xi’an 710069, China.
Xiaowei Wang, Shaanxi Institute of Zoology, Shaanxi Province Academy of Sciences, Xi’an 710032, China.
Haitao Zhao, Shaanxi Key Laboratory for Animal Conservation, College of Life Sciences, Northwest University, Xi’an 710069, China; Shaanxi Institute of Zoology, Shaanxi Province Academy of Sciences, Xi’an 710032, China.
Chengliang Wang, Shaanxi Key Laboratory for Animal Conservation, College of Life Sciences, Northwest University, Xi’an 710069, China; Shaanxi Institute of Zoology, Shaanxi Province Academy of Sciences, Xi’an 710032, China.
Baoguo Li, Shaanxi Key Laboratory for Animal Conservation, College of Life Sciences, Northwest University, Xi’an 710069, China.
Pei Zhang, Shaanxi Key Laboratory for Animal Conservation, College of Life Sciences, Northwest University, Xi’an 710069, China.
References
- Albers CH, de Vries H, 2001. Elo-rating as a tool in the sequential estimation of dominance strengths. Anim Behav 61:489–495. [Google Scholar]
- Alberts SC, Buchan JC, Altmann J, 2006. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim Behav 72:1177–1196. [Google Scholar]
- Allen M, Engstrom AS, Meyers S, Handt O, Saldeen T et al. , 1998. Mitochondrial DNA sequencing of shed hairs and saliva on robbery caps: sensitivity and matching probabilities. J Forensic Sci 43:453–464. [PubMed] [Google Scholar]
- Andersson M, 1994. Sexual Selection. Princeton University Press. [Google Scholar]
- Akinwande MO, Dikko HG, Samson A, 2015. Variance inflation factor: as a condition for the inclusion of suppressor variable(s) in regression analysis. Open J Statist 5:754–767. [Google Scholar]
- Baniel A, Cowlishaw G, Huchard E, 2018. Context dependence of female reproductive competition in wild chacma baboons. Anim Behav 139:37–49. [Google Scholar]
- Baudouin A, Gatti S, Levrero F, Genton C, Cristescu RH et al. , 2019. Disease avoidance, and breeding group age and size condition the dispersal patterns of western lowland gorilla females. Ecology 100:e02786. [DOI] [PubMed] [Google Scholar]
- Berard J, 1999. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques Macaca mulatta. Primates 40:159–175. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Nurnberg P, 1996. Socioendocrine and morphological correlates of paternity in rhesus macaques Macaca mulatta. J Reprod Fertil 107:59–68. [DOI] [PubMed] [Google Scholar]
- Briefer EF, Farrell ME, Hayden TJ, McElligott AG, 2013. Fallow deer polyandry is related to fertilization insurance. Behav Ecol Sociobiol 67:657–665. [Google Scholar]
- Clutton-Brock TH, Isvaran K, 2007. Sex differences in ageing in natural populations of vertebrates. Proc R Soc B 274:3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltman DW, Festa-Bianchet M, Jorgenson JT, Strobeck C, 2002. Age-dependent sexual selection in bighorn rams. Proc R Soc B 269:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C, 1871. The Descent of Man, and Selection in Relation to Sex. New York: D. Appleton. [Google Scholar]
- Evans SR, Gustafsson L, Sheldon BC, 2011. Divergent patterns of age-dependence in ornamental and reproductive traits in the collared flycatcher. Evolution 65:1623–1636. [DOI] [PubMed] [Google Scholar]
- Fang G, Chen J, Pan RL, Qi XG, Li BG, 2018. Female choice impacts residential male takeover in golden snub-nosed monkeys Rhinopithecus roxellana. Zool Res 39:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman-Gallant CR, Taff CC, Morin DF, Dunn PO, Whittingham LA et al. , 2010. Sexual selection, multiple male ornaments, and age- and condition-dependent signaling in the common yellowthroat. Evolution 64:1007–1017. [DOI] [PubMed] [Google Scholar]
- Gao LF, Zhang HY, Zhang W, Sun YH, Liang MJ et al. , 2019. Effects of extra-pair paternity and maternity on the provisioning strategies of the Azure-winged magpie Cyanopica cyanus. Int J Avia Sci 162:627–636. [Google Scholar]
- Garcia-Berro A, Yliportimo J, Lindstrom K, Kvarnemo C, 2019. Understanding resource driven female–female competition: ovary and liver size in sand gobies. R Soc Open Sci 6:190886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini C, Devigili A, Pilastro A, 2019. Sexual selection and ageing: interplay between pre- and post-copulatory traits senescence in the guppy. Proc Biol Sci 286:20182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith SC, Owens IPF, Thuman KA, 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212. [DOI] [PubMed] [Google Scholar]
- Grueter CC, Qi X, Li B, Li M, 2017. Multilevel societies. Curr Biol 27:R984–R986. [DOI] [PubMed] [Google Scholar]
- Guo ST, Ji WH, Li M, Chang HL, Li BG, 2010. The mating system of the Sichuan snub-nosed monkey Rhinopithecus roxellana. Am J Primatol 72:25–32. [DOI] [PubMed] [Google Scholar]
- Guo ST, Huang K, Ji WH, Garber PA, Li BG, 2015. The role of kinship in the formation of a primate multilevel society. Am J Phys Anthropol 156:606–613. [DOI] [PubMed] [Google Scholar]
- Hammers M, Richardson DS, Burke T, Komdeur J, 2012. Age-dependent terminal declines in reproductive output in a wild bird. PLoS ONE 7:e40413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WE, Moore PJ, 2005. Female mate preference and sexual conflict: females prefer males that have had fewer consorts. Am Nat 165:S64–S71. [DOI] [PubMed] [Google Scholar]
- Hayward AD, Moorad J, Regan CE, Berenos C, Pilkington JG et al. , 2015. Asynchrony of senescence among phenotypic traits in a wild mammal population. Exp Gerontol 71:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Zhao H, Qi X, Wang X, Guo S et al. , 2013. Dominance rank of adult females and mating competition in Sichuan snub-nosed monkeys Rhinopithecus roxellana in the Qinling Mountains, China. Chin Sci Bull 58:2205–2211. [Google Scholar]
- Huang K, Ritland K, Dunn DW, Qi XG, Guo ST et al. , 2016. Estimating relatedness in the presence of null alleles. Genetics 202:247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OR, Gaillard JM, Tuljapurkar S, Alho JS, Armitage KB et al. , 2008. Senescence rates are determined by ranking on the fast-slow life-history continuum. Ecol Lett 11:664–673. [DOI] [PubMed] [Google Scholar]
- Jones TM, Balmford A, Quinnell RJ, 2000. Adaptive female choice for middle-aged mates in a lekking sandfly. Proc R Soc B 267:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler PM, Schaik CP, 2004. Sexual Selection in Primates. New York (NY: ): Cambridge University Press. [Google Scholar]
- Kalinowski ST, M L, 2006. Maximum likelihood estimation of the frequency of null alleles at microsatellite loci. Conserv Genet 7:991–995. [Google Scholar]
- Kervinen M, Lebigre C, Soulsbury CD, 2016. Simultaneous age-dependent and age-independent sexual selection in the lekking black grouse Lyrurus tetrix. J Anim Ecol 85:715–725. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick RC, Grueter CC, 2010. Snub-nosed monkeys: multilevel societies across varied environments. Evol Anthropol 19:98–113. [Google Scholar]
- Kirkwood TB, Rose MR, 1991. Evolution of senescence: late survival sacrificed for reproduction. Phil Trans R Soc Lond B Biol Sci 332:15–24. [DOI] [PubMed] [Google Scholar]
- Kokita T, , TakahashiS, , KumadaH, 2013. Molecular signatures of lineage-specific adaptive evolution in a unique sea basin: the example of an anadromous goby Leucopsarion petersii. Molecular Ecology 22:1341–1355. [DOI] [PubMed] [Google Scholar]
- Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guinness F et al. , 2002. Antler size in red deer: heritability and selection but no evolution. Evolution 56:1683–1695. [DOI] [PubMed] [Google Scholar]
- Lawler RR, 2009. Monomorphism, male–male competition, and mechanisms of sexual dimorphism. J Hum Evol 57:321–325. [DOI] [PubMed] [Google Scholar]
- Li BG, Li HQ, Zhao DP, Zhang YH, 2006. Study on dominance hierarchy of Sichuan snub-nosed monkey Rhinopithecus roxellana in the Qinling Mountains. Acta Theriol Sin 26:18–25. [Google Scholar]
- Li YL, Huang K, Tang SY, Feng L, Yang J, et al. 2021. Genetic structure and evolutionary history of Rhinopithecus roxellana in Qinling Mountains, Central China. Front Genet 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Qi HJ, Zhang SY, Ren BP, 2001. Developmental traits of captive Sichuan snub-nosed monkeys Rhinopithecus roxellana at different age stages. Acta Zool Sin 47:381–387. [Google Scholar]
- Lukas D, Huchard E, 2014. The evolution of infanticide by males in mammalian societies. Science 346:841–844. [DOI] [PubMed] [Google Scholar]
- Hill K, Boesch C, Goodall J, Pusey A, Williams J et al. , 2001. Mortality rates among wild chimpanzees. J Hum Evol 40:437–450. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton EA, 2020. Many loci make light work: high individual diversity despite low population diversity and random mating at class I MHC in a critically endangered island songbird. Mol Ecol 29:3575–3577. [DOI] [PubMed] [Google Scholar]
- Mainguy J, Côté SD, 2007. Age- and state-dependent reproductive effort in male mountain goats Oreamnos americanus. Behav Ecol Sociobiol 62:935–943. [Google Scholar]
- Martin JG, Festa-Bianchet M, 2011. Age-independent and age-dependent decreases in reproduction of females. Ecol Lett 14:576–581. [DOI] [PubMed] [Google Scholar]
- McCleery RH, Perrins CM, Sheldon BC, Charmantier A, 2008. Age-specific reproduction in a long-lived species: the combined effects of senescence and individual quality. Proc Biol Sci 275:963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AP, Jennions MD, 2001. How important are direct fitness benefits of sexual selection? Sci Nat 88:401–415. [DOI] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW, 2009. Evolution of Sexual Coercion with Respect to Sexual Selection and Sexual Conflict Theory. Cambridge: Harvard University Press. [Google Scholar]
- Muller MN, Thompson ME, Wrangham RW, 2006. Male chimpanzees prefer mating with old females. Curr Biol 16:2234–2238. [DOI] [PubMed] [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, 2011. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim Behav 82:911–921. [Google Scholar]
- Nussey DH, Froy H, Lemaitre JF, Gaillard JM, 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res Rev 12:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussey DH, Kruuk LEB, Morris A, Clements MN, Pemberton JM, 2009. Inter- and intrasexual variation in aging patterns across reproductive traits in a wild red deer population. Am Nat 174:342–357. [DOI] [PubMed] [Google Scholar]
- Perlman RF, Borries C, Koenig A, 2016. Dominance relationships in male Nepal gray langurs Semnopithecus schistaceus. Am J Phys Anthropol 160:208–219. [DOI] [PubMed] [Google Scholar]
- Pines M, Chowdhury S, Saunders J, Swedell L, 2015. The rise and fall of leader males in a multi-level society: takeovers and tenures of male Hamadryas baboons. Am J Primatol 77:44–55. [DOI] [PubMed] [Google Scholar]
- Qi XG, Grueter CC, Fang G, Huang PZ, Zhang J et al. , 2020. Multilevel societies facilitate infanticide avoidance through increased extrapair matings. Anim Behav 161:127–137. [Google Scholar]
- Qi XG, Garber PA, Ji W, Huang ZP, Huang K et al. , 2014. Satellite telemetry and social modeling offer new insights into the origin of primate multilevel societies. Nat Commun 5:5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XG, Li BG, Garber PA, Ji W, Watanabe K, 2009. Social dynamics of the golden snub-nosed monkey Rhinopithecus roxellana: female transfer and one-male unit succession. Am J Primatol 71:670–679. [DOI] [PubMed] [Google Scholar]
- Raveh S, Heg D, Dobson FS, Coltman DW, Gorrell JC et al. , 2010. Mating order and reproductive success in male Columbian ground squirrels Urocitellus columbianus. Behav Ecol 21:537–547. [Google Scholar]
- Reddy RB, Mitani JC, 2020. Adolescent and young adult male chimpanzees form affiliative, yet aggressive, relationships with females. J Hum Evol 144:102813. [DOI] [PubMed] [Google Scholar]
- Ren Y, Huang K, Guo S, Pan R, Derek DW et al. , 2018. Kinship promotes affiliative behaviors in a monkey. Curr Zool 64:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE, 1998. Evolutionary theories of aging: confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am Nat 152:24–44. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Wickings EJ, Knapp LA, 2006. Life history in male mandrills Mandrillus sphinx: physical development, dominance rank, and group association. Am J Phys Anthropol 131:498–510. [DOI] [PubMed] [Google Scholar]
- Silk JB, Stadele V, Roberts EK, Vigilant L, Strum SC, 2020. Shifts in male reproductive tactics over the life course in a polygynandrous mammal. Curr Biol 30:1716–1720.e1713. [DOI] [PubMed] [Google Scholar]
- Smuts BB, Smuts RW, 1993. Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv Behav 1:63. [Google Scholar]
- Stervander M, Dierickx EG, Thorley J, Brooke ML, Westerdahl H, 2020. High MHC gene copy number maintains diversity despite homozygosity in a critically endangered single-island endemic bird, but no evidence of MHC-based mate choice. Mol Ecol 29:3578–3592. [DOI] [PubMed] [Google Scholar]
- Steenbeek R, 1999. Tenure related changes in wild Thomas’s langurs I: between-group interactions. Behaviour 136:595–625. [Google Scholar]
- Sutherland WJ, 1989. Reproductive success: studies of individual variation in contrasting breeding systems. Trends Ecol Evol 4:218. [Google Scholar]
- Vuarin P, Bouchard A, Lesobre L, Levêque G, Chalah T et al. , 2019. Post-copulatory sexual selection allows females to alleviate the fitness costs incurred when mating with senescing males. Proc R Soc B 286:1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CL, , PanRL, , WangXW, , QiXG, , Zhao HT et al. , 2020. Decision-making process during collective movement initiation in golden snub-nosed monkeys (Rhinopithecus roxellana). Sci Rep10:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikberg EC, Jack KM, Fedigan LM, Campos FA, Yashima AS et al. , 2017. Inbreeding avoidance and female mate choice shape reproductive skew in capuchin monkeys Cebus capucinus imitator. Mol Ecol 26:653–667. [DOI] [PubMed] [Google Scholar]
- Zhang P, Watanabe K, Li BG, Qi XG, 2008. Dominance relationships among one-male units in a provisioned free-ranging band of the Sichuan snub-nosed monkeys Rhinopithecus roxellana in the Qinling Mountains, China. Am J Primatol 70:634–641. [DOI] [PubMed] [Google Scholar]
- Zhang P, Watanabe K, Li BG, Tan CL, 2006. Social organization of Sichuan snub-nosed monkeys Rhinopithecus roxellana in the Qinling Mountains, Central China. Primates 47:374–382. [DOI] [PubMed] [Google Scholar]
- Zhao DP, , LiBG, , GrovesCP, , WatanabeK, 2008. Impact of Male Takeover on Intra-Unit Sexual Interactions and Subsequent Interbirth Interval in Wild Rhinopithecus roxellana. Folia Primatol 79: 93–102. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Tan CL, 2011. Inter-unit contests within a provisioned troop of Sichuan snub-nosed monkeys Rhinopithecus roxellana in the Qinling Mountains, China. Am J Primatol 73:262–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.