Abstract
Rescue behavior is observed when 1 individual provides help to another individual in danger. Most reports of rescue behavior concern ants (Formicidae), in which workers rescue each other from various types of entrapment. Many of these entrapment situations can be simulated in the laboratory using an entrapment bioassay, in which ants confront a single endangered nest mate entrapped on a sandy arena by means of an artificial snare. Here, we compared numerous characteristics of rescue actions (contact between individuals, digging around the entrapped individual, pulling at its body parts, transport of the sand covering it, and biting the snare entrapping it) in Formica cinerea ants. We performed entrapment tests in the field and in the laboratory, with the latter under varying conditions in terms of the number of ants potentially engaged in rescue actions and the arena substrate (marked or unmarked by ants’ pheromones). Rescue actions were more probable and pronounced in the field than in the laboratory, regardless of the type of test. Moreover, different test types in the laboratory yielded inconsistent results and showed noteworthy variability depending on the tested characteristic of rescue. Our results illustrate the specifics of ant rescue actions elicited in the natural setting, which is especially important considering the scarcity of field data. Furthermore, our results underline the challenges related to the comparison of results from different types of entrapment tests reported in the available literature. Additionally, our study shows how animal behavior differs in differing experimental setups used to answer the same questions.
Keywords: ants; animal behavior; entrapment bioassay, Formica cinerea; rescue behavior
Altruistic behavior in animals draws high scientific interest (Pennisi 2005). A form of altruistic behavior, rescue, is a behavior of 1 individual helping a related individual in grave danger, not bringing any direct reward to the rescuer, except for the benefits from reciprocal altruism and kin selection (Nowbahari and Hollis 2010). Numerous cases of nonhuman animals rescuing each other can be found in the literature. In capuchin monkeys, males rescue females from harassment by distracting the aggressors (Vogel and Fuentes-Jiménez 2006). In bottlenose dolphins, injured members of the group receive help in reaching the water surface (Siebenaler and Caldwell 1956). In birds, flock members remove flight preventing bird-catcher tree seeds from each other’s bodies (Hammers and Brouwer 2017). By far, the most commonly studied in this context among all vertebrates are rats due to the vast opportunities to study their rescue behavior in terms of ensuring appropriate laboratory conditions (Bartal et al. 2011; Vasconcelos et al. 2012; Silberberg et al. 2014; Silva et al. 2020). Notably, however, most reports of rescue behavior concern animals with a much less complex nervous system than that in vertebrates, namely, ants (e.g., Czechowski et al. 2002; Nowbahari et al. 2009; Taylor et al. 2013; Miler 2016). However, the similarities and differences in ant rescue actions observed under different field and laboratory setups are entirely unstudied. Using several tests to examine the same behavior may help uncover the role of context in animal behavior (Watanabe 2012; Scharf and Martin 2013). It has been suggested, for example, that relatively similar tests devoted to the study of boldness in crabs lead to different responses of tested individuals (Watanabe 2012). Moreover, as showed by Scharf and Martin (2013), frequencies of same-sex mating in insects and arachnids are higher in the laboratory conditions than in the field. Feeding strategy in desert gerbils, and their response to predation risk, also differs strikingly between the natural and laboratory contexts (Ovadia et al. 2001). Plausibly, the response of ants to a risky situation, such as another individual in need of rescue, can also vary between contexts.
The simplest forms of ant rescue behavior, such as helping a nest mate move from under pebbles, were documented for the first time by Belt (1874). Afterward, similar observations of ants’ digging behavior around individuals covered by sand were reported several times by different authors (Lafleur 1940; Wilson 1958; Markl 1965; Blum and Warter 1966; Spangler and Kansas 1968; Hangartner 1969). More advanced forms of rescue behavior were found by Czechowski et al. (2002) in sand-dwelling ants (Formica sanguinea, Formica fusca, and Formica cinerea) trying to set imperiled individuals free from traps of predatory antlions. Other recent examples of risky rescue operations included those described in Matabele ants Megaponera analis saving wounded nest mates after direct confrontation with termites (Frank et al. 2017) and in weaver ants Oecophylla smaragdina and harvester ants Veromessor pergandei trying to save endangered individuals who became stuck on spider webs (Uy et al. 2018; Kwapich and Hölldobler 2019). Rescue may be considerably more widespread in nature than these reports demonstrate (Nowbahari and Hollis 2010).
Ant societies are thought to largely dominate the Earth (Hölldobler and Wilson 1990). One of the essential components of their success, beyond advanced nest construction, foraging behavior and complex division of labor, seems to be rescue behavior (Andras et al. 2020). The potential meaning of this altruistic behavior to the overall success of the colony is suggested by 2 previous studies. As shown by Frank et al. (2017), rescue behavior of M. analis ants prevents mortality in 32% of cases and allows for up to 28.7% higher colony size. In a study conducted by Kwapich and Hölldobler (2019), V. pergandei ants not only saved endangered nest mates that became stuck in spider webs but also removed the webs to reduce future risks and ensure better foraging performance, which largely determined the success of the colony. Further studies devoted to rescue in ant species with well-known ecology will surely allow to assess the value of rescue actions for ants’ success in nature (Taylor et al. 2013).
To date, although only 20 species have been studied out of over 16,000 known species of ants (Bolton 2020), rescue has already been demonstrated in several different subfamilies, that is, Dolichoderinae, Formicinae, Myrmicinae, and Ponerinae. With some exceptions in which rescue was studied in an ecological context in the field (e.g., Czechowski et al. 2002; Frank et al. 2017; Uy et al. 2018), it was most frequently triggered in the laboratory with the use of the so-called “entrapment bioassay,” the most universal type of rescue test in ants so far (Hollis and Nowbahari 2013; Miler et al. 2017b). In the bioassay, ants confront a single endangered nest mate entrapped on a sandy arena by means of an artificial snare, as was described for the first time by Nowbahari et al. (2009) and then subsequently adapted by multiple authors (e.g., Taylor et al. 2013; Andras et al. 2020).
This test is thought to provide the laboratory setup for drawing conclusions on entrapment situations in which ant workers of various species may find themselves in the field. However, several alternative versions of the entrapment bioassay can be found in the literature. The main variations include the type of substrate used on the test arena (i.e., marked or unmarked by ants’ pheromones), the number of potential rescuer ants (i.e., 1 or 5), and the method used to code behavior during analysis (i.e., time or interval recording procedures). Thus, despite the recognized universality of the entrapment bioassay, it is unclear how comparable are the results of various independent studies utilizing its different versions. Moreover, considering the overall scarcity of comparable data on ant rescue behavior in the natural setting (Czechowski et al. 2002; Hollis and Nowbahari 2013; Taylor et al. 2013), it is also unclear how well each of these versions corresponds to field-based rescues.
Here, we decided to compare numerous characteristics of rescue actions in F. cinerea workers, one of the species most often chosen in research on ant rescue behavior (Czechowski et al. 2002; Miler 2016; Miler and Kuszewska 2017; Miler et al. 2017a, 2017b; Turza et al. 2020), under field- and laboratory-based entrapment bioassays of various types. Furthermore, we conducted comparisons of the data obtained using various coding procedures. We hypothesized that 1) rescue actions would be more pronounced in the field than in laboratory tests, 2) laboratory tests would yield overall similar rescue actions, and 3) coding procedures would not severely affect the conclusions. Regarding the first hypothesis, data from experiments conducted in the field may differ drastically from the results of similar experiments performed under laboratory conditions (e.g., Calisi and Bentley 2009). In the case of second and third hypothesis, many variations of the entrapment bioassay and coding procedures can be found in the literature, all regarded as appropriate for testing all rescue components (e.g., Nowbahari et al. 2009; Taylor et al. 2013; Miler 2016).
Materials and Methods
Tests of rescue behavior were performed in the field near Klucze (Błędowska Desert, Poland, 50°21′22″N 19°31′03″E) and in the laboratory in Kraków (Institute of Environmental Sciences, Jagiellonian University, Poland) in July 2020. Formica cinerea ants, which are sand-dwelling ants naturally exposed to different types of entrapment, were selected as the model organism. According to Czechowski et al. (2012), F. cinerea ants often create extensive polycalic colonies (i.e., multi-nest colonies between which workers move freely without aggression). Therefore, we decided to test 2 independent polycalic colonies of F. cinerea ants from 2 areas (southern and northern parts of the Błędowska Desert) 2 km apart and well separated geographically. First, we performed tests in the field and then we collected ants from the 2 focal colonies and tested them in different laboratory tests.
Entrapment tests in the field
The testing procedure was similar to those of Hollis and Nowbahari (2013) and Taylor et al. (2013). For each test, using clean forceps, 1 ant (the victim) was captured close to the entrance to the nest and then inserted into a nylon thread loop so that the thread was located between its thorax and abdomen (i.e., on the petiole) and then tied to a small piece of filter paper (2 cm in diameter). The ant was placed within a plastic ring (7 cm in diameter) that was previously positioned on an ant-free area 5–10 cm away from the nest entrance. The plastic ring was smeared with fluon (Sigma–Aldrich, Germany) on the outside to prevent nearby ants from entering the test area before the test began. Then, the plastic ring was removed and a 5-min video recording started. After the test ended, the tested ant worker was not released but placed in a temporary container. The procedure was repeated 50 times for each of the 2 colonies (a total of 100 tests under field conditions). A new nylon thread and filter paper were used for each test. Forceps were sterilized after each test in 98% ethanol to avoid the transfer of cuticular hydrocarbons between tested ants. The position of the plastic ring was changed for each test but was always within 5–10 cm of the nest entrance. All tests were performed during 2 days: on 4 July 2020 (23 °C, 48% relative humidity) and on 5 July 2020 (25 °C, 52% relative humidity) (1 day per colony), between 9 AM and 6 PM. After all tests for a given colony were completed, the tested ants were untied and released, with the exception of a few individuals collected for later taxonomic identification. The taxonomic identity of ants was confirmed in the laboratory with reference to a taxonomic key (Czechowski et al. 2012).
Entrapment tests in the laboratory
On a day after the entrapment tests in the field were completed, we collected approximately 1,000 active F. cinerea foragers from each of the 2 colonies previously tested in the field and transported them to the laboratory where they were housed separately in plastic boxes (28 × 15 × 6 cm) at a constant temperature of 24 °C, 40–60% relative humidity, and a 12:12 day:night cycle, each with an attached foraging arena of the same size. Both the nest part and the foraging arena were half-filled with sand from the original habitat. Ants were provided with ad libitum water and protein–carbohydrate–vitamin–mineral food recommended by Czechowski and Pisarski (1992) in their foraging arena. The edges of the plastic boxes were smeared with fluon (Sigma–Aldrich, Germany) to prevent escape of ants. After 2 days habituation period, during which time the ants were allowed to move freely inside both boxes and accumulate pheromones on the substrate (Heyman et al. 2017), the experimental part started.
The laboratory simulations of entrapment followed standard protocols (e.g., Nowbahari et al. 2009; Miler 2016). In each test, using clean forceps, 1 ant (the victim) was captured on the foraging arena and inserted into a nylon thread loop so that the thread was located between its thorax and abdomen (i.e., on the petiole) and then tied to a small piece of filter paper (2 cm in diameter). Depending on the type of test, the victim was placed into a plastic ring (7 cm in diameter) on an ant-free area of the foraging arena or inside a test cup (7 cm in diameter, 8 cm high) half-filled with dry (unmarked) sand. Immediately after, the potential rescuer(s) was(were) introduced into the test area (Table 1). Then, a 5-min video recording started. No ant was used twice. A new nylon thread and filter paper were used for each test. Forceps were sterilized after each test in 98% ethanol. In the case of tests on the substrate marked by ants’ pheromones (M1 and M5), the position of the plastic ring was changed for each test within 5–10 cm of the entrance to the foraging arena. In the case of tests on the substrate unmarked by ants’ pheromones (NM1 and NM5), unmarked sand in the test cup was renewed for each test. The procedure was repeated 50 times for each test type and colony (a total of 400 tests under laboratory conditions). The order of testing was counterbalanced by test type and colony. Tests were performed over several days, always between 9 AM and 6 PM. See Figure 1 for the scheme of the experiment.
Table 1.
Detailed procedures for the different types of tests conducted under field and laboratory conditions
| Type of test | Detailed procedure |
|---|---|
| One victim and potential rescuer(s) on marked substrate under field conditions (F) | The plastic ring is placed on an ant-free area 5–10 cm away from the nest entrance (i.e., sand marked by ant pheromones). Then, the victim is placed in the center of the ring. Then, the plastic ring is removed. |
| One victim and 1 potential rescuer on marked substrate under laboratory conditions (M1) | The plastic ring is placed on an ant-free area of the foraging arena attached to the nest (i.e., sand marked by ant pheromones). Then, the victim is placed in the center of the ring. Then, a randomly chosen nest mate from the foraging arena is placed into the ring. |
| One victim and 5 potential rescuers on marked substrate under laboratory conditions (M5) | The plastic ring is placed on an ant-free area of the foraging arena attached to the nest (i.e., sand marked by ant pheromones). Then, the victim is placed in the center of the ring. Then, 5 randomly chosen nest mates from the foraging arena are placed into the ring. |
| One victim and 1 potential rescuer on unmarked substrate under laboratory conditions (NM1) | The victim is placed into the plastic cup filled with dry (unmarked) sand. Then, a randomly chosen nest mate from the foraging arena is placed inside the cup. |
| One victim and 5 potential rescuers on unmarked substrate under laboratory conditions (NM5) | The victim is placed into the plastic cup filled with dry (unmarked) sand. Then, 5 randomly chosen nest mates from the foraging arena are placed inside the cup. |
Figure 1.
Scheme of the experiment.
Analysis of the recordings
Recorded videos were analyzed using BORIS software, which enables coding behavior of animals in an accurate and quantitative way (Friard and Gamba 2016). The videos were analyzed in 2 ways, namely, using the time and interval recording procedures described by Martin and Bateson (2007). For the time recording procedure, each occurrence of the given behavior was recorded, together with information about the time the behavior started and its total duration of occurrence. For the interval recording procedure, each test (lasting 5 min in total) was divided into 10 s intervals (in total 30 intervals), and behaviors were noted for each interval, providing information on the number of intervals in which a given behavior occurred (e.g., 10 out of 30). Both procedures were previously used in ant rescue behavior research (e.g., Hollis and Nowbahari 2013; Miler et al. 2017b).
We obtained data on the dependent variables common to both procedures, which included the number of tests with contact with the victim, the number of tests with any rescue actions among those tests with contact as well as the number of rescuers and the latency to the first episode of rescue in those tests with rescue. Additionally, we obtained data on the duration of (the number of intervals with) contacts in tests with at least 1 contact as well as the duration of (the number of intervals with) rescue actions and the durations of (the number of intervals with) selected rescue categories in tests with at least 1 rescue action. Operational definitions of contact and various rescue categories considered in this study were described in previous research (Table 2).
Table 2.
Definitions of rescue categories used in the study (based on Hollis and Nowbahari 2013)
| Behavior | Operational definition |
|---|---|
| Contact | The ant touches any part of the body of the victim, using antennae |
| Digging | The ant stands in front of the victim and repels sand backward, using legs |
| Pulling | The ant grabs any part of the body of the victim and drags it backward, using mandibles |
| Sand transport | The ant picks up a pebble near the victim and moves it away, using mandibles |
| Snare biting | The ant tugs on the nylon thread holding the victim, using mandibles |
Statistics
Statistical analyses were performed using the statistical programming language R (R Core Team 2020). We used generalized linear mixed models (lme4 package) with binomial distribution, logit link function and 2 factors, including random “colony” (N versus S) and fixed “type of test” (F versus M1 versus M5 versus NM1 versus NM5). Colonies “N” and “S” refer to the parts of the Błędowska Desert from where they have been collected, that is, northern and southern part, respectively. Types of test include “F,” which refers to the field entrapment tests, and other 4 types, which refer to laboratory entrapment tests. “M” and “NM” refer to marked and unmarked substrate and the numbers “1” and “5” refer to the number of freely moving workers (potential rescuers) in the test type. These models were used to compare the number of tests with contact with the victim (1—contact, 0—no contact) and to compare the number of tests with any rescue actions (1—rescue, 0—no rescue). We used a similar model to compare whether the number of rescuers differed between tests (1—more than 1 rescuer, 0—1 rescuer), but in this analysis, M1 and NM1 tests were not included, as there was no possibility for more than 1 rescuer to occur in those tests (levels of “type of test” included only F versus M5 versus NM5). Furthermore, we used generalized linear mixed models (lme4 package) with Poisson distribution, log link function and 2 factors, random “colony” (N versus S) and fixed “type of test” (F versus M1 versus M5 versus NM1 versus NM5), to compare the duration of (the number of intervals with) contact and rescue. Finally, we used generalized zero inflated linear mixed models (glmmTMB package) with Poisson distribution, log link function, and 2 factors, random “colony” (N versus S) and fixed “type of test” (F versus M1 versus M5 versus NM1 versus NM5), to compare the durations of (the number of intervals with) rescue categories. Models were chosen based on deviations from normality and homogeneity of variance (stats package) and zero inflation (performance package). In all models, we performed post hoc Tukey comparisons for “type of test” (emmeans package). All figures were produced in R as visualizations of model outputs (sjPlot package).
Results
Contact probability differed between test types (χ2 = 31.34, P < 0.001). In the field, the probability of contact was as high as ∼90% (Figure 2). Only the laboratory test with the use of 1 potential rescuer on marked substrate (the M1 test type) showed a clearly lower probability of contact (ca. 70%). A more detailed analysis revealed that among tests with at least 1 contact with the victim, the duration of contact and the number of intervals with contact depended on the type of test (χ2 = 5074.10, P < 0.001, χ2 = 377.95, P < 0.001, respectively) and were both lowest in the M1 test type (Figure 3). Indeed, contact duration lasted for about 2 min in the field, but only for about 40 s in the M1 tests, with in-between durations in the other test types (Figure 3A). The analysis of the number of intervals with contact revealed a very similar pattern (Figure 3B).
Figure 2.
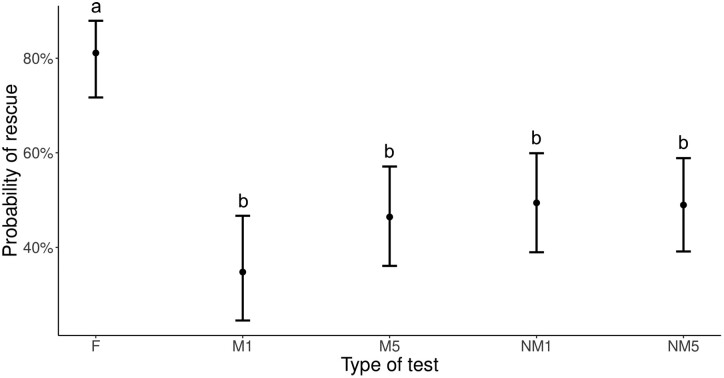
Probability of contact in different types of tests (field entrapment tests, “F,” and 4 types of laboratory entrapment bioassays). See Table 1 for differences between the “M1,” “M5,” “NM1,” and “NM5” tests. Dots represent model predictions and whiskers indicate estimated 95% confidence intervals. Small letters above upper whiskers indicate significance with Tukey’s post hoc contrasts after Bonferroni correction < 0.0125.
Figure 3.
Duration of contacts (A) and number of intervals with contact (B) in different types of tests (field entrapment tests, “F,” and 4 types of laboratory entrapment bioassays). See Table 1 for differences between the “M1,” “M5,” “NM1,” and “NM5” tests. Dots represent model predictions and whiskers indicate estimated 95% confidence intervals. Small letters above upper whiskers indicate significance with Tukey’s post hoc contrasts after Bonferroni correction < 0.0125.
Rescue probability differed between test types (χ2 = 42.92, P < 0.001) in such a way that all 4 types of laboratory tests had lower probabilities of eliciting rescue (with a mean below 50%) than the field tests (∼80%) (Figure 4). In those tests in which rescue was observed, the number of rescuers only exceeded one occasionally, although there were significant differences between test types (χ2 = 18.92, P < 0.001). In the field, the mean probability of observing more than 1 rescuer was lower than 50%, but in the 2 laboratory tests with the use of 5 potential rescuers, it was below 20% (Figure 5). Furthermore, latency to the first episode of rescue differed between test types (χ2 = 2555.00, P < 0.001) and was highest for those conducted on unmarked substrates (the NM1 and NM5 test types). Indeed, in their case, the mean latency was over 2 min, whereas in the field in was below 1 min, with in-between results in tests conducted on marked substrates (the M1 and M5 test types) (Figure 6). The results for the duration of rescue and the number of intervals with rescue were consistent in the detected differences between test types (χ2 = 3078.20, P < 0.001, χ2 = 354.02, P < 0.001, respectively, Figure 7), namely, being highest in the field tests (with a mean of almost 2 min and over a half of all intervals). Among laboratory tests, the duration of rescue and the number of intervals with rescue were closest to that in the M5 test with the use of 5 potential rescuers on marked substrate (with a mean of almost 1 min and about 9 intervals). The other test types yielded poorer results, that is, even lower rescue durations and numbers of intervals with rescue (Figure 7).
Figure 4.
Probability of rescue in different types of tests (field entrapment tests, “F,” and 4 types of laboratory entrapment bioassays). See Table 1 for differences between the “M1,” “M5,” “NM1,” and “NM5” tests. Dots represent model predictions and whiskers indicate estimated 95% confidence intervals. Small letters above upper whiskers indicate significance with Tukey’s post hoc contrasts after Bonferroni correction < 0.0125.
Figure 5.
Probability of observing more than 1 rescuer in different types of tests (field entrapment tests, “F,” and 2 types of laboratory entrapment bioassays). See Table 1 for differences between the “M5” and “NM5” tests. Dots represent model predictions and whiskers indicate estimated 95% confidence intervals. Small letters above upper whiskers indicate significance with Tukey’s post hoc contrasts after Bonferroni correction < 0.0250.
Figure 6.
Latency to first episode of rescue in different types of tests (field entrapment tests, “F,” and 4 types of laboratory entrapment bioassays). See Table 1 for differences between the “M1,” “M5,” “NM1,” and “NM5” tests. Dots represent model predictions and whiskers indicate estimated 95% confidence intervals. Small letters above upper whiskers indicate significance with Tukey’s post hoc contrasts after Bonferroni correction < 0.0125.
Figure 7.
Duration of rescue (A) and number of intervals with rescue (B) in different types of tests (field entrapment tests, “F,” and 4 types of laboratory entrapment bioassays). See Table 1 for differences between the “M1,” “M5,” “NM1,” and “NM5” tests. Dots represent model predictions and whiskers indicate estimated 95% confidence intervals. Small letters above upper whiskers indicate significance with Tukey’s post hoc contrasts after Bonferroni correction < 0.0125.
Rescue categories (digging, pulling, sand transport, and snare biting) in terms of both the duration and number of intervals all depended on the type of test (Table 3 and Figure 8). Digging and pulling behaviors were most pronounced in the field and their levels seemed to be closest in the M5 test type conducted in the laboratory with the use of 5 potential rescuers on marked substrate (Figure 8). Sand transport was generally very rare in all types of tests (Figure 8). Interestingly, snare biting seemed even more pronounced in the M5 test type in the laboratory than in the field tests (Figure 8). In the analysis of rescue categories, differences between test types were more visible when analyzed in terms of the duration than number of intervals. In other words, the duration was a better measure than the number of intervals for the fine-scale analysis of behavioral categories. This was especially clear for snare biting, in which there were differences between types of tests in the duration (highest in the M5 test type, lower in the F and NM1 test types, and lowest in the M1 and NM5 test types, Figure 8) but less so in the number of intervals (similar in the F, M1, M5, and NM1 test types and lower in the NM5 test type, Figure 8).
Table 3.
Results of the models for the duration of (the number of intervals with) rescue categories depending on the test type (F versus M1 versus M5 versus NM1 versus NM5)
| Behavior | Procedure | χ 2 | P-value |
|---|---|---|---|
| Digging | Time recording | 497.01 | < 0.001 |
| Interval recording | 98.73 | < 0.001 | |
| Pulling | Time recording | 1836.90 | < 0.001 |
| Interval recording | 242.60 | < 0.001 | |
| Sand transport | Time recording | 9.84 | 0.043 |
| Interval recording | 26.45 | < 0.001 | |
| Snare biting | Time recording | 179.26 | < 0.001 |
| Interval recording | 24.53 | <0.001 |
Figure 8.
Duration of selected rescue categories (A–D) and number of intervals within rescue categories (E–H) in different types of tests (field entrapment tests, “F,” and 4 types of laboratory entrapment bioassays). See Table 1 for differences between the “M1,” “M5,” “NM1,” and “NM5” tests. Dots represent model predictions and whiskers indicate estimated 95% confidence intervals. Small letters above upper whiskers indicate significance with Tukey’s post hoc contrasts after Bonferroni correction < 0.0125.
Discussion
Our results reveal for the first time the similarities and differences in rescue actions elicited in the field and various laboratory tests conducted on ants. We compared different versions of the entrapment bioassay, considered to be a highly universal test of rescue behavior in ants (Hollis and Nowbahari 2013; Miler et al. 2017b). Our first hypothesis, that 1) rescue actions would be more pronounced in the field than in laboratory tests, was confirmed. This illustrates the difficulty of reproducing appropriate rescue contexts in the laboratory. Our second hypothesis, that 2) laboratory tests would yield overall similar rescue actions, was not confirmed. Although we expected considerable similarities between the results of the different laboratory test types, and these results were indeed always closer to each other than to those conducted in the field, we detected some marked differences. Our third hypothesis, that 3) coding procedures would not severely affect the conclusions, was only partly confirmed. On the one hand, major issues of interest, such as the assessment of the contact and rescue proneness in different types of tests, were generally unaffected by the coding procedure. On the other hand, however, conclusions on a fine-scale analysis of different rescue categories differed depending on the procedure, with generally higher resolution of the analysis focused on duration rather than on intervals (and, thus, more detailed conclusions in the former case).
In this study, we measured contact with the victim and examined its importance in the context of later elicitation of rescue behavior. Laboratory tests with the use of 1 potential rescuer on marked substrate (the M1 test type) were least effective in eliciting contact with the victim (Figure 2). Moreover, contacts within this M1 test, expressed as either the duration or number of intervals, differed the most from contacts in the field (Figure 3). It is reasonable to expect that contact generally impacts the occurrence of rescue because it is a prerequisite of this more advanced behavior (Taylor et al. 2013; Silberberg et al. 2014). However, the probability of rescue was much lower in all laboratory tests compared with the field (Figure 4). Indeed, this probability in the laboratory was approximately 50%, which was in line with earlier results obtained on F. cinerea ants (Miler and Kuszewska 2017) and much lower than that in the field (∼80%). This general difference between the field and the laboratory might stem from the fact that the field study, in contrast to artificial conditions, provides many more natural environmental cues and close proximity of the whole nest, leading to a more natural behavior and stimulation of risky behavior (Czechowski et al. 2002, 2012; Hollis and Nowbahari 2013). Based on these results alone, it is obvious that whenever possible, field tests should be preferred when studying ant rescue behavior. Of note, however, the present results do not mean that laboratory tests are useless. All types of tests triggered rescue actions and may be suitable for many studies on rescue behavior. In general, however, tests might be enhanced under laboratory conditions by, for example, collecting full ant colonies (including queen and brood), which would provide more natural conditions and probably intensify the occurrence of rescue behaviors (Nowbahari et al. 2009).
It was previously suggested that at least 5 nest mates (potential rescuers) must be present to trigger any rescue action in the laboratory (Nowbahari et al. 2009, 2012). Our data showed that, even in tests with multiple potential rescuers, the number of ants actually performing rescue was rarely more than 1, even in the field (Figure 5). Notably, the maximum number of active rescuers did not exceed 3 in the laboratory and 5 in the field, although more than 1 rescuer in any test was rare. One possible explanation for this is related to energy limitations as ants might have evolved ways to prevent the elicitation of potentially costly rescue behaviors simultaneously in many individuals (Nowbahari and Hollis 2010). In many cases, we observed active rescuers to cease their rescue activity during a test to interact with other freely moving workers, possibly exchanging signals and changing behavior of both the rescuer and the other surroundings ants (Mallon and Franks 2000; Pratt et al. 2001; McLeman et al. 2002).
Of note, when considering latency to the first episode of rescue in our study, it clearly depended on the familiarity of the substrate (Figure 6). Marked substrate enabled ants to identify the rescue context faster and react appropriately to the trapped individual. Also, when analyzing more detailed data, that is, the duration of rescue and the number of intervals with rescue, the laboratory test with the use of 5 potential rescuers on marked substrate (the M5 test type) differed the least from that performed in the field (Figure 7). Thus, the use of laboratory tests performed on familiar substrates and/or with the use of several potential rescuers should be considered in further studies especially if, for instance, the total number of tests to be performed is to be low and cannot be increased. Doing so might facilitate rescue observations, even in its more advanced forms, such as pulling or snare biting (Figure 8).
Snare biting is considered to be precision rescue, that is, the ability to target the object holding the victim directly (Nowbahari et al. 2009, 2012). Indeed, a study by Hollis and Nowbahari (2013) showed that some species can perform rescue actions with digging, sand transport, and pulling, but not with precision rescue behavior. These differences between species suggest that rescue behavior categories (or at least precision rescue behavior) are not necessarily common among ants. Indeed, ant species may differ strikingly in their rescue behavior. For instance, ants show division of labor, influenced by age and/or body size (Hölldobler and Wilson 1990), and different species might differ in the type of workers responding to rescue-associated stimuli. As pointed out by Nowbahari et al. (2012), temporal polyethism can regulate the expression of rescue behavior. In Cataglyphis cursor ants, which are characterized by this type of polyethism, older foragers both rescue and are being rescued more frequently than younger nurses. However, polyethism is not present in all species of ants (Traniello 1978), and for those in which it occurs, it is diverse in its forms (Hölldobler and Wilson 1990). Similarly, rescue in ants can be under genetic control (Andras et al. 2020). Apparently, in C. cursor ants, 34% of the variation in propensity for rescue behavior is explained by paternity. This means that the relatedness of workers within a colony, which often differs drastically between ant species, might affect the ease of rescue elicitation. Indeed, Nowbahari et al. (2009) showed that the behavior of C. cursor rescuers is preferentially aimed at providing help to more related individuals. This means that multifaceted analyses of rescue actions in ants are indispensable.
Here, we also indicate the need for careful interpretation of results depending on the examined behavioral component of rescue (Figure 8). In this context, we found pronounced differences between both the test types and coding procedures. In the case of coding procedures, differences between test types in some behaviors were more difficult to detect when measured by interval coding procedure than by time coding procedure (compare, e.g., differences between tests in digging when analyzed via time duration, Figure 8A, and interval duration, Figure 8E, or differences between tests in pulling when analyzed via time duration, Figure 8B and interval duration, Figure 8F). When analyzing the results, one has to keep in mind to fit the resolution of the coding method to the amount of gathered data. In the case of test types, on the other hand, in line with what was mentioned above, the M5 test type with the use of 5 potential rescuers on marked substrate was closest to field tests in behavioral categories of digging, pulling, and snare biting (Figure 8). It needs to be stressed that choosing a category to study is highly important and may significantly affect the conclusions. In different test types, the expression of behavioral categories may differ. Moreover, the detected behavioral categories may differ between species. Here, for example, the most common behavior was pulling the victim (Figure 8), which was in line with previous results obtained on F. cinerea ants (Miler 2016). Interestingly, in the case of C. cursor, digging around the victim is considered to be most common rescue category (Duhoo et al. 2017). In a further study, it would be interesting to examine the differences in the intensity of behavioral categories in different ant species more closely. Such differences may suggest that rescue actions evolved in various contexts, depending on the selective pressure of the environment and ecology of given species (Hollis and Nowbahari 2013).
Rescue behavior plausibly plays an essential role in ecological success of ants by increasing individual survival and, ultimately, benefiting the ant colony. However, studies assessing the benefits of rescue actions in ants are generally scarce (Frank et al. 2017; Kwapich and Hölldobler 2019). As mentioned in the introduction, rescue actions at least in some cases decrease mortality of individuals (Frank et al. 2017). How important each and every individual is for the colony is demonstrated by Kwapich and Hölldobler (2019), which calculated that the loss of 5 individuals a day in a colony of V. pergandei seed harvester ants causes the loss of 65,700 seeds per year. This suggests that rescue actions translate into real benefits. Here, during field entrapment tests, F. cinerea ants carrying resources (i.e., nest material or prey item) were observed several times to abandon it and start a rescue action of the entrapped nest mate (F. Turza, personal observation). Attempts to measure the benefits of rescuing a nest mate in F. cinerea would contribute to a better understanding of its generally high propensity for rescue behavior.
In further research, it would be interesting to examine rescue propensity in the field, depending on how far away from the nest the entrapped individual needs help. This would supplement our current suggestions that field studies yield highest probabilities of rescue actions due to familiarity of the surroundings. Such research could also compare rescue propensity on and off the foraging trails (see Kwapich and Hölldobler 2019) or in areas with a few or many nest mates. In addition, connection between rescue and activity cycle seems worthwhile of investigation too (Fujioka et al. 2017; Kay et al. 2018). It could provide information on the specificity of rescue behavior, which may contribute to a better understanding of the unknown variability in the expression of such behavior among Formicidae (Hollis and Nowbahari 2013; Miler et al. 2017b).
Data from experiments utilizing laboratory entrapment tests (Nowbahari et al. 2009, 2012, 2016; Duhoo et al. 2017; Miler and Kuszewska 2017; Miler et al. 2017a, 2017b; Andras et al. 2020) may strongly underestimate rescue proneness in comparison to field conditions. Therefore, regarding ant species tested only in the laboratory, that showed no rescue behavior, such as Camponotus korthalsiae, Anoplolepis gracilipes, and Myrmica ruginodis (Miler et al. 2017b), or that showed low-level rescue behavior, such as Camponotus aethiops (Nowbahari et al. 2016), Formica polyctena, and Iridomyrmex anceps (Miler et al. 2017b), their inability to perform rescue actions should be considered carefully. Even species showing high levels of rescue behavior in laboratory tests, such as C. cursor (Nowbahari et al. 2009, 2012, 2016; Duhoo et al. 2017; Andras et al. 2020) and F. cinerea (Miler and Kuszewska 2017; Miler et al. 2017a, 2017b), can potentially be even more prone to express rescue behavior than so far thought. Overall, the choice of a test variant in a study should be carefully considered depending on the specifics of the research questions. Here, we agree with Duhoo et al. (2017) that ants, despite common belief, are not “the hardwired reflex automatons” and might be highly sensitive to the behavioral context. Our data support a growing number of studies showing that methods of assessing animal behavior, even if differing only slightly, may lead to significantly different results (Ovadia et al. 2001; Watanabe 2012; Scharf and Martin 2013).
Authors’ Contributions
K.M. conceived the study. F.T. and K.M. designed the study, analyzed the data, and wrote the manuscript. F.T. collected the data.
Data Availability
The datasets generated and analyzed during the current study are available in the Supplementary Excel file.
Funding
The study was funded by the National Science Centre, Poland [grant PRELUDIUM number 2018/31/N/NZ8/02312].
Conflict of Interest
The authors declare no conflict of interests.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
Supplementary Material
Contributor Information
Filip Turza, Institute of Environmental Sciences, Faculty of Biology, Jagiellonian University, Gronostajowa 7, Kraków 30-387, Poland.
Krzysztof Miler, Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska 17, Kraków 31-016, Poland.
References
- Andras JP, Hollis KL, Carter KA, Couldwell G, Nowbahari E, 2020. Analysis of ants’ rescue behavior reveals heritable specialization for first responders. J Exp Biol 223:jeb212530. [DOI] [PubMed] [Google Scholar]
- Bartal IB-A, Decety J, Mason P, 2011. Empathy and pro-social behavior in rats. Science 334:1427–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt T, 1874. The Naturalist in Nicaragua. London: John Murray. [Google Scholar]
- Blum MS, Warter SL, 1966. Chemical releasers of social behaviour. VII. The isolation of 2-heptanone from Conomyrma pyramica (Hymenoptera: Formicidae: Dolichoderinae) and its modus operandi as a releaser of alarm and digging behaviour. Ann Entomol Soc Am 59:774–779. [Google Scholar]
- Bolton B, 2020. AntWeb: Ants of Bolton World Catalog. Available from https://www.antweb.org/project.do?name=worldants.
- Calisi RM, Bentley GE, 2009. Lab and field experiments: Are they the same animal? Horm Behav 56:1–10. [DOI] [PubMed] [Google Scholar]
- Czechowski W, Godzińska EJ, Kozłowski MW, 2002. Rescue behavior shown by workers of Formica sanguinea Latr., F. fusca L. and F. cinerea Mayr (Hymenoptera: Formicidae) in response to their nestmates caught by an ant lion larva. Ann Zool 52:423–431. [Google Scholar]
- Czechowski W, Pisarski B, 1992. Laboratory methods for rearing ants (Hymenoptera, Formicoidea). Memorabilia Zool 45:1–32. [Google Scholar]
- Czechowski W, Radchenko A, Czechowska W, 2012. The Ants (Hymenoptera, Formicidae) of Poland. Warsaw: Museum and Institute of Zoology PAS. [Google Scholar]
- Duhoo T, Durand J-L, Hollis KL, Nowbahari E, 2017. Organization of rescue behaviour sequences in ants Cataglyphis cursor reflects goal-directedness, plasticity and memory. Behav Process 139:12–18. [DOI] [PubMed] [Google Scholar]
- Frank ET, Schmitt T, Hovestadt T, Mitesser O, Stiegler J et al. , 2017. Saving the injured: Rescue behavior in the termite-hunting ant Megaponera analis. Sci Adv 3:e160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friard O, Gamba M, 2016. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. [Google Scholar]
- Fujioka H, Abe MS, Fuchikawa T, Tsuji K, Shimada M et al. , 2017. Ant circadian activity associated with brood care type. Biol Lett 13:20160743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangartner W, 1969. Carbon dioxide, a releaser for digging behavior in Solenopsis geminata (Hymenoptera: Formicidae). Psyche 76:58–67. [Google Scholar]
- Hammers M, Brouwer L, 2017. Rescue behaviour in a social bird: Removal of sticky ‘bird catcher tree’ seeds by group members. Behaviour 154:403–411. [Google Scholar]
- Heyman Y, Shental N, Brandis A, Hefetz A, Feinerma O, 2017. Ants regulate colony spatial organization using multiple chemical road-signs. Nat Commun 8:15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO, 1990. The Ants. Cambridge: Harvard University Press. [Google Scholar]
- Hollis KL, Nowbahari E, 2013. A comparative analysis of precision rescue behavior in sand-dwelling ants. Anim Behav 85:537–544. [Google Scholar]
- Kay J, Menegazzi P, Mildner S, Roces F, Helfrich-Förster C, 2018. The circadian clock of the ant Camponotus floridanus is localized in dorsal and lateral neurons of the brain. J Biol Rhythms 33:255–271. [DOI] [PubMed] [Google Scholar]
- Kwapich CL, Hölldobler B, 2019. Destruction of spiderwebs and rescue of ensnared nestmates by a granivorous desert ant Veromessor pergandei. Am Nat 194:395–404. [DOI] [PubMed] [Google Scholar]
- Lafleur LJ, 1940. Helpfulness in ants. J Comp Psychol 30:23–29. [Google Scholar]
- Nowbahari E, Hollis KL, 2010. Rescue behavior: Distinguishing between rescue, cooperation, and other forms of altruistic behavior. Commun Integr Biol 3:1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowbahari E, Hollis KL, Durand J, 2012. Division of labor regulates precision rescue behavior in sand-dwelling Cataglyphis cursor ants: To give is to receive. PLoS ONE 7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowbahari E, Scohier A, Durand J, Hollis KL, 2009. Ants Cataglyphis cursor use precisely directed rescue behavior to free entrapped relatives. PLoS ONE 4:e6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowbahari E, Amirault C, Hollis KL, 2016. Rescue of newborn ants by older Cataglyphis cursor adult workers. Anim Cogn 19:543–553. [DOI] [PubMed] [Google Scholar]
- Mallon EB, Franks NR, 2000. Ants estimate area using Buffon’s needle. Proc R Soc B 267:765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markl H, 1965. Stridulation in leaf-cutting ants. Science 149:1392–1393. [DOI] [PubMed] [Google Scholar]
- Martin P, Bateson P, 2007. Measuring Behavior: An Introductory Guide. Cambridge: Cambridge University Press. [Google Scholar]
- McLeman MA, Pratt SC, Franks NR, 2002. Navigation using visual landmarks by the ant Leptothorax albipennis. Insect Soc 49:203–208. [Google Scholar]
- Miler K, Kuszewska K, 2017. Secretions of mandibular glands are not involved in the elicitation of rescue behaviour in Formica cinerea ants. Insect Soc 64:303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miler K, 2016. Moribund ants do not call for help. PLoS ONE 11:e0151925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miler K, Symonowicz B, Godzińska EJ, 2017a. Increased risk proneness or social withdrawal? The effects of shortened life expectancy on the expression of rescue behavior in workers of the ant Formica cinerea (Hymenoptera: Formicidae). J Insect Behav 30:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miler K, Yahya BE, Czarnoleski M, 2017b. Pro-social behaviour of ants depends on their ecological niche: Rescue actions in species from tropical and temperate regions. Behav Proc 144:1–4. [DOI] [PubMed] [Google Scholar]
- Ovadia O, Ziv Y, Abramsky Z, Pinshow B, Kotler B, 2001. Harvest rates and foraging strategies in Negev Desert gerbils. Behav Ecol 12:219–226. [Google Scholar]
- Pennisi E, 2005. How did cooperative behavior evolve? Science 309:93. [DOI] [PubMed] [Google Scholar]
- Pratt SC, Brooks SE, Franks NR, 2001. The use of edges in visual navigation by the ant Leptothorax albipennis. Ethology 107:1125–1136. [Google Scholar]
- R Core Team, 2020. R: A Language and Environment for Statistical Computing. Vienna (Austria: ): R Foundation for Statistical Computing. Available from https://www.R-project.org. [Google Scholar]
- Siebenaler JB, Caldwell DK, 1956. Cooperation among adult dolphins. J Mammal 37:126–128. [Google Scholar]
- Silberberg A, Allouch C, Sandfort S, Kearns D, Karpel H et al. , 2014. Desire for social contact, not empathy, may explain “rescue” behavior in rats. Anim Cogn 17:609–618. [DOI] [PubMed] [Google Scholar]
- Silva PRR, Silva RH, Lima RH, Meurer YS, Ceppi B et al. , 2020. Are there multiple motivators for helping behavior in rats? Front Psychol 11:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler HG, Kansas J, 1968. Stimuli releasing digging behavior in the western harvester ant (Hymenoptera: Formicidae). J Kans Entomol Soc 41:318–323. [Google Scholar]
- Scharf I, Martin OY, 2013. Same-sex sexual behavior in insects and arachnids: Prevalence, causes, and consequences. Behav Ecol Sociobiol 67:1719–1730. [Google Scholar]
- Taylor K, Visvader A, Nowbahari E, Hollis KL, 2013. Precision rescue behavior in North American ants. Evol Psychol 11:665–677. [Google Scholar]
- Traniello JF, 1978. Caste in a primitive ant: Absence of age polyethism in amblyopone. Science 202:770–772. [DOI] [PubMed] [Google Scholar]
- Turza F, Zuber G, Bzoma M, Prus M, Filipiak M et al. , 2020. Ants co-occurring with predatory antlions show unsuccessful rescue behavior towards captured nestmates. J Insect Behav 33:1–6. [Google Scholar]
- Vasconcelos M, Hollis K, Nowbahari E, Kacelnik A, 2012. Pro-sociality without empathy. Biol Lett 8:910–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel ER, Fuentes-Jiménez A, 2006. Rescue behavior in white-faced capuchin monkeys during an intergroup attack: Support for the infanticide avoidance hypothesis. Am J Primatol 68:1012–1016. [DOI] [PubMed] [Google Scholar]
- Watanabe NM, Stahlman WD, Blaisdell AP, Garlick D, Fast CD et al. , 2012. Quantifying personality in the terrestrial hermit crab: different measures, different inferences. Behav Proc 91 133–140. [DOI] [PubMed] [Google Scholar]
- Wilson EO, 1958. A chemical releaser of alarm and digging behavior in the ant Pogonomyrmex badius (Latreille). Psyche 65:41–51. [Google Scholar]
- Uy FMK, Adcock JD, Jeffries SF, Pepere E, 2018. Intercolony distance predicts the decision to rescue or attack conspecifics in weaver ants. Insect Soc 66:1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available in the Supplementary Excel file.