Abstract
Background/Aim
The aim of this study was to investigate the outcomes of atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma (HCC), including those with disease refractory to lenvatinib, in clinical practice.
Patients and Methods
Of 34 patients treated with atezolizumab plus bevacizumab, a total of 23, including 16 with lenvatinib failure, were enrolled in this retrospective study. The adverse events, changes in liver function and antitumor responses at 6 weeks after starting therapy were evaluated.
Results
The incidence of grade 3 adverse events was low, at 13.0%. Albumin–bilirubin scores did not worsen at 3 and 6 weeks compared to baseline. The objective response rate and disease control rate at 6 weeks were 17.4% and 78.3% according to Response Evaluation Criteria in Solid Tumors (RECIST), and 30.4% and 78.3% according to modified RECIST, respectively.
Conclusion
Our results suggest that atezolizumab plus bevacizumab might have potential therapeutic safety and efficacy in patients with advanced HCC, including those with disease refractory to lenvatinib. Further studies are needed to confirm the outcomes of atezolizumab plus bevacizumab after lenvatinib failure.
Keywords: Atezolizumab, bevacizumab, hepatocellular carcinoma, lenvatinib, liver function
In 2020, based on the positive results of the IMbrave150 trial (1), atezolizumab (an antibody to programmed cell death ligand 1) plus bevacizumab (an antibody to vascular endothelial growth factor A) has been approved as a new first-line treatment for advanced hepatocellular carcinoma (HCC) (2-5). Before this combination treatment became available, clinical practice guidelines for HCC management recommended both sorafenib and lenvatinib as the standard first-line systemic therapy for advanced HCC (6,7). Lenvatinib has been increasingly administered in real-world practice because the REFLECT trial showed significantly better antitumor efficacy and progression-free survival with lenvatinib than sorafenib (8-12). However, at present, no effective second-line therapy has been established after failure of lenvatinib (13,14). The clinical outcomes of atezolizumab plus bevacizumab for HCC in a real-world setting, including in patients with lenvatinib failure, are unknown. The aim of this study was to investigate the safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice.
Patients and Methods
Patients. According to the inclusion criteria of the IMbrave150 trial (1), atezolizumab plus bevacizumab treatment was initiated for patients with a HCC stage that was equivalent to Barcelona Clinic Liver Cancer stage C or B and who were not eligible for surgical resection or locoregional therapy, and those with Eastern Cooperative Oncology Group performance status scores of 0 or 1 and Child-Pugh class A. Between October 2020 and February 2021, 34 consecutive patients with advanced HCC were initiated on atezolizumab plus bevacizumab at our hospital. Patients who were observed for <6 weeks were excluded (n=11). The remaining 23 patients were enrolled in this study and underwent retrospective evaluation of their outcomes. The protocol of this study was approved by the Ethics Committee of Fujita Health University School of Medicine (HM17-152) and the study was carried out in compliance with the 1975 Declaration of Helsinki.
Atezolizumab plus bevacizumab treatment and evaluation of adverse events and changes in liver function. All patients received 1200 mg of atezolizumab plus 15 mg bevacizumab/kg body weight intravenously every 3 weeks. Adverse events (AEs) were evaluated according to the Common Terminology Criteria for Adverse Events version 5.0 (15). When drug-related AEs occurred, temporary interruption of both atezolizumab and bevacizumab or either alone was performed until the symptoms improved to grade 1 or 2, according to the guidelines provided by the manufacturer. Atezolizumab plus bevacizumab treatment was continued until the occurrence of potentially fatal AEs or until clinical tumor progression. For evaluation of changes in liver function, the albumin–bilirubin (ALBI) score (16) was investigated at baseline and at 1, 2, 3, and 6 weeks after starting therapy.
Evaluation of antitumor responses. Antitumor responses were evaluated according to both the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (17) and modified RECIST (mRECIST) (18). Dynamic computed tomographic examination was performed according to a predetermined schedule at baseline and at 6 weeks after atezolizumab plus bevacizumab initiation, and every 4 to 10 weeks thereafter.
Statistical analysis. All statistical analyses were performed using Easy R version 1.29 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (19). Changes in ALBI scores were evaluated by the Wilcoxon signed-rank test. Differences with a p-value of less than 0.05 were considered statistically significant.
Results
Baseline patient characteristics. Table I shows the baseline characteristics of the 23 patients with HCC enrolled in this study. The study cohort comprised 18 men and five women, with a median age of 74 years (range=38-85 years). There were 21 patients with an Eastern Cooperative Oncology Group performance score of 0 and 15 patients with a Child–Pugh score of 5. The median alpha fetoprotein (AFP) level at baseline was 173.6 ng/ml (range=2.6-231724 ng/ml). Atezolizumab plus bevacizumab was initiated as first-line therapy in seven patients (30.4%), second-line therapy in 15 patients (65.2%), and third-line therapy in one patient (4.3%). All 16 patients who had previously received systemic therapy had radiologically confirmed progressive disease (PD) after lenvatinib therapy. The median observation period was 95 days (range=53-123 days).
Table I. Baseline characteristics of the patients with hepatocellular carcinoma enrolled in this study.
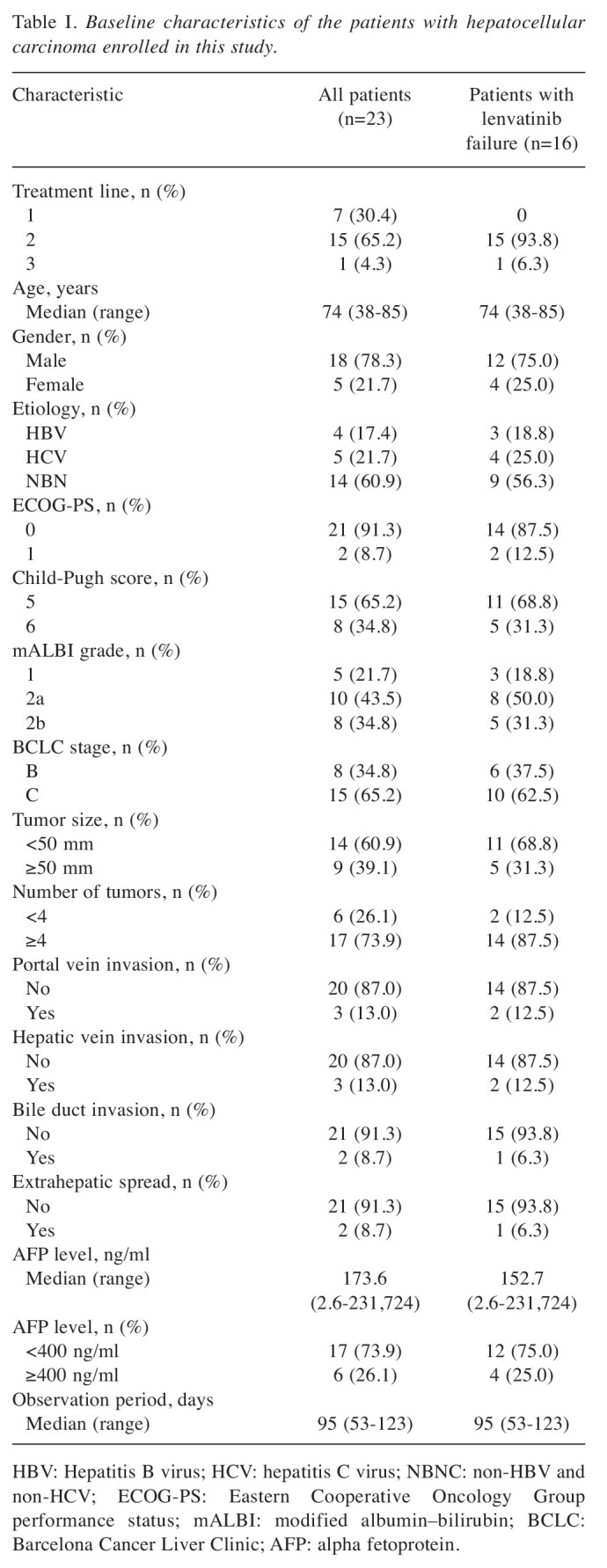
HBV: Hepatitis B virus; HCV: hepatitis C virus; NBNC: non-HBV and non-HCV; ECOG-PS: Eastern Cooperative Oncology Group performance status; mALBI: modified albumin–bilirubin; BCLC: Barcelona Cancer Liver Clinic; AFP: alpha fetoprotein.
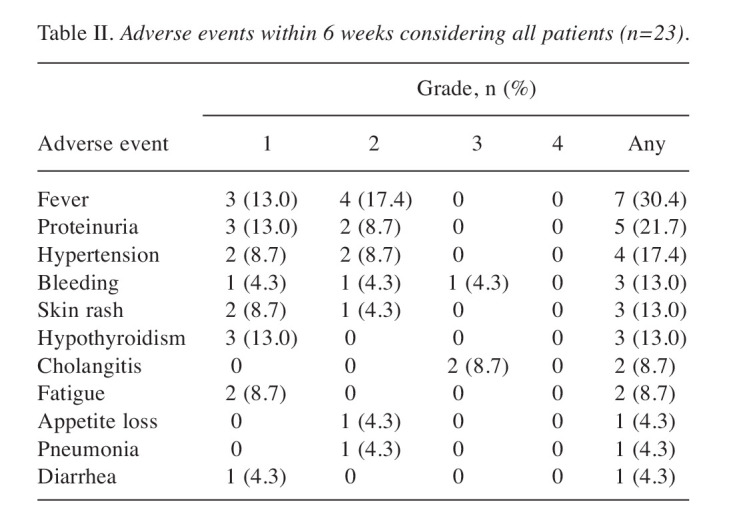
Safety. Table II shows the frequency of AEs within 6 weeks after atezolizumab plus bevacizumab initiation. In all 23 patients, the most common AEs were fever, proteinuria, hypertension, bleeding, skin rash and hypothyroidism. The incidence of grade 3 AEs within 6 weeks was 13.0% (n=3) [including acute obstructive cholangitis due to bile duct invasion (n=2) and esophageal variceal bleeding (n=1)]. These three patients needed temporary interruption of both atezolizumab and bevacizumab. Two patients with cholangitis were able to resume atezolizumab and bevacizumab after improvement with endoscopic retrograde biliary drainage (ERBD) tube stent replacement, and one patient with esophageal variceal bleeding was able to resume atezolizumab after improvement with endoscopic variceal ligation. One patient who developed grade 2 pneumonia discontinued both atezolizumab and bevacizumab. Grade 4 AEs were not observed. The median treatment duration of atezolizumab plus bevacizumab was 81 days (range=41-116 days). No patient died during the observation period.
Table II. Adverse events within 6 weeks considering all patients (n=23).
Changes in the ALBI scores within 6 weeks were evaluated in 21 of the patients, excluding two patients who developed acute obstructive cholangitis due to bile duct invasion. ALBI scores (median±standard error) at baseline and at 1, 2, 3, and 6 weeks were −2.42±0.06, −2.44±0.07, −2.30±0.10, −2.33±0.10, and −2.36±0.10, respectively. Compared to ALBI scores at baseline, ALBI scores were significantly worse at 2 weeks (p=0.0156), while those at 1, 3 and 6 weeks were not. In the 15 patients with lenvatinib failure, ALBI grades (median±standard error) at baseline and at 1, 2, 3 and 6 weeks were −2.42±0.07, −2.47±0.08, −2.38±0.08, −2.34±0.11, and −2.36±0.10, respectively; there was no significant worsening of ALBI scores at each week versus baseline.
Efficacy. With regard to the antitumor response at 6 weeks according to RECIST, among the 23 patients, none of them had complete response (CR), five had partial response (PR), 14 had stable disease (SD), and five had PD. The objective response rate (ORR) was 17.4%, and the disease control rate (DCR) was 78.3%. According to mRECIST, there were one CR, six PR, eleven SD and five PD designations. The ORR was 30.4%, and the DCR was 78.3%. Among the 16 patients treated after lenvatinib failure, in terms of the antitumor response at 6 weeks according to RECIST, there were no cases with CR, two PR, 10 SD and four PD designations. The ORR was 12.5%, and the DCR was 75.0%. According to mRECIST, there were zero CR, four PR, eight SD and four PD designations. The ORR was 25.0%, and the DCR was 75.0%.
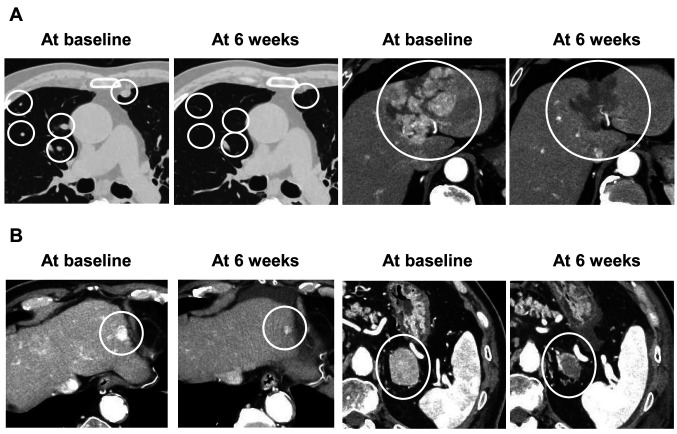
Two representative cases are shown in Figure 1. Case 1 (first-line systemic treatment) was judged as PR by RECIST and as CR by mRECIST (Figure 1A), and had an AFP level change from 21 ng/ml at baseline to 3.2 ng/ml at 6 weeks. Case 2 (second-line systemic treatment) was judged as PR by both RECIST and mRECIST (Figure 1B). His AFP level at baseline was 7.5 ng/ml (within the normal range).
Figure 1. Dynamic computed tomographic images in the late arterial phase in two representative hepatocellular carcinoma cases. A: Case 1 (firstline systemic treatment) was judged as having a partial response by Response Evaluation Criteria in Solid Tumors (RECIST) and as complete response by modified (m)RECIST at 6 weeks. B: Case 2 (second-line systemic treatment) was judged as having a partial response by both RECIST and mRECIST at 6 weeks.
Discussion
In the IMbrave150 trial, immunotherapy with a combination of atezolizumab and bevacizumab resulted in better efficacy and safety than treatment with sorafenib (1). To the best of our knowledge, ours is the first study to investigate the outcomes of atezolizumab plus bevacizumab for HCC in clinical practice including patients who had previously received systemic therapy with lenvatinib.
In the IMbrave150 trial, the frequency of grade 3 or 4 AEs of any cause was 56.5% in the atezolizumab plus bevacizumab group (1). Grade 3 or 4 hypertension of any cause occurred in 15.2% of patients in the atezolizumab plus bevacizumab group, while other high-grade AEs were infrequent. In the present study, the incidence of grade 3 AEs within 6 weeks was low, at 13.0% (n=3) (including acute obstructive cholangitis due to bile duct invasion in two and esophageal variceal bleeding in one). In the two patients with cholangitis, an ERBD tube stent was inserted for bile duct invasion prior to the initiation of atezolizumab plus bevacizumab, so the cause of acute cholangitis was thought to be ERBD tube stent problems rather than AEs from this combination therapy. In the IMbrave150 trial, the frequencies of AEs leading to interruption or withdrawal of any trial drug were 49.5% and 15.5%, respectively, in the atezolizumab plus bevacizumab group (1). Gastrointestinal disorders were reported to be the most common reason for drug discontinuation in the atezolizumab plus bevacizumab group. In particular, the frequency of upper gastrointestinal bleeding observed in the combination therapy group was 7%. In the present study, gastrointestinal bleeding occurred in two patients (8.7%). The patient with grade 3 esophageal variceal bleeding underwent endoscopic variceal ligation 5 months before the initiation of atezolizumab plus bevacizumab but variceal bleeding occurred during this combination therapy. Bleeding is a well-known side-effect of bevacizumab (20). Since upper gastrointestinal bleeding is a life-threatening AE, it is important for physicians to regularly monitor and manage varices, not only before the initiation of atezolizumab plus bevacizumab, but also during treatment.
Some reports documented deterioration of liver function in the early period after initiation of sorafenib or lenvatinib therapy (21,22). Changes in liver function of atezolizumab plus bevacizumab are unknown because the IMbrave150 trial did not document such changes. In the present study, although there was a statistically significant deterioration in ALBI scores at 2 weeks compared to baseline, they improved at 3 and 6 weeks without any special treatment. Our results indicate that atezolizumab plus bevacizumab treatment might be well tolerated with no clinical deterioration of liver function.
In terms of radiological antitumor response, the IMbrave150 trial reported confirmed ORR of 27.3% by RECIST and 33.2% by mRECIST, respectively, in the atezolizumab plus bevacizumab group (1). Similarly, our study showed that RECIST and mRECIST ORRs at 6 weeks were 17.4% and 30.4%, respectively. In the IMbrave150 trial, the CR rates were reported to be 5.5% by RECIST and 10.2% by mRECIST, respectively, in the atezolizumab plus bevacizumab group (1). The high CR rate suggests that the combination of atezolizumab and bevacizumab has the potential to cure HCC. In the present study, one patient (4.3%) was judged as exhibiting CR according to mRECIST at 6 weeks.
There are several limitations to the present study. Firstly, it was the retrospective and nonrandomized design. Secondly the sample size was small and the observation period short. Therefore, confirmation of our findings would require future large-scale prospective studies.
In conclusion, our present results suggest that atezolizumab plus bevacizumab might have potential therapeutic safety and efficacy in patients with advanced HCC, including those whose disease is refractory to lenvatinib. Further studies are needed to confirm the outcomes of atezolizumab plus bevacizumab after lenvatinib failure.
Conflicts of Interest
Teiji Kuzuya received lecture fees from Eisai, Bayer and Eli Lilly. All the other Authors declare no competing interests.
Authors’ Contributions
Conceptualization: Teiji Kuzuya; methodology: Teiji Kuzuya; formal analysis and investigation: Teiji Kuzuya and Naoto Kawabe; data curation: Teiji Kuzuya, Naoto Kawabe, Senju Hashimoto, Ryoji Miyahara, Takuji Nakano, Kazunori Nakaoka, Hiroyuki Tanaka, Yohei Miyachi, Arisa Mii, Yoshinao Tanahashi, Yutaro Kato and Atsushi Sugioka; writing - original draft preparation: Teiji Kuzuya; writing - review and editing: Naoto Kawabe; supervision: Atsushi Sugioka and Yoshiki Hirooka. All Authors approved the final draft of the article.
References
- 1.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, IMbrave150 Investigators Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, Lencioni R, Greten TF, Kudo M, Mandrekar SJ, Zhu AX, Finn RS, Roberts LR, AASLD Panel of Experts on Trial Design in HCC Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73(Suppl 1):158–191. doi: 10.1002/hep.31327. [DOI] [PubMed] [Google Scholar]
- 3.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic Therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers (Basel) 2020;12(5) doi: 10.3390/cancers12051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo M. A new era in systemic therapy for hepatocellular carcinoma: Atezolizumab plus bevacizumab combination therapy. Liver Cancer. 2020;9(2):119–137. doi: 10.1159/000505189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address easloffice@easloffice.eu. , European Association for the Study of the Liver EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 9.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol Res. 2020;50(3):374–381. doi: 10.1111/hepr.13452. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi A, Moriguchi M, Seko Y, Ishikawa H, Yo T, Kimura H, Fujii H, Shima T, Mitsumoto Y, Ishiba H, Takashima H, Nagao Y, Jo M, Arai M, Hara T, Okajima A, Muramatsu A, Morita A, Yoshinami N, Nakajima T, Mitsuyoshi H, Umemura A, Nishikawa T, Yamaguchi K, Itoh Y. Impact of relative dose intensity of early-phase lenvatinib treatment on therapeutic response in hepatocellular carcinoma. Anticancer Res. 2019;39(9):5149–5156. doi: 10.21873/anticanres.13710. [DOI] [PubMed] [Google Scholar]
- 11.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Sorafenib vs. Lenvatinib as first-line therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Anticancer Res. 2020;40(4):2283–2290. doi: 10.21873/anticanres.14193. [DOI] [PubMed] [Google Scholar]
- 12.Chuma M, Uojima H, Hiraoka A, Kobayashi S, Toyoda H, Tada T, Hidaka H, Iwabuchi S, Numata K, Itobayashi E, Itokawa N, Kariyama K, Ohama H, Hattori N, Hirose S, Shibata H, Tani J, Imai M, Tajiri K, Moriya S, Wada N, Iwasaki S, Fukushima T, Ueno M, Yasuda S, Atsukawa M, Nouso K, Fukunishi S, Watanabe T, Ishikawa T, Nakamura S, Morimoto M, Kagawa T, Sakamoto M, Kumada T, Maeda S. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: A multicenter analysis. Hepatol Res. 2021;51(2):201–215. doi: 10.1111/hepr.13592. [DOI] [PubMed] [Google Scholar]
- 13.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Initial experience of Ramucirumab treatment after Lenvatinib failure for patients with advanced hepatocellular carcinoma. Anticancer Res. 2020;40(4):2089–2093. doi: 10.21873/anticanres.14167. [DOI] [PubMed] [Google Scholar]
- 14.Tomonari T, Sato Y, Tanaka H, Tanaka T, Taniguchi T, Sogabe M, Okamoto K, Miyamoto H, Muguruma N, Takayama T. Sorafenib as second-line treatment option after failure of lenvatinib in patients with unresectable hepatocellular carcinoma. JGH Open. 2020;4(6):1135–1139. doi: 10.1002/jgh3.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health , National Cancer Institute , U.S. Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. [Last accessed on June 20, 2020]
- 16.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 19.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79(1-2):27–38. doi: 10.1159/000314980. [DOI] [PubMed] [Google Scholar]
- 21.Terashima T, Yamashita T, Sunagozaka H, Arai K, Kawaguchi K, Kitamura K, Yamashita T, Sakai Y, Mizukoshi E, Honda M, Kaneko S. Analysis of the liver functional reserve of patients with advanced hepatocellular carcinoma undergoing sorafenib treatment: Prospects for regorafenib therapy. Hepatol Res. 2018;48(12):956–966. doi: 10.1111/hepr.13196. [DOI] [PubMed] [Google Scholar]
- 22.Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Koizumi Y, Hiasa Y, Michitaka K, On behalf of the Real-Life Practice Experts for HCC (RELPEC) Study Group , HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) Early relative change in hepatic function with Lenvatinib for unresectable hepatocellular carcinoma. Oncology. 2019;97(6):334–340. doi: 10.1159/000502095. [DOI] [PubMed] [Google Scholar]