Abstract
Background/Aim
This study aimed to investigate the effects of the combination of alkalization therapy (an alkaline diet and bicarbonate therapy) and intravenous vitamin C treatment on chemotherapy outcomes in patients with small-cell lung cancer (SCLC) (study registration: UMIN000043056).
Patients and Methods
Twelve patients with SCLC in the intervention group (receiving both alkalization therapy and vitamin C treatment together with chemotherapy) were retrospectively compared to 15 patients with SCLC in the control group (receiving chemotherapy only).
Results
The mean urine pH of the intervention group was significantly higher than that of the control group (7.32±0.45 vs. 6.44±0.74, respectively; p<0.005). The median overall survival for the intervention group was 44.2 months (95% confidence interval=22.0−not reached), as compared with 17.7 months for the control group (95% confidence intervaI=13.5−not reached; p<0.05).
Conclusion
The combination of alkalization therapy and intravenous vitamin C treatment may be associated with favorable outcomes in patients with SCLC receiving chemotherapy.
Keywords: Small-cell lung cancer, alkalization therapy, vitamin C, tumor microenvironment, urinary pH, bicarbonate
Small-cell lung cancer (SCLC) accounts for 10% to 15% of all lung cancer in Japan (1,2). For nonSCLC, treatment methods, such as molecular-targeted drugs and immune checkpoint inhibitors, have recently been developed and have substantially improved treatment outcomes. On the other hand, for the treatment of SCLC, although immune checkpoint inhibitors are used, their therapeutic effects are limited, and hence the development of more effective treatment methods is urgently required.
The acidic tumor microenvironment, which is created by the highly activated glycolysis that occurs in cancer cells, is reported to be associated with cancer progression and resistance to cancer treatments (3,4). Although in normal cells, adenosine triphosphate is usually generated via oxidative phosphorylation, cancer cells have unique characteristics of energy metabolism in which adenosine triphosphate is produced mainly using aerobic glycolysis (5,6). The pH of the tumor microenvironment is regulated by acid–base transporters, such as Na+/H+ exchangers and monocarboxylate transporters, and protons resulting from the production of lactic acid during glycolysis are exported from cancer cells, resulting in a decrease in extracellular pH and an increase in intracellular pH (4,7). The approach of neutralizing the acidic extracellular pH of cancer cells, such as by bicarbonate administration, has suggested more favorable outcomes of cancer treatment in several in vivo and in vitro studies (8,9). Our group has reported some clinical studies on the effects of alkalization therapy for cancer treatment. Firstly, in patients with advanced-stage epidermal growth factor receptor mutation-positive nonSCLC, prolonged progression-free survival (19.5 months) was achieved by a regimen of low-dose epidermal growth factor receptor tyrosine kinase inhibitor (56%±22% of the standard dosage) and an alkaline diet (eating fruit and vegetables and limiting meat and milk). We also reported that an alkaline diet increased the urine pH of patients from 6.00±0.38 to 6.95±0.55 (10). Secondly, in a retrospective study of patients with advanced pancreatic cancer undergoing alkalization therapy, a prolonged median overall survival (OS) was observed in patients with a urine pH of more than 7.0 compared with those with a urine pH of 7.0 or less (16.1 vs. 4.7 months; p<0.05). Furthermore, a prolonged median OS for the alkalization group compared with the control group (15.4 vs. 10.8 months; p<0.005), and a significantly prolonged median OS of patients with high urine pH (pH >7.0 or ΔpH >1.0, where ΔpH was the mean urine pH before alkalization therapy subtracted from the mean urine pH after alkalization therapy) for the alkalization group compared with the control group were observed in a case–control study (11,12).
In recent years, there have been many reports on intravenous vitamin C treatment for cancer therapy in clinical settings which have suggested that chemotherapy combined with intravenous vitamin C treatment may improve cancer treatment outcomes and reduce the toxicities of chemotherapy and radiation therapy (13-16). Regarding the mechanisms underlying the therapeutic effects of intravenous vitamin C treatment for cancer, it has been reported that dehydroascorbate (DHA), which is the oxidized form of vitamin C, inhibits glyceraldehyde 3-phosphate dehydrogenase and leads to anticancer effects. Furthermore, the suppression of inflammation by vitamin C in patients with cancer may improve cancer treatment effects (17,18).
Based on the above, we hypothesized that the combination of alkalization therapy and intravenous vitamin C treatment may improve outcomes of patients with SCLC being treated with chemotherapy. Therefore, we conducted a retrospective study on the chemotherapy outcomes of such patients comparing the intervention group, in which patients were treated with both alkalization therapy and intravenous vitamin C with chemotherapy, with the control group, in which patients were treated with only chemotherapy.
Patients and Methods
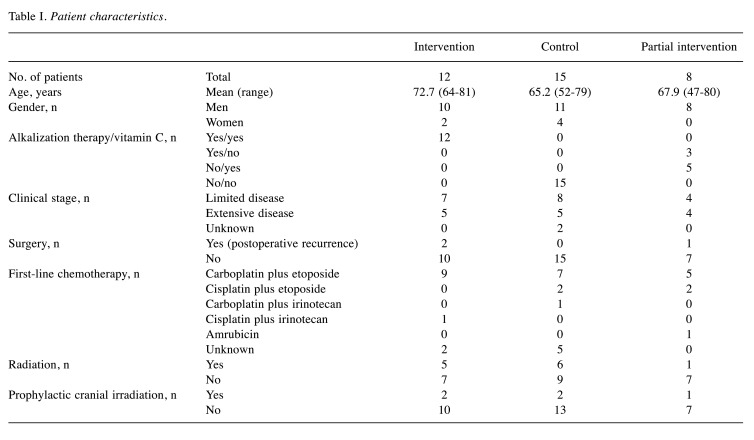
Study design. This study was retrospectively conducted to investigate the effects of the combination of alkalization therapy and intravenous vitamin C treatment on chemotherapy outcomes in patients with SCLC. Patients with SCLC who were treated at Karasuma Wada Clinic between January 1, 2011 and December 31, 2018 were retrospectively analyzed, using medical records from Karasuma Wada Clinic. Patients were divided into two groups according to alkalization therapy and intravenous vitamin C treatment, as described below. The intervention group was defined as patients with SCLC who received both alkalization therapy and intravenous vitamin C treatment together with standard chemotherapy, and the control group was defined as patients who received chemotherapy only, with no alkalization therapy or intravenous vitamin C treatment. The rest of the patients, who received only alkalization therapy or only intravenous vitamin C treatment together with standard chemotherapy, were defined as the partial intervention group. A flowchart of the study is shown in Figure 1. All procedures were performed in accordance with the ethical principles stated in the 1995 Declaration of Helsinki. Written informed consent was obtained from each patient. This study was approved by the Institutional Review Board of the Japan-Multinational Trial Organization and was registered with UMIN Clinical Trials (UMIN000043056).
Figure 1. Flowchart of this study. Flowchart showing the number of patients included in the study groups.
Intervention methods. Alkalization therapy was defined as a combination of an alkaline diet and bicarbonate therapy. An alkaline diet was meals comprising a large amount of vegetables and fruit, and minimal meat and dairy products. All patients who received alkalization therapy were instructed to take at least 400 g of fruit and vegetables a day and not to take any meat and dairy products, and they recorded their daily meals for at least the first 4 weeks from the start of the alkaline diet. Their records were reviewed to confirm whether the meals were appropriate or not by a doctor or nurse at every visit, and they were given advice according to their records, but the actual diet was decided by the patients at their homes. Patients who received alkalization therapy also received oral bicarbonate (3.0−5.0 g/day). Patients who agreed to receive vitamin C treatment were given vitamin C (25-50 g/day by infusion every 1 or 2 weeks). Patients were allowed to receive all appropriate concomitant chemotherapies in addition to the alkalization therapy and intravenous vitamin C treatment.
Assessment procedures. The OS from the time of diagnosis in the intervention group and control group were calculated. Urine pH data in both groups were also collected at the patients’ regular visits, which were at least once every 2 months, and up to twice a month.
Statistical analyses. Mean values of urine pH for each patient were calculated from all data on the pH of urine samples collected from each patient’s first visit to Karasuma Wada Clinic until March 31, 2021. Mean urine pH values were compared between the intervention group and the control group using unpaired t-test. The OS from the time of diagnosis for the intervention and the control groups was compared using Kaplan–Meier estimates. Standard deviations of mean dataset values were calculated. All p-values were two-sided and a p-value of less than 0.05 was considered to indicate a statistically significant difference between two groups. All statistical analyses were performed with EZR (version 1.54; Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface that is a modified version of R (The R Foundation for Statistical Computing, Vienna, Austria) (19); more precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Results
Patient characteristics. Fifty patients with SCLC visited Karasuma Wada Clinic between January 1, 2011 and December 31, 2018. Of these patients, 15 who visited fewer than three times were excluded. Of the 35 remaining patients, 12 were assigned to the intervention group, 15 were assigned to the control group, and eight were assigned to the partial intervention group. The mean age at diagnosis of patients in the intervention group was 72.7 years (range=64-81 years), and in the control group was 65.2 years (range=52-79). Five patients out of 12 in the intervention group, and five out of 15 in the control group had extensive disease. Two patients out of 12 in the intervention group had postoperative recurrent disease, and no patients in the control group underwent surgery. Regimens of the first-line chemotherapies that the patients received in both groups did not differ remarkably. Patient characteristics are shown in Table I.
Table I. Patient characteristics.
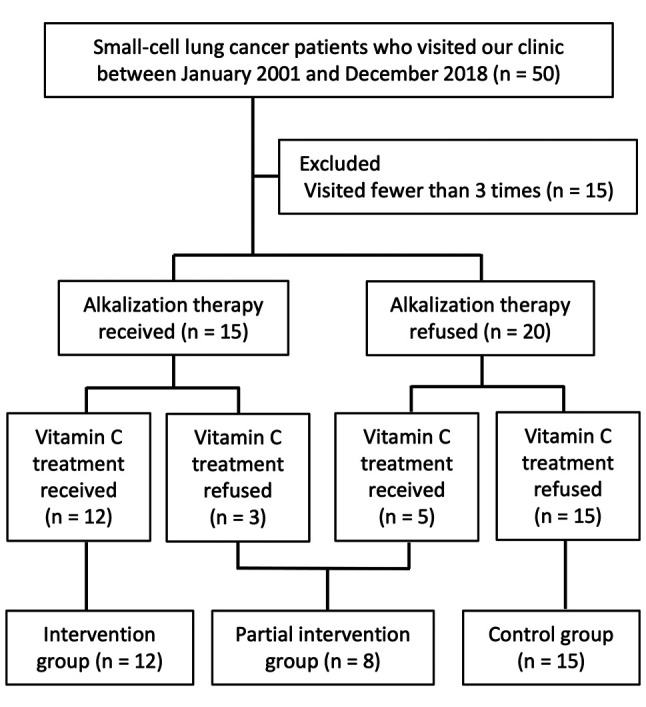
Urine pH analysis. The mean urine pH during the period from each patient’s first visit until March 31, 2021 for the intervention group and the control group are shown in Figure 2. The mean urine pH of the intervention group was significantly higher than that of the control group (7.32±0.45 vs. 6.44±0.74; p<0.005).
Figure 2. Urine pH in patients of the intervention and the control groups for the study period. The mean urine pH of the intervention group (n=12) and the control group (n=12) are shown. The lines indicate the median values, the error bars indicate the maximum and minimum values, and the boxes indicate the values between the upper and the lower quartiles.
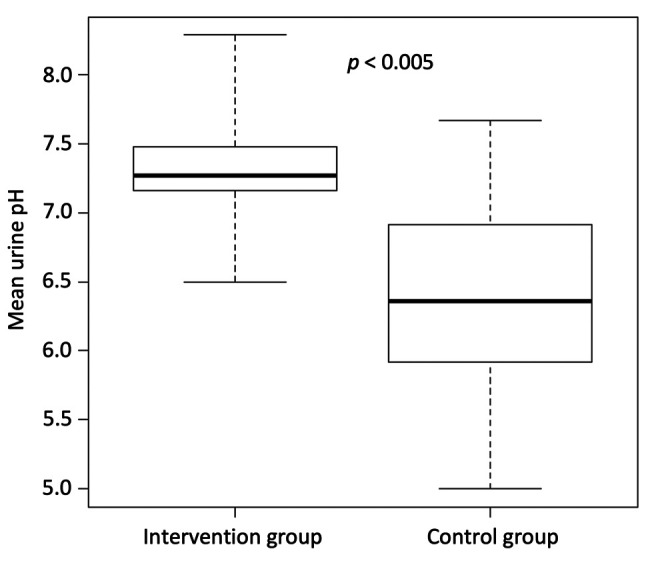
Overall survival. The median OS from the time of diagnosis for the intervention group was 44.2 months (n=12, 95% confidence interval=22.0−not reached) as compared with 17.7 months for patients in the control group (n=15, 95% confidence intervaI=13.5−not reached; p<0.05). The Kaplan–Meier curves of both groups are shown in Figure 3.
Figure 3. Overall survival of the intervention and control groups. Kaplan–Meier curves of the overall survival from diagnosis of smallcell lung cancer in patients in the intervention group and the control group are shown.
Discussion
SCLC is a highly malignant tumor with a rapid growth rate and early metastasis, and is one of the most difficult tumors to treat. The 5-year relative survival rate of patients who were diagnosed as having SCLC between 2009 and 2011 in Japan was 17.3% (1). In the present retrospective study, we demonstrated that the median OS of patients with SCLC who received a combination of alkalization therapy and intravenous vitamin C treatment together with chemotherapy was significantly longer than that of those who received only chemotherapy. The combination of alkalization therapy and intravenous vitamin C treatment may exert synergetic effects that contribute to the favorable outcomes of patients with SCLC, because the median OS was not prolonged in those who received only alkalization therapy or only intravenous vitamin C treatment together with chemotherapy. However, we were not able to compare the median OS of the partial intervention group and the control group in this study, owing to the small number of patients who received only alkalization therapy or only intravenous vitamin C treatment in addition to chemotherapy, and hence further investigation is needed.
Similar to our previous studies (10-12), a significant increase in mean urine pH was observed in patients who received alkalization therapy and intravenous vitamin C treatment compared with the control group in the present study. The effects of diet on the acid−base balance can be predicted through calculating the potential renal acid load of foods, and it is known that fruit and vegetables have an alkalizing effect, and meat and dairy products have an acidifying effect on urine pH (20). Acid from the daily diet is neutralized or ‘stored’ within the body, and also accumulates within cells, and results in a decrease in the level of serum bicarbonate (21). A clinical study has shown the safety and tolerability of the long-term consumption of bicarbonate (0.5 g/kg/day, i.e., 25 g/50 kg body weight), and bicarbonate is also known to have buffering effects and to increase urine pH levels (22). Although the effects of chemotherapeutic drugs on urine pH are not well known, a clinical study reported that cisplatin did not affect urine pH (23). We believe empirically that without alkalization therapy, chemotherapy with vitamin C does not affect urine pH; however, we have no clinical data regarding this point, and therefore further studies are required to clarify the effects of other chemotherapeutic drugs or intravenous vitamin C on urine pH. Consequently, we suggest that alkalization therapy, which consists of an alkaline diet and bicarbonate, has an alkalizing effect on the body and results in an increase in urine pH.
Although the association between the pH of the tumor microenvironment and urine pH was not clarified in this study, computer simulation studies reported that bicarbonate consumption leads to an increase in the pH of the tumor microenvironment (24,25). Moreover, an in vivo study demonstrated that bicarbonate administration in mouse models of metastatic breast cancer increased the pH of tumor cells and resulted in the suppression of cancer progression (8). Neutralization of the acidic pH of the extracellular tumor microenvironment has been shown to lead to improvements in multi-chemotherapeutic drug resistance in several in vivo and in vitro studies (4,9,26,27). It was reported that in an acidic environment, weak-base chemotherapeutic drugs are positively charged and become trapped in extracellular compartments, leading to a reduction in their cellular uptake and efficacy (28-30). In addition, an acidic tumor microenvironment is also associated with the mechanisms of multidrug efflux. Firstly, P-glycoprotein, which is a multidrug transporter, is activated and expressed in an acidic environment, and results in a decrease in the level of chemotherapeutic drugs in the body (31,32). Secondly, an increase in the number of exosomes is observed in an acidic tumor microenvironment, which assist in removing chemotherapeutic drugs from cancer cells (33,34). In the present study, a prolonged median OS and increased urine pH was observed in patients in the intervention group compared with those in the control group. Similarly, our previous study demonstrated that a urine pH of higher than 7.0, or a urine ΔpH of more than 1.0 was significantly associated with prolonged OS in patients with advanced pancreatic cancer compared with a urine pH of 7.0 or lower, or a urine ΔpH of 1.0 or less (11,12). Therefore, we believe that alkalization therapy has a neutralizing effect on the acidic tumor microenvironment, and is associated with favorable outcomes of patients with SCLC.
In this study, a prolonged median OS was observed in patients with SCLC who received both alkalization therapy and intravenous vitamin C treatment together with chemotherapy, compared with those who received only chemotherapy. Although clinical reports on intravenous vitamin C as a supplementary treatment for various cancer therapies are increasing, there are still very few reports regarding SCLC (13-16). SCLC typically occurs in heavy smokers and is characterized by poor patient survival owing to its aggressive growth and frequent metastases. About 90% or more patients with SCLC have somatic mutations in the tumor suppressor gene TP53 which are associated with the overexpression of mutant P53 protein in tumor cells (35). The P53 protein binds to glucose-6-phosphate dehydrogenase, which is the first and rate-limiting enzyme of the pentose phosphate pathway and suppresses the overactivation of glycolysis. On the other hand, mutant P53 proteins lack this glucose-6-phosphate dehydrogenase-inhibitory activity, which may result in activated glycolysis via the pentose phosphate pathway (36). Activation of aerobic glycolysis is an essential characteristic of cancer cell metabolism, and hence the regulation of activated glycolysis may be a potential therapy for cancer (6). Vitamin C is transported into cancer cells via the glucose transporter, is oxidized to DHA, and then DHA inhibits glyceraldehyde 3-phosphate dehydrogenase, which may result in the suppression of activated glycolysis, particularly in cancer cells with high glycolytic metabolism (17). Therefore, intravenous vitamin C treatment may have favorable effects on the treatment outcomes of patients with SCLC.
We acknowledge that this study has several limitations. Firstly, this was a retrospective study analyzing a small number of patients from a single center. Secondly, we recognize that the timing of the start of alkalization therapy and intravenous vitamin C treatment from the time of diagnosis in the intervention group was not consistent and we were not able meticulously to control the details of the patients’ daily diet. Therefore, a prospective randomized study is necessary to further clarify the effects of the combination of alkalization therapy and intravenous vitamin C treatment. Moreover, we acknowledge that alkalization therapy may have some other potential effects besides an alkalization effect. An alkaline diet with more vegetables and fruit and less meat and dairy products may result in caloric restriction and anti-inflammatory effects in the body, which may affect cancer metabolism. Hence, methods to measure the pH of the tumor microenvironment, such as acido-chemical exchange saturation transfer magnetic resonance imaging, which can measure the extracellular pH of the tumor microenvironment using the ratio of two pH-dependent signals from such imaging may be useful for clarifying the association between the combination of alkalization therapy plus intravenous vitamin C treatment and pH changes of the tumor microenvironment (37,38).
Conclusion
We demonstrated that the combination of alkalization therapy and intravenous vitamin C treatment results in an increase in the urine pH of patients with SCLC, and may improve their outcomes from chemotherapy treatment. An alkaline urine pH may hence be associated with favorable outcomes in patients with SCLC.
Conflicts of Interest
The Authors declare that they have no conflicts of interest associated with this study.
Authors’ Contributions
Reo Hamaguchi performed the literature review, analyzed the data, and wrote the article. Ryoko Narui and Hiromasa Morikawa performed the acquisition of data. Hiromi Wada supervised the study. All Authors conceived and designed the study and gave final approval for publication.
Acknowledgements
The Authors thank Dr. Helena Akiko Popiel of Tokyo Medical University for her editing of this article.
References
- 1.Foundation for Promotion of Cancer Research (FPCR), edited by The Editorial Board of Cancer Statistics in Japan Cancer Statistics in Japan, 2019. Available at: https://ganjoho.jp/en/professional/statistics/brochure/2019_en.html. [Last accessed on May 18, 2021]
- 2.Guidelines for the Diagnosis and Treatment of Lung Cancer 2020, edited by The Japan Lung Cancer Society. Tokyo, Kanehara Publ Corp, 2021 (in Japanese). Available at: https://www.haigan.gr.jp/modules/guideline/index.php?content_id=3. [Last accessed on May 13, 2021]
- 3.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 4.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—one single nature. Biochim Biophys Acta. 2005;1756(1):1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 6.Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599(6):1745–1757. doi: 10.1113/JP278810. [DOI] [PubMed] [Google Scholar]
- 7.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 8.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, Gillies RJ. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim-Hashim A, Estrella V. Acidosis and cancer: from mechanism to neutralization. Cancer Metastasis Rev. 2019;38(1-2):149–155. doi: 10.1007/s10555-019-09787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamaguchi R, Okamoto T, Sato M, Hasegawa M, Wada H. Effects of an alkaline diet on EGFR-TKI therapy in EGFR mutation-positive NSCLC. Anticancer Res. 2017;37(9):5141–5145. doi: 10.21873/anticanres.11934. [DOI] [PubMed] [Google Scholar]
- 11.Hamaguchi R, Narui R, Wada H. Effects of alkalization therapy on chemotherapy outcomes in metastatic or recurrent pancreatic cancer. Anticancer Res. 2020;40(2):873–880. doi: 10.21873/anticanres.14020. [DOI] [PubMed] [Google Scholar]
- 12.Hamaguchi R, Ito T, Narui R, Morikawa H, Uemoto S, Wada H. Effects of alkalization therapy on chemotherapy outcomes in advanced pancreatic cancer: a retrospective case-control study. In Vivo. 2020;34(5):2623–2629. doi: 10.21873/invivo.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr AC, Cook J. Intravenous vitamin C for cancer therapy - Identifying the current gaps in our knowledge. Front Physiol. 2018;9:1182. doi: 10.3389/fphys.2018.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6(222):222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 15.Alexander MS, Wilkes JG, Schroeder SR, Buettner GR, Wagner BA, Du J, Gibson-Corley K, O’Leary BR, Spitz DR, Buatti JM, Berg DJ, Bodeker KL, Vollstedt S, Brown HA, Allen BG, Cullen JJ. Pharmacologic ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Cancer Res. 2018;78(24):6838–6851. doi: 10.1158/0008-5472.CAN-18-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polireddy K, Dong R, Reed G, Yu J, Chen P, Williamson S, Violet PC, Pessetto Z, Godwin AK, Fan F, Levine M, Drisko JA, Chen Q. High dose parenteral ascorbate inhibited pancreatic cancer growth and metastasis: mechanisms and a phase I/IIa study. Sci Rep. 2017;7(1):17188. doi: 10.1038/s41598-017-17568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik J, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikirova N, Casciari J, Rogers A, Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. 2012;10:189. doi: 10.1186/1479-5876-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 21.Passey C. Reducing the dietary acid load: How a more alkaline diet benefits patients with chronic kidney disease. J Ren Nutr. 2017;27(3):151–160. doi: 10.1053/j.jrn.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Robey IF, Lopez AM, Roe DJ. Safety and tolerability of long-term sodium bicarbonate consumption in cancer care. J Integr Oncol. 2015;4(1) doi: 10.4172/2329-6771.1000128. [DOI] [Google Scholar]
- 23.Arunkumar PA, Viswanatha GL, Radheshyam N, Mukund H, Belliyappa MS. Science behind cisplatin-induced nephrotoxicity in humans: a clinical study. Asian Pac J Trop Biomed. 2012;2(8):640–644. doi: 10.1016/S2221-1691(12)60112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin NK, Robey IF, Gaffney EA, Gillies RJ, Gatenby RA, Maini PK. Predicting the safety and efficacy of buffer therapy to raise tumour pHe: an integrative modelling study. Br J Cancer. 2012;106(7):1280–1287. doi: 10.1038/bjc.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva AS, Yunes JA, Gillies RJ, Gatenby RA. The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res. 2009;69(6):2677–2684. doi: 10.1158/0008-5472.CAN-08-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5(5):1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 27.Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8(6):2032–2038. doi: 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66(7):1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 29.Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol. 2003;66(7):1219–1229. doi: 10.1016/s0006-2952(03)00468-4. [DOI] [PubMed] [Google Scholar]
- 30.Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev. 2014;33(4):1095–1108. doi: 10.1007/s10555-014-9531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia. 2006;8(2):143–152. doi: 10.1593/neo.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O. Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncol Rep. 2007;17(1):239–244. [PubMed] [Google Scholar]
- 33.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D’Ilio S, Lugini L, Violante N, Azzarito T, Majorani C, Brambilla D, Fais S. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One. 2014;9(2):e88193. doi: 10.1371/journal.pone.0088193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, Müller C, Dahmen I, Jahchan NS, Park KS, Yang D, Karnezis AN, Vaka D, Torres A, Wang MS, Korbel JO, Menon R, Chun SM, Kim D, Wilkerson M, Hayes N, Engelmann D, Pützer B, Bos M, Michels S, Vlasic I, Seidel D, Pinther B, Schaub P, Becker C, Altmüller J, Yokota J, Kohno T, Iwakawa R, Tsuta K, Noguchi M, Muley T, Hoffmann H, Schnabel PA, Petersen I, Chen Y, Soltermann A, Tischler V, Choi CM, Kim YH, Massion PP, Zou Y, Jovanovic D, Kontic M, Wright GM, Russell PA, Solomon B, Koch I, Lindner M, Muscarella LA, la Torre A, Field JK, Jakopovic M, Knezevic J, Castaños-Vélez E, Roz L, Pastorino U, Brustugun OT, Lund-Iversen M, Thunnissen E, Köhler J, Schuler M, Botling J, Sandelin M, Sanchez-Cespedes M, Salvesen HB, Achter V, Lang U, Bogus M, Schneider PM, Zander T, Ansén S, Hallek M, Wolf J, Vingron M, Yatabe Y, Travis WD, Nürnberg P, Reinhardt C, Perner S, Heukamp L, Büttner R, Haas SA, Brambilla E, Peifer M, Sage J, Thomas RK. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13(3):310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones KM, Randtke EA, Yoshimaru ES, Howison CM, Chalasani P, Klein RR, Chambers SK, Kuo PH, Pagel MD. Clinical translation of tumor acidosis measurements with AcidoCEST MRI. Mol Imaging Biol. 2017;19(4):617–625. doi: 10.1007/s11307-016-1029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldenberg JM, Pagel MD. Assessments of tumor metabolism with CEST MRI. NMR Biomed. 2019;32(10):e3943. doi: 10.1002/nbm.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]