Abstract
The in vitro antifungal activity and spectrum of FK463 were compared with those of amphotericin B, fluconazole, and itraconazole by using a broth microdilution method specified by National Committee for Clinical Laboratory Standards document M27-A (National Committee for Clinical Laboratory Standards, Wayne, Pa., 1997). FK463 exhibited broad-spectrum activity against clinically important pathogens including Candida species (MIC range, ≦0.0039 to 2 μg/ml) and Aspergillus species (MIC range, ≦0.0039 to 0.0313 μg/ml), and its MICs for such fungi were lower than those of the other antifungal agents tested. FK463 was also potently active against azole-resistant Candida albicans as well as azole-susceptible strains, and there was no cross-resistance with azoles. FK463 showed fungicidal activity against C. albicans, i.e., a 99% reduction in viability after a 24-h exposure at concentrations above 0.0156 μg/ml. The minimum fungicidal concentration (MFC) assays indicated that FK463 was fungicidal against most isolates of Candida species. In contrast, the MFCs of FK463 for A. fumigatus isolates were much higher than the MICs, indicating that its action is fungistatic against this species. FK463 had no activity against Cryptococcus neoformans, Trichosporon species, or Fusarium solani. Neither the test medium (kind and pH) nor the inoculum size greatly affected the MICs of FK463, while the addition of 4% human serum albumin increased the MICs for Candida species and A. fumigatus more than 32 times. Results from preclinical in vitro evaluations performed thus far indicate that FK463 should be a potent parenteral antifungal agent.
The currently available antifungal drugs for the treatment of deep-seated mycoses are limited to amphotericin B (AMPH-B), azole compounds, and flucytosine. AMPH-B remains the drug of choice for the treatment of most fungal diseases because it has broad-spectrum and potent fungicidal activity, but it is well known to be toxic (16). Although the azole antifungal agents are considered to be less toxic than AMPH-B, their efficacies against deep-seated, life-threatening mycoses are not satisfactory. In addition, it has been reported that the frequency of isolation of multiazole-resistant strains of Candida species other than Candida albicans is increasing (6). Therefore, there is a critical need for new antifungal agents which are fungicidal, have a broad spectrum of activity and have fewer side effects. Inhibition of glucan synthesis is an attractive target for antifungal agents, since the absence of homologous enzymes in humans may afford a high degree of selectivity for fungi (5). Moreover, an inhibitor of glucan synthesis could possess activity against fungi resistant to other antifungal agents (7, 17, 19). These considerations have led to the development of the echinocandin LY303366 by Eli Lilly & Company and the related pneumocandin MK-0991 by Merck Research Laboratories. Both of these compounds have been introduced into clinical trials (2, 14). The MICs of these compounds for various yeast isolates were determined in accordance with the standard reference method, recently developed by consensus through the National Committee for Clinical Laboratory Standards (9, 12). The guidelines for antifungal susceptibility testing of yeasts were applied to in vitro susceptibility testing of various filamentous fungi (8, 13, 15, 19). Under standard conditions, both LY303366 and MK-0991 displayed substantial activities against Candida and Aspergillus species.
FK463 is a semisynthetic derivative of FR901379, a water-soluble echinocandin-like lipopeptide with a sulfonate moiety, isolated from the culture broth of Coleophoma empedri (T. Iwamoto, N. Sakamoto, M. Yamashita, M. Ezaki, S. Hashimoto, T. Furuta, M. Okuhara, and M. Kohsaka, Program Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 371, 1993). In order to define clearly the in vitro antifungal activity of FK463, we determined the susceptibilities of several clinically important pathogenic fungi under the conditions mentioned above.
MATERIALS AND METHODS
Compounds.
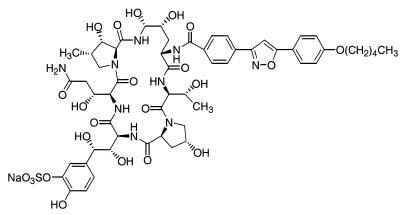
FK463 (Fig. 1) was synthesized at Fujisawa Pharmaceutical Co., Ltd. (AMPH-B), fluconazole (FLCZ), and itraconazole (ITCZ) were purchased from Bristol-Myers Squibb (Tokyo, Japan), Pfizer (Tokyo, Japan), and Janssen-Kyowa (Tokyo, Japan), respectively.
FIG. 1.
Chemical structure of FK463.
Organisms.
The antifungal agents were evaluated against a large battery of clinical isolates from the culture collection in our laboratories. FLCZ-resistant C. albicans isolates were graciously provided by K. Shimada of Tokyo University. Some strains were obtained from the American Type Culture Collection (ATCC). IFM and TIMM strains were graciously provided by M. Miyaji of Chiba University and H. Yamaguchi of Teikyo University, respectively.
MIC assays.
Antifungal susceptibility assays were performed by the broth microdilution method according to the guidelines recommended by the National Committee for Clinical Laboratory Standards in document M27-A (12) to determine the MICs of FK463 and other reference antifungal agents. RPMI 1640 medium with l-glutamine and without sodium bicarbonate was buffered with 165 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.0) and was used as a test medium. All test compounds except ITCZ were solubilized in distilled water at 1,280 μg/ml. ITCZ was solubilized in dimethyl sulfoxide at 1,280 μg/ml. The compounds were then diluted to 64 μg/ml in RPMI 1640 medium and were serially diluted twofold, yielding final drug concentrations ranging from 64 to 0.0078 μg/ml. Inoculum suspensions of 106 cells/ml were prepared by a hemocytometric procedure and were diluted to obtain an inoculum size of approximately 1.0 × 103 to 2.5 × 103 cells/ml. Microplates were incubated at 35°C for Candida species, Saccharomyces cerevisiae, Cryptococcus neoformans, Aspergillus species, and Trichosporon species and at 30°C for Fusarium solani; and readings were taken when good growth in the growth control well was observed. The MICs of FK463 and AMPH-B for yeasts were defined as the lowest concentrations at which no visible growth was observed, and the MICs of FLCZ and ITCZ were defined as the lowest concentrations at which a prominent decrease in turbidity was observed. The MICs of all the compounds tested for filamentous fungi were defined as the lowest concentrations at which a prominent decrease in turbidity compared with that for the growth control was observed.
Influence of experimental conditions on MIC.
The influences of medium, initial pH, inoculum size, and the addition of human serum albumin (HSA) on MICs were determined analogously by the broth microdilution method with RPMI 1640 medium, yeast nitrogen base-dextrose (YNBD) medium, Sabouraud dextrose (SD) medium, or PYG (0.5% yeast extract-containing SD) medium as a test medium, a pH range of 5 to 8, cell concentrations of 102 to 105 CFU/ml, and HSA concentrations of 0 to 4%.
Fungicidal activity.
A culture of C. albicans FP633 was diluted with RPMI 1640 medium buffered with 165 mM MOPS buffer (pH 7.0) to a concentration of 104 CFU/ml. After preincubation for 1 h at 35°C, the antifungal agents were added at various concentrations. The number of viable organisms was determined at 24 h after the addition of drugs by plate counts.
MFC assays.
After the MIC was measured, the microtiter plates were shaken and a 100-μl sample from each well of the microtiter plate was transferred to a single-reservoir plate containing SD agar, and these plates were incubated for more than 72 h at 35°C. The minimum fungicidal concentration (MFC) was defined as the minimum concentration of compound which resulted in the growth of less than 2 CFU. This represents killing of >99% of the original inoculum.
RESULTS
Antifungal spectrum.
Table 1 shows the spectrum of activity of FK463 and other reference antifungal agents against various yeasts and molds. FK463 had a broad-spectrum and potent activity against a variety of fungal species. FK463 was more active than AMPH-B, FLCZ, and ITCZ against most Candida species and all Aspergillus species tested. However, FK463 was inactive against C. neoformans, Trichosporon cutaneum, Trichosporon asahii, and F. solani.
TABLE 1.
Antifungal spectrum of FK463a
| Organism | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| FK463 | AMPH-B | ITCZ | FLCZ | |
| Candida albicans ATCC90028 | 0.0156 | 0.5 | 0.0313 | 0.5 |
| Candida tropicalis TIMM0313 | 0.0313 | 0.5 | 0.125 | 4 |
| Candida glabrata ATCC90030 | 0.0156 | 0.5 | 1 | 16 |
| Candida kefyr ATCC28838b | 0.125 | 0.5 | 0.0625 | 0.5 |
| Candida krusei ATCC6258 | 0.125 | 1 | 0.25 | 32 |
| Candida guilliermondii ATCC9390 | 0.125 | 0.5 | 0.25 | 4 |
| Candida parapsilosis ATCC22019 | 2 | 0.5 | 0.25 | 2 |
| Candida stellatoidea IFM5491 | 0.0313 | 0.0625 | 0.0078 | 0.125 |
| Saccharomyces cerevisiae ATCC9763 | 0.125 | 0.5 | 0.25 | 2 |
| Cryptococcus neoformans TIMM0354b | >64 | 0.25 | 0.0313 | 0.5 |
| Trichosporon cutaneum IFM40104 | >64 | 2 | 0.5 | 8 |
| Trichosporon asahii TIMM3144 | >64 | 0.25 | 0.25 | 2 |
| Aspergillus fumigatus TIMM0063b | 0.0078 | 0.5 | 0.5 | >64 |
| Aspergillus niger ATCC6275b | 0.0078 | 0.25 | 0.5 | >64 |
| Aspergillus nidulans IFM5369b | 0.0078 | 1 | 0.0625 | 32 |
| Aspergillus flavus ATCC9643b | 0.0156 | 1 | 0.25 | 64 |
| Aspergillus terreus IFM40852b | 0.0156 | 1 | 0.125 | >64 |
| Aspergillus versicolor IFM41406b | 0.0156 | 0.5 | 0.0625 | 32 |
| Fusarium solani IFM41532c | >64 | 0.25 | >8 | >64 |
MICs were determined by the microdilution method described in National Committee for Clinical Laboratory Standards document M27-A (12). The methods used to determine the MICs for the yeasts or the molds are described in the text. Unless otherwise noted, incubation was for 48 h at 35°C.
Incubation was for 72 h at 35°C.
Incubation was for 72 h at 30°C.
MICs for clinical isolates of fungi.
Table 2 shows the MICs of FK463 and other antifungal agents for clinical isolates of yeast. The FK463 MICs at which 90% of isolates are inhibited (MIC90s) for C. albicans, including FLCZ-resistant strains, Candida tropicalis, Candida glabrata, and Candida krusei were 0.125 μg/ml or lower; and FK463 was more potent than the other antifungal agents tested. Against Candida parapsilosis and Candida guilliermondii isolates, the MIC90s of FK463 were 1 and 2 μg/ml, respectively, which was slightly less than those of ITCZ and AMPH-B. FK463 had no activity in vitro against C. neoformans and T. cutaneum isolates. Table 3 shows the MICs of FK463 for clinical isolates of Aspergillus species. The MIC90s of FK463 for the four species of Aspergillus were 0.0078 to 0.0156 μg/ml, and its MICs were lower than those of the other antifungal agents tested.
TABLE 2.
MICs of FK463 for clinical isolates of yeastsa
| Organism (no. of isolates) | Compound | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Candida albicans (37) | FK463 | ≤0.0039–0.0156 | 0.0078 | 0.0156 |
| FLCZ | 0.125–4 | 0.25 | 0.5 | |
| ITCZ | 0.0156–0.25 | 0.0313 | 0.0313 | |
| AMPH-B | 0.25–1 | 0.25 | 0.5 | |
| Candida albicans, FLCZ resistant (4) | FK463 | 0.0156–0.0313 | 0.0156 | 0.0313 |
| FLCZ | 16–>64 | 32 | >64 | |
| ITCZ | 0.5–>8 | 0.5 | >8 | |
| AMPH-B | 0.25–0.5 | 0.5 | 0.5 | |
| Candida tropicalis (20) | FK463 | 0.0156–0.0313 | 0.0313 | 0.0313 |
| FLCZ | 0.25–>64 | 0.5 | 8 | |
| ITCZ | 0.0156–>8 | 0.125 | 0.5 | |
| AMPH-B | 0.0313–0.25 | 0.125 | 0.125 | |
| Candida glabrata (20) | FK463 | 0.0078–0.0156 | 0.0156 | 0.0156 |
| FLCZ | 4–>64 | 4 | 64 | |
| ITCZ | 0.5–>8 | 0.5 | 8 | |
| AMPH-B | 0.0625–1 | 0.5 | 1 | |
| Candida krusei (11) | FK463 | 0.125–0.25 | 0.125 | 0.125 |
| FLCZ | 16–64 | 32 | 64 | |
| ITCZ | 0.25–1 | 0.5 | 1 | |
| AMPH-B | 0.5–1 | 1 | 1 | |
| Candida parapsilosis (17) | FK463 | 0.5–2 | 1 | 1 |
| FLCZ | 0.125–4 | 0.5 | 1 | |
| ITCZ | 0.0313–0.5 | 0.125 | 0.5 | |
| AMPH-B | 0.125–0.5 | 0.25 | 0.5 | |
| Candida guilliermondii (12) | FK463 | 0.25–2 | 0.5 | 2 |
| FLCZ | 2–16 | 2 | 4 | |
| ITCZ | 0.25–4 | 0.5 | 1 | |
| AMPH-B | 0.0625–0.5 | 0.0625 | 0.25 | |
| Cryptococcus neoformansb (5) | FK463 | >64 | >64 | >64 |
| FLCZ | 1–8 | 4 | 8 | |
| ITCZ | 0.0313–0.5 | 0.25 | 0.5 | |
| AMPH-B | 0.25–0.5 | 0.25 | 0.5 | |
| Trichosporon cutaneumb (5) | FK463 | >64 | >64 | >64 |
| FLCZ | 2–16 | 8 | 16 | |
| ITCZ | 0.5 | 0.5 | 0.5 | |
| AMPH-B | 0.5–32 | 1 | 32 | |
MICs were determined by the microdilution method described in National Committee for Clinical Laboratory Standards document M27-A (12). The methods used to determine the MICs for the yeasts are described in the text. Unless otherwise noted, incubation was for 24 to 48 h at 35°C.
Incubation was for 72 h at 35°C.
TABLE 3.
MICs of FK463 for clinical isolates of Aspergillus speciesa
| Organism (no. of isolates) | Compound | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Aspergillus fumigatus (29) | FK463 | 0.0078–0.0313 | 0.0078 | 0.0156 |
| FLCZ | 8–>64 | >64 | >64 | |
| ITCZ | 0.0156–1 | 0.5 | 0.5 | |
| AMPH-B | 0.125–1 | 0.5 | 1 | |
| Aspergillus niger (15) | FK463 | ≤0.0039–0.0156 | 0.0078 | 0.0078 |
| FLCZ | 64–>64 | >64 | >64 | |
| ITCZ | 0.5–1 | 0.5 | 1 | |
| AMPH-B | 0.5 | 0.5 | 0.5 | |
| Aspergillus flavus (13) | FK463 | 0.0078–0.0156 | 0.0078 | 0.0156 |
| FLCZ | 8–>64 | >64 | >64 | |
| ITCZ | 0.125–0.5 | 0.5 | 0.5 | |
| AMPH-B | 0.5–1 | 1 | 1 | |
| Aspergillus terreus (7) | FK463 | ≤0.0039–0.0078 | ≤0.0039 | 0.0078 |
| FLCZ | 4–>64 | 32 | >64 | |
| ITCZ | 0.0313–0.125 | 0.125 | 0.125 | |
| AMPH-B | 0.0625–0.5 | 0.5 | 0.5 | |
MICs were determined by the microdilution method described in National Committee for Clinical Laboratory Standards document M27-A. The methods used to determine the MICs for Aspergillus species are described in the text. Incubation was for 72 h at 35°C.
Fungicidal activity.
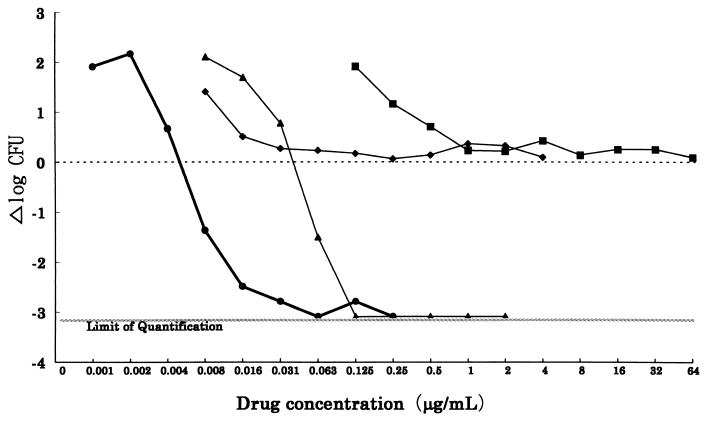
Figure 2 shows the relationship between the change in viable cell counts and drug concentration when C. albicans FP633 was exposed to FK463 and other antifungal agents for 24 h. A 99% or more reduction in viability was observed after 24 h of exposure to FK463 at concentrations above 0.0156 μg/ml. FK463 exhibited fungicidal activity at concentrations lower than those at which AMPH-B exhibited fungicidal activity, and its activity was superior to those of FLCZ and ITCZ, which had only fungistatic activities.
FIG. 2.
Fungicidal activity against C. albicans FP633 after a 24-h exposure. The number of viable organisms was determined at 24 h after the addition of compounds by plate counts. Δlog CFU, logarithm of CFU after 24 h of exposure − logarithm of CFU at time zero. ●, FK463; ▴, AMPH-B; ■, FLCZ; ⧫, ITCZ.
MFCs for clinical isolates of Candida species and Aspergillus fumigatus.
Table 4 shows the MFCs (killing of >99% of the original inoculum) of FK463 and other antifungal agents for clinical isolates of six Candida species (including FLCZ-resistant C. albicans) and A. fumigatus. The FK463 MFCs at which 90% of isolates are inhibited (MFC90s) for C. albicans, including FLCZ-resistant strains, C. glabrata, and C. krusei were 0.5 μg/ml or lower; and FK463 was more potent than the other antifungal agents tested. Against C. tropicalis, C. parapsilosis, and C. guilliermondii isolates, the MFC90s of FK463 were >64, 8, and >64 μg/ml, respectively, which were less than those of AMPH-B. The MFCs of FK463 for A. fumigatus isolates were much higher than the MICs, indicating that its action is fungistatic against this species.
TABLE 4.
MFCs of FK463 for clinical isolates of Candida species and A. fumigatus
| Organism (no. of isolates) | Compound | MFC (μg/ml)a
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Candida albicans (12) | FK463 | 0.0156–4 | 0.0313 | 0.25 |
| FLCZ | >64 | >64 | >64 | |
| ITCZ | >8 | >8 | >8 | |
| AMPH-B | 0.5–1 | 0.5 | 1 | |
| Candida albicans, FLCZ resistant (4) | FK463 | 0.0156–0.5 | 0.0313 | 0.5 |
| FLCZ | >64 | >64 | >64 | |
| ITCZ | >8 | >8 | >8 | |
| AMPH-B | 0.5–2 | 0.5 | 2 | |
| Candida tropicalis (12) | FK463 | 0.0313–>64 | 0.0625 | >64 |
| FLCZ | 0.25–>64 | >64 | >64 | |
| ITCZ | 0.0625–>8 | >8 | >8 | |
| AMPH-B | 0.25–2 | 1 | 2 | |
| Candida glabrata (15) | FK463 | 0.0156–0.0313 | 0.0156 | 0.0313 |
| FLCZ | 4–>64 | >64 | >64 | |
| ITCZ | 0.5–>8 | >8 | >8 | |
| AMPH-B | 1–2 | 1 | 2 | |
| Candida krusei (10) | FK463 | 0.125–0.25 | 0.125 | 0.25 |
| FLCZ | 64–>64 | >64 | >64 | |
| ITCZ | 1–8 | 1 | 8 | |
| AMPH-B | 1–2 | 1 | 2 | |
| Candida parapsilosis (10) | FK463 | 2–16 | 4 | 8 |
| FLCZ | 16–>64 | >64 | >64 | |
| ITCZ | 0.5–>8 | 8 | >8 | |
| AMPH-B | 1–4 | 2 | 2 | |
| Candida guilliermondii (10) | FK463 | 1–>64 | 8 | >64 |
| FLCZ | >64 | >64 | >64 | |
| ITCZ | >8 | >8 | >8 | |
| AMPH-B | 0.5–2 | 1 | 1 | |
| Aspergillus fumigatus (19) | FK463 | >64 | >64 | >64 |
| FLCZ | 64–>64 | >64 | >64 | |
| ITCZ | 0.25–4 | 1 | 2 | |
| AMPH-B | 1–4 | 2 | 4 | |
Microtiter plates were shaken and 0.1-ml samples were transferred to SD agar plates, which were incubated for more than 72 hours at 35°C.
Influence of experimental conditions on activity of FK463.
Table 5 shows the influence of the kind and pH of the medium, inoculum size, and addition of HSA on the MIC of FK463. The kind and initial pH of the medium and inoculum size did not significantly affect the MIC of FK463 for C. albicans, C. glabrata, and A. fumigatus. In contrast, the addition of 4% HSA increased the MICs for these strains more than 32 times.
TABLE 5.
Influence of culture conditions on MICa
| Culture condition | MIC (μg/ml)
|
||
|---|---|---|---|
| C. albicans ATCC 90028b | C. glabrata ATCC 90030b | A. fumigatus TIMM0063c | |
| Kind of medium | |||
| RPMI 1640 | 0.0156 | 0.0156 | 0.0039 |
| YNBD | 0.0625 | 0.0156 | ≤0.002 |
| SD | 0.0625 | 0.0313 | ≤0.002 |
| PYG | 0.0313 | 0.0313 | ≤0.002 |
| pH of medium | |||
| 5 | 0.0313 | 0.0156 | 0.0078 |
| 6 | 0.0156 | 0.0156 | 0.0039 |
| 7 | 0.0078 | 0.0156 | 0.0039 |
| 8 | 0.0313 | 0.0078 | 0.25 |
| Inoculum size (cells/ml) | |||
| 102 | 0.0156 | 0.0313 | ≤0.002 |
| 103 | 0.0156 | 0.0313 | ≤0.002 |
| 104 | 0.0156 | 0.0625 | 0.0156 |
| 105 | 0.0156 | 0.0625 | 0.0156 |
| Addition of HSA | |||
| 0% | 0.0156 | 0.0156 | 0.0078 |
| 0.04% | 0.0156 | 0.0625 | 0.0078 |
| 0.4% | 0.25 | 0.25 | 0.0313 |
| 4.0% | 2 | 4 | 0.25 |
Broth microdilution testing was used. The MICs for Candida species were determined as the minimum concentration resulting in at least 80% inhibition of growth of Candida species measured as a decrease in turbidity compared with the turbidities of the growth controls. The MICs for A. fumigatus were determined as the minimum concentration resulting in a prominent decrease in turbidity compared with the turbidity of the growth control.
Incubation was for 48 h at 35°C.
Incubation was for 72 h at 35°C.
DISCUSSION
FK463 is a semisynthetic derivative of FR901379, which is a water-soluble echinocandin-like lipopeptide isolated from the culture broth of C. empedri (Iwamoto et al., 33rd ICAAC). FR901379 and related compounds were shown to have inhibitory activities on 1,3-β-d-glucan synthase (Iwamoto et al., 33rd ICAAC), and these activities were similar to those of echinocandin B analogs and the pneumocandins (4, 5, 10). In this study, we determined the activity of FK463 by the broth microdilution methods specified in document M27-A, a new reference standard recently developed by consensus through the National Committee for Clinical Laboratory Standards (12). We defined the MICs of FK463 for yeasts as the lowest concentration that inhibited visible growth completely because trailing end points with FK463 were rarely encountered. FK463 showed potent in vitro activity against a broad spectrum of Candida species. The results indicate that FK463 possesses the best activity with the lowest MICs for C. albicans, C. tropicalis, and C. glabrata and is more potent than AMPH-B. FK463 showed less activity against C. krusei, although the MIC90 was lower than those of the other drugs tested. An outstanding feature of FK463 was the good activity against strains of C. albicans and non-C. albicans Candida species which are resistant to FLCZ. In contrast to the potent activity against the Candida species described above, FK463 had lower levels of activity against C. parapsilosis and C. guilliermondii and was slightly less active than ITCZ and AMPH-B. Cell wall 1,3-β-d-glucan-inhibitory compounds are reported to possess fungicidal activity (3). The results obtained by the MFC assays demonstrated that FK463 has fungicidal activity against most isolates of Candida species. The in vitro antifungal activity of FK463 was not significantly affected by experimental conditions except that the addition of HSA lowered the activity. This reduction in activity is due to the high level of protein binding of FK463 (S. Suzuki, M. Terakawa, F. Yokobayashi, T. Fujiwara, and T. Hata, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F144, 1998).
FK463 and other 1,3-β-d-glucan synthase inhibitors exhibit good in vivo efficacy against A. fumigatus (1, 11, 14, 18) (S. Matsumoto, Y. Wakai, K. Maki, E. Watabe, T. Ushitani, K. Otomo, N. Nakai, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F142, 1998; Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, K. Otomo, T. Nakai, K. Hatano, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F143, 1998; D. Zeckner, T. Butler, C. Boylan, B. Boyll, Y. Lin, P. Raab, J. Schmidtke, and W. Current, Program Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 364, 1993), but complete growth inhibition is not observed by the standard broth dilution method (2, 3, 7). Therefore, we evaluated the anti-Aspergillus activity of FK463 by determining the concentration at which a prominent decrease in turbidity compared with the turbidity of the growth control was observed. The MIC90s of FK463 for four species of Aspergillus determined by this method were much lower than those of AMPH-B. The validity of this methodology was proven by the good in vivo efficacy of FK463 against A. fumigatus (K. Maki, Y. Morishita, Y. Iguchi, E. Watabe, K. Otomo, N. Teratani, Y. Watanabe, F. Ikeda, S. Tawara, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, H. Tanaka, K. Sakane, F. Matsumoto, and S. Kuwahara, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F141, 1998; Matsumoto et al., 38th ICAAC; Wakai et al., 38th ICAAC).
FK463 was ineffective against C. neoformans, T. cutaneum, T. asahii, and F. solani, as was MK-0991, a pneumocandin B derivative (2, 8, 9, 15). One possible explanation is that these species may possess more 1,6-β-d-glucan or other non-1,3-β-d-glucans in their cell walls and smaller amounts of 1,3-β-d-glucan. Other possibilities are that poor penetration or access of the compound to the target may be related to relative resistance, as pointed out by Bartizal et al. (3).
In conclusion, these results suggest that FK463 is a promising compound for further evaluation as a new antifungal candidate.
ACKNOWLEDGMENT
We are grateful to David Barrett, Medicinal Chemistry Research Laboratories, for kind help and advice.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartizal K, Gill C J, Abruzzo G K, Flattery A M, Kong L, Scott P M, Smith J G, Leighton C E, Bouffard A, Dropinski J F, Balkovec J M. Antimicrob. Agents Chemother. 1997;41:2326–2332. doi: 10.1128/aac.41.11.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartizal K, Scott T, Abruzzo G K, Gill C J, Pacholok C, Lynch L, Kropp H. In vitro evaluation of the pneumocandin antifungal agent L-733,560, a new water-soluble hybrid of L-705,589 and L-731,373. Antimicrob Agents Chemother. 1995;39:1070–1076. doi: 10.1128/aac.39.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaulieu D, Tang J, Zeckner D J, Parr T R., Jr Correlation of cilofungin in vivo efficacy with its activity against Aspergillus fumigatus (1,3)-β-d-glucan synthase. FEMS Microbiol Lett. 1993;108:133–138. doi: 10.1111/j.1574-6968.1993.tb06088.x. [DOI] [PubMed] [Google Scholar]
- 5.Debono M, Gordee R S. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 6.Hitchcock C A, Pye G W, Troke D F, Johnson E M, Warnock D W. Fluconazole resistance in Candida glabrata. Antimicrob Agents Chemother. 1993;37:1962–1965. doi: 10.1128/aac.37.9.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang A, Edwards F, Bernard E M, Armstrong D, Schmitt H J. In vitro activity of the new semisynthetic polypeptide cilofungin ( LY121019) against Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 1990;9:697–699. doi: 10.1007/BF01964276. [DOI] [PubMed] [Google Scholar]
- 8.Ingroff A E. Comparison of the in vitro activities of the new triazole SCH56592 and the echinocandin MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnarao T V, Galgiani J N. Comparison of the in vitro activities of the echinocandin LY303366, the pneumocandin MK-0991, and fluconazole against Candida species and Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41:1957–1960. doi: 10.1128/aac.41.9.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz M B, Douglas C, Marrinan J, Nollstadt K, Onishi J, Dreikorn S, Millican J, Mandara S, Thompson J, Balkovec J M, Bouffard F A, Dropinski J F, Hammond M L, Zambias R A, Abruzzo G, Bartizal K, Mcmanus O B, Garcia M L. Increased antifungal activity of L-733,560, a water-soluble, semisynthetic pneumocandin, is due to enhanced inhibition of cell wall synthesis. Antimicrob Agents Chemother. 1994;38:2750–2757. doi: 10.1128/aac.38.12.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtz M B, Bernard E M, Edwards F F, Marrinan J A, Dropinski J, Douglas C M, Armstrong D. Aerosol and parenteral pneumocandins are effective in a rat model of pulmonary aspergillosis. Antimicrob Agents Chemother. 1995;39:1784–1789. doi: 10.1128/aac.39.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Oakley K L, Moore C B, Denning D W. In vitro activity of the echinocandin antifungal agent LY303,366 in comparison with itraconazole and amphotericin B against Aspergillus spp. Antimicrob Agents Chemother. 1998;42:2726–2730. doi: 10.1128/aac.42.10.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petraitis V, Petraitiene R, Groll A H, Bell A, Callender D P, Sein T, Schufele R L, McMillian C L, Bacher J, Walsh T J. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 1998;42:2898–2905. doi: 10.1128/aac.42.11.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poeta M D, Schell W A, Perfect J R. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother. 1997;41:1835–1836. doi: 10.1128/aac.41.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas A H. Suggested mechanisms for the antimycotic activity of the polyene antibiotics and the N-substituted imidazoles. J Antimicrob Chemother. 1986;17:269–279. doi: 10.1093/jac/17.3.269. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez J A, Lynch M, Sobel J D. In vitro activity of a new pneumocandin antifungal agent, L-733,560, against azole-susceptible and -resistant Candida and Torulopsis species. Antimicrob Agents Chemother. 1995;39:2689–2691. doi: 10.1128/aac.39.12.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verweiji P E, Oakley K L, Morrissey J, Morrissey G, Denning D W. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob Agents Chemother. 1998;42:873–878. doi: 10.1128/aac.42.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhanel G G, Karlowsky J A, Harding G A J, Balko T V, Zelenitsky S A, Friesen M, Kobani A, Turik M, Hoban D J. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatidis, and Aspergillus species. Antimicrob Agents Chemother. 1997;41:863–865. doi: 10.1128/aac.41.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]