Abstract
Background
Some prognostic factors for pancreatic neuroendocrine neoplasms (PanNENs) have been reported; however, the significance of lymphatic, microvascular, and perineural invasion remains unclear. We aimed to clarify the role of these factors in PanNEN recurrence.
Patients and Methods
We analyzed 138 patients who underwent curative pancreatectomy and were pathologically diagnosed with PanNEN. We evaluated the association between clinicopathological factors and the recurrence of PanNENs.
Results
The numbers of patients with lymphatic, microvascular, and perineural invasion were 34 (25%), 43 (31%) and 17 (12%), respectively. Twenty-four patients (17%) had recurrences, and the 3, 5, and 10-year recurrence-free survival (RFS) rates were 88%, 84%, and 76%, respectively. The recurrence sites (with duplication) were mainly the liver (twenty-two patients), followed by the lymph nodes (seven patients), and bone (two patients). In multivariate analyses, grade 2-3 and the presence of microvascular invasion were significant risk factors for RFS (hazard ratio=7.5 and 7.9, respectively). When examining outcomes according to these factors, the 5-year RFS rates of patients with risk scores of 0, 1, and 2 were 100%, 91%, and 32%, respectively (p<0.001). Even in patients with grade 1 (n=97) or limited resection (enucleation, splenic-preserving distal pancreatectomy, central pancreatectomy, and duodenum-preserving pancreatic head resection, n=62), the presence of microvascular invasion was a significant risk factor for RFS (hazard ratio=13.4 and 18.0, respectively).
Conclusion
The presence of microvascular invasion is an independent risk factor for recurrence in patients with PanNEN.
Keywords: Lymphatic invasion, microvascular invasion, perineural invasion, risk factor, pancreatic neuroendocrine neoplasm
Although pancreatic neuroendocrine neoplasms (PanNENs) are comparatively rare neoplasms, recently, their incidence and prevalence have been increasing steadily (1-3). Particularly, it has been pointed out that the number of well-differentiated and localized PanNENs is increasing (2). This may be due to the spread of the concept of PanNEN and advancements in diagnostic imaging studies (2,3). Surgical resection is currently the only curative treatment for PanNENs (4,5). Pancreatic resection with lymph node dissection (LND) is the standard surgical procedure, but limited resection may be considered for small and/or less likely malignant PanNENs (6-8). It has been reported that the World Health Organization (WHO) grading (9), lymph node metastasis (LNM), liver metastasis, and some immune-inflammatory markers are associated with prognosis in patients with PanNEN (10-15), but the association between other clinicopathological factors and prognosis remains unclear.
In gastroenterological cancers, if lymphatic and/or microvascular invasion is detected histopathologically after endoscopic treatment, radical surgery with LND is recommended as an additional treatment for improving prognosis (16,17). Similarly, some reports have stated that additional resection is recommended for gastrointestinal neuroendocrine neoplasms with lymphatic and/or microvascular invasion (18,19). This is because these lymphatic and/or microvascular invasions suggest potential LNM (20). It is conceivable that hematogenous and lymphatic metastases cannot be established without the presence of lymphatic and microvascular invasion.
However, the oncological importance of lymphatic, microvascular, and perineural invasion in patients with PanNEN remains unclear. Particularly, there are no reports on the role of these factors in patients with low-malignant PanNEN or in those who underwent limited surgery. In addition, the frequency of lymphatic, microvascular, and perineural invasion in various PanNEN backgrounds is unknown. Therefore, we aimed to clarify the details and significance of lymphatic, microvascular, and perineural invasion in patients with PanNEN.
Patients and Methods
Study design. This study was approved by the Institutional Review Board of Tokyo Women’s Medical University (approval number: 3954). The requirement for informed consent was waived owing to the retrospective nature of the study.
In this retrospective study, we analyzed the medical records of 138 patients who underwent curative pancreatectomy for PanNEN at the Department of Surgery, Institute of Gastroenterology, Tokyo Women’s Medical University, between 2000 and 2019. These patients did not receive preoperative or postoperative adjuvant treatment. We evaluated the clinicopathological factors to assess their association with recurrence-free survival (RFS).
Preoperative parameters included age, sex, tumor location, existence of genetic heredity, existence of functionality, preoperative body mass index (BMI), hemoglobin A1c (HbA1c) level, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), prognostic nutrition index (PNI), C-reactive protein/albumin ratio (CAR), controlling nutritional status (CONUT) score, and Glasgow prognostic score (GPS). Intraoperative parameters included the type of surgical procedure, number of dissected lymph nodes, amount of blood loss and duration of surgery. Postoperative parameters included the postoperative complication grade, number of Ki-67 index of tumor tissues, 2017 WHO classification (9), tumor size, existence of LNM, presence of lymphatic, microvascular, and perineural invasion as described in a later section, existence of residual tumor, recurrence and surgical outcomes.
Univariate and multivariate analyses were performed to identify the independent predictors of RFS. Survival rates were compared according to the presence or absence of risk factors identified in the univariate or multivariate analyses. A risk score was applied for this purpose, consisting of the number of risk factors present in each patient.
Definition of the pancreatic neuroendocrine neoplasm. PanNEN was defined by cord-like, rosette-like and alveolar-like structures that were detected by hematoxylin and eosin staining, positive immunohistochemical staining for chromogranin A and synaptophysin. We considered PanNEN without clinical symptoms of hormone production as a non-functioning NEN (NF-PanNEN).
Assessment of lymphatic, microvascular and perineural invasion. Lymphatic invasion was suggested by the presence of cancer cells and cancer cell nests in the interstitial space. A space filled with lymph and lymphocytes was especially likely to be a lymphatic vessel. When endothelial cells were identified around the space, the space was concluded to represent a lymphatic vessel. When it was difficult to evaluate lymphatic vessels, D2-40 immunohistochemical staining was applied. Microvascular invasion was highly likely when a circular, semicircular, or oblong cancer cell nest with regular margins was located in the vicinity of vessels and distant from the main lesion. If such a cancer cell nest was surrounded by venous wall structures (such as internal elastic membrane or perivascular smooth muscle), it was concluded to represent microvascular invasion. When it was difficult to identify vessels, Victoria blue staining was applied to elucidate elastic fibers in vessel walls. Perineural invasion was detected by the finding of cancer cells in the perineural space and nerve fiber bundles. We updated the data on the prior diagnosis by previous pathologists, by an independent board-certified pathologist (TF) using the abovementioned unified definition.
Surgical procedure. Distal pancreatectomy (DP), pancreaticoduo-denectomy (PD), and total pancreatectomy (TP) were considered standard resection procedures. Enucleation, spleen-preserving DP, central pancreatectomy and duodenum-preserving pancreatic head resection were defined as limited resection procedures. Regional LND was performed using standard resection. In patients who underwent DP, LND regions included the area along the left gastric artery, common hepatic artery (CHA), celiac artery, splenic hilum, splenic artery, superior mesenteric artery (SMA) and inferior margin of the pancreas. In patients who underwent PD, the LND region included the area around the subpyloric, infrapyloric, CHA, hepatoduodenal ligament, anterior and posterior surface of the pancreatic head and SMA. In patients who underwent TP, the LND regions included all those covered for patients who underwent DP and PD. By contrast, in limited resection, LND was only partially performed around the PanNEN, or LND was not performed. By principle, we performed a standard resection with regional LND for large tumors and a grade 2 or 3 preoperative diagnosis using endoscopic ultrasonography-guided fine-needle aspiration. Conversely, limited resection was performed in patients with a small tumor size in whom LNM was not suspected. Endoscopic ultrasonography-guided fine-needle aspiration for PanNEN was introduced at our institution in 2011. Before 2011, the operative procedure was determined based on the tumor size and location.
Follow-up. After surgery, the patients underwent laboratory examinations and imaging studies every 3 to 6 months as a standard follow-up strategy. Disease-free survival was measured from the time of surgery until recurrence or the last follow-up day if there was no recurrence. A tumor initially identified on postoperative imaging studies was considered a recurrence. When two sites of recurrence were observed simultaneously, all sites were counted.
Miscellaneous definitions. Preoperative laboratory and imaging data were acquired within 21 days of the surgery. Postoperative complications were rated according to the Clavien-Dindo classification (21). The cutoff values for the preoperative BMI, HbA1c level, NLR, PLR, LMR, PNI, CAR, CONUT score, GPS score, number of dissected lymph nodes, surgery duration, amount of blood loss, Ki-67 index and tumor size were determined using the receiver operating characteristic (ROC) curve analysis and designated as the point at which the area under the ROC curve was the largest for predicting RFS.
Statistical analyses. In this study, factors that seemed to be clinically relevant to prognosis were selected as the examined factors, excluding some confounding factors. The number of deaths from PanNENs was small, and the same number of deaths was observed from another disease. Therefore, univariate and multivariate analyses were performed to identify the independent predictors of RFS instead of disease-specific survival (DSS) and overall survival (OS). We only performed a univariate analysis since we determined that obtaining effective results in the multivariate analysis was not possible owing to insufficient statistical data. Thus, only a univariate analysis was performed to identify the independent predictors for DSS. Survival analyses were performed using the Kaplan–Meier method, log-rank test and Cox proportional hazards model. Factors showing statistical significance in univariate analysis were subjected to multivariate analysis. p<0.05 was considered statistically significant. All analyses were performed using JMP 12.1.0 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
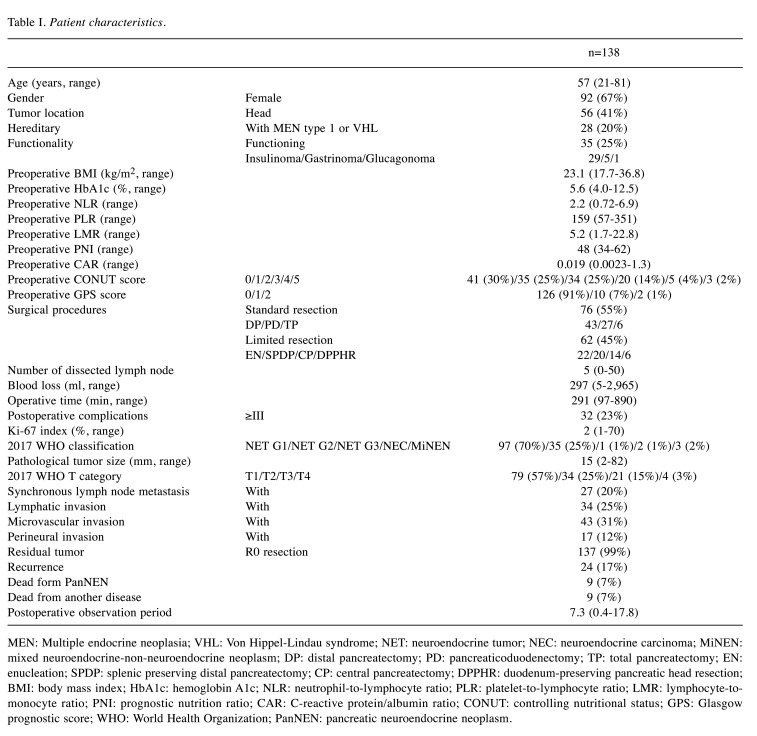
Patient characteristics are shown in Table I. The median and average postoperative observation period was 7.3 (0.4-17.8) and 6.4 years, respectively. Moreover, 25% of the patients had functioning PanNENs. Furthermore, 55% of the patients underwent standard resection, while 45% underwent limited resection. According to the 2017 WHO classification, 70% of the patients had a neuroendocrine G1 tumor (NET). A total of 25% of the patients had synchronous LNM. The number of patients with lymphatic, microvascular and perineural invasion was 34 (25%), 43 (31%), and 17 (12%), respectively. Furthermore, 17% of the patients had recurrence, and the recurrence sites (with duplication) were mainly in the liver (twenty-two patients, 92%), followed by the lymph nodes (seven patients, 29%), and bone (two patients, 8%). Only 7% of the patients died from PanNENs, and 7% of the patients died from another disease.
Table I. Patient characteristics.
MEN: Multiple endocrine neoplasia; VHL: Von Hippel-Lindau syndrome; NET: neuroendocrine tumor; NEC: neuroendocrine carcinoma; MiNEN: mixed neuroendocrine-non-neuroendocrine neoplasm; DP: distal pancreatectomy; PD: pancreaticoduodenectomy; TP: total pancreatectomy; EN: enucleation; SPDP: splenic preserving distal pancreatectomy; CP: central pancreatectomy; DPPHR: duodenum-preserving pancreatic head resection; BMI: body mass index; HbA1c: hemoglobin A1c; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-tomonocyte ratio; PNI: prognostic nutrition ratio; CAR: C-reactive protein/albumin ratio; CONUT: controlling nutritional status; GPS: Glasgow prognostic score; WHO: World Health Organization; PanNEN: pancreatic neuroendocrine neoplasm.
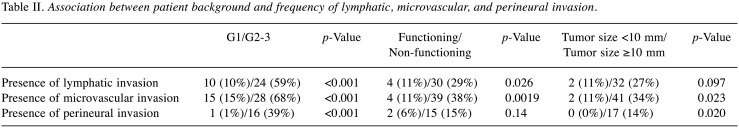
Association between patient background and frequency of lymphatic, microvascular, and perineural invasion. The association between patient background and frequency of lymphatic, microvascular, and perineural invasion is shown in Table II. Although G1 had significantly less lymphatic, microvascular, and perineural invasion than G2 or G3, even in patients with G1, these were found in 10%, 15% and 1% of patients, respectively. NF-PanNENs had significantly more invasion than functioning PanNENs. Even in patients with tumor size <10 mm, lymphatic and microvascular invasion was found in 11% of patients, in both cases.
Table II. Association between patient background and frequency of lymphatic, microvascular, and perineural invasion.
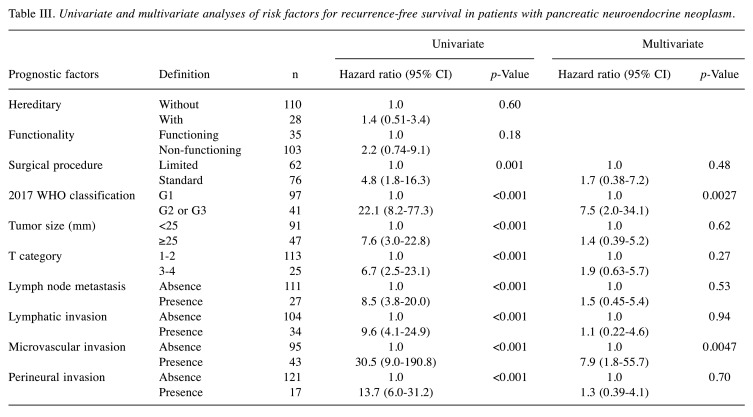
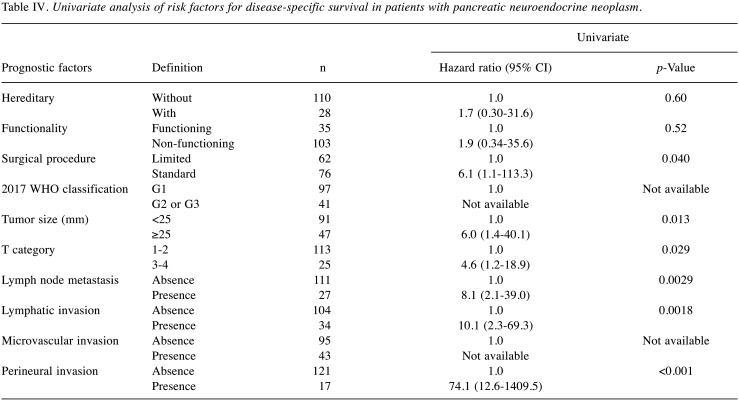
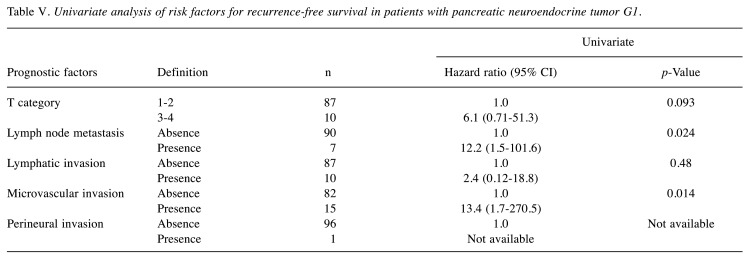
Risk factors for recurrence-free survival in all patients. In all patients, the recurrence rates were 17% (n=24), and the 3, 5, and 10-year RFS rates were 88%, 84% and 76%, respectively. In multivariate analyses, the 2017 WHO classification G2 or G3 [vs G1, hazard ratio (HR)=7.5] and the presence of microvascular invasion (vs absence, HR=7.9) were the independent risk factors for RFS (Table III). When examining outcomes according to these risk factors, the 3, 5, and 10-year RFS rates of patients with risk scores of 0 (n=82), 1 (n=28), and 2 (n=28) were 100%, 100%, and 97% (median RFS time: not achieved); 96%, 91%, and 78% (median RFS time: not achieved); and 47%, 32%, and 12% (median survival time: 2.9 years) (p<0.001), respectively (Figure 1). The HRs of risk score groups 1 and 2 were 12.6 and 120.3 times higher than that of risk score group 0 (p<0.05). In the univariate analysis, the independent risk factors for DSS are shown in Table IV.
Table III. Univariate and multivariate analyses of risk factors for recurrence-free survival in patients with pancreatic neuroendocrine neoplasm.
Figure 1. Kaplan–Meier analyses of recurrence-free survival rates in patients with pancreatic neuroendocrine neoplasm according to risk score. The risk score indicates the number of risk factors for recurrencefree survival, such as G2–G3 and the presence of microvascular invasion, derived via a multivariate analysis. The 3, 5, and 10-year recurrence-free survival rates of patients with risk scores of 0 (n=82), 1 (n=28) and 2 (n=28) were 100%, 100% and 97%; 96%, 91% and 78%; and 47%, 32% and 12%, respectively (p<0.001).
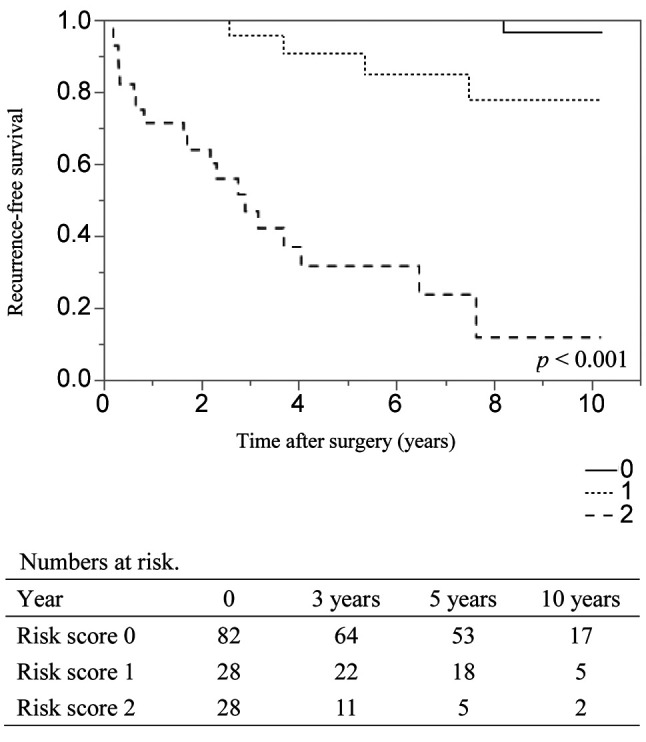
Table IV. Univariate analysis of risk factors for disease-specific survival in patients with pancreatic neuroendocrine neoplasm.
Risk factors for recurrence-free survival in patients with G1. In patients with G1 (n=97), the recurrence rate was 4% (n=4), and the 3, 5 and 10-year RFS rates were 99%, 97% and 93%, respectively. In univariate analysis, the presence of LNM (vs. absence, HR=12.2) and the presence of microvascular invasion (vs absence, HR=13.4) were the independent risk factors for RFS (Table V). When examining outcomes according to these risk factors, the 3-, 5-, and 10-year RFS rates of patients with risk scores of 0 (n=50) and 1-2 (n=12) were 100%, 100%, and 100% (median RFS time: not achieved) and 94%, 88%, and 71% (median RFS time: not achieved) (p<0.001), respectively (Figure 2).
Table V. Univariate analysis of risk factors for recurrence-free survival in patients with pancreatic neuroendocrine tumor G1.
Figure 2. Kaplan–Meier analyses of recurrence-free survival rates in patients with pancreatic neuroendocrine tumor G1 according to risk score. The risk score indicates the number of risk factors for recurrencefree survival, such as the presence of lymph node metastasis and the presence of microvascular invasion, as determined via a univariate analysis. The 3, 5, and 10-year recurrence-free survival rates of patients with risk scores of 0 (n=79) and 1-2 (n=18) were 100%, 100%, and 100% and 94%, 88%, and 71%, respectively (p<0.001).
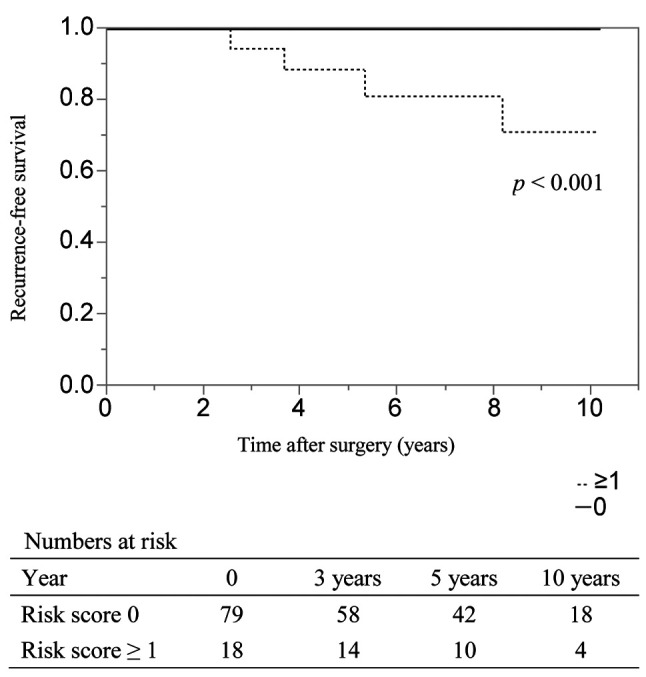
Risk factors for recurrence-free survival in patients with limited resection. In patients with limited resection (n=62), the recurrence rate was 6% (n=4), and the 3, 5, and 10-year RFS rates were 100%, 98%, and 88%, respectively. In univariate analysis, the 2017 WHO classification of G2 or G3 (vs. G1, HR=16.5) and the presence of microvascular invasion (vs. absence, HR=18.0) were the independent risk factors for RFS (Table VI). When examining each of these risk factors, the 3, 5, and 10-year RFS rates of patients with risk scores of 0 (n=50) were 100% in all cases (median RFS time: not achieved); and those of patients with risk scores of 1-2 (n=12), they were 100%, 90% and 40%, respectively (median RFS time: 7.6 years) (Figure 3). The differences between these risk score groups were significant (p<0.001).
Table VI. Univariate analysis of risk factors for recurrence-free survival in patients with pancreatic neuroendocrine neoplasm who underwent limited resection.
Figure 3. aplan–Meier analyses of recurrence-free survival rates in patients with pancreatic neuroendocrine neoplasm who underwent limited resection according to risk score. The risk score suggests the number of risk factors for recurrence-free survival, such as G2-G3 and the presence of microvascular invasion, derived via a univariate analysis. The 3, 5, and 10-year recurrence-free survival rates of patients with risk scores of 0 (n=50) and 1-2 (n=12) were 100%, 100%, 100% and 100%, 90%, and 40%, respectively (p<0.001).
Discussion
This study revealed the frequency of lymphatic, microvascular and perineural invasion depending on the patients’ backgrounds, and even patients with G1 PanNET had 10% and 15% of lymphatic and microvascular invasion, respectively. In addition, we found that the presence of microvascular invasion was an important factor in predicting recurrence in patients with PanNENs. These results are new findings and may be useful for decision-making regarding treatment strategies in patients with such neoplasms.
PanNENs originate from pancreatic neuroendocrine cells and were formerly called “carcinoids” (22). In 2017, the WHO clarified the definition of PanNEN and classified it into NET G1, NET G2, NET G3, and neuroendocrine carcinoma (NEC) (9). NET G1, NET G2, NET G3 and NEC were defined as <3%, 3%-20%, >20%, and >20% of the Ki-67 proliferation index, respectively. NET is defined as a well-differentiated type, NEC is defined as a poorly differentiated type, and the prognosis is considered to be different for each. Although PanNENs were previously thought to be rare neoplasm, their incidence has gradually increased in recent years (1-3). In particular, the consistent advancements in imaging diagnostics have led to an increase in the incidence of well-differentiated and localized types of PanNENs (1). It has also been reported that contrast-enhanced computed tomography can be used to distinguish between NET G1 and NET G2 during preoperative examination (23). The curative treatment is surgical resection (4,5), and pancreatectomy with regional LND is the standard treatment (6-8). However, as mentioned above, as the frequency of small or less likely malignant lesions of PanNENs increases, limited resection, which preserves pancreatic function and omits LND, may also be performed.
We set the cut-off value of the tumor size as 10 mm in this study. In the ENETS and NCCN guideline, the cut-off value of the size was 20 mm (6,7). On the other hand, in the Japanese 2019 guidelines, the cut-off value was 10 mm (8). We have chosen the smaller number since no clear conclusions have been reached as to what the cut-off value should be. The WHO grading and synchronous LNM have been reported as poor prognostic factors for PanNEN (10-13). These results were reported in a meta-analysis as well (24). Furthermore, several immune-inflammatory markers were recently proposed as poor prognostic factors in patients with PanNEN (14,15) and pancreatic ductal carcinoma (25-27). A simplified prognostic scoring system for predicting recurrence after resection using these factors has been proposed (10. 28, 29). On the other hand, although there are some previous reports about the significance of lymphatic, microvascular, and perineural invasion (30-32), these studies have an insufficient number of patients and examination items. Thus, the role of lymphatic, microvascular, and perineural invasion as predictive factors for prognosis remains unclear.
In the field of gastroenterological cancers and neuroendocrine neoplasms, if pathological lymphatic and/or microvascular invasion is detected after endoscopic local resection, radical surgery with LND is recommended as an additional treatment for improving prognosis (16-18). Additional radical resection with LND is recommended when lymphatic and/or microvascular invasion is noted after minimally invasive endoscopic treatment because lymphatic and microvascular invasion are potential risk factors for hematogenous and/or lymphatic metastasis. By contrast, in patients with PanNENs, currently, it is clinically unrealistic to perform additional treatment (standard pancreatectomy with regional LND) based on the results of pathological examination after limited resection. The reason is that pancreatectomy has certain complications and high mortality rates (33,34), and the significance of additional resection for improving prognosis has not been established. Therefore, it is desirable that lymphatic, microvascular, and perineural invasion be used as predictors of recurrence, not as indicators of additional resection, and for the availability of postoperative adjuvant treatment and strict postoperative observation.
Although lanreotide, streptozocin, sunitinib and everolimus are used for unresectable PanNENs (35-38), the effects of these drugs as postoperative adjuvant therapies have not been established (18). In the future, clinical trials showing the significance of adjuvant treatments will be necessary, but at that time, extracting cases with a high risk of recurrence will be important. The results of this study may help identify patients who are eligible for adjuvant therapy.
Generally, computed tomography and magnetic resonance imaging are often used as regular postoperative imaging studies, and somatostatin receptor scintigraphy is recommended every 2 years for G1 and every year for G2-G3 (39). However, all imaging studies involve costs and are physically invasive, and there is no clear definition of duration in these studies. In this study, no recurrence was observed in patients with NET G1 without LNM or microvascular invasion. Additional studies are needed in the future, but the results may be useful in determining the intervals and duration of regular postoperative examinations.
Information on the presence or absence of LNM may be lacking when limited surgery is performed. Izumo et al. have reported that the optimal range of LND in NF-PanNEN and LND in this area was considered necessary when performing limited resection (10). However, we found that patients with NET G1 and without microvascular invasion had no recurrence even after limited surgery; thus, it may be possible to predict recurrence more easily.
In this study, WHO grading and the presence of microvascular invasion were extracted as predictors of recurrence from various factors; LNM, lymphatic invasion, and surgical margin were not extracted. This is probably because most of the recurrence sites in this study were in the liver, and not in the lymph nodes, while nearly all the patients (99%) underwent R0 resection. Owing to the small number of lymph node recurrences in this study, our indication of the LND seems to have been appropriate. Recurrence of liver metastasis is thought to be a factor that correlates with prognosis, and microvascular invasion is considered a more important early predictor of liver recurrence than LNM.
This study has some limitations. Patients from different periods over the 19-year study period underwent different diagnostic and treatment modalities owing to the advancements in these techniques that have occurred over time; these variations may have skewed the outcomes of patients treated during the different periods of the study. In particular, the 10-year RFS rate was considered a reference value. Because there were few deaths from PanNENs and the number of deaths from another disease was the same as that of deaths from PanNENs, and because it was difficult to evaluate DSS and OS, we investigated RFS alone. Moreover, our investigation was retrospective and conducted at a single institution, and the biases inherent to such settings cannot be completely excluded.
In conclusion, we were able to clearly determine the details and significance of lymphatic, microvascular and perineural invasion in patients with PanNENs. The presence of microvascular invasion plays an important role in predicting recurrence. This information is useful for decision-making regarding treatment strategies in patients with PanNENs.
Conflicts of Interest
The Authors declare that they have no competing interests.
Authors’ Contributions
WI and RH contributed to conception and design. WI, RH, TF, TY, SU, YM, MS, YT, JT, KS, TK, and MY contributed to development of methodology and data acquisition. WI, RH, TF, and MY contributed to analysis and interpretation of data, writing, review, and/or revision of the manuscript. All Authors have read and approved the final manuscript.
Acknowledgements
This study was supported by a NAKAYAMA Komei Research Fellowship Grant.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, Takayanagi R, Shimatsu A. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50(1):58–64. doi: 10.1007/s00535-014-0934-2. [DOI] [PubMed] [Google Scholar]
- 4.Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, Tseng JF. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115(4):741–751. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 5.Doi R. Determinants of surgical resection for pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci. 2015;22(8):610–617. doi: 10.1002/jhbp.224. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network clinical practice guidelines in oncology. Neuroendocrine Tumors. Version 1.2015. Available at: https://www.spg.pt/wp-content/uploads/Guidelines/NCCN/2015%20NET%20neuroendocrine%20(1).pdf. [Last accessed on January 21, 2022]
- 7.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT, Vienna Consensus Conference participants ENETS Consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103(2):153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Japan Neuroendocrine Tumor Society . Kanehara Publishing Co. Ltd. 2019. Committee for guidelines for the diagnosis of pancreatic and gastrointestinal neuroendocrine tumors: Clinical Practice Guidelines for Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NEN) 2019. [Google Scholar]
- 9.Lloyd RV, Osamura RY, Kloppel G, Rosai J. Geneva, IARC Press. 2017. WHO classification of tumors of endocrine organs. [Google Scholar]
- 10.Izumo W, Higuchi R, Furukawa T, Yazawa T, Uemura S, Shiihara M, Yamamoto M. Evaluation of the site and frequency of lymph node metastasis with non-functioning pancreatic neuroendocrine tumor. Eur Surg Res. 2019;60(5-6):219–228. doi: 10.1159/000504410. [DOI] [PubMed] [Google Scholar]
- 11.Song KB, Kim SC, Kim JH, Hong SM, Park KM, Hwang DW, Lee JH, Lee YJ. Prognostic factors in 151 patients with surgically resected non-functioning pancreatic neuroendocrine tumours. ANZ J Surg. 2016;86(7-8):563–567. doi: 10.1111/ans.12738. [DOI] [PubMed] [Google Scholar]
- 12.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A, Doglioni C, Delle Fave G, Fischer L, Fusai G, de Herder WW, Jann H, Komminoth P, de Krijger RR, La Rosa S, Luong TV, Pape U, Perren A, Ruszniewski P, Scarpa A, Schmitt A, Solcia E, Wiedenmann B. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104(10):764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 13.Fischer L, Bergmann F, Schimmack S, Hinz U, Prieß S, Müller-Stich BP, Werner J, Hackert T, Büchler MW. Outcome of surgery for pancreatic neuroendocrine neoplasms. Br J Surg. 2014;101(11):1405–1412. doi: 10.1002/bjs.9603. [DOI] [PubMed] [Google Scholar]
- 14.Miura T, Ohtsuka H, Aoki T, Aoki S, Hata T, Takadate T, Maeda S, Ariake K, Kawaguchi K, Masuda K, Ishida M, Mizuma M, Nakagawa K, Morikawa T, Fujishima F, Kamei T, Sasano H, Unno M. Increased neutrophil-lymphocyte ratio predicts recurrence in patients with well-differentiated pancreatic neuroendocrine neoplasm based on the 2017 World Health Organization classification. BMC Surg. 2021;21(1):176. doi: 10.1186/s12893-021-01178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harimoto N, Hoshino K, Muranushi R, Hagiwara K, Yamanaka T, Ishii N, Tsukagoshi M, Igarashi T, Tanaka H, Watanabe A, Kubo N, Araki K, Hosouchi Y, Suzuki H, Arakawa K, Hirai K, Fukazawa T, Ikota H, Shirabe K. Prognostic significance of neutrophil-lymphocyte ratio in resectablepancreatic neuroendocrine tumors with special referenceto tumor-associated macrophages. Pancreatology. 2019;19(6):897–902. doi: 10.1016/j.pan.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Japanese Society for Cancer of the Colon and Rectum . Tokyo, Kanehara Publishing Co. Ltd. 2016. JSCCR Guidelines 2016 for the Treatment of Colorectal Cancer. [Google Scholar]
- 17.Japanese Gastric Cancer Association . Kanehara Publishing Co. Ltd. 2018. Gastric cancer treatment guideline. 5th ed. [Google Scholar]
- 18.Japan Neuroendocrine Tumor Society . Kanehara publishing Co. Ltd. 2015. Committee for guidelines for the diagnosis of pancreatic and gastrointestinal neuroendocrine tumors. Gastrointestinal neuroendocrine tumor (NET) clinical practice guidelines. [Google Scholar]
- 19.Zhou X, Xie H, Xie L, Li J, Fu W. Factors associated with lymph node metastasis in radically resected rectal carcinoids: a systematic review and meta-analysis. J Gastrointest Surg. 2013;17(9):1689–1697. doi: 10.1007/s11605-013-2249-7. [DOI] [PubMed] [Google Scholar]
- 20.Hashim YM, Trinkaus KM, Linehan DC, Strasberg SS, Fields RC, Cao D, Hawkins WG. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs) Ann Surg. 2014;259(2):197–203. doi: 10.1097/SLA.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberndorfer S. Karzinoide Tumoren des Dunndarms. Frankf Z Pathol. 1907;1:426–432. [Google Scholar]
- 23.Belousova E, Karmazanovsky G, Kriger A, Kalinin D, Mannelli L, Glotov A, Karelskaya N, Paklina O, Kaldarov A. Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumours: correlation with histological findings and diagnostic performance in differentiation between tumour grades. Clin Radiol. 2017;72(2):150–158. doi: 10.1016/j.crad.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreasi V, Ricci C, Partelli S, Guarneri G, Ingaldi C, Muffatti F, Crippa S, Casadei R, Falconi M. Predictors of disease recurrence after curative surgery for nonfunctioning pancreatic neuroendocrine neoplasms (NF-PanNENs): a systematic review and meta-analysis. J Endocrinol Invest. 2021 doi: 10.1007/s40618-021-01705-2. [DOI] [PubMed] [Google Scholar]
- 25.Kawai M, Hirono S, Okada KI, Miyazawa M, Shimizu A, Kitahata Y, Kobayashi R, Ueno M, Hayami S, Tanioka K, Yamaue H. Low lymphocyte monocyte ratio after neoadjuvant therapy predicts poor survival after pancreatectomy in patients with borderline resectable pancreatic cancer. Surgery. 2019;165(6):1151–1160. doi: 10.1016/j.surg.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe J, Otani S, Sakamoto T, Arai Y, Hanaki T, Amisaki M, Tokuyasu N, Honjo S, Ikeguchi M. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduo-denectomy for pancreatic cancer. Surg Today. 2016;46(11):1258–1267. doi: 10.1007/s00595-016-1308-6. [DOI] [PubMed] [Google Scholar]
- 27.Izumo W, Higuchi R, Furukawa T, Yazawa T, Uemura S, Matsunaga Y, Shiihara M, Yamamoto M. Evaluation of the significance of adjuvant chemotherapy in patients with stage ⅠA pancreatic ductal adenocarcinoma. Pancreatology. 2021;21(3):581–588. doi: 10.1016/j.pan.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Wang WQ, Zhang WH, Gao HL, Huang D, Xu HX, Li S, Li TJ, Xu SS, Li H, Long J, Ye LY, Wu CT, Han X, Wang XH, Liu L, Yu XJ. A novel risk factor panel predicts early recurrence in resected pancreatic neuroendocrine tumors. J Gastroenterol. 2021;56(4):395–405. doi: 10.1007/s00535-021-01777-0. [DOI] [PubMed] [Google Scholar]
- 29.Zou S, Jiang Y, Wang W, Zhan Q, Deng X, Shen B. Novel scoring system for recurrence risk classification of surgically resected G1/2 pancreatic neuroendocrine tumors - Retrospective cohort study. Int J Surg. 2020;74:86–91. doi: 10.1016/j.ijsu.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Masui T, Sato A, Nakano K, Uchida Y, Yogo A, Anazawa T, Nagai K, Kawaguchi Y, Takaori K, Uemoto S. Comparison of recurrence between pancreatic and duodenal neuroendocrine neoplasmsafter curative resection: a single-institution analysis. Ann Surg Oncol. 2018;25(2):528–534. doi: 10.1245/s10434-017-6260-1. [DOI] [PubMed] [Google Scholar]
- 31.Genç CG, Jilesen AP, Partelli S, Falconi M, Muffatti F, van Kemenade FJ, van Eeden S, Verheij J, van Dieren S, van Eijck CHJ, Nieveen van Dijkum EJM. A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann Surg. 2018;267(6):1148–1154. doi: 10.1097/SLA.0000000000002123. [DOI] [PubMed] [Google Scholar]
- 32.Kim JY, Lee SH, An S, Kim SJ, Sung YN, Song KB, Hwang DW, Kim SC, Hong SM. Carbonic anhydrase 9 expression in well-differentiated pancreatic neuroendocrine neoplasms might be associated with aggressive behavior and poor survival. Virchows Arch. 2018;472(5):739–748. doi: 10.1007/s00428-018-2353-x. [DOI] [PubMed] [Google Scholar]
- 33.Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, Baba H, Tomita N, Nakagoe T, Sugihara K, Mori M. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014;259(4):773–780. doi: 10.1097/SLA.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 34.Izumo W, Higuchi R, Yazawa T, Uemura S, Shiihara M, Yamamoto M. Evaluation of preoperative risk factors for postpancreatectomy hemorrhage. Langenbecks Arch Surg. 2019;404(8):967–974. doi: 10.1007/s00423-019-01830-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P, CLARINET Investigators Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 36.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326(8):519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 37.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 38.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K, RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold R, Chen YJ, Costa F, Falconi M, Gross D, Grossman AB, Hyrdel R, Kos-Kudła B, Salazar R, Plöckinger U, Mallorca Consensus Conference participants , European Neuroendocrine Tumor Society ENETS Consensus guidelines for the standards of care in neuroendocrine tumors: follow-up and documentation. Neuroendocrinology. 2009;90(2):227–233. doi: 10.1159/000225952. [DOI] [PubMed] [Google Scholar]