Abstract
Background
Urinary continence after radical prostatectomy (RP) is an important determinant of patient quality of life. Anatomic measures at prostate MRI have been previously associated with continence outcomes, but their predictive ability and interrater agreement are unclear in comprehensive clinical models.
Purpose
To evaluate the predictive ability and interrater agreement of MRI-based anatomic measurements of post-RP continence when combined with clinical multivariable models.
Materials and Methods
In this retrospective cohort study, continence outcomes were evaluated in men who underwent RP from August 2015 to October 2019. Preoperative MRI-based anatomic measures were obtained retrospectively by four abdominal radiologists. Before participation, these radiologists completed measure-specific training. Logistic regression models were developed with clinical variables alone, MRI variables alone, and combined variables for predicting continence at 3, 6, and 12 months after RP; some patient data were missing at each time point. Interrater agreement of MRI variables was assessed by using intraclass correlation coefficients (ICCs).
Results
A total of 586 men were included (mean age ± standard deviation: 63 years ± 7). The proportion of patients with incontinence was 0.2% (one of 589) at baseline, 27% (145 of 529) at 3 months, 14% (63 of 465) at 6 months, and 9% (37 of 425) at 12 months. Longer coronal membranous urethra length (MUL) improved the odds of post-RP continence at all time points (odds ratio per 1 mm: 0.86 [95% CI: 0.80, 0.93], P < .001; 0.86 [95% CI: 0.78, 0.95], P = .003; and 0.79 [95% CI: 0.67, 0.91], P = .002, respectively) in models that incorporated both clinical and MRI predictors. No other MRI variables were predictive. Age and baseline urinary function score were the only other predictive clinical variables at every time point. Interrater agreement was moderate (ICC, 0.62) for MUL among readers with measure-specific prostate MRI training and poor among those without the training (ICC, 0.38).
Conclusion
Preoperative MRI-measured coronal membranous urethra length was an independent predictor of urinary continence after prostatectomy.
© RSNA, 2022
Summary
Coronal membranous urethra length on preoperative prostate MRI scans was an independent predictor of post-prostatectomy continence; interrater agreement of this measure was higher after measure-specific training.
Key Results
■ In a retrospective review of 586 men, membranous urethra length (MUL) was a multivariable predictor of continence at 3 months (odds ratio [OR]: 0.86; P < .001), 6 months (OR: 0.86; P = .003), and 12 months (OR: 0.79; P = .002) after prostatectomy.
■ Interrater agreement for MUL was moderate (intraclass correlation coefficient [ICC], 0.62) with measure-specific training but poor among those without the training (ICC, 0.38).
Introduction
Urinary continence after radical prostatectomy (RP) is an important determinant of patient quality of life (1). Incidence of urinary incontinence at 1 year after RP ranged from 4% to 31% in one meta-analysis evaluating the robot-assisted approach (2) and from 8% to 21% in another evaluating retropubic, laparoscopic, and robot-assisted approaches (3). The Prostate Cancer Outcomes Study found that only 35% of men had total urinary control at 12 months after RP (4). The ability to predict continence after RP could substantially aid physicians and patients when making treatment recommendations or decisions.
A variety of patient characteristics have been used to predict recovery of post-RP continence, including younger age, lower body mass index, and lower clinical tumor stage (5–8). Several anatomic measures at preoperative MRI have also been associated with continence recovery, including smaller prostate volume (5,9,10), a longer membranous urethra length (MUL) (8,11–18), closer proximity of the levator muscles to the membranous urethra (12), and the angle between the membranous urethra and prostatic axis (aMUP) (19). The pubourethral angle has been associated with stress and mixed incontinence in women but has not been evaluated in men (20,21).
The membranous urethra is hypothesized to be a major contributor to post-RP continence because there is a striated sphincter surrounding the membranous urethra (22,23). The sphincter and its nerve supply may be damaged during surgical dissection, resulting in sphincter weakness and incontinence. Although MUL has been shown to possibly enable prediction of post-RP continence, most studies evaluating MUL do not report interrater agreement (11,14–18). In the few studies that have reported agreement, there was poor to fair agreement between radiologists in training and supervising radiologists (24), excellent agreement among specialized genitourinary radiologists (25), and moderate agreement among trained experts (12). A comprehensive assessment of MUL in conjunction with clinical parameters and other potential MRI-based anatomic measures of continence prediction is needed to determine the usefulness of MUL in routine clinical care.
Our purpose was to evaluate the inclusion of MRI-based anatomic measures of post-RP continence prediction (coronal MUL, inner levator distance, outer levator distance, aMUP, pubourethral angle, prostate volume) into multivariable clinical models using data from the Michigan Urological Surgery Improvement Collaborative Patient Reported Outcomes (MUSIC-PRO) database. The secondary objective was to determine the interrater agreement of the anatomic measures.
Materials and Methods
This Health Insurance Portability and Accountability Act–compliant retrospective cohort study received institutional review board approval. The requirement for informed consent was waived. This study followed Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Patient Characteristics
The study population included adult men who underwent RP at one quaternary care facility and had available longitudinal patient-reported continence data in the MUSIC-PRO database. MUSIC-PRO comprises 45 diverse community and academic urology practices representing approximately 90% of the urologists in the state of Michigan. For all men seen in MUSIC-PRO practices who undergo a prostate biopsy, trained data abstractors prospectively enter a standardized set of demographic and clinical-pathologic data elements into the registry database. Prior reports have described the data acquisition and quality control activities for MUSIC-PRO (26,27). Each MUSIC-PRO practice obtained an exemption or approval for collaborative participation from a local institutional review board. The MUSIC-PRO database has been used to create more than 50 publications from 2014 to present (28). The MRI-based anatomic measures we collected were obtained outside of the MUSIC-PRO database and have not been previously reported.
Inclusion criteria for this study were as follows: (a) adult men who underwent retropubic robot-assisted RP between August 2015 and October 2019; (b) completion of the baseline MUSIC-PRO survey within 3 months before RP; (c) completion of at least one MUSIC-PRO survey at 3, 6, or 12 months after RP; and (d) diagnostic preoperative prostate MRI performed within 12 months before RP. Exclusion criteria were as follows: (a) missing or incomplete baseline prostate MRI data and (b) missing continence data at follow-up (Fig 1). If there was missing continence data at follow-up, then those patients were excluded only from the time points with the missing data. Different subsets were included in each model due to missing continence data at the 3-, 6-, or 12-month time points (Fig 1).
Figure 1:
Study flowchart. MUSIC = Michigan Urological Surgery Improvement Collaborative.
Data Extraction
The following data were extracted from the MUSIC-PRO database and the electronic medical record system: age at diagnosis, race, body mass index, grade group, maximum whole-gland Prostate Imaging Data and Reporting System (PI-RADS) version 2 score, TNM stage, clinical stage, presence of seminal vesicle invasion, presence of extracapsular extension, index lesion size (ie, maximum diameter of largest mass in prostate), prostate-specific antigen level at diagnosis, whether nerve-sparing operative technique was used, preoperative urinary function scores, postoperative urinary function scores, and reported MUL in the preoperative MRI report.
Imaging Procedures
All multiparametric prostate MRI examinations were performed with a 3.0-T scanner (Ingenia [Philips Healthcare] or Vida [Siemens Healthineers]) without an endorectal coil. Sequences were obtained according to PI-RADS version 2 technical specifications. All measurements were performed with axial, coronal, or sagittal small field-of-view two-dimensional T2-weighted fast spin-echo sequences. A variable-channel surface coil was used.
Continence Evaluation
Patient functional outcomes were measured by using the Expanded Prostate Cancer Index Composite 26-question short form (EPIC-26) survey before RP and at 3, 6, 12, and 24 months after RP (29–31). The survey includes questions regarding continence, urinary symptoms, and sexual function. EPIC-26 domain scores range from 0 to 100, with higher scores representing better health-related quality of life (32). EPIC-26 domain scores before RP—representing baseline urinary and sexual function—were used as clinical predictor variables in the regression models. Patient use of adult diapers or more than one urinary pad per day was used to define incontinence. For patients participating in MUSIC-PRO before 2016, their baseline EPIC-26 urinary continence domain score was calculated using a previously published crosswalk (33).
Image Analysis
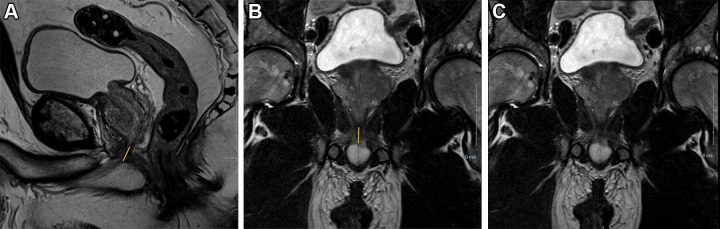
Coronal MUL and prostate volume were prospectively recorded in a structured report as part of routine clinical care. In addition, coronal and sagittal MUL, inner and outer levator distance, aMUP, and the pubourethral angle were obtained retrospectively from the same MRI study as the prospectively obtained MUL (Figs 2–3). The retrospective measurements were made by three fellowship-trained abdominal radiologists (M.S.D., P.R.S., and E.M.C., with 10, 5, and 20 years of experience, respectively) and one abdominal radiology fellow (C.H., with 1 year of experience). Each radiologist interpreted either 163 or 164 MRI examinations (ie, to evenly divide the reading assignment), with 20 overlapping interpretations for all radiologists to facilitate assessment of interrater agreement. Raters were blinded to all clinical and pathologic data. Raters were provided an atlas detailing the measurement techniques and were trained on these techniques in two sessions with example cases.
Figure 2:
T2-weighted fast spin-echo MRI scans in (A) sagittal view and (B, C) coronal views with (B) and without (C) annotation illustrate membranous urethra length (MUL) measurement technique. MUL was defined as the distance from the prostate apex to the urethral entry into the penile bulb. The yellow line in A and B represents the MUL.
Figure 3:
(A) Axial and (B, C) sagittal T2-weighted fast spin-echo MRI scans demonstrate anatomic measurement techniques. (A) Axial image shows the inner levator distance (dots) and outer levator distance (arrows). (B, C) Sagittal images show the angle between membranous urethra and prostatic axis (B) and pubourethral angle (C). Inner levator distance was measured in the axial plane and was defined as the narrowest distance between the inner borders of the levator muscles just below the caudal margin of the prostatic apex. Outer levator distance was measured in the axial plane and was defined as the distance between the outer borders of the levator muscles at the same level as the inner levator distance measurement. The angle between the membranous urethra and prostatic axis was measured in the sagittal plane and defined as the angle between the membranous urethra length and a line drawn through the prostatic axis. The pubourethral angle was measured in the sagittal plane and defined as the angle between a line drawn from the anterior bladder neck to the lower border of the pubic symphysis and a line drawn from the upper to lower border of the pubic symphysis.
MUL was measured in the coronal and sagittal planes. MUL was defined as the distance from the prostate apex to the urethral entry into the penile bulb (Fig 2) (11,12,34). Inner levator distance was measured in the axial plane and defined as the narrowest distance between the inner borders of the levator muscles, just below the caudal margin of the prostatic apex. Outer levator distance was measured in the axial plane and defined as the distance between the outer borders of the levator muscles at the same level as the inner levator distance measurement (Fig 3A) (12). The aMUP was measured in the sagittal plane and defined as the angle between the MUL and a line drawn through the prostatic axis (Fig 3B) (12). The pubourethral angle was measured in the sagittal plane and defined as the angle between a line drawn from the anterior bladder neck to the lower border of the pubic symphysis and a line drawn from the upper to lower border of the pubic symphysis (Fig 3C) (21). Prostate volume was calculated using the formula for an ellipse (length × width × height × 0.52).
Primary Outcome
The primary outcome was urinary continence at 3 months, 6 months, and 12 months following RP. Continence was assessed by using the third question from the prospectively reported short-form EPIC-26 questionnaire.
Statistical Analysis
Logistic regression models including clinical variables (eg, patient age, grade group) and MRI-based anatomic measures of continence (eg, coronal MUL, inner levator distance) were developed for 3, 6, and 12 months after RP. We compared models using baseline clinical variables alone, MRI variables alone, and combined clinical and MRI variables. The retrospective coronal MUL measured by the trained radiologists was used in the models. Sagittal MUL was not used because it was collinear with coronal MUL (ie, it measured the same structure), and the coronal measure is more commonly reported in the literature. All other retrospectively gathered MRI variables, as well as the prospectively reported prostate volume, PI-RADS version 2 score, and median lobe enlargement, were modeled. Both the prospectively and retrospectively recorded coronal MUL measurements were evaluated for interrater agreement. We assessed model discrimination using the area under the receiver operating characteristic curve (AUC). The DeLong test was used to compare AUCs between the three models at each time point (35).
P < .017 (.05 of three models) was used for significance for the three multivariable models (primary outcome). P < .05 was used for significance for all secondary analyses. Statistical analyses of the regression models were all performed using R version 3.6.0 software (R Core Team).
Interrater agreement of the MRI variables obtained from the preoperative MRI examinations were assessed using intraclass correlation coefficients (ICCs). ICCs were calculated with two-way random single measures for absolute agreement for retrospective research measures and one-way random single measures for absolute agreement when comparing research measures to clinical measures (36). ICCs were calculated for the four trained radiologists for the overlapping 20 patients for all retrospectively gathered variables. An a priori sample size calculation determined that to detect an ICC of at least 0.3, an overlap of 20 patients was needed to achieve 90% power and α of .05 (37). Agreement was also calculated between the prospectively reported MUL (no study-related training, measurement made as part of clinical care) and the retrospectively measured MUL (trained blinded reader from this study). ICC analysis was performed using software (IBM SPSS Statistics for Macintosh, version 27.0; SPSS).
Results
Patient Characteristics
The study sample is described in Table 1. Because of variable data availability at each time point, subsets of the entire population were used in each model (Fig 1). The median EPIC-26 urinary function baseline score in our study population was 100. Based on our definition, only one of the 589 patients (0.2%) was incontinent at baseline before surgery. The proportion of patients with incontinence was 27% (145 of 529) at 3 months, 14% (63 of 465) at 6 months, and 9% (37 of 425) at 12 months. Median annualized prostatectomy volume for the surgeons in this cohort was 59 prostatectomies per year (interquartile range, 38–153 prostatectomies per year).
Table 1:
Patient Demographic and Clinical Characteristics

Univariable and Multivariable Analyses
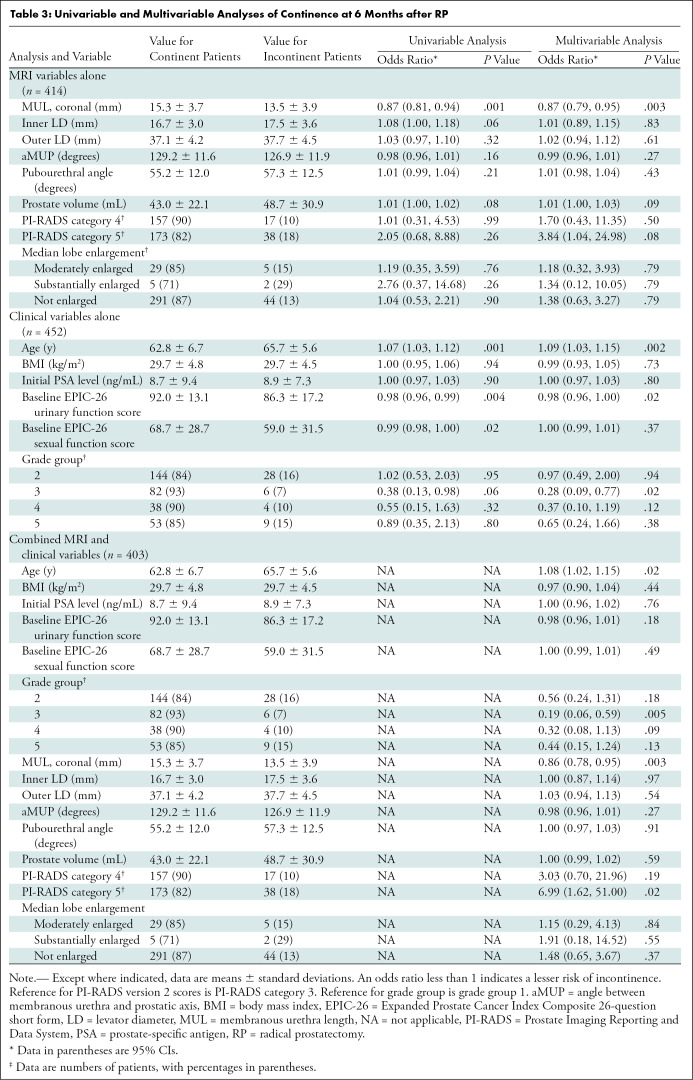
Univariable and multivariable analyses for clinical variables alone, MRI variables alone, and combined clinical and MRI variables are reported for 3, 6, and 12 months after RP (Tables 2–4, respectively). At each time point, and in both the MRI alone and combined multivariable models, a longer coronal MUL was a predictor of post-RP continence, with the effect size increasing over time (odds ratio [OR] per 1 mm for combined multivariable models: 0.86 [95% CI: 0.80, 0.93; P < .001] at 3 months, 0.86 [95% CI: 0.78, 0.95; P = .003] at 6 months, and 0.79 [95% CI: 0.67, 0.91; P = .002] at 12 months).
Table 2:
Univariable and Multivariable Analyses of Continence at 3 Months after RP
Table 4:
Univariable and Multivariable Analyses of Continence at 12 Months after RP
Table 3:
Univariable and Multivariable Analyses of Continence at 6 Months after RP
Increased prostate volume conferred a lower likelihood of continence only at 3 months after RP, but with a small effect size (combined MRI and clinical model: OR, 1.01 per milliliter gland volume [95% CI: 1.00, 1.03]). Age and EPIC-26 urinary function baseline score were the only statistically significant clinical variables at every time point, both in the univariable and multivariable clinical models (except for EPIC-26 urinary function baseline domain score at 6 months in the combination model). Regarding age in the combined multivariable models, OR per year of age was 1.07 (95% CI: 1.03, 1.12; P = .001) at 3 months, 1.08 (95% CI: 1.02, 1.15; P = .02) at 6 months, and 1.12 (95% CI: 1.03, 1.22; P = .008) at 12 months. Regarding EPIC-26 urinary function baseline scores in the combined multivariable models, OR per point was 0.98 (95% CI: 0.96, 1.00; P = .02) at 3 months and 0.95 (95% CI: 0.92, 0.97; P < .001) at 12 months.
Significant predictors at only one or two time points included inner levator distance at 3 months (univariable analysis only), PI-RADS category 4 at 3 months (univariable analysis only), PI-RADS category 5 score at 6 months (combined multivariable analysis only), baseline EPIC-26 sexual function score at 3 and 6 months (univariable analysis only), and grade group 3 at 6 months (multivariable clinical and combined analysis only) (Tables 2–4).
Model Performance Comparisons
Table E1 (online) shows the comparisons between each model (AUC at 3 months: 0.69 [clinical variables alone], 0.70 [MRI variables alone], and 0.75 [combined MRI and clinical variables]; AUC at 6 months: 0.71 [clinical variables alone], 0.71 [MRI variables alone], and 0.77 [combined MRI and clinical variables]; AUC at 12 months: 0.81 [clinical variables alone], 0.71 [MRI variables alone], and 0.82 [combined MRI and clinical variables]). There was no evidence of a difference between the combined models, the clinical variables–only models, or the MRI variables–only models (Table E1 [online]). The discriminatory function of the combined models was 0.75 (3 months), 0.77 (6 months), and 0.82 (12 months).
Interrater Agreement
In the acquired MRI measurements, ICC was assessed between the four readers. Agreement for the retrospectively acquired MRI data was fair for outer levator distance (ICC, 0.50; n = 20) and aMUP (ICC, 0.50; n = 20), good for sagittal MUL (ICC, 0.69; n = 20) and coronal MUL (ICC, 0.62; n = 19), and excellent for inner levator distance (ICC, 0.77; n = 20) and pubourethral angle (ICC, 0.82; n = 20). Agreement was poor (ICC, 0.38; n = 564) between the prospectively reported coronal MUL (clinical interpretation without study-specific training; 18 radiologists with 1–22 years of experience) and moderate (ICC, 0.62) for the retrospectively measured coronal MUL (trained reader pool). In addition to interrater agreement, the average absolute difference between the coronal and sagittal MUL was calculated (average difference ± standard deviation, 1.8 mm ± 2.0; n = 586).
Discussion
Urinary continence after radical prostatectomy (RP) is an important determinant of patient quality of life. In this single-center analysis of prospectively reported continence outcomes and retrospectively abstracted MRI data, we used multivariable logistic regression models to predict patient-reported continence after RP. Multivariable models with and without comprehensive clinical data demonstrated that a longer membranous urethra length (MUL) was a significant independent predictor of continence at 3 (odds ratio [OR]: 0.86 per 1 mm; P < .001), 6 (OR: 0.86 per 1 mm; P = .003), and 12 (OR: 0.79 per 1 mm; P = .002) months after RP. These results indicate the durability of the finding (ie, internal validity), and that for each 1-mm increase in coronal MUL, the odds of continence increased by 14% (3 months) to 21% (12 months). In other words, a longer preoperative MUL increases the likelihood of post-RP continence recovery. Among the clinical variables, older age and lower baseline urinary function scores were the most consistent predictors of incontinence.
The association of MUL with continence outcomes in men after RP has been observed in other studies (8,11–14,16–18). In the largest prior study, von Bodman et al (12) studied 600 men and found greater MUL-predicted continence recovery at 6 and 12 months, while larger outer levator distance was associated with incontinence at 12 months and larger inner levator distance was associated with incontinence at 6 months. They also found that MUL and levator muscle distance improved discrimination of a clinical regression model when MRI variables were included. Although MUL was a significant predictor in all our models, we did not find inner or outer levator distance to have a significant multivariable effect (inner levator distance at 3 months only was significant in univariable analysis). Our study differed in that we evaluated continence outcomes at 3 months after RP, and we report multivariable ORs for all variables. Tienza et al (16) and others (9–11) found a larger prostate volume to be associated with an increased risk of incontinence after RP. In our models, prostate volume had a minor effect at 3 months but was not significant at 6 and 12 months. Unlike Regis et al (19), we did not find aMUP to be predictive of continence after RP. We also did not find a significant predictive effect for the pubourethral angle (21). Of the six MRI-based anatomic measures of continence we studied, only MUL was found to be an important predictor of post-RP continence.
Following reader training, we observed fair or greater interrater agreement for all retrospectively obtained MRI measures. For coronal MUL, interrater agreement was moderate following training (ICC, 0.62) but poor between the trained readers and the original prospective interpretation (ICC, 0.38). This implies that if MUL is going to be incorporated into clinical practice, specific training will be needed to ensure that the measurements are being performed accurately and consistently. The study-related interrater agreement we observed was superior to agreements observed by Curci et al (24) (among trained experts and general radiologists, ICCs ranged from 0.29 to 0.42 [poor to fair]) and von Bodman et al (12) (among trained experts, they obtained a moderate weighted κ statistic of 0.48). We observed a 1.8-mm average difference between MUL in the coronal versus sagittal plane. Although we do not know which is the “true” MUL length, we have shown that standardized MUL in the coronal plane is useful for continence prediction.
Our study had several limitations. First, each of our multivariable models contains a slightly different cohort of patients, dependent on the availability of data at each time point. Rather than longitudinally following up one cohort over time, we instead have multiple cross-sectional cohorts that each contain a large degree of overlap. Second, although we used prospectively reported measures of longitudinal post-RP urinary continence, the MRI anatomic measures we studied were obtained retrospectively following specific training. It is likely MUL would be less predictive if the measurements were obtained by untrained radiologists. Third, inclusion of a continence nomogram would have created more practical clinical value, but we did not have the sample size available to create a validation cohort. A larger sample size at each time point would have improved power to detect other significant predictors of continence. Finally, we defined incontinence as use of adult diapers or greater than one urinary pad per day, which was more liberal than what has been reported previously. However, continence data were obtained prospectively using a validated questionnaire and was consistent across our patient population (30).
In conclusion, our findings support the use of coronal membranous urethra length (MUL) in combination with clinical variables to predict post–radical prostatectomy urinary continence. Our data also support the need for specific training of radiologists in performing MUL measurements to improve interrater agreement. Development of a continence nomogram in an independent validation cohort that incorporates clinical and MRI measures could guide patients and providers considering prostate cancer treatment options.
Acknowledgments
Acknowledgment
We thank Rodney Dunn, MS, senior biostatistician and director of the analytic core in the Dow Division of Health Services Research in the Department of Urology at Michigan Medicine (Ann Arbor, Mich), for his advice on the statistical methodology.
Disclosures of conflicts of interest: H.L. No relevant relationships. P.R.S. No relevant relationships. K.S. Payments from Blue Cross Blue Shield of Michigan to institution for work done as part of the Michigan Urological Surgery Collaborative; payments from National Institute of Diabetes and Digestive and Kidney Diseases, University of Michigan internal grants to institution for K12 grant; payments from Harvard Medical School for lectures given as part of the Safety, Quality, Informatics, and Leadership program. E.M.C. No relevant relationships. A.K.G. No relevant relationships. C.H. No relevant relationships. A.J. Support from Blue Cross Blue Shield of Michigan. M.S.D. Royalties from Wolters Kluwer for an unrelated book review; member of the Radiology editorial board.
Abbreviations:
- aMUP
- angle between the membranous urethra and prostatic axis
- AUC
- area under the receiver operating characteristic curve
- EPIC-26
- Expanded Prostate Cancer Index Composite 26-question short form
- ICC
- intraclass correlation coefficient
- MUL
- membranous urethra length
- MUSIC-PRO
- Michigan Urological Surgery Improvement Collaborative Patient Reported Outcomes
- OR
- odds ratio
- PI-RADS
- Prostate Imaging Data and Reporting System
- RP
- radical prostatectomy
References
- 1. Bernardes MFVG , Chagas SC , Izidoro LCR , Veloso DFM , Chianca TCM , Mata LRFP . Impact of urinary incontinence on the quality of life of individuals undergoing radical prostatectomy . Rev Lat Am Enfermagem 2019. ; 27 e3131 . [Published correction appears in Rev Lat Am Enfermagem. 2019 Dec 5;27:e3244.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ficarra V , Novara G , Rosen RC , et al . Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy . Eur Urol 2012. ; 62 ( 3 ): 405 – 417 . [DOI] [PubMed] [Google Scholar]
- 3. Coelho RF , Rocco B , Patel MB , et al . Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a critical review of outcomes reported by high-volume centers . J Endourol 2010. ; 24 ( 12 ): 2003 – 2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Penson DF , McLerran D , Feng Z , et al . 5-year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study . J Urol 2008. ; 179 ( 5 Suppl ): S40 – S44 . [DOI] [PubMed] [Google Scholar]
- 5. Konety BR , Sadetsky N , Carroll PR ; CaPSURE Investigators . Recovery of urinary continence following radical prostatectomy: the impact of prostate volume–analysis of data from the CaPSURE Database . J Urol 2007. ; 177 ( 4 ): 1423 – 1425 ; discussion 1425–1426 . [DOI] [PubMed] [Google Scholar]
- 6. Kim JJ , Ha YS , Kim JH , et al . Independent predictors of recovery of continence 3 months after robot-assisted laparoscopic radical prostatectomy . J Endourol 2012. ; 26 ( 10 ): 1290 – 1295 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim JH , Ha YS , Jeong SJ , Lee DH , Kim WJ , Kim IY . Impact of robot-assisted radical prostatectomy on lower urinary tract symptoms and predictive factors for symptom changes: a longitudinal study . Urology 2013. ; 81 ( 4 ): 787 – 793 . [DOI] [PubMed] [Google Scholar]
- 8. Mendoza PJ , Stern JM , Li AY , et al . Pelvic anatomy on preoperative magnetic resonance imaging can predict early continence after robot-assisted radical prostatectomy . J Endourol 2011. ; 25 ( 1 ): 51 – 55 . [DOI] [PubMed] [Google Scholar]
- 9. Link BA , Nelson R , Josephson DY , et al . The impact of prostate gland weight in robot assisted laparoscopic radical prostatectomy . J Urol 2008. ; 180 ( 3 ): 928 – 932 . [DOI] [PubMed] [Google Scholar]
- 10. Song C , Doo CK , Hong JH , Choo MS , Kim CS , Ahn H . Relationship between the integrity of the pelvic floor muscles and early recovery of continence after radical prostatectomy . J Urol 2007. ; 178 ( 1 ): 208 – 211 . [DOI] [PubMed] [Google Scholar]
- 11. Coakley FV , Eberhardt S , Kattan MW , Wei DC , Scardino PT , Hricak H . Urinary continence after radical retropubic prostatectomy: relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging . J Urol 2002. ; 168 ( 3 ): 1032 – 1035 . [DOI] [PubMed] [Google Scholar]
- 12. von Bodman C , Matsushita K , Savage C , et al . Recovery of urinary function after radical prostatectomy: predictors of urinary function on preoperative prostate magnetic resonance imaging . J Urol 2012. ; 187 ( 3 ): 945 – 950 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grivas N , van der Roest R , Schouten D , et al . Quantitative assessment of fascia preservation improves the prediction of membranous urethral length and inner levator distance on continence outcome after robot-assisted radical prostatectomy . Neurourol Urodyn 2018. ; 37 ( 1 ): 417 – 425 . [DOI] [PubMed] [Google Scholar]
- 14. Paparel P , Akin O , Sandhu JS , et al . Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging . Eur Urol 2009. ; 55 ( 3 ): 629 – 637 . [DOI] [PubMed] [Google Scholar]
- 15. Kim M , Park M , Pak S , et al . Integrity of the Urethral Sphincter Complex, Nerve-sparing, and Long-term Continence Status after Robotic-assisted Radical Prostatectomy . Eur Urol Focus 2019. ; 5 ( 5 ): 823 – 830 . [DOI] [PubMed] [Google Scholar]
- 16. Tienza A , Hevia M , Benito A , Pascual JI , Zudaire JJ , Robles JE . MRI factors to predict urinary incontinence after retropubic/laparoscopic radical prostatectomy . Int Urol Nephrol 2015. ; 47 ( 8 ): 1343 – 1349 . [DOI] [PubMed] [Google Scholar]
- 17. Jeong CW , Oh JJ , Jeong SJ , et al . Effect of dorsal vascular complex size on the recovery of continence after radical prostatectomy . World J Urol 2013. ; 31 ( 2 ): 383 – 388 . [DOI] [PubMed] [Google Scholar]
- 18. Hakimi AA , Faleck DM , Agalliu I , Rozenblit AM , Chernyak V , Ghavamian R . Preoperative and intraoperative measurements of urethral length as predictors of continence after robot-assisted radical prostatectomy . J Endourol 2011. ; 25 ( 6 ): 1025 – 1030 . [DOI] [PubMed] [Google Scholar]
- 19. Regis L , Cuadras M , Miret E , et al . MP05-04 Anatomical Benchmarks in Preoperative Magnetic Resonance Imaging Predict Early Continence Recovery Following Robotic Radical Prostatectomy . J Urol 2018. ; 199 ( 4S ): e43 . [Google Scholar]
- 20. Hu Y , Lou Y , Liao L , et al . Comparison of Urodynamics and Perineal Ultrasonography for the Diagnosis of Mixed Urinary Incontinence in Women . J Ultrasound Med 2018. ; 37 ( 11 ): 2647 – 2656 . [DOI] [PubMed] [Google Scholar]
- 21. Tunn R , Schaer G , Peschers U , et al . Updated recommendations on ultrasonography in urogynecology . Int Urogynecol J Pelvic Floor Dysfunct 2005. ; 16 ( 3 ): 236 – 241 . [DOI] [PubMed] [Google Scholar]
- 22. Myers RP , Cahill DR , Devine RM , King BF . Anatomy of radical prostatectomy as defined by magnetic resonance imaging . J Urol 1998. ; 159 ( 6 ): 2148 – 2158 . [DOI] [PubMed] [Google Scholar]
- 23. Dubbelman YD , Bosch JLHR . Urethral sphincter function before and after radical prostatectomy: Systematic review of the prognostic value of various assessment techniques . Neurourol Urodyn 2013. ; 32 ( 7 ): 957 – 963 . [DOI] [PubMed] [Google Scholar]
- 24. Curci NE , Gartland P , Shankar PR , et al . Long-distance longitudinal prostate MRI quality assurance: from startup to 12 months . Abdom Radiol (NY) 2018. ; 43 ( 9 ): 2505 – 2512 . [DOI] [PubMed] [Google Scholar]
- 25. Sauer M , Tennstedt P , Berliner C , et al . Predictors of short- and long-term urinary incontinence after radical prostatectomy in prostate MRI: Significance and reliability of standardized measurements . Eur J Radiol 2019. ; 120 108668 . [DOI] [PubMed] [Google Scholar]
- 26. Womble PR , Montie JE , Ye Z , et al . Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer . Eur Urol 2015. ; 67 ( 1 ): 44 – 50 . [DOI] [PubMed] [Google Scholar]
- 27. Womble PR , Linsell SM , Gao Y , et al . A Statewide Intervention to Reduce Hospitalizations after Prostate Biopsy . J Urol 2015. ; 194 ( 2 ): 403 – 409 . [DOI] [PubMed] [Google Scholar]
- 28. Publications . Michigan Urological Surgery Improvement Collaborative (MUSIC) . https://musicurology.com/publications/. Accessed February 15, 2021 . [Google Scholar]
- 29. PRO - Patient Reported Outcomes . Michigan Urological Surgery Improvement Collaborative (MUSIC) . https://musicurology.com/pro/. Accessed May 26, 2020 . [Google Scholar]
- 30. Wei JT , Dunn RL , Litwin MS , Sandler HM , Sanda MG . Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer . Urology 2000. ; 56 ( 6 ): 899 – 905 . [DOI] [PubMed] [Google Scholar]
- 31. EPIC-26 Short Form . https://medicine.umich.edu/sites/default/files/content/downloads/EPIC-SF-6.2002_0.pdf. Accessed January 22, 2021 .
- 32. Sanda MG , Wei JT , Litwin MS . Scoring Instructions for the Expanded Prostate cancer Index Composite Short Form (EPIC-26) . https://medicine.umich.edu/sites/default/files/content/downloads/Scoring%20Instructions%20for%20the%20EPIC%2026.pdf. Published 2002. Accessed May 20, 2020 .
- 33. Singh K , Tin AL , Dunn RL , Kim T , Vickers AJ . Development and Validation of Crosswalks for Patient-reported Sexual and Urinary Outcomes Between Commonly Used Instruments . Eur Urol 2019. ; 75 ( 5 ): 723 – 730 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mungovan SF , Sandhu JS , Akin O , Smart NA , Graham PL , Patel MI . Preoperative Membranous Urethral Length Measurement and Continence Recovery Following Radical Prostatectomy: A Systematic Review and Meta-analysis . Eur Urol 2017. ; 71 ( 3 ): 368 – 378 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeLong ER , DeLong DM , Clarke-Pearson DL . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach . Biometrics 1988. ; 44 ( 3 ): 837 – 845 . [PubMed] [Google Scholar]
- 36. Landers R . Computing Intraclass Correlations (ICC) as Estimates of Interrater Reliability in SPSS . Winnower 2015. ; 2 : e143518.81744 . [Google Scholar]
- 37. Bujang MA , Baharum N . A simplified guide to determination of sample size requirements for estimating the value of intraclass correlation coefficient: a review . Arch Orofac Sci 2017. ; 12 ( 1 ): 1 – 11 . http://mymedr.afpm.org.my/publications/55349 . [Google Scholar]