Abstract
Background/Aim
To evaluate the relationship between treatment period and overall survival (OS) and to identify clinical factors associated with OS in patients with metastatic renal cell carcinoma (mRCC).
Patients and Methods
Two hundred thirteen consecutive patients with mRCC receiving systemic therapy between 2008 and 2020 were divided into two groups: those starting first-line therapy in 2008-2015 (n=133) and those in 2016-2020 (n=80). Clinical factors associated with OS were retrospectively and statistically analyzed.
Results
Median OS and one-, three- and five-year OS rates were not reached and 88.7%, 64.9%, and 64.9% in patients treated in 2016-2020; 31.4 months and 78.5%, 42.8% and 34.2% in 2008-2015 (p=0.0013). Multivariate analysis identified the period in which first-line therapy was started as the strongest predictor for OS (p=0.0002).
Conclusion
OS was significantly better in mRCC patients treated in 2016-2020 than in 2008-2015. Treatment period was the strongest predictor for OS.
Keywords: Systemic therapy, ICI therapy, treatment period, metastatic renal cell carcinoma
The ongoing improvement of our understanding of molecular biology has led to major breakthroughs in medical treatment for patients with metastatic renal cell carcinoma (mRCC) (1).
Nivolumab, a fully human IgG4 programmed death 1 antibody, is the first immune checkpoint inhibitor (ICI) therapy that has been available for the treatment of mRCC in clinical practice since 2016 (2,3). Currently, the ICI combination therapy and the ICI plus tyrosine kinase inhibitor (TKI) combination therapy, a category that includes the nivolumab plus ipilimumab, pembrolizumab plus axitinib, and avelumab plus axitinib therapies, have become the mainstays of medical treatment of mRCC in clinical practice in Japan. Various clinical studies have demonstrated that upgraded systemic therapies improve patient outcomes including overall survival (OS) (4). Yet, there are few reports showing the effect on mRCC prognosis of the recent replacement of previous systemic therapies with upgraded versions in real-world clinical practice in Japan (5). The present study aimed to confirm whether the therapeutic results of mRCC have truly been improved by the recent upgrades. We conducted a single-institutional retrospective study comparing therapeutic outcomes in Japanese patients with mRCC treated between 2008 and 2015 with those treated between 2016 and 2020. We also aimed to identify the clinical factors associated with OS in patients with mRCC in real-world clinical practice.
Patients and Methods
Study population. The clinical and laboratory data from 213 consecutive patients with mRCC who started treatment with TKI or ICI therapy between April 2008 and December 2020 at our institution were retrospectively investigated. The patients were divided into two groups based on the year in which they started first-line therapy: 2008-2015 (n=133) or 2016-2020 (n=80). This study was approved by the institutional review board at the Cancer Institute Hospital, Japanese Foundation for Cancer Research.
We recorded each patient’s medical history; physical examination data, Karnofsky performance status (KPS), laboratory data, and chest radiography findings both before initiating and during TKI or ICI therapy; TKI or ICI was administered to each patient based on the attending physician’s decision. We evaluated the objective response by computed tomography (CT) every two or three months according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.1 (6).
Statistical analysis. Descriptive statistics for continuous variables were presented as the median and interquartile range (IQR), and categorical variables were reported as frequencies and percentages. OS period was defined as the time from initiation of first-line targeted or ICI therapy to final follow-up or death from any cause. In addition, we investigated the following variables as candidate predictors associated with OS: age, sex, KPS, duration from diagnosis to treatment, blood hemoglobin concentration, corrected serum calcium, platelet count, serum lactate dehydrogenase (LDH), neutrophil count, lymphocyte count, history of cytoreductive nephrectomy, histology (clear cell vs. non-clear cell cancer), metastasis at the time of diagnosis, period in which first-line therapy was started, International Metastatic Renal Cell Cancer Database Consortium (IMDC) risk classification and duration of first-line therapy. OS curves were estimated according to the Kaplan–Meier method and compared among the categorical covariates with the log-rank test for univariate analysis. Using a univariate and multivariate Cox proportional hazards regression analysis, any significant association between OS and clinical factors was investigated. The hazard ratio (HR) and 95% confidence interval (CI) were calculated for each predictor of OS. All of the statistical analyses were performed using JMP software version 14.0 (SAS Institute Inc., Cary, NC, USA) and p-values <0.05 were considered statistically significant.
Results
Patient characteristics. Median follow-up period was 23.9 (IQR=9.6-44.4) months from initiation of first-line therapy. Patient characteristics are presented in Table I. No patient characteristics were significantly different between the two groups (all, p>0.05) except for ICI therapy (p<0.001) (Table I). Of the patients treated in 2008-2015 and 2016-2020, 96 (72%) and 16 (20%) patients, respectively, had died because of disease progression, while the remaining 37 (28%) and 64 (80%) patients, respectively, were alive at the time of this writing. Twenty-five (19%) of the patients treated in 2008-2015 and 55 (69%) of the patients treated in 2016-2020 received ICI therapy (p<0.001). One, 11, 10, and 3 patients treated in 2008-2015 received ICI as a first-line, second-line, third-line, and fourth-line therapy, respectively, while 20, 32, and 3 patients treated in 2016-2020 received ICI as a first-line, second-line and third-line therapy, respectively. According to the IMDC risk classification2, the numbers of patients with favorable, intermediate, poor risk, and unknown were 37 (28%), 65 (49%), 24 (18%), and 7 (5%) in the 2008-2015 group and 34 (42%), 27 (34%), 14 (18%), and 5 (6%) in the 2016-2020 group. Although a higher proportion of patients had favorable risk in the 2016-2020 group compared to the 2008-2015 group, this difference was not significant (p=0.053).
Table I. Patient characteristics (n=213).
IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; ICI: immune checkpoint inhibitor.
Comparison of overall survival between the 2008-2015 and 2016-2020 groups. From the initiation of first-line therapy, median OS and five- and 10-year OS rates in all patients were 35.2 months, 40.9%, and 20.7%, respectively (Figure 1). Median OS and one-, three-, and five-year OS rates in patients treated in 2016-2020 were not reached and 88.7%, 64.9%, and 64.9%, respectively; the corresponding figures for patients treated in 2008-2015 were 31.4 months and 78.5%, 42.8%, and 34.2%, respectively (p=0.0013) (Figure 2). ICI therapy was discontinued for 19 (76%) patients treated in 2008-2015 and 27 (49%) patients treated in 2016-2020. After ICI therapy, 13, 1, 1, and 4 patients treated in 2008-2015 switched to axitinib, switched to sunitinib, enrolled in a clinical trial of another therapy, and died, respectively, while 18, 2, 1, and 6 patients treated in 2016-2020 switched to axitinib, switched to cabozantinib, switched to sunitinib, and died, respectively.
Figure 1. Overall survival from initiation of first-line therapy in all patients with metastatic renal cell carcinoma (mRCC) treated between 2008 and 2020 (n=213).
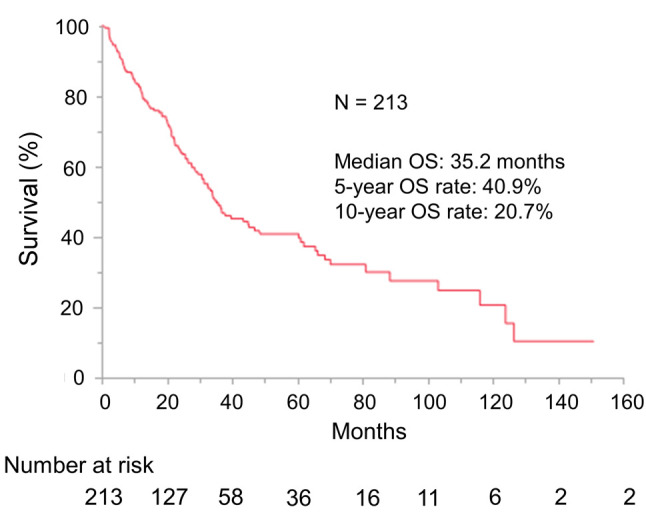
Figure 2. Comparison of overall survival from initiation of first-line therapy in metastatic renal cell carcinoma (mRCC) patients treated in 2016-2020 (n=80) and those treated in 2008-2015 (n=133). Overall survival was significantly improved in the period of 2016-2020 compared with 2008-2015.
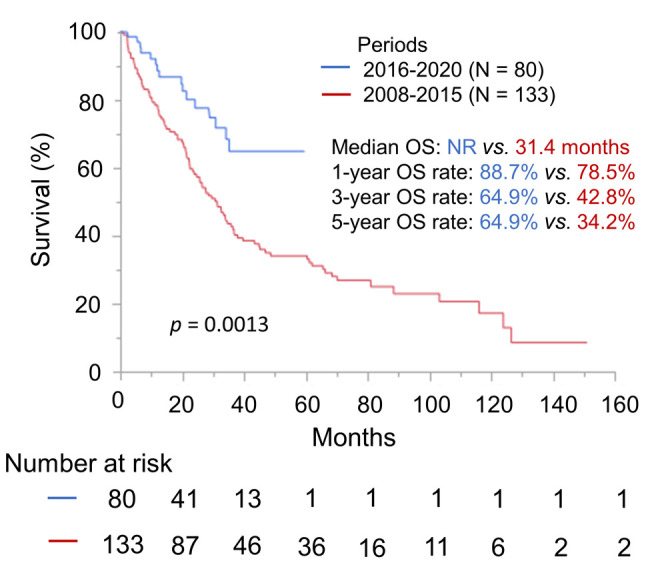
Next, we investigated which of the clinical factors were predictors of OS. In multivariate analysis, period in which first-line therapy was started (HR=0.38, 95%CI=0.21-0.65, p=0.0002) was extracted as the strongest predictor for OS followed by corrected serum calcium (p=0.0006), KPS (p=0.0014), hemoglobin (p=0.0043), neutrophil count (p=0.0063), and history of nephrectomy (p=0.015) in that order. Although ICI therapy was not significantly associated with OS, OS did tend to be longer in ICI therapy recipients (HR=0.69, 95%CI=0.44-1.06, p=0.089) (Table II).
Table II. Comparison of clinical factors as predictors of overall survival.
HR: Hazard ratio; CI: confidence interval; LLN: lower limit of normal range; ULN: upper limit of normal range; NS: not significant; LDH: lactate dehydrogenase; IMDC: International Metastatic Renal Cell Cancer Database Consortium classification.
Discussion
In this study, we confirmed that the therapeutic outcomes of real-world clinical practice treatment for mRCC were significantly better among patients who started treatment in 2016-2020 than among those who started treatment in 2008-2015. Likewise, multivariate analyses identified the period in which first-line therapy was started as the factor that most strongly predicted OS.
Targeted therapies (TT) including TKI and mTOR inhibitors, which were approved for use in Japan in 2008, have dramatically changed the mainstream of mRCC treatment, and greatly improved patient outcomes including OS compared with the previous treatment, that is cytokine-based [interleukin 2 and interferon (IFN)-α] treatment (7-10). In real-world clinical practice at the start of the TKI era, Heng et al. demonstrated the satisfactory treatment outcomes and safety profiles of these targeted agents in a retrospective study in a large cohort. In that study, 396, 200, and 49 patients were treated with sunitinib, sorafenib, and bevacizumab, respectively, as a first-line therapy. The median OS period was 22 months (95%CI=20.2-26.5) (11). Heng et al. also identified higher neutrophil count, higher platelet count, lower hemoglobin concentration, higher serum corrected calcium, poorer KPS, and a duration of less than one year between diagnosis and treatment as the risk variables for short OS in the TKI era; these are currently known as the IMDC risk factors (11). In a previous single-institutional retrospective study (12), we likewise reported an estimated median PFS of 9.3 months (95%CI=5.0-13.7) and an OS of 32.2 months (95%CI=24.4-40.0).
Various clinical trials (13-15) have reported that ICI therapies improve the clinical outcomes of patients with mRCC. Some other clinical trials have reported the efficacy of ICI therapies among Japanese patients with advanced RCC (aRCC). Japanese patients with aRCC in the IMDC intermediate/poor-risk group tended to have longer OS when they were treated with nivolumab plus ipilimumab rather than sunitinib (16). In real-world clinical practice in the early years of the ICI era, most patients received ICI therapy as a second- or later-line therapy (17,18). In the first large retrospective study of ICI therapy including 90% of patients with clear cell and 10% with non-clear cell carcinoma, conducted by the IMDC study group, the median OS was not reached (95%CI=NR-NR) in the favorable-risk group, was 26.7 months (95%CI=20.6-48.5) in the intermediate-risk group, and 7.4 months (95%CI=4.8-16.7) in the poor-risk group (p<0.0001), in the second-line setting. These findings confirmed that the IMDC classification also divided patients into appropriate risk groups in the ICI era (17). Likewise, we have previously demonstrated that, among patients treated with nivolumab as a second- or later-line therapy, the estimated OS period and one-year and two-year OS rates from initiation of nivolumab were not reached and 91.1% and 86.2%, respectively (18).
Nevertheless, there are still few real-world reports evaluating prognosis for mRCC patients receiving systemic therapy in the ICI era in Japan (5). To address this gap, we compared the treatment outcomes of systemic therapies started in 2016-2020 with those started in 2008-2015, because ICI therapy was introduced into clinical practice in Japan in 2016, while TTs were introduced in 2008. In 2016-2020, ICI therapy was administered to a significantly larger proportion of patients with mRCC, and OS was significantly better in our study. It was previously reported that nivolumab administration appeared to improve OS in patients with metastatic non-clear cell RCC (5). In our study, although this trend was not significant, ICI therapy was associated with longer OS.
A larger number of treatment lines is reported to be associated with prolonged OS (5,19). In our study, however, there was no significant difference in the number of treatment lines between patients treated in 2016-2020 and those treated in 2008-2015 (mean±SD; 1.9±1.1 vs. 2.0±1.2, p=0.667). We previously reported that objective response rate (ORR) and tumor shrinkage rate were significantly better in patients who received axitinib after nivolumab therapy than in patients who received axitinib after other therapies (p=0.026 and p=0.012) (20). Another report also demonstrated the efficacy of axitinib as a third-line therapy after the failure of second-line nivolumab monotherapy (21). Sequencing therapies in which ICI is followed by TKI may also contribute to better OS in patients who started treatment in 2016-2020. Recently, some clinical trials have suggested that new regimens such as cabozantinib plus nivolumab and lenvatinib plus pembrolizumab may contribute to better clinical outcomes in mRCC patients (22,23). It is our hope that treatment outcomes for mRCC patients will continue to improve year by year.
We conducted this retrospective study to confirm that OS was better among mRCC patients who started treatment in 2016-2020 compared with those who started treatment in 2008-2015. A major limitation of our study is that it was a small, retrospective study at a single institution. However, no general clinical trials or no large prospective study have been performed on mRCC patients in Japan. Our study, showing the improvement of therapeutic outcomes associated with the transition to new drug therapies for patients with mRCC in real-world clinical practice in Japan will therefore be useful.
In conclusion, OS was significantly better in mRCC patients who started treatment in 2016-2020 than in those who started treatment in 2008-2015, and the period in which first-line therapy was started was the strongest predictor of OS. Further study is necessary to evaluate the effect of ICI therapy on OS in patients with mRCC.
Conflicts of Interest
T. Yuasa has received remuneration for lectures from Pfizer Japan (Tokyo, Japan), Novartis Pharma Japan (Tokyo, Japan), Ono Pharma (Osaka, Japan), Bristol-Myers Squibb Japan (Tokyo, Japan), and MSD Japan (Tokyo, Japan). The other Authors have declared no conflicts of interest.
Authors’ Contributions
Ryo Fujiwara: Methodology, Formal analysis, Investigation, Data Curation, Writing-Original draft, Visualization. Yoshinobu Komai: Writing-Review and Editing. Tomohiko Oguchi: Writing-Review and Editing. Noboru Numao: Writing-Review and Editing. Shinya Yamamoto: Writing-Review and Editing. ,Junji Yonese: Supervision. Takeshi Yuasa: Conceptualization, Writing-Review and Editing, Project administration. All Authors discussed the results and contributed to the final manuscript.
Acknowledgements
This work was partly supported by JSPS KAKENHI Grant Number 16K11035 (T.Y.).
References
- 1.Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V, Eisen T, Horwich A, ESMO Guidelines Working Group Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii49–iii56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 2.Yuasa T, Masuda H, Yamamoto S, Numao N, Yonese J. Biomarkers to predict prognosis and response to checkpoint inhibitors. Int J Clin Oncol. 2017;22(4):629–634. doi: 10.1007/s10147-017-1122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 4.Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin. 2017;67(6):507–524. doi: 10.3322/caac.21411. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara H, Tachibana H, Takagi T, Yoshida K, Kondo T, Tanabe K. Effect of improved systemic therapy on patient survival in metastatic non-clear-cell renal cell carcinoma. Int J Urol. 2021;28(5):605–607. doi: 10.1111/iju.14523. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ, Global ARCC Trial Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A, RECORD-1 Study Group Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Soerensen AV, Donskov F, Hermann GG, Jensen NV, Petersen A, Spliid H, Sandin R, Fode K, Geertsen PF. Improved overall survival after implementation of targeted therapy for patients with metastatic renal cell carcinoma: results from the Danish Renal Cancer Group (DARENCA) study-2. Eur J Cancer. 2014;50(3):553–562. doi: 10.1016/j.ejca.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Lindskog M, Wahlgren T, Sandin R, Kowalski J, Jakobsson M, Lundstam S, Ljungberg B, Harmenberg U. Overall survival in Swedish patients with renal cell carcinoma treated in the period 2002 to 2012: Update of the RENCOMP study with subgroup analysis of the synchronous metastatic and elderly populations. Urol Oncol. 2017;35(9):541.e15–541.e22. doi: 10.1016/j.urolonc.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 12.Yuasa T, Tsuchiya N, Urakami S, Horikawa Y, Narita S, Inoue T, Saito M, Yamamoto S, Yonese J, Fukui I, Nakano K, Takahashi S, Hatake K, Habuchi T. Clinical efficacy and prognostic factors for overall survival in Japanese patients with metastatic renal cell cancer treated with sunitinib. BJU Int. 2012;109(9):1349–1354. doi: 10.1111/j.1464-410X.2011.10534.x. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B, CheckMate 214 Investigators Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T, KEYNOTE-426 Investigators Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH Jr, Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomita Y, Kondo T, Kimura G, Inoue T, Wakumoto Y, Yao M, Sugiyama T, Oya M, Fujii Y, Obara W, Motzer RJ, Uemura H. Nivolumab plus ipilimumab versus sunitinib in previously untreated advanced renal-cell carcinoma: analysis of Japanese patients in CheckMate 214 with extended follow-up. Jpn J Clin Oncol. 2020;50(1):12–19. doi: 10.1093/jjco/hyz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip SM, Wells C, Moreira R, Wong A, Srinivas S, Beuselinck B, Porta C, Sim HW, Ernst DS, Rini BI, Yuasa T, Basappa NS, Kanesvaran R, Wood LA, Canil C, Kapoor A, Fu SYF, Choueiri TK, Heng DYC. Checkpoint inhibitors in patients with metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer. 2018;124(18):3677–3683. doi: 10.1002/cncr.31595. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara R, Inamura K, Yuasa T, Numao N, Yamamoto S, Masuda H, Kawauchi A, Takeuchi K, Yonese J. Efficacy and safety profile of nivolumab for Japanese patients with metastatic renal cell cancer. Int J Clin Oncol. 2020;25(1):151–157. doi: 10.1007/s10147-019-01542-7. [DOI] [PubMed] [Google Scholar]
- 19.Ko JJ, Choueiri TK, Rini BI, Lee JL, Kroeger N, Srinivas S, Harshman LC, Knox JJ, Bjarnason GA, MacKenzie MJ, Wood L, Vaishampayan UN, Agarwal N, Pal SK, Tan MH, Rha SY, Yuasa T, Donskov F, Bamias A, Heng DY. First-, second-, third-line therapy for mRCC: benchmarks for trial design from the IMDC. Br J Cancer. 2014;110(8):1917–1922. doi: 10.1038/bjc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasuoka S, Yuasa T, Fujiwara R, Komai Y, Numao N, Yamamoto S, Kondo Y, Yonese J. Efficacy and safety of axitinib therapy after nivolumab for patients with metastatic renal cell cancer. Anticancer Res. 2020;40(11):6493–6497. doi: 10.21873/anticanres.14671. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara H, Takagi T, Kondo T, Fukuda H, Tachibana H, Yoshida K, Iizuka J, Okumi M, Ishida H, Tanabe K. Efficacy of axitinib after nivolumab failure in metastatic renal cell carcinoma. In Vivo. 2020;34(3):1541–1546. doi: 10.21873/invivo.11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, Suárez C, Hamzaj A, Goh JC, Barrios C, Richardet M, Porta C, Kowalyszyn R, Feregrino JP, Żołnierek J, Pook D, Kessler ER, Tomita Y, Mizuno R, Bedke J, Zhang J, Maurer MA, Simsek B, Ejzykowicz F, Schwab GM, Apolo AB, Motzer RJ, CheckMate 9ER Investigators Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ, Kozlov V, Alyasova A, Hong SH, Kapoor A, Alonso Gordoa T, Merchan JR, Winquist E, Maroto P, Goh JC, Kim M, Gurney H, Patel V, Peer A, Procopio G, Takagi T, Melichar B, Rolland F, De Giorgi U, Wong S, Bedke J, Schmidinger M, Dutcus CE, Smith AD, Dutta L, Mody K, Perini RF, Xing D, Choueiri TK, CLEAR Trial Investigators Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]