Abstract
Background/Aim
To investigate the utility of peripheral blood biomarkers – absolute lymphocyte count (ALC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) – for predicting outcomes in eribulin-treated patients with metastatic human epidermal growth factor receptor type 2 (HER2)-negative breast cancer.
Patients and Methods
ALC, NLR, and PLR were retrospectively obtained from pre-treatment blood sampling results of 120 patients and stratified according to means. Univariate and multivariate analyses were performed to investigate the association of clinicopathological factors, including these values, with overall survival (OS) and progression-free survival (PFS).
Results
The ALC, NLR, and PLR cut-off points were 1,285/μl, 3.3, and 235, respectively. No biomarkers were associated with PFS. However, univariate analysis showed ALC (p=0.044) and PLR (p=0.044) to be significantly associated with OS.
Conclusion
ALC and PLR can predict eribulin efficacy in terms of OS, reflecting the antitumour immune response in the microenvironment and indicating eribulin’s effectiveness.
Keywords: Eribulin, metastatic breast cancer, absolute lymphocyte count, platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, overall survival, prognosis
Eribulin methylate (Halaven®, Esai Co. Ltd., Tokyo, Japan) is a cytotoxic anticancer drug with a non-taxane microtubule inhibitory effect. Thus far, it has been approved for breast cancer treatment in approximately 60 countries. In Japan, eribulin has been available since 2011 for the management of inoperable and recurrent human epidermal growth factor receptor type 2 (HER2)-negative breast cancer after treatment with anthracyclines and taxanes (1). The phase 3 open-label randomized trial EMBRACE showed significantly prolonged overall survival (OS) linked to eribulin treatment compared to that associated with the treatment of physician’s choice (TPC), despite no inter-treatment difference in progression-free survival (PFS) (2). In addition to inhibiting mitosis, eribulin has been shown to have various effects. The drug affects the immune system and enhances the antitumour immune response by reducing the expression of programmed death ligand-1 and forkhead box P3 and increasing the expression of cluster of differentiation 8. Furthermore, in the tumour microenvironment, it improves blood circulation by remodelling tumour vasculature by reducing the expression of vascular endothelial growth factor (VEGF), inducing the epithelialization of breast cancer cells by reducing the expression of transforming growth factor-β (TGF-β), and reducing the metastatic potential of breast cancer cells (3-6).
In recent years, peripheral blood biomarkers, namely absolute lymphocyte count (ALC), as a factor that reflects immune regulation of the tumour, neutrophil-to-lymphocyte ratio (NLR), as a factor that reflects systemic inflammation, and platelet-to-lymphocyte ratio (PLR), which is related to systemic immunity, have been reported as prognostic or therapeutic effect predictive factors for breast cancer (7-18). Hence, in the present study, we retrospectively evaluated the utility of ALC, NLR, and PLR as predictors of the therapeutic effect of eribulin in patients with metastatic and recurrent HER2-negative breast cancer.
Patients and Methods
This study was approved by the ethics committee of Tokyo Medical University (#2021-0163). In all, 120 patients who were diagnosed with metastatic and recurrent HER2-negative breast cancer and received eribulin monotherapy at Tokyo Medical University or Tokyo Medical University Hachioji Medical Centre from September 2011 to December 2018 were investigated. Patients’ background and outcome data were retrospectively collected. Eribulin was intravenously administered at 1.4 mg/m² on days 1 and 8, every 21 days. The dose was adjusted according to adverse events severity. In several cases, eribulin was administered bi-weekly without dose reduction due to neutropenia. None of the patients received granulocyte-colony-stimulating factor agents. Treatment was continued until disease progression, intolerable toxicity, patient or physician request to discontinue treatment, or death from any cause. Disease progression was determined by attending doctors based on radiological findings, symptoms, laboratory data, and/or other supplementary findings.
Outcomes. PFS and OS were evaluated as the study endpoints, and their correlation with background factors was explored. PFS was defined as the duration from eribulin treatment initiation until disease progression, intolerable toxicity, or death from any cause. OS was defined as the duration from eribulin treatment initiation to death from any cause.
Evaluation of peripheral blood biomarkers. ALC, NLR, and PLR were calculated from the baseline laboratory data collected most recently before the first eribulin administration. NLR and PLR were calculated by dividing the neutrophil/platelet count by the lymphocyte count. To evaluate PFS- and OS-related factors, because no absolute cut-off values have been reported for these biomarkers, the mean value of each parameter was taken as the cut-off value, distinguishing between high and low groups. The values obtained for ALC, NLR, and PLR were 1,285/μl, 3.3, and 235, respectively.
Statistical analysis. All analyses included data from patients with evaluable baseline ALC, NLR, and PLR. Patients whose baseline blood test data were unavailable were excluded. Baseline characteristics, eribulin treatment status, and peripheral blood biomarkers were collected retrospectively. To analyse the PFS and OS, Kaplan–Meier curves were used, and the median PFS and OS [95% confidence interval (CI)] were estimated. The hazard ratios (HRs) and 95%CIs for each baseline factor were estimated using the Cox proportional hazard model. Univariate and multivariate Cox regression analyses were performed to explore potential factors affecting PFS and OS. HRs and 95%CIs were calculated for each factor by using univariate models. Multivariate Cox regression analysis included only factors that were significant in the univariate analysis. Factors with p<0.05 were considered statistically significant. Because the present study was conducted for exploratory purposes, no adjustments were made for multiple testing. All analyses were performed using the IBM SPSS Statistics Version 27 software package (IBM Corp., Armonk, NY, USA).
Results
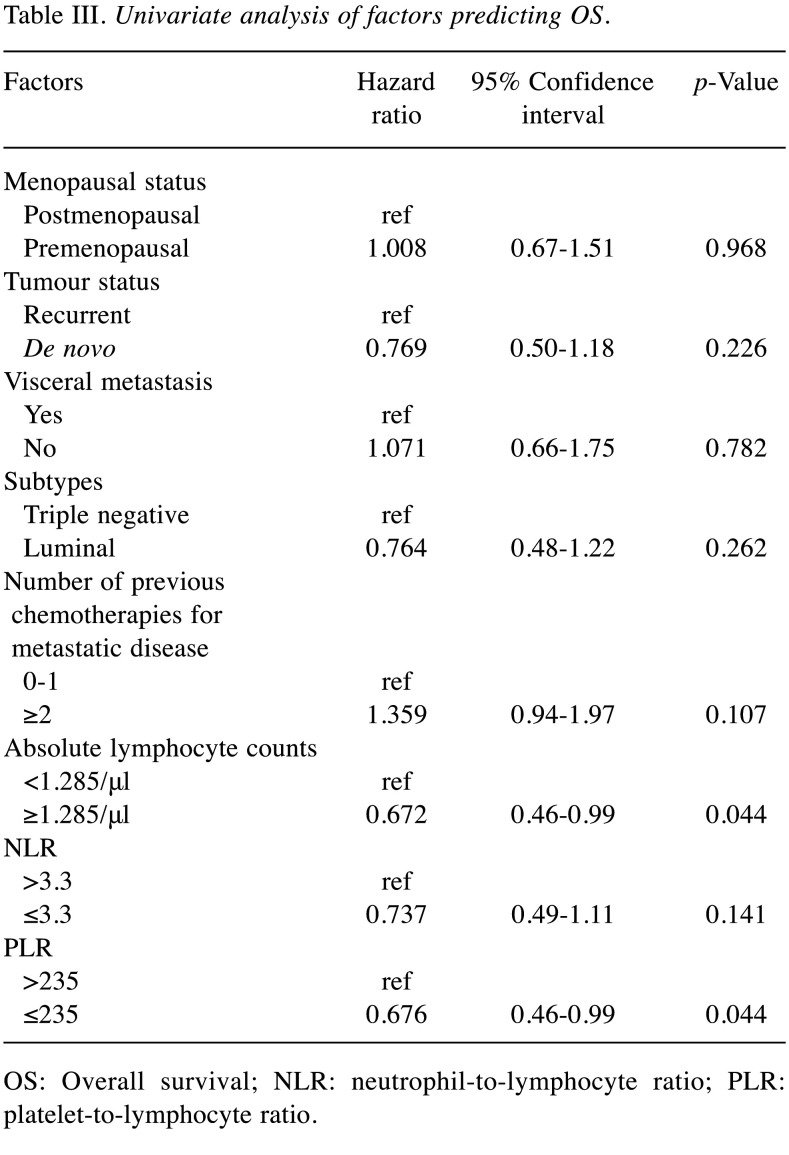
Clinicopathological features and treatment outcomes. We identified 120 patients (97 oestrogen receptor-positive [ER+] and 23 ER-) with HER2-negative metastatic breast cancer who received eribulin treatment during the study period. The patient characteristics are summarized in Table I. The patients’ median age and follow-up period were 61 years and 15.2 months (range=0.9-65.9 months), respectively; 84 (70%) patients were postmenopausal. One hundred and one (84%) patients showed recurrence; 19 (16%) were diagnosed with de novo stage IV disease. Seventy (58%) patients received anthracycline and/or taxane antineoplastic agents as adjuvant or neoadjuvant chemotherapy. At eribulin initiation, 100 (83%) and 20 (17%) patients had visceral and non-visceral metastasis, respectively. Before eribulin treatment, the patients received a median of one line of chemotherapy (range 0-8) for metastatic treatment: 62 (52%), 48 (40%), and 10 (8%) patients received no or one, 2-4, and >4 lines of chemotherapy, respectively. Regarding treatment discontinuation, most patients, 110 (92%), stopped treatment because of disease progression. Only 10 (8%) discontinued treatment because of adverse events (asthenia and fatigue in five cases; neutropenia and alopecia in two cases; and liver dysfunction, constipation, and interstitial lung disease in one case each). Among the 120 patients, the overall response rate [ORR; defined as the rate of partial response (PR) and complete response (CR)] was only 10% (12 cases; PR and CR in 12 and 0 cases, respectively). However, the clinical benefit rate [defined as the rate of long-term stable disease (SD), i.e., over 24 weeks, PR, and CR] was 39% (47 cases; long-term SD in 35 cases). The median PFS and OS were 4.9 months (95%CI=4.2-5.7) and 15.2 months (95%CI=13.4-17.0), respectively.
Table I. Clinicopathological features of 120 patients.
ER: Oestrogen receptor; PR: progesterone receptor; NLR: neutrophilto-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; PFS: progression-free survival; OS: overall survival; 95%CI: 95% confidence interval. 1At the time of starting eribulin. 2Defined as the rate of partial or complete response afforded by eribulin treatment. 3Defined as the rate of long-term stable disease (over 24 weeks) and partial or complete response afforded by eribulin treatment.
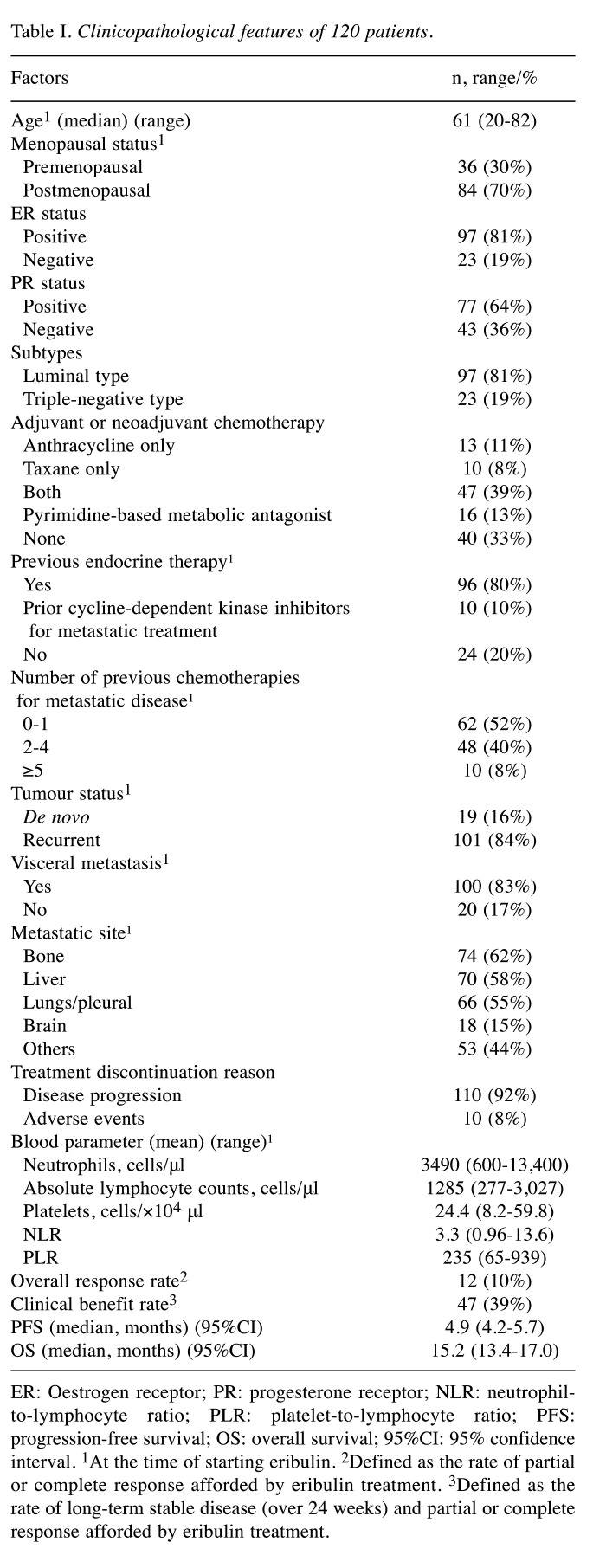
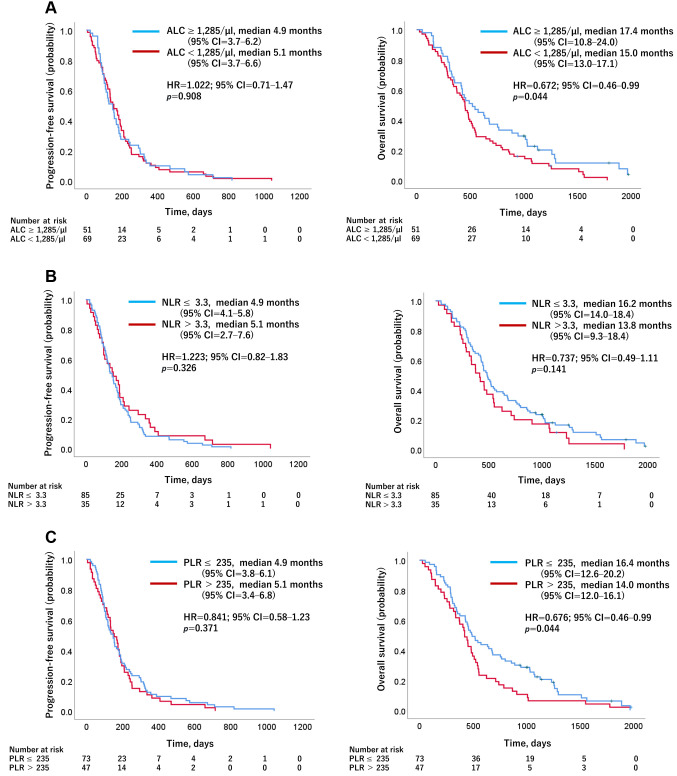
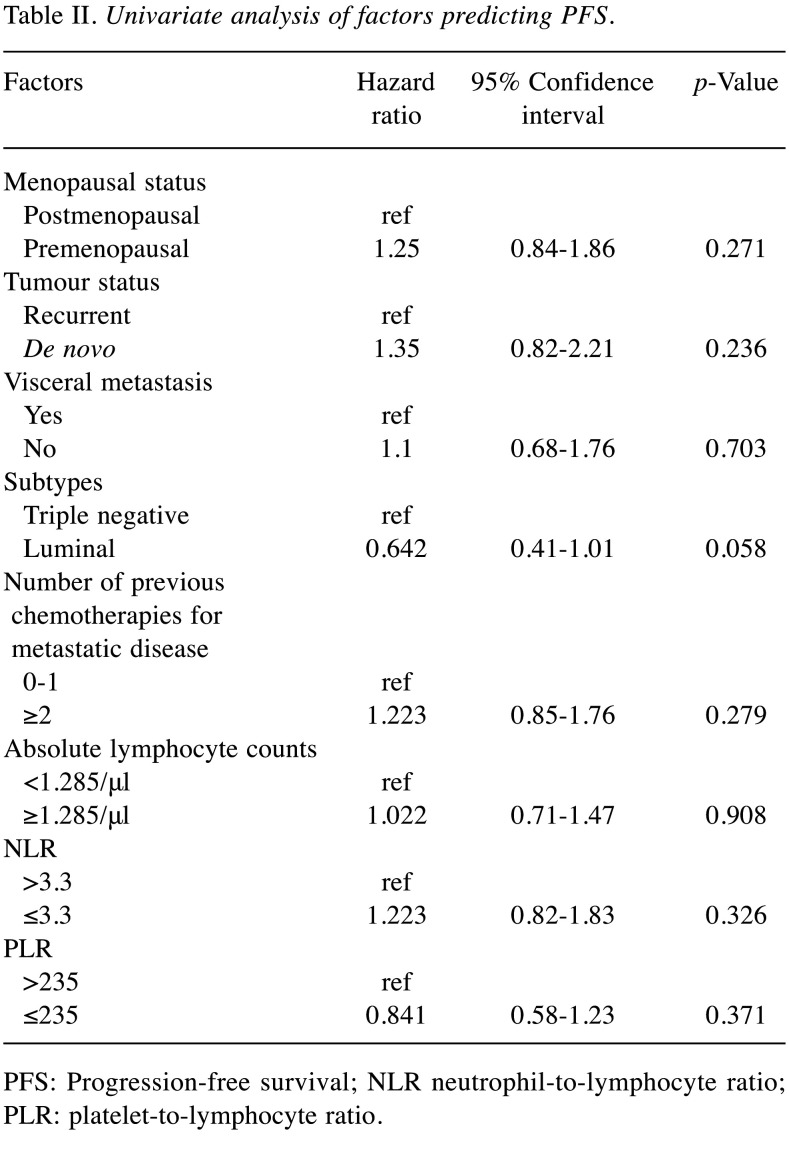
Effect of baseline ALC (cut-off value, 1,285/μl), NLR (cut-off value, 3.3), and PLR (cut-off value, 235) on PFS/OS. Figure 1 shows the Kaplan–Meier curves for PFS and OS with regard to baseline ALC, NLR, and PLR. The median OS was significantly longer in patients with an ALC≥1,285/μl and a PLR≤235 than in patients with an ALC<1,285/μl and PLR>235 (ALC≥1,285/μl vs. ALC<1,285/μl: 17.4 vs. 15.0 months, HR=0.672, 95%CI=0.46-0.99; PLR≤235 vs. PLR>235: 16.4 vs. 14.0 months, HR=0.676, 95%CI=0.46-0.99) (Figure 1A and C). Regarding baseline NLR, there was no significant difference in OS between those with an NLR≤3.3 and NLR>3.3 (NLR≤3.3 vs. NLR>3.3: 16.2 vs. 13.8 months, HR=0.737, 95%CI=0.49-1.11) (Figure 1B). No significant difference was found in peripheral blood biomarkers with regard to PFS.
Figure 1. Kaplan–Meier curves for progression-free survival and overall survival predicted by the biomarkers. Stratification according to (A) absolute lymphocyte counts [ALC: a; ALC≥1,285/μl vs. ALC<1,285/μl (n=51 vs. 69)], (B) neutrophil-to-lymphocyte ratio [NLR: b; NLR≤3.3 vs. NLR>3.3 (n=85 vs. 35)], and (C) platelet-to-lymphocyte ratio [PLR: c; PLR≤235 vs. PLR>235 (n=73 vs. 47)]. No significant difference was found in peripheral blood biomarkers for progression-free survival (PFS). However, patients with ALC≥1,285/μl and PLR≤235 had significantly better overall survival (OS) than those with ALC<1,285/μl and PLR>235. HR: Hazard ratio; 95%CI: 95% confidence interval; ALC: absolute lymphocyte counts; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio.
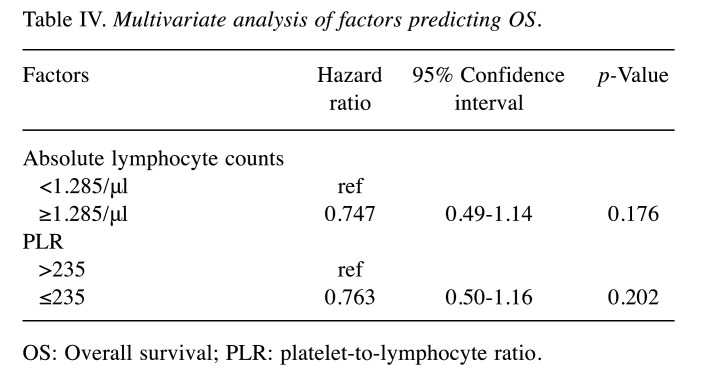
Univariate and multivariate analyses of baseline factors for PFS/OS. Tables II and III show the results of univariate Cox regression analysis for factors affecting PFS and OS. Univariate Cox regression analysis was performed to evaluate menopausal and tumour statuses, visceral metastasis, subtypes, number of previous chemotherapies for metastatic disease; and baseline ALC, NLR, and PLR. No factor was identified as influencing PFS (Table II). Baseline ALC (95%CI=0.46-0.99, p=0.044) and PLR (95%CI=0.46-0.99, p=0.044) were identified by univariate Cox regression analysis as factors affecting OS (Table III); however, no significant independent factor affecting OS was found by multivariate Cox regression analysis (Table IV).
Table II. Univariate analysis of factors predicting PFS.
PFS: Progression-free survival; NLR neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio.
Table III. Univariate analysis of factors predicting OS.
OS: Overall survival; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio.
Table IV. Multivariate analysis of factors predicting OS.
OS: Overall survival; PLR: platelet-to-lymphocyte ratio.
Discussion
In the present study, we found that ALC and PLR were associated with OS, but not PFS, in eribulin-treated patients with metastatic breast cancer. Higher ALC and lower PLR were significant predictors of eribulin efficacy. The pre-treatment cut-off points for ALC and PLR in this study were 1,285/μl and 235, respectively. One of the landmark studies in this field is a post-hoc analysis based on the EMBRACE study (7). The post-hoc analysis clearly demonstrated that baseline ALC at a cut-off value of 1,500/μl was an independent predictor for longer OS in eribulin-treated patients with metastatic breast cancer, while NLR was a general prognostic marker rather than a specific predictor of OS in these patients.
Another study with 144 patients having HER2-negative metastatic breast cancer also showed that ALC at a cut-off value of 1000/μL was a significant predictor of OS in patients treated with eribulin in the oestrogen receptor (ER)-positive subgroup but not in the ER-negative subgroup (19). The background characteristics of the patients in the current study, including the limited number of patients, differed from those of patients in the aforementioned studies. The OS in this study was longer than that in the EMBRACE study, because eribulin was administered mostly in patients with ER-positive breast cancer and in relatively early lines of treatment. However, the association of baseline ALC with OS remained significant, as indicated by the univariate analysis, suggesting the reliability of ALC in predicting eribulin efficacy.
Eribulin has been shown to have immunomodulatory activity in the tumour microenvironment. Lymphocytes play an important role in antitumour immune responses and tumour immunological surveillance, reflecting a state of T-cell function (20-24). Although the cut-off values varied across studies, ALC was consistently associated with OS in eribulin-treated patients. Thus, ALC predicted eribulin’s therapeutic effect, likely reflecting the antitumour immune response produced by the drug.
PLR, but not NLR, was also a significant predictor of the effectiveness of eribulin in this study. A post-hoc analysis based on the EMBRACE study revealed that NLR was a prognostic marker in patients with metastatic breast cancer. NLR was associated with prolonged OS in both the eribulin and TPC groups (7). Neutrophils have been reported to relate to cancer-associated inflammation, suppress the activities of lymphocytes and promote tumour progression (25,26). In addition to cancer biology, neutrophils have a shorter blood half-life and are susceptible to lifestyle-related diurnal variations (27), temporary inflammation due to factors such as infection, and bone marrow exhaustion due to pre-treatment.
Platelets have been reported to be related to cancer progression by promoting tumour angiogenesis, extracellular matrix degradation, release of adhesion molecules and facilitation of inflammatory cell migration (28-30). Regarding their immune functions, platelets store immunomodulatory molecules such as TGF-β and interleukin-1 and affect the immune response. Platelets also express and secrete cluster of differentiation 40 and 154, which affect dendritic cell maturation and T cell activation (31). Regarding neutrophils and platelets in cancer patients, platelets are less dispersed compared to neutrophils (27). Thus, PLR may reflect ALC better than NLR because ALC may be the best predictor of OS afforded by eribulin treatment (7). However, all these studies were retrospective, and prospective studies are required to confirm the significance of ALC in the treatment with eribulin.
In conclusion, ALC and PLR predicted the efficacy of eribulin in terms of OS but not PFS. PLR may be better associated with ALC than NLR. ALC proved to serve as an immunological predictive index, specifically of the improvement in OS in eribulin-treated patients by the modulation of the immune regulation system. Taken together with the results of previous studies, the findings in this study indicate that ALC has a significant impact on eribulin efficacy. This finding needs to be confirmed in prospective studies.
Conflicts of Interest
All Authors have no conflicts of interest in relation to this study.
Authors’ Contributions
SK, NU, MO, MA, ST, AU, KM, TK, and HK have approved the submitted version (and any substantially modified version that involves the authors’ contributions to the study) and have agreed to be personally accountable for the Authors’ own contributions and ensure that questions related to the accuracy or integrity of any part of the work, even those in which the Author(s) was/were not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
YK is the main Author who was involved in the supervision and implementation of the study and the final review. YK performed data collection and analyses. YK, KY, and TI contributed to the study conception and design. All Authors have read and approved the final manuscript.
Acknowledgements
The Authors would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Inoue K, Takahashi M, Mukai H, Yamanaka T, Egawa C, Sakata Y, Ikezawa H, Matsuoka T, Tsurutani J. Effectiveness and safety of eribulin in Japanese patients with HER2-negative, advanced breast cancer: a 2-year post-marketing observational study in a real-world setting. Invest New Drugs. 2020;38(5):1540–1549. doi: 10.1007/s10637-019-00890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C, EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389) investigators Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 3.Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, Tohyama O, Uehara T, Kimura T, Watanabe H, Asano M, Kawano S, Tizon X, McCracken PJ, Matsui J, Aoshima K, Nomoto K, Oda Y. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014;105(10):1334–1342. doi: 10.1111/cas.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, Uesugi M, Agoulnik S, Taylor N, Funahashi Y, Matsui J. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer. 2014;110(6):1497–1505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goto W, Kashiwagi S, Asano Y, Takada K, Morisaki T, Fujita H, Takashima T, Ohsawa M, Hirakawa K, Ohira M. Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res. 2018;38(5):2929–2938. doi: 10.21873/anticanres.12541. [DOI] [PubMed] [Google Scholar]
- 6.Fujii T, Tokuda S, Nakazawa Y, Kurozumi S, Obayashi S, Yajima R, Shirabe K. Eribulin suppresses new metastases in patients with metastatic breast cancer. In Vivo. 2020;34(2):917–921. doi: 10.21873/invivo.11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyoshi Y, Yoshimura Y, Saito K, Muramoto K, Sugawara M, Alexis K, Nomoto K, Nakamura S, Saeki T, Watanabe J, Perez-Garcia JM, Cortes J. High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin-but not with treatment of physician’s choice-in the EMBRACE study. Breast Cancer. 2020;27(4):706–715. doi: 10.1007/s12282-020-01067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyagawa Y, Araki K, Bun A, Ozawa H, Fujimoto Y, Higuchi T, Nishimukai A, Kira A, Imamura M, Takatsuka Y, Miyoshi Y. Significant association between low baseline neutrophil-to-lymphocyte ratio and improved progression-free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab-paclitaxel. Clin Breast Cancer. 2018;18(5):400–409. doi: 10.1016/j.clbc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Kashiwagi S, Asano Y, Goto W, Takada K, Takahashi K, Noda S, Takashima T, Onoda N, Tomita S, Ohsawa M, Hirakawa K, Ohira M. Use of Tumor-infiltrating lymphocytes (TILs) to predict the treatment response to eribulin chemotherapy in breast cancer. PLoS One. 2017;12(2):e0170634. doi: 10.1371/journal.pone.0170634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krenn-Pilko S, Langsenlehner U, Thurner EM, Stojakovic T, Pichler M, Gerger A, Kapp KS, Langsenlehner T. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi: 10.1038/bjc.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh CH, Bhoo-Pathy N, Ng KL, Jabir RS, Tan GH, See MH, Jamaris S, Taib NA. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–158. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muñoz-Montaño W, Cabrera-Galeana P, Alvarado-Miranda A, Villarreal-Garza C, Mohar A, Olvera A, Bargallo-Rocha E, Lara-Medina F, Arrieta O. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in different phenotypes of locally advanced breast cancer during neoadjuvant systemic treatment. Clin Breast Cancer. 2020;20(4):307–316.e1. doi: 10.1016/j.clbc.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, Guastalla JP, Bachelot T, Perol D, Chabaud S, Hogendoorn PC, Cassier P, Dufresne A, Blay JY, European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W, Deng M. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 2019;8(9):4135–4148. doi: 10.1002/cam4.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lusho S, Durando X, Mouret-Reynier MA, Kossai M, Lacrampe N, Molnar I, Penault-Llorca F, Radosevic-Robin N, Abrial C. Platelet-to-lymphocyte ratio is associated with favorable response to neoadjuvant chemotherapy in triple negative breast cancer: a study on 120 patients. Front Oncol. 2021;11:678315. doi: 10.3389/fonc.2021.678315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamoto S, Ikeda M, Kubo S, Yamamoto M, Yamashita T. Dynamic changes in absolute lymphocyte counts during eribulin therapy are associated with survival benefit. Anticancer Res. 2021;41(6):3109–3119. doi: 10.21873/anticanres.15095. [DOI] [PubMed] [Google Scholar]
- 17.Oba T, Maeno K, Ono M, Ito T, Kanai T, Ito KI. Prognostic nutritional index is superior to neutrophil-to-lymphocyte ratio as a prognostic marker in metastatic breast cancer patients treated with eribulin. Anticancer Res. 2021;41(1):445–452. doi: 10.21873/anticanres.14794. [DOI] [PubMed] [Google Scholar]
- 18.Sata A, Fukui R, Miyagawa Y, Bun A, Ozawa H, Fujimoto Y, Higuchi T, Imamura M, Miyoshi Y. C-reactive protein and absolute lymphocyte count can predict overall survival of patients treated with eribulin. Anticancer Res. 2020;40(7):4147–4156. doi: 10.21873/anticanres.14414. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe J, Saito M, Horimoto Y, Nakamoto S. A maintained absolute lymphocyte count predicts the overall survival benefit from eribulin therapy, including eribulin re-administration, in HER2-negative advanced breast cancer patients: a single-institutional experience. Breast Cancer Res Treat. 2020;181(1):211–220. doi: 10.1007/s10549-020-05626-1. [DOI] [PubMed] [Google Scholar]
- 20.Kono SA, Heasley LE, Doebele RC, Camidge DR. Adding to the mix: fibroblast growth factor and platelet-derived growth factor receptor pathways as targets in non-small cell lung cancer. Curr Cancer Drug Targets. 2012;12(2):107–123. doi: 10.2174/156800912799095144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopewell EL, Zhao W, Fulp WJ, Bronk CC, Lopez AS, Massengill M, Antonia S, Celis E, Haura EB, Enkemann SA, Chen DT, Beg AA. Lung tumor NF-ĸB signaling promotes T cell-mediated immune surveillance. J Clin Invest. 2013;123(6):2509–2522. doi: 10.1172/JCI67250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wick DA, Webb JR, Nielsen JS, Martin SD, Kroeger DR, Milne K, Castellarin M, Twumasi-Boateng K, Watson PH, Holt RA, Nelson BH. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin Cancer Res. 2014;20(5):1125–1134. doi: 10.1158/1078-0432.CCR-13-2147. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Liu T, Tang W, Deng B, Chen Y, Zhu J, Shen X. Hepatocellular carcinoma cells induce regulatory T cells and lead to poor prognosis via production of transforming growth factor-β1. Cell Physiol Biochem. 2016;38(1):306–318. doi: 10.1159/000438631. [DOI] [PubMed] [Google Scholar]
- 24.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10(15):4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- 26.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139(7):2406–2413. [PubMed] [Google Scholar]
- 27.Sennels HP, Jørgensen HL, Hansen AL, Goetze JP, Fahrenkrug J. Diurnal variation of hematology parameters in healthy young males: the Bispebjerg study of diurnal variations. Scand J Clin Lab Invest. 2011;71(7):532–541. doi: 10.3109/00365513.2011.602422. [DOI] [PubMed] [Google Scholar]
- 28.Ghasemzadeh M, Hosseini E. Intravascular leukocyte migration through platelet thrombi: directing leukocytes to sites of vascular injury. Thromb Haemost. 2015;113(6):1224–1235. doi: 10.1160/TH14-08-0662. [DOI] [PubMed] [Google Scholar]
- 29.Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22(9):913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 30.Egan K, Crowley D, Smyth P, O’Toole S, Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, McEvoy L, Ffrench B, Stordal B, Keegan H, Finn S, McEneaney V, Laios A, Ducrée J, Dunne E, Smith L, Berndt M, Sheils O, Kenny D, O’Leary J. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011;6(10):e26125. doi: 10.1371/journal.pone.0026125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]