Abstract
Background
A variety of minimally invasive surgical approaches are available as an alternative to transurethral resection of the prostate (TURP) for management of lower urinary tract symptoms (LUTS) in men with benign prostatic hyperplasia (BPH). Prostatic arterial embolization (PAE) is a relatively new, minimally invasive treatment approach.
Objectives
To assess the effects of PAE compared to other procedures for treatment of LUTS in men with BPH.
Search methods
We performed a comprehensive search the Cochrane Library, MEDLINE, Embase, three other databases, trials registries, other sources of grey literature, and conference proceedings with no restrictions on language of publication or publication status, up to 8 November 2021.
Selection criteria
We included parallel‐group randomized controlled trials (RCTs), as well as non‐randomized studies (NRS, limited to prospective cohort studies with concurrent comparison groups) enrolling men over the age of 40 years with LUTS attributed to BPH undergoing PAE versus TURP or other surgical interventions.
Data collection and analysis
Two review authors independently classified studies for inclusion or exclusion and abstracted data from the included studies. We performed statistical analyses by using a random‐effects model and interpreted them according to the Cochrane Handbook for Systematic Reviews of Interventions. We used GRADE guidance to rate the certainty of evidence of RCTs and NRSs.
Main results
We found data to inform two comparisons: PAE versus TURP (six RCTs and two NRSs), and PAE versus sham (one RCT). Mean age was 66 years, International Prostate Symptom Score (IPSS) was 22.8, and prostate volume of participants was 72.8 mL. This abstract focuses on the comparison of PAE versus TURP as the primary topic of interest.
Prostatic arterial embolization versus transurethral resection of the prostate
We included six RCTs and two NRSs with short‐term (up to 12 months) follow‐up, and two RCTs and one NRS with long‐term follow‐up (13 to 24 months).
Short‐term follow‐up: based on RCT evidence, there may be little to no difference in urologic symptom score improvement measured by the International Prostatic Symptom Score (IPSS) on a scale from 0 to 35, with higher scores indicating worse symptoms (mean difference [MD] 1.72, 95% confidence interval [CI] –0.37 to 3.81; 6 RCTs, 360 participants; I² = 78%; low‐certainty evidence). There may be little to no difference in quality of life as measured by the IPSS‐quality of life question on a scale from 0 to 6, with higher scores indicating worse quality of life between PAE and TURP, respectively (MD 0.28, 95% CI –0.28 to 0.84; 5 RCTs, 300 participants; I² = 63%; low‐certainty evidence). While we are very uncertain about the effects of PAE on major adverse events (risk ratio [RR] 0.75, 95% CI 0.19 to 2.97; 4 RCTs, 250 participants; I² = 24%; very low‐certainty evidence), PAE likely increases retreatments (RR 3.20, 95% CI 1.41 to 7.27; 4 RCTs, 303 participants; I² = 0%; moderate‐certainty evidence). PAE may make little to no difference in erectile function measured by the International Index of Erectile Function‐5 on a scale from 1 to 25, with higher scores indicating better function (MD –0.50 points, 95% CI –5.88 to 4.88; 2 RCTs, 120 participants; I² = 68%; low‐certainty evidence). Based on NRS evidence, PAE may reduce the occurrence of ejaculatory disorders (RR 0.51, 95% CI 0.35 to 0.73; 1 NRS, 260 participants; low‐certainty evidence).
Long‐term follow‐up: based on RCT evidence, PAE may result in little to no difference in urologic symptom scores (MD 2.58 points, 95% CI –1.54 to 6.71; 2 RCTs, 176 participants; I² = 73%; low‐certainty evidence) and quality of life (MD 0.50 points, 95% CI –0.03 to 1.04; 2 RCTs, 176 participants; I² = 29%; low‐certainty evidence). We are very uncertain about major adverse events (RR 0.91, 95% CI 0.20 to 4.05; 2 RCTs, 206 participants; I² = 72%; very low‐certainty evidence). PAE likely increases retreatments (RR 3.80, 95% CI 1.32 to 10.93; 1 RCT, 81 participants; moderate‐certainty evidence). While PAE may result in little to no difference in erectile function (MD 3.09 points, 95% CI –0.76 to 6.94; 1 RCT, 81 participants; low‐certainty evidence), PAE may reduce the occurrence of ejaculatory disorders (RR 0.67, 95% CI 0.45 to 0.98; 1 RCT, 50 participants; low‐certainty evidence).
Authors' conclusions
Compared to TURP, PAE may provide similar improvement in urologic symptom scores and quality of life. While we are very uncertain about major adverse events, PAE likely increases retreatment rates. While erectile function may be similar, PAE may reduce ejaculatory disorders. Certainty of evidence for the outcomes of this review was low or very low except for retreatment (moderate‐certainty evidence), signaling that our confidence in the reported effect size is limited or very limited, and that this topic should be better informed by future research.
Plain language summary
Prostatic arterial embolization for treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia
Review question
What are the effects of a procedure that reduces blood flow to the prostate (called prostatic arterial embolization) in men with symptoms caused by an enlarged prostate?
Background
An enlarged prostate may cause difficulty with urination such as a weak stream or the need to urinate often during the day or at night. This can be treated by medications or by different types of surgery. One main type of surgery is called transurethral resection of the prostate. This involves going inside the urethra through the penis and removing prostate tissue. Prostatic arterial embolization is another form of treatment that works by stopping blood flow to parts of the prostate. We did this study to compare how prostatic arterial embolization compares to transurethral resection of the prostate and other procedures used in men with an enlarged prostate.
Study characteristics
We found eight studies that compared prostatic arterial embolization to transurethral resection of the prostate. In six of eight studies, so‐called randomized trials, chance decided which group people were in. In the other two studies, the men themselves and their doctors decided. We also included one study that compared prostatic arterial embolization to a sham procedure (men were made to believe that they had received treatment, but in reality, they did not). We found no evidence comparing prostatic arterial embolization to treatments other than transurethral resection of the prostate.
Key results
Prostatic arterial embolization compared to transurethral resection of the prostate
Based on up to 24 months' follow‐up, prostatic arterial embolization and transurethral resection of the prostate may work similarly well in helping to relieve symptoms. Men's quality of life may be also improved similarly. We are very uncertain about differences in major unwanted effects. Prostatic arterial embolization likely increases the need for being treated again for the same problem. Prostatic arterial embolization may work similarly with regard to erection problems, but may reduce problems with ejaculation.
Certaintyof evidence
The certainty of evidence for the outcomes was mainly low or very low. This means that the true effect can be very different from what this review shows. Better designed, larger studies with longer follow‐up are needed to answer the question of how prostatic arterial embolization compares to other treatments.
Summary of findings
Summary of findings 1. PAE compared to TURP for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia (short term).
| Patient or population: men with lower urinary tract symptoms suggesting benign prostatic hyperplasia Setting: RCTs (likely single center) and NRSs (including multicenter registry‐based study)/China, Brazil, Egypt, and Europe Intervention: PAE Comparison: TURP | ||||||
| Outcomes | No of participants (studies) | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | What happens? | |
| Risk with TURP (short term) | Risk difference with PAE | |||||
|
Urologic symptom scoresa
assessed with International Prostate Symptom Score
Scale from 0 (best; not at all) to 35 (worst; almost always) Follow‐up: range 12 weeks to 12 months MCID: 3 points |
360 (6 RCTs) | ⊕⊕⊝⊝ Lowb,c,d | — | Urologic symptom scores of RCTs ranged from 6.1 to 10.2 | MD 1.72 higher (0.37 lower to 3.81 higher) | There may be little to no difference in urologic symptom score improvement between PAE and TURP. |
|
Quality of lifea
assessed with International Prostate Symptom Score – Quality of Life
Scale from 0 (best; delighted) to 6 (worst; terrible) Follow‐up: range 12 weeks to 12 months MCID: 0.5 points |
300 (5 RCTs) | ⊕⊕⊝⊝ Lowb,c,d | — | Quality of life of RCTs ranged from 0.9 to 2.91 | MD 0.28 higher (0.28 lower to 0.84 higher) | There may be little to no difference in quality of life improvement between PAE and TURP.
|
|
Major adverse events Follow‐up: range 12 weeks to 12 months MCID: relative risk reduction/increase of 0.25 |
250 (4 RCTs) | ⊕⊝⊝⊝ Very lowb,e |
RR 0.75
(0.19 to 2.97) |
Study population | We are very uncertain whether PAE results in more or fewer major adverse events than TURP. | |
| 59 per 1000 | 15 fewer per 1000 (48 fewer to 116 more) | |||||
| 305 (1 NRS) | ⊕⊝⊝⊝ Very lowb,f | Not estimableg | Study population | |||
| — | — | |||||
|
Retreatmenta Follow‐up: range 6–12 months MCID: relative risk reduction/increase of 0.25 |
303 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | RR 3.20 (1.41 to 7.27) | Study population | PAE likely increases retreatment rates. | |
| 37 per 1000 | 81 more per 1000 (15 more to 231 more) | |||||
|
Erectile functiona assessed with International Index of Erectile Function‐5 Scale from 1 (worst; severe) to 25 (best; normal) Follow‐up: 12 months MCID: 5 points |
120 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c,d | — | Erectile function of RCTs ranged from 12.47 to 16.1 | MD 0.50 lower (5.88 lower to 4.88 higher) | There may be little to no difference in erectile function between PAE and TURP. |
|
Ejaculatory disordersh Follow‐up: range 12 weeks to 12 months MCID: relative risk reduction/increase of 0.25 |
260 (1 NRS) | ⊕⊕⊝⊝ Lowb | RR 0.51 (0.35 to 0.73) | Study population | PAE may reduce ejaculatory disorder compared to TURP. | |
| 475 per 1000 | 233 fewer per 1000 (309 fewer to 128 fewer) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; MD: mean difference; NRS: non‐randomized study; PAE: prostatic arterial embolization; RCT: randomized controlled trial; RR: risk ratio; TURP: transurethral resection of prostate. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aCertainty of evidence of RCTs was higher than NRSs (Appendix 1). bDowngraded for study limitations: RCTs, unclear or high risk of bias in half or more domains in the included studies (–1)/NRS, overall serious or critical risk of bias according to risk of bias tool to assess non‐randomized studies of interventions (–2). cDowngraded one level for inconsistency due to clinical important heterogeneity with high I2 values. dNot downgraded further for imprecision; wide confidence intervals attributed to observed inconsistency (for which we rated down). eDowngraded two levels for imprecision: wide confidence intervals crossed assumed threshold of clinically important difference or large risk difference in absolute effects, or both. fDowngraded two levels for imprecision: very rare event. gNo event in group. hCertainty of evidence of NRSs was higher than RCTs (Appendix 1).
Summary of findings 2. PAE compared to TURP for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia (long term).
|
Participants: men with lower urinary tract symptoms suggesting benign prostatic hyperplasia Setting: RCT (likely single center) and NRS (multicenter registry‐based study)/China and Europe Intervention: PAE Comparator: TURP | ||||||
| Outcomes | No of participants (studies) | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | What happens? | |
| Risk with TURP (long term) | Risk difference with PAE | |||||
|
Urologic symptom scores
assessed with International Prostate Symptom Score
Scale from 0 (best; not at all) to 35 (worst; almost always) Follow‐up: 24 months MCID: 3 points |
176 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | — | Urologic symptom scores of RCTs ranged from 5.19 to 8.4 | MD 2.58 higher (1.54 lower to 6.71 higher) | There may be little to no difference in urologic symptom score improvement from PAE compared to TURP. |
|
Quality of life
assessed with International Prostate Symptom Score – Quality of Life
Scale from 0 (best; delighted) to 6 (worst; terrible) Follow‐up: 24 months MCID: 0.5 points |
176 (2 RCTs) | ⊕⊕⊝⊝ Lowa,d | — | Quality of life of RCTs ranged from 0.96 to 1.4 | MD 0.50 higher (0.03 lower to 1.04 higher) | There may be little to no difference in quality of life improvement from PAE compared to TURP. |
|
Major adverse events Follow‐up: 24 months MCID: relative risk reduction/increase of 0.25 |
206 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,e | RR 0.91 (0.20 to 4.05) | Study population | We are very uncertain whether PAE results in more or fewer major adverse events than TURP. | |
| 135 per 1000 | 12 fewer per 1000 (108 fewer to 411 more) | |||||
|
Retreatmentf Follow‐up: after 24 months MCID: relative risk reduction/increase of 0.25 |
81 (1 RCT) | ⊕⊕⊕⊝ Moderatea | RR 3.80 (1.32 to 10.93) | Study population | PAE likely increases retreatment rates. | |
| 85 per 1000 | 238 more per 1000 (27 more to 845 more) | |||||
|
Erectile function assessed with International Index of Erectile Function‐5 Scale from 1 (worst; severe) to 25 (best; normal) Follow‐up: 12 months MCID: 5 points |
81 (1 RCT) | ⊕⊕⊝⊝ Lowa,d | — | Erectile function of RCT was 11.28 | MD 3.09 higher (0.76 lower to 6.94 higher) | There may be little to no difference in erectile function between PAE and TURP. |
|
Ejaculatory disorders Follow‐up: 24 months MCID: relative risk reduction/increase of 0.25 |
50 (1 RCT) | ⊕⊕⊝⊝ Lowa,d | RR 0.67 (0.45 to 0.98) | Study population | PAE may reduce ejaculatory disorder compared to TURP. | |
| 840 per 1000 | 277 fewer per 1000 (462 fewer to 17 fewer) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; MD: mean difference; NRS: non‐randomized study; PAE: prostatic arterial embolization; RCT: randomized controlled trial; RR: risk ratio; TURP: transurethral resection of prostate. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations: RCT, unclear risk of selection and reporting bias/high risk of performance or detection bias (–1) /NRS, overall serious or critical risk of bias according to risk of bias tool to assess non‐randomized studies of interventions (–2). bDowngraded one level for inconsistency due to clinical important heterogeneity with high I2 values. cNot downgraded further for imprecision; wide confidence intervals attributed to observed inconsistency (for which we rated down). dDowngraded one level for imprecision: confidence intervals crossed assumed threshold of clinically important difference. eDowngraded two levels for imprecision: wide confidence intervals crossed assumed threshold of clinically important difference. fCertainty evidence of RCTs was higher than NRSs (Appendix 1).
Summary of findings 3. PAE compared to sham for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia (short term).
| Patient or population: men with lower urinary tract symptoms suggesting benign prostatic hyperplasia Setting: RCT/single center/Portugal Intervention: PAE Comparison: sham | ||||||
| Outcomes | No of participants (studies) | Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | What happens? | |
| Risk with sham | Risk difference with PAE | |||||
|
Urologic symptom scores
assessed with International Prostate Symptom Score
Scale from 0 (best; not at all) to 35 (worst; almost always)
Follow‐up: 6 months MCID: 3 points |
80 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — | Change in urologic symptom scores was –5.03 | MD 12.07 lower (15.45 lower to 8.69 lower) | PAE likely improves urologic symptom scores compared to sham. |
|
Quality of life
assessed with International Prostate Symptom Score – Quality of Life
Scale from 0 (best; delighted) to 6 (worst; terrible)
Follow‐up: 6 months MCID: relative risk reduction/increase of 0.5 |
80 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — | Change in quality of life was –1.03 | MD 1.97 lower (2.48 lower to 1.46 lower) | PAE likely improves quality of life compared to sham. |
|
Major adverse events Follow‐up: 6 months MCID: relative risk reduction/increase of 0.25 |
80 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Not estimablec | Study population | We are very uncertain about the effects of PAE on major adverse events. | |
| — | — | |||||
|
Retreatment Follow‐up: 6 months MCID: relative risk reduction/increase of 0.25 |
80 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
Not estimablec | Study population | We are very uncertain about effects of PAE on retreatment. | |
| — | — | |||||
| Erectile function | — | — | — | — | — | Not reported. |
|
Ejaculatory disorders Follow‐up: 6 months MCID: relative risk reduction/increase of 0.25 |
80 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Not estimablec | Study population | We are very uncertain about effects of PAE on major adverse events. | |
| — | — | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; MD: mean difference; PAE: prostatic arterial embolization; RCT: randomized controlled trial. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for study limitations: high risk of performance and detection bias. bDowngraded by two levels for imprecision: very rare event. cNo event in both groups.
Background
Description of the condition
Benign prostatic hyperplasia (BPH) is histologically defined as an increased number of epithelial and stromal cells in the periurethral area of the prostate, which may cause prostate enlargement (Roehrborn 2008). Prostate enlargement may constrict urine flow and cause lower urinary tract symptoms (LUTS) (Dunphy 2015). The development of LUTS resulting from BPH is associated with increasing age, and is most commonly encountered in men over the age of 45 years (Barry 1997; Dunphy 2015; Egan 2016). LUTS consist of storage symptoms (such as urinary frequency, urgency, and nocturia) and voiding symptoms (such as urinary hesitancy, weak urinary stream, straining to void, and prolonged voiding). LUTS severity was positively correlated with men's overall distress based on patient perception of bladder condition, which can be measured by a single‐item global question (ranging from 1 [no problems at all] to 6 [causes severe problems]) (Chapple 2017). However, LUTS are relatively non‐specific and may also be associated with bladder disorders, such as detrusor overactivity. This review specifically considers the term BPH as prostatic enlargement with LUTS by which to define the disease condition and the potential need for intervention (Dunphy 2015; Roehrborn 2008).
The histologic prevalence of BPH is reported to be 8% in the fourth decade of life, and up to 40% in the sixth decade and 70% in the eighth decade of life (Barry 1995; Roehrborn 2008; Yoo 2012). Aside from LUTS, untreated BPH can result in other serious medical consequences, such as acute urinary retention, urinary tract infection, and upper urinary tract deterioration. Subsequently, BPH results in a negative impact on public health and reduction in a person's quality of life (Martin 2014; Yoo 2012). BPH results in a significant economic burden as well, with an estimated cost to the USA of USD 4 billion annually (Taub 2006). In 2013, the fee‐for‐service costs excluding medication costs for BPH/LUTS in USA were estimated at USD 785 million (USD 285–301/patient/year) (Feinsten 2018). It is reasonable to assume that the cost will escalate further in the future with increasing life expectancy in men over the age of 65 years (Centers for Disease Control and Prevention 2003).
Treatment decisions for men with BPH are typically based on severity of symptoms and subjectively perceived bother, presence of complications such as acute urinary retention, risk of progression, and treatment‐related morbidity. Self‐administered questionnaires, namely, the International Prostate Symptom Score (IPSS), which consists of eight questions (seven symptom questions plus one quality of life question) to evaluate symptom severity and relative degree of bother, have been used to guide management of LUTS (Barry 1995; EAU 2021; Lerner 2021a). Watchful waiting and behavioral management are appropriate first‐line options in men with mild or non‐bothersome symptoms. Additional medical treatment options in men with more bothersome symptoms consist of alpha‐blockers, 5‐alpha reductase inhibitors, or a combination of the two (EAU 2021; Lerner 2021a). If symptoms progress despite medical therapy, or if BPH‐related complications such as acute urinary retention, recurrent urinary tract infection, bladder stones, hematuria, or renal insufficiency occur, surgical options are considered (EAU 2021; Lerner 2021b).
A wide variety of surgical options are available for treatment of BPH, from open simple prostatectomy to minimally invasive surgeries, such as transurethral resection of the prostate (TURP), laser ablation, or enucleation of the prostate. According to current guidelines, TURP remains the "gold standard" surgical procedure for men over 40 years of age with various forms of non‐neurogenic benign LUTS (EAU 2021; Lerner 2021b). Although TURP resulted in a mean decrease in LUTS of 70% and a mean increase in maximum flow rate (Qmax) of 162%, considerable rates of perioperative and long‐term complications, such as bleeding requiring blood transfusion (2%), transurethral resection syndrome (0.8%), acute urinary retention (4.5%), clot retention (4.9%), urinary tract infection (4.1%), bladder neck stenosis (4.7%), urethral stricture (3.8%), retrograde ejaculation (65.4%), and erectile dysfunction (6.5%), have been reported (Ahyai 2010). TURP also commonly requires a period of temporary catheterization or hospital admission, or both. Reducing treatment‐related morbidity and patient burden has therefore motivated the development of new, minimally invasive alternatives. Minimally invasive surgeries, such as those using electrode, laser, transurethral thermal ablation of prostate (needle ablation, microwave therapy, and radiofrequency ablative techniques), and mechanical stents, have been introduced and are widely recognized as alternatives to TURP in select patients (EAU 2021; Lerner 2021b). Prostatic arterial embolization (PAE) represents a relatively new, minimally invasive treatment option that is particularly suitable for men who are at high risk to undergo anesthesia (Wang 2015).
Description of the intervention
Embolization of the prostatic arteries has been used historically to control persistent or massive prostatic bleeding not otherwise amenable to treatment, with typical causes of BPH or locally advanced prostate cancer, or occurring after transurethral prostatectomy (Mitchell 1976). DeMeritt 2000 reported a case of PAE performed with polyvinyl alcohol particles for BPH‐induced hematuria, in which hematuria was immediately stopped and the patient reported symptomatic improvement of his BPH symptoms. These researchers also found that prostate size was reduced by 52% of the initial size in the initial five‐month follow‐up and 62% of the initial size at 12‐month follow‐up. Carnevale 2010 reported positive preliminary results of PAE procedures with microspheres as a primary treatment in two men with acute urinary retention due to BPH. For elderly men with symptomatic BPH, PAE can be an alternative treatment, which is performed by a femoral artery puncture and use of conscious sedation instead of general anesthesia. The procedure is typically performed on an outpatient basis and usually does not require catheterization, unless the man has urinary retention (Wang 2015).
In preparation for PAE, preoperative computed tomography or magnetic resonance angiography is typically performed to evaluate the pelvic artery anatomy. Digital subtraction angiography of the right and left internal iliac arteries is performed to assess the prostatic blood supply (Martins Pisco 2012). Super‐selective microcatheterization and embolization are then performed on the prostatic arteries. Embolization is typically performed to complete stasis (Carnevale 2010; Martins Pisco 2012; Wang 2015). Particle embolics are used almost exclusively, with wide variation in the type and size of particles (Carnevale 2010; DeMeritt 2000). Vasodilators to mitigate vasospasm once the prostatic artery is catheterized are recommended by some authors to avoid premature stasis (Martins Pisco 2012).
Adverse effects of the intervention
Although major complications were low (less than 1%) (Pisco 2016), perineal pain (9.4%), hematuria (9%), and acute urinary retention (7%) were commonly reported as complications of PAE (Feng 2017). The highest prevalence of acute urinary retention was 28.4% among the included studies (Wang 2015). Minor complications, such as hematospermia, rectal bleeding, urinary tract infection, inguinal hematoma, and transient urinary frequency, were also reported (Feng 2017; Kuang 2017; Pyo 2017; Shim 2017). However, there was inconsistency in reporting or classifying the adverse events.
How the intervention might work
The underlying mechanism of PAE is ischemia or hypoxia that induces apoptosis, necrosis, sclerosis, and prostatic shrinkage with cystic transformation of part, or all, of the gland, resulting in a softer gland with reduced compression of the urethra (DeMeritt 2000; Sun 2008). In addition, PAE may decrease the plasma concentration of free testosterone that enters prostate cells, thereby lowering dihydrotestosterone levels in the prostate. This may result in secondary inhibition of prostate growth (Sun 2008). Furthermore, ischemia or hypoxia may induce prostate cell death and necrosis with decreased numbers of some receptors, such as alpha‐adrenergic receptors. Therefore, the neuromuscular tone may be decreased, resulting in improvement in clinical symptoms associated with the dynamic pathologic component of BPH (Zlotta 1997).
Why it is important to do this review
Despite reported relative advantages of PAE, it remains unclear how this procedure compares to the numerous surgical alternatives that are available. Although existing systematic reviews have compared PAE to other therapies used to treat BPH (Feng 2017; Kuang 2017; Pyo 2017; Shim 2017; Xu 2020; Zumstein 2019), none so far has used the same rigorous methods as Cochrane Reviews, which include application of the GRADE approach with focus on patient‐important outcomes (Guyatt 2008). In this era, with the availability of numerous minimally invasive procedures to treat LUTS suggestive of BPH, the findings of this Cochrane Review will be relevant to policymakers, healthcare providers, and patients alike.
Objectives
To assess the effects of PAE compared to other procedures for treatment of LUTS in men with BPH.
Methods
Criteria for considering studies for this review
Types of studies
We considered parallel‐group randomized controlled trials (RCTs) and cluster‐RCTs for inclusion. We excluded cross‐over studies as they were not applicable. We also included non‐randomized studies (NRSs), limited to prospective cohort studies with concurrent comparison groups, which is similar to relevant RCTs, as a source of complementary, sequential, or replacement evidence for RCTs if RCTs provided low‐certainty evidence for a given outcome and comparison (e.g. limited information about adverse events and long‐term effects) (Schünemann 2013). We excluded single‐armed studies. We included studies regardless of their publication status or language of publication.
Types of participants
We defined the eligible population as men over the age of 40 years with a prostate volume of 20 mL or greater (as assessed by ultrasound or cross‐sectional imaging), with LUTS as determined by an IPSS of 8 or over, and with Qmax less than 15 mL/second, as measured by non‐invasive uroflowmetry, invasive pressure flow studies, or both (EAU 2021; Lerner 2021a). The age limitation was based on the observation that the prevalence of BPH increases among middle‐aged and older men, and that BPH is infrequent in younger men (Barry 1997; EAU 2021; Egan 2016).
We excluded trials including men with chronic renal failure; untreated bladder calculi or large diverticula; a diagnosis of prostate cancer; urethral stricture disease; or prior prostate, bladder neck, or urethral surgery. We also excluded studies including men with other conditions that affect urinary symptoms, such as neurogenic bladder due to spinal cord injury, multiple sclerosis, or central nervous system disease.
Types of interventions
We compared experimental and comparator interventions for the following outcomes. Concomitant interventions had to be the same in experimental and comparator groups to establish fair comparisons.
Experimental interventions
PAE.
Comparator interventions
Sham control (or no intervention).
TURP (monopolar or bipolar).
Laser ablation of the prostate (e.g. photoselective vaporization of the prostate [PVP]).
Laser enucleation of the prostate (e.g. holmium laser enucleation of the prostate).
Other minimally invasive therapies (e.g. transurethral incision of the prostate, transurethral thermal ablation of the prostate [needle ablation, microwave therapy, and radiofrequency ablative techniques], prostate stent, and prostatic urethral lift [PUL]).
Comparisons
PAE versus sham control (or no intervention).
PAE versus TURP.
PAE versus laser ablation of the prostate.
PAE versus laser enucleation of the prostate.
PAE versus other minimally invasive therapies.
Types of outcome measures
We did not use measurement of the outcomes assessed in this review as an eligibility criterion.
Primary outcomes
Urologic symptom scores.
Quality of life.
Major adverse events.
Secondary outcomes
Retreatment.
Erectile function.
Ejaculatory disorders.
Minor adverse events.
Acute urinary retention.
Indwelling urinary catheter.
Hospital stay.
Method and timing of outcome measurement
We considered clinically important differences for review outcomes to rate the certainty of the evidence for imprecision in the summary of findings tables (Johnston 2010).
Urologic symptom scores
Final value or change from baseline measured as IPSS.
We considered improvement in the IPSS score of 3 points as a minimal clinically important difference (MCID) to assess efficacy and comparative effectiveness (Barry 1995).
Quality of life
Final value or change from baseline measured as IPSS‐quality of life.
No threshold was established for IPSS‐quality of life. We used an MCID of 0.5 to assess efficacy and comparative effectiveness (Brasure 2016; Rees 2015).
Major adverse events
For example, postoperative hemorrhage requiring admission or intervention.
We used the Clavien‐Dindo Classification System to assess surgical complications (Dindo 2004), and we categorized grade III, IV, and V complications as major.
We judged the adverse events by severity using the available information described in the studies.
Retreatment
Participants undergoing the same or other surgical treatment modalities due to insufficient treatment response.
Erectile function
Final value or change from baseline measured by International Index of Erectile Function‐5 questionnaire (IIEF‐5) (Rosen 1997).
We considered improvement in IIEF‐5 over 5 points as an MCID (Spaliviero 2010).
Ejaculatory disorders
We intended to measure the outcome of ejaculatory function based on the Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ‐EjD; Rosen 2007).
Due to lack of data based on the questionnaire, we used the incidence rate of ejaculatory disorders such as postoperative retrograde ejaculation or reduction in ejaculation volume as summarized under the outcome ejaculatory disorder.
Minor adverse events
For example, postoperative fever or pain requiring medication.
We used the Clavien‐Dindo Classification System to assess surgical complications (Dindo 2004), and we categorized grade I and II complications as minor.
We judged the adverse events by severity using the available information described in the studies.
Acute urinary retention
Events requiring catheterization after intervention.
Indwelling urinary catheter
Measured in days from intervention to urinary catheter removal.
Hospital stay
Measured in days from admission to discharge.
There is no reported threshold for adverse events, retreatment, ejaculatory function (based on the questionnaire), acute urinary retention, indwelling urinary catheter, or hospital stay. We considered the clinically important difference for adverse events, retreatment, acute urinary retention, and ejaculatory disorders (based on the events) as a relative risk reduction of at least 25% (Guyatt 2011a). We used an MCID of 25% improvement from baseline on the MSHQ‐EjD for ejaculatory function (Nickel 2015). We used a clinically important difference of one day to assess efficacy and comparative effectiveness for indwelling urinary catheter and hospital stay; this was informed by the clinical expertise of urologists on the review author team. We did not seek other stakeholder feedback.
We considered outcomes measured up to and including 12 months after randomization as short term, and beyond 12 months as long term, for urologic symptom scores, quality of life, major adverse events, retreatment, erectile function, ejaculatory disorders, minor adverse events, and acute urinary retention. We assessed indwelling urinary catheter and hospital stay only at short term.
Main outcomes for summary of findings tables
We present summary of findings tables reporting the following outcomes listed according to priority.
Urologic symptom scores.
Quality of life.
Major adverse events.
Retreatment.
Erectile function.
Ejaculatory disorders.
Search methods for identification of studies
We searched the following sources from inception of each database to 8 November 2021 (Appendix 2).
Electronic searches
Cochrane Library via Wiley (from 1991).
MEDLINE via Ovid (from 1946).
Embase via Ovid (from 1947).
Latin American and Caribbean Health Sciences Literature (LILACS; www.bireme.br/; from 1982).
Scopus (from 1966).
Web of Science (from 1900).
Google Scholar.
We also searched the following.
ClinicalTrials.gov (www.clinicaltrials.gov/).
World Health Organization (WHO) International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/).
Grey literature repository from the current Grey Literature Report (www.greylit.org/).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses, and health technology assessment reports. We also contacted study authors of included trials to identify any further studies that we may have missed. We searched for unpublished studies by handsearching abstract proceedings of annual meetings of the American Urological Association, the European Association of Urology, and the Radiological Society of North America.
Data collection and analysis
Selection of studies
We used reference management software to identify and remove potentially duplicate records (EndNote 2016). Two review authors (JHJ and KAM) independently scanned the abstract, title, or both, of remaining records retrieved, to determine which studies should be assessed further using Covidence 2017. Two review authors (JHJ and KAM) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We resolved any discrepancies through consensus or recourse to a third review author (PD). We documented reasons for exclusion of studies that may have reasonably been expected to be included in the review in the Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We developed a dedicated data abstraction form that we pilot‐tested ahead of time.
For studies that fulfilled our inclusion criteria, two review authors (JHJ and KAM) independently abstracted the following information, which we provided in the Characteristics of included studies table.
Study design.
Study dates.
Study settings and countries.
Participant inclusion and exclusion criteria.
Participant details, baseline demographics (age, prostate volume, prostate‐specific antigen, IPSS, and Qmax) including confounders listed in Assessment of risk of bias in included studies.
Numbers of participants by study and study arm.
Details of relevant experimental and comparator interventions, such as embolization, catheterization approach (unilateral or bilateral), and characteristics of the embolization agent used (polyvinyl alcohol particle size) including co‐intervention listed in Assessment of risk of bias in included studies.
Definitions of relevant outcomes and methods (type of instrument, such as IPSS) and timing of outcome measurement (in months).
Study funding sources.
Declarations of conflicts of interest by primary investigators.
We extracted outcome data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we obtained numbers of events and totals for populations in a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we obtained means and standard deviations (SDs) or data necessary to calculate this information.
We resolved any disagreements by discussion or, if required, by consultation with a third review author (PD).
We provided information, including trial identifier, about potentially relevant ongoing studies in the Characteristics of ongoing studies table.
We contacted authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (JHJ and KAM) assessed the risk of bias of each included study independently. We resolved disagreements by consensus, or by consultation with a third review author (PD). We presented a risk of bias summary figure to illustrate these findings. We further summarized risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome, in accordance with the approach for summary assessments of risk of bias as presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Sterne 2016a).
Assessment of risk of bias in randomized controlled trials
We assessed risk of bias using Cochrane's risk of bias assessment tool (Higgins 2011). We assessed the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
We judged risk of bias domains as 'low risk', 'high risk', or 'unclear risk', and we evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For selection bias (random sequence generation and allocation concealment), we evaluated risk of bias at a trial level.
For performance bias (blinding of participants and personnel), we considered all outcomes similarly susceptible to performance bias.
For detection bias (blinding of outcome assessment), we grouped outcomes as susceptible to detection bias (subjective outcomes) or not susceptible to detection bias (objective outcomes).
We defined the following endpoints as subjective outcomes.
Urologic symptom scores.
Quality of life.
Major adverse events.
Erectile function.
Minor adverse events.
We defined the following endpoints as objective outcomes.
Retreatment.
Acute urinary retention.
Indwelling urinary catheter.
Hospital stay.
We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and we presented the judgment for each outcome separately when reporting our findings in the risk of bias tables. We collapsed reporting for identical judgments.
For reporting bias (selective reporting), we evaluated risk of bias at a trial level. We assessed the risk as low if there was an a priori protocol, and if outcome reporting and planned analyses actually performed matched.
We further summarized risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome, in accordance with the approach for summary assessments of risk of bias as presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in non‐randomized studies
We assessed risk of bias in NRS with ROBINS‐I (Sterne 2016a). We assessed the following domains on outcome‐specific basis for each study and outcome.
Bias due to confounding.
Bias in selection of participants into the study.
Bias in classification of interventions.
Bias due to deviations from intended interventions.
Bias due to missing data.
Bias in measurement of outcomes.
Bias in selection of the reported result.
We judged risk of bias domains as 'low risk', 'moderate risk', 'serious risk', 'critical risk', or 'no information', and we evaluated individual bias items as described in Sterne 2016a.
Based on a particular level of risk of bias for an individual domain, we made an overall judgment about risk of bias.
Low risk of bias (the study is comparable to a well‐performed RCT).
Moderate risk of bias (the study provides sound evidence for an NRS but cannot be considered comparable to a well‐performed RCT).
Serious risk of bias (the study has some important problems).
Critical risk of bias (the study is too problematic to provide any useful evidence and should not be included in any synthesis).
No information on which to base a judgment about risk of bias.
The effect of interest in the NRS was that of assigning intervention at baseline (start of follow‐up), regardless of the extent to which the intervention was received during follow‐up (sometimes referred to as the 'intention‐to‐treat' effect in the context of RCTs).
List of confounding factors and co‐interventions
We considered the following as baseline confounding factors and co‐interventions.
Confounding factors
Age.
Co‐morbidities such as hypertension and diabetes mellitus.
Prostate volume.
Severity of LUTS based on baseline questionnaire score (such as IPSS, IPSS‐quality of life, IIEF‐5, MSHQ‐EjD).
We did not consider time‐varying confounding, as these instances of confounding were not relevant in this setting (Sterne 2016b).
Co‐interventions
Medications such as alpha‐blockers, 5‐alpha reductase inhibitors, or anticholinergic drugs.
The listed confounding factors and co‐interventions can affect a participant's preference for each surgical intervention (both experimental and control) based on the recent guideline (EAU 2021; Lerner 2021b).
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence interval (CIs). We expressed continuous data as mean differences (MDs) with 95% CIs. If studies used different measures to assess the same outcome, we expressed data as standardized MDs with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant. Should we identify cluster‐RCTs, or trials with more than two intervention groups for inclusion in next update, we will manage these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Dealing with missing data
We obtained missing data from study authors and performed intention‐to‐treat analyses if data were available. We investigated attrition rates (e.g. dropouts, losses to follow‐up, withdrawals), and we critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) through visual inspection of forest plots to assess the amount of overlap of CIs and the I² statistic, which quantified inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); we interpreted the I² statistic as follows (Deeks 2011).
0% to 40%: may not be important.
30% to 60%: may indicate moderate heterogeneity.
50% to 90%: may indicate substantial heterogeneity.
75% to 100%: considerable heterogeneity.
When we found heterogeneity, we determined possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We obtained study protocols to assess for selective outcome reporting. Given the fact that we included nine studies in analyses, we could not use funnel plots to assess small‐study effects.
Data synthesis
We summarized data using a random‐effects model in accordance with Cochrane Urology Editorial as likely to provide the more conservative effect size estimate (in most cases). We performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes, we used the inverse variance method. We reported effect estimates for RCTs and NRSs separately when both were included in the review. We used Review Manager 5 software to perform analyses by pooling studies only when appropriate (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity. We planned to carry out subgroup analyses with investigation of interactions, but did not find any studies reported relevant data. If we have sufficient data, we will perform subgroup analysis accordingly.
Patient age (younger than 65 years versus 65 years of age or older).
Prostate volume (40 mL or less versus greater than 40 mL).
Severity of LUTS based on IPSS (score 19 or less [moderately symptomatic] versus score greater than 19 [severely symptomatic]).
These planned subgroup analyses were based on the following observations.
Age is a well‐known risk factor for BPH surgery. Elderly men have a higher rate of postoperative complications compared with younger men (Bhojani 2014; Pariser 2015). The age cut‐off is based on the WHO definition of old age (WHO 2002).
Outcomes and complications of ablative procedures, such as TURP, correlate with prostate volume (Reich 2008). The prostate volume cut‐off of greater than 40 mL is based on this being the most commonly used threshold to distinguish 'small' from 'large' for the indication of treatment with a 5‐alpha reductase inhibitor (EAU 2021).
The relationship between changes in IPSS scores and patient global ratings of improvement is influenced by baseline scores (Barry 1995).
Sensitivity analysis
We planned to perform sensitivity analyses only for RCTs (not NRSs) and limited to primary outcomes to explore the influence of the following factor (when applicable) on effect sizes.
Restricting the analysis by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk'.
Summary of findings and assessment of the certainty of the evidence
We presented the certainty of the evidence for each outcome according to the GRADE approach (Guyatt 2008). For each comparison, two review authors (JHJ and PD) independently rated the certainty of the evidence for each outcome as 'high', 'moderate', 'low', or 'very low' using GRADEpro GDT 2015 (Guyatt 2011a; Guyatt 2011b). We resolved any discrepancies by consensus.
For RCTs, we considered criteria related to internal validity (risk of bias, inconsistency, imprecision, and publication bias) and to external validity, such as directness of results, when downgrading the certainty of the evidence for a specific outcome (Schünemann 2011a; Schünemann 2011b). For NRS, we additionally considered three criteria for upgrading the certainty of the evidence (large magnitude of effects, all plausible confounding that would reduce a demonstrated effect or suggest a spurious effect when results show no effect, and the dose–response gradient) (Schünemann 2011a; Schünemann 2011b). Based on recent guidance to rate the certainty of the evidence of NRS in the context of GRADE, we noted that an initial rating of 'high' was used, with appropriate consideration of the impact of lack of randomization leading to down rating for risk of bias according to the ROBINS‐I tool (Schünemann 2019).
When RCTs and NRSs were considered together, we followed current GRADE guidance; if certainty of evidence differed in a body of RCTs and a body of NRSs, we presented summary of findings tables only with higher‐certainty evidence; If certainty ratings were the same, we presented results from the two bodies of evidence separately. In addition, if results were consistent, then the overall certainty assessment was that of the two bodies of evidence. If results were inconsistent, and we believed both bodies of evidence should be taken into consideration, then we rated down further for this inconsistency (Schünemann 2019). We did not pool across bodies of evidence from RCTs and NRSs.
Results
Description of studies
Details of included studies are presented elsewhere (Characteristics of included studies table; Table 4; Table 5; Table 6).
1. Baseline characteristics of included studies.
| Study name | Trial period (year to year) | Study design/setting/country | Description of participants | Intervention(s) and comparator(s) | Duration of follow‐up | Age (years) | IPSS | Prostate volume (mL) |
| Abt 2021 | 2014–2017 | RCT/single center/Switzerland | Men aged ≥ 40 years, TURP indicated, refractory to medical treatment or not willing to undergo or continue medical treatment, with prostate size 25–80 mL as measured by transabdominal ultrasound, with IPSS of at least 8, with IPSS‐related quality of life of ≥ 3, with Qmax < 12 mL/second or urinary retention, and who provided written informed consent | PAE | 24 months | 65.7 (SD 9.3) | 19.38 (SD 6.37) | 52.8 (SD 32.0) |
| TURP | 66.1 (SD 9.8) | 17.59 (SD 6.17) | 56.5 (SD 31.1) | |||||
| Carnevale 2016 | 2010–2012 | RCT/single center/Brazil | Men aged > 45 years; IPSS > 19; symptoms refractory to medical treatment for ≥ 6 months; negative screening for prostate cancer; prostate volume 30–90 mL on magnetic resonance imaging; and bladder outlet obstruction confirmed by urodynamic exam | PAE | 12 months | 63.5 (SD 8.7) | 25.3 (SD 3.6) | 63.0 (SD 17.8) |
| TURP | 66.4 (SD 5.6) | 27.6 (SD 3.2) | 56.6 (SD 21.5) | |||||
| Gao 2014 | 2007–2012 | RCT/not defined/China | Men with IPSS > 7 after failed medical therapy with a washout period of ≥ 2 weeks, prostate volume 20–100 mL on transrectal ultrasonographic or magnetic resonance imaging, Qmax < 15 mL/second, and negative prostate biopsy if PSA > 4 ng/mL or abnormal digital rectal exam | PAE | 24 months | 67.7 (SD 8.7) | 22.8 (SD 5.9) | 64.7 (SD 19.7) |
| TURP | 66.4 (SD 7.8) | 23.1 (SD 5.8) | 63.5 (SD 18.6) | |||||
| Insausti 2020 | 2014–2017 | RCT/single center/Spain | Men aged > 60 years; BPH‐related LUTS refractory to medical treatment for ≥ 6 months or the patient could not tolerate medical treatment; TURP was indicated; IPSS ≥ 8; quality of life related to LUTS ≥ 3; Qmax ≤ 10 mL/second or urinary retention | PAE | 12 months | 72.4 (SD 6.2) | 25.8 (SD 4.64) | 60.0 (SD 21.6) |
| TURP | 71.8 (SD 5.5) | 26.0 (SD 7.29) | 62.8 (SD 23.8) | |||||
| Pisco 2020 | 2014–2018 | RCT/single center/Portugal | Men aged > 45 years; diagnosis of LUTS/BPH based on clinical history, digital rectal exam, urinalysis, transrectal ultrasound, and PSA; severe LUTS defined, at screening and at a baseline visit 2 weeks apart, by IPSS of 20 and quality of life score of 3 after a minimum of 6 months' treatment with alpha‐blockers for LUTS/BPH; Qmax < 12 mL/second; prostate volume 40 mL | PAE | 6 months | Median 64 (IQR 59 to 67.5) | Median 25.5 (IQR 22.5 to 29) | Median 63.5 (IQR 55.5 to 100) |
| Sham | Median 64 (IQR 60 to 68.5) | Median 27.5 (IQR 24 to 30.5) | Median 66 (IQR 55.5 to 94.5) | |||||
| Radwan 2020 | 2016–2018 | RCT/single center/Egypt | Men with LUTS with IPSS score 8–35 (8 being moderate and 35 being severe), uroflowmetry with a mean flow ≤ 10 mL/second, and a prostate volume < 100 mL by TRUS | PAE | 6 months | 63.0 (SD 7.2) | 27.0 (SD 5.0) | 58.7 (SD 23.4) |
| TURP | 62.0 (SD 9.0) | 26.5 (SD 4.0) | 60.1 (SD 21.5) | |||||
| Ray 2018 | 2014–2016 | NRS/multicenter/UK | Men with LUTS who had consented to undergo PAE, TURP, open prostatectomy, or holmium enucleation of the prostate at 1 of the United Kingdom Register of Prostate Embolization collaborating centers; were able to read, write, and understand English; and were capable of giving informed written consent | PAE | 12 months | 66 (SD 7.4) | 21.3 (SD 6.7) | 101.2 (SD 57.1) |
| TURP | 70 (SD 7.5) | 21.63 (SD 5.8) | 68.7 (SD 9.2) | |||||
| Soluyanov 2018 | 2016 | NRS/not reported/Russia | BPH with 2 or 3 stages (stage was not defined). | PAE | 6 months | Median 68 (IQR 63 to 75) | Median 23 (IQR 22 to 24) | Median 53 (IQR 37.5 to 56.5) |
| TURP | Median 67 (IQR 62 to 75) | Median 22 (IQR 21 to 24) | Median 43.1 (IQR 36.5 to 50) | |||||
| Zhu 2018 | 2016 | RCT/single center/China | Men with comprehensive diagnosis of BPH through ultrasound prostate exam, digital rectal exam, IPSS, etc.; no absolute contraindication for surgery; no previous history of surgery; not taking 5‐alpha reductase inhibitors | PAE | 12 months | 61.1 (SD 4.4) | 25.63 (SD 4.28) | 81.21 (SD 6.34) |
| TURP | 62.4 (SD 4.9) | 26.22 (SD 4.35) | 82.09 (SD 6.47) |
BPH: benign prostatic hyperplasia; IPSS: International Prostate Symptom Score; IQR: interquartile range; LUTS: lower urinary tract symptoms; NRS: non‐randomized study; PAE: prostatic arterial embolization; PSA: prostate‐specific antigen; Qmax: maximum flow rate; RCT: randomized controlled trial; SD: standard deviation; TRUS: transrectal ultrasound; TURP: transurethral resection of prostate.
2. Participants in included randomized controlled trials.
| Study name | Intervention(s) and comparator(s) | Screened/eligible, n | Randomized, n | Analyzed, n: efficacya | Analyzed, n: safetyb | Finishing trial, n (%) |
| Abt 2021 | PAE | 144/103 | 51 | 34 | 48 | 34 (66.6) |
| TURP | 52 | 47 | 51 | 47 (90.3) | ||
| Total | 103 | 81 | 99 | 81 (78.6) | ||
| Carnevale 2016 | PAE | NR/30 | 15 | 15 | 15 | 15 (100.0) |
| TURP | 15 | 15 | 15 | 15 (100.0) | ||
| Total | 30 | 30 | 30 | 30 (100.0) | ||
| Gao 2014 | PAE | 120/114 | 57 | 47 | 54 | 47 (82.4) |
| TURP | 57 | 48 | 53 | 48 (84.2) | ||
| Total | 114 | 95 | 107 | 95 (83.3) | ||
| Insausti 2020 | PAE | 81/61 | 31 | 23 | 31 | 23 (74.1) |
| TURP | 30 | 22 | 30 | 22 (73.3) | ||
| Total | 61 | 45 | 61 | 45 (73.7) | ||
| Pisco 2020 | PAE | 677/80 | 40 | 40 | 40 | 39 (97.5) |
| Sham | 40 | 40 | 40 | 38 (95.0) | ||
| Total | 80 | 80 | 80 | 77 (96.2) | ||
| Zhu 2018 | PAE | NR/40 | 20 | 20 | 20 | 20 (100.0) |
| TURP | 20 | 20 | 20 | 20 (100.0) | ||
| Total | 40 | 40 | 40 | 40 (100.0) | ||
| Radwan 2020 | PAE | NR/60 | 20 | 20 | 20 | 20 (100.0) |
| TURP | 40 | 40 | 40 | 40 (100.0) | ||
| Total | 60 | 60 | 60 | 60 (100.0) | ||
| Overall total | Intervention: PAE | 234 | 199 | 228 | 198 (84.6) | |
| Comparator: TURP | 214 | 192 | 209 | 192 (89.7) | ||
| Comparator: sham | 40 | 40 | 40 | 38 (95.0) | ||
| Overall | 488 | 431 | 477 | 428 (87.7) | ||
n: number of participants; NR: not reported; PAE: prostatic arterial embolization; TURP: transurethral resection of prostate.
aNumber of participants analyzed for urologic symptom scores. bNumber of participants with adverse events.
3. Participants in included non‐randomized studies.
| Study name | Intervention(s) and comparator(s) | eligible, n | Analyzed, n: efficacya | Analyzed, n: safetyb | Finishing study, n (%) |
| Ray 2018 | PAE | 216 | 132 | 216 | 189 (87.5) |
| TURP | 89 | 29 | 89 | 65 (73.0) | |
| Total | 161 | 305 | 254 (83.2) | ||
| Soluyanov 2018 | PAE | 8 | 8 | NR | 8 (100.0) |
| TURP | 19 | 19 | NR | 19 (100.0) | |
| Total | 27 | NR | 27 (100.0) | ||
| Overall total | Intervention: PAE | 224 | 140 | 216 | 197 (87.9) |
| Comparator: TURP | 108 | 48 | 89 | 84 (82.4) | |
| Overall | 188 | 305 | 281 (84.6) | ||
n: number of participants; NR: not reported; PAE: prostatic arterial embolization; TURP: transurethral resection of prostate. aNumber of participants analyzed for urologic symptom scores. bNumber of participants with adverse events.
Results of the search
We identified 2980 records through electronic database searching, including 96 records in trials registers. We found no records in the grey literature repository. We further identified one record through other sources by searching the reference lists of included study (protocol of Abt 2021 published in BMC Urology). After removing duplicates, we screened the titles and abstracts of 1248 records, and we excluded 1198 records. We screened 50 full‐text articles and excluded 13 studies (16 records) that did not meet the inclusion criteria or were not relevant to the question under trial. We found one study awaiting classification. Six studies (six records) are ongoing. We included nine studies (seven RCTs: 21 records; two NRSs: six records) in the review. The flow of literature through the assessment process is shown in the PRISMA flow chart (Figure 1).
1.
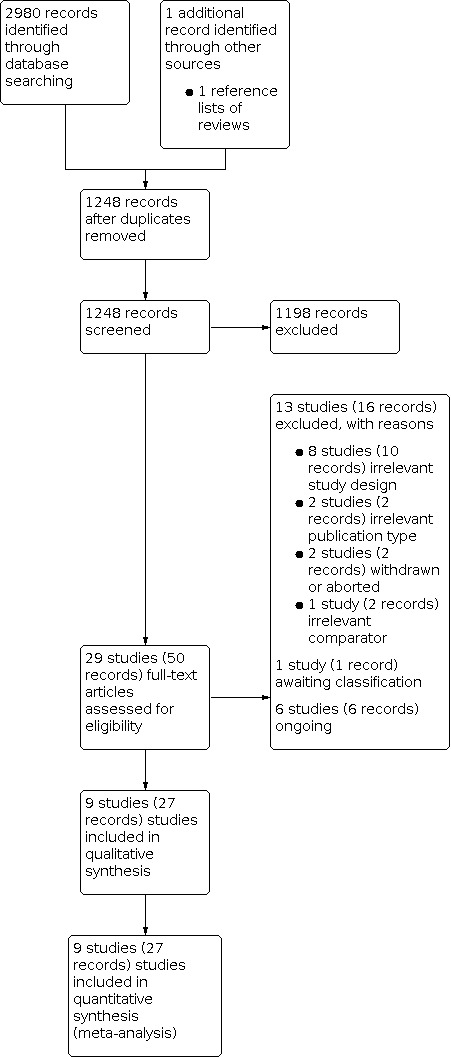
Study flow diagram.
Included studies
1. Randomized controlled trials
Sources of data
We identified the seven published full‐text studies (Abt 2021; Carnevale 2016; Gao 2014; Insausti 2020; Pisco 2020; Radwan 2020; Zhu 2018). Six trials were published in English, and Zhu 2018 was published in Chinese. We attempted to contact all corresponding authors of included trials to obtain additional information on study methods and results, and we received replies from three (Abt 2021; Pisco 2020; Radwan 2020; see Appendix 3).
Study design and settings
All trials were likely single‐center parallel RCTs that were conducted in various countries, namely, Brazil (Carnevale 2016), China (Gao 2014; Zhu 2018), Egypt (Radwan 2020), Portugal (Pisco 2020), Spain (Insausti 2020), and Switzerland (Abt 2021). Abt 2021 and Insausti 2020 were reported as "open label". Four studies did not provide information regarding blinding (Carnevale 2016; Gao 2014; Radwan 2020; Zhu 2018). Pisco 2020 blinded study participants only. The included studies were performed between 2007 and 2018.
Participants
The seven studies included 488 randomized participants (PAE 234, TURP 214, sham 40). Mean age was 65 years, IPSS was 23.8, and Qmax was 7.89 mL/second. Mean prostate volume was 62.6 mL.
Most studies included participants with LUTS as defined by an IPSS score greater than 7 despite medical treatment, and prostate volume between 20 mL and 100 mL. Five trials used uroflowmetry as an inclusion criterion (Qmax less than 15 mL/second: Abt 2021; Gao 2014; Insausti 2020; Pisco 2020; mean flow less than 10 mL/second: Radwan 2020). Carnevale 2016 included participants based on bladder outlet obstruction confirmed by urodynamic evaluation (Bladder Outlet Obstruction Index greater than 40).
Major exclusion criteria relevant to all trials included urethral (e.g. urethral stricture) or bladder disorders (e.g. neurogenic bladder, bladder calculi, diverticula); renal failure; history of prostate, bladder neck, or urethral surgery; and suspected prostate cancer.
Intervention(s) and comparator(s)
All PAE procedures were conducted via a femoral approach under local anesthesia. The studies obtained an initial pelvic arteriogram to evaluate the iliac vessels and the prostatic arteries. Selective angiography of the internal iliac arteries was performed to better assess the blood supply to the prostate. After super‐selective catheterization of the inferior vesicle arteries was performed to ensure that the tip of the microcatheter was inside or at the ostium of the prostatic arteries, embolization using microspheres (Abt 2021: 250 μm to 400 μm microspheres [Embozene, Boston Scientific, USA]; Carnevale 2016 and Zhu 2018: calibrated 300 μm to 500 µm tris‐acryl gelatin microspheres [Embosphere Microspheres, Merit Medical, USA]; Gao 2014: 355 μm to 500 µm polyvinyl alcohol microspheres [Ivalon, Cook, USA]; Insausti 2020 and Pisco 2020: 300 μm to 500 µm poly(vinyl alcohol) microspheres [Bead Block BTG plc, Boston Scientific, USA]; Radwan 2020: not specified) was performed. Embolization was terminated when there was complete stasis, without reflux of the mixture to undesired arteries.
Six studies used TURP as a comparator. Monopolar or bipolar TURP (Abt 2021; Carnevale 2016: monopolar TURP; Gao 2014; Insausti 2020: bipolar TURP; Radwan 2020: both TURP techniques; Zhu 2018: not specified) was performed under spinal or general anesthesia.
One study used a sham procedure as a comparator (Pisco 2020). In the sham group, there were no embolization particles injected after catheterization of the prostatic arteries.
Comparisons
Six RCTs compared PAE to TURP (Abt 2021; Carnevale 2016; Gao 2014; Insausti 2020; Radwan 2020; Zhu 2018); one study compared PAE to sham (Pisco 2020); no study compared PAE to laser ablation or enucleation of the prostate, or other minimally invasive therapies.
Outcomes
We identified reporting of all primary and secondary outcomes in each of the included studies. All studies reported urologic symptom scores and quality of life outcomes except Radwan 2020 (only reported urologic symptom scores). Urologic symptom scores were reported by IPSS (scale 0 to 35; higher scores indicating worse urologic symptoms) and quality of life by IPSS‐quality of life (scale 0 to 6; higher scores indicating worse quality of life). Adverse events were classified by National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0 (Carnevale 2016), or by the Clavien‐Dindo Classification System (Abt 2021; Gao 2014; Insausti 2020; Pisco 2020). The remaining studies did not specify the adverse events classification system. Abt 2021 reported all primary and secondary outcomes. Two studies reported erectile function using the IIEF‐5 (scale 1 to 25; higher scores indicating better erectile function) (Abt 2021; Carnevale 2016). Although we found no studies using a questionnaire to assess ejaculatory function, all studies except Gao 2014 (outcome not measured) reported data on ejaculatory disorders as reduction in ejaculate volume or retrograde ejaculation. Abt 2021 reported the duration (days) of indwelling catheter placement, and Gao 2014 provided the proportion of participants with indwelling catheter after intervention. Four studies reported hospital stay (days) (Abt 2021; Carnevale 2016; Gao 2014; Insausti 2020), but three studies reported data that we were unable to use for meta‐analysis (Abt 2021; Gao 2014; Insausti 2020).
Gao 2014 and Abt 2021 reported both short‐term and long‐term follow‐up outcomes (up to 24 months), and the remaining studies reported only short‐term follow‐up outcomes (Carnevale 2016; Insausti 2020; Pisco 2020; Radwan 2020; Zhu 2018: up to 12 months).
Funding sources and conflicts of interest
Abt 2021 was supported by a grant from the research committee of St Gallen Cantonal Hospital. Device manufacturers supported two studies (Insausti 2020; Pisco 2020). One study reported no external funding (Carnevale 2016), and the others did not report the funding source (Gao 2014; Radwan 2020; Zhu 2018).
Study authors of five studies reported that they had no relevant conflicts of interest (Abt 2021; Carnevale 2016; Gao 2014; Pisco 2020; Radwan 2020). One study reported conflicts of interest of members of the investigative team with the device manufacturer (Insausti 2020), and the other study did not report the conflicts of interest (Zhu 2018).
2. Non‐randomized studies (prospective comparative studies)
Sources of data
We identified two published studies (Ray 2018; Soluyanov 2018). Ray 2018 was published in English and Soluyanov 2018 in Russian. We attempted to contact all corresponding authors to obtain additional information on study methods and results, and we received replies from Ray 2018 (see Appendix 3).
Study design and settings
Ray 2018 was a multicenter registry‐based NRS (UK‐ROPE) with a propensity‐matched pair analysis as a joint initiative between the British Society of Interventional Radiologists, the British Association of Urological Surgeons, and the National Institute for Health and Care Excellence (NICE). Soluyanov 2018 was a single center‐based prospective NRS conducted in Russia.
Participants
We included 332 participants (PAE 224, TURP 108) (Ray 2018; Soluyanov 2018). Mean age was 67 years, prostate volume was 87.1 mL and IPSS was 21.4. Baseline characteristics of participants who underwent PAE versus TURP were significantly different in age, prostate volume, and postvoid residual in UK‐ROPE (Ray 2018). Neither study reported its inclusion and exclusion criteria in detail (Ray 2018; Soluyanov 2018).
Intervention(s) and comparator(s)
Ray 2018 did not report its PAE technique in any detail, and Soluyanov 2018 performed PAE using 300 μm to 500 μm microspheres (product manufacturer: not described) under local anesthesia.
Both studies used TURP as a comparator (Ray 2018: monopolar or bipolar TURP; Soluyanov 2018: bipolar TURP). Ray 2018 did not provide information with regard to anesthesia, and Soluyanov 2018 performed TURP under spinal anesthesia.
Comparisons
Both studies compared PAE to TURP (Ray 2018; Soluyanov 2018). Soluyanov 2018 included more than two intervention groups – PAE, TURP, and transvesical adenectomy.
We found no studies that compared PAE to sham (no treatment), laser ablation or enucleation of the prostate, or other minimally invasive therapies. UK‐ROPE planned to report multiple comparisons with PAE and holmium laser enucleation of the prostate, but these data were not available.
Outcomes
We identified reporting of all review outcomes except indwelling urinary catheter outcomes in each of the studies for comparisons with TURP (Ray 2018; Soluyanov 2018).
Urologic symptom scores were reported using IPSS and quality of life using IPSS‐quality of life. Ray 2018 used Clavien‐Dindo Classification to report adverse events, and Soluyanov 2018 did not provide details on measuring this outcome. Ray 2018 reported retreatment, erectile function by IIEF‐5, and the event of retrograde ejaculation during the follow‐up period.
All NRSs reported short‐term outcomes only except retreatment (Ray 2018 reported the outcome after 12 months [long term]).
Funding sources and conflicts of interest
UK‐ROPE was supported by a medical device company, the British Society of Interventional Radiologists, and the British Association of Urological Surgeons. The NICE funded an independent academic unit to run the registry through a competitive tender (Ray 2018). The other study did not mention a funding source (Soluyanov 2018).
Ray 2018 reported having relationships with medical device companies, and Soluyanov 2018 did not indicate any conflicts of interest.
Excluded studies
We excluded 13 studies (16 records) after evaluating the full‐text publications. Eight studies used the wrong study design (Abt 2019; Bagla 2017; Brown 2019; Mullhaupt 2019; NCT01835860; Pereira 2018; Qiu 2017; Wu 2019). Two studies were reported as a letter to the editor (Bilhim 2015) and narrative review (Steurer 2018). Two studies were withdrawn or aborted (NCT02006303; NCT02566551). Russo 2015 compared PAE to simple prostatectomy, which was outside the scope of this review (wrong comparator). Further details of the excluded studies are presented in the Characteristics of excluded studies table.
Studies awaiting classification and ongoing trials
We found one study awaiting classification (Ng 2020; Characteristics of studies awaiting classification). Six studies including four RCTs (ACTRN12617001235392; NCT04084938; NCT04236687; NCT04807010) and two NRS (ChiCTR1800014818; NCT01789840) are ongoing. Details of these trials are presented in the Characteristics of ongoing studies table.
Risk of bias in included studies
1. Randomized controlled trials
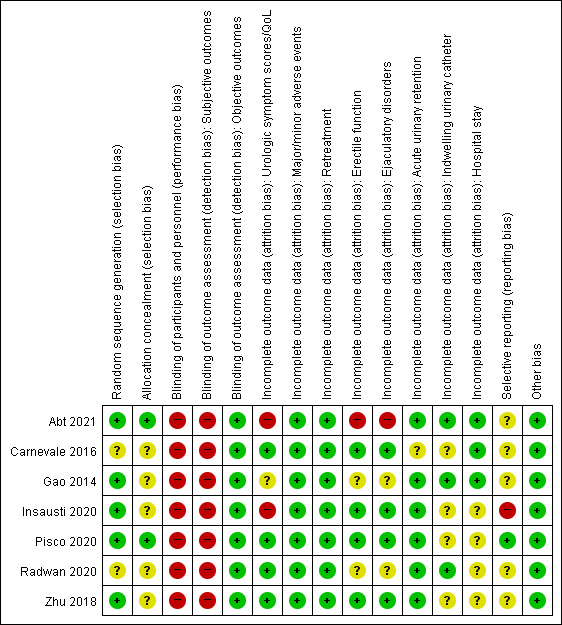
We found seven RCTs comparing PAE to TURP (Abt 2021; Carnevale 2016; Gao 2014; Insausti 2020; Radwan 2020; Zhu 2018) or sham (Pisco 2020). Only Gao 2014 reported anything beyond short‐term outcomes. See Figure 2 and Figure 3.
2.
Risk of bias summary: review authors' judgments about each risk of bias item for randomized controlled studies. Categories: green point (+) = low risk of bias; yellow point (?) = unclear risk of bias; red point (‐) = high risk of bias.
3.
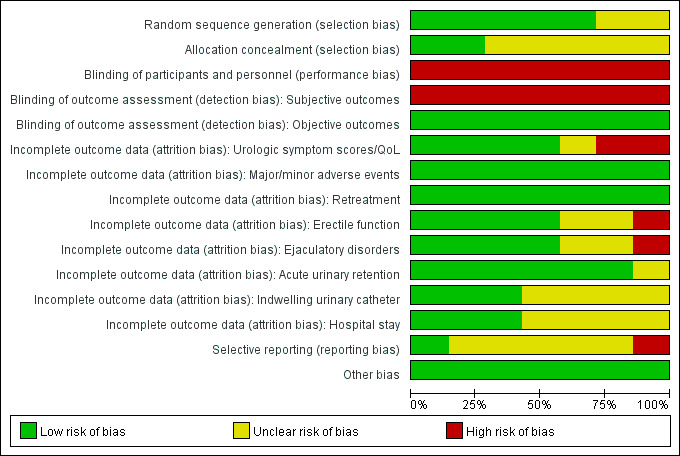
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included randomized controlled studies.
Allocation
Random sequence generation
We rated five studies at low risk of bias (Abt 2021; Gao 2014; Insausti 2020; Pisco 2020; Zhu 2018), and two studies at unclear risk of bias (Carnevale 2016; Radwan 2020).
Allocation concealment
We rated two studies at low risk of bias (Abt 2021; Pisco 2020), and the remaining studies at unclear risk of bias due to lack of information on the allocation method (Carnevale 2016; Gao 2014; Insausti 2020; Radwan 2020; Zhu 2018).
Blinding
Blinding of participants and personnel
We rated all studies at high risk of bias. Abt 2021 and Insausti 2020 were open‐label studies. Pisco 2020 was a single‐blind study. Although Carnevale 2016, Radwan 2020, and Zhu 2018 did not report any information on blinding, blinding appeared highly unlikely to have taken place in a surgical trial without specific measures, which would have been noted. In Gao 2014, study authors included participants after informing them about details of the procedure, thereby indicating lack of blinding.
Blinding of outcome assessment
Subjective outcomes (urologic symptom scores, quality of life, major adverse events, erectile function, ejaculatory disorders, and minor adverse events): we judged all studies at high risk of bias given lack of assurance of appropriate methods of blinding.
Objective outcomes (retreatment, acute urinary retention, indwelling urinary catheter, and hospital stay): we rated all studies at low risk of bias for these outcomes as they were unlikely to be affected by lack of blinding (ascertaining this does not involve judgment).
Incomplete outcome data
Two studies only reported both short‐term and long‐term (longer than 12 months' follow‐up) outcome data (Abt 2021; Gao 2014), but the remaining studies reported only short‐term outcomes (up to 12 months' follow‐up). We rated the risk of bias separately for all outcomes in Abt 2021 and Gao 2014 according to the timing of outcome measurement (short‐term or long‐term), but judgments were the same for all outcomes; therefore, reporting was collapsed.
Urologic symptom scores and quality of life: we rated four studies at low risk of bias (Carnevale 2016; Pisco 2020; Radwan 2020; Zhu 2018); we judged the others as having unclear (Gao 2014), or high (Abt 2021; Insausti 2020) risk of bias.
Major and minor adverse events: we rated all studies at low risk of bias.
Retreatment: we rated all studies at low risk of bias (Abt 2021; Carnevale 2016; Gao 2014; Insausti 2020; Pisco 2020; Radwan 2020; Zhu 2018).
Erectile function: we rated four studies at low risk of bias (Carnevale 2016; Insausti 2020; Pisco 2020; Zhu 2018); we judged the remaining studies as having unclear (Gao 2014; Radwan 2020) or high (Abt 2021) risk of bias.
Ejaculatory disorders: we rated four studies at low risk of bias (Carnevale 2016; Insausti 2020; Pisco 2020; Zhu 2018); we judged the others at unclear (Gao 2014; Radwan 2020), or high (Abt 2021) risk of bias.
Acute urinary retention: we rated six studies at low risk of bias (Abt 2021; Gao 2014; Insausti 2020; Pisco 2020; Radwan 2020; Zhu 2018); we judged Carnevale 2016 at unclear risk of bias.
Indwelling urinary catheter: we rated three studies at low risk of bias (Abt 2021; Gao 2014; Radwan 2020); we judged four studies at unclear risk of bias (Carnevale 2016; Insausti 2020; Pisco 2020; Zhu 2018).
Hospital stay: we rated three studies at low risk of bias (Abt 2021; Carnevale 2016; Gao 2014); we judged the remaining studies at unclear risk of bias (Insausti 2020; Pisco 2020; Radwan 2020; Zhu 2018).
Selective reporting
We rated one study at low risk of bias (Pisco 2020). We rated four studies at unclear risk of bias given lack of available protocols (Carnevale 2016; Gao 2014; Radwan 2020; Zhu 2018), or reporting of study outcomes that were not predefined in the protocol (Abt 2021). We judged one study at high risk of bias due to deviation in study outcomes from the protocol (Insausti 2020).
Other potential sources of bias
We rated all studies at low risk of bias; we identified no other sources of bias.
2. Non‐randomized studies (prospective comparative studies)
We found two prospective comparative studies comparing PAE to TURP for short‐term only (Ray 2018; Soluyanov 2018). For reporting purposes, we split the risk of bias assessments for the outcomes into three sets. Within each set of outcomes the risk of bias assessments were the same across all domains. Set 1: urologic symptom scores; set 2: quality of life, erectile function, ejaculatory disorders, and hospital stay; set 3: major adverse events, retreatment, minor adverse events, and acute urinary retention. No study reported indwelling catheter (no information). Overall, we judged outcomes in set 1 (urologic symptom scores) to be at critical risk of bias for Soluyanov 2018 and serious risk of bias overall for Ray 2018 (Figure 4; Table 7). Only Ray 2018 reported outcome sets 2 and 3 and we judged these at serious risk of bias (Figure 4; Table 8). Details of risk of bias from NRSs using ROBINS‐I are presented in Figure 4, Table 7, Table 8, and Appendix 4.
4.
Risk of bias summary: ROBINS‐I set 1 includes outcome: urologic symptom scores; ROBINS‐I set 2 includes outcomes: quality of life, erectile function, ejaculatory disorders, hospital stay; ROBINS‐I set 3 includes outcomes: adverse events, retreatment, acute urinary retention; ROBINS‐I set 4 includes outcome (not reported in either study): indwelling catheter measured at up to 12 months (short term). Figure created using robvis: www.riskofbias.info/welcome/robvis-visualization-tool.
4. ROBINS‐I assessment by study: Ray 2018.
| Study name:Ray 2018 | |||
| Risk of bias domain | Assessments by outcome | Support for judgment | Conclusion |
| Bias due to confounding | All outcomesa | Quote: "multivariate analysis was performed in R version 3.3.2 (2016‐10‐31). We applied a combination of multiple imputation and propensity‐matched pairing in the comparative between‐group analysis. Propensity matching was based on a logistic regression model and yielded 65 matched pairs. Background variables used for matching were age at procedure; length of time with LUTS; baseline IPSS; IPSS QoL; IIEF; Qmax; and PVR". Judgment: although authors likely used an appropriate analysis method to control confounding factors, concerns for confounding may remain. In addition, multivariate analysis including propensity‐matched pairing was reported only for IPSS and IPSS QoL. For all other outcomes in the review, risk of bias due to confounding could be considerable. |
Serious |
| Bias in selection of participants into the study | Judgment: selection of participants into the study was not based on participant characteristics observed after the start of the intervention and the start of follow‐up and the start of the intervention likely coincided for most participants. As inclusion criteria were not reported in detail in protocol as well as in publication, there are concerns for postintervention variables that influenced selection likely to be associated with intervention (e.g. prostate volume). | Moderate | |
| Bias in classification of interventions | Quote: "the British Society of Interventional Radiologists and the British Association of Urological Surgeons co‐funded the online UK Register of Prostate Embolization (UK‐ROPE), which was built and hosted by Dendrite Clinical Systems Ltd". Judgment: this study was based on the ongoing authorized registry (UK‐ROPE) that predefined the interventions. |
Moderate | |
| Bias due to deviations from intended interventions | Judgment: although this study was based on the prospective enrolled registry (UK‐ROPE), no information was provided with regard to co‐intervention. | No information | |
| Bias due to missing data | Urologic symptom scores, QoL, erectile function, ejaculatory disorders, and hospital stay | Judgment: although the proportion of participants with missing data was similar across interventions, about 2/3 participants in each group were included in the analysis. | Serious |
| Major adverse events, retreatment, minor adverse events, and AUR | Judgment: all participants were included in the analysis. | Low | |
| Bias in measurement of outcomes | Subjective outcomesb | Quote: "there was no blinding (either clinician or participant) in this single‐arm observational study". Judgment: given that study outcomes were subjective, outcome measures were likely influenced by knowledge of the intervention received. |
Serious |
| Objective outcomesc | Judgment: although objective outcomes are unlikely influenced by knowledge of the intervention received in outcome assessment, participants and personnel were not blinded. | Serious | |
| Bias in selection of the reported result | All outcomesa | Judgment: protocol was published and study outcomes were well predefined and described. In addition, study author provided unreported data via email. | Low |
| Overall | — | Judgment: serious risk of bias in ≥ 1 domain, but not at critical risk of bias in any domain. | Serious |
AUR: acute urinary retention; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; PVR: postvoid residual; Qmax: maximum flow rate; QoL: quality of life; ROBINS‐I: risk of bias tool to assess non‐randomized studies of interventions. aAll review outcomes reported in study: urologic symptom scores, QoL, major adverse events, retreatment, minor adverse events, erectile function, AUR, ejaculatory disorders, and hospital stay. bUrologic symptom scores, QoL, major adverse events, erectile function, ejaculatory disorders, and minor adverse events. cRetreatment, AUR, and hospital stay.
5. ROBINS‐I assessment by study: Soluyanov 2018.
| Study name:Soluyanov 2018 | |||
| Risk of bias domain | Assessments by outcome | Support for judgment | Conclusion |
| Bias due to confounding | Urologic symptom scoresa | Quote: "patients were assigned to one of three groups (i.e., planning one of three operations) taking into account the volume of the prostate gland and the presence of concomitant chronic diseases". Judgment: participants were selected based on participant characteristics and post intervention and study author did not use an appropriate analysis method that controlled for confounding. |
Critical |
| Bias in selection of participants into the study | Quote: "patients were assigned to one of three groups (i.e., planning one of three operations) taking into account the volume of the prostate gland and the presence of concomitant chronic diseases". Judgment: participants were selected based on prostate volume related to the results of outcomes. |
Critical | |
| Bias in classification of interventions | Judgment: likely prospective comparative trial with predefined criteria for the intervention. | Moderate | |
| Bias due to deviations from intended interventions | Judgment: no information with regard to co‐intervention and analysis used to estimate the effects of starting and adhering to the intervention. | No information | |
| Bias due to missing data | Judgment: all participants were included in the analysis. | Low | |
| Bias in measurement of outcomes | Judgment: given that study outcomes were subjective, outcome measures were likely influenced by knowledge of the intervention received. | Serious | |
| Bias in selection of the reported result | Judgment: study outcomes were not well predefined and described, and the protocol was not found. | No information | |
| Overallb | — | Judgment: critical risk of bias in ≥ 1 domain. | Critical |
ROBINS‐I: risk of bias tool to assess non‐randomized studies of interventions. aThe review outcome reported in study.
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1; Table 2; and Table 3.
Prostatic arterial embolization versus transurethral resection of the prostate (short term)
Primary outcomes
1. Urologic symptom scores
Six RCTs with 360 participants (PAE 165, TURP 195) reported short‐term urologic symptom scores (Abt 2021; Carnevale 2016; Gao 2014; Insausti 2020; Radwan 2020; Zhu 2018). There may be little to no difference between PAE and TURP in improvement of IPSS (MD 1.72, 95% CI –0.37 to 3.81; I² = 78%; low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and serious inconsistency (–1); we did not downgrade further for imprecision, since we attributed the wide CIs to the observed inconsistency.
One prospective NRS with 161 participants (PAE 132, TURP 29) (Ray 2018) reported short‐term urologic symptom scores. We are very uncertain about the effect on urologic symptom scores (MD 2.80, 95% CI 0.04 to 5.56; very low‐certainty evidence). We downgraded the certainty of evidence for very serious study limitations (–2) and serious imprecision (–1).
Based on evidence from RCTs that provided evidence of higher certainty, there may be little to no difference between these procedures in the improvement of short‐term urologic symptom scores (low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 1: Urologic symptom scores
2. Quality of life
Five RCTs with 300 participants (PAE 145, TURP 155) reported short‐term quality of life (Abt 2021; Carnevale 2016; Gao 2014; Insausti 2020; Zhu 2018). There may be little to no difference between PAE and TURP in IPSS‐quality of life improvement (MD 0.28, 95% CI –0.28 to 0.84; I² = 63%; low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and serious inconsistency (–1); we did not downgrade further for imprecision, since we attributed the wide CIs to the observed inconsistency.
One prospective NRS with 164 participants (PAE 133, TURP 31) reported short‐term quality of life (Ray 2018). We are very uncertain about the effect on quality of life (MD 0.50, 95% CI –0.03 to 1.03; very low‐certainty evidence). We downgraded the certainty of evidence for very serious study limitations (–2) and serious imprecision (–1).
Based on the evidence from RCTs that provided evidence of higher certainty, there may be little to no difference between PAE and TURP in short‐term quality of life (low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 2: Quality of life
3. Major adverse events
Four RCTs with 250 participants (PAE 114, TURP 136) reported short‐term major adverse events (Abt 2021; Carnevale 2016; Insausti 2020; Radwan 2020). We are very uncertain about the effects of PAE on major adverse events (RR 0.75, 95% CI 0.19 to 2.97; I² = 24%; very low‐certainty evidence); this corresponds to 15 fewer (95% CI 48 fewer to 116 more) major adverse events per 1000 participants. We rated the certainty of evidence as very low, downgrading for serious study limitations (–1) and very serious imprecision (–2).
One prospective NRS with 305 participants (PAE 216, TURP 89) reported short‐term major adverse events (Ray 2018). There were no major adverse events in either study group (very low‐certainty evidence). We rated the certainty of evidence as very low, after downgrading for very serious study limitations (–2) and very serious imprecision (–2).
Based on the entire body of evidence that included both RCTs and NRSs, we are very uncertain whether PAE results in fewer or more short‐term major adverse events than TURP (very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 3: Major adverse events
Secondary outcomes
1. Retreatment
Four RCTs with 303 participants (PAE 140, TURP 163) reported short‐term retreatment (Abt 2021; Carnevale 2016; Gao 2014; Radwan 2020). PAE likely increases retreatment rates (RR 3.20, 95% CI 1.41 to 7.27; I² = 0%; moderate‐certainty evidence); this corresponds to 81 more (95% CI 15 more to 231 more) retreatments per 1000 participants. We downgraded the certainty of evidence for serious study limitations (–1).
We are very uncertain about the effects of PAE compared with TURP on retreatment based on one prospective NRS (RR 1.51, 95% CI 0.43 to 5.29; very low‐certainty evidence); this corresponds to 17 more (95% CI 19 fewer to 145 more) retreatments per 1000 participants (Ray 2018). We downgraded the certainty of evidence for very serious study limitations (–2) and very serious imprecision (–2).
Based on evidence from RCTs that provided evidence of higher certainty, PAE likely increases short‐term retreatment rates (moderate‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 4: Retreatment
2. Erectile function
Two RCTs with 120 participants (PAE 55, TURP 65) reported short‐term erectile function (Abt 2021; Carnevale 2016). There may be little to no difference between PAE and TURP in improvement of IIEF‐5 (MD –0.50, 95% CI –5.88 to 4.88; I² = 68%; low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and serious inconsistency (–1); we did not downgrade further for imprecision, since we attributed the wide CIs to the observed inconsistency.
One prospective NRS with 122 participants (PAE 102, TURP 20) reported short‐term erectile function (Ray 2018). We are very uncertain about the effects of PAE on erectile function (MD 1.50, 95% CI –2.01 to 5.01; very low‐certainty evidence). We downgraded the certainty of evidence for very serious study limitations (–2) and for serious imprecision (–1).
Based on evidence from RCTs that provided evidence of higher certainty, there may be little to no difference between PAE and TURP in short‐term erectile function (low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 5: Erectile function
3. Ejaculatory disorders
Three RCTs with 141 participants (PAE 71, TURP 70) reported short‐term ejaculatory disorders (Abt 2021; Carnevale 2016; Insausti 2020). We are uncertain how PAE affects this outcome (RR 0.26, 95% CI 0.06 to 1.19; I² = 83%; very low‐certainty evidence); this would correspond to 476 fewer (95% CI 604 fewer to 122 more) ejaculatory disorders per 1000 men. We rated the certainty of evidence as very low, downgrading for serious study limitations (–1) and for very serious imprecision (–2).
One prospective NRS with 260 participants (PAE 199, TURP 61) reported short‐term ejaculatory disorders (Ray 2018). PAE may reduce ejaculatory disorders (RR 0.51, 95% CI 0.35 to 0.73; low‐certainty evidence); this would correspond to 233 fewer ejaculatory disorders per 1000 men (95% CI 309 fewer to 128 fewer). We rated the certainty of evidence as low, downgrading for very serious study limitations (–2).
Based on the body of evidence from the NRS that provided evidence of higher certainty, PAE may reduce short‐term ejaculatory disorders (low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 6: Ejaculatory disorder
4. Minor adverse events
Three RCTs with 189 participants (PAE 83, TURP 106) reported minor adverse events (Abt 2021; Carnevale 2016; Radwan 2020). We are very uncertain about the effects of PAE on minor adverse events (RR 0.86, 95% CI 0.42 to 1.73; I² = 74%; very low‐certainty evidence); this would correspond to 67 fewer (95% CI 279 fewer to 351 more) minor adverse events per 1000 men. We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2).
One prospective NRS with 305 participants (PAE 216, TURP 89) reported minor adverse events (Ray 2018). We are very uncertain about the effects of PAE on minor adverse events (RR 2.27, 95% CI 0.51 to 10.02; very low‐certainty evidence); this would correspond to 74 fewer (95% CI 180 more to 115 fewer) minor adverse events per 1000 men. We downgraded the certainty of evidence for very serious study limitations (–2) and very serious imprecision (–2).
Based on the entire body of evidence, we are very uncertain about the effects of PAE on short‐term minor adverse events (very low‐certainty evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 7: Minor adverse events
5. Acute urinary retention
Five RCTs with 367 participants (PAE 173, TURP 194) reported short‐term acute urinary retention (Abt 2021; Gao 2014; Insausti 2020; Radwan 2020; Zhu 2018). We are very uncertain about the effects of PAE on acute urinary retention (RR 1.65, 95% CI 0.54 to 5.07; I² = 44%; very low‐certainty evidence). PAE may result in 37 more (95% CI 26 fewer to 231 more) acute urinary retention events per 1000 men. We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2).
One prospective NRS with 305 participants (PAE 216, TURP 89) reported short‐term acute urinary retention (Ray 2018). There were no acute urinary retention episodes in either group (very low‐certainty evidence). We downgraded the certainty of evidence for very serious study limitations (–2) and very serious imprecision (–2).
Based on the entire body of evidence, we are very uncertain about effects of these procedures on short‐term acute urinary retention (very low‐certainty evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 8: Acute urinary retention
6. Indwelling urinary catheter
One RCT with 99 participants (PAE 48, TURP 51) reported short‐term indwelling urinary catheter (Abt 2021). PAE likely reduces time with an indwelling urinary catheter (MD –2.00 days, 95% CI –2.55 to –1.45; moderate‐certainty evidence; Analysis 1.9). We downgraded the certainty of evidence for study limitations (–1).
1.9. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 9: Indwelling urinary catheter
No NRS reported short‐term indwelling urinary catheter.
7. Hospital stay
Three RCTs with 260 participants (PAE 129, TURP 131) reported short‐term hospital stay (Abt 2021; Gao 2014; Insausti 2020). PAE may reduce hospital stay (MD –1.51 days, 95% CI –2.44 to –0.58; I² = 90%; low‐certainty evidence; Analysis 1.10). We downgraded the certainty of evidence for study limitations (–1) and serious imprecision (–1). We did not downgrade for inconsistency despite substantial heterogeneity given that likely not clinically meaningful.
1.10. Analysis.

Comparison 1: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term), Outcome 10: Hospital stay
No NRS reported short‐term hospital stay.
Subgroup and sensitivity analyses
We were unable to perform any predefined secondary analyses because there were no relevant short‐term data and the included studies had a similar risk of bias.
Prostatic arterial embolization versus transurethral resection of the prostate (long term)
Primary outcomes
1. Urologic symptom scores
Two RCTs with 176 participants (PAE 81, TURP 95) reported long‐term urologic symptom scores (Abt 2021; Gao 2014). PAE may result in little to no difference in improvement of IPSS (MD 2.58, 95% CI –1.54 to 6.71; I² = 73%; low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and serious inconsistency (–1); we did not downgrade further for imprecision, since we attributed the wide CIs to the observed inconsistency (Analysis 2.1).
2.1. Analysis.

Comparison 2: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term), Outcome 1: Urologic symptom scores
2. Quality of life
Two RCTs with 176 participants (PAE 81, TURP 95) reported long‐term quality of life (Abt 2021; Gao 2014). PAE may result in little to no difference in IPSS‐quality of life (MD 0.50, 95% CI –0.03 to 1.04; I² = 29%; low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and serious imprecision (–1) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term), Outcome 2: Quality of life
3. Major adverse events
Two RCTs with 206 participants (PAE 102, TURP 104) reported long‐term adverse events (Abt 2021; Gao 2014). We are very uncertain about the effects of PAE on major adverse events (RR 0.91, 95% CI 0.20 to 4.05; I² = 72%; very low‐certainty evidence). PAE would result in 12 fewer (95% CI 108 fewer to 411 more) major adverse events per 1000 men. We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2) (Analysis 2.3).
2.3. Analysis.

Comparison 2: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term), Outcome 3: Major adverse events
Secondary outcomes
1. Retreatment
One RCT with 81 participants (PAE 34, TURP 47) reported long‐term retreatment (Abt 2021). PAE likely increases retreatment rates (RR 3.80, 95% CI 1.32 to 10.93; moderate‐certainty evidence); this corresponds to 238 more (95% CI 27 more to 845 more) retreatments per 1000 men. We downgraded the certainty of evidence for serious study limitations (–1).
One NRS with 305 participants (PAE 216, TURP 89) reported long‐term retreatment (Ray 2018). PAE may increase retreatment rates (RR 3.54, 95% CI 1.45 to 8.65; low‐certainty evidence); this corresponds to 47 more (95% CI 0 fewer to 214 more) retreatments per 1000 men. We downgraded the certainty of evidence for serious study limitations (–2).
Based on evidence from RCTs that provided evidence of higher certainty, PAE likely increases long‐term retreatment rates compared to TURP (moderate‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term), Outcome 4: Retreatment
2. Erectile function
One RCT with 81 participants (PAE 34, TURP 47) reported long‐term erectile function (Abt 2021). PAE may result in little to no difference in improvement of IIEF‐5 (MD 3.09, 95% CI –0.76 to 6.94; low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and serious imprecision (–1) (Analysis 2.5).
2.5. Analysis.

Comparison 2: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term), Outcome 5: Erectile function
3. Ejaculatory disorders
One RCT with 50 participants (PAE 25, TURP 25) reported long‐term ejaculatory disorders (Abt 2021). PAE may reduce ejaculatory disorders compared to TURP (RR 0.67, 95% CI 0.45 to 0.98; low‐certainty evidence); this would correspond to 277 fewer (95% CI 462 fewer to 17 fewer) ejaculatory disorders per 1000 men. We downgraded the certainty of evidence for serious study limitations (–1) and serious imprecision (–1) (Analysis 2.6).
2.6. Analysis.

Comparison 2: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term), Outcome 6: Ejaculatory disorder
4. Minor adverse events
Two RCTs with 206 participants (PAE 102, TURP 104) reported long‐term minor adverse events (Abt 2021; Gao 2014). We are very uncertain about the effects of PAE on minor adverse events (RR 1.15, 95% CI 0.60 to 2.22; I² = 76%; very low‐certainty evidence). PAE would result in 68 more (95% CI 181 fewer to 551 more) minor adverse events per 1000 men. We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2) (Analysis 2.7).
2.7. Analysis.

Comparison 2: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term), Outcome 7: Minor adverse events
5. Acute urinary retention
One RCT with 99 participants (PAE 48, TURP 51) reported long‐term acute urinary retention (Abt 2021). We are very uncertain about the effects of PAE on acute urinary retention (RR 0.71, 95% CI 0.12 to 4.06; very low‐certainty evidence); this would correspond to 17 (95% CI 52 fewer to 180 more) acute urinary retention events per 1000 men. We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2) (Analysis 2.8).
2.8. Analysis.

Comparison 2: Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term), Outcome 8: Acute urinary retention
6. Indwelling urinary catheter
No studies reported long‐term indwelling urinary catheter.
7. Hospital stay
No studies reported long‐term hospital stay.
Subgroup and sensitivity analyses
We were unable to perform any predefined secondary analyses because there were no relevant data and the included studies had a similar risk of bias.
Prostatic arterial embolization versus sham (short term)
One RCT compared PAE versus sham treatment (Pisco 2020). We included 80 participants (PAE 40, sham 40) in the analysis for all review outcomes.
Primary outcomes
1. Urologic symptom scores
PAE likely improves urologic symptom scores compared with sham (MD –12.07, 95% CI –15.45 to –8.69; moderate‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Prostatic arterial embolization (PAE) versus sham (short term), Outcome 1: Urologic symptom scores
2. Quality of life
PAE likely improves quality of life compared with sham (MD –1.97, 95% CI –2.48 to –1.46; moderate‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Prostatic arterial embolization (PAE) versus sham (short term), Outcome 2: Quality of life
3. Major adverse events
There were no major adverse events in either PAE or sham groups (very low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2) (Analysis 3.3).
3.3. Analysis.

Comparison 3: Prostatic arterial embolization (PAE) versus sham (short term), Outcome 3: Major adverse events
Secondary outcomes
1. Retreatment
There were no retreatments in either PAE or sham groups (very low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2) (Analysis 3.4).
3.4. Analysis.

Comparison 3: Prostatic arterial embolization (PAE) versus sham (short term), Outcome 4: Retreatment
2. Erectile function
The RCT did not compare PAE versus sham for erectile function.
3. Ejaculatory disorders
We are very uncertain about the effects of PAE compared with sham on ejaculatory disorders; given there were no events in either group, no effect size could be calculated (very low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2) (Analysis 3.5).
3.5. Analysis.

Comparison 3: Prostatic arterial embolization (PAE) versus sham (short term), Outcome 5: Ejaculatory disorder
4. Minor adverse events
We are very uncertain about effects of PAE compared with sham on minor adverse events (RR 1.08, 95% CI 0.58 to 1.99; very low‐certainty evidence). PAE would result in 26 more (95% CI 137 fewer to 322 fewer) minor adverse events per 1000 men. We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2) (Analysis 3.6).
3.6. Analysis.

Comparison 3: Prostatic arterial embolization (PAE) versus sham (short term), Outcome 6: Minor adverse events
5. Acute urinary retention
We are very uncertain about the effects of PAE compared with sham on acute urinary retention; given there were no events in either group no effect size could be calculated (very low‐certainty evidence). We downgraded the certainty of evidence for serious study limitations (–1) and very serious imprecision (–2) (Analysis 3.7).
3.7. Analysis.

Comparison 3: Prostatic arterial embolization (PAE) versus sham (short term), Outcome 7: Acute urinary retention
6. Indwelling urinary catheter
The RCT did not compare PAE versus sham for indwelling urinary catheter.
7. Hospital stay
The RCT did not compared PAE versus sham for hospital stay.
Subgroup and sensitivity analyses
We were unable to perform any predefined secondary analyses because there were no relevant data and the included studies had a similar risk of bias.
Discussion
Summary of main results
We found evidence to inform two comparisons, namely, PAE versus TURP and PAE versus a sham procedure. Mean age was 66 years, IPSS was 22.8, and prostate volume of participants was 72.8 mL.
Prostatic arterial embolization versus transurethral resection of the prostate
Based on short‐term data (up to 12 months' follow‐up) from both RCTs and prospective comparative NRSs, PAE may result in a somewhat lesser but overall similar improvement in urologic symptom score and quality of life. While we are very uncertain as to whether PAE results in more or fewer major adverse events, PAE likely increases retreatment rates. Although there were similar effects on erectile function, PAE may reduce ejaculatory disorders.
For longer‐term outcomes (greater than 12 months' follow‐up), we found that urologic symptom score and quality of life may be similarly improved between these procedures. We are very uncertain whether PAE results in more or fewer major adverse events. PAE also likely increases retreatment rates. While there was no difference in erectile dysfunction between the two procedures, PAE may reduce ejaculatory disorders.
Prostatic arterial embolization versus sham
PAE likely improves urologic symptom scores and quality of life compared with sham. There were no major adverse events or retreatment reported in either group. We found no evidence to inform the outcomes of erectile function, and there were no ejaculatory disorders in either group.
We were unable to perform any of the predefined secondary analyses for both comparisons based on patient age, prostate volume, or severity of LUTS.
Overall completeness and applicability of evidence
The studies included in this review have important limitations.
Although the included studies were performed across the world (Asia, Europe, and Latin America), these studies were likely each conducted at single‐center locations. Given our focus on comparative effectiveness versus other treatment modalities, and in accordance with our published protocol, we excluded single‐armed NRSs and included only comparative studies. This forms a fairly narrow evidence base. Several prospective trials appear ongoing (see Characteristics of ongoing studies table); their findings may be highly valuable in improving our understanding of the role of PAE in the armamentarium to treat LUTS secondary to BPH.
We found additional retrospective case‐control studies (not included, in accordance with our protocol) to inform the two comparisons of PAE versus PUL (Pereira 2018) and PAE versus PVP (NCT02006303); however as expected, these studies provided only evidence of very low certainty, mainly due to very serious study limitations. Given the rapid pace of change in the surgical treatment of BPH (e.g. continuing decline of TURP, increased use of laser vaporization and other techniques) in routine clinical practice, more studies comparing PAE to other modalities are needed (Malaeb 2012).
We were unable to conduct any of our predefined subgroup analyses for factors such as patient age, prostate volume, or LUTS severity, which may be important effect modifiers.
Although the studies in this Cochrane Review included men with a large prostate (ranging from 80 mL to 100 mL) as a subset, most participants had smaller prostate volumes (less than 80 mL). Currently, simple prostatectomy and laser enucleation procedures remain the standard treatments for men with prostate gland size greater than 80 mL to 100 mL; PAE may have a potential role in treating men with a very large prostate (greater than 80 mL) (Bhatia 2018; Wang 2015). Therefore, studies about effects of PAE in this population would be of particular interest.
Six studies did not report on the technical success rate of PAE (Abt 2021; Insausti 2020; Pisco 2020; Radwan 2020; Ray 2018; Zhu 2018). Given that the technical success of PAE depends on the expertise of intervention radiologists, this would be a topic of interest. Widespread adoption of PAE (as for any other newer surgical treatment modality) would likely require specialized training and quality assurance.
Each included study used a different TURP method (monopolar or bipolar) as a comparator. Given the reported lower rate of adverse events with bipolar TURP (Omar 2014), studies comparing monopolar TURP versus bipolar TURP may overestimate the risk of adverse events.
Three studies did not report how they categorized the severity of adverse events (Carnevale 2016; Soluyanov 2018; Zhu 2018), and Young 2017 expressed concerns that the classified numbers of participants with adverse events used in Gao 2014 were not accurate. Although Gao 2014 chose to label technical and clinical failures as major complications in the PAE group, these researchers did not consider hemorrhage requiring blood transfusion as a major complication in the TURP group.
The existing body of evidence was limited to relatively short‐term outcomes (up to 12 months' follow‐up); only two studies provided outcomes up to 24 months in duration (Abt 2021; Gao 2014). This appears insufficient to provide assurance of long‐term effectiveness, namely, with regard to comparative retreatment rates. However, the same is unfortunately true for many other surgical techniques to treat BPH. More high‐quality studies with long‐term follow‐up are needed to address these limitations.
In accordance with our published and peer‐reviewed Cochrane Review protocol (Jung 2017), this review focused on outcomes of direct patient importance; therefore, it does not provide information on maximum urinary flow or on postvoid residuals.
Quality of the evidence
For evidence from RCTs, we downgraded the certainty of evidence for study limitations and imprecision.
Study limitations: we downgraded for unclear risk of selection bias and high risk of blinding of participants, personnel, and outcome assessors.
Inconsistency: we downgraded for inconsistency due to clinical important heterogeneity with high I2 values.
Imprecision: we downgraded for imprecision due to wide CIs that crossed the assumed threshold of a clinically important difference or very rare event.
For evidence from NRSs, we downgraded the certainty of evidence for study limitations and imprecision.
Study limitations: we judged studies to be at critical risk of bias due to known or unknown of confounding variables even though Ray 2018 made some attempt to (incompletely) adjust for these using statistical methods. In addition, we had major concerns about detection bias in the absence of any efforts to blind outcome assessors.
Imprecision: CIs were wide and crossed the assumed threshold of a clinically important difference.
Potential biases in the review process
Despite a comprehensive search strategy with no publication or language restrictions, we may have missed additional RCTs that may be unpublished or were published in languages other than English, or both. The small number of studies included in this review was insufficient to generate funnel plots; therefore, the risk of publication bias may have been underestimated.
Agreements and disagreements with other studies or reviews
One systematic review (by the authors of an included trial [Abt 2021]) found that PAE may not be as effective as TURP in improving urologic symptom score but may have a more favorable adverse effect profile (Zumstein 2019). Study authors called for additional high‐quality trials with longer‐term follow‐up, and we concur.
One more‐recent review that of nine studies including RCTs and comparative NRSs also reported similar results (Xu 2020). The review authors found that IPSS (MD 2.50, 95% CI 0.78 to 4.21) and quality of life (MD 0.40, 95% CI 0.09 to 0.71) were more improved after TURP than PAE but did not take minimal clinical important differences in consideration in their interpretation. They also found that PAE was associated with a lower sexual dysfunction rate (odds ratio [OR] 0.24, 95% CI 0.15 to 0.39) and fewer complications (OR 0.57, 95% CI 0.21 to 1.55) compared with TURP. One systematic review by Malling 2019 based their conclusions on indiscriminate pooling of RCTs and NRSs including comparative and non‐comparative studies. Other systematic reviews and meta‐analyses based on single‐arm studies have also consistently reported significant improvement in urologic symptom scores and in quality of life after PAE (Kuang 2017; Pyo 2017). However, we advise caution with interpretation of these findings, which included all study designs including case series, given their major risk of bias.
Shim 2017, which is another systematic review that included comparative and non‐comparative studies, was criticized by Narayan 2017 for considerable shortcomings in its assessment of risk of bias and data synthesis, thus questioning the validity of its findings. These review authors found that PAE improved IPSS (MD –12.77, 95% CI –15.04 to –10.50) and quality of life (MD –2.34, 95% CI –2.72 to –1.97). This review also reported that PAE had inferior effectiveness with regard to IPSS (standardized mean difference [SMD] 0.88, 95% CI 0.10 to 1.66) yet a similar effect on quality of life (SMD 0.25, 95% CI –0.28 to 0.77) when compared to control (TURP or simple prostatectomy) based on three comparative studies (Carnevale 2016; Gao 2014; Russo 2015). The incidence rate of adverse events was higher for PAE (41.6%) when compared to control (30.4%).
In terms of individual studies other than RCTs and NRSs, single‐armed cohort studies should have a limited role in informing comparative effectiveness in settings such as this, where several effective treatment modalities exist and define the standard of care. Pisco 2016 reported a single‐armed cohort study with 630 consecutive men with BPH and moderate‐to‐severe LUTS refractory to medical therapy who were followed for a median of two years. Participants reported a large reduction in IPSS (long‐term: mean change –16.94, SD 8.70) and quality of life (long‐term: mean change –1.74, SD 1.45). A cumulative clinical success rate, defined as improved symptoms (IPSS 15 points or less and a decrease 25% or greater from the baseline score), improved quality of life (quality of life score 3 points or less or a decrease of at least 1 point from baseline), and no need for any medical or other therapy after PAE at long‐term follow‐up, was met by 76.3% (95% CI 68.6% to 82.4%) of participants. This study reported two major complications – bladder wall ischemia and persistent perineal pain – in addition to 555 minor adverse events (Pisco 2016).
We found one study comparing PAE to open simple prostatectomy (Russo 2015). PAE was inferior to open simple prostatectomy in terms of symptoms (IPSS: 10.4 with PAE versus 4.31 with open simple prostatectomy) and Qmax (16.89 with PAE versus 23.82 with open simple prostatectomy) one year after the procedures. PAE had a lower rate of adverse events compared to open surgery (8.25% with PAE versus 32.25% with open simple prostatectomy). We excluded this trial from the present review comparing PAE to open simple prostatectomy as open surgery, as we did not consider open simple prostatectomy as a comparator of relevance given its considerable morbidity and fading appeal compared to less‐invasive surgical alternatives (Parsons 2015).
Guideline recommendations based on this evidence are currently contradictory and potentially in flux, thereby emphasizing the importance of this up‐to‐date Cochrane Review. Specifically, one current American Urological Association guideline recommends against the use of PAE outside of clinical trials (Lerner 2021b). Meanwhile, guidance provided by NICE indicates that PAE is a treatment option for LUTS caused by BPH (NICE 2018). This guidance was in part informed by the UK‐ROPE study, which was run by UK interventional radiologists and urologic surgeons (Ray 2018). In addition, one Society of Interventional Radiology multisociety consensus position statement that recommends PAE as an acceptable minimally invasive treatment option for appropriately selected men with BPH was published in 2019 (McWilliams 2019). One more‐recent guideline of European Association of Urology also recommends that PAE can be offered to men with moderate‐to‐severe LUTS who wish to consider minimally invasive treatment options and accept less‐optimal objective outcomes (e.g. urologic symptoms and urodynamic parameters such as flow rate) when compared to TURP (EAU 2021).
Authors' conclusions
Implications for practice.
The main implications for clinical practice can be drawn from the comparison to transurethral resection of the prostate (TURP) that has long been considered the treatment reference standard. Compared to TURP and based on short‐term and long‐term follow‐up, the impact on urologic symptoms and quality of life improvement as perceived by patients appears to be similar. This review did reveal major uncertainty as to how major adverse events compare. Prostatic arterial embolization (PAE) likely increases retreatment rates. PAE may have similar effects on erectile function.
This review found that PAE may reduce the incidence of ejaculatory disorders compared to TURP, which is an important consideration for some men. The rate of ejaculatory disorders in the largest, non‐randomized study by Ray 2018, which is also known as the UK‐ROPE study, was 24.1% (48/199 men). One Cochrane Review on convective radiofrequency water vapor thermal therapy (REZUM) found that it may not adversely impact ejaculatory function compared to sham at three months (Kang 2020), but no longer‐term studies with an active control exist, which represents a major limitation. One Cochrane Review on the prostatic urethral lift procedure (Urolift) found that it probably preserved ejaculatory function better at both short‐term (up to 12 months) and long‐term assessment (up to 24 months) (Jung 2019).
Compared to a sham procedure with short‐term follow‐up, PAE likely improves urologic symptom score and quality of life. There were no major adverse events or retreatments in either study group. Although we found no evidence to inform the outcome of erectile function, there were no ejaculatory problems in either study group. This analysis was based on one study (Pisco 2020), in which these outcomes were compared with those for convective radiofrequency water vapor thermal therapy (Kang 2020), as well as the prostatic urethral lift procedure (Jung 2019), and it should be noted that enrolled men with severe LUTS (median IPSS 25.5) and quite a large prostate (median 63.5 mL) limit comparability.
Implications for research.
A variety of minimally invasive surgeries such as prostatic urethral lift and convective radiofrequency water vapor thermal therapy have recently become available (McVary 2018; Roehrborn 2017). In addition, less‐invasive techniques than open simple prostatectomy for very large prostates, such as robotic‐assisted laparoscopic prostatectomy and laser enucleation of the prostate, are increasingly accepted as appropriate treatment approaches by current evidence‐based guidelines (EAU 2021; Lerner 2021b). Given the low and very low certainty of evidence found for PAE, additional research studies of better quality comparing PAE to TURP and newer evolving treatment alternatives appear essential. Future trials should be conducted according to higher methodologic standards with regard to allocation concealment and blinding to minimize concerns about selection, performance, and detection bias. These studies also need to provide long‐term data across treatment modalities.
Given that PAE outcomes are hampered by technical issues related to variations in arterial anatomy, PAE techniques should be standardized for indication, preoperative evaluation, approach method (e.g. transfemoral, transbrachial), and type of embolization material.
What's new
| Date | Event | Description |
|---|---|---|
| 13 April 2022 | Amended | Author order corrected. |
History
Protocol first published: Issue 11, 2017 Review first published: Issue 12, 2020
| Date | Event | Description |
|---|---|---|
| 2 March 2022 | New search has been performed | Review updated. |
| 2 March 2022 | New citation required and conclusions have changed | Results and conclusion were revised based on updated search. |
Notes
We based parts of the Methods section of this review on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which was modified and adapted for use by Cochrane Urology.
Acknowledgements
We are very grateful to Bhaskar Somani, Charalampos Mamoulakis, and Marcelino Rivera for their assistance in preparation of this review in their role as external reviewers. We thank Cochrane Urology, Cochrane Cancer Network, Cochrane Methods (methods.cochrane.org), and our contact editor Mari Imamura for supporting this review.
We based parts of the Assessment of risk of bias in included studies, Risk of bias in included studies, Figure 4, Table 7, Table 8, and Appendix 4 with regard to NRS on a guidance under the Cochrane Methods (methods.cochrane.org). We used the robvis app (www.riskofbias.info/welcome/robvis-visualization-tool: free, online and recommended by the ROBINS‐I team) to create Figure 4 as Cochrane Methods recommended.
We appreciate the efforts of Balaji Reddy and Tae Young Shin in a prior published version of this review. We thank Anne Lawson for copy editing the review.
Appendices
Appendix 1. Certainty of evidence decisions (PAE versus TURP [short term])
| Outcomes | Study design | Certainty of evidence (GRADE) |
| Urologic symptom scoresa | RCT | Low |
| NRS | Very low | |
| Quality of lifea | RCT | Low |
| NRS | Very low | |
| Major adverse events | RCT | Very low |
| NRS | Very low | |
| Retreatmenta | RCT | Moderate |
| NRS | Very low | |
| Erectile functiona | RCT | Low |
| NRS | Very low | |
| Ejaculatory disordera | RCT | Very low |
| NRS | Low |
NRS: non‐randomized study; PAE: prostatic arterial embolization; RCT: randomized controlled trial; TURP: transurethral resection of prostate. aHigher Certainty of evidence only shown in Table 1 due to the difference in a body of RCTs and a body of non‐RCTs.
Appendix 2. Search strategy
| Cochrane Library (via Wiley) |
| 1 MeSH descriptor: [Prostatic Hyperplasia] explode all trees 2 (prostat* near/3 hyperplasia*):ti,ab,kw (Word variations have been searched) 3 (prostat* near/3 hypertroph*):ti,ab,kw (Word variations have been searched) 4 (prostat* near/3 adenoma*):ti,ab,kw (Word variations have been searched) 5 (BPH or BPO or BPE):ti,ab,kw (Word variations have been searched) 6 (prostat* near/3 enlarg*):ti,ab,kw (Word variations have been searched) 7 MeSH descriptor: [Prostatism] explode all trees 8 prostatism:ti,ab,kw (Word variations have been searched) 9 MeSH descriptor: [Urinary Bladder Neck Obstruction] explode all trees 10 ("bladder outlet obstruction" or BOO):ti,ab,kw (Word variations have been searched) 11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 12 MeSH descriptor: [Embolization, Therapeutic] this term only 13 emboli?ation*:ti,ab,kw (Word variations have been searched) 14 Embolotherap*:ti,ab,kw (Word variations have been searched) 15 #12 or #13 or #14 16 #11 and #15 |
| MEDLINE (via Ovid) |
| 1 exp Prostatic Hyperplasia/ 2 (Prostat* adj3 hyperplasia*).tw. 3 (Prostat* adj3 hypertroph*).tw. 4 (Prostat* adj3 adenoma*).tw. 5 (BPH or BPO or BPE).tw. 6 (prostat* adj3 enlarg*).tw. 7 exp Prostatism/ 8 Prostatism.tw. 9 exp Urinary Bladder Neck Obstruction/ 10 (Bladder* adj3 obstruct*).tw. 11 BOO.tw. 12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13 Embolization, Therapeutic/ 14 emboli#ation$.tw. 15 Embolotherap*.tw. 16 13 or 14 or 15 17 12 and 16 18 (animals not (humans and animals)).sh. 19 17 not 18 |
| Embase (via Elsevier) |
| 1 'prostate hypertrophy'/exp 2 (Prostat* NEAR/3 hyperplasia*):ab,ti 3 (Prostat* NEAR/3 hypertroph*):ab,ti 4 (Prostat* NEAR/3 adenoma*):ab,ti 5 'bph':ab,ti OR 'bpo':ab,ti OR 'bpe':ab,ti 6 (prostat* NEAR/3 enlarg*):ab,ti 7 'prostatism'/exp 8 'prostatism':ab,ti 9 'bladder obstruction'/exp 10 (bladder* NEAR/3 obstruct*):ab,ti 11 'BOO':ab,ti 12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 13 'artificial embolization'/de 14 embolisation*:ab,ti 15 embolization*:ab,ti 16 Embolotherap*:ab,ti 17 #13 OR #14 OR #15 OR #16 18 #12 AND #17 19 ('animals'/exp) NOT ('humans'/exp and 'animals'/exp) 20 #18 NOT #19 |
| LILACS |
| 1 (mh:("Prostatic Hyperplasia" or Prostatism or "Urinary Bladder Neck Obstruction")) 2 (tw:("Prostatic Hyperplasia" or "Prostatic Adenoma" or "Prostatic Hypertrophy" or "Prostatic Enlargement" or BPH or BPO or BPE or Prostatism or "Bladder Neck Obstruction" or "Bladder Outlet Obstruction" or BOO)) 3 1 OR 2 4 tw:(embolisation$ OR embolization$ OR embolotherap$) 5 3 AND 4 |
| Scopus |
| 1 TITLE‐ABS‐KEY((hyperplasia* W/3 prostat*) OR (hypertroph* W/3 prostat*) OR (adenoma* W/3 prostat*) OR (prostat* W/3 enlarg*) OR (bph OR bpo OR bpe OR boo) OR prostatism OR (bladder* W/3 obstruct*)) 2 TITLE‐ABS‐KEY(embolisation* OR embolization* OR Embolotherap*) 3 1 AND 2 |
| Web of Science |
| 1 TS= ((hyperplasia* NEAR/3 prostat*) OR (hypertroph* NEAR/3 prostat*) OR (adenoma* NEAR/3 prostat*) OR (prostat* NEAR/3 enlarg*) OR (bph OR bpo OR bpe OR boo) OR prostatism OR (bladder* NEAR/3 obstruct*)) 2 TS= (embolisation* OR embolization* OR Embolotherap*) 3 1 AND 2 |
| Google Scholar |
| 1 allintitle: ("Prostatic Hyperplasia" OR "prostatic hypertrophy" OR prostatism OR "bladder obstruction" OR "bladder outlet obstruction" OR bph OR bpo OR bpe OR boo) AND (embolisation OR embolisations OR embolization OR embolizations OR embolotherapy OR embolotherapies)) |
| ClinicalTrials.gov |
| 1 ("Prostatic Hyperplasia" OR "Prostatic Hypertrophy" OR "Prostatic Adenoma" OR BPH OR BPO OR BPE OR Prostatism OR "Bladder Neck Obstruction" OR "Bladder Outlet Obstruction" or BOO) 2 (embolisation OR embolisations OR embolization OR embolizations OR embolotherapy OR embolotherapies) 3 1 AND 2 |
| World Health Organization International Clinical Trials Registry Platform search portal |
| 1 In the title = ("Prostatic Hyperplasia" OR "Prostatic Hypertrophy" OR "Prostatic Adenoma" OR BPH or BPO or BPE OR Prostatism OR "Bladder Neck Obstruction" or "Bladder Outlet Obstruction" or BOO) AND In the intervention= (embolisation OR embolisations OR embolization OR embolizations OR embolotherapy OR embolotherapies) |
| Grey literature (Open Grey) |
| 1 ("Prostatic Hyperplasia" OR "Prostatic Hypertrophy" OR "Prostatic Adenoma" OR BPH or BPO or BPE OR Prostatism OR "Bladder Neck Obstruction" or "Bladder Outlet Obstruction" or BOO) 2 (embolisation OR embolisations OR embolization OR embolizations OR embolotherapy OR embolotherapies) 3 1 AND 2 |
Appendix 3. Survey of trial investigators providing information on included trials
| Study name | Date trial author contacted (first) | Date trial author provided data (latest) |
Data trial author provided short summary |
| Abt 2021 | 13 October 2018 | 25 October 2018 | Standard deviations of IPSS, QoL, IIEF, Qmax, and PVR at baseline and 12 weeks/number of participants with AEs and retreatment |
| 7 July 2021 | 18 August 2021 | Standard deviations of IPSS, QoL, IIEF at 12 and 24 months/number of participants with major and minor AEs, ejaculatory disorder, and AUR at 12 months and 24 months | |
| Ray 2018 | 19 October 2018 | 1 November 2018 | Standard deviations at endpoint and changes from baseline in IPSS, QoL, and IIEF/number of participants with AEs, acute urinary retention, and re‐operation/mean length of hospital stay |
| Pisco 2020 | 28 March 2020 | 3 April 2020 | Number of participants with major and minor AEs, and reoperation rate at 6 months (blinded period) |
| Radwan 2020 | 7 October 2020 | 15 October 2020 | Baseline characteristics (age, IPSS, QoL, prostate volume, Qmax, PVR)/number of participants analyzed at 6 months (study endpoint)/means and standard deviations for IPSS, AEs, retreatment, and acute urinary retention |
Footnotes
AEs: adverse events; AUR: acute urinary retention; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; PVR: post void residual; Qmax: maximum flow rate; QoL: quality of life.
Appendix 4. Assessment for risk of bias for NRS using ROBINS‐I
| Bias domain | Outcome | Author's judgment | Support for judgment |
| Bias due to confounding | Set 1 |
Ray 2018: serious risk of bias Soluyanov 2018: critical risk of bias |
Although Ray 2018 used a statistical method to adjust confounding factors, residual or unmeasured confounding can occur. Soluyanov 2018 did not perform any such method to adjust for potential confounding. |
| Set 2 and 3 | Ray 2018: serious risk of bias | Although Ray 2018 used a statistical method to adjust confounding factors, residual or unmeasured confounding can occur. | |
| Bias in selection of participants into the study | Set 1 |
Ray 2018: moderate risk of bias Soluyanov 2018: critical risk of bias |
As Ray 2018 recruited the participants based on predefined protocol, selection based on participant characteristics appears unlikely to have occurred. In Soluyanov 2018, participants were selected to each intervention based on prostate volume. |
| Set 2 and 3 | Ray 2018: moderate risk of bias | As Ray 2018 recruited the participants based on predefined protocol, selection based on participant characteristics appears unlikely to have occurred. | |
| Bias in classification of interventions | Set 1 | Ray 2018; Soluyanov 2018: moderate risk of bias | Both studies used predefined criteria for the intervention (Ray 2018: ongoing authorized registry, Soluyanov 2018: prospective study design). |
| Set 2 and 3 | Ray 2018: moderate risk of bias | Ray 2018 used predefined criteria for the intervention (ongoing authorized registry). | |
| Bias due to deviations from intended interventions | All review outcomes | Ray 2018; Soluyanov 2018: no information | Both studies reported no information on whether there was deviation from the intended intervention. |
| Bias due to missing data | Set 1 |
Ray 2018: serious risk of bias Soluyanov 2018: low risk of bias |
Ray 2018 showed a large proportion of missing data, while Soluyanov 2018 reported the data of all participants who were assigned to each intervention completed follow‐up by the end of the study. |
| Set 2 | Ray 2018: serious risk of bias | Ray 2018 showed a large proportion of missing data. | |
| Set 3 | Ray 2018: low risk of bias | All participants were included in the analysis. | |
| Bias in measurement of outcomes | Set 1 (subjective outcome) | Ray 2018; Soluyanov 2018: serious risk of bias | Lack of blinding for participants, personnel, outcome assessors, or a combination. |
| Set 2 and 3 (other subjective outcomesa) | Ray 2018: serious risk of bias | Lack of blinding for participants, personnel, outcome assessors, or a combination. | |
| Set 2 and 3 (objective outcomesb) | Ray 2018: serious risk of bias | Although objective outcomes are unlikely influenced by knowledge of the intervention received in outcome assessment, participants and personnel were not blinded. | |
| Bias in selection of the reported result | Set 1 |
Ray 2018: low risk of bias Soluyanov 2018: no information |
Ray 2018 was based on a published protocol, while Soluyanov 2018 did not reported any protocol available. |
| Set 2 and 3 | Ray 2018: low risk of bias | Ray 2018 was based on a published protocol. | |
| Overall bias | — |
Ray 2018: serious risk of bias Soluyanov 2018: critical risk of bias |
— |
NRS: non‐randomized study; ROBINS‐I: risk of bias tool to assess non‐randomized studies of interventions. Set 1: urologic symptom scores; Set 2: quality of life, erectile function, ejaculatory disorders, and hospital stay; Set 3: major adverse events, retreatment, minor adverse events, and acute urinary retention. aQuality of life, major adverse events, erectile function, ejaculatory disorders, and minor adverse events. bRetreatment, acute urinary retention, and hospital stay.
Appendix 5. Certainty of evidence decisions (PAE versus TURP [long term])
| Outcomes | Study design | Certainty of evidence (GRADE) |
| Retreatmenta | RCT | Moderate |
| NRS | Low |
NRS: non‐randomized study; PAE: prostatic arterial embolization; RCT: randomized controlled trial; TURP: transurethral resection of prostate. aHigher Certainty of evidence only shown in Table 2 due to the difference in a body of RCTs and a body of non‐RCTs.
Data and analyses
Comparison 1. Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Urologic symptom scores | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 RCTs | 6 | 360 | Mean Difference (IV, Random, 95% CI) | 1.72 [‐0.37, 3.81] |
| 1.1.2 NRSs | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 2.80 [0.04, 5.56] |
| 1.2 Quality of life | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 RCTs | 5 | 300 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.28, 0.84] |
| 1.2.2 NRSs | 1 | 164 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.03, 1.03] |
| 1.3 Major adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 RCTs | 4 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.19, 2.97] |
| 1.3.2 NRSs | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4 Retreatment | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 RCTs | 4 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 3.20 [1.41, 7.27] |
| 1.4.2 NRSs | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.43, 5.29] |
| 1.5 Erectile function | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 RCTs | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐5.88, 4.88] |
| 1.5.2 NRSs | 1 | 122 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐2.01, 5.01] |
| 1.6 Ejaculatory disorder | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 RCTs | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.19] |
| 1.6.2 NRSs | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.35, 0.73] |
| 1.7 Minor adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 RCTs | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.73] |
| 1.7.2 NRSs | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 2.27 [0.51, 10.02] |
| 1.8 Acute urinary retention | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 RCTs | 5 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.54, 5.07] |
| 1.8.2 NRSs | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9 Indwelling urinary catheter | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 RCTs | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐2.55, ‐1.45] |
| 1.10 Hospital stay | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 RCTs | 3 | 260 | Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐2.44, ‐0.58] |
Comparison 2. Prostatic arterial embolization (PAE) versus transurethral resection of the prostate (TURP) (long term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Urologic symptom scores | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 RCTs | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐1.54, 6.71] |
| 2.2 Quality of life | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 RCTs | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.03, 1.04] |
| 2.3 Major adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 RCTs | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.20, 4.05] |
| 2.4 Retreatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 RCTs | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [1.32, 10.93] |
| 2.4.2 NRSs | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 3.54 [1.45, 8.65] |
| 2.5 Erectile function | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 RCTs | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 3.09 [‐0.76, 6.94] |
| 2.6 Ejaculatory disorder | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.6.1 RCTs | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 0.98] |
| 2.7 Minor adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.7.1 RCTs | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.22] |
| 2.8 Acute urinary retention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.8.1 RCTs | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.12, 4.06] |
Comparison 3. Prostatic arterial embolization (PAE) versus sham (short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Urologic symptom scores | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 RCTs | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐12.07 [‐15.45, ‐8.69] |
| 3.2 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 RCTs | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.97 [‐2.48, ‐1.46] |
| 3.3 Major adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.3.1 RCTs | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.4 Retreatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.4.1 RCTs | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.5 Ejaculatory disorder | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.5.1 RCTs | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.6 Minor adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.6.1 RCTs | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.58, 1.99] |
| 3.7 Acute urinary retention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.7.1 RCTs | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abt 2021.
| Study characteristics | ||
| Methods |
Study design: open‐label, randomized controlled trial Setting/country: single center/Switzerland Dates when study was conducted: February 2014 to May 2017 |
|
| Participants |
Inclusion criteria: men aged ≥ 40 years, TURP indicated, refractory to medical treatment or not willing to undergo or continue medical treatment, with prostate size 25–80 mL as measured by transabdominal US, with IPSS ≥ 8, with IPSS‐related QoL of ≥ 3 points, with a maximum urinary flow rate < 12 mL/second or urinary retention, and who provided written informed consent Exclusion criteria: men with severe atherosclerosis, aneurysmatic changes or severe tortuosity in the aortic bifurcation or internal iliac arteries, acontractile detrusor, neurogenic lower urinary tract dysfunction, urethral stenosis, bladder diverticulum, bladder stone, allergy to intravenous contrast media, contraindication for magnetic resonance imaging, pre‐interventionally confirmed carcinoma of the prostate, and renal failure (glomerular filtration rate < 60 mL/minute) Total number of participants randomly assigned: 103 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: monopolar TURP Follow‐up: 2 years |
|
| Outcomes |
Primary outcome
How measured: IPSS questionnaire Time points measured: at baseline and 12 weeks Time points reported: at baseline, 1 week, 6 weeks, 12 weeks, 6 months, 12 months, and 24 months Secondary outcomes
How measured: IPSS questionnaire Time points measured: at baseline, 1 week, 6 weeks, 12 weeks, 6 months, 12 months, and 24 months Time points reported: at baseline, 1 week, 6 weeks, 12 weeks, 6 months, 12 months, and 24 months
How measured: uroflowmetry/transabdominal US/IPSS questionnaire//Chronic Prostatitis Symptoms Index questionnaire/IIEF‐5 questionnaire Time points measured: at baseline, 1 week, 6 weeks, 12 weeks, 6 months, 12 months, and 24 months Time points reported: at baseline, 1 week, 6 weeks, 12 weeks, 6 months, 12 months, and 24 months Safety outcomes: adverse events How measured: modified Clavien system and common terminology criteria for adverse events Time points measured: before intervention (baseline), during participants' stay in hospital, and at 1 week, 6 weeks, 12 weeks, 6 months, 12 months, and 24 months after surgery Time points reported: likely cumulative incidence Subgroup: none |
|
| Funding sources | Grant from the research committee of St Gallen Cantonal Hospital | |
| Declarations of interest | None | |
| Notes |
Protocol: NCT02054013 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using the data management software SecuTrial, stratifying for patient age (< 70 or ≥ 70 years) and prostate volume (< 50 or ≥ 50 mL) through minimisation. SecuTrial was programmed by the clinical trials unit’s data manager, and automatic treatment allocation by SecuTrial was determined for individual patients without a predefined sequence after inclusion and entry of baseline characteristics by the investigators". |
| Allocation concealment (selection bias) | Low risk | Quote: "using the data management software SecuTrial, stratifying for patient age (< 70 or ≥ 70 years) and prostate volume (< 50 or ≥ 50 mL) through minimisation. SecuTrial was programmed by the clinical trials unit’s data manager, and automatic treatment allocation by SecuTrial was determined for individual patients without a predefined sequence after inclusion and entry of baseline characteristics by the investigators". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "randomised, open‐label trial". |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "randomised, open‐label trial". |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgment: objective outcomes were likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/QoL | High risk | Judgments Short term: 40/51 (78.4%) participants randomized in PAE and 50/52 (96.1%) in TURP were included in the analysis. Long term: 34/51 (66.6%) participants randomized in PAE and 47/52 (90.3%) in TURP were included in the analysis. |
| Incomplete outcome data (attrition bias) Major/minor adverse events | Low risk | Judgment: 48/51 (92.3%) participants randomized in PAE and 51/52 (98.0%) in TURP were included in the analysis. |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgment: 48/51 (92.3%) participants randomized in PAE and 51/52 (98.0%) in TURP were included in the analysis. |
| Incomplete outcome data (attrition bias) Erectile function | High risk | Judgments Short term: 40/51 (78.4%) participants randomized in PAE and 50/52 (96.1%) in TURP were included in the analysis. Long term: 34/51 (66.6%) participants randomized in PAE and 47/52 (90.3%) in TURP were included in the analysis. |
| Incomplete outcome data (attrition bias) Ejaculatory disorders | High risk | Judgment: 25/51 (49.0%) participants randomized in PAE and 25/52 (48.0%) in TURP were included in the analysis. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgment: 48/51 (92.3%) participants randomized in PAE and 51/52 (98.0%) in TURP were included in the analysis. |
| Incomplete outcome data (attrition bias) Indwelling urinary catheter | Low risk | Judgment: 48/51 (92.3%) participants randomized in PAE and 51/52 (98.0%) in TURP were included in the analysis. |
| Incomplete outcome data (attrition bias) Hospital stay | Low risk | Judgment: 48/51 (92.3%) participants randomized in PAE and 51/52 (98.0%) in TURP were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Judgment: protocol was published and study author shared the data (not shown in the article). But results that were not predefined in the protocol were reported. Data from bladder diary were not described in the methods section but they were described in the protocol. |
| Other bias | Low risk | Judgment: not detected. |
Carnevale 2016.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized, controlled study Setting/country: single center/Brazil Dates when study was conducted: November 2010 to December 2012 |
|
| Participants |
Inclusion criteria: men aged > 45 years; IPSS > 19; symptoms refractory to medical treatment for ≥ 6 months; negative screening for prostate cancer; prostate volume 30–90 mL on magnetic resonance imaging; and bladder outlet obstruction confirmed by urodynamic exam Exclusion criteria: men with renal failure, bladder calculi or diverticula, suspected prostate cancer, urethral stenosis, or neurogenic bladder disorders Total number of participants randomly assigned: 30 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: monopolar TURP Follow‐up: 12 months |
|
| Outcomes |
How measured: IPSS and IIEF questionnaires/non‐invasive uroflowmetry/not reported/magnetic resonance imaging Time points measured: at baseline and 1 year Time points reported: at baseline and 1 year
How measured: invasive pressure flow study Time points measured: at baseline Time points reported: at baseline Safety outcomes: adverse events How measured: National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0 Time points measured: not reported Time points reported: not reported Subgroup: none |
|
| Funding sources | No financial disclosure | |
| Declarations of interest | None | |
| Notes |
Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgment: not described. |
| Allocation concealment (selection bias) | Unclear risk | Judgment: not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgment: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgment: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgment: objective outcomes are likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/QoL | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Major/minor adverse events | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Ejaculatory disorders | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Indwelling urinary catheter | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Hospital stay | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Selective reporting (reporting bias) | Unclear risk | Judgment: study outcomes were well predefined and described, but protocol was not found. |
| Other bias | Low risk | Judgment: statistical differences in baseline IIEF and Qmax, but those likely underestimate the effect size of PAE (more conservative). |
Gao 2014.
| Study characteristics | ||
| Methods |
Study design: prospective parallel randomized controlled study Setting/country: not defined/China Dates when study was conducted: January 2007 to January 2012 |
|
| Participants |
Inclusion criteria: men with IPSS ≥ 7 after failed medical therapy with a washout period of ≥ 2 weeks, prostate volume 20–100 mL on transrectal ultrasonographic or magnetic resonance imaging, Qmax < 15 mL/second, and negative prostate biopsy if PSA > 4 ng/mL or abnormal digital rectal exam Exclusion criteria: men with detrusor hyperactivity or hypocontractility at urodynamic study, urethral stricture, prostate cancer, diabetes mellitus, and previous prostate, bladder neck, or urethral surgery, or positive prostate biopsy Total number of participants randomly assigned: 114 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: bipolar TURP Follow‐up: 24 months |
|
| Outcomes |
How measured: IPSS questionnaire/uroflowmetry/transabdominal US Time points measured: at baseline, 1 month, 3 months, 6 months, 1 year, and 2 years Time points reported: at baseline, 1 month, 3 months, 6 months, 1 year, and 2 years
How measured: intraoperative, perioperative, and postoperative study data Time points measured: not reported Time points reported: early (< 30 days), late (≤ 2 years) Safety outcomes: adverse events How measured: modified Clavien Classification System Time points measured: not reported Time points reported: early (< 30 days), late (≤ 2 years) Subgroup: none |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated simple random tables". |
| Allocation concealment (selection bias) | Unclear risk | Judgment: not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgment: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgment: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgment: objective outcomes likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/QoL | Unclear risk | Judgment: 47/57 (82.5%) randomized participants in PAE and 48/57 (84.3%) in TURP were included in the analysis (short and long term). |
| Incomplete outcome data (attrition bias) Major/minor adverse events | Low risk | Judgment: 54/57 (94.8%) randomized participants in PAE and 53/57 (93.0%) in TURP were included in the analysis (short and long term). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Erectile function | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Ejaculatory disorders | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgment: 54/57 (94.8%) randomized participants in PAE and 53/57 (93.0%) in TURP were included in the analysis (long term). |
| Incomplete outcome data (attrition bias) Indwelling urinary catheter | Low risk | Judgment: 54/57 (94.8%) randomized participants in PAE and 53/57 (93.0%) in TURP were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Hospital stay | Low risk | Judgment: 54/57 (94.8%) randomized participants in PAE and 53/57 (93.0%) in TURP were included in the analysis (short term). |
| Selective reporting (reporting bias) | Unclear risk | Judgment: study outcomes were well predefined and described, but protocol was not found. |
| Other bias | Low risk | Judgment: not detected. |
Insausti 2020.
| Study characteristics | ||
| Methods |
Study design: prospective randomized non‐inferiority clinical trial Setting/country: single center/Spain Dates when study was conducted: November 2014 and January 2017 |
|
| Participants |
Inclusion criteria: men aged > 60 years; BPH‐related LUTS refractory to medical treatment for ≥ 6 months, or the patient could not tolerate medical treatment; TURP was indicated; IPSS ≥ 8; QoL related to LUTS ≥ 3; Qmax ≤ 10 mL/second or urinary retention Exclusion criteria: men with advanced atherosclerosis and tortuosity of the iliac arteries, non‐visualization of the prostatic artery or other accessory arteries supplying the prostate on computed tomography angiography, urethral stenosis, detrusor failure or neurogenic bladder, glomerular filtration rate < 30 mL/minute, and the presence of prostate cancer Total number of participants randomly assigned: 61 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: bipolar TURP Follow‐up: 12 months |
|
| Outcomes |
Primary outcomes
How measured: uroflowmetry/IPSS questionnaire Time points measured: at baseline, 3 months, 6 months, and 12 months Time points reported: at baseline, 3 months, 6 months, and 12 months Secondary outcomes
How measured: IPSS questionnaire/transabdominal US/transabdominal US/IIEF‐5 questionnaire Time points measured: at baseline, 3 months, 6 months, and 12 months Time points reported: at baseline, 3 months, 6 months, and 12 months
How measured: blood test Time points measured: at baseline, 3 months, and 12 months Time points reported: at baseline, 3 months, and 12 months Safety outcomes: adverse events How measured: modified Clavien Classification System Time points measured: at all follow‐up visits Time points reported: likely cumulative incidence Subgroup: none |
|
| Funding sources | Biocompatibles UK Ltd | |
| Declarations of interest | Biocompatibles UK Ltd | |
| Notes |
Protocol: NCT01963312 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "principal Investigator randomly selected a number from a table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the individual enrolling participants were unaware of the allocation of the next participants". Judgment: the method was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "there was no blinding of clinicians or patients due to the nature of the trial". |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "there was no blinding of clinicians or patients due to the nature of the trial". |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgment: objective outcomes likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/QoL | High risk | Judgment: 23/31 (74.1%) participants randomized to PAE and 22/30 (73.3%) to TURP were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Major/minor adverse events | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Ejaculatory disorders | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Indwelling urinary catheter | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Hospital stay | Unclear risk | Judgment: 27/31 (87.0%) participants randomized to PAE and 27/30 (90.0%) to TURP were included in the analysis (short term). |
| Selective reporting (reporting bias) | High risk | Judgment: protocol was published, but study outcomes were not identical to the outcomes prespecified in the protocol. |
| Other bias | Low risk | Judgment: BPH medication was prescribed longer for the PAE group; however, it seemed this did not affect results 12 months after treatment. |
Pisco 2020.
| Study characteristics | ||
| Methods |
Study design: parallel randomized controlled study Setting/country: single center/Portugal Dates when study was conducted: September 2014 to March 2018 |
|
| Participants |
Inclusion criteria: men aged > 45 years; diagnosis of LUTS/BPH based on clinical history, digital rectal exam, urinalysis, TRUS, and PSA; severe LUTS defined, at screening and at a baseline visit 2 weeks apart, by IPSS of 20 and QoL score of 3 after a minimum of 6 months' treatment with alpha‐blockers for LUTS/BPH; Qmax < 12 mL/second; prostate volume 40 mL Exclusion criteria: men with computed tomography angiography showing that prostatic arteries were not feasible for PAE; previous surgical or invasive prostate treatments such as TURP, transurethral microwave therapy, transurethral needle ablation, laser, or any other minimally invasive treatment; acute or chronic prostatitis or suspected prostatitis including chronic pain, intermittent pain, or abnormal sensation in the penis, testis, or anal or pelvic area in the previous 12 months; history of prostate or bladder cancer or pelvic irradiation; active or recurrent urinary tract infections (more than 1 episode in the previous 12 months); history of neurogenic bladder or LUTS secondary to neurologic disease; advanced atherosclerosis and tortuosity of iliac and prostatic arteries; secondary renal insufficiency (due to prostatic obstruction); large bladder diverticula or stones; detrusor failure; history of acute urinary retention; current severe, significant, or uncontrolled disease; bleeding disorder such as hemophilia, clotting factor deficiency, anticoagulation, or bleeding diathesis; hypersensitivity or contraindication to tamsulosin use; mental condition or disorder that would interfere with the man's ability to provide informed consent; participation in a study of any investigational drug or device in the previous 3 months; and administration of the 5‐alpha reductase inhibitors finasteride in the previous 6 months and dutasteride in the previous 3 months. The latter criterion was changed by a protocol amendment to administration of the 5‐alpha reductase inhibitors finasteride in the previous 2 weeks and dutasteride in the previous 4 months (these men may be included if they stop those medications and replace them for tamsulosin, alfuzosin, or silodosin for ≥ 2 weeks for finasteride and ≥ 4 months for dutasteride) Total number of participants randomly assigned: 80 Group A (PAE)
Group B (sham)
|
|
| Interventions |
Group A: PAE Group B: sham (after catheterization of 1 prostatic artery, the catheter was removed and no particles were injected) Follow‐up: 6 months |
|
| Outcomes |
Primary outcome
How measured: IPSS questionnaires Time points measured: at baseline, 1 month, 3 months, and 6 months Time points reported: at baseline, 1 month, 3 months, and 6 months Secondary outcomes
How measured: BPH Impact Index/IIEF‐15/TRUS/not reported/not reported/not reported Time points measured: at baseline, 1 month, 3 months, and 6 months Time points reported: at baseline, 1 month, 3 months, and 6 months
How measured: not reported/not reported/visual analog scale Time points measured: during procedure, at discharge, and the next morning Time points reported: during procedure, at discharge, and the next morning Safety outcomes: adverse events How measured: Clavien‐Dindo Classification Time points measured: at baseline, 1 month, 3 months, and 6 months Time points reported: likely cumulative incidence Subgroup: none |
|
| Funding sources | Partially funded by an unrestricted grant from BTG plc (London, UK) | |
| Declarations of interest | None | |
| Notes |
Protocol: NCT02074644 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a randomisation list consisting of permuted blocks of size varying between 4 and 8 was prepared by the trial biostatistician". |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation sequence was concealed using opaque envelopes numbered sequentially". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "patients were blinded to the intervention received until end of single‐blind period". Judgment: single‐blind study (participants). |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgment: single‐blind study (participants). |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgment: objective outcomes likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/QoL | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Major/minor adverse events | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgment: no information given (not reported): author reply – all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Ejaculatory disorders | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Indwelling urinary catheter | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Hospital stay | Unclear risk | Judgment: no information given (not measured). |
| Selective reporting (reporting bias) | Low risk | Judgment: protocol was published and study outcomes were well predefined and described. |
| Other bias | Low risk | Judgment: tamsulosin was prescribed longer for the sham group. However, it made the difference between groups much smaller (more conservative). |
Radwan 2020.
| Study characteristics | ||
| Methods |
Study design: parallel randomized controlled study Setting/Country: single center/Egypt Dates when study was conducted: January 2016 to January 2018 |
|
| Participants |
Inclusion criteria: men with LUTS with an IPSS score 8–35 (8 being moderate and 35 being severe), uroflowmetry with a mean flow ≤ 10 mL/second, and a prostate volume < 100 mL by TRUS Exclusion criteria: men with elevated kidney functions (1.5 mg/dL), with allergy to intravenous contrast media, unfit for surgery, with prostatic adenocarcinoma, with history of prostatic or urethral operations, with signs of the decompensated bladder (e.g. bladder diverticulum), with signs of upper urinary tract infection revealed by pelvic abdominal US were excluded Total number of participants randomly assigned: 60 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: TURP (monopolar or bipolar) Follow‐up: 6 months |
|
| Outcomes |
How measured: IPSS questionnaire/uroflowmetry/TRUS/not reported Time points measured: at baseline, 1 month, and 6 months Time points reported: at baseline, 1 month, and 6 months
How measured: uroflowmetry/TRUS/NR Time points measured: at baseline, 1 month, and 6 months Time points reported: at baseline and postoperatively (not defined) Safety outcomes: How measured: TUR syndrome, acute urinary retention, postembolization syndrome Time points measured: not reported Time points reported: likely cumulative incidence Subgroup: none |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgment: not described. |
| Allocation concealment (selection bias) | Unclear risk | Judgment: not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgment: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgment: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgment: objective outcomes likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/QoL | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Major/minor adverse events | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Erectile function | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Ejaculatory disorders | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgment: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Indwelling urinary catheter | Low risk | Judgment: all randomized participants were included in the analysis (catheter removal time: TURP [third postoperative day], PAE [fifth postoperative day]). |
| Incomplete outcome data (attrition bias) Hospital stay | Unclear risk | Judgment: no information given (not measured). |
| Selective reporting (reporting bias) | Unclear risk | Judgment: protocol was not found, the outcomes at prespecified time point (likely 1 month) were omitted |
| Other bias | Low risk | Judgment: not detected. |
Ray 2018.
| Study characteristics | ||
| Methods |
Study design: prospective cohort study (United Kingdom Register of Prostate Embolization) Setting/country: multicenter/UK Dates when study was conducted: July 2014 to January 2016 |
|
| Participants |
Inclusion criteria: men with LUTS who had consented to undergo PAE, TURP, open prostatectomy, or holmium enucleation of the prostate at 1 of the United Kingdom Register of Prostate Embolization collaborating centers; were able to read, write, and understand English; and were capable of giving informed written consent Exclusion criteria: men who were unable to read, write, or understand English; unable/unwilling to provide informed written consent Total number of participants analyzed: 305 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: monopolar and bipolar TURP Follow‐up: 12 months |
|
| Outcomes |
Primary outcome
How measured: IPSS questionnaire Time points measured: at baseline, 1 month, 3 months, 6 months, and 12 months Time points reported: at baseline, 1 month, 3 months, 6 months, and 12 months Secondary outcomes
How measured: IPSS questionnaire/IIEF questionnaire Time points measured: at baseline, 1 month, 3 months, 6 months, and 12 months Time points reported: at baseline, 3 months, and 12 months
How measured: not reported/flow study Time points measured: at baseline, 3 months, and 12 months Time points reported: at baseline, 3 months, and 12 months Safety outcomes: adverse events How measured: Clavien Dindo Classification (by patients and clinicians) and retreatment (not defined in the methods section) Time points measured: at baseline, 1 month, 3 months, 6 months, and 12 months (by mail)/within 12 months and after 12 months Time points reported: likely cumulative incidence Subgroup: none |
|
| Funding sources | Cook Medical, British Society of Interventional Radiologists, and British Association of Urological Surgeons. National Institute for Health and Care Excellence funded an independent academic unit (the Cardiff and Vale UHB/Cardiff University‐based unit, Cedar) to run the registry through a competitive tender. | |
| Declarations of interest | The study included the coauthors who worked part‐time as a Consultant Clinical Advisor to the Interventional Procedures Programme at NICE and held a Consultant Contract with Boston Scientific, Terumo, Cook Medical, and Celonova. 1 coauthor was President of British Association of Urological Surgeons for 2014–2016. | |
| Notes |
Protocol: NCT02434575 Language of publication: English |
|
Soluyanov 2018.
| Study characteristics | ||
| Methods |
Study design: prospective comparative study Setting/country: not reported/Russia Dates when study was conducted: 2016 |
|
| Participants |
Inclusion criteria: BPH with 2–3 stages (stage not defined) Exclusion criteria: not reported Total number of participants analyzed: 27 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: bipolar TURP Follow‐up: 6 months |
|
| Outcomes |
How measured: IPSS questionnaire/not reported/TRUS Time points measured: at baseline, 3 months, and 6 months Time points reported: at baseline, 3 months, and 6 months Safety outcomes: not reported Subgroup: none |
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Protocol: not available Language of publication: Russian |
|
Zhu 2018.
| Study characteristics | ||
| Methods |
Study design: parallel randomized controlled study Setting/country: single center/China Dates when study was conducted: January–October 2016 |
|
| Participants |
Inclusion criteria: comprehensive diagnosis of BPH through US prostate exam, digital rectal exam, IPSS, etc.; no absolute contraindication for surgery; no history of surgery; not taking 5‐alpha reductase inhibitors Exclusion criteria: men with severe liver and kidney disorders, severe urethral strictures; prostate tumors, bladder neck stenosis, urinary infections, and neurogenic bladder; severe heart and brain diseases, coagulopathy, systemic organ low functionality Total number of participants randomly assigned: 40 Group A (PAE)
Group B (sham)
|
|
| Interventions |
Group A: PAE Group B: TURP (not defined) Follow‐up: 12 months |
|
| Outcomes |
How measured: IPSS questionnaires/IPSS questionnaires/TRUS/US/uroflowmetry/blood sampling Time points measured: at baseline, 3 months, 6 months, and 12 months Time points reported: at baseline, 3 months, 6 months, and 12 months
How measured: follow‐up by telephone (erectile dysfunction and retrograde ejaculation) Time points measured: at baseline, 3 months, and 12 months Time points reported: at baseline, 3 months, and 12 months Safety outcomes: adverse events How measured: not reported Time points measured: within 12 months Time points reported: likely cumulative incidence Subgroup: none |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Protocol: not available Language of publication: Chinese |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgment: random numbers table method. |
| Allocation concealment (selection bias) | Unclear risk | Judgment: not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgment: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgment: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgment: objective outcomes likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/QoL | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Major/minor adverse events | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Ejaculatory disorders | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgment: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Indwelling urinary catheter | Unclear risk | Judgment: no information given (not measured). |
| Incomplete outcome data (attrition bias) Hospital stay | Unclear risk | Judgment: no information given (not measured). |
| Selective reporting (reporting bias) | Unclear risk | Judgment: study outcomes were well predefined and described, but protocol not found. |
| Other bias | Low risk | Judgment: not detected. |
BPH: benign prostatic hyperplasia; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; IQR: interquartile range; LUTS: lower urinary tract symptoms; PAE: prostatic arterial embolization; PSA: prostate‐specific antigen; PVR: postvoid residual; Qmax: maximum flow rate; QoL: quality of life; SD: standard deviation; TRUS: transrectal ultrasound; TURP: transurethral resection of prostate; US: ultrasound.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abt 2019 | Irrelevant study design (post‐hoc analysis). |
| Bagla 2017 | Irrelevant study design (retrospective chart review for cost analysis). |
| Bilhim 2015 | Letter to editor. |
| Brown 2019 | Irrelevant study design (retrospective comparative study). |
| Mullhaupt 2019 | Irrelevant study design (cost analysis). |
| NCT01835860 | Irrelevant study design (single group assignment). |
| NCT02006303 | Aborted. |
| NCT02566551 | Withdrawn. |
| Pereira 2018 | Irrelevant study design (retrospective comparative study). |
| Qiu 2017 | Irrelevant study design (retrospective comparative study). |
| Russo 2015 | Irrelevant comparator (open simple prostatectomy). We focused on effects of prostatic arterial embolization compared to minimal invasive therapies (Jung 2017). |
| Steurer 2018 | Review. |
| Wu 2019 | Irrelevant study design (retrospective comparative study). |
Characteristics of studies awaiting classification [ordered by study ID]
Ng 2020.
| Methods |
Study design: prospective cohort study Setting/Country: single center/Hong Kong |
| Participants | Inclusion criteria: American Society of Anesthesiology Class 3/4; obstructive uropathy or refractory urinary retention with prostate size > 50 mL |
| Interventions |
Group A: prostatic arterial embolization Group B: transurethral resection of prostate |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Abstract only |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617001235392.
| Study name | PAE for patients with LUTS due to BPH |
| Methods |
Study design: parallel randomized controlled trial (open label) Setting/country: single center/New Zealand |
| Participants | Inclusion criteria: men were willing, able, and mentally competent to provide written consent; aged ≥ 40 years; with LUTS (IPSS > 8, QoL > 3); prostate gland > 40 mL on transabdominal ultrasound; vascular anatomy that in the opinion of the interventional radiologist is amenable to PAE as assessed on CTA; adequate laboratory parameters: platelets > 100/μL, INR < 1.5, bilirubin < 2 μmol/L, albumin > 2.5 g/dL, estimated glomerular filtration rate > 60 mL/minute |
| Interventions |
Group A: PAE Group B: TURP |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | August 2017 |
| Contact information | martin.krauss@cdhb.health.nz |
| Notes | Sponsor: Christchurch hospital |
ChiCTR1800014818.
| Study name | PAE as a primary treatment for BPH |
| Methods |
Study design: prospective non‐randomized study (cohort study) Setting/country: single center/China |
| Participants | Inclusion criteria: men diagnosed with BPH by the 2014 Chinese urologic disease diagnosis and treatment guideline |
| Interventions |
Group A: PAE Group B: TURP |
| Outcomes |
|
| Starting date | February 2018 |
| Contact information | wjh9877@163.com |
| Notes | Sponsor: Tianjin First Center Hospital |
NCT01789840.
| Study name | PAE with embosphere microspheres compared to TURP for BPH |
| Methods |
Study design: prospective non‐randomized study Setting/country: multicenter/USA |
| Participants | Inclusion criteria: ages 50–79 years inclusive; signed informed consent; LUTS secondary to BPH for ≥ 6 months before study treatment; baseline IPSS score > 13; prostate size ≥ 50 g and < 90 g measured by MRI; BPH symptoms refractory to medical treatment or for whom medication is contraindicated, not tolerated, or refused; candidate for TURP; must meet 1 of the following criteria: baseline PSA < 2.5 ng/mL (no prostate biopsy required), baseline PSA > 2.5 ng/mL and ≤ 10 ng/mL and free PSA > 25% of total PSA (no prostate biopsy required), baseline PSA > 2.5 ng/mL and ≤ 10 ng/mL and free PSA < 25% of total PSA and a negative prostate biopsy result (minimum 12‐core biopsy), baseline PSA >10 ng/mL, and a negative prostate biopsy (minimum 12‐core biopsy) |
| Interventions |
Group A: PAE Group B: TURP |
| Outcomes |
Primary outcome
Secondary outcomes
Other outcomes
|
| Starting date | July 2013 |
| Contact information | Not provided but we contacted Dr Francisco C Carnevale (who is listed as principal investigator) using fcarnevale@uol.com.br on 31 August 2020. |
| Notes | Study completed in December 2017 Sponsor: Merit Medical Systems, Inc. |
NCT04084938.
| Study name | Artery embolization vs operation of benign prostate hyperplasia (NORTAPE) |
| Methods |
Study design: parallel randomized controlled trial (open label) Setting/country: single center/Norway |
| Participants | Inclusion criteria: LUTS from BPH with moderate and severe IPSS score (IPSS > 8) and QoL ≥ 3; refractory to medical treatment for ≥ 6 months or the patient is unwilling to accept medical treatment; BPH using permanent or intermittent catheterization; prostate volume > 50 mL; signed informed consent |
| Interventions |
Group A: PAE Group B: prostate operation through a catheter into the penis or through an incision in lower abdomen |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | September 2019 |
| Contact information | fagreda.germanstrias@gencat.cat |
| Notes | Sponsor: Oslo University Hospital |
NCT04236687.
| Study name | PAE compared to Holmium laser enucleation of the prostate for BPH |
| Methods |
Study design: parallel randomized controlled trial (open label) Setting/country: single center/Spain |
| Participants | Inclusion criteria: patients evaluated in the urology department and candidates to surgical treatment; age > 45 years; IPSS ≥ 10; Qmax < 12 mL/second; PVR < 300 mL; prostatic volume 20–250 mL assessed by ultrasound; signed informed consent |
| Interventions |
Group A: PAE Group B: Holmium laser enucleation of the prostate |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | February 2020 |
| Contact information | thihag@ous‐hf.no |
| Notes | Sponsor: Hospital Universitari Germans Trias i Pujol |
NCT04807010.
| Study name | PROARTE – PROstate ARTery to reduce the symptoms of benign prostatic hyperplasia |
| Methods |
Study design: randomized double blinded crossover trial Setting/country: not available/USA |
| Participants | Inclusion criteria: men ages ≥ 45 and ≤ 90 years presenting with BPH with symptoms for ≥ 6 months that are refractory to medical management or in whom medications are contraindicated, not tolerated, or refused; IPSS ≥ 14; QoL ≥ 3; Qmax ≤ 12 mL/second; PVR > 125 mL; prostate volume > 30 mL as determined by ultrasound, MRI, or computed tomography; personal risk < 40% based on the University of Texas San Antonio prostate cancer risk calculator or having a negative prostate biopsy for cancer within the last 24 months; able to provide written consent; not participating in any other investigational drug or device studies |
| Interventions |
Group A: PAE Group B: sham |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | August 2021 |
| Contact information | pdoshi@sirweb.org |
| Notes | Sponsor: Society of Interventional Radiology Foundation |
BPH: benign prostatic hyperplasia; CTA: computer tomography angiography; IIEF: International Index of Erectile Function; INR: international normalized ratio; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; MRI: magnetic resonance imaging; PAE: prostatic arterial embolization; PSA: prostate‐specific antigen; PVP: photovaporization of the prostate; PVR: postvoid residual; Qmax: maximum flow rate; QoL: quality of life; TURP: transurethral resection of prostate.
Differences between protocol and review
This review was based on a published protocol (Jung 2017), and differences are described here.
Types of studies: we included only NRSs designed as prospective comparative studies, as other studies were very unlikely to provide evidence other than evidence of very low certainty.
Types of outcome measures: we used a minimal clinically important difference (MCID) of 0.5 to assess the quality of life outcome based on Rees 2015. In addition, we used final values instead of changes from baseline to make the fullest use of the results (half or more studies reported only final values).
Types of outcome measures: we changed the outcome of ejaculatory function to ejaculatory disorder due to lack of data based on the questionnaire. Therefore, we used incidence rate of ejaculatory disorders such as postoperative retrograde ejaculation or reduction of ejaculation volume.
We revised the definition of 'retreatment' to "Participants undergoing the same or other surgical treatment modalities due to insufficient treatment response" for clarity, also omitting the time horizon of up to six months since later retreatments would also be of interest.
Electronic searches: we additionally searched Google Scholar.
Assessment of risk of bias in included studies: we listed baseline confounding factors and co‐interventions to assess risk of bias in NRSs.
Summary of findings table: we referenced GRADE guidance to rate the certainty of the evidence in RCTs and NRSs (Schünemann 2019).
Contributions of authors
JHJ: conceived, designed, and wrote the protocol and performed all aspects of data abstraction, analysis, risk of bias assessment, and certainty of evidence ratings.
KAM: provided clinical and methodologic input to the protocol and the review.
MB: provided critical content expertise input to the protocol and review from a urology perspective.
SY: provided critical content expertise input to the protocol and review from an interventional radiology perspective.
JG: provided critical content expertise input to the protocol and review from an interventional radiology perspective.
MHK: created search strategies and executed the searches.
VN: provided critical content expertise input to the protocol and the review.
PD: conceived, designed, and wrote the protocol, reviewed critical content, and gave final approval.
Sources of support
Internal sources
-
Department of Urology, Yonsei University Wonju College of Medicine, Korea, South
Salary support for Jae Hung Jung
-
Minneapolis VA Health Care System, USA
Salary support for Philipp Dahm
-
Department of Urology, University of Minnesota, USA
Salary support for Philipp Dahm
External sources
-
N/A, USA
No external support was received for this review
Declarations of interest
JHJ: none.
MB: Boston Scientific (consultant for endourology and stone management), Auris Health (consultant for robotic surgery and endourology).
KAM: none.
SY: none.
JG: none.
MHK: none.
VN: none.
PD: none.
Edited (no change to conclusions)
References
References to studies included in this review
Abt 2021 {published data only}
- Abt D, Hechelhammer L, Müllhaupt G, Kessler T, Schmid HP, Engeler DS, et al.Prostatic artery embolization vs conventional TUR-P in the treatment of benign prostatic hyperplasia: first results of a prospective, randomized non-inferiority trial. European Urology Supplement 2016;15(3):e1080. [DOI: 10.1016/S1569-9056(16)61081-3] [DOI] [Google Scholar]
- Abt D, Hechelhammer L, Müllhaupt G, Markart S, Güsewell S, Kessler TM, et al.Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ 2018;361:k2338. [DOI: 10.1136/bmj.k2338] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt D, Müllhaupt G, Hechelhammer L, Markart S, Güsewell S, Schmid HP, et al.Prostatic artery embolisation versus transurethral resection of the prostate for benign prostatic hyperplasia: 2-yr outcomes of a randomised, open-label, single-centre trial. European Urology 2021;80(1):34-42. [DOI: 10.1016/j.eururo.2021.02.008] [DOI] [PubMed] [Google Scholar]
- Abt D, Mordasini L, Hechelhammer L, Kessler TM, Schmid HP, Engeler DS.Prostatic artery embolization versus conventional TUR-P in the treatment of benign prostatic hyperplasia: protocol for a prospective randomized non-inferiority trial. BMC Urology 2014;14:94. [DOI: 10.1186/1471-2490-14-94] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt D, Mullhaupt G, Hechelhammer L, Markart S, Gusewell S, Schmid HP, et al.Prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: two-year outcomes of a randomised, open label, single-centre trial. European Urology 2021;79(Suppl 1):S83. [DOI] [PubMed] [Google Scholar]
- Müllhaupt G, Hechelhammer L, Diener PA, Engeler DS, Güsewell S, Schmid HP, et al.Ejaculatory disorders after prostatic artery embolization: a reassessment of two prospective clinical trials. World Journal of Urology 2020;38(10):2595-9. [DOI: 10.1007/s00345-019-03036-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02054013.Prostatic artery embolization vs. conventional transurethral prostatectomy in the treatment of benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT02054013 (first received 4 February 2014).
- NCT03521648.Database for the assessment of efficacy and safety of BPH treatment. clinicaltrials.gov/show/NCT03521648 (first received 11 May 2018).
Carnevale 2016 {published data only}
- Carnevale FC, Iscaife A, Yoshinaga EM, Moreira AM, Antunes AA, Srougi M.Transurethral resection of the prostate (TURP) versus original and perfected prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovascular and Interventional Radiology 2016;39(1):44-52. [DOI: 10.1007/s00270-015-1202-4] [DOI] [PubMed]
- Yoshinaga EM, Nakano E, Marchini GS, Galvao O, Baroni R, Carnevale FC, et al.A prospective and randomized trial comparing transurethral resection of the prostate (TURP) to prostate artery embolization (PAE) for treatment of bladder outlet obstruction due to benign prostatic hyperplasia (BPH). Journal of Urology 2014;191(4 Suppl):e793. [DOI: 10.1016/j.juro.2014.02.2168] [DOI]
Gao 2014 {published data only}
Insausti 2020 {published data only}
- Capdevila F, Insausti I, Galbete A, Sanchez-Iriso E, Montesino M.Prostatic artery embolization versus transurethral resection of the prostate: a post hoc cost analysis of a randomized controlled clinical trial. Cardiovascular and Interventional Radiology 2021;44(11):1771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giral Villalta PJ, Aguilar Guevara JF, Lopez Ubillos G, Lacarra Fernandez S, Zabalo San Juan A, Asiáin Urmeneta M, et al.Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: 12 month results of a clinical trial. European Urology, Supplements 2019;18(1):e1494-5. [DOI: 10.1016/S1569-9056(19)31075-9] [DOI]
- Insausti I, Sáez de Ocáriz García A, Galbete A, Capdevila F, Solchaga S, Giral P, et al.Randomized comparison of prostatic arterial embolization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology 2020;31(6):882-90. [DOI: 10.1016/j.jvir.2019.12.810] [DOI] [PubMed]
- Napal Lecumberri S, Insausti Gorbea I, Sáez de Ocáriz García A, Solchaga Álvarez S, Cebrián Lostal JL, Monreal Beortegui R, et al.Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: protocol for a non-inferiority clinical trial. Research and Reports in Urology 2018;10:17-22. [DOI: 10.2147/RRU.S139086] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01963312.Clinical trial to evaluate the efficacy and safety of the transarterial supraselective embolization of the prostate to treat the urinary symptoms. clinicaltrials.gov/ct2/show/NCT01963312 (first received 16 October 2013).
- Saez De Ocariz Garcia A, Insausti Gorbea I, Solchaga Alvarez S, Monreal Beortegui R, Giral Villalta PJ, Napal Lecumberri S, et al.Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: 6-month results of a clinical trial. Cardiovascular and Interventional Radiology 2017;40(2):S117-8. [Google Scholar]
Pisco 2020 {published data only}
- NCT02074644.Clinical trial of prostatic arterial embolization versus a sham procedure to treat benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT02074644 (first received 28 February 2014).
- Pisco JM, Bilhim T, Costa NV, Torres D, Pisco J, Pinheiro LC, et al.Randomised clinical trial of prostatic artery embolisation versus a sham procedure for benign prostatic hyperplasia. European Urology 2020;77(3):354-62. [DOI: 10.1016/j.eururo.2019.11.010] [DOI] [PubMed] [Google Scholar]
Radwan 2020 {published data only}
- Radwan A, Farouk A, Higazy A, Samir YR, Tawfeek AM, Gamal MA.Prostatic artery embolization versus transurethral resection of the prostate in management of benign prostatic hyperplasia. Prostate International 2020;8(3):130-3. [DOI: 10.1016/j.prnil.2020.04.001] [DOI] [PMC free article] [PubMed]
Ray 2018 {published data only}
- Dasgupta R, Speakman M, Ray A, Powell J, Modi S, Carolan-Rees G, et al.Prostate artery embolisation versus TURP; a multicentric prospective comparison: the UK-ROPE study. Journal of Urology 2018;199(4 Suppl):e835. [DOI: 10.1016/j.juro.2018.02.2010] [DOI] [Google Scholar]
- Modi S, Bryant TJ, Ray AF, Hacking N.UK-ROPE: preliminary findings. Cardiovascular and Interventional Radiology 2016;39(3):S152. [Google Scholar]
- NCT02434575.UK ROPE Register Study. clinicaltrials.gov/show/NCT02434575 (first received 5 May 2015).
- NCT02849522.ROPE registry project to determine the safety and efficacy of prostate artery embolisation (PAE) for lower urinary tract symptoms secondary to benign prostatic enlargement (LUTS BPE). clinicaltrials.gov/show/NCT02849522 (first received 29 July 2016).
- Ray AF, Powell J, Speakman MJ, Longford NT, DasGupta R, Bryant T, et al.Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU International 2018;122(2):270-82. [DOI: 10.1111/bju.14249] [DOI] [PubMed] [Google Scholar]
Soluyanov 2018 {published data only}
- Soluyanov MY, Shumkov OA, Smagin MA, Nimaev VV.First experience with prostate artery embolization for benign prostatic hyperplasia. Urologia 2018;4:33-7. [PubMed] [Google Scholar]
Zhu 2018 {published data only}
- Zhu C, Lin W, Huang Z, Cai J.Prostate artery embolization and transurethral resection of prostate for benign prostatic hyperplasia: a prospective randomized controlled trial. Chinese Journal of Interventional Imaging and Therapy 2018;15(3):134-8. [DOI: 10.13929/j.1672-8475.201711043] [DOI] [Google Scholar]
References to studies excluded from this review
Abt 2019 {published data only}
- Abt D, Mullhaupt G, Mordasini L, Gusewell S, Markart S, Zumstein V, et al.Outcome prediction of prostatic artery embolization: post hoc analysis of a randomized, open-label, non-inferiority trial. BJU International 2019;124(1):134-44. [DOI] [PubMed] [Google Scholar]
Bagla 2017 {published data only}
- Bagla S, Smirniotopoulos J, Orlando J, Piechowiak R.Cost analysis of prostate artery embolization (PAE) and transurethral resection of the prostate (TURP) in the treatment of benign prostatic hyperplasia. Cardiovascular and Interventional Radiology 2017;40(11):1694-7. [DOI: 10.1007/s00270-017-1700-7] [DOI] [PubMed] [Google Scholar]
- Bagla S, Vadlamudi V, Orlando J, Smirniotopoulos J.Cost analysis of prostate artery embolization (PAE) and transurethral resection of the prostate (TURP) in the treatment of benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology 2016;27(3):S56. [DOI: 10.1016/j.jvir.2015.12.154] [DOI] [PubMed] [Google Scholar]
Bilhim 2015 {published data only}
- Bilhim T, Bagla S, Sapoval M, Carnevale FC, Salem R, Golzarian J.Prostatic arterial embolization versus transurethral resection of the prostate for benign prostatic hyperplasia. Radiology 2015;276(1):310-1. [DOI] [PubMed] [Google Scholar]
Brown 2019 {published data only}
Mullhaupt 2019 {published data only}
- Mullhaupt G, Hechelhammer L, Engeler DS, Gusewell S, Betschart P, Zumstein V, et al.In-hospital cost analysis of prostatic artery embolization compared with transurethral resection of the prostate: post hoc analysis of a randomized controlled trial. BJU International 2019;123(6):1055-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01835860 {unpublished data only}
- NCT01835860.Prostatic artery embolization for benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT01835860 (first received 19 April 2013).
NCT02006303 {unpublished data only}
- NCT02006303.Prostatic artery embolization versus 532 nm green light PVP for catheterized patients. clinicaltrials.gov/ct2/show/NCT02006303 (first received 10 December 2013).
NCT02566551 {unpublished data only}
- NCT02566551.Prospective controlled randomized study of PAE vs TURP for BPH treatment. clinicaltrials.gov/ct2/show/NCT02566551 (first received 2 October 2015).
Pereira 2018 {published data only}
- NCT03043222.Innovative minimally invasive options in treatment of urinary problems related to prostate enlargement (BPH) in men. clinicaltrials.gov/show/NCT03043222 (first received 3 February 2017).
- Pereira K, Ford-Glanton S, Johar R, Xu P, Pham K, Gadani S, et al.Prostatic artery embolization (PAE) and prostatic urethral lift (PUL) procedures for symptomatic benign prostatic enlargement (BPH): a retrospective, single-center comparison of outcomes. Journal of Vascular and Interventional Radiology 2018;29(4 Suppl 1):S6. [DOI: 10.1016/j.jvir.2018.01.010] [DOI]
Qiu 2017 {published data only}
- Qiu ZL, Zhang CC, Wang XS, Cheng K, Liang X, Wang DW, et al.Clinical evaluation of embolization of the superior vesical prostatic artery for treatment of benign prostatic hyperplasia: a single-center retrospective study. Wideochir Inne Tech Maloinwazyjne 2017;12(4):409-16. [DOI: 10.5114/wiitm.2017.72324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Russo 2015 {published data only}
- Russo GI, Kurbatov D, Sansalone S, Lepetukhin A, Dubsky S, Sitkin I, et al.Prostatic arterial embolization vs open prostatectomy: a 1-year matched-pair analysis of functional outcomes and morbidities. Urology 2015;86(2):343-8. [DOI] [PubMed] [Google Scholar]
- Russo GI, Kurbatov D, Sansalone S, Lepetukhin A, Dubsky S, Sitkin I, et al.Prostatic arterial embolization vs open prostatectomy: a matched-pair analysis of functional outcomes and morbidities after 1 year of follow-up. European Urology Supplement 2015;14(2):e570. [DOI] [PubMed] [Google Scholar]
Steurer 2018 {published data only}
- Steurer J.Benign prostatic hyperplasia: transurethral resection or embolization of prostate arteries? Praxis 2018;107(20):1115-6. [DOI] [PubMed] [Google Scholar]
Wu 2019 {published data only}
- Wu S, Cai S, Yi T, Cai W, Zhou Y, He J, et al.Interventional embolization vs transurethral resection for the treatment of benign prostatic hyperplasia in elderly patients: a comparison study. Journal of Interventional Radiology 2019;28(2):179-83. [Google Scholar]
References to studies awaiting assessment
Ng 2020 {published data only}
- Ng CM, Chung K, Cheng B, Chak H, Chan W, Ming S, et al.Comparison of prostatic artery embolisation with transurethral resection of the prostate for high-risk surgical candidates in obstructive uropathy or refractory retention: a prospective cohort study. International Journal of Urology 2020;27(Suppl 1):113. [Google Scholar]
References to ongoing studies
ACTRN12617001235392 {unpublished data only}
- ACTRN12617001235392.Prostate artery embolization for patients with lower urinary tract symptoms due to benign prostate hyperplasia. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373427 (first received 24 August 2017).
ChiCTR1800014818 {unpublished data only}
- ChiCTR1800014818.Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia. www.chictr.org.cn/com/25/showprojen.aspx?proj=25226 (first received 7 February 2018).
NCT01789840 {unpublished data only}
- NCT01789840.Prostate artery embolization with embosphere microspheres compared to TURP for benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT01789840 (first received 12 February 2013).
NCT04084938 {unpublished data only}
- NCT04084938.Artery embolization vs operation of benign prostate hyperplasia (NORTAPE). clinicaltrials.gov/ct2/show/NCT04084938 (first received 10 September 2019).
NCT04236687 {unpublished data only}
- NCT04236687.Prostate artery embolization compared to holmium laser enucleation of the prostate for benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT04236687 (firs received 22 January 2020).
NCT04807010 {unpublished data only}
- NCT04807010.PROARTE -PROstate ARTery to reduce the symptoms of benign prostatic hyperplasia. clinicaltrials.gov/ct2/show/NCT04807010 (first received 19 March 2021). [NCT04807010]
Additional references
Ahyai 2010
- Ahyai SA, Gilling P, Kaplan SA, Kuntz RM, Madersbacher S, Montorsi F, et al.Meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic enlargement. European Urology 2010;58(3):384-97. [DOI] [PubMed] [Google Scholar]
Barry 1995
- Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al.Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? Journal of Urology 1995;154(5):1770-4. [DOI] [PubMed] [Google Scholar]
Barry 1997
- Barry MJ, Fowler FJ Jr, Bin L, Pitts JC 3rd, Harris CJ, Mulley AG Jr.The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. Journal of Urology 1997;157(1):10-4; discussion 14-5. [PubMed] [Google Scholar]
Bhatia 2018
- Bhatia S, Sinha VK, Harward S, Gomez C, Kava BR, Parekh DJ.Prostate artery embolization in patients with prostate volumes of 80 mL or more: a single-institution retrospective experience of 93 patients. Journal of Vascular and Interventional Radiology 2018;29(10):1392-8. [DOI: 10.1016/j.jvir.2018.05.012] [DOI] [PubMed] [Google Scholar]
Bhojani 2014
- Bhojani N, Gandaglia G, Sood A, Rai A, Pucheril D, Chang SL, et al.Morbidity and mortality after benign prostatic hyperplasia surgery: data from the American College of Surgeons national surgical quality improvement program. Journal of Endourology 2014;28(7):831-40. [DOI] [PubMed] [Google Scholar]
Brasure 2016
- Brasure M, MacDonald R, Dahm P, Olson CM, Nelson VA, Fink HA, et al.AHRQ comparative effectiveness reviews. In: Newer Medications for Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: a Review. Rockville (MD): Agency for Healthcare Research and Quality (US), 2016. [PubMed] [Google Scholar]
Carnevale 2010
- Carnevale FC, Antunes AA, da Motta Leal Filho JM, Oliveira Cerri LM, Baroni RH, Marcelino AS, et al.Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovascular and Interventional Radiology 2010;33(2):355-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Centers for Disease Control and Prevention 2003
- Centers for Disease Control and Prevention.Public health and aging: trends in aging – United States and worldwide. JAMA 2003;289(11):1371-3. [PubMed] [Google Scholar]
Chapple 2017
- Chapple C, Castro-Diaz D, Chuang YC, Lee KS, Liao L, Liu SP, et al.Prevalence of lower urinary tract symptoms in China, Taiwan, and South Korea: results from a cross-sectional, population-based study. Advances in Therapy 2017;34(8):1953-65. [DOI] [PMC free article] [PubMed]
Covidence 2017 [Computer program]
- Covidence.Version accessed 18 August 2017. Melbourne, Australia: Veritas Health Innovation, 2013. Available at www.covidence.org.
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s).Chapter 9. Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
DeMeritt 2000
- DeMeritt JS, Elmasri FF, Esposito MP, Rosenberg GS.Relief of benign prostatic hyperplasia-related bladder outlet obstruction after transarterial polyvinyl alcohol prostate embolization. Journal of Vascular and Interventional Radiology 2000;11(6):767-70. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA.Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunphy 2015
- Dunphy C, Laor L, Te A, Kaplan S, Chughtai B.Relationship between depression and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Reviews in Urology 2015;17(2):51-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
EAU 2021
- European Association of Urology.Management of non-neurogenic male LUTS. uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ (accessed 3 November 2021).
Egan 2016
- Egan KB.The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urologic Clinics of North America 2016;43(3):289-97. [DOI] [PubMed] [Google Scholar]
EndNote 2016 [Computer program]
- EndNote.Version 7.5. Clarivate Analytics, 2016.
Feinsten 2018
- Feinsten L, Matlaga B.Urologic diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office, 2018; NIH Publication No. 12-7865.
Feng 2017
- Feng S, Tian Y, Liu W, Li Z, Deng T, Li H, et al.Prostatic arterial embolization treating moderate-to-severe lower urinary tract symptoms related to benign prostate hyperplasia: a meta-analysis. Cardiovascular and Interventional Radiology 2017;40(1):22-32. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT.Version accessed 18 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ, et al.GRADE: what is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed) 2008;336(7651):995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al.GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG.Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed) 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA.Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Johnston 2010
- Johnston BC, Thorlund K, Schunemann HJ, Xie F, Murad MH, Montori VM, et al.Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health and Quality of Life Outcomes 2010;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2019
- Jung JH, Reddy B, McCutcheon KA, Borofsky M, Narayan V, Kim MH, et al.Prostatic urethral lift for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD012832. [DOI: 10.1002/14651858.CD012832.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2020
- Kang TW, Jung JH, Hwang EC, Borofsky M, Kim MH, Dahm P.Convective radiofrequency water vapour thermal therapy for lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013251. [DOI: 10.1002/14651858.CD013251.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuang 2017
- Kuang M, Vu A, Athreya S.A systematic review of prostatic artery embolization in the treatment of symptomatic benign prostatic hyperplasia. Cardiovascular and Interventional Radiology 2017;40(5):655-63. [DOI] [PubMed] [Google Scholar]
Lerner 2021a
- Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, et al.Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART I – initial work-up and medical management. Journal of Urology 2021;206(4):806-17. [DOI] [PubMed] [Google Scholar]
Lerner 2021b
- Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, et al.Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART II – surgical evaluation and treatment. Journal of Urology 2021;206(4):818-26. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malaeb 2012
- Malaeb BS, Yu X, McBean AM, Elliott SP.National trends in surgical therapy for benign prostatic hyperplasia in the United States (2000–2008). Urology 2012;79(5):1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malling 2019
- Malling B, Røder MA, Brasso K, Forman J, Taudorf M, Lönn L.Prostate artery embolisation for benign prostatic hyperplasia: a systematic review and meta-analysis. European Radiology 2019;29:287-98. [DOI] [PubMed] [Google Scholar]
Martin 2014
- Martin S, Lange K, Haren MT, Taylor AW, Wittert G.Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. Journal of Urology 2014;191(1):130-7. [DOI] [PubMed] [Google Scholar]
Martins Pisco 2012
- Martins Pisco J, Pereira J, Rio Tinto H, Fernandes L, Bilhim T.How to perform prostatic arterial embolization. Techniques in Vascular and Interventional Radiology 2012;15(4):286-9. [DOI] [PubMed] [Google Scholar]
McVary 2018
- McVary KT, Roehrborn CG.Three-year outcomes of the prospective, randomized controlled Rezūm System study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology 2018;111:1-9. [DOI] [PubMed] [Google Scholar]
McWilliams 2019
- McWilliams JP, Bilhim TA, Carnevale FC, Bhatia S, Isaacson AJ, Bagla S, et al.Society of Interventional Radiology multisociety consensus position statement on prostatic artery embolization for treatment of lower urinary tract symptoms attributed to benign prostatic hyperplasia: from the Society of Interventional Radiology, the Cardiovascular and Interventional Radiological Society of Europe, Société Française de Radiologie, and the British Society of Interventional Radiology: endorsed by the Asia Pacific Society of Cardiovascular and Interventional Radiology, Canadian Association for Interventional Radiology, Chinese College of Interventionalists, Interventional Radiology Society of Australasia, Japanese Society of Interventional Radiology, and Korean Society of Interventional Radiology. Journal of Vascular and Interventional Radiology 2019;30(5):627-37. [DOI] [PubMed] [Google Scholar]
Mitchell 1976
- Mitchell ME, Waltman AC, Athanasoulis CA, Kerr WS Jr, Dretler SP.Control of massive prostatic bleeding with angiographic techniques. Journal of Urology 1976;115(6):692-5. [DOI] [PubMed] [Google Scholar]
Narayan 2017
- Narayan V, Jung JH, Dahm P.Re: efficacy and safety of prostatic arterial embolization: systematic review with meta-analysis and meta-regression: SR Shim, KJ Kanhai, YM Ko, JH Kim. Journal of Urology 2017;197:465-79. Journal of Urology 2017;198(1):215-6. [DOI] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence.Prostate artery embolisation for lower urinary tract symptoms caused by benign prostatic hyperplasia. Interventional procedures guidance. www.nice.org.uk/guidance/ipg611 (accessed 4 December 2018).
Nickel 2015
- Nickel JC, Brock GB, Herschorn S, Dickson R, Henneges C, Viktrup L.Proportion of tadalafil-treated patients with clinically meaningful improvement in lower urinary tract symptoms associated with benign prostatic hyperplasia – integrated data from 1,499 study participants. BJU International 2015;115(5):815-21. [DOI] [PubMed] [Google Scholar]
Omar 2014
- Omar MI, Lam TB, Alexander CE, Graham J, Mamoulakis C, Imamura M, et al.Systematic review and meta-analysis of the clinical effectiveness of bipolar compared with monopolar transurethral resection of the prostate (TURP). BJU International 2014;113(1):24-35. [DOI] [PubMed] [Google Scholar]
Pariser 2015
- Pariser JJ, Pearce SM, Patel SG, Bales GT.National trends of simple prostatectomy for benign prostatic hyperplasia with an analysis of risk factors for adverse perioperative outcomes. Urology 2015;86(4):721-5. [DOI] [PubMed] [Google Scholar]
Parsons 2015
- Parsons JK, Rangarajan SS, Palazzi K, Chang D.A national, comparative analysis of perioperative outcomes of open and minimally invasive simple prostatectomy. Journal of Endourology 2015;29(8):919-24. [DOI] [PubMed] [Google Scholar]
Pisco 2016
- Pisco JM, Bilhim T, Pinheiro LC, Fernandes L, Pereira J, Costa NV, et al.Medium- and long-term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. Journal of Vascular and Interventional Radiology 2016;27(8):1115-22. [DOI] [PubMed] [Google Scholar]
Pyo 2017
- Pyo JS, Cho WJ.Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clinical Radiology 2017;72(1):16-22. [DOI] [PubMed] [Google Scholar]
Rees 2015
- Rees J.Patients not P values. BJU international 2015;115(5):678-9. [DOI] [PubMed] [Google Scholar]
Reich 2008
- Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al.Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. Journal of Urology 2008;180(1):246-9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roehrborn 2008
- Roehrborn CG.Pathology of benign prostatic hyperplasia. International Journal of Impotence Research 2008;20 Suppl 3:S11-8. [DOI] [PubMed] [Google Scholar]
Roehrborn 2017
- Roehrborn CG, Barkin J, Gange SN, Shore ND, Giddens JL, Bolton DM, et al.Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Canadian Journal of Urology 2017;24(3):8802-13. [PubMed] [Google Scholar]
Rosen 1997
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A, et al.The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822-30. [DOI] [PubMed] [Google Scholar]
Rosen 2007
- Rosen RC, Catania JA, Althof SE, Pollack LM, O'Leary M, Seftel AD, et al.Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007;69(5):805-9. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH.Chapter 11. Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al.Chapter 12. Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2013
- Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al.Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013;4(1):49-62. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al.GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2019;111:105-14. [DOI] [PMC free article] [PubMed]
Shim 2017
- Shim SR, Kanhai KJ, Ko YM, Kim JH.Efficacy and safety of prostatic arterial embolization: systematic review with meta-analysis and meta-regression. Journal of Urology 2017;197(2):465-79. [DOI] [PubMed] [Google Scholar]
Spaliviero 2010
- Spaliviero M, Strom KH, Gu X, Araki M, Culkin DJ, Wong C.Does Greenlight HPS(™) laser photoselective vaporization prostatectomy affect sexual function? Journal of Endourology 2010;24(12):2051-7. [DOI] [PubMed] [Google Scholar]
Sterne 2016a
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al.ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016b
- Sterne JA, Higgins JP, Elbers RG, Reeves BC, Development group for ROBINS-I.Risk of bias In non-randomized studies of interventions (ROBINS-I): detailed guidance, updated 12 October 2016. www.riskofbias.info (accessed 9 November 2018).
Sun 2008
- Sun F, Sanchez FM, Crisostomo V, Lima JR, Luis L, Garcia-Martinez V, et al.Benign prostatic hyperplasia: transcatheter arterial embolization as potential treatment – preliminary study in pigs. Radiology 2008;246(3):783-9. [DOI] [PubMed] [Google Scholar]
Taub 2006
- Taub DA, Wei JT.The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Current Urology Reports 2006;7(4):272-81. [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang MQ, Guo LP, Zhang GD, Yuan K, Li K, Duan F, et al.Prostatic arterial embolization for the treatment of lower urinary tract symptoms due to large (> 80 mL) benign prostatic hyperplasia: results of midterm follow-up from Chinese population. BMC Urology 2015;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization.Proposed working definition of an older person in Africa for the MDS project. www.who.int/healthinfo/survey/ageingdefnolder/en (accessed 17 August 2017).
Xu 2020
- Xu XJ, Li J, Huang XZ, Liu Q.An updated meta-analysis of prostatic arterial embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia. World Journal of Urology 2020;38(8):2069-70. [DOI] [PubMed] [Google Scholar]
Yoo 2012
- Yoo TK, Cho HJ.Benign prostatic hyperplasia: from bench to clinic. Korean Journal of Urology 2012;53(3):139-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2017
- Young S, Golzarian J.Prostate arterial embolization is a viable option for treating symptoms of benign prostatic hyperplasia: pro. Journal of Urology 2017;198(1):9-11. [DOI] [PubMed] [Google Scholar]
Zlotta 1997
- Zlotta AR, Raviv G, Peny MO, Noel JC, Haot J, Schulman CC.Possible mechanisms of action of transurethral needle ablation of the prostate on benign prostatic hyperplasia symptoms: a neurohistochemical study. Journal of Urology 1997;157(3):894-9. [PubMed] [Google Scholar]
Zumstein 2019
- Zumstein V, Betschart P, Vetterlein MW, Kluth LA, Hechelhammer L, Mordasini L, et al.Prostatic artery embolization versus standard surgical treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. European Urology Focus 2019;5(6):1091-100. [DOI] [PubMed]
References to other published versions of this review
Jung 2017
- Jung JH, Shin TY, McCutcheon KA, Borofsky M, Narayan V, Young S, et al.Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012867. [DOI: 10.1002/14651858.CD012867] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2020
- Jung JH, McCutcheon KA, Borofsky M, Young S, Golzarian J, Reddy B, et al.Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD012867. [DOI: 10.1002/14651858.CD012867.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


