Abstract
The increasing problem of antibiotic resistance among pathogenic bacteria requires development of new antimicrobial agents. One line of investigation is the synthesis of antimicrobial hybrid peptides. The aim of the present investigation was to determine the in vitro activities of 16 cecropin-melittin hybrid peptides (CAMEL analogues) against 60 anaerobic bacterial strains, to compare their activities with those of seven clinically used antimicrobial agents, and to compare different methods for anaerobic susceptibility testing of these peptides. The stability of one of the peptides, temporin B, with different stereoisomeric configurations was investigated in a fecal milieu. The CAMEL analogues showed antimicrobial activity against the anaerobic bacteria, with MICs ranging from 0.125 to 32 μg/ml. The overall activities (the MICs at which 90% of isolates are inhibited) of the CAMEL analogues against anaerobic bacteria were mainly inferior to those of imipenem, clindamycin, and piperacillin but were equal to or superior to those of metronidazole, cefoxitin, ciprofloxacin, and chloramphenicol. The agarose dilution method was found to be an accurate method for the testing of large numbers of bacterial strains. The d isomer of temporin B was inactivated more slowly in feces than the l isomer. This study shows that the CAMEL analogues are potential agents for the treatment of anaerobic infections.
During the last 15 to 20 years, resistance among clinical bacterial strains against currently available antimicrobial agents has emerged at an alarming rate. The evolution and rapid spread of resistant strains are now significant nosocomial problems and are of increasing importance in community-acquired infections. The resistance threat demands an increased effort to search for antimicrobial agents with new mechanisms of action. Anaerobic bacteria are a common cause of serious infections. The anaerobic species which predominate in clinical infections include the Bacteroides fragilis group, Clostridium spp., Fusobacterium nucleatum, and Peptostreptococcus spp. Treatment of anaerobic infections is often difficult, since many anaerobes harbor intrinsic or required resistance against several antimicrobial agents (14, 19). Pseudomembranous colitis caused by Clostridium difficile is a fearful complication associated with previous antimicrobial therapy. Treatment with vancomycin or metronidazole is often successful, although about 20% of the patients suffer recurrences after treatment (13).
Antimicrobial peptides are a new group of active antibiotics with a unique mechanism of action (7, 25). These peptides are part of an innate immune system that is widely distributed in nature and that has been found in many different animal species (4, 18). Genes encoding these peptides are immediately expressed after injury or invasion of the host (21). Their effect against bacteria is probably due to their positive charge and their ability to adopt amphipathic conformations (22). A suggested model is as follows: (i) a direct, electrostatic interaction with the negatively charged bacterial cytoplasmic membrane, (ii) interaction of the peptide with the hydrocarbon core of the membrane and (iii) subsequent peptide conformational change into alpha-helical peptides that form membrane-spanning pores that disrupt the ionic homeostasis of the bacterium and lead to cell lysis (31). Several antimicrobial peptides have also been reported to act against fungi (8), parasites (42), viruses (2, 3, 34, 39), and tumors (43). In addition to the cytolytic capacities, other mechanisms are probably involved.
One line of investigation for the detection of new antimicrobial agents and for the development of more active and stable variants of naturally occurring peptides is the design and chemical synthesis of analogues of the natural antimicrobial peptides (6, 41). The synthesis of hybride peptides containing portions of the amino acid sequences of two peptides with different antibiotic properties has been a way of optimizing these compounds. CAMEL0, or CA(1–7)M(2–9)NH2, is a 15-residue hybride peptide amide with seven amino acids that are derived from the sequence of cecropin A, which comes from the larvae of the silk moth Hyalophora cecropia, and eight amino acids that are derived from the sequence of melittin, which comes from honey bee venom (1, 23, 40). It has been found that CAMEL0 is more active than the native molecules and also lacks the undesirable hemolytic properties of melittin (1). A previous study indicated that the range of antimicrobial activities of CAMEL0 was not restricted to aerobic microorganisms but also included several gram-negative and gram-positive anaerobic microorganisms (11). Various methods have been used to screen for active antimicrobial peptides. Agar-containing media have been shown to give higher inhibitory concentrations compared to those in agarose-based media, probably due to binding of the basic peptides to components of the agar (6). Therefore, a comparison of agarose to agar media was made in this study.
The main aim of the present investigation was to determine the in vitro activities of a set of CAMEL analogues (16 analogues, including CAMEL0) against 60 anaerobic clinical bacterial isolates and to compare the activities to those of piperacillin, cefoxitin, imipenem, ciprofloxacin, clindamycin, metronidazole, and chloramphenicol. Three different methods for susceptibility testing of CAMEL0 against anaerobic bacteria were compared. Previous studies have shown that antimicrobial agents can be inactivated inside the digestive tract under certain conditions (10). Thus, a secondary aim of the present study was to investigate the stability in the fecal milieu of two different enantiomers of temporin B (LLPIVGNLLKSLL), a 13-residue synthetic antimicrobial peptide amide first isolated from the European frog Rana temporaria (36). These experiments were performed in a simulated fecal model.
MATERIALS AND METHODS
Antimicrobial peptides and antimicrobial agents.
The CAMEL analogues were a gift from Philip J. Morgan of Proteus International PLC, Macclesfield, United Kingdom, and the temporins were purchased from Interactiva, Ulm, Germany. The peptides had been synthesized by solid-phase methods by use of 9-fluorenymethoxycarbonyl chemistry and purified by reverse-phase high-performance liquid chromatography (HPLC), and the concentrations of the peptides in aqueous solution were estimated by absorption spectroscopy at neutral pH and a wavelength of 280 nm by using the molar absorption coefficient for tryptophan (ɛ280 = 5.6 × 103 M−1 cm−1) (17). The purities of the CAMEL analogues and the temporins varied between >95 and >99%, as measured by HPLC. The concentrations of the different peptides were also verified by amino acid analysis. The amino acid sequences and the molecular weights of the peptides are shown in Table 1.
TABLE 1.
Amino acid sequences of peptides tested and number of mutations compared with sequence of CAMEL0a
| Peptide label | Amino acid sequenceb | No. of mutationsc | Mol wt |
|---|---|---|---|
| CAMEL0 | KWKLFKKIGAVLKVL | 0 | 1,770.3 |
| CAMEL9 | KWrLFKnIGAVLKVL | 2 | 1,784.2 |
| CAMEL24 | KWKLFKhIGAVLKVL | 1 | 1,779.4 |
| CAMEL42 | hWKLFKKIGAVLKVL | 1 | 1,779.3 |
| CAMEL46 | KWKLFKgIrAVLKVL | 2 | 1,798.4 |
| CAMEL48 | KWKLgKKIlAVLKVL | 2 | 1,736.4 |
| CAMEL48D | KWKLgKKIlAVLKVL | 2 | 1,736.3 |
| CAMEL101 | KWKLgKKIlrVLKVL | 3 | 1,821.4 |
| CAMEL102 | gWKLgKKIlrVLKVL | 4 | 1,750.7 |
| CAMEL108 | KWKLgKKIlnVLKVL | 3 | 1,779.7 |
| CAMEL109 | gWrLgKKIlrVLKVL | 5 | 1,778.7 |
| CAMEL110 | gWKLgKKIlnVLKVL | 4 | 1,708.2 |
| CAMEL123 | lWKLFKKIrrVLrVL | 4 | 1,967.5 |
| CAMEL129 | lWKLFKKInrVLKVL | 3 | 1,897.4 |
| CAMEL135 | gWRLiKKIlrVfKgl | 7 | 1,826.4 |
| CAMEL136 | vWrLiKKIlrVfKgL | 7 | 1,868.4 |
| Temporin B | LLPIVGNLLKSLL | 1,406.7 | |
| Temporin B, D | LLPIVGNLLKSLL | 1,406.7 |
The following antimicrobial agents were obtained from the indicated manufacturers: piperacillin, Lederle Laboratories, Wayne, N.J.; cefoxitin and imipenem, Merck Sharp & Dohme, Rahway, N.J.; ciprofloxacin, Bayer AG, Wuppertal, Germany; clindamycin, Upjohn, Kalamazoo, Mich.; metronidazole, Rhône-Poulenc Rorer Inc., Collegeville, Pa.; and chloramphenicol, Parke-Davis Pharmaceutical Research Division, Warner-Lambert Company, Ann Arbor, Mich.
Bacterial strains.
A total of 60 anaerobic clinical isolates including Peptostreptococcus spp. (10 strains), Propionibacterium spp. (10 strains), C. difficile (10 strains), B. fragilis (10 strains), Prevotella spp. (10 strains), and F. nucleatum (10 strains) collected at the Swedish Institute for Infectious Disease Control, Stockholm, Sweden, were investigated. All strains were identified by biochemical tests and gas-liquid chromatography analysis of metabolic end products by the techniques described by Summanen et al. (38). Three reference strains, Staphylococcus aureus ATCC 2921, B. fragilis NTCC 9343, and Escherichia coli ATCC 25922, were included in each run.
Antibiotic susceptibility tests. (i) Agarose and agar dilution method.
The MICs of the 16 CAMEL peptides were determined by a modified agar dilution method (11). Fresh dilutions of the peptides, prepared in sterile water in twofold serial dilutions, were incorporated into 1% agarose (type I; low electroendosmosis [EEO]; Sigma, St. Louis, Mo.) with tryptic soy broth (TSB; Difco, Detroit, Mich.) to final concentrations of 0.125 to 32 μg/ml. Antimicrobial susceptibility tests with piperacillin, cefoxitin, imipenem, ciprofloxacin, metronidazole, and chloramphenicol were performed by the agar dilution method with PDM-ASM agar (Biodisk, Stockholm, Sweden) and 5% defibrinated horse blood. The antimicrobial agents were suspended and diluted according to the manufacturer's instructions. Antimicrobial concentrations from 0.008 to 128 mg/liter were obtained by incorporation of each substance when preparing the agar plates. A plate without an antimicrobial agent was always included as a growth control. The inocula were prepared by suspending colonies from a 48-h blood agar plate directly into 0.1 M sterile phosphate buffer (PB; pH 7.3) to achieve a density equivalent to that of a 0.5 McFarland standard and were then adjusted to give a final inoculum of 105 CFU per spot applied with a Steers replicator (29). The plates were incubated anaerobically at 37°C for 48 h in GasPak jars (BBL Microbiology Systems, Cockeysville, Md.). The MIC was defined as the lowest concentration of the drug that produced the most significant reduction of growth compared to that of the growth control. The agarose dilution method was compared to the agar dilution method by assaying the antimicrobial activity of cefoxitin against the 60 strains by both methods.
(ii) Broth microdilution test.
A twofold serial dilution of CAMEL0 was prepared in TSB to give a concentration range of 0.06 to 32 μg/ml. To each well of sterile microtiter plates (96-well cell culture cluster; Costar, Cambridge, Mass.), 100-μl portions of CAMEL0 dilutions were added. The inocula were prepared by suspending colonies from a 48-h blood agar plate directly into TSB to achieve a density approximately equivalent to that of a 0.5 McFarland standard. After further dilution in TSB, 100-μl portions were then added to each well, giving a final inoculum of 5 × 104 CFU/well. The National Committee for Clinical Laboratory Standards (29) specifies 106 CFU/ml for broth microdilution tests. The microtiter plates were incubated anaerobically at 37°C for 48 h, and each concentration was run in duplicate. Growth controls without an antimicrobial peptide were included in each run. The MICs were defined as the lowest concentration of the peptide that produced the most significant reduction of growth compared to that of the growth control. The minimum bactericidal concentration (MBC) was determined by subculturing on blood agar plates.
(iii) Inhibition zone assay.
The inhibition zone assay described by Hultmark and colleagues (20, 35) is a commonly used method for determining the antimicrobial activities of synthetic peptides. A volume of 10 μl of bacterial dilution prepared from 48-h cultures was added to 6 ml of sterile agarose in TSB, and the mixture was spread on 9-cm petri dishes (Labora; A/S Heger, Rjukan, Norway), giving an agarose depth of 1 mm. After settling, 3-mm wells were punched in the agarose (eight wells per plate). Three microliters of CAMEL0 diluted to different concentrations in sterile H2O was added to each well in duplicate, giving total amounts of 0.028 to 3.6 μg per well. The plates were incubated anaerobically at 37°C for 48 h. The squared diameter (in centimeters) of the inhibition zone was plotted against the log amount of peptide (in nanomoles), and the lethal concentration (LC) was calculated from the slope (k), the intercept (l), and the agarose depth (a; in centimeters) by using a diffusion equation. The LC is considered to be almost equal to the MIC, as follows: LC = (4 ln 10/π a k 10l/k) = 2.93/(a k 10l/k).
The three different methods (the agarose dilution, broth microdilution, and inhibition zone assays) were all run on two separate occasions under the same conditions. In each run all strains were tested in duplicate.
Stability study.
Portions of 0.4 g of feces collected from a healthy volunteer were diluted in 0.6 ml of PB in sterile plastic tubes in duplicate. Synthetic l and d isomers of temporin B were added to final concentrations of 130 μg/ml, respectively. Controls with only PB and peptide were included. The tubes were incubated anaerobically at 37°C. After 30 min and 24, 48, and 72 h, samples from each tube were collected and frozen at −70°C until they were assayed. The active concentrations of the temporins were analyzed by a microbiological method (modified agar diffusion method) by using agarose plates as the test medium and S. aureus ATCC 29213 as the indicator strain. The plates were incubated aerobically at 37°C for 24 h. The peptide concentrations were calculated by relating the diameters of the inhibition zones to a standard series of known concentrations. This model was also used to determine the stability of vancomycin by using Bacillus subtilis ATCC 6633 as the indicator strain. The detection limits were 16 mg/liter for the l isomer of temporin B, 4.0 mg/liter for the d isomer of temporin B, and 2.0 mg/liter for vancomycin.
RESULTS
Antimicrobial activities.
The antimicrobial activities of the 16 CAMEL analogues and the seven antimicrobial agents against the anaerobic bacterial strains are shown in Table 2. C. difficile and B. fragilis were the species most susceptible to the CAMEL analogues, with the MICs at which 90% of isolates are inhibited (MIC90s) being consistent and ranging from 1 to 4 μg/ml. For only 1 of the 10 B. fragilis strains tested the MIC was >4 μg/ml. The lowest activity was shown against the Peptostreptococcus group, for which 10 of the 16 analogues had MIC90s of ≥8 μg/ml. Among the different peptide analogues, CAMEL24 and CAMEL42 showed the highest overall levels of antimicrobial activities against the 60 anaerobic strains, with overall MIC90s of 2 μg/ml for CAMEL24 and 4 μg/ml for CAMEL42. All strains tested with the exception of three Peptostreptococcus spp. were susceptible to ≤2 μg of CAMEL24 per ml. CAMEL24 and CAMEL42 were the only CAMEL analogues containing the amino acid histidine.
TABLE 2.
Activities of 16 cecropin-melittin peptides and seven other antimicrobial agents against 60 clinical anaerobic strains
| Antimicrobial agent |
Peptostreptococcus spp. (n = 10)
|
Propionibacterium (n = 10)
|
C. difficile(n = 10)
|
B. fragilis(n = 10)
|
Prevotella spp. (n = 10)
|
F. nucleatum(n = 10)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (μg/ml) | MIC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | |
| CAMEL0 | 4 | 4 | 4 | 8 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 8 |
| CAMEL9 | 8 | 8 | 2 | 2 | 2 | 2 | 2 | 2 | 0.25 | 1 | 4 | 4 |
| CAMEL24 | 2 | 4 | 0.5 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| CAMEL42 | 2 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 4 |
| CAMEL46 | 4 | 4 | 2 | 2 | 4 | 4 | 2 | 4 | 4 | 4 | 4 | 4 |
| CAMEL48 | 4 | 32 | 2 | 2 | 1 | 2 | 1 | 2 | 8 | 8 | 4 | 4 |
| CAMEL48D | 4 | 8 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| CAMEL101 | 8 | 8 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 8 | 8 |
| CAMEL102 | 4 | 16 | 8 | 8 | 4 | 4 | 2 | 2 | 2 | 4 | 2 | 2 |
| CAMEL108 | 4 | 32 | 8 | 8 | 2 | 2 | 2 | 2 | 4 | 4 | 2 | 2 |
| CAMEL109 | 8 | 16 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 16 | 4 | 4 |
| CAMEL110 | 16 | 16 | 8 | 8 | 2 | 2 | 2 | 2 | 2 | 4 | 4 | 4 |
| CAMEL123 | 2 | 2 | 2 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 4 |
| CAMEL129 | 8 | 16 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 8 | 16 | 16 |
| CAMEL135 | 4 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 4 | 8 | 8 |
| CAMEL136 | 4 | 4 | 2 | 4 | 4 | 4 | 4 | 4 | 2 | 4 | 16 | 16 |
| Imipenem | 0.032 | 0.032 | 0.032 | 0.032 | 8 | 8 | 0.064 | 0.125 | 0.032 | 0.032 | 0.032 | 0.032 |
| Clindamycin | 0.064 | 0.064 | 0.016 | 0.032 | 4 | 8 | 0.5 | 1 | 0.016 | 0.016 | 0.016 | 0.016 |
| Cefoxitin | 2 | 4 | 0.125 | 0.125 | 64 | 64 | 4 | 16 | 0.5 | 1 | 0.5 | 0.5 |
| Ciprofloxacin | 0.5 | 1 | 0.5 | 0.5 | 8 | 16 | 2 | 8 | 4 | 16 | 2 | 2 |
| Metronidazole | 2 | 4 | >64 | >64 | 0.125 | 0.125 | 0.5 | 1 | 1 | 1 | 4 | 4 |
| Piperacillin | 0.125 | 0.25 | 0.25 | 0.25 | 0.5 | 4 | 4 | 16 | 1 | 4 | 0.064 | 0.064 |
| Chloramphenicol | 4 | 4 | 1 | 1 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
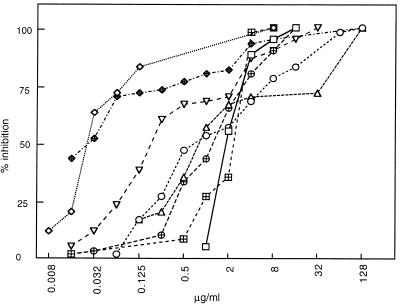
The overall activities (MIC90s) of the different agents against all 60 anaerobic strains are shown in Fig. 1. The activity of CAMEL0 was mainly inferior to those of imipenem, clindamycin, and piperacillin but was equal to or superior to those of metronidazole, cefoxitin, ciprofloxacin, and chloramphenicol.
FIG. 1.
In vitro activities of CAMEL0 and seven other antimicrobial agents against 60 anaerobic bacterial strains: Peptostreptococcus spp. (n = 10), Propionibacterium acnes (n = 10), C. difficile (n = 10), B. fragilis (n = 10), Prevotella spp. (n = 10), and F. nucleatum (n = 10). □, CAMEL0; ◊, imipenem; ○, cefoxitin; ▵, metronidazole; ⊞, chloramphenicol;  , clindamycin ⊕, ciprofloxacin; ▿, piperacillin.
, clindamycin ⊕, ciprofloxacin; ▿, piperacillin.
Comparison of antimicrobial susceptibility tests.
The agarose dilution method was found to be an accurate method after running each of the three methods in two independent experiments performed in duplicate, yielding identical results (Table 3). The agarose dilution method often resulted in MICs 1 to 2 dilution steps higher than those obtained by the broth microdilution method and the inhibition zone assay. The use of agarose compared to the use of PDM-ASM agar for assaying the antimicrobial activity of cefoxitin against the 60 anaerobic strains yielded almost identical results. The MICs were all within 1 dilution step (data not shown).
TABLE 3.
Comparison of the antibacterial activity of CAMEL0 assayed by agarose dilution method, broth microdilution method, or inhibition zone assay
| Strain and parameter | MIC (μg/ml) by agarose dilution method | Broth microdilution method
|
LC (μg/ml) by inhibition zone assay | |
|---|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | |||
| Peptostreptococcus spp. (n = 10) | ||||
| Range | 2–16 | 0.5–8 | 1–16 | 0.59–1.36 |
| 50% | 4 | 4 | 4 | 0.71 |
| 90% | 4 | 8 | 8 | 1.25 |
| P. acnes(n = 10) | ||||
| Range | 4–8 | 0.5–8 | 1–8 | 0.38–0.55 |
| 50% | 4 | 2 | 4 | 0.47 |
| 90% | 8 | 4 | 8 | 0.54 |
| C. difficile(n = 10) | ||||
| Range | 2–2 | 0.5–4 | 1–8 | 0.53–0.82 |
| 50% | 2 | 1 | 2 | 0.57 |
| 90% | 2 | 4 | 2 | 0.66 |
| B. fragilis(n = 10) | ||||
| Range | 2–2 | 0.5–2 | 1–4 | 0.62–1.05 |
| 50% | 2 | 0.5 | 1 | 0.75 |
| 90% | 2 | 1 | 2 | 0.90 |
| Prevotella spp. (n = 10) | ||||
| Range | 1–8 | 0.125–8 | 0.25–16 | 0.42–0.64 |
| 50% | 2 | 1 | 2 | 0.53 |
| 90% | 2 | 2 | 4 | 0.64 |
| F. nucleatum(n = 10) | ||||
| Range | 2–16 | 1–2 | 2–4 | 0.38–0.91 |
| 50% | 4 | 1 | 2 | 0.65 |
| 90% | 8 | 2 | 4 | 0.71 |
| Reference strains | ||||
| S. aureus ATCC 2921 | 8 | 8 | 16 | 2.7 |
| B. fragilis NTCC 9343 | 2 | 2 | 4 | 0.93 |
| E. coli ATCC 25922 | 4 | 4 | 8 | 1.16 |
Stability study.
The stability study showed that the l form of temporin B quickly lost its antimicrobial activity in the fecal milieu. The antimicrobial activity of the d form of temporin B was detected after 30 min (20 μg/ml), but no activity was detected after 24, 48, and 72 h in the fecal milieu. In the control samples, which contained PB, the original antimicrobial activity of 130 μg/ml was detected at all time points for both isomers. When assaying the stability of vancomycin with the fecal model, an original concentration of ≥32 μg of vancomycin per ml yielded activities after 0.5, 24, 48, and 72 h of incubation that corresponded to concentrations in feces of 3.2, 2.0, 3.4, and 3.4 μg/ml, respectively, while an original concentration of ≤16 μg of vancomycin per ml yielded no detectable activity after 30 min.
DISCUSSION
Anaerobic bacteria are an important class of human pathogens (14, 33). Hybrid peptides have received increasing attention recently because of their antimicrobial effects in vitro. In previous studies antimicrobial peptides have mainly been tested against aerobic microorganisms. In a study by Mee et al. (23), a set of CAMEL analogues was assayed against 24 aerobic bacterial strains, resulting in a range of MICs of 2 to 100 μg/ml, and the MIC of CAMEL0 was 4 μg/ml. The present study demonstrates that several antimicrobial peptide analogues of CAMEL0 also exhibit good in vitro activities against a wide range of gram-positive and gram-negative anaerobic microorganisms, which is in accordance with previous results for the parent substance, CAMEL0 (11). The inhibitory effect of CAMEL0 seems to be similar for all 60 strains when a certain concentration is reached. The importance of the amino acid histidine in the two most active peptides, CAMEL24 and CAMEL42, remains to be clarified. The narrow range of activity of the CAMEL analogues may be due to the mechanism of action of the peptides, involving disruption of the plasma membrane. As shown in Fig. 1, the curve is remarkably steep compared to those for the conventional antibiotics; ≥50% of the strains were inhibited by 2 μg/ml, and ≥90% of the strains were inhibited by 8 μg/ml. This implies that CAMEL0 has a wide range of activity against anaerobic organisms.
The most appropriate method for assaying the antimicrobial activities of peptides has been discussed previously (5). The results of the present study show that the agarose dilution method is an accurate method for susceptibility testing of peptides with activities against anaerobic species. A disadvantage of this method is that relatively large amounts of the antimicrobial agent are needed, and it is therefore preferable that several strains be assayed simultaneously. In contrast, the inhibition zone assay requires only minimal amounts of peptide and would be the method of choice when screening the activities of large numbers of antimicrobial peptides against a few strains. Somewhat higher MICs were obtained by the agarose dilution method compared to those obtained by the broth microdilution method and the inhibition zone assay, which is in accordance with the situation when the conventional agar dilution method is compared to the broth microdilution method (39). High-quality agarose is the recommended medium for peptide assays (5, 6). The agarose dilution method was further validated by assaying the antimicrobial activity of cefoxitin and comparing the results obtained by this method with those obtained by the conventional agar dilution method. The results indicate that agarose is a reliable test medium for assaying antimicrobial activity.
The clinical applications of the CAMEL analogues are hard to interpret due to the lack of pharmacokinetic studies with antimicrobial peptides. In vivo experimental trials with synthetic antimicrobial peptides describe intravenous injection (3, 9, 16, 28) and topical application (30, 37). Oral administration of peptides in general is limited by the low level of bioavailability due to degradation by enzymes in the gastrointestinal tract and a lack of permeation through the intestinal mucosal membranes (15). Research is ongoing to overcome delivery problems for peptide drugs (12, 32). Treatment of C. difficile-associated diarrhea with orally administered, poorly absorbed antimicrobial agents like vancomycin may be considered topical or local treatment. There is an urgent need for new treatment strategies for recurrent C. difficile infections. The antimicrobial activities of the CAMEL0 hybrids against C. difficile seems promising, although their stability in feces may be crucial. The stability study with the fecal model showed that the activity of the d form of temporin B lasted longer than the activity of the l form in a fecal milieu. Previous studies have shown that the d isomers are not only more resistant to hydrolysis by enzymes but are also more active than the l isomers (24, 27). This is also in accordance with the higher level of activity of d-CAMEL48 compared to that of l-CAMEL48 against the 60 anaerobic bacterial strains recorded in the present study (Table 2).
Earlier studies on the activities of antimicrobial peptides against anaerobic bacteria have focused on the activities of protegrins, isolated from porcine leukocytes, against bacteria associated with periodontitis, including Porphyromonas gingivalis, Prevotella intermedia, F. nucleatum, Actinobacillus actinomycetemcomitans, and Capnocytophaga spp. (26, 27). Those results as well as the results of the present study indicate a possible use of peptides for the topical treatment of periodontitis, since topical delivery of antimicrobial peptides diminishes the degradation problems associated with peptide drugs.
The results on peptide stability presented here, together with those obtained by Merrifield et al. (24) and Miyasaki et al. (27) suggest that the d isomers of peptides are more stable than the l isomers, which is encouraging for further studies on the in vivo activities of antimicrobial peptides with d configurations. In conclusion, the results of the present study show that CAMEL analogues have good in vitro activities against anaerobic bacteria. These and other antimicrobial peptides merit further investigation, especially investigations on their bioavailabilities, toxicities, and stabilities, as treatments for bacterial infections.
REFERENCES
- 1.Andreu D, Ubach J, Wade D, Wåhlin B, Merrifield R B, Boman H G. Shortened cecropin A-melittin hybrids: significant size reduction retains potent antibiotic activity. FEBS Lett. 1992;296:190–194. doi: 10.1016/0014-5793(92)80377-s. [DOI] [PubMed] [Google Scholar]
- 2.Baghian A, Jaynes J, Enright F, Kousolas K G. An amphipathic alpha-helical synthetic peptide analogue of melittin inhibits herpes simplex virus-1 (HSV-1)-induced cell fusion and virus spread. Peptides. 1997;18:177–183. doi: 10.1016/s0196-9781(96)00290-2. [DOI] [PubMed] [Google Scholar]
- 3.Barr S C, Rose D, Jaynes J M. Activity of lytic peptides against intracellular Trypanosoma cruzi amastigotes in vitro and parasitemias in mice. J Parasitol. 1995;81:974–978. [PubMed] [Google Scholar]
- 4.Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 5.Boman H G. Antimicrobial peptides. Chairman opening remarks. Ciba Found Symp. 1994;186:1–4. [PubMed] [Google Scholar]
- 6.Boman H G, Wade D, Boman I A, Wåhlin B, Merrifield R B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989;259:103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- 7.Boman H G. Gene encoded peptide antibiotics and the concept of innate immunity. An updated review. Scand J Immunol. 1998;48:15–25. doi: 10.1046/j.1365-3083.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 8.Coote P J, Holyoak C D, Bracey D, Ferdinando D P, Pearce J A. Inhibitory action of a truncated derivative of the amphibian skin peptide dermaseptin s3 on Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1998;42:2160–2170. doi: 10.1128/aac.42.9.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darveau R P, Cunningham M D, Seachord C L, Cassiano-Clough L, Cosand W L, Blake J, Watkins C S. Beta-lactam antibiotics potentiate magainin 2 antimicrobial activity in vitro and in vivo. Antimicrob Agents Chemother. 1991;35:1153–1159. doi: 10.1128/aac.35.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlund C. Non-specific inactivation of antimicrobial agents. Old Herborn Univ Seminar Monogr. 1993;3:55–61. [Google Scholar]
- 11.Edlund C, Hedberg M, Engström Å, Flock J I, Wade D. Antianaerobic activity of a cecropin-melittin peptide. Clin Microbiol Infect. 1998;4:181–185. doi: 10.1111/j.1469-0691.1998.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 12.Fasano A. Innovative strategies for the oral delivery of drugs and peptides. Trends Biotechnol. 1998;16:152–157. doi: 10.1016/s0167-7799(97)01170-0. [DOI] [PubMed] [Google Scholar]
- 13.Fekety R, Shah B A. Diagnosis and treatment of Clostridium difficile colitis. JAMA. 1993;269:71–75. [PubMed] [Google Scholar]
- 14.Finegold S. Anaerobic infections in humans: an overview. Anaerobe. 1995;1:3–9. doi: 10.1016/s1075-9964(95)80340-8. [DOI] [PubMed] [Google Scholar]
- 15.Fricker G, Drewe J. Current concepts in intestinal peptide absorption. J Pept Sci. 1996;2:195–211. doi: 10.1002/psc.66. [DOI] [PubMed] [Google Scholar]
- 16.Gough M, Hancock R E, Kelly N M. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun. 1996;64:4922–4927. doi: 10.1128/iai.64.12.4922-4927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratzer W B. In: CRC practical handbook of biochemistry and molecular Biology. Fasman G D, editor. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 8–18. [Google Scholar]
- 18.Hancock R E W. Peptide antibiotics. Lancet. 1997;349:419–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 19.Hedberg M, Nord C E. Antimicrobial-resistant anaerobic bacteria in human infections Med. Microbiol Lett. 1996;5:295–304. [Google Scholar]
- 20.Hultmark D, Engström Å, Andersson K, Steiner H, Bennich H, Boman H G. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 1983;2:571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaitre B, Reichart J M, Hoffmann J A. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuzaki K. Molecular action mechanisms and membrane recognition of membrane-acting antimicrobial peptides. J Pharm Soc Jpn. 1997;117:253–264. doi: 10.1248/yakushi1947.117.5_253. [DOI] [PubMed] [Google Scholar]
- 23.Mee R P, Auton T R, Morgan P J. Design of active analogues of a 15-residue peptide using d-optimal design, QSAR and a combinatorial search algorithm. J Pept Res. 1997;49:89–102. doi: 10.1111/j.1399-3011.1997.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 24.Merrifield E L, Mitchell S A, Ubach J, Boman H G, Andreu D, Merrifield R B. d-enantiomers of 15-residue cecropin A-melittin hybrids. Int J Pept Prot Res. 1995;46:214–220. doi: 10.1111/j.1399-3011.1995.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 25.Merrifield R B, Juvvadi P, Andreu D, Ubach J, Boman A, Boman H G. Retro and retroenantioanalogs of cecropin-melittin hybrids. Proc Natl Acad Sci USA. 1995;92:3449–3453. doi: 10.1073/pnas.92.8.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyasaki K T, Lehrer R I. Beta-sheet antibiotic peptides as potential dental therapeutics. Int J Antimicrob Agents. 1998;9:269–280. doi: 10.1016/s0924-8579(98)00006-5. [DOI] [PubMed] [Google Scholar]
- 27.Miyasaki K T, Iofel R, Oren A, Huyhn T, Lehrer R. Killing of Fusobacterium nucleatum, Porphyromonas gingivalis and Prevotella intermedia by protegrins. J Periodont Res. 1998;33:91–98. doi: 10.1111/j.1600-0765.1998.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima Y, Alvares-Bravo J, Cho J, Homma K, Kanegasaki S, Natori S. Chemotherapeutic activity of synthetic antimicrobial peptides: correlation between chemotherapeutic activity and neutrophil activating activity. FEBS Lett. 1997;415:64–66. doi: 10.1016/s0014-5793(97)01101-0. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Sixth information supplement M100-s7. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 30.Nos-Barbera S, Portoles M, Morilla A, Ubach J, Andreu D, Paterson C A. Effect of hybrid peptides of cecropin A and melittin in an experimental model of bacterial keratitis. Cornea. 1997;16:101–106. [PubMed] [Google Scholar]
- 31.Oren Z, Shai Y. Selective lysis of bacteria but not mammalian cells by diastomeres of melittin: structure-function study. Biochemistry. 1997;36:1826–1835. doi: 10.1021/bi962507l. [DOI] [PubMed] [Google Scholar]
- 32.Pettit D K, Gombotz W R. The development of site-specific drug-delivery systems for protein and peptide biopharmaceuticals. Trends Biotechnol. 1998;16:343–349. doi: 10.1016/s0167-7799(98)01186-x. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen B A, Bush K, Tally F P. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997;24:110–120. doi: 10.1093/clinids/24.supplement_1.s110. [DOI] [PubMed] [Google Scholar]
- 34.Robinson W E, Jr, McDougall B, Tran D, Selstedt M E. Anti-HIV-1 activity of indocilin, an antimicrobial peptide from neutrophils. J Leukoc Biol. 1998;63:91–100. doi: 10.1002/jlb.63.1.94. [DOI] [PubMed] [Google Scholar]
- 35.Samakovlis C A, Kimbrell D, Kylsten P, Engström Å, Hultmark D. The immune response in Drosophila: pattern of cecropin expression and biological activity. EMBO J. 1990;9:2969–2976. doi: 10.1002/j.1460-2075.1990.tb07489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmaco M, Mignogna G, Canofeni S, Miele R, Mangoni, L. M, Barra D. Temporins, antimicrobial peptides from the European red frog Rana temporaria. Eur J Biochem. 1996;242:788–792. doi: 10.1111/j.1432-1033.1996.0788r.x. [DOI] [PubMed] [Google Scholar]
- 37.Soballe P W, Maloy W L, Myrga M L, Jacob L S, Herlyn M. Experimental local therapy of human melanoma with lytic magainin peptides. Int J Cancer. 1995;60:280–284. doi: 10.1002/ijc.2910600225. [DOI] [PubMed] [Google Scholar]
- 38.Summanen P. Susceptibility testing of anaerobic bacteria. In: Baron E J, Citron D M, Strong C A, Wexler H M P, Finegold S M, editors. Wadsworth anaerobic bacteriology manual. 5th ed. Belmont, Calif: Star Publishing Company; 1993. pp. 111–127. [Google Scholar]
- 39.Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, Holle R, Salmons B, Erfle V, Brack-Wernwe R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol. 1998;79:731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- 40.Wade D, Andreu D, Mitchell S A, Silveria A M, Boman A, Boman H G, Merrifield R B. Antibacterial peptides designed as analogs on hybrids of cecropins and melittin. Int J Pept Prot Res. 1992;40:429–436. doi: 10.1111/j.1399-3011.1992.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 41.Wade D, Boman A, Wåhlin B, Drain C M, Andreu D, Boman H G, Merrifield R B. All d-amino acid containing channel-forming antibiotic peptides. Proc Natl Acad Sci USA. 1990;87:4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wakabayashi H, Abe S, Teraguchi S, Hayasawa H, Yamaguchi H. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin-related compounds. Antimicrob Agents Chemother. 1998;42:1587–1591. doi: 10.1128/aac.42.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winder D, Gunzburg W H, Erfle W, Salmons B. Expression of antimicrobial peptides has an antitumour effect in human cells. Biochem Biophys Res Commun. 1998;242:608–612. doi: 10.1006/bbrc.1997.8014. [DOI] [PubMed] [Google Scholar]