Abstract
Background and Objectives
Leisure activity engagement (LAE) may reduce the risk of incident dementia. However, cognitive performance may predict LAE change. We evaluated the temporal ordering of overall and subtypes of LAE (intellectual, physical, and social) and cognitive performance (global, language, memory, and visuospatial function) among non-demented older adults.
Research Design and Methods
The Washington Heights–Inwood Columbia Aging Project concurrently administered a survey measure of 13 leisure activities and a neuropsychological battery every 18–24 months for up to 14 years to 5,384 racially and ethnically diverse participants. We used parallel process conditional latent growth curve models to examine temporal ordering in the overall sample and within baseline diagnostic groups (mild cognitive impairment [MCI] vs. cognitively normal).
Results
Levels and changes of overall and subtypes of LAE were positively correlated with cognitive performance in the overall sample and within each diagnostic group. In the overall sample, higher initial memory was associated with slower declines in social LAE (estimate = 0.019, 95% confidence interval [95% CI]: 0.001–0.037). Among MCI, higher initial physical LAE was associated with slower declines in memory (estimate = 0.034, 95% CI: 0.001–0.067), but higher initial intellectual LAE was related to steeper declines in visuospatial function (estimate = −0.028, 95% CI: −0.052 to −0.004). Among cognitively normal, higher initial memory was associated with slower declines in intellectual LAE (estimate = 0.012, 95% CI: 0.002−0.022).
Discussion and Implications
Dynamic interplay of LAE with cognitive performance was observed across diagnostic groups. Levels of LAE subtypes could be more predictive of change in certain cognitive domains within older adults with MCI.
Keywords: Cognitive aging, Intellectual activity engagement, Mild cognitive impairment, Physical activity engagement, Social activity engagement
Higher levels of educational attainment, employment in mentally stimulating jobs, and leisure activity engagement (LAE) may delay or prevent Alzheimer’s disease (AD) (Alzheimer’s Association, 2017). The protective effects of educational attainment, occupation, and LAE may reflect increased cognitive reserve, a hypothetical construct that prevents clinical decline in the face of pathological injury (Stern, 2012). LAE and its subtypes, that is, intellectual, social, and physical, are potentially modifiable in later life. A systematic review of 12 longitudinal studies concluded that late-life cognitive activity may delay or prevent AD and related dementias, with limited consideration of reverse causation as a possible or partial explanatory factor (Sajeev et al., 2016). Cognitive performance may affect the willingness and/or ability to engage in leisure activities such that cognitive changes may precede subsequent patterns of activity engagement in older adults (Gow et al., 2012; Wilson et al., 2007, 2012). Better characterization of the temporal relationship between LAE and cognition would inform the implementation and precision of preclinical AD interventions as well as improve our understanding of the aging mind and brain.
Among community-dwelling older adults, greater LAE has been associated with lower AD incidence (Hebert et al., 2013) and dementia incidence, albeit in a short timeframe (Scarmeas et al., 2001; Sommerlad et al., 2020). Most studies consider the relationship between LAE at one timepoint and cognitive change or diagnostic status over time, generally demonstrating the positive relationship noted above (Helzner et al., 2007; Sajeev et al., 2016; Scarmeas et al., 2001), but these studies did not examine if initial cognitive performance predicted LAE changes.
Other studies examined the interplay of subtypes of LAE, that is, intellectual and social LAE (Bielak et al., 2012) and physical LAE (Bielak, Gerstorf et al., 2014), and cognitive performance longitudinally. Greater average LAE was associated with higher baseline cognitive performance, not with change across adulthood (Bielak et al., 2012; Bielak, Cherbuin et al., 2014). Significant correlations between changes in intellectual LAE and perceptual speed as well as changes in social LAE and immediate episodic memory, but not between baseline LAE and cognitive change, were reported in another study (Bielak, Gerstorf et al., 2014). Similarly, across the Integrative Analysis of Longitudinal Studies on Aging (IALSA), changes in intellectual (Mitchell et al., 2012) and physical (Lindwall et al., 2012) LAE were correlated with cognitive change, but baseline LAE was not associated with cognitive change.
Results for correlations between change in social LAE and cognitive performance are mixed among studies. Social LAE change was associated with the change in memory performance in two IALSA studies (Brown et al., 2012). Meanwhile, findings from another study showed that there were correlations of social LAE change with change in semantic decision making, episodic memory, and semantic memory and that initial cognitive performance predicted social LAE changes (Small et al., 2012).
Two possible explanations for these associations include differential preservation or preserved differentiation (Salthouse et al., 1990). Differential preservation suggests that LAE changes subsequent trajectories of age-related cognitive performance, so those with more LAE would have slower rates of cognitive decline as they age (Helzner et al., 2007; Sajeev et al., 2016; Scarmeas et al., 2001). Preserved differentiation signifies that a higher level of cognitive performance is correlated with more LAE, so that this association does not affect cognitive change. Evidence for preserved differentiation has been supported previously, such that adults with greater LAE have higher initial cognitive ability (Bielak et al., 2012; Bielak, Cherbuin et al., 2014).
To study both potential explanations, we tested the temporal ordering of the concurrent longitudinal relationship between LAE and cognition in a diverse prospective cohort of older adults. We evaluated the association of initial level and change over time in overall LAE with level and subsequent change in cognition, and the converse. We hypothesized that preserved differentiation would be supported, such that we expect baseline correlations and slope correlations, but not associations between the initial level of one and subsequent change in the other. As exploratory analyses, we used LAE subtypes to determine whether a specific subtype was a driver in main associations. Lastly, as a secondary analysis, we examined these relationships stratified by baseline diagnostic status (mild cognitive impairment [MCI] vs. cognitively normal). We hypothesized that patterns of correlations differed by diagnostic status in that those with baseline MCI may not engage in a variety of activities if cognitive performance affected engagement.
Method
Participants
Washington Heights–Inwood Columbia Aging Project (WHICAP) is a prospective, community-based study of aging and dementia in a racially and ethnically diverse sample of Medicare-eligible residents of Northern Manhattan (Manly et al., 2005; Tang et al., 2001). Participants were recruited in three waves (1992/1999/2009). Ongoing follow-up in-person visits occurred at 18- to 24-month intervals and include a battery of cognitive, functional, and health measures administered in the participant’s preferred language (English or Spanish). Acceptable measurement invariance of the neuropsychological battery across English and Spanish speakers and across race/ethnicity has been demonstrated (Avila et al., 2020; Siedlecki et al., 2010).
The current sample included 5,384 dementia-free participants at study initiation. Dementia was determined by a consensus of a group of neurologists and neuropsychologists who reviewed neuropsychological, medical, and functional interviews to adjudicate a diagnosis based on standard research criteria (Manly et al., 2008; McKhann et al., 1984). We included individuals with baseline MCI, assigned as previously described (Manly et al., 2008). Briefly, MCI was defined as having (a) memory complaint, (b) impairment in one cognitive domain, (c) preserved activities of daily living, and (d) no dementia diagnosis. Those missing baseline diagnostic status (n = 207) were excluded from the stratified analyses.
Leisure Activities
A survey measure including participation in 13 leisure activities during the preceding month was collected at each visit. These activities were grouped into four categories based on previously published factor analysis (Scarmeas et al., 2001): (a) intellectual—three activities: reading magazines, newspapers, or books; going to classes; and playing cards, games, or bingo; (b) social—six activities: doing unpaid volunteer work; going to a club or center; going to movies, restaurants, or sporting events; attending church, synagogue, or temple; visiting friends or relatives; and being visited by friends or relatives; (c) physical—two activities: physical conditioning and walking for pleasure or excursion; and (d) other—two activities: knitting, music, or other hobby and watching television or listening to the radio. One point was given for participation in each activity, and an aggregate score (range, 0–13) was assigned to each participant.
In exploratory analyses, we examined three domains of leisure activities: intellectual, physical, and social. Other category was excluded, because it would be difficult to qualify what level and change in the other category meant as a function of cognition level and change. LAE scores were standardized to baseline mean and SD.
Neuropsychological Measures
At each visit, participants were administered a comprehensive neuropsychological battery evaluating the cognitive domains of memory, language, and visuospatial functioning (Stern et al., 1992). All interview questions, test instructions, and stimuli were translated into Spanish by a committee of Spanish speakers and then backtranslated to ensure comparability of the English and Spanish measures. Composite cognitive domain scores were derived for each domain based on a previously published factor structure (Siedlecki et al., 2010). Memory composite scores include immediate, delayed, and recognition trials from the Selective Reminding Test (Buschke & Fuld, 1974). Language scores include measures of naming, letter and category fluency, verbal abstract reasoning, repetition, and comprehension (Kaplan et al., 1983; Wechsler, 1981). Visuospatial scores include recognition and matching trials from the Benton Visual Retention Test (Benton, 1955), the Rosen Drawing Test (Rosen, 1981), and the Identities and Oddities subtest of the Dementia Rating Scale (Mattis, 1976).
All scores were standardized to z-scores using the larger WHICAP sample’s means and SDs at their initial visit and averaged within domains and across tests for global cognitive performance (GCP). Higher composite scores indicated better cognitive performance.
Adjustment Covariates
Baseline adjustment covariates included age, sex, years of education, monthly income, race (African American vs. non-African American), ethnicity (Hispanic vs. non-Hispanic), occupation, WHICAP recruitment cohort (1992/1999/2009), chronic disease burden, and depressive symptoms. Monthly income was defined as more than $1,000 vs. $1,000 or less, based on the median split of the sample. Race and ethnicity, mutually exclusive categories, were self-reported using the format of the 2000 U.S. Census. Lifetime occupation was defined as three categories: high (professional/technical and business/government manager), low (unskilled/semiskilled worker, skilled trade/craft, clerical/office worker), and homemaker (Scarmeas et al., 2001). Chronic disease burden was a sum score of the following: hypertension, diabetes, myocardial infarction, stroke, arthritis, chronic obstructive pulmonary disease, thyroid disease, liver disease, renal disease, ulcer, peripheral vascular disease, cancer, and essential tremors (Zahodne et al., 2019). Depressive symptoms were defined as the sum of depressive symptoms from the 10-item Center for Epidemiologic Studies—Depression scale, ranging from 0 to 10 (Irwin et al., 1999).
Statistical Analyses
Means and standard deviations for continuous variables and numbers and percentages for categorical variables were reported. Two-sample t tests for continuous variables and chi-squared tests for categorical variables were conducted to determine differences in baseline diagnostic status of the sample. Mplus 8.0 (Muthén & Muthén, 1998–2017) and Stata 15.0 (StataCorp, 2017) were used for the analyses. We did not use multiple comparisons adjustment as the secondary analyses were performed as exploratory analyses (Streiner & Norman, 2011). Significance was determined by the 95% confidence interval not overlapping 0.
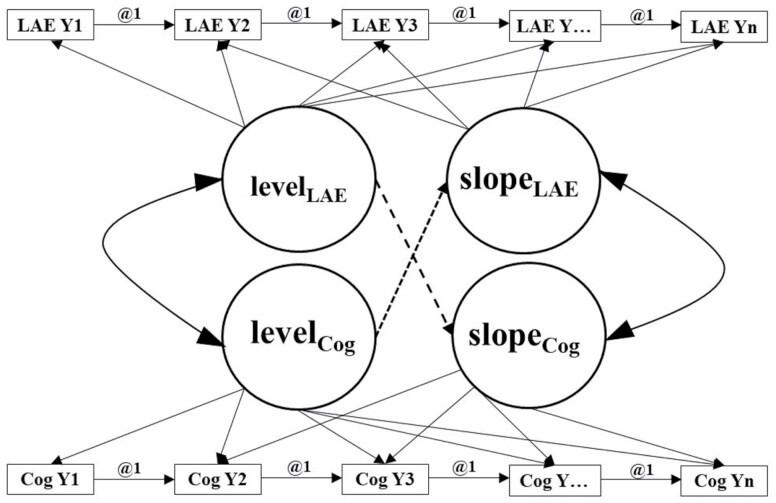
Figure 1 illustrates the parallel process latent growth curve model used in the analyses. These analyses enabled us to test hypotheses corresponding to (a) concurrent relationship between initial cognitive performance and initial LAE, (b) initial cognitive performance predicting LAE change, (c) initial LAE predicting cognitive change, and (d) a dynamic relationship between slopes of cognition and LAE. Concurrent relationships were defined by correlated initial levels. Dynamic relationships were defined by correlated slopes. Directional relationships were defined by regressions involving the initial level of one construct and the slope of the other. The timescale was years in the study since baseline. Due to the uneven spacing of the time intervals, we used time scores for the seven visits, which were computed as years between visits and differed across participants. Using time scores accounts for the uneven spacing of time intervals by treating the individually varying times of observation as data, not fixed parameters, and providing flexibility of individually varying times of observation within a single-level model in a wide format. Follow-up was truncated at seven visits to maximize covariance coverage, as there were few participants with more than seven visits. Right censoring occurred if dementia, death, or end of study occurred. Associations of predictors of cognitive change with the level of and change in cognitive performance are not sensitive to practice effects (Vivot et al., 2016), so we did not adjust by these.
Figure 1.
Framework for the unconditional parallel process latent growth curve model. Notes: LAE = leisure activity engagement; Cog = cognitive performance; i = intercept; s = slope; Y = year. This figure represents an unconditional parallel process latent growth curve model that can test four hypotheses simultaneously. These four hypotheses are (a) concurrent relationship between initial cognitive performance and LAE, (b) initial cognitive performance predicting rates of change in LAE over the follow-up period, (c) initial LAE predicting rates of change in cognitive performance over the follow-up period, and (d) a dynamic, bidirectional relationship between cognitive performance and LAE over the follow-up period. The boxes indicate the assessments for both cognitive performance and LAE at each year from Year 1 to Year 7. The circles are the latent variables representing either LAE or cognitive performance at the initial level and slope or rate of change. The hypotheses correspond to the latent variables (circles) that are in the middle of the figure.
To build the model, we first examined the growth processes for each cognitive outcome (GCP, memory, language, and visuospatial functioning) and LAE separately. We characterized these outcomes with latent variables corresponding to an initial intercept, a linear slope or rate of decline, and quadratic growth (Curran & Hussong, 2009; McArdle & Bell, 2000; Muthén, 1997; Muthén & Curran, 1997; Stull, 2008). We used goodness of fit statistics, that is, Akaike’s Information Criteria, Bayesian Information Criteria, Root Mean Square Error of Approximation, Comparative Fit Index, Tucker–Lewis Index, and Standardized Root Mean Square Residual, to determine the best shape for each latent growth curve model. Then, we estimated unconditional parallel process latent growth curve models in which levels and rates of change of both LAE and cognition were allowed to correlate, and each rate of change was regressed onto each level. Lastly, we added adjustment covariates to the model in a backward elimination procedure. The maximum likelihood estimator uses all available data to estimate parameters. Missingness was handled using full information maximum likelihood under the missingness at random assumption.
We conducted additional analyses. As an exploratory analysis, we examined the associations of the LAE subtypes with cognitive performance to determine whether any LAE component drove the pattern of main findings between overall LAE and cognition. As a secondary analysis, we stratified the analyses by initial diagnostic status, because inclusion of this covariate could have induced regression to the mean bias in the main analysis. We also examined the associations of LAE subtypes with cognitive performance by initial diagnostic status.
Results
Characteristics of Study Sample
Table 1 displays the baseline sample characteristics. The mean age of the overall sample was 75.8 ± 6.5 years, 67.1% (n = 3,610) were female, 30.3% (n = 1,632) were African American, and 43.0% (n = 2,313) were Hispanic. The average follow-up time was 4.4 ± 4.2 years. Approximately 22.0% (n = 1,186) had baseline MCI. Compared to cognitively normal participants, on average, participants with MCI were older, had fewer years of education, were female, reported fewer leisure activities, and were more likely to be Hispanic.
Table 1.
Baseline Characteristics of Study Sample (N = 5,384)
| Overall | Baseline MCI | Cognitively normal | |
|---|---|---|---|
| Baseline characteristics | N = 5,384 | n = 1,186a | n = 3,991a |
| Age, mean (SD) | 75.8 (6.5) | 76.6 (6.4) | 75.5 (6.4) |
| Education, in years, mean (SD) | 10.3 (5.0) | 9.2 (5.0) | 10.6 (4.9) |
| Female, n (%) | 3,610 (67.1) | 808 (68.1) | 2,677 (67.1) |
| Enrollment year, n (%) | |||
| 1992 | 1,427 (26.5) | 379 (32.0) | 948 (23.8) |
| 1999 | 1,955 (36.3) | 426 (35.9) | 1,485 (37.2) |
| 2009 | 2,002 (37.2) | 381 (32.1) | 1,558 (39.0) |
| African American, n (%) | 1,632 (30.3) | 338 (28.5) | 1,249 (31.3) |
| Hispanic, n (%) | 2,313 (43.0) | 573 (48.3) | 1,690 (42.4) |
| Occupation, n (%) | |||
| Low | 1,094 (20.3) | 175 (14.8) | 874 (21.9) |
| High | 3,759 (69.8) | 899 (75.8) | 2,731 (68.4) |
| Homemaker | 314 (5.8) | 79 (6.7) | 226 (5.7) |
| Chronic disease sum score, mean (SD) | 2.1 (1.6) | 2.2 (1.6) | 2.1 (1.6) |
| 10-item CES-D score, mean (SD) | 1.8 (2.1) | 2.2 (2.2) | 1.7 (2.0) |
| Monthly income greater than $1,000, n (%) | 2,631 (48.9) | 714 (60.2) | 1,828 (45.8) |
| Overall number of leisure activities, mean (SD) | 7.0 (2.5) | 6.4 (2.4) | 7.2 (2.5) |
| Intellectual, mean (SD) | 1.3 (0.8) | 1.1 (0.7) | 1.3 (0.8) |
| Social, mean (SD) | 3.1 (1.5) | 2.8 (1.4) | 3.2 (1.5) |
| Physical, mean (SD) | 1.2 (0.8) | 1.1 (0.8) | 1.2 (1.8) |
| Overall deaths, n (%) | 2,043 (38.0) | 510 (43.0) | 1,432 (35.9) |
| Age at death, mean (SD) | 85.4 (7.5) | 85.9 (7.4) | 85.2 (7.5) |
| Global cognitive performance, in SD, mean (SD) | 0.28 (0.57) | −0.11 (0.46) | 0.39 (0.54) |
| Memory performance, in SD, mean (SD) | 0.28 (0.70) | −0.14 (0.66) | 0.41 (0.67) |
| Language performance, in SD, mean (SD) | 0.26 (0.67) | −0.16 (0.58) | 0.38 (0.64) |
| Visuospatial performance, in SD, mean (SD) | 0.29 (0.61) | −0.06 (0.64) | 0.39 (0.57) |
| Baseline amnestic MCI, n (%) | — | 248 (21.9) | — |
| Incident dementia, n (%) | 724 (13.4) | 245 (20.7) | 442 (11.1) |
| Time to incident dementia, mean (SD) | 5.8 (4.6) | 5.6 (4.7) | 6.2 (4.6) |
| Follow-up time, in years, mean (SD) | 4.4 (4.2) | 4.2 (4.1) | 4.4 (4.3) |
| Follow-up time, in years, among those with ≥2 visits (n = 3,864), mean (SD) | 5.8 (3.9) | 5.6 (3.8) | 5.9 (3.9) |
| Number of visits, n (%) | |||
| 1 | 5,384 (100.0) | 1,186 (100.0) | 3,991 (100.0) |
| 2 | 3,864 (71.8) | 856 (72.2) | 2,928 (73.4) |
| 3 | 2,496 (46.4) | 548 (46.2) | 1,905 (47.7) |
| 4 | 1,452 (27.0) | 297 (25.0) | 1,132 (28.4) |
| 5 | 908 (16.9) | 189 (15.9) | 707 (17.7) |
| 6 | 602 (11.2) | 120 (10.1) | 474 (11.9) |
| 7 | 209 (3.9) | 40 (3.4) | 167 (4.2) |
Notes: CES-D = Center for Epidemiological Studies—Depression scale; MCI = mild cognitive impairment; SD = standard deviation. Missing n = 22 on education, missing n = 641 on baseline income, missing n = 217 on baseline occupation, missing n = 81 on ethnicity, missing n = 81 on race, missing n = 807 on baseline depressive symptoms, and missing n = 207 on baseline MCI.
aMissing n = 207 on MCI diagnosis; these individuals were included in the overall analysis, not in the stratified analyses.
Longitudinal Relationship Between LAE and Cognition
First, we determined the shape of the univariate growth curve models, that is, linear, quadratic, and logarithmic, through the goodness of fit statistics (Supplementary Table 1). For LAE and all cognitive domains, the shape of the univariate growth curve models was linear. Although the goodness of fit statistics for quadratic growth curve models were similar to those of the linear growth curve models, there was no interindividual variance associated with the quadratic term (Supplementary Table 2).
Next, we examined the unadjusted associations of overall LAE with GCP, memory, language, and visuospatial functioning before (Supplementary Table 3) and after covariate adjustment (Table 2). Initial levels and slopes of LAE and GCP were correlated. Higher initial GCP was associated with slower rates of GCP decline. Adjusted relationships were similar to the unadjusted findings (Supplementary Table 3). In contrast, higher initial LAE was associated with steeper rates of LAE decline. Higher initial GCP was associated with slower rates of LAE decline, but initial LAE was not associated with GCP change. Similar patterns were observed for associations of LAE with memory, language, and visuospatial function with one exception. Initial language abilities and LAE change were unrelated. No associations were observed after covariate adjustment (Table 2).
Table 2.
Relationships Between LAE and Cognitive Domains Using Conditional Parallel Process Latent Growth Curve Models Adjusted for Covariates
| Global cognitive performance | Memory | Language | Visuospatial function | |||||
|---|---|---|---|---|---|---|---|---|
| n = 4,463 | n = 4,412 | n = 4,105 | n = 4,363 | |||||
| Types of relationships | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Autoregressive associations | ||||||||
| Initial cognition → Rate of cognitive change | 0.025 | 0.017 to 0.033 | 0.028 | 0.018 to 0.038 | 0.010 | 0.004 to 0.016 | 0.013 | 0.005 to 0.021 |
| Initial LAE → Rate of change in LAE | −0.015 | −0.023 to −0.007 | −0.016 | −0.024 to −0.008 | −0.015 | −0.023 to −0.007 | −0.016 | −0.024 to −0.008 |
| Cross-lagged associations | ||||||||
| Initial cognition → Rate of change in LAE | 0.013 | −0.001 to 0.027 | 0.010 | 0.000 to 0.020 | 0.000 | −0.014 to 0.014 | 0.013 | −0.003 to 0.029 |
| Initial LAE → Rate of change in cognition | 0.001 | −0.003 to 0.005 | 0.003 | −0.005 to 0.011 | −0.002 | −0.006 to 0.002 | −0.003 | −0.007 to 0.001 |
| Correlations | ||||||||
| Initial cognition → Initial LAE | 0.055 | 0.045 to 0.065 | 0.058 | 0.042 to 0.074 | 0.064 | 0.052 to 0.076 | 0.047 | 0.035 to 0.059 |
| Rate of cognitive change → Rate of change in LAE | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.000 | 0.000 to 0.000 |
Notes: LAE = leisure activity engagement; CI = confidence interval. Adjusted by baseline age, education in years, sex (female vs. male), enrollment year, race/ethnicity, occupation, baseline chronic disease sum score, and baseline income. Bolded results signify that the 95% CIs do not overlap with 0. Overall LAE scores have been standardized to baseline mean and standard deviation.
Table 3 provides the results from models stratified by baseline diagnostic status to examine within-group associations. Among cognitively normal individuals, initial LAE was associated with initial cognition across outcomes. In contrast, among those with MCI, initial LAE was only significantly associated with initial GCP and episodic memory, and all concurrent associations between initial LAE and initial cognition were weaker in the MCI group. Regarding dynamic associations between rates of change in LAE and rates of change in cognition, associations were largely similar in both groups, although LAE change was not associated with the change in visuospatial functioning among cognitively normal individuals. Regarding directional associations, there were no associations between initial LAE and subsequent changes in cognition or between initial cognition and subsequent changes in LAE in either group. Associations between initial cognition and subsequent changes in LAE appeared to be numerically larger among those with MCI.
Table 3.
Relationships Between Overall LAE and Cognitive Domains Using Conditional Parallel Process Latent Growth Curve Models by Baseline Cognitive Status
| Global cognitive performance | Memory | Language | Visuospatial function | |||||
|---|---|---|---|---|---|---|---|---|
| Types of relationships | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Baseline MCI only (n = 1,017) | ||||||||
| Autoregressive associations | ||||||||
| Initial cognition → Rate of cognitive change | 0.055 | 0.028 to 0.082 | 0.031 | 0.007 to 0.055 | 0.009 | −0.011 to 0.029 | 0.022 | −0.003 to 0.047 |
| Initial LAE → Rate of change in LAE | −0.032 | −0.052 to −0.012 | −0.032 | −0.054 to −0.01 | −0.032 | −0.052 to −0.012 | −0.032 | −0.052 to −0.012 |
| Cross-lagged associations | ||||||||
| Initial cognition → Rate of change in LAE | 0.030 | −0.019 to 0.079 | 0.010 | −0.017 to 0.037 | 0.018 | −0.019 to 0.055 | 0.017 | −0.020 to 0.054 |
| Initial LAE → Rate of change in cognition | 0.001 | −0.013 to 0.015 | 0.010 | −0.008 to 0.028 | 0.000 | −0.012 to 0.012 | −0.010 | −0.022 to 0.002 |
| Correlations | ||||||||
| Initial cognition → Initial LAE | 0.032 | 0.012 to 0.052 | 0.056 | 0.021 to 0.091 | 0.021 | −0.004 to 0.046 | 0.026 | 0.001 to 0.051 |
| Rate of cognitive change → Rate of change in LAE | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 |
| Cognitively normal at baseline only (n = 3,392) | ||||||||
| Autoregressive associations | ||||||||
| Initial cognition → Rate of cognitive change | 0.030 | 0.020 to 0.040 | 0.033 | 0.021 to 0.045 | 0.017 | 0.009 to 0.025 | 0.018 | 0.008 to 0.028 |
| Initial LAE → Rate of change in LAE | −0.011 | −0.021 to −0.001 | −0.011 | −0.021 to −0.001 | −0.010 | −0.020 to 0.000 | −0.011 | −0.021 to −0.001 |
| Cross-lagged associations | ||||||||
| Initial cognition → Rate of change in LAE | 0.015 | −0.003 to 0.033 | 0.013 | −0.001 to 0.027 | −0.004 | −0.022 to 0.014 | 0.017 | −0.021 to 0.019 |
| Initial LAE → Rate of change in cognition | 0.000 | −0.006 to 0.006 | 0.001 | −0.007 to 0.009 | −0.002 | −0.008 to 0.004 | −0.001 | 0.037 to 0.049 |
| Correlations | ||||||||
| Initial cognition → Initial LAE | 0.049 | 0.039 to 0.059 | 0.043 | 0.027 to 0.059 | 0.061 | 0.049 to 0.073 | 0.043 | 0.031 to 0.055 |
| Rate of cognitive change → Rate of change in LAE | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.000 | 0.000 to 0.000 |
Notes: LAE = leisure activity engagement; CI = confidence interval; MCI = mild cognitive impairment. Adjusted by baseline age, education in years, sex (female vs. male), enrollment year, race/ethnicity, occupation, baseline chronic disease sum score, and baseline income. Bolded results signify that the 95% CIs do not overlap with 0. Overall LAE scores have been standardized to baseline mean and standard deviation.
Associations of Components of LAE With Cognition
Table 4 presents the associations of the LAE components, that is, intellectual, physical, and social, with GCP, memory, language, and visuospatial functioning after covariate adjustment. All autoregressive and concurrent relationships were significant across LAE components and cognitive domains. For the intellectual subscale, higher initial GCP, memory, and visuospatial ability were associated with slower rates of intellectual LAE, while higher LAE was associated with steeper declines in GCP, memory, language, and visuospatial ability. The associations of the physical subscales with GCP, memory, language, and visuospatial function were identical to those from the main adjusted findings. For the social subscale, there was an additional directional association indicating that higher initial GCP and memory scores were associated with slower rates of decline in social LAE independent of covariates, which was not observed with other cognitive domains.
Table 4.
Relationships Between Components of LAE and Cognitive Domains Using Conditional Parallel Process Latent Growth Curve Models
| Global cognitive performance | Memory | Language | Visuospatial function | |||||
|---|---|---|---|---|---|---|---|---|
| n = 4,463 | n = 4,412 | n = 4,105 | n = 4,363 | |||||
| Types of relationships | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Intellectual subscale | ||||||||
| Autoregressive associations | ||||||||
| Initial cognition → Rate of cognitive change | 0.026 | 0.016 to 0.036 | 0.028 | 0.018 to 0.038 | 0.012 | 0.000 to 0.024 | 0.015 | 0.003 to 0.027 |
| Initial LAE → Rate of change in LAE | −0.025 | −0.035 to −0.015 | −0.025 | −0.035 to −0.015 | −0.024 | −0.036 to −0.012 | −0.026 | −0.038 to −0.014 |
| Cross-lagged associations | ||||||||
| Initial cognition → Rate of change in LAE | 0.025 | 0.015 to 0.035 | 0.028 | 0.018 to 0.038 | 0.010 | 0.000 to 0.020 | 0.012 | 0.002 to 0.022 |
| Initial LAE → Rate of change in cognition | −0.025 | −0.035 to −0.015 | −0.026 | −0.036 to −0.016 | −0.024 | −0.036 to −0.012 | −0.026 | −0.036 to −0.016 |
| Correlations | ||||||||
| Initial cognition → Initial LAE | 0.051 | 0.041 to 0.061 | 0.038 | 0.022 to 0.054 | 0.055 | 0.043 to 0.067 | 0.057 | 0.045 to 0.069 |
| Rate of cognitive change → Rate of change in LAE | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.000 | 0.000 to 0.000 | 0.000 | 0.000 to 0.000 |
| Physical subscale | ||||||||
| Autoregressive associations | ||||||||
| Initial cognition → Rate of cognitive change | 0.025 | 0.015 to 0.035 | 0.028 | 0.018 to 0.038 | 0.010 | 0.000 to 0.020 | 0.012 | 0.002 to 0.022 |
| Initial LAE → Rate of change in LAE | −0.025 | −0.035 to −0.015 | −0.026 | −0.036 to −0.016 | −0.024 | −0.036 to −0.012 | −0.026 | −0.036 to −0.016 |
| Cross-lagged associations | ||||||||
| Initial cognition → Rate of change in LAE | 0.002 | −0.014 to 0.018 | −0.004 | −0.016 to 0.008 | 0.001 | −0.013 to 0.015 | 0.008 | −0.008 to 0.024 |
| Initial LAE → Rate of change in cognition | 0.002 | −0.004 to 0.008 | 0.003 | −0.007 to 0.013 | 0.003 | −0.003 to 0.009 | 0.000 | −0.006 to 0.006 |
| Correlations | ||||||||
| Initial cognition → Initial LAE | 0.025 | 0.015 to 0.035 | 0.029 | 0.013 to 0.045 | 0.023 | 0.011 to 0.035 | 0.018 | 0.006 to 0.03 |
| Rate of cognitive change → Rate of change in LAE | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.000 | 0.000 to 0.000 | 0.000 | 0.000 to 0.000 |
| Social subscale | ||||||||
| Autoregressive associations | ||||||||
| Initial cognition → Rate of cognitive change | 0.025 | 0.015 to 0.035 | 0.028 | 0.018 to 0.038 | 0.011 | 0.001 to 0.021 | 0.012 | 0.002 to 0.022 |
| Initial LAE → Rate of change in LAE | −0.011 | −0.019 to −0.003 | −0.011 | −0.019 to −0.003 | −0.012 | −0.022 to −0.002 | −0.012 | −0.02 to −0.004 |
| Cross-lagged associations | ||||||||
| Initial cognition → Rate of change in LAE | 0.016 | 0.000 to 0.032 | 0.014 | 0.002 to 0.026 | 0.005 | −0.009 to 0.019 | 0.014 | −0.002 to 0.03 |
| Initial LAE → Rate of change in cognition | 0.002 | −0.002 to 0.006 | 0.003 | −0.003 to 0.009 | −0.001 | −0.005 to 0.003 | −0.001 | −0.005 to 0.003 |
| Correlations | ||||||||
| Initial cognition → Initial LAE | 0.036 | 0.026 to 0.046 | 0.041 | 0.025 to 0.057 | 0.042 | 0.028 to 0.056 | 0.023 | 0.011 to 0.035 |
| Rate of cognitive change → Rate of change in LAE | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.001 | 0.001 to 0.001 | 0.000 | 0.000 to 0.000 |
Notes: LAE = leisure activity engagement; CI = confidence interval. Adjusted by baseline age, education in years, sex (female vs. male), enrollment year, race/ethnicity, occupation, baseline chronic disease sum score, and baseline income. Bolded results signify that the 95% CIs do not overlap with 0. All LAE measures have been standardized to baseline mean and standard deviation.
When we stratified the models by baseline diagnostic status, we found different patterns of associations (Supplementary Table 4). First, those with MCI showed concurrent associations of initial intellectual LAE with initial GCP and visuospatial functioning, as well as associations of initial social LAE with initial GCP and memory. In contrast, there were more concurrent associations between initial LAE and initial cognition among cognitively normal individuals. Specifically, initial intellectual, physical, and social LAE were each associated with initial cognitive performance across all domains in this group (Supplementary Table 4). Individuals with MCI only showed concurrent associations between initial physical LAE and initial memory. Similarly, individuals with MCI showed dynamic associations between rates of social LAE change and rates of GCP change, while cognitively normal individuals showed dynamic associations between rates of LAE change and rates of change in nearly all cognitive domains. Regarding directional associations, cognitively normal individuals showed an association between the initial level of memory performance and slower rates of decline in intellectual LAE (Supplementary Table 4). Greater intellectual LAE was associated with the faster subsequent visuospatial decline among individuals with MCI.
Discussion
The overall study objective was to clarify the longitudinal relationship between LAE and cognition among older adults. Both concurrent and dynamic correlations between overall LAE and cognition were noted. LAE was tightly coupled with cognition in a time-dependent manner, such that levels and rates of change in LAE and cognition were positively correlated with one another, largely irrespective of the cognitive domain, activity type, or baseline diagnostic status. Greater initial LAE was not strongly related to subsequent cognitive changes except for associations between initial physical LAE and less memory decline and initial intellectual LAE and more memory decline among the baseline MCI group. In the overall sample, higher initial memory scores were associated with slower rates of decline in social LAE. A similar pattern was found for initial memory performance and subsequent intellectual LAE only among cognitively normal individuals.
This study attempted to establish whether LAE is associated with longitudinal declines in cognitive performance and/or vice versa. Our findings suggest that both level and change in LAE and cognition are concurrent. Other studies found that higher LAE is a protective factor against incident cognitive impairment (Helzner et al., 2007; Sajeev et al., 2016; Scarmeas et al., 2001). However, these studies did not jointly model the relationship between cognition and LAE initially and longitudinally. Our findings may suggest that LAE and cognition are influenced by some external common factor (e.g., age-related brain changes) that drives individual differences in levels and changes in both, even after stratifying by diagnostic status. Moreover, it could be that cognitive performance is maintained if LAE is maintained, which is suggestive of preserved differentiation (Salthouse et al., 1990). Evidence for preserved differentiation has been found in other studies examining LAE and cognitive performance (Bielak et al., 2012; Bielak, Cherbuin et al., 2014; Bielak, Gerstorf et al., 2014).
Only when we examined the components of LAE in exploratory analyses did we observe directional associations that represent hypotheses to be confirmed in future work. Specifically, initial memory was associated with subsequent social LAE, but not vice versa, in the overall sample. This pattern of results differs from previous studies that have reported associations between social LAE and subsequent memory functioning (Arbuckle et al., 1986, 1992; Christensen et al., 1996). In one study, social disengagement, defined as social connections and activities, was significantly associated with incident cognitive decline among cognitively normal older adults (Bassuk et al., 1999). In contrast, our study suggests that poorer memory performance may lead to subsequent declines in social LAE (Green et al., 2008; Small et al., 2012). The stigma associated with poorer memory performance may lead to social withdrawal (Ayalon et al., 2016) or reduced confidence and/or ability to function socially (Green et al., 2008). Older adults may experience embarrassment or social exclusion as a result of memory failures. This social stigma may explain why social activities appeared to be more vulnerable to age-related memory impairment than other leisure activities in the current study.
The current study found no evidence of directional associations between intellectual or physical LAE and cognitive performance. In other studies of community-dwelling older adults with annual visit schedules, intellectual, but not physical, LAE was most strongly associated with lower risk of incident cognitive impairment (Verghese et al., 2006; Wang et al., 2006). While the lack of association between baseline physical LAE and cognitive trajectories in the current study is in line with this previous work, the lack of association between initial intellectual LAE and cognitive trajectories in the current study contrasted with these two studies. This might be due to the differences in methods, because these studies utilized survival analyses with dichotomous outcomes, not a growth model approach with continuous cognitive outcomes that controls for previous cognitive level. Findings from a survival analysis suggested that high levels of intellectual, physical, and social LAE were associated with reduced risk of incident cognitive impairment (Wang et al., 2013). Because analyses of the associations of components with cognitive domains in the current study were exploratory, these results could generate hypotheses to study these patterns in other studies.
When we stratified the models by diagnostic status, better memory performance predicted slower rates of decline in intellectual LAE among cognitively normal older adults, but not among older adults with baseline MCI. It could be that a higher level of engagement promotes positive cognitive performance and higher levels of cognitive performance promote a lifestyle with more LAE (Stern, 2002). Additionally, the association of greater intellectual LAE with steeper visuospatial decline among individuals with MCI was surprising. Given that initial intellectual LAE was only associated with better initial visuospatial performance, it may be that those with greater intellectual LAE had more “room” to decline. These stratified associations could be further explored in other studies as well.
There were several study strengths and limitations. We used an ethnically and racially diverse, community-based sample to increase generalizability. We also used longitudinal measures of both cognition and LAE to attend to the notion of reverse causality and model longitudinal change of both cognition and LAE jointly. Some limitations were the self-reported LAE scale and the limited number of leisure activities defining the LAE subtypes. However, Carlson et al. (2012) observed, over a 10-year period, that greater LAE variety was more predictive of healthy cognitive aging than the frequency of activities or level of cognitive challenge. Additionally, leisure activities, especially intellectual ones, are difficult to quantify. Intellectual activities, representing multiple domains, are less amenable to factor analytic methods. Furthermore, an impediment to LAE intervention studies is the topic of dosage (Carlson, 2011; Fallahpour et al., 2016), and the ability to quantify how much stimulation is being provided. LAE may act as a surrogate for unmeasured variables associated with cognitive performance, or there could have been an unmeasured variable that confounded the associations, that is, stressful events.
In conclusion, we found dynamic associations between overall LAE and cognitive performance in the overall sample of older adults and by diagnostic status, which further supports preserved differentiation. The patterns of the correlations of levels and change were similar throughout the analyses. Interestingly, the only evidence for directionality in the relationship between LAE and cognition in the overall sample was that better baseline memory performance was prospectively associated with slower rates of decline in subsequent social LAE. Future research may elucidate ways in which social engagement can be promoted among older adults in line with their preferences and cognitive abilities. Finally, physical and intellectual LAE among those with MCI could be predictive of change in certain cognitive domains, which may lead to targeted interventions.
Supplementary Material
Acknowledgments
We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study.
Funding
Data collection and sharing for this project was supported by the Washington Heights–Inwood Columbia Aging Project (WHICAP; P01AG07232, R01AG037212, and RF1AG054023) funded by the National Institute on Aging (NIA), and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR001873). This abstract has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. This work was also supported by the Advanced Psychometrics Methods in Cognitive Aging Research Conference (R13AG030995). E. M. Simonsick and N. M. Armstrong were supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. A. Harrati was supported by NIA R01AG02629. S. E. Tom was supported by NIA K01AG050723. K. B. Casaletto was supported by NIA K23AG058752 and the Larry L Hillblom Foundation (2017-A-004-FEL). J. Pa was supported by NIA RF1AG054617. L. B. Zahodne was supported by NIA K99/R00 AG047963 and AG054520.
Conflict of Interest
None declared.
References
- Alzheimer’s Association. (2017). 2017 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 13(4), 325–373. doi:10.1016/j.jalz.2017.02.001 [Google Scholar]
- Arbuckle, T. Y., Gold, D., & Andres, D. (1986). Cognitive functioning of older people in relation to social and personality variables. Psychology and Aging, 1(1), 55–62. doi:10.1037/0882-7974.1.1.55 [DOI] [PubMed] [Google Scholar]
- Arbuckle, T. Y., Gold, D. P., Andres, D., Schwartzman, A., & Chaikelson, J. (1992). The role of psychosocial context, age, and intelligence in memory performance of older men. Psychology and Aging, 7(1), 25–36. doi:10.1037//0882-7974.7.1.25 [DOI] [PubMed] [Google Scholar]
- Avila, J. F., Rentería, M. A., Witkiewitz, K., Verney, S. P., Vonk, J. M. J., & Manly, J. J. (2020). Measurement invariance of neuropsychological measures of cognitive aging across race/ethnicity by sex/gender groups. Neuropsychology, 34(1), 3–14. doi:10.1037/neu0000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon, L., Shiovitz-Ezra, S., & Roziner, I. (2016). A cross-lagged model of the reciprocal associations of loneliness and memory functioning. Psychology and Aging, 31(3), 255–261. doi:10.1037/pag0000075 [DOI] [PubMed] [Google Scholar]
- Bassuk, S. S., Glass, T. A., & Berkman, L. F. (1999). Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine, 131(3), 165–173. doi:10.7326/0003-4819-131-3-199908030-00002 [DOI] [PubMed] [Google Scholar]
- Benton, A. (1955). The Visual Retention Test. The Psychological Corporation. [Google Scholar]
- Bielak, A. A., Anstey, K. J., Christensen, H., & Windsor, T. D. (2012). Activity engagement is related to level, but not change in cognitive ability across adulthood. Psychology and Aging, 27(1), 219–228. doi:10.1037/a0024667 [DOI] [PubMed] [Google Scholar]
- Bielak, A. A., Cherbuin, N., Bunce, D., & Anstey, K. J. (2014). Preserved differentiation between physical activity and cognitive performance across young, middle, and older adulthood over 8 years. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69(4), 523–532. doi:10.1093/geronb/gbu016 [DOI] [PubMed] [Google Scholar]
- Bielak, A. A. M., Gerstorf, D., Anstey, K. J., & Luszcz, M. A. (2014). Longitudinal associations between activity and cognition vary by age, activity type, and cognitive domain. Psychology and Aging, 29(4), 863–872. doi:10.1037/a0036960 [DOI] [PubMed] [Google Scholar]
- Brown, C. L., Gibbons, L. E., Kennison, R. F., Robitaille, A., Lindwall, M., Mitchell, M. B., Shirk, S. D., Atri, A., Cimino, C. R., Benitez, A., MacDonald, S. W. S., Zelinski, E. M., Willis, S. L., Schaie, K. W., Johansson, B., Dixon, R. A., Mungas, D. M., Hofer, S. M., & Piccinin, A. M. (2012). Social activity and cognitive functioning over time: A coordinated analysis of four longitudinal studies. Journal of Aging Research, 2012, 287438. doi:10.1155/2012/287438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke, H., & Fuld, P. A. (1974). Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology, 24(11), 1019–1025. doi:10.1212/wnl.24.11.1019 [DOI] [PubMed] [Google Scholar]
- Carlson, M. C. (2011). Introduction: A life course perspective on activity and neurocognitive health. Journal of the International Neuropsychological Society, 17(6), 970–974. doi:10.1017/s1355617711001366 [DOI] [PubMed] [Google Scholar]
- Carlson, M. C., Parisi, J. M., Xia, J., Xue, Q. L., Rebok, G. W., Bandeen-Roche, K., & Fried, L. P. (2012). Lifestyle activities and memory: Variety may be the spice of life. The women’s health and aging study II. Journal of the International Neuropsychological Society, 18(2), 286–294. doi:10.1017/S135561771100169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, H., Korten, A., Jorm, A., Henderson, A., Scott, R., & Mackinnon, A. (1996). Activity levels and cognitive functioning in an elderly community sample. Age and Ageing, 25(1), 72–80. doi:10.1093/ageing/25.1.72 [DOI] [PubMed] [Google Scholar]
- Curran, P. J., & Hussong, A. M. (2009). Integrative data analysis: The simultaneous analysis of multiple data sets. Psychological Methods, 14(2), 81–100. doi:10.1037/a0015914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallahpour, M., Borell, L., Luborsky, M., & Nygård, L. (2016). Leisure-activity participation to prevent later-life cognitive decline: A systematic review. Scandinavian Journal of Occupational Therapy, 23(3), 162–197. doi:10.3109/11038128.2015.1102320 [DOI] [PubMed] [Google Scholar]
- Gow, A. J., Corley, J., Starr, J. M., & Deary, I. J. (2012). Reverse causation in activity–cognitive ability associations: The Lothian Birth Cohort 1936. Psychology and Aging, 27(1), 250–255. doi:10.1037/a0024144 [DOI] [PubMed] [Google Scholar]
- Green, A. F., Rebok, G., & Lyketsos, C. G. (2008). Influence of social network characteristics on cognition and functional status with aging. International Journal of Geriatric Psychiatry, 23(9), 972–978. doi:10.1002/gps.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, L. E., Weuve, J., Scherr, P. A., & Evans, D. A. (2013). Alzheimer’s disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. doi:10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner, E. P., Scarmeas, N., Cosentino, S., Portet, F., & Stern, Y. (2007). Leisure activity and cognitive decline in incident Alzheimer disease. Archives of Neurology, 64(12), 1749–1754. doi:10.1001/archneur.64.12.1749 [DOI] [PubMed] [Google Scholar]
- Irwin, M., Artin, K. H., & Oxman, M. N. (1999). Screening for depression in the older adult: Criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Archives of Internal Medicine, 159(15), 1701–1704. doi:10.1001/archinte.159.15.1701 [DOI] [PubMed] [Google Scholar]
- Kaplan, E., Goodglass, H., & Weintraub, S. (Eds.). (1983). The Boston Naming Test (2nd ed.). Lea & Febiger. [Google Scholar]
- Lindwall, M., Cimino, C. R., Gibbons, L. E., Mitchell, M. B., Benitez, A., Brown, C. L., Kennison, R. F., Shirk, S. D., Atri, A., Robitaille, A., Macdonald, S. W., Zelinski, E. M., Willis, S. L., Schaie, K. W., Johansson, B., Praetorius, M., Dixon, R. A., Mungas, D. M., Hofer, S. M., … Piccinin, A. M. (2012). Dynamic associations of change in physical activity and change in cognitive function: Coordinated analyses of four longitudinal studies. Journal of Aging Research, 2012, 493598. doi:10.1155/2012/493598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly, J. J., Bell-McGinty, S., Tang, M. X., Schupf, N., Stern, Y., & Mayeux, R. (2005). Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Archives of Neurology, 62(11), 1739–1746. doi:10.1001/archneur.62.11.1739 [DOI] [PubMed] [Google Scholar]
- Manly, J. J., Tang, M.-X., Schupf, N., Stern, Y., Vonsattel, J.-P. G., & Mayeux, R. (2008). Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology, 63(4), 494–506. doi:10.1002/ana.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis, S. (1976). Mental status examination for organic mental syndrome in the elderly patient. In Bellack L. & Karusu T. B. (Eds.), Geriatric psychiatry (pp. 77–121). Grune & Stratton. [Google Scholar]
- McArdle, J. J., & Bell, R. Q. (2000). An introduction to latent growth models for developmental data analysis. In Little T., Schnabel K., & Baumert J. (Eds.), Modeling longitudinal and multilevel data: Practical issues, applied approaches, and specific examples (pp.69–107, 269–281). Lawrence Erlbaum Associates Publishers. [Google Scholar]
- McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34(7), 939–939. doi:10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- Mitchell, M. B., Cimino, C. R., Benitez, A., Brown, C. L., Gibbons, L. E., Kennison, R. F., Shirk, S. D., Atri, A., Robitaille, A., Macdonald, S. W., Lindwall, M., Zelinski, E. M., Willis, S. L., Schaie, K. W., Johansson, B., Dixon, R. A., Mungas, D. M., Hofer, S. M., & Piccinin, A. M. (2012). Cognitively stimulating activities: Effects on cognition across four studies with up to 21 years of longitudinal data. Journal of Aging Research, 2012, 461592. doi:10.1155/2012/461592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, B. (1997). Latent variable modeling of longitudinal and multilevel data. Sociological Methodology, 27(1), 453–480. doi:10.1111/1467-9531.271034 [Google Scholar]
- Muthén, B. O., & Curran, P. J. (1997). General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychological Methods, 2(4), 371–402. doi:10.1037/1082-989X.2.4.371 [Google Scholar]
- Muthén, L., & Muthén, B. (1998–2017). Mplus user’s guide (8th ed.). Muthén & Muthén. [Google Scholar]
- Rosen, W. (1981). The Rosen Drawing Test. Veterans Administration Medical Center. [Google Scholar]
- Sajeev, G., Weuve, J., Jackson, J. W., VanderWeele, T. J., Bennett, D. A., Grodstein, F., & Blacker, D. (2016). Late-life cognitive activity and dementia: A systematic review and bias analysis. Epidemiology, 27(5), 732–742. doi:10.1097/EDE.0000000000000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse, T. A., Babcock, R. L., Skovronek, E., Mitchell, D. R. D., & Palmon, R. (1990). Age and experience effects in spatial visualization. Developmental Psychology, 26, 128–136. doi:10.1037/00121649.26.1.128 [Google Scholar]
- Scarmeas, N., Levy, G., Tang, M. X., Manly, J., & Stern, Y. (2001). Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology, 57(12), 2236–2242. doi:10.1212/wnl.57.12.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlecki, K. L., Manly, J. J., Brickman, A. M., Schupf, N., Tang, M. X., & Stern, Y. (2010). Do neuropsychological tests have the same meaning in Spanish speakers as they do in English speakers? Neuropsychology, 24(3), 402–411. doi:10.1037/a0017515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, B. J., Dixon, R. A., McArdle, J. J., & Grimm, K. J. (2012). Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology, 26(2), 144–155. doi:10.1037/a0026579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerlad, A., Sabia, S., Livingston, G., Kivimäki, M., Lewis, G., & Singh-Manoux, A. (2020). Leisure activity participation and risk of dementia: An 18-year follow-up of the Whitehall II Study. Neurology, 95(20), e2803–e2815. doi:10.1212/WNL.0000000000010966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. (2017). Stata Statistical Software: Release 15. StataCorp LLC. [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460. doi:10.1017/S1355617702813248 [PubMed] [Google Scholar]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology, 11(11), 1006–1012. doi:10.1016/s1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Andrews, H., Pittman, J., Sano, M., Tatemichi, T., Lantigua, R., & Mayeux, R. (1992). Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives of Neurology, 49(5), 453–460. doi:10.1001/archneur.1992.00530290035009 [DOI] [PubMed] [Google Scholar]
- Streiner, D. L., & Norman, G. R. (2011). Correction for multiple testing: Is there a resolution? Chest, 140(1), 16–18. doi:10.1378/chest.11-0523 [DOI] [PubMed] [Google Scholar]
- Stull, D. E. (2008). Analyzing growth and change: Latent variable growth curve modeling with an application to clinical trials. Quality of Life Research, 17(1), 47–59. doi:10.1007/s11136-007-9290-5 [DOI] [PubMed] [Google Scholar]
- Tang, M.-X., Cross, P., Andrews, H., Jacobs, D. M., Small, S., Bell, K., Lantigua, M. R., Costa, R., Stern, Y., & Mayeux, R. (2001). Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology, 56(1), 49–56. doi:10.1212/wnl.56.1.49 [DOI] [PubMed] [Google Scholar]
- Verghese, J., LeValley, A., Derby, C., Kuslansky, G., Katz, M., Hall, C., Buschke, H., & Lipton, R. B. (2006). Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology, 66(6), 821–827. doi:10.1212/01.wnl.0000202520.68987.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivot, A., Power, M. C., Glymour, M. M., Mayeda, E. R., Benitez, A., Spiro, A.3rd, Manly, J. J., Proust-Lima, C., Dufouil, C., & Gross, A. L. (2016). Jump, hop, or skip: Modeling practice effects in studies of determinants of cognitive change in older adults. American Journal of Epidemiology, 183(4), 302–314. doi:10.1093/aje/kwv212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. X., Jin, Y., Hendrie, H. C., Liang, C., Yang, L., Cheng, Y., Unverzagt, F. W., Ma, F., Hall, K. S., Murrell, J. R., Li, P., Bian, J., Pei, J. J., & Gao, S. (2013). Late life leisure activities and risk of cognitive decline. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 68(2), 205–213. doi:10.1093/gerona/gls153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. Y. J., Zhou, D. H. D., Li, J., Zhang, M., Deng, J., Tang, M., Gao, C., Li, J., & Chen, M. (2006). Leisure activity and risk of cognitive impairment: The Chongqing aging study. Neurology, 66(6), 911–913. doi:10.1212/01.wnl.0000192165.99963.2a [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1981). Wechsler Adult Intelligence Scale-revised (Vol. 1). Psychological Corporation. [Google Scholar]
- Wilson, R. S., Scherr, P. A., Schneider, J. A., Tang, Y., & Bennett, D. A. (2007). Relation of cognitive activity to risk of developing Alzheimer’s disease. Neurology, 69(20), 1911–1920. doi:10.1212/01.wnl.0000271087.67782.cb [DOI] [PubMed] [Google Scholar]
- Wilson, R. S., Segawa, E., Boyle, P. A., & Bennett, D. A. (2012). Influence of late-life cognitive activity on cognitive health. Neurology, 78(15), 1123–1129. doi:10.1212/WNL.0b013e31824f8c03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne, L. B., Mayeda, E. R., Hohman, T. J., Fletcher, E., Racine, A. M., Gavett, B., Manly, J. J., Schupf, N., Mayeux, R., Brickman, A. M., & Mungas, D. (2019). The role of education in a vascular pathway to episodic memory: Brain maintenance or cognitive reserve? Neurobiology of Aging, 84, 109–118. doi:10.1016/j.neurobiolaging.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.