Abstract
Objective
Pregnant women with autoimmune (subclinical) hypothyroidism have an increased risk of developing gestational diabetes mellitus (GDM). However, this association remains controversial in euthyroid women with thyroid autoimmunity (TAI). Therefore, the aim of the study was to determine the association between TAI and GDM in euthyroid women in a logistic regression analysis with adjustments for baseline/demographic parameters.
Methods
Cross-sectional study in 1447 euthyroid women who performed their entire clinical/biological workup and oral glucose tolerance test (OGTT) in our center. At median 13 (11–17) weeks of gestation, thyroid-stimulating hormone, free T4, and thyroid peroxidase antibodies (TPOAb) were measured, baseline characteristics were recorded, and an OGTT was performed between 24 and 28 weeks of pregnancy. Exclusion criteria were pre-pregnancy diabetes, assisted pregnancies, and women with (treated) thyroid dysfunction before or after screening. The diagnosis of GDM was based on 2013 World Health Organization criteria, and TAI was defined as TPOAb levels ≥60 kIU/L.
Results
Two hundred eighty women were diagnosed with GDM (19.4%), 26.1% in women with TAI, and 18.9% in women without TAI (P = 0.096). In the logistic regression analysis, TAI was associated with GDM in women older than 30 years (adjusted odds ratio 1.68 (95% CI, 1.01–2.78); P = 0.048). Maternal age >30 years, pre-pregnancy BMI ≥30 kg/m2, and other than Caucasian background were also associated with GDM; aOR 1.93 (95% CI, 1.46–2.56); P < 0.001, 2.03 (95% CI, 1.46–2.81); P < 0.001 and 1.46 (95% CI, 1.03–2.06); P = 0.034, respectively.
Conclusions
In older pregnant women, the presence of TAI in euthyroid women was associated with GDM. In line with the literature data, (higher) age and BMI were strongly associated with GDM. Future investigations should focus on treatments that might prevent the development of GDM in euthyroid women with TAI.
Keywords: gestational diabetes, pregnancy, thyroid autoimmunity, euthyroid
Introduction
Thyroid autoimmunity (TAI) and (subclinical) hypothyroidism (SCH) have been associated with adverse pregnancy outcomes, such as miscarriage, preterm birth, and gestational diabetes mellitus (GDM) (1). TAI and GDM have been linked with each other via two pathways. One is by the development of (sub)clinical hypothyroidism (TAI is the most frequent cause of hypothyroidism) and another via inflammatory pathways involving IL-6 and TNF-α; both pathways can lead to insulin resistance (IR) (2, 3).
In a meta-analysis pooling studies from the period, 2000 to 2014, no increased risk of GDM was reported in euthyroid women with TAI (relative risk (RR) 1.07 (95% CI, 0.96–1.19)) (2). However, in original studies published since 2015 in euthyroid Chinese populations, a significant association between TAI and GDM was observed (odds ratios (ORs) between 1.65 and 2.54) (4, 5, 6). Also in the most recent meta-analysis, increased thyroid peroxidase antibodies (TPOAb) were associated with GDM (OR 1.65 (95% CI, 1.13-2.40); P < 0.001). However, it should be noted that the index of heterogeneity (I2) was high with 74% (7). The reason for that heterogeneity was not specified and might be due to different definitions of thyroid dysfunction, changes in the criteria for the diagnosis of GDM (before and after the criteria of The International Association of Diabetes and Pregnancy Study Groups), the inclusion of different populations (cosmopolitan vs regional), and different exclusion criteria. Furthermore, in most studies, it is not clear to what extent the impact of TAI on GDM was adjusted for other variables such as (over)weight and (high) age (8).
Therefore, the aim of this study was to investigate in an adjusted logistic regression the association between TAI and GDM in euthyroid women that performed their biological workup and obstetric follow-up in a single center throughout the entire pregnancy.
Materials and methods
Overall study design/definitions
The obstetric clinic of the CHU Saint-Pierre is part of a downtown public university hospital in Brussels, Belgium. In this cross-sectional analysis (period February 1, 2013/December 12, 2014), we included women with ongoing pregnancies, that performed their biological work-up and obstetric follow-up in our center throughout the entire pregnancy. The oral glucose tolerance test (OGTT) is done systematically in all pregnant women in our center, and therefore also in all women included in this study.
Exclusion criteria were women with known diabetes mellitus before pregnancy, pregnancies resulting from assisted reproduction, multiple pregnancies, and (treated) thyroid disorders (with LT4/antithyroid drugs) before or after screening. These exclusions were based on previous studies on the impact of thyroid disorders and other variables on glucose metabolism as reviewed by Biondi et al.in 2019 (3).
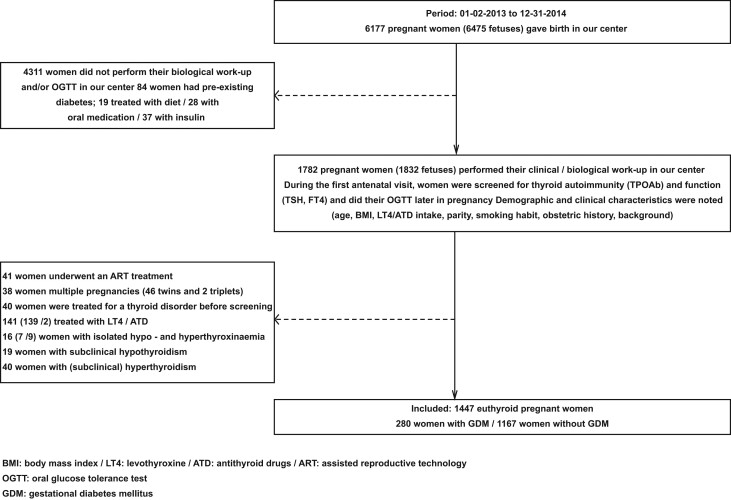
Finally, 1447 women were included to investigate the association between TAI and GDM in euthyroid women. In Fig. 1, we illustrate in a flowchart the study selection process (noteworthy is that among all excluded women, 16.7% were TAI+ (56/335)).
Figure 1.
Flowchart of the study selection process.
During the first antenatal consultation, demographic and obstetrical data are noted and systematically completed with a biological analysis including serum thyroid-stimulating hormone (TSH), free thyroxine (FT4), TPOAb, and ferritin measurement. The ethnic background of the women is based on a history taken by the social workers that includes systematically the nationality at birth and the ethnic origin of the women (9). Gestational age is based on ultrasound findings and expressed in full weeks and days of amenorrhea. Smoking is stratified as yes/no (yes meant a minimum of five cigarettes daily and women who stopped smoking during pregnancy were also considered as smokers).
TAI is present when TPOAb levels were ≥60 kIU/L. In a previous study, we determined the first-trimester reference range (2.5–97.5th percentile) for serum TSH (0.06–3.74 mIU/L) and FT4 (10.29–18.02 pmol/L) (10). GDM is diagnosed after the administration of 75 g glucose during an OGTT performed between 24 and 28 weeks of pregnancy when fasting glucose ≥92 mg/dL or 1-h postprandial glycemia ≥180 mg/dL or 2 h ≥153 mg/dL) (11, 12). Iron deficiency is defined as serum ferritin levels <15 µg/L.
The study was approved by the institutional review board (AK/15-11-114/4568); no written consent was obtained from the participants.
Serum assay
All provisions were implemented by the laboratory of hormonology of our institution.
Serum TSH, FT4, TPOAbs, and ferritin levels were measured using the Chemiluminescence Centaur XP Siemens immunoanalyzer. The reference values were 0.3–4.0 mIU/L, 10.3–25.7 pmol/L (0.8–2.0 ng/dL), <60 kIU/L, and 15–300 ug/L for TSH, FT4, TPOAb, and ferritin, respectively. The total imprecision CVs were 6.9, 4.2, 7.6, and 3.7% for TSH, FT4, TPOAb, and for ferritin, respectively. Plasma glucose was measured by an automated colorimetric-enzymatic method on a Hitachi/Roche-Modular P analyzer; CV is 1%.
Statistical analysis
Data were stored in a Microsoft Excel database and statistical analyses were performed using Stata 11.2 software (StataCorp LLC, College Station, TX, USA). Differences between groups were analyzed by Fisher’s exact tests for categorical data and by a t-test or Mann–Whitney U test for continuous data. A multivariable logistic regression analysis was performed with GDM as a dependent outcome. As independent outcomes, we included variables with a proven impact on GDM (6, 8). These are: maternal age (whole range and as higher age (>30 years)), pre-pregnancy BMI (whole range and as obesity (≥30 kg/m2)), background other than Caucasian (e.g. Sub-Saharan and North African), tobacco use, ferritin levels (whole range and as iron deficiency (ferritin levels (<15 µg/L)), parity (whole range and as high parity rate (>2)), the fetal gender (female) and TPOAb (whole range and as TAI (TPOAb ≥60 kIU/L)). The regression analysis was done ones with the independent variables as continuous data and ones with the independent variables as categorical data (the results are shown in one table). Results are expressed as adjusted odds ratios (95% CI), and statistical tests were considered significant whenever P < 0.05.
Results
Table 1 shows the demographic and obstetric parameters in all women and according to the presence (GDM+)/absence (GDM−) of gestational diabetes.
Table 1.
Demographic and obstetric parameters in all women and according to the presence (GDM+)/absence (GDM−) of gestational diabetes.
| Continuous dataa | All women | GDM+ | GDM− | P |
|---|---|---|---|---|
| Categorical data, n (%) | n= 1447 | n= 280 (19%) | n= 1167 (81%) | |
| Maternal age (years) | 30.0 ± 5.8 | 31.7 ± 5.4 | 29.5 ± 5.8 | <0.001 |
| Maternal age >30 years | 665 (46.0%) | 170 (60.7%) | 495 (42.4%) | <0.001 |
| Pre-pregnancy BMI (kg/m2) | 25.0 (22.3–28.2) | 26.8 (23.3–30.0) | 24.6 (22.1–27.6) | <0.001 |
| Obesity (BMI ≥30 kg/m2) | 228 (15.8%) | 71 (25.4%) | 157 (13.5%) | <0.001 |
| Other than Caucasian background | 1103 (76.2%) | 230 (82.1%) | 873 (74.8%) | 0.010 |
| Parity (n) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.007 |
| Multiparity (>2) | 177 (12.2%) | 43 (15.4%) | 134 (11.5%) | 0.076 |
| History of ≥2 first-trimester MC | 96 (6.6%) | 22 (7.9%) | 74 (6.3%) | 0.360 |
| Smoking during pregnancy | 218 (15.1%) | 36 (12.9%) | 182 (15.6%) | 0.250 |
| Ferritin levels (μg/L) | 20 (12–37) | 23 (13–40) | 20 (12–36) | 0.122 |
| Iron deficiency (Ferritin <15 μg/L) | 503 (34.8%) | 87 (31.1%) | 416 (35.7%) | 0.149 |
| Fetal gender (% female) | 738 (51.0%) | 147 (52.5%) | 591 (50.6%) | 0.577 |
aContinuous data are expressed as mean ± s.d. or median (Q1–Q3).
GDM, gestational diabetes mellitus; MC, miscarriage.
Bold indicates statistical significance.
Of the 1447 pregnant women included, 280 (19%) had GDM and 1167 (81%) did not.
Women with GDM were older (mean maternal age 31.7 ± 5.4 years vs 29.5 ± 5.8 years; P < 0.001), more often obese before pregnancy (25.4% vs 13.5%; P < 0.001) and had more often another than Caucasian background (82.1% vs 74.8%; P = 0.010). Parity was higher in the GDM group, median (interquartile range (IQR)): 1 (0–2) vs 1 (0–2); P = 0.007. However, when expressed as multiparty rate >2, no difference was present; 15.4% vs 11.5%; P = 0.076.
Table 2 shows thyroid parameters in all women and according to the presence (GDM+)/absence (GDM−) of gestational diabetes.
Table 2.
Thyroid parameters in all women and according to the presence (GDM+)/absence (GDM−) of gestational diabetes.
| Continuous dataa | All women | GDM+ | GDM− | P |
|---|---|---|---|---|
| Categorical data, n (%) | n = 1447 | n = 280 (19%) | n= 1167 (81%) | |
| TSH (mIU/L) | 1.37 (0.88–1.89) | 1.40 (0.93–1.91) | 1.36 (0.88–1.89) | 0.706 |
| TSH >2.50 mIU/L | 140 (9.7%) | 25 (8.9%) | 115 (9.9%) | 0.638 |
| FT4 (pmol/L) | 14.2 (12.9–15.4) | 13.5 (12.9–14.2) | 14.2 (12.9–15.4) | 0.441 |
| TPOAb (kIU/L) | 28 (28–37) | 29 (28–38) | 28 (28–37) | 0.337 |
| TAI (TPOAb ≥60 kIU/L) | 88 (6.1%) | 23 (8.2%) | 65 (5.6%) | 0.096a |
aContinuous data are expressed as median (Q1–Q3).
FT4, free thyroxine; TAI, thyroid autoimmunity; TSH, thyrotropin; TPOAb, thyroid peroxidase autoantibodies.
Serum TSH levels (median (IQR)) were comparable between both study groups: 1.40 (0.93–1.91) mIU/L vs 1.36 (0.88–1.89) mIU/L; P = 0.706. The prevalence of high-normal serum TSH levels (>2.50 mIU/L) was comparable between both groups: 8.9% vs 9.9%; P = 0.638. Serum FT4 levels (median (IQR)) were comparable between both groups: 13.5 (12.9–14.2) pmol/L vs 14.2 (12.9–15.4) pmol/L; P = 0.441. The prevalence of TAI was 8.2% in the GDM+ group and 5.6% in the GDM− group; P = 0.096.
Table 3 shows the multivariable logistic regression analysis with demographic, obstetric/pregnancy parameters, and thyroid parameters as independent variables, and GDM as a dependent outcome.
Table 3.
Logistic regression analysis with demographic, obstetric/pregnancy parameters as independent variables, and gestational diabetes as a dependent outcome.
| Independent variablesa | Dependent Outcome (GDM) | |
|---|---|---|
| aOR (95% CI) | P | |
| Age (whole range) | 1.06 (1.04–1.09) | <0.001 |
| Age >30 years | 1.93 (1.46–2.56) | <0.001 |
| BMI (whole range) | 1.07 (1.04–1.09) | <0.001 |
| BMI ≥30 kg/m2 | 2.03 (1.46–2.81) | <0.001 |
| Background (other than Caucasian) | 1.46 (1.03–2.06) | 0.034 |
| Tobacco use | 0.90 (0.60–1.34) | 0.593 |
| Ferritin levels | 1.00 (0.99–1.01) | 0.328 |
| Iron deficiency (<15 µg/L) | 0.81 (0.60–1.07) | 0.140 |
| Parity (whole range) | 0.95 (0.84–1.08) | 0.437 |
| High parity (>2) | 1.26 (0.94–1.68) | 0.122 |
| Fetal gender (female) | 1.13 (0.86–1.47) | 0.384 |
| TSH (all normal) range | 1.07 (0.89–1.28) | 0.497 |
| TSH >2.50 mIU/L | 1.00 (0.62–1.60) | 0.990 |
| FT4 (all normal range) | 1.00 (0.92–1.09) | 0.959 |
| TPOAb whole range | 1.00 (0.99–1.00) | 0.811 |
| TAIb | 1.58 (0.94–2.66) | 0.084 |
| TAIc | 1.68 (1.01–2.78) | 0.048 |
aGiven as continuous or categorical values; bAge and BMI included as continuous variables; cAge and BMI included as categorical variables older age and obesity.
aOR, adjusted odds ratio; GDM, gestational diabetes mellitus; POAb, thyroid peroxidase antibodies; TAI, thyroid autoimmunity (TPOAb ≥60 kIU/L).
Bold indicates statistical significance.
Significantly associated with GDM were age in the whole range, aOR 1.06 (95% CI, 1.04-1.09); P < 0.001 and as higher age (>30 years), aOR 1.93 (95% CI, 1.46–2.56); P < 0.001, pre-pregnancy BMI in the whole range, aOR (95% CI, 1.04–1.09); P < 0.001 and as obesity (BMI ≥30 kg/m2), aOR 2.03 (95% CI, 1.46–2.81); P < 0.001, and another other than a Caucasian background, 1.46 (95% CI, 1.03–2.06); P = 0.034.
In the model with age and BMI as continuous variables, TAI was not associated with GDM, aOR 1.58 (95% CI, 0.94-2.66); P = 0.084. In the model with age and BMI as categorical variables (>30 years and BMI ≥30 kg/m2), TAI was associated with GDM, aOR 1.68 (95% CI, 1.01-2.78); P = 0.048.
Discussion
The main observation in our study is the significant association between increased TPOAb levels in early pregnancy and the occurrence of GDM later on during pregnancy in women older than 30 years. Our results are in line with recently published studies performed in Chinese populations and a meta-analysis in euthyroid women with TAI in which the OR was 1.65 (95% CI, 1.13–2.40) (4, 5, 6, 7). Noteworthy concerning that recent meta-analysis is the high index of heterogeneity (I2of 70%), which was not specified more in detail (7). Furthermore, in many studies included, it was not clear to what extent adjustments for confounders such as age, BMI, ethnic background, subclinical thyroid dysfunction were made. Therefore, we adjusted our results for these confounders, selected patients to avoid treatments with an impact on thyroid function that were started before or after the screening, defined euthyroidism according to our institutional cut-off, and finally, included pregnant women of our cosmopolitan area. After adjustments, the association between TAI in early pregnancy and GDM persisted, but only in older pregnant women.
In a US study, it was reported that the prevalence of GDM was the highest among Filipina (10.9 %) and Asian women (10.2%), and the lowest among Caucasian (4.5%) and African American women (4.4%) (13). In our study, background other than Caucasian was associated with a higher prevalence of GDM. However, since these women had mainly a North African and sub-Saharan background (<1% far Asian), we could not specify which one was associated with a higher prevalence of GDM (10). The reasons why the prevalence of GDM differs between women according to their background are multi-faceted, including another lifestyle (physical activity/alimentary), BMI, genes associated with IR, and healthcare systems access.
A difference between our study and recent Chinese studies is the pre-pregnancy BMI that was 20–21 kg/m2 in theirs compared with 25.5 kg/m2 in ours. This might explain the higher prevalence of GDM in general in our cohort (~19%) compared with ~14% in mainland China (14). However, and despite the difference in the baseline prevalence of GDM, positivity for TPOAb remained significantly increased with GDM, independent of BMI. Obesity is a variable known to be associated with IR and GDM, as it was also the case in our regression analysis (8, 15, 16). In one study, obesity, high LDL and hyperuricemia were positively correlated with TAI in euthyroid subjects, and the so-called ‘immunometabolism’ highlights the relationship between the immune and hormonal system (17). Obesity might increase the susceptibility to harbor TAI with leptin as a peripheral determinant, which can decrease the function of regulatory T cells and increase the percentage of T helper 1 cells (18, 19). However, in a multivariable analysis in pregnant women, BMI was a poor predictor for the development of TAI (20). In line with those results, also in our study, obesity was no predictor of TAI (data not shown).
Mean age in our cohort was somewhat higher (30 years) compared with that in recent Chinese studies in which it was 28 and 27 years, respectively (5, 6). Age is a well-known variable associated with an increased risk of GDM, which we confirm in our study (4, 5, 6). When age was included as a continuous variable in the multivariable analysis, TAI was not associated anymore. Due to our strict selection criteria, 335 women were excluded of whom 16.7% were TAI+, and a type II error cannot be excluded; a priori, TAI is less frequent in younger women. Despite those exclusions, TAI remained an independent variable associated with GDM in older pregnant women. Moreover, age >30 was no predictor of TAI as such (data not shown).
The prevalence of TAI in the recent Chinese studies was higher compared with that in our cohort were 15, 9.7, and 12%, respectively, while in our study it was 6.1%; the latter maybe lower due to our strict exclusion criteria and/or the heterogeneity in the backgrounds of the women included in our cohort (4, 5, 6, 10, 21). Studies in the US and the Netherlands also showed differences in the prevalence of TAI according to different backgrounds, with the highest in white women (22, 23).
In our cohort, the prevalence of GDM increased from 18.9 to 26.1% in women with TAI, and several mechanisms have been suggested that could link TAI with GDM through IR, a key element in GDM (3, 24). One pathway is via higher serum TSH levels, since TAI is the most frequent cause of hypothyroidism, and the latter has been associated with IR as reviewed in detail by Biondi et al.(3). Already in infants, higher serum TSH levels have been associated with IR through a reduction in blood flow in the skeletal muscle and adipose tissue and decreased glucose uptake via lesser expression of the GLUT-4 glucose transporter (25). In a recent study, another hypothesis was brought forward to explain the association between thyroid disorders and GDM. The authors observed that lower serum human chorionic gonadotrophin (hCG) levels during the first trimester were associated with a higher prevalence of GDM, with FT4 as a mediator (26). However, in the sensitivity analysis restricted to TPOAb-positive women, hCG was not associated with GDM. In our study, we included only euthyroid women, and furthermore, the impact of TAI on GDM was adjusted for high-normal serum TSH (>2.5 mIU/L) indicating other mechanisms beyond that of a (subtle) thyroid dysfunction must be involved.
Another pathway that can link TAI and GDM is via inflammatory pathways that are common in both conditions (24, 25, 27). In one study, levels of serum interleukin‐6 (IL‐6), tumor necrosis factor‐α, IL‐12, IL‐10, and IR (HOMA‐index) were investigated in women with Hashimoto’s disease, in euthyroid women with TAI and a control group (27). These inflammatory parameters were the highest in the Hashimoto’s group but were also higher in euthyroid patients with TAI compared with those in the controls. In women with GDM, some of these parameters (IL-6, TNF-α) were also involved in the pathogenesis, and therefore, a link with TAI is plausible (24).
Finally, is it noteworthy, that in another recent meta-analysis on the association between thyroid disorders and GDM, the presence of TAI together with SCH led to the highest OR; 2.04 (95% CI, 1.32–3.13) compared to the presence of each risk factor separately (28).
An altered iron metabolism can also play a role in an association between TAI and GDM. In a previous study, we and others showed that women with low ferritin levels had a higher prevalence of TAI and mean serum TSH levels (29, 30). However, in the current analysis, ferritin levels and iron deficiency were not associated with GDM. This can be explained by the fact that high ferritin levels have been associated with GDM too, since it may serve as a marker of inflammation (associated with IR) (31, 32).
Limitations of our study are the absence TgAb levels and the lack of longitudinal measurement of thyroid function tests. In a study in the Brussels region, published a few years ago, it was shown that in infertile women, 5% had positivity for TgAb without TPOAb, and in another study in pregnant women in the same region, it was 5.9% (33, 34). Therefore, if we would have been able to include TgAb positive women, the group with TAI might have become more important and the association stronger. Indeed, in the meta-analysis discussed earlier, it was shown that the presence of increased TgAb levels only was associated with a higher prevalence of GDM compared with that in case of positivity for TPOAb; OR 1.88 (95% CI, 1.13–3.12) vs OR 1.65 (95% CI, 1.13–2.40) (28).
Concerning women with subclinical hyperthyroidism (à priori due to the high hCG levels), they might have normalized their TSH levels later in pregnancy, which cannot be confirmed due to the absence of repeated measures, and therefore, they were excluded from the study. Also, concerning euthyroid women at the beginning of pregnancy (the screening period), data in the literature suggest that they might develop SCH later during the second and third trimester (the period when the OGTT is done). In one study by Glinoer et al., involving 87 euthyroid (TSH ≤4mU/L) women, TPOAb or TgAb positive, it was shown that 20% developed a serum TSH >4.0mU/L during pregnancy (35). In 2006, Negro et al. reported that in TPOAb-positive euthyroid women, TSH levels increased as gestation progressed, with 19% of women having supranormal (>4.0 mU/L) TSH values at delivery (36). In our series, we only had 52 repeated measures at median (Q1–Q3) 25 (23–27) weeks out of the 88 TPO+ women (first measurement 12 (11–15). At the second measurement, no woman had a TSH >3.74 mIU/L (data not shown).
Finally, the familial history of diabetes and previous episodes of GDM were not always documented in our files since some women had their GDM followed up by an endocrinologist in another center.
Strengths of the study were the exclusion of women treated with thyroid hormone or antithyroid drugs before and after the screening for thyroid disorders, and as such, avoiding their impact on the results of the OGTT performed weeks after the initial screening (at 24–28 weeks). Indeed, it has been shown in the past and in a recent study that women with TSH levels in the range 2.5–4.0 mIU/L and treated with thyroid hormones had increased rates of GDM compared with untreated women (37, 38, 39).
Conclusion
In our cohort, applying strict inclusion and exclusion criteria, TAI in euthyroid women was associated with gestational diabetes in women older than 30 years. Furthermore, we observed a strong association with (higher) age and pre-pregnancy BMI, in line with literature data. Further research on this association is necessary, to predict better which patients with TAI in early pregnancy will develop gestational diabetes, ultimately aiming to treat them and prevent the development of gestational diabetes.
Declaration of interest
Kris G Poppe had no conflict of interest in relation to the current study but received in the period 2018–2021 lecture fees from the Berlin-Chemie, Merck and IBSA company. The other authors have nothing to disclose.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
G S collected data and revized the manuscript; F V collected data and revized the manuscript; P K revized the manuscript; M I revized the manuscript; J P P revized the manuscript; S R revized the manuscript and approved the final version; K G P designed and performed the study, acquired and analyzed the data, drafted and revized the manuscript, and approved the final version.
References
- 1.Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nature Reviews: Endocrinology 201713610–622. ( 10.1038/nrendo.2017.93) [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Li Q, Wang Q, Ma X. Thyroid antibodies and gestational diabetes mellitus: a meta-analysis. Fertility and Sterility 20171046, 65.e3–6. ( 10.1016/j.fertnstert.2015.06.003) [DOI] [PubMed] [Google Scholar]
- 3.Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocrine Reviews 201940789–824. ( 10.1210/er.2018-00163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ying H, Tang YP, Bao YR, Su XJ, Cai X, Li YH, Wang DF. Maternal TSH level and TPOAb status in early pregnancy and their relationship to the risk of gestational diabetes mellitus. Endocrine 201654742–750. ( 10.1007/s12020-016-1022-6) [DOI] [PubMed] [Google Scholar]
- 5.Huang K, Xu Y, Yan S, Li T, Xu Y, Zhu P, Tao F. Isolated effect of maternal thyroid-stimulating hormone, free thyroxine and antithyroid peroxidase antibodies in early pregnancy on gestational diabetes mellitus: a birth cohort study in China. Endocrine Journal 201966223–231. ( 10.1507/endocrj.EJ18-0340) [DOI] [PubMed] [Google Scholar]
- 6.Li F, Hu Y, Zeng J, Zheng L, Ye P, Wei D, Chen D. Analysis of risk factors related to gestational diabetes mellitus. Taiwanese Journal of Obstetrics and Gynecology 202059718–722. ( 10.1016/j.tjog.2020.07.016) [DOI] [PubMed] [Google Scholar]
- 7.Luo J, Wang X, Yuan L, Guo L. Association of thyroid disorders with gestational diabetes mellitus: a meta-analysis. Endocrine 202173550–560. ( 10.1007/s12020-021-02712-2) [DOI] [PubMed] [Google Scholar]
- 8.Li G, Wei T, Ni W, Zhang A, Zhang J, Xing Y, Xing Q. Incidence and risk factors of gestational diabetes mellitus: a prospective cohort study in Qingdao, China. Frontiers in Endocrinology 202011 636. ( 10.3389/fendo.2020.00636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paquier L, Barlow P, Paesmans M, Rozenberg S. Do recent immigrants have similar obstetrical care and perinatal complications as long-term residents? A retrospective exploratory cohort study in Brussels. BMJ Open 202010 e029683. ( 10.1136/bmjopen-2019-029683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veltri F, Belhomme J, Kleynen P, Grabczan L, Rozenberg S, Pepersack T, Poppe K. Maternal thyroid parameters in pregnant women with different ethnic backgrounds: do ethnicity-specific reference ranges improve the diagnosis of subclinical hypothyroidism? Clinical Endocrinology 201786830–836. ( 10.1111/cen.13340) [DOI] [PubMed] [Google Scholar]
- 11.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod Met al. International Association of Diabetes and Pregnancy Study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 201033676–682. ( 10.2337/dc09-1848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Research and Clinical Practice 2014103341–363. ( 10.1016/j.diabres.2013.10.012) [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Current Diabetes Reports 201616 7. ( 10.1007/s11892-015-0699-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. Journal of Diabetes Investigation 201910154–162. ( 10.1111/jdi.12854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Retnakaran R, Kramer CK, Ye C, Kew S, Hanley AJ, Connelly PW, Sermer M, Zinman B. Fetal sex and maternal risk of gestational diabetes mellitus: the impact of having a boy. Diabetes Care 201538844–851. ( 10.2337/dc14-2551) [DOI] [PubMed] [Google Scholar]
- 16.Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends in Endocrinology and Metabolism 201829743–754. ( 10.1016/j.tem.2018.09.004) [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Shi X, Tang X, Li Y, Tong N, Wang G, Zhang JA, Wang Y, Ba J, Chen B.et al. The correlation between metabolic disorders and Tpoab/Tgab: a cross-sectional population-based study. Endocrine Practice 202026869–882. ( 10.4158/EP-2020-0008) [DOI] [PubMed] [Google Scholar]
- 18.Marzullo P, Minocci A, Tagliaferri MA, Guzzaloni G, Di Blasio A, De Medici C, Aimaretti G, Liuzzi A. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. Journal of Clinical Endocrinology and Metabolism 2010953965–3972. ( 10.1210/jc.2009-2798) [DOI] [PubMed] [Google Scholar]
- 19.Song RH, Wang B, Yao QM, Li Q, Jia X, Zhang JA. The impact of obesity on thyroid autoimmunity and dysfunction: a systematic review and metaanalysis. Frontiers in Immunology 201910 2349. ( 10.3389/fimmu.2019.02349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korevaar TI, Nieboer D, Bisschop PH, Goddijn M, Medici M, Chaker L, de Rijke YB, Jaddoe VW, Visser TJ, Steyerberg EW.et al. Risk factors and a clinical prediction model for low maternal thyroid function during early pregnancy: two population-based prospective cohort studies. Clinical Endocrinology 201685902–909. ( 10.1111/cen.13153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veltri F, Poppe K. Variables contributing to thyroid (Dys)function in pregnant women: more than thyroid antibodies? European Thyroid Journal 20187120–128. ( 10.1159/000488279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La'ulu SL, Roberts WL. Second-trimester reference intervals for thyroid tests: the role of ethnicity. Clinical Chemistry 2007531658–1664. ( 10.1373/clinchem.2007.089680) [DOI] [PubMed] [Google Scholar]
- 23.Korevaar TI, Medici M, de Rijke YB, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VW, Hofman A, Ross HA, Visser WE, Hooijkaas H.et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. Journal of Clinical Endocrinology and Metabolism 2013983678–3686. ( 10.1210/jc.2013-2005) [DOI] [PubMed] [Google Scholar]
- 24.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. International Journal of Molecular Sciences 201819 3342. ( 10.3390/ijms19113342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Deng S, Sena C, Zhou C, Thaker VV. Relationship of TSH levels with cardiometabolic risk factors in US youth and reference percentiles for thyroid function. Journal of Clinical Endocrinology and Metabolism 2021106 e1221–e1230. ( 10.1210/clinem/dgaa900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Guo F, Maraka S, Zhang Y, Zhang C, Korevaar TIM, Fan J. Associations between human chorionic gonadotropin, maternal free thyroxine, and gestational diabetes mellitus. Thyroid 2021311282–1288. ( 10.1089/thy.2020.0920) [DOI] [PubMed] [Google Scholar]
- 27.Lei Y, Yang J, Li H, Zhong H, Wan Q. Changes in glucose-lipid metabolism, insulin resistance, and inflammatory factors in patients with autoimmune thyroid disease. Journal of Clinical Laboratory Analysis 201933 e22929. ( 10.1002/jcla.22929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent NL, Young SL, Akison LK, Cuffe JSM. Is the link between elevated TSH and gestational diabetes mellitus dependant on diagnostic criteria and thyroid antibody status: a systematic review and meta-analysis. Endocrine 20217438–49. ( 10.1007/s12020-021-02733-x) [DOI] [PubMed] [Google Scholar]
- 29.Veltri F, Decaillet S, Kleynen P, Grabczan L, Belhomme J, Rozenberg S, Pepersack T, Poppe K. Prevalence of thyroid autoimmunity and dysfunction in women with iron deficiency during early pregnancy: is it altered? European Journal of Endocrinology 2016175191–199. ( 10.1530/EJE-16-0288) [DOI] [PubMed] [Google Scholar]
- 30.Zhang HY, Teng XC, Shan ZY, Wang ZJ, Li CY, Yu XH, Mao JY, Wang WW, Xie XC, Teng WP. Association between iron deficiency and prevalence of thyroid autoimmunity in pregnant and non-pregnant women of childbearing age: a cross-sectional study. Chinese Medical Journal 20191322143–2149. ( 10.1097/CM9.0000000000000409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kataria Y, Wu Y, Horskjær PH, Mandrup-Poulsen T, Ellervik C. Iron status and gestational diabetes – a meta-analysis. Nutrients 201810 621. ( 10.3390/nu10050621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannakou K, Evangelou E, Yiallouros P, Christophi CA, Middleton N, Papatheodorou E, Papatheodorou SI. Risk factors for gestational diabetes: an umbrella review of meta-analyses of observational studies. PLoS ONE 201914 e0215372. ( 10.1371/journal.pone.0215372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unuane D, Velkeniers B, Anckaert E, Schiettecatte J, Tournaye H, Haentjens P, Poppe K. Thyroglobulin autoantibodies: is there any added value in the detection of thyroid autoimmunity in women consulting for fertility treatment? Thyroid 2013231022–1028. ( 10.1089/thy.2012.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno-Reyes R, Glinoer D, Van Oyen H, Vandevijvere S. High prevalence of thyroid disorders in pregnant women in a mildly iodine-deficient country: a population-based study. Journal of Clinical Endocrinology and Metabolism 2013983694–3701. ( 10.1210/jc.2013-2149) [DOI] [PubMed] [Google Scholar]
- 35.Glinoer D, Riahi M, Grün JP, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. Journal of Clinical Endocrinology and Metabolism 199479197–204. ( 10.1210/jcem.79.1.8027226) [DOI] [PubMed] [Google Scholar]
- 36.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. Journal of Clinical Endocrinology and Metabolism 2006912587–2591. ( 10.1210/jc.2005-1603) [DOI] [PubMed] [Google Scholar]
- 37.Wikner BN, Sparre LS, Stiller CO, Källén B, Asker C. Maternal use of thyroid hormones in pregnancy and neonatal outcome. Acta Obstetricia et Gynecologica Scandinavica 200887617–627. ( 10.1080/00016340802075103) [DOI] [PubMed] [Google Scholar]
- 38.Maraka S, Mwangi R, McCoy RG, Yao X, Sangaralingham LR, Singh Ospina NM, O'Keeffe DT, De Ycaza AE, Rodriguez-Gutierrez R, Coddington CC., 3rdet al. Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ 2017356 i6865. ( 10.1136/bmj.i6865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM, Brito JP. Levothyroxine overuse: time for an about face? Lancet: Diabetes and Endocrinology 20175246–248. ( 10.1016/S2213-8587(1630276-5) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a