Abstract
Scarring is a dire consequence of acne vulgaris. Particularly, atrophic acne scarring is highly prevalent among young adults, and its physical and psychological effects can persist throughout their lives if left untreated. This literature review will analyze various non-energy-based approaches to treating atrophic acne scarring, emphasizing recent advances within the last 5 to 10 years. To accomplish this, we performed a PubMed search for various acne scar treatments such as chemical peels, dermabrasion, microdermabrasion, subcision, microneedling, punch techniques, dermal fillers, and thread lifting. Our findings and analysis show that there is no panacean solution to treating atrophic acne scars, which explains the evolving trend towards developing unique combinatorial treatments. Although a fair comparison of each treatment approach is difficult to achieve due to the studies’ varying sample sizes, strength of evidence, treatment execution, etc, there still remains a level of consensus on what treatments are well suited for particular scar types.
Keywords: TCA, subcision, microdermabrasion, microneedling, fillers, excision
Introduction
Acne vulgaris is a prevalent dermatological condition that afflicts a vast majority of adolescents.1 It pathogenesis begins with follicular hyperkeratinization and androgen-mediated overproduction of sebum in the pilosebaceous unit (PSU).2 The presence of excess sebum contributes to the proliferation of Propionibacterium acnes in the PSU, triggering a potent inflammatory response that can potentially lead to scarring.2 The probability of scarring is highly influenced by the intensity and duration of the inflammatory response and an unbalanced ratio of matrix metalloproteinases (MMPs) to tissue inhibitors of MMPs (TIMPs) during the extracellular matrix remodeling (ECM) process.3 Specifically, an increased ratio of TIMPs to MMPs weakens collagen synthesis during the healing process and leads to the development of atrophic scars, while the opposite leads to the formation of hypertrophic or keloid scars.3
Overall, studies estimate that >80% of acne scars are atrophic (Table 1). Within this category, ice pick scars are the predominant clinical manifestation of atrophic scarring (~60%), followed by boxcar scars (~25%), and rolling scars (~15%).4
Table 1.
Atrophic Acne Scar Subtypes
| Scar Type | Morphology |
|---|---|
| Ice Pick |
Diameter: <2 mm Depth: Lower dermis/subcutis Visual: V-shaped, pitted appearance |
| Boxcar |
Diameter: 1–4 mm Depth: Upper dermis (shallow) or lower dermis (deep) Visual: Round or geometric appearance with vertical walls and flat base |
| Rolling |
Diameter: ≥4 mm Depth: Upper dermis Visual: Slopy, undulating appearance caused by fibrotic tether of dermis to subcutaneous layer |
Notes: Adapted from J Am Acad Dermatol, 45(1), Jacob CI, Dover JS, Kaminer MS. Acne scarring: A classification system and review of treatment options. 109–117, copyright 2001, with permission from Elsevier.4
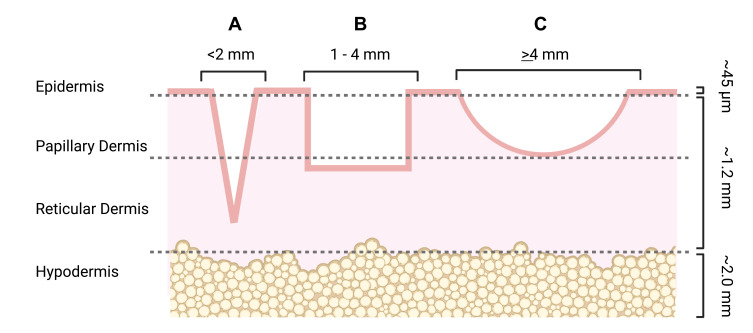
Of all the different types of atrophic acne scars, ice pick scars are known to be the most challenging to treat. Ice pick scars are vertical, “V-shaped”, <2 mm depressions in the skin that can penetrate through the entire thickness of the dermis.4 Boxcar scars are round depressions in the skin with vertical walls and a flat base, with a wider diameter of 1 to 4 mm and a depth of 0.1 to 0.5 mm.4 Rolling scars have the largest diameter (4 to 6 mm) but are more shallow. Their unique undulating, sloped appearance on the skin is caused by tethering from the dermis to the subcutaneous layer (Figure 1).4
Figure 1.
Visual representation of atrophic acne scar subtypes.
Notes: (A) Ice pick scars are narrow atrophic scars that can extend into the reticular dermis and, in some cases, down to the hypodermis. (B) Boxcar scars are oval- or rectangular-shaped depressions with vertical walls and a flat base. Shallow boxcar scars can extend into the papillary dermis but more severe boxcar scars can penetrate through the reticular dermis. (C) Rolling scars are the widest but shallowest scar type, rarely reaching down to the reticular dermis. They have a wavy or slope-like appearance on the skin. Created with Biorender.com.
Because each atrophic acne scar type has its own unique characteristics, achieving optimal outcomes requires both appropriate treatment selection and proper execution. These treatments can be generally categorized as energy-based or non-energy-based. Energy-based approaches consist broadly of laser therapy and radiofrequency, whereas non-energy-based approaches consist of a series of chemical- and surgical-based treatments. In this review, we will specifically discuss non-energy based treatment modalities such as chemical peels, dermabrasion, microdermabrasion, subcision, microneedling, punch techniques, fillers, and thread lifting, with particular emphasis on noteworthy advances within the last decade.
Chemical Peels
Chemical peels are a popular technique utilized by aestheticians and medical professionals for skin rejuvenation. These peels consist of acids that cause controlled injury to the epidermal or dermal layers of the skin, promoting exfoliation and neocollagenesis during the ECM remodeling process.5 Chemical peels come in different grades (Table 2), and depending on their strength, can be used to improve a variety of skin disorders, such as photoaging, dyschromia, precancerous lesions, acne vulgaris, and acne scarring.6
Table 2.
Categorical Overview of Chemical Peels
| Type | Depth of Penetration | Common Examples |
|---|---|---|
| Superficial | Stratum corneum to stratum basale/upper papillary dermis | Glycolic acid, salicylic acid, lactic acid, retinoic acid, mandelic acid, pyruvic acid, Jessner’s peel, <35% TCA |
| Medium | Papillary dermis/upper reticular dermis | 35–50% TCA, Glycolic acid + 35% TCA Jessner’s peel + 35% TCA |
| Deep | Upper reticular dermis/mid-reticular dermis | >50% TCA, 50–88% phenol, phenol-croton oil peels |
Notes: Adapted from O’Connor AA, Lowe PM, Shumack S, Lim AC. Chemical peels: A review of current practice. Australas J Dermatol. 2018;59(3):171–181, © 2017 The Australasian College of Dermatologists.7
Superficial peels are used to promote destruction of the outermost layer of skin, causing necrosis through the entire epidermal layer to the dermal-epidermal junction.7 These peels have potent keratolytic properties that reduce corneocyte adhesion, promote epidermolysis and squamation, and induce neocollagenesis.7 Some of the most common superficial peels include those listed in Table 2. Studies show that sequential application of these peeling agents can improve the appearance of superficial acne scars and treat dyspigmentation, especially at higher concentrations and frequency of treatment.3,7–12 Application of superficial combination peels, such as salicylic-mandelic acid peels (SMPs) and retinoic acid-glycolic acid (RAGA) peels, have shown to be even more effective for treating acne scars when compared to chemical peels used as monotherapies.13,14 Combining Jessner’s peel or glycolic acid with 35% TCA can further enhance penetration to treat more severe acne scars, effectively serving as an enhanced medium-depth peel.15,16
Due to their limited depth of penetration, superficial and medium-depth peels have largely been limited to mild atrophic scarring or acne-related hyperpigmentation.17 Treatment for deeper atrophic scarring, notably ice pick scars, requires stronger peels, such as TCA (>50%), that can penetrate to the mid-reticular dermis.7 When applied to the skin, a characteristic white frost appears, which indicates the onset of protein precipitation and coagulative necrosis. In the several days following treatment, the necrotic layers of the skin are shed and the treated area is re-epithelialized by the surrounding healthy skin.18 In recent years, a new application technique, termed the chemical reconstruction of skin scarring (CROSS), has been used to focally apply controlled amounts of deep peeling agents to individual scars, reducing the risk of scarring and dyspigmentation associated with full-face application.19
In the CROSS method, sharp applicator tools, such as toothpicks or needles, are used to apply the chemical to the base of the scar while sparing the surrounding healthy skin.19 Currently, TCA is the most popular chemical peel for treating atrophic acne scars using this technique.20 Published data have shown that the degree of acne scar correction is positively correlated with the concentration of TCA applied.21 However, multiple independent studies overall have demonstrated that a broad range of concentrations of TCA can significantly improve the appearance of acne scars, including relatively lower percentages such as 50%, with fewer complications.19–21
Currently, the most common adverse reaction associated with TCA is post-inflammatory hyperpigmentation (PIH).22 Risk of PIH increases with darker skin types (Fitzpatrick skin types IV–VI), strength of peel (> 50%), and lack of compliance with aftercare instructions (avoiding sun exposure, applying sunscreen, etc).23 Most studies, however, have shown that PIH is temporary and even preventable by pretreatment and posttreatment with topical hydroquinone.24 Hypopigmentation, erythema, pain, and irritation are also common complications that can occur immediately following treatment but are also either transient or shown to continually resolve on their own.25 Pretreatment and posttreatment with topical tretinoin for two weeks is recommended to exfoliate the skin and promote faster re-epithelialization to enhance not only the depth of the deep peeling agent but also the speed of recovery.7,26
Of greater concern, however, is the repeated finding that TCA can worsen scars if not applied properly to the skin.27 More recent studies suggest that this could be possibly due to TCA being accidentally applied to adjacent healthy skin, even when using precise applicators.24,27 Additional studies need to be conducted to determine what the causes are of such complications.
Phenol is another deep chemical peel that has recently come into favor for CROSS treatment.21 Currently, it is considered the deepest yet riskiest peel on the market.7 Full-face application of this peel can lead to systemic toxicity, cardiac arrhythmias, kidney and liver damage, scarring, and permanent dyspigmentation.7,19 However, these complications have yet to be reported when using the CROSS technique. Interestingly, Dalpizzol et al demonstrated that 88% phenol could be a viable alternative to TCA.27 In their comparative study, phenol was found to be as efficacious as TCA in reducing the appearance of acne scars. Additionally, phenol did not cause widening of scars, as observed for several patients treated with TCA in this study.27 Future studies should determine if these adverse outcomes can really be attributed to differences in chemical peel composition or to differences in precision of application.
Chemical peeling is a safe and economical treatment approach, whose efficacy for acne scar revision is dependent on the depth of the peel used and the frequency of application. Nevertheless, more studies are needed for the CROSS technique. While studies on the efficacy of TCA CROSS on deep atrophic acne scarring are abundant, there is a lack of published work on CROSS application of different concentrations or formulations of phenol, including well-known phenol-croton oil peels such as the Baker-Gordon peel and Hetter peels.7
Dermabrasion
Dermabrasion is a surgical skin-resurfacing procedure that mechanically ablates the outer layers of skin through the use of a motorized wire brush or diamond fraise.17 Over time, re-epithelialization and repigmentation initiated by spared adnexal structures as well as dermal neocollagenesis produce a smoother complexion.28 Because dermabrasion was originally designed to induce controlled injury to the skin but not surpass the level of the reticular dermis, it is particularly effective for superficial rolling and boxcar scars but provides minimal clinical improvement for deep ice pick scars.29,30
Noticeable correction of acne scars can be achieved through a single session of dermabrasion.17 However, dermabrasion requires either local or general anesthesia to alleviate intraoperative pain.28 In addition, this approach is highly operator-dependent and abrasion of the skin must be carefully limited to the reticular dermis or otherwise risk causing additional scarring.30 Fortunately, the shift from using aluminum oxide or sodium bicarbonate crystals as abrasive material on the handpiece to the diamond fraise and wire brush has enabled finer control of this technique.3
Nevertheless, there have been numerous reports of complications associated with dermabrasion, notably persistent postoperative pain, erythema, and permanent dyschromia.17 Additionally, Rubenstein et al reported keloid scarring in six patients who had recently used oral isotretinoin prior to undergoing dermabrasion in their study.31 Interestingly, however, in another study, seven patients who underwent dermabrasion while taking oral isotretinoin did not present hypertrophic scarring at 6- and 12-month follow-up evaluations.32 Therefore, whether recent use of oral isotretinoin is a contraindication for dermabrasion is unclear but still remains an important consideration upon review of the patient’s medication history.
Dermabrasion is still regarded as a simple and effective technique for acne scar treatment. However, given the many disadvantages of dermabrasion which are often not redeemable by their therapeutic capacity for more severe cases of acne scarring, dermabrasion has gradually been replaced with other treatment modalities. These include chemical peels and lasers, both of which have similar, if not greater, resurfacing capacity but less extensive recovery.17
Microdermabrasion
Microdermabrasion is a simple outpatient procedure that exfoliates the skin by brushing aluminum oxide crystals across the epidermis.33 The rapid passage of these fine particles across the skin effectively removes the stratum corneum and can reach as far down as the papillary dermis with higher speeds.34 The rejuvenating effect of microdermabrasion can be likened to that of very light chemical peels due to their non-invasive and mild destruction of the skin. This method was designed as a more conservative and less invasive alternative to regular dermabrasion, which causes significantly greater damage to the skin with a large rotating wire brush and thus entails more risks and complications.35
Marini and Lo Brutto first introduced microdermabrasion in 1985, and the first microdermabrasion machine was approved in 1994.33 Although microdermabrasion has gained popularity in beauty salons, medical spas, and medical offices, its efficacy for acne scarring is not well supported. An early study on microdermabrasion treatment for acne, traumatic, surgical, and burn scars had “good to excellent” results for all 41 cases, although acne scars required more sessions to achieve such results compared to the other scar types.36 Conversely, another more standardized split-face study comparing the efficacy of a 20% glycolic acid peel and microdermabrasion showed no significant difference between the two treatment modalities and no significant improvement overall from baseline measurements based on physician evaluation.37 Moreover, patient self-assessment scores showed that they preferred glycolic acid peels over microdermabrasion, although the latter presented less discomfort during the procedure.37
Histological studies show that macroscopic changes in the epidermis and dermis are dependent on the intensity of microdermabrasion. Microdermabrasion performed with more aggressive settings to penetrate the papillary dermis produced greater change in epidermal thickness, higher increase in collagen production, more obvious change in elastin production, and overall better clinical improvement, although this was not observed in every case.38
Microdermabrasion is a relatively safe procedure that has little downtime and only mild intraoperative discomfort when performed more aggressively.39 Evidence for its effectiveness, however, is still sparse, and this can be reasonably attributed to its limited ablative capacity that cannot remodel scars located deeper in the dermis.
Subcision
Subcutaneous incisionless surgery, also known as subcision, was invented by Orentreich and Orentreich in 1995.40 They described this procedure as a manual technique in which a hypodermic needle is inserted underneath the skin and maneuvered in a fan-like motion. This motion detaches the fibrotic strands that tether the scar to the subcutis, effectively liberating the dell from the underlying tissue. The resulting blood clot formation in between the subcised layers promotes new tissue formation, also known as fibrosis, which produces a noticeable lifting of the regenerated skin tissue.40
Alam et al were the first among many other clinicians in the past two decades to demonstrate the efficacy of subcision using objective physician assessment and subjective patient self-assessment measures.41 Subcision, overall, has consistently shown to improve primarily rolling scars in the majority of patients, with some studies reporting a 100% success rate using standardized acne scar scoring assessment scales, such as the Goodman and Baron Qualitative grading system.42
On the other hand, subcision alone has proven to be ineffective for boxcar scars and almost entirely ineffective for ice pick scars due to the absence of tethered scarring that is characteristic of rolling scars.41,43,44 Some studies have reported a “partial leveling” of boxcar and ice pick scars utilizing subcision, but these studies combined subcision with other non-energy-based treatment modalities.44–47
Indeed, combinatorial therapy involving subcision and other well-known acne scar treatments has gained tremendous momentum to provide a synergistic improvement to acne scars. One reason for this shift is the repeated observation that the effects of subcision are time-sensitive. After acne scars are successfully subcised, there is a considerable chance that tethering of the dermis to the subcutaneous layer could reoccur and diminish results.47
In one early interesting split-face study, the authors inserted absorbable surgical sutures underneath subcised acne scars to further promote neocollagenesis but with no significant additional improvement.48 In another study, combining subcision with other manual methods, notably facial suctioning, was shown to improve patient outcomes after subcision.47 The author noted that suctioning everyday for two weeks following subcision was more effective than suctioning every other day, although the former reportedly had higher rates of ecchymosis and other complications.47
Subcision has also been combined with other commonly used scar treatment devices, such as microneedling dermarollers and cryorollers.49 In a split-face randomized controlled trial (RCT), patients and physicians reported that the side treated with subcision followed by cryorolling improved significantly more than the side treated with subcision followed by dermarolling.49 The authors note in their discussion that their results were comparable to those of patients treated with fractional CO2 or erbium:YAG (er:YAG) lasers, which are known to carry higher risk for epidermal damage and PIH.50
In more recent studies, applying 50% TCA after subcision also produced favorable outcomes for patients.51,52 In other studies, combining subcision either with microneedling, platelet-rich plasma (PRP), or both synergistically reduced the appearance of acne scars.44,46,53 Interestingly, PRP not only enhanced the results of manual scar revision techniques but also seemed to reduce complication rates.53
Not surprisingly, the majority of studies revolving around subcision done in isolation or in combination with other therapies have noted considerable risks, such as hematoma formation, ecchymosis, erythema, and hyperpigmentation.54 Due to the nature of the procedure and the surgical tools involved, subcision always runs the risk of damaging neurovascular structures that can cause the aforementioned side effects.45 Taylor et al, for instance, recently developed a novel surgical instrument consisting of a long metal rod with a “W-shaped” blunt end.55 The sharp notches at the tip are effective at capturing the fibrotic tethers in rolling scars and severing them as the rod is advanced through the subdermal plane.55,56 While this improves the overall efficacy of subcision and limits the need for multiple sessions, risk of damage to deep facial structures is higher.55 As such, there has been an increased popularity of injecting tumescent anesthesia in the treatment area, known as hydrodissection, to lift dermal structures from the underlying tissue to add to the safety profile of subcision.54
Additionally, many authors over the years have shifted away from using the sharp 1.5-inch Nokor needle or hollow-bore needles to safer alternatives, notably blunt cannulas42,45,57 and surgical wires,58 all of which provide the same or even better patient outcomes. In one very innovative study, Nilforoushzadeh et al used a 300-micron laser fiber to perform energy- and wire-based subcision, noting 100% improvement according to clinical photographic data evaluation.59 The authors noted that inflammation and postoperative erythema were less severe than what is usually observed with cannula-based subcision and lasers.59
Subcision has also been known to cause hypertrophic scarring if done too superficially due to the formation of a subepidermal hematoma.47 Therefore, it has been warned that subcision must be done precisely in the dermal-subcutis junction to prevent hypertrophic scarring from occurring.47
Overall, subcision is an effective surgical procedure that can be easily performed in an outpatient setting. Proper execution requires meticulous handling of instruments and thorough understanding of facial anatomy to maximize clinical and safety outcomes for patients. Subcision can be combined with a myriad of other treatment modalities and remains an excellent option for patients afflicted with rolling scars or boxcar scars.
Microneedling
Percutaneous collagen induction (PCI) therapy, also known more commonly as microneedling, is another non-energy-based treatment that is widely used to treat not only facial scars, such as acne scars, but also fine lines, wrinkles, rhytids, melasma, and other age-related dermatological conditions.60,61 Microneedling entails creating hundreds of pin-sized holes in the skin to stimulate rejuvenation of healthy skin.62 This technique was first developed in the early 21st century and many histometric studies since have reported increased epidermal thickness, upregulated elastin fiber and collagen lattice formation, and release of various regenerative growth factors in days and weeks following treatment.60,63–65 Due to the relative simplicity, low downtime, and minor complication rates of PCI, microneedling has been touted as a practical and efficacious modality of treatment for acne scarring.
Microneedling is most often performed with a standard medical dermaroller.66,67 The dermaroller consists of a rolling barrel with studded needles that is applied to the patient’s skin until pinpoint bleeding is observed.66 Other devices, such as the dermastamp, a similar device that features a handpiece attached to a needle-studded block, has been used in outpatient settings.68 Much more recently, automated microneedling stamp devices, such as the Dermapen, have come into favor for their increased safety and efficiency.69–71
As a monotherapy, microneedling has been shown to be most effective with rolling scars followed by boxcar scars and then ice pick scars.61,70,72,73 Although most studies show very minimal improvement in ice pick scars, a study by Gupta et al achieved mildly significant improvement in ice pick scars.74 The authors suggested that using a dermaroller with 2.0-mm needles could be responsible for these results because of the depth of ice pick scars, which would otherwise be harder to replicate with the more commonly used 1.5-mm needles.73
On the other hand, one interesting study demonstrated that 0.1% topical tazarotene therapy provided equal clinical benefit to microneedling.75 One possible explanation for this is that tazarotene, a retinoid, has been shown to increase epidermal thickness,76 similar to microneedling.60 More studies will need to be conducted to better understand if tazarotene, a simple at-home therapy, can be a suitable replacement for microneedling.
Because microneedling is an effective transdermal delivery adjunct, microneedling has been studied most commonly in combination with PRP therapy. Numerous RCTs, however, have reported mixed results regarding this combination therapy, with some reporting a significant increase in efficacy with the addition of PRP77,78 and more recent studies reporting no significant difference at all.64,74,79 It is important to note that all these studies have some variation in the frequency, application, and duration of combined therapy.
Other alternatives to PRP have also been explored over the years. Using topical insulin was shown to be more effective than PRP when combined with microneedling, with boxcar scars showing a significantly better response to the former.80 Amniotic fluid topical application also improved patient outcomes according to objective physician measures and histometric analysis noting an increase in epidermal thickness and neocollagenesis.81 Finally, various studies have shown that combining microneedling with chemical peels, including TCA and glycolic acid, have improved patient outcomes,64,72,82 while one study noted that Jessner’s solution did not enhance the effects of microneedling.70 More specifically, El-Domyati et al noted that increased epidermal thickness was especially apparent for skin treated with TCA and microneedling while PRP seemed to better augment collagen and elastin production.64
In all these studies, erythema, edema, and pain are the most commonly reported side effects of microneedling, although they normally resolve on their own.83 PIH was also not a point of concern, as it had a very low occurrence rate and persisted temporarily.84,85 Interestingly, hypertrophic scarring in the same needle distribution of microneedling devices used, resembling a “tram-trek” pattern, was noted by several authors over bony facial features (temples, forehead, etc).86,87 Extra caution is highly recommended over these treatment areas.
Microneedling has demonstrated the greatest success with rolling scars and boxcar scars but little improvement for ice pick scars. It has the ability to promote transdermal delivery of topical agents, which further adds to its versatility, and thus plays a significant role in combinatorial therapies.
Punch Techniques
Punch surgical techniques for acne scarring entails the use of a circular blade that is inserted through the skin and down to the subcutaneous tissue.4 The column of tissue can either be completely removed through punch excision, replaced with a skin graft through punch grafting, or elevated by lifting the tissue to the level of the surrounding healthy skin through punch elevation.4 Because punch excision approaches penetrate down to the subcutaneous fat, they are particularly useful for deep ice pick and boxcar scars, which are more resistant to alternative treatment methods, such as microneedling and subcision.73
Punch Excision
Punch excision is the simplest approach out of all punch techniques. Ice pick scars can be identified and subsequently removed with a punch biopsy tool whose diameter encompasses the entirety of the scar.88 The resulting 2 to 3-mm hole is then closed with sutures.88 For scars that are greater than 3 to 3.5 mm in diameter, elliptical closure or punch elevation, as described below, should be performed to prevent facial contour abnormalities.4 Although many scars can be excised at the same time, it is recommended that excisions be performed at least 4 to 5 mm apart to prevent excess skin tension following suture repair of all the punch sites.89 Any residual scars that need to be excised can then be done several weeks later after the previous sites have healed.4 Furthermore, it has been shown that residual scarring or textural irregularities can be further diminished with fractional laser resurfacing.90
Punch Grafting
While punch excision is an effective technique for treating ice pick scars, residual linear scars, although less noticeable than the original acne scars, are left behind at the punch sites.16 Punch grafting addresses this limitation with the added step of taking graft tissue, most commonly from the postauricular skin, and inserting it snugly into the punch site.91 Care must be taken to ensure that the proper graft size is obtained prior to insertion and the proper elevation after. Studies report that graft depression can occur, resulting in an uneven skin topography that can be correctable through dermal fillers, such as collagen, or skin resurfacing techniques, such as CO2 laser or dermabrasion, 4 to 6 weeks after punch excision is performed.91,92
Punch Elevation
Lastly, punch elevation is another punch technique that elevates the cylinder of tissue rather than completely excising it. Because of this, there is no concern for skin textural irregularities as seen with punch grafting.93 This technique provides the best results for boxcar scars due to their flat base, which can become flush with the surrounding skin upon elevation.94 One disadvantage is that scars can retract and impede healing around the margins of the punched tissue and leave a noticeable ring scar.73 Uneven skin topography can also result if not elevated optimally but can be corrected with laser resurfacing techniques.90,92
Punch techniques are particularly advantageous for deep ice pick and boxcar scars that are resistant to other treatment approaches and thus serve as a last resort. There are not many recent advances in this field, aside from the laser technologies that serve as adjuvant therapies.
Dermal Fillers
Dermal fillers are another popular mainstay option for patients with atrophic acne scars. The breadth of dermal filler types and brands is vast but easily distinguishable by their durability. They can be broadly categorized as short-lasting (temporary), semi-permanent, or long-lasting (permanent) (Table 3).95 While many filler brands have demonstrated highly efficacious results, patients’ varying preferences or qualms regarding permanence, adverse effects, mechanism of action, and economic value are important factors to consider when deciding which filler to administer.
Table 3.
Categorical Overview of Dermal Fillers
| Type | Estimated Longevity | Common Examples |
|---|---|---|
| Short-Lasting (Temporary) | 6–18 months | Hyaluronic acid (HA) Bovine/Porcine-sourced collagen |
| Semipermanent | 20–24 months | Poly-L-lactic acid (PLLA) Calcium hydroxylapatite (CaHA) |
| Long-Lasting (Permanent) | >5 years | Polymethylmethacrylate (PMMA) |
Notes: Adapted with permission from Dove Medical Press. Wollina U, Goldman A. Fillers for the improvement in acne scars. Clinical, Cosmetic and Investigational Dermatology. 2015;8:493–499.122
Short-Lasting Fillers
The varying durability of fillers can be attributed to their biodegradability.96 Hyaluronic acid (HA), for instance, is known to be a highly biodegradable substance that is naturally broken down by hyaluronidase in the body.97 Contemporary technological advances have stabilized HA through cross-linking, extending its clinical longevity to upwards of 6 to 18 months.98,99 The distinguishing characteristic of HA is that its effects are both immediate and gradual. Its hydrophilic properties enable immediate tissue volumization via water retention in the dermis upon injection.100 In the months to follow, fibroblast activation by HA leads to increased collagen production, promoting additional soft-tissue augmentation.95,100 HA can be further categorized as either monophasic (uniform mix of low-molecular-weight and high-molecular-weight HA) or biphasic (cross-linked HA in non-crosslinked HA suspension).101 In one study, microdose injections of biphasic HA in patients who had residual ice pick scarring even after 2 to 5 rounds of fractional laser resurfacing yielded high patient satisfaction scores.102 Similarly, injection of monophasic HA with a pneumatic injector in two patients led to 1 full-grade improvement in each patient.103 Interestingly, while biphasic fillers have shown to be more resistant to hyaluronidase degradation, monophasic fillers have a greater volumizing effect.104
Another short-lasting filler is exogenous collagen fillers. Collagen fillers can be derived from porcine or bovine sources and are injected directly in the deep dermal plane to restore volume loss caused by facial acne scarring.105 A major disadvantage with collagen fillers is their allergenic potential, which must be tested for every patient several weeks prior to injection.106 In a pilot study, bovine-sourced collagen was injected superficially in 18 patients, ages ranging 12 to 37 years old.107 Gradual cumulative improvement was noted across 6 sessions of injections, with the exception of ice pick scars. Shortly after, a more durable glutaraldehyde-linked collagen filler was developed and demonstrated not only better correction of facial acne scarring but also no allergenic reaction at the injection sites, unlike the previous version of bovine-sourced collagen.108 In a much more recent study, administration of porcine-derived collagen in one patient led to substantial correction of severe facial acne scarring with no adverse reactions.109
Semipermanent Fillers
Poly-L-lactic acid (PLLA) is another popular class of fillers that boasts a semipermanent biostimulatory effect.110 PLLA exerts its action by directly stimulating fibroblasts to synthesize collagen in the dermis.111 Unlike HA, results are not immediate and require several weeks to months for optimal collagen production and ECM remodeling.110 Results are reported to last around 24 months; however, a case report has shown that correction of rolling acne scars with PLLA can be maintained for up to 4 years.112 More importantly, PLLA has proven to be a more efficacious alternative to bovine collagen at 2-, 4-, and 6-month follow-ups, with greater patient satisfaction and no need for allergenic testing.113 As expected, however, PLLA yields very minimal correction for ice pick scars, as often seen with other acne scar treatment modalities.114 In addition, PLLA also runs the risk of bruising, erythema, pain, and nodule formation, especially if administered in the wrong dermal plane.101,115
Calcium hydroxylapatite (CaHA) is another semipermanent biostimulatory filler that similarly stimulates collagen production and fibrous tissue formation.116 Similar to PMMA fillers, CaHA fillers consist of microspheres, which are suspended in a carboxymethyl cellulose gel.117 Upon injection, the gel carrier is rapidly degraded, and the remaining microspheres are able to provide instant volume replacement.118 More significantly, long-term volume restoration is achieved through fibroblast activation, which upregulates collagen production for up to 24 months or longer.119 In one study, nine out of ten acne scar patients were injected with 0.1 to 0.3 mL of CaHA filler and displayed >50% improvement in their rolling scars at a 12-month follow-up evaluation. As observed with other fillers, ice pick scars displayed no improvement.117 Although only minor transient erythema and extrusions were reported in this study, transient bruising, ecchymosis, hematoma, and few reports of nodule formation have been noted in several studies.120
Permanent Fillers
In efforts to improve the outcome, safety, and longevity of collagen fillers, polymethylmethacrylate (PMMA) fillers were developed. Polymethylmethacrylate is a nonbiodegradable, synthetic polymer that was originally designed for dental and ophthalmic implants but was later adapted for soft-tissue augmentation.96 A histological study on the first commercially available PMMA filler, consisting of PMMA combined with Tween 80 medium, demonstrated fibrous capsule formation surrounding the microspheres along with neocollagenesis, resulting in substantial soft-tissue augmentation.121 Although results were substantial, the presence of nonuniform microsphere size and texture as well as unfiltered microscopic sediments in this PMMA filler led to a 2.5% rate of granuloma formation.96 Since then, more stringent purification steps, including ultrasonic bathing and wet sieving, have been incorporated to improve the uniformity of PMMA microspheres, resulting in better clinical outcomes for acne scar patients.122 The most commonly used PMMA filler today consists of 20% (v/v) PMMA suspended in bovine-sourced collagen and lidocaine. In the most recent clinical trial testing the efficacy of this PMMA filler for acne scarring, it was noted that 67 out of 97 patients had significant correction.123 In another independent study, the same filler had a 90% patient satisfaction.114 Both studies reported transient bruising, ecchymosis, erythema, and pain on the injection site.114 Surprisingly, granuloma formation was not reported in either study at 12-month and 7-month follow-ups, respectively. However, longer-term follow-up is necessary to better assess the safety profile of this PMMA filler, since granulomas are known to have a delayed occurrence.96
Fillers provide immediate and gradual correction of acne scars, particularly rolling scars. PMMA and collagen fillers have had a longer history of development, which has led to more published data regarding its efficacy for acne scar revision. However, there is still a dearth of studies evaluating the efficacy of more contemporary biostimulatory fillers for acne scars.
Thread Lifting
Thread lifting is a simple non-surgical facelift procedure in which a long barbed suture is inserted through the face and into the subcutaneous layer. Implantation of the suture suspends the underlying tissue, which not only mechanically lifts the skin but also triggers collagen synthesis through fibroblast activation. Originally, this was achieved with nonabsorbable threads, whose resistance to biodegradation and high tensile strength provide long-term cosmetic improvement. Despite their high duration of clinical efficacy, however, nonabsorbable threads have largely been replaced by less permanent absorbable threads, which yield shorter-term correction but offer greater biocompatibility and lower risk of infection.
Currently, there is only one study discussing the application of thread lifting for acne scar treatment. In this study, Donnarumma et al performed a thread lift procedure using an absorbable polydioxanone (PDO) barbed suture on five patients with predominantly boxcar scars, reporting an improvement score of 2 (“Very improved patient”) for 2 patients and a score of 3 (“Improved patient”) for 3 patients. Additionally, the authors observed via reflectance confocal microscopy the appearance of a more regular, fibrillar network of dermal fibers at 6 months following treatment. No adverse events were reported in the study.124
Further studies are needed to evaluate the effectiveness of thread lifting for acne scar treatment. Specifically, there should be further analysis of how acne scars respond to different types of threads available and varying surgical techniques. Despite the absence of complications in the study described above, the sample size is small. Known risks of thread lifting include bruising, bleeding, infection, skin dimpling, salivary and parotid gland injury, which calls for greater emphasis on the surgical experience and expertise of the physician.
Discussion
Acne scarring is a sequela of acne vulgaris and carries a heavy psychological burden for individuals suffering from this condition. To this day, treating acne scars still poses a significant challenge to physicians because of both its widespread prevalence and the absence of an all-encompassing clinical solution. Discovering treatment modalities that are both efficacious and highly customizable to the patient’s needs remains an important development in the field of cosmetic dermatology.
A closer analysis of available treatment modalities reveals that scar type is one of the most important factors for dermatologists to consider when assessing patient candidacy for various treatments (Table 4). This is because the depth and mechanism of controlled injury induced by each treatment modality must correlate with the depth and histological characteristics unique to each scar type.
Table 4.
Clinical Efficacy of Acne Scar Treatments per Atrophic Scar Subtype
| Scar Type | C/P | DA | MDA | SUB | MN | P/E | Filler | TL |
|---|---|---|---|---|---|---|---|---|
| Ice Pick | +++ | + | + | + | + | + | + | * |
| Deep Boxcar | ++ | + | + | ++ | + | +++ | ++ | * |
| Shallow Boxcar | ++ | ++ | ++ | ++ | ++ | - | ++ | * |
| Rolling | ++ | + | + | +++ | ++ | - | +++ | * |
Notes: +++, Good; ++, Moderate; +, Poor; -, Not recommended; *, Lack of evidence.
Abbreviations: C/P, chemical peel; DA, dermabrasion; MDA, microdermabrasion; SUB, subcision; MN, microneedling; P/E, punch excision; TL, thread lifting.
For rolling scars, published data have shown that chemical peels, microneedling, and fillers can lead to a noticeable reduction in their appearance. However, they do not address the underlying cause of the atrophic appearance of rolling scars, which is the presence of fibrotic tethers that attach the dermis to the subcutaneous layer. Subcision is the only approach that yields the mechanical force necessary to break the tethers and significantly raise the scar closer to the level of the surrounding tissue. Combining subcision with the aforementioned treatments can further optimize the effect of subcision and obviate the need for multiple subcision treatments.
Conversely, boxcar scars and ice pick scars are less responsive to subcision because they both lack tethering at the dermal-subcutaneous junction. While they still undergo moderate correction from neocollagenesis induced by mechanical abrasion of the dermis, other less invasive and more economical alternatives can provide similar, if not greater, clinical benefit. Shallow boxcar scars, for instance, can benefit from superficial chemical peeling, microdermabrasion, and microneedling, which target the epidermis and upper papillary dermis. Ice pick scars are particularly more difficult to treat, and a review of the literature shows that the most consistent and successful clinical outcomes are achieved with multiple sessions of TCA or phenol CROSS. Furthermore, more severe cases of deep boxcar and ice pick scarring that reach the deep reticular dermis or subcutaneous layer, require more invasive approaches, such as punch techniques, which replace the atrophic scar with a much less noticeable linear scar.
Of all the treatment modalities reviewed, thread lifting lacks the most evidence regarding its clinical efficacy in the context of acne scarring. Nevertheless, the one report124 that exists describing its application for acne scar correction provides a renewed perspective on the use of absorbable sutures. As discussed earlier, Balighi et al attempted to enhance the effect of subcision by using absorbable cat gut sutures as subdermal implants under subcised scars.48 While this study reported no added benefit with this additional step, contrasting results between these two studies highlight that this could be due to differences in suture biomaterial and design, surgical technique, and combinatorial treatment approaches. Greater evidence supporting the clinical feasibility of suture implants could potentially expand the armamentarium of acne scar treatments.
Clinical success with the treatments discussed requires extensive knowledge of facial skin anatomy, experience, and meticulous technical execution by the surgeon. For instance, invasive procedures, such as subcision, filler injections and thread lifting, are prone to complications if not done in the proper dermal plane, whereas simple field treatment with superficial chemical peels requires a much less complicated approach. Furthermore, patients’ genetics, age, and Fitzpatrick skin type, all of which affect the skin’s ability to respond favorably to controlled injury and produce collagen, can heavily influence treatment outcomes.125 Therefore, “test” treatments on scars should be performed when feasible to assess efficacy and safety prior to full implementation.
Other factors to consider when optimizing treatment plans fall outside clinical consideration. Patient expectations, downtime, and cost can be influential in clinical decision-making when it comes to acne scar treatment. For instance, patients with rolling scars that desire instantaneous results may decide to pursue HA fillers but will have to repeat treatments every 1 to 1.5 years to maintain correction. On the other hand, patients with ice pick scars can decide to undergo multiple rounds of slow chemical-based correction or undergo invasive punch excisions to remove scars instantly while still running the risk of slight skin textural differences.
It is important to note that the papers discussed in this review are highly variable in terms of clinical methodology, expertise, physician and patient assessment, strength of evidence, grading scales, sample size, follow-up intervals, and more. Therefore, it is impossible to compare studies in a completely objective manner given that these studies employ their own approach to scientific inquiry. Nevertheless, a thorough review of studies in the past few decades still sheds light on comparative treatments with various efficacies for acne scarring and recent advances in the field.
Conclusion
The continual strive for improving existing non-energy-based acne scar treatments has been evident in the past few decades and still remains a goal in clinical and cosmetic dermatology. As seen across countless studies discussed in this review, there is no universal remedy for acne scarring because every patient’s acne scar profile is unique and requires a tailored treatment approach. The studies nevertheless shed light on the strengths and weaknesses of each approach done alone or in strategic combination with each other. Although the authors differ in their scientific execution and interpretation, a review of the existing literature provides a broad yet flexible clinical framework for dermatologists seeking to participate in and advance the field of acne scar revision.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Oge’ LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100(8):475–484. [PubMed] [Google Scholar]
- 2.Thiboutot DM. An overview of acne and its treatment. Cutis. 1996;57(1Suppl):8–12. [PubMed] [Google Scholar]
- 3.Fabbrocini G, Annunziata MC, D’Arco V, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010:1–13. doi: 10.1155/2010/893080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45(1):109–117. doi: 10.1067/mjd.2001.113451 [DOI] [PubMed] [Google Scholar]
- 5.Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11(8):21–28. [PMC free article] [PubMed] [Google Scholar]
- 6.Rendon MI, Berson DS, Cohen JL, Roberts WE, Starker I, Wang B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3(7):32–43. [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor AA, Lowe PM, Shumack S, Lim AC. Chemical peels: a review of current practice. Australas J Dermatol. 2018;59(3):171–181. doi: 10.1111/ajd.12715 [DOI] [PubMed] [Google Scholar]
- 8.Erbagci Z, Akcali C. Biweekly serial glycolic acid peels vs. long-term daily use of topical low-strength glycolic acid in the treatment of atrophic acne scars. Int J Dermatol. 2000;39(10):789–794. doi: 10.1046/j.1365-4362.2000.00076.x [DOI] [PubMed] [Google Scholar]
- 9.Harris DWS, Buckley CC, Ostlere IS, Rustin MHA. Topical retinoic acid in the treatment of fine acne scarring. Br J Dermatol. 1991;125(1):81–82. doi: 10.1111/j.1365-2133.1991.tb06048.x [DOI] [PubMed] [Google Scholar]
- 10.Knor T. Flattening of atrophic acne scars by using tretinoin by iontophoresis. Acta Dermatovenerol Croat. 2004;12(2):84–91. [PubMed] [Google Scholar]
- 11.Sachdeva S. Lactic acid peeling in superficial acne scarring in Indian skin. J Cosmet Dermatol. 2010;9(3):246–248. [DOI] [PubMed] [Google Scholar]
- 12.Berardesca E, Cameli N, Primavera G, Carrera M. Clinical and instrumental evaluation of skin improvement after treatment with a new 50% pyruvic acid peel. Dermatol Surg. 2006;32(4):526–531. doi: 10.1111/j.1524-4725.2006.32106.x [DOI] [PubMed] [Google Scholar]
- 13.Garg VK, Sinha S, Sarkar R. Glycolic acid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: a comparative study. Dermatol Surg. 2009;35(1):59–65. doi: 10.1111/j.1524-4725.2008.34383.x [DOI] [PubMed] [Google Scholar]
- 14.Chandrashekar B, Ashwini K, Vasanth V, Navale S. Retinoic acid and glycolic acid combination in the treatment of acne scars. Indian Dermatol Online J. 2015;6(2):84. doi: 10.4103/2229-5178.153007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puri N. Efficacy of modified Jessner′s peel and 20% TCA versus 20% TCA peel alone for the treatment of acne scars. J Cutan Aesthet Surg. 2015;8(1):42. doi: 10.4103/0974-2077.155082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gozali MV, Zhou B, Luo D. Effective treatments of atrophic acne scars. J Clin Aesthet Dermatol. 2015;8(5):33–40. [PMC free article] [PubMed] [Google Scholar]
- 17.Boen M, Jacob C. A review and update of treatment options using the acne scar classification system. Dermatol Surg. 2019;45(3):411–422. doi: 10.1097/DSS.0000000000001765 [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Khachemoune A, Rashid RM. Chemical burn following 50% trichloroacetic acid for acne: presentation of a case and a focused review. J Dermatol Dermatol Surg. 2016;20(1):71–74. doi: 10.1016/j.jdds.2015.06.001 [DOI] [Google Scholar]
- 19.Fabbrocini G, Cacciapuoti S, Fardella N, Pastore F, Monfrecola G. CROSS technique: chemical reconstruction of skin scars method. Dermatol Ther. 2008;21(SUPPL. 3):29–32. doi: 10.1111/j.1529-8019.2008.00239.x [DOI] [PubMed] [Google Scholar]
- 20.Handog E, Singzon I, Datuin MS. Chemical peels for acne and acne scars in asians: evidence based review. J Cutan Aesthet Surg. 2012;5(4):239. doi: 10.4103/0974-2077.104911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JB, Chung WG, Kwahck H, Lee KH. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg. 2002;28(11):1017–21; discussion 1021. doi: 10.1046/j.1524-4725.2002.02095.x [DOI] [PubMed] [Google Scholar]
- 22.Khunger N, Bhardwaj D, Khunger M. Evaluation of CROSS technique with 100% TCA in the management of ice pick acne scars in darker skin types. J Cosmet Dermatol. 2011;10(1):51–57. doi: 10.1111/j.1473-2165.2010.00526.x [DOI] [PubMed] [Google Scholar]
- 23.Nikalji N, Patil S, Sakhiya J, Godse K, Nadkarni N. Complications of medium depth and deep chemical peels. J Cutan Aesthet Surg. 2012;5(4):254. doi: 10.4103/0974-2077.104913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veenstra JJ, Fakhoury JW, Ozog D. Worsening of acne scars from trichloroacetic acid CROSS delivered via micropipette: a case report. J Clin Aesthet Dermatol. 2021;14(4):41–42. [PMC free article] [PubMed] [Google Scholar]
- 25.Chung HJ, Al Janahi S, Cho SB, Chang YC. Chemical reconstruction of skin scars (CROSS) method for atrophic scars: a comprehensive review. J Cosmet Dermatol. 2021;20(1):18–27. doi: 10.1111/jocd.13556 [DOI] [PubMed] [Google Scholar]
- 26.Hevia O, Nemeth AJ, Taylor JR. Tretinoin accelerates healing after trichloroacetic acid chemical peel. Arch Dermatol. 1991;127(5):678. doi: 10.1001/archderm.1991.01680040086008 [DOI] [PubMed] [Google Scholar]
- 27.Dalpizzol M, Weber MB, Mattiazzi APF, Manzoni APD. Comparative study of the use of trichloroacetic acid and phenolic acid in the treatment of atrophic-type acne scars. Dermatol Surg. 2016;42(3):377–383. doi: 10.1097/DSS.0000000000000606 [DOI] [PubMed] [Google Scholar]
- 28.Levy LL, Zeichner JA. Management of acne scarring, part II a comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol. 2012;13(5):331–340. [DOI] [PubMed] [Google Scholar]
- 29.Aronsson A, Eriksson T, Jacobsson S, Salemark L. Effects of dermabrasion on acne scarring. A review and a study of 25 cases. Acta Derm Venereol. 1997;77(1):39–42. doi: 10.2340/0001555577039042 [DOI] [PubMed] [Google Scholar]
- 30.Hirsch RJ, Lewis AB. Treatment of acne scarring. Semin Cutan Med Surg. 2001;20(3):190–198. doi: 10.1053/sder.2001.27557 [DOI] [PubMed] [Google Scholar]
- 31.Rubenstein R, Roenigk HH, Stegman SJ, Hanke CW. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2):280–285. doi: 10.1016/S0190-9622(86)70167-9 [DOI] [PubMed] [Google Scholar]
- 32.Bagatin E, dos Santos Guadanhim LR, Yarak S, Kamamoto CSL, de Almeida FA. Dermabrasion for acne scars during treatment with oral isotretinoin. Dermatol Surg. 2010;36(4):483–489. doi: 10.1111/j.1524-4725.2010.01474.x [DOI] [PubMed] [Google Scholar]
- 33.Bhalla M, Thami GP. Microdermabrasion: reappraisal and brief review of literature. Dermatol Surg. 2006;32(6):809–814. doi: 10.1111/j.1524-4725.2006.32165.x [DOI] [PubMed] [Google Scholar]
- 34.El‐Domyati M, Hosam W, Abdel‐Azim E, Abdel‐Wahab H, Mohamed E. Microdermabrasion: a clinical and histopathologic study. J Cosmet Dermatol. 2016;15(4):503–513. [DOI] [PubMed] [Google Scholar]
- 35.Alkhawam L, Alam M. Dermabrasion and microdermabrasion. Facial Plast Surg. 2009;25(5):301–310. doi: 10.1055/s-0029-1243078 [DOI] [PubMed] [Google Scholar]
- 36.Tsai RY, Wang CN, Chan HL. Aluminum oxide crystal microdermabrasion. Dermatol Surg. 1995;21(6):539–542. doi: 10.1111/j.1524-4725.1995.tb00258.x [DOI] [PubMed] [Google Scholar]
- 37.Alam M, Omura NE, Dover JS, Arndt KA. Glycolic acid peels compared to microdermabrasion: a right-left controlled trial of efficacy and patient satisfaction. Dermatol Surg. 2002;28(6):475–479. doi: 10.1046/j.1524-4725.2002.01144.x [DOI] [PubMed] [Google Scholar]
- 38.Shim EK, Barnette D, Hughes K, Greenway HT. Microdermabrasion: a clinical and histopathologic study. Dermatol Surg. 2001;27(6):524–530. doi: 10.1046/j.1524-4725.2001.01001.x [DOI] [PubMed] [Google Scholar]
- 39.Andrews SN, Zarnitsyn V, Bondy B, Prausnitz MR. Optimization of microdermabrasion for controlled removal of stratum corneum. Int J Pharm. 2011;407(1–2):95–104. doi: 10.1016/j.ijpharm.2011.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21(6):543–549. doi: 10.1111/j.1524-4725.1995.tb00259.x [DOI] [PubMed] [Google Scholar]
- 41.Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg. 2005;31(3):310–317. doi: 10.1097/00042728-200503000-00011 [DOI] [PubMed] [Google Scholar]
- 42.Nilforoushzadeh MA, Lotfi E, Heidari-kharaji M, Nickhah N, Alavi S, Mahmoudbeyk M. Comparing cannula-based subcision with the common needle method: a clinical trial. Skin Res Technol. 2020;26(1):39–44. doi: 10.1111/srt.12761 [DOI] [PubMed] [Google Scholar]
- 43.Ramadan SA, El-Komy MHM, Bassiouny DA, El-Tobshy SA. Subcision versus 100% trichloroacetic acid in the treatment of rolling acne scars. Dermatol Surg. 2011;37(5):626–633. doi: 10.1111/j.1524-4725.2011.01954.x [DOI] [PubMed] [Google Scholar]
- 44.Bhargava S, Kumar U, Varma K. Subcision and microneedling as an inexpensive and safe combination to treat atrophic acne scars in dark skin: a prospective study of 45 patients at a tertiary care center. J Clin Aesthet Dermatol. 2019;12(8):18–22. [PMC free article] [PubMed] [Google Scholar]
- 45.Barikbin B, Akbari Z, Yousefi M, Dowlati Y. Blunt blade subcision: an evolution in the treatment of atrophic acne scars. Dermatol Surg. 2017;43:S57–S63. doi: 10.1097/DSS.0000000000000650 [DOI] [PubMed] [Google Scholar]
- 46.Bhargava S, Kroumpouzos G, Varma K, Kumar U. Combination therapy using subcision, needling, and platelet-rich plasma in the management of grade 4 atrophic acne scars: a pilot study. J Cosmet Dermatol. 2019;18(4):1092–1097. doi: 10.1111/jocd.12935 [DOI] [PubMed] [Google Scholar]
- 47.Aalami Harandi S, Balighi K, Lajevardi V, Akbari E. Subcision-suction method: a new successful combination therapy in treatment of atrophic acne scars and other depressed scars. J Eur Acad Dermatol Venereol. 2011;25(1):92–99. doi: 10.1111/j.1468-3083.2010.03711.x [DOI] [PubMed] [Google Scholar]
- 48.Balighi K, Robati RM, Moslehi H, Robati AM. Subcision in acne scar with and without subdermal implant: a clinical trial. J Eur Acad Dermatol Venereol. 2008;22(6):707–711. doi: 10.1111/j.1468-3083.2008.02583.x [DOI] [PubMed] [Google Scholar]
- 49.Gadkari R, Nayak C. A split-face comparative study to evaluate efficacy of combined subcision and dermaroller against combined subcision and cryoroller in treatment of acne scars. J Cosmet Dermatol. 2014;13(1):38–43. doi: 10.1111/jocd.12071 [DOI] [PubMed] [Google Scholar]
- 50.Al-Niaimi F. Laser and energy-based devices complications in dermatology. J Cosmet Laser Ther. 2016;18(1):25–30. doi: 10.3109/14764172.2015.1052511 [DOI] [PubMed] [Google Scholar]
- 51.Kaur J, Kalsy J. Subcision plus 50% trichloroacetic acid chemical reconstruction of skin scars in the management of atrophic acne scars: a cost-effective therapy. Indian Dermatol Online J. 2014;5(1):95. doi: 10.4103/2229-5178.126053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fulchiero GJ, Parham-Vetter PC, Obagi S. Subcision and 1320-Nm Nd:YAG nonablative laser resurfacing for the treatment of acne scars: a simultaneous split-face single patient trial. Dermatol Surg. 2004;30(10):1356–1360. doi: 10.1111/j.1524-4725.2004.30411.x [DOI] [PubMed] [Google Scholar]
- 53.Deshmukh NS, Belgaumkar VA. Platelet-rich plasma augments subcision in atrophic acne scars: a split-face comparative study. Dermatol Surg. 2019;45(1):90–98. doi: 10.1097/DSS.0000000000001614 [DOI] [PubMed] [Google Scholar]
- 54.Dadkhahfar S, Robati RM, Gheisari M, Moravvej H. Subcision: indications, adverse reactions, and pearls. J Cosmet Dermatol. 2020;19(5):1029–1038. doi: 10.1111/jocd.13308 [DOI] [PubMed] [Google Scholar]
- 55.Taylor MB, Zaleski-Larsen L, McGraw TA. Single session treatment of rolling acne scars using tumescent anesthesia, 20% trichloracetic acid extensive subcision, and fractional CO2 laser. Dermatol Surg. 2017;43:S70–S74. doi: 10.1097/DSS.0000000000000895 [DOI] [PubMed] [Google Scholar]
- 56.Taylor MB, Koron N. Combined treatment of rolling acne scars in ethnic skin using extensive subcision, trichloracetic acid peel, and fractional ablative erbium laser. Dermatol Surg. 2021;47(4):496–499. doi: 10.1097/DSS.0000000000002858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gheisari M, Iranmanesh B, Saghi B. Blunt cannula subcision is more effective than Nokor needle subcision for acne scars treatment. J Cosmet Dermatol. 2019;18(1):192–196. doi: 10.1111/jocd.12523 [DOI] [PubMed] [Google Scholar]
- 58.Graivier M. Wire subcision for complete release of depressions, subdermal attachments, and scars. Aesthet Surg J. 2006;26(4):387–394. doi: 10.1016/j.asj.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 59.Nilforoushzadeh MA, Fakhim T, Heidari-Kharaji M, Hanifnia AR, Hejazi S, Torkamaniha E. Efficacy evaluation of Endolift-based Subcision on acne scar treatment. J Cosmet Dermatol. 2021;20(8):2579–2582. doi: 10.1111/jocd.13876 [DOI] [PubMed] [Google Scholar]
- 60.Aust MC, Fernandes D, Kolokythas P, Kaplan HM, Vogt PM. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121(4):1421–1429. doi: 10.1097/01.prs.0000304612.72899.02 [DOI] [PubMed] [Google Scholar]
- 61.Bandral MR, Padgavankar PH, Japatti SR, Gir PJ, Siddegowda CY, Gir RJ. Clinical evaluation of microneedling therapy in the management of facial scar: a prospective randomized study. J Maxillofac Oral Surg. 2019;18(4):572–578. doi: 10.1007/s12663-018-1155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iriarte C, Awosika O, Rengifo-Pardo M, Ehrlich A. Review of applications of microneedling in dermatology. Clin Cosmet Investig Dermatol. 2017;10:289–298. doi: 10.2147/CCID.S142450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aust MC, Reimers K, Repenning C, et al. Percutaneous collagen induction: minimally invasive skin rejuvenation without risk of hyperpigmentation-fact or fiction? Plast Reconstr Surg. 2008;122(5):1553–1563. doi: 10.1097/PRS.0b013e318188245e [DOI] [PubMed] [Google Scholar]
- 64.El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison. J Cosmet Dermatol. 2018;17(1):73–83. doi: 10.1111/jocd.12459 [DOI] [PubMed] [Google Scholar]
- 65.El-Domyati M, Barakat M, Awad S, Medhat W, El-fakahany H, Farag H. Microneedling therapy for atrophic acne scars an objective evaluation. J Clin Aesthet Dermatol. 2015;8(7):36. [PMC free article] [PubMed] [Google Scholar]
- 66.Doddaballapur S. Microneedling with dermaroller. J Cutan Aesthet Surg. 2009;2(2):110. doi: 10.4103/0974-2077.58529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7(4):244. doi: 10.4103/2229-5178.185468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dsouza L, Ghate VM, Lewis SA. Derma rollers in therapy: the transition from cosmetics to transdermal drug delivery. Biomed Microdevices. 2020;22(4). doi: 10.1007/s10544-020-00530-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saadawi AN, Esawy AM, Kandeel AH, El-Sayed W. Microneedling by dermapen and glycolic acid peel for the treatment of acne scars: comparative study. J Cosmet Dermatol. 2019;18(1):107–114. doi: 10.1111/jocd.12827 [DOI] [PubMed] [Google Scholar]
- 70.Ali B, ElMahdy N, Elfar NN. Microneedling (Dermapen) and Jessner’s solution peeling in treatment of atrophic acne scars: a comparative randomized clinical study. J Cosmet Laser Ther. 2019;21(6):357–363. doi: 10.1080/14764172.2019.1661490 [DOI] [PubMed] [Google Scholar]
- 71.Khalid FA, Ahmad S, Mehrose MY, et al. Efficacy of micro-needling on post acne scars. J Ayub Med Coll Abbottabad. 2019;31(3):336–339. [PubMed] [Google Scholar]
- 72.Rana S, Mendiratta V, Chander R. Efficacy of microneedling with 70 % glycolic acid peel vs microneedling alone in treatment of atrophic acne scars — a randomized controlled trial. J Cosmet Dermatol. 2017;16(4):454–459. doi: 10.1111/jocd.12377 [DOI] [PubMed] [Google Scholar]
- 73.Gupta A, Kaur M, Patra S, Khunger N, Gupta S. Evidence-based surgical management of post-acne scarring in skin of color. J Cutan Aesthet Surg. 2021;13(2):124–141. doi: 10.4103/JCAS.JCAS_154_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta M, Barman KD, Sarkar R. A comparative study of microneedling alone versus along with platelet-rich plasma in acne scars. J Cutan Aesthet Surg. 2021;14(1):64–71. doi: 10.4103/JCAS.JCAS_190_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Afra TP, Razmi MT, Narang T, Dogra S, Kumar A. Topical tazarotene gel, 0.1%, as a novel treatment approach for atrophic postacne scars: a randomized active-controlled clinical trial. JAMA Facial Plast Surg. 2019;21(2):125–132. doi: 10.1001/jamafacial.2018.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machtinger LA, Kaidbey K, Lim J, et al. Histological effects of tazarotene 0.1% cream vs. vehicle on photodamaged skin: a 6-month, multicentre, double-blind, randomized, vehicle-controlled study in patients with photodamaged facial skin. Br J Dermatol. 2004;151(6):1245–1252. doi: 10.1111/j.1365-2133.2004.06186.x [DOI] [PubMed] [Google Scholar]
- 77.Fabbrocini G, de Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24(4):177–183. [Google Scholar]
- 78.Porwal S, Chahar YS, Singh PK. A comparative study of combined dermaroller and platelet-rich plasma versus dermaroller alone in acne scars and assessment of quality of life before and after treatment. Indian J Dermatol. 2018;63(5):403–408. doi: 10.4103/ijd.IJD_118_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatol Treat. 2018;29(3):281–286. doi: 10.1080/09546634.2017.1365111 [DOI] [PubMed] [Google Scholar]
- 80.Pawar M, Singh M. Microneedling with autologous platelet-rich plasma versus microneedling with topical insulin in the treatment of postacne atrophic scars: a simultaneous split-face comparative study. J Am Acad Dermatol. 2021;84(3):810–811. doi: 10.1016/j.jaad.2020.05.152 [DOI] [PubMed] [Google Scholar]
- 81.El-Domyati M, Moftah NH, Nasif GA, Ragaie MH, Ibrahim MR, Ameen SW. Amniotic fluid-derived mesenchymal stem cell products combined with microneedling for acne scars: a split-face clinical, histological, and histometric study. J Cosmet Dermatol. 2019;18(5):1300–1306. doi: 10.1111/jocd.13039 [DOI] [PubMed] [Google Scholar]
- 82.Sharad J. Combination of microneedling and glycolic acid peels for the treatment of acne scars in dark skin. J Cosmet Dermatol. 2011;10(4):317–323. doi: 10.1111/j.1473-2165.2011.00583.x [DOI] [PubMed] [Google Scholar]
- 83.Gowda A, Healey B, Ezaldein H, Merati M. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14(1):45–54. [PMC free article] [PubMed] [Google Scholar]
- 84.Leheta T, El Tawdy A, Abdel Hay R, Farid S. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37(2):207–216. doi: 10.1111/j.1524-4725.2010.01854.x [DOI] [PubMed] [Google Scholar]
- 85.Thi Kim CN, Thi LP, Van TN, et al. Successful treatment of facial atrophic acne scars by fractional radiofrequency microneedle in Vietnamese patients. Open Access Maced J Med Sci. 2019;7(2):192–194. doi: 10.3889/oamjms.2019.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13(3):180–187. doi: 10.1111/jocd.12095 [DOI] [PubMed] [Google Scholar]
- 87.Pahwa M, Pahwa P, Zaheer A. “Tram track effect” after treatment of acne scars using a microneedling device. Dermatol Surg. 2012;38(7PART 1):1107–1108. doi: 10.1111/j.1524-4725.2012.02441.x [DOI] [PubMed] [Google Scholar]
- 88.Fife D. Practical evaluation and management of atrophic acne scars: tips for the general dermatologist. J Clin Aesthet Dermatol. 2011;4(8):50–57. [PMC free article] [PubMed] [Google Scholar]
- 89.Callaghan DJ. Review on the treatment of scars. Plast Aesthet Res. 2020;2020. doi: 10.20517/2347-9264.2020.166 [DOI] [Google Scholar]
- 90.Kim HS, Cho EJ, Park YM, Kim HO, Lee JY. Punch excision combined with erbium: yAGfractional laser: its application on different types of scars in Asian patients (a pilot study). J Cosmet Laser Ther. 2011;13(4):196–199. doi: 10.3109/14764172.2011.594064 [DOI] [PubMed] [Google Scholar]
- 91.Johnson WC. Treatment of pitted scars: punch transplant technique. J Dermatol Surg Oncol. 1986;12(3):260–265. [DOI] [PubMed] [Google Scholar]
- 92.Grevelink JM, White VR. Concurrent use of laser skin resurfacing and punch excision in the treatment of facial acne scarring. Dermatol Surg. 1998;24(5):527–530. doi: 10.1111/j.1524-4725.1998.tb04201.x [DOI] [PubMed] [Google Scholar]
- 93.Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59(4):659–676. doi: 10.1016/j.jaad.2008.05.029 [DOI] [PubMed] [Google Scholar]
- 94.Nouraei S, Asilian A, Keyvan S, et al. Efficacy of punch elevation combined with fractional carbon dioxide laser resurfacing in facial atrophic acne scarring: a randomized split-face clinical study. Indian J Dermatol. 2015;60(5):473. doi: 10.4103/0019-5154.159616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wollina U, Goldman A. Hyaluronic acid dermal fillers: safety and efficacy for the treatment of wrinkles, aging skin, body sculpturing and medical conditions. Clin Med Rev Ther. 2011;3:107–121. doi: 10.4137/cmrt.s6928 [DOI] [Google Scholar]
- 96.Gold MH, Sadick NS. Optimizing outcomes with polymethylmethacrylate fillers. J Cosmet Dermatol. 2018;17(3):298–304. doi: 10.1111/jocd.12539 [DOI] [PubMed] [Google Scholar]
- 97.Buhren BA, Schrumpf H, Bölke E, Kammers K, Gerber PA. Standardized in vitro analysis of the degradability of hyaluronic acid fillers by hyaluronidase. Eur J Med Res. 2018;23(1). doi: 10.1186/s40001-018-0334-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prasetyo AD, Prager W, Rubin MG, Moretti EA, Nikolis A. Hyaluronic acid fillers with cohesive polydensified matrix for soft-tissue augmentation and rejuvenation: a literature review. Clin Cosmet Investig Dermatol. 2016;9:257–280. doi: 10.2147/CCID.S106551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hussain SN, Goodman GJ, Rahman E. Treatment of a traumatic atrophic depressed scar with hyaluronic acid fillers: a case report. Clin Cosmet Investig Dermatol. 2017;10:285–287. doi: 10.2147/CCID.S132626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turlier V, Delalleau A, Casas C, et al. Association between collagen production and mechanical stretching in dermal extracellular matrix: in vivo effect of cross-linked hyaluronic acid filler. A randomised, placebo-controlled study. J Dermatol Sci. 2013;69(3):187–194. doi: 10.1016/j.jdermsci.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 101.da Costa A, Biccigo DGZ, de Souza Weimann ET, et al. Durability of three different types of hyaluronic acid fillers in skin: are there differences among biphasic, monophasic monodensified, and monophasic polydensified products? Aesthet Surg J. 2017;37(5):573–581. doi: 10.1093/asj/sjw161 [DOI] [PubMed] [Google Scholar]
- 102.Halachmi S, Ben Amitai D, Lapidoth M. Treatment of acne scars with hyaluronic acid: an improved approach. J Drugs Dermatol. 2013;12(7):e121–3. [PubMed] [Google Scholar]
- 103.Patel T, Tevet O. Effective treatment of acne scars using pneumatic injection of hyaluronic acid. J Drugs Dermatol. 2015;14(1):74–76. [PubMed] [Google Scholar]
- 104.Park KY, Kim HK, Kim BJ. Comparative study of hyaluronic acid fillers by in vitro and in vivo testing. J Eur Acad Dermatol Venereol. 2014;28(5):565–568. doi: 10.1111/jdv.12135 [DOI] [PubMed] [Google Scholar]
- 105.Lee JH, Choi YS, Kim SM, Kim YJ, Rhie JW, Jun YJ. Efficacy and safety of porcine collagen filler for nasolabial fold correction in Asians: a prospective multicenter, 12 months follow-up study. J Korean Med Sci. 2014;29:S217–S221. doi: 10.3346/jkms.2014.29.S3.S217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. doi: 10.2147/CCID.S50546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Varnavides CK, Forster RA, Cunliffe WJ. The role of bovine collagen in the treatment of acne scars. Br J Dermatol. 1987;116(2):199–206. doi: 10.1111/j.1365-2133.1987.tb05812.x [DOI] [PubMed] [Google Scholar]
- 108.Elson ML. Clinical assessment of Zyplast implant: a year of experience for soft tissue contour correction. J Am Acad Dermatol. 1988;18(4):707–713. doi: 10.1016/S0190-9622(88)70094-8 [DOI] [PubMed] [Google Scholar]
- 109.Smith KC. Repair of acne scars with dermicol-P35. Aesthet Surg J. 2009;29(3SUPPL):S16–S18. doi: 10.1016/j.asj.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 110.Fitzgerald R, Bass LM, Goldberg DJ, Graivier MH, Lorenc ZP. Physiochemical characteristics of Poly-L-Lactic Acid (PLLA). Aesthet Surg J. 2018;38:S13–S17. doi: 10.1093/asj/sjy012 [DOI] [PubMed] [Google Scholar]
- 111.Kim SA, Kim HS, Jung JW, Suh SI, Ryoo YW. Poly-l-lactic acid increases collagen gene expression and synthesis in cultured dermal fibroblast (Hs68) through the p38 MAPK pathway. Ann Dermatol. 2019;31(1):97–100. doi: 10.5021/ad.2019.31.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sapra S, Stewart JA, Mraud K, Schupp R. A Canadian study of the use of poly-l-lactic acid dermal implant for the treatment of hill and valley acne scarring. Dermatol Surg. 2015;41(5):587–594. doi: 10.1097/DSS.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 113.Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of restylane versus zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29(6):588–595. doi: 10.1046/j.1524-4725.2003.29150.x [DOI] [PubMed] [Google Scholar]
- 114.Joseph JH, Shamban A, Eaton L, et al. Polymethylmethacrylate collagen gel–injectable dermal filler for full face atrophic acne scar correction. Dermatol Surg. 2019;45(12):1558–1566. doi: 10.1097/DSS.0000000000001863 [DOI] [PubMed] [Google Scholar]
- 115.Beer K. A single-center, open-label study on the use of injectable poly-L-lactic acid for the treatment of moderate to severe scarring from acne or varicella. Dermatol Surg. 2007;33(SUPPL. 2):159–167. doi: 10.1111/j.1524-4725.2007.33356.x [DOI] [PubMed] [Google Scholar]
- 116.Bagal A, Dahiya R, Tsai V, Adamson PA. Clinical experience with polymethylmethacrylate microspheres (artecoll) for soft-tissue augmentation: a retrospective review. Arch Facial Plast Surg. 2007;9(4):275–280. doi: 10.1001/archfaci.9.4.275 [DOI] [PubMed] [Google Scholar]
- 117.Goldberg DJ, Amin S, Hussain M. Acne scar correction using calcium hydroxylapatite in a carrier-based gel. J Cosmet Laser Ther. 2006;8(3):134–136. doi: 10.1080/14764170600891632 [DOI] [PubMed] [Google Scholar]
- 118.Jacoveila PF. Use of calcium hydroxylapatite (Radiesse®) for facial augmentation. Clin Interv Aging. 2008;3(1):161–174. doi: 10.2147/CIA.S2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Loghem J, Yutskovskaya A, Werschler WP. Calcium hydroxylapatite over a decade of clinical experience. J Clin Aesthet Dermatol. 2015;8(1):38. [PMC free article] [PubMed] [Google Scholar]
- 120.Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16(2):152–161. doi: 10.1111/jocd.12326 [DOI] [PubMed] [Google Scholar]
- 121.Lemperle G, Ott H, Charrier U, Hecker J, Lemperle M. PMMA microspheres for intradermal implantation: part I. Animal research. Ann Plast Surg. 1991;26(1):57–63. doi: 10.1097/00000637-199101000-00009 [DOI] [PubMed] [Google Scholar]
- 122.Wollina U, Goldman A. Fillers for the improvement in acne scars. Clin Cosmet Investig Dermatol. 2015;8:493–499. doi: 10.2147/CCID.S86478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.US Food and Drug Administration. FDA; 2014. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020012S009b.pdf. Accessed September 24, 2021.
- 124.Donnarumma M, Vastarella M, Ferrillo M, Cantelli M, D’andrea M, Fabbrocini G. An innovative treatment for acne scars with thread-lift technique: our experience. G Ital Dermatol Venereol. 2019. doi: 10.23736/S0392-0488.19.06313-2 [DOI] [PubMed] [Google Scholar]
- 125.English RS, Shenefelt PD. Keloids and hypertrophic scars. Dermatol Surg. 1999;25(8):631–638. doi: 10.1046/j.1524-4725.1999.98257.x [DOI] [PubMed] [Google Scholar]