Abstract
Sources of exposure to per- and polyfluorinated alkyl substances (PFAS) include food, water, and given that humans spend typically 90% of our time indoors, air and dust. Quantifying PFAS prevalent indoors, such as neutral, volatile PFAS, and estimating their exposure risk to humans is thus important. To accurately measure these compounds indoors, polyethylene (PE) sheets were employed and validated as passive detection tools, and analyzed by gas chromatography-mass spectrometry. Air concentrations were compared to dust and carpet concentrations reported elsewhere. Partitioning between PE sheets of different thicknesses suggested that interactions of the PEs with the compounds are occurring by absorption. Volatile PFAS, specifically fluorotelomer alcohols (FTOHs), were ubiquitous in indoor environments. For example, in carpeted Californian kindergarten classrooms, 6:2 FTOH dominated with concentrations ranging from 9-600 ng m−3, followed by 8:2 FTOH. Concentrations of volatile PFAS from air, carpet and dust were closely related to each other, indicating that carpets and dust are major sources of FTOHs in air. Nonetheless, air posed the largest exposure risk of FTOHs and biotransformed perfluorinated alkyl acids (PFAA) in young children. This research highlights inhalation of indoor air as an important exposure pathway and the need for further reduction of precursors to PFAA.
Keywords: Passive sampling, Polyethylene sheets, PFAS precursors, gas-phase, Carpet, Dust, Risk Assessment
Graphical Abstract
Introduction
Human exposure to fluorotelomer alcohols (FTOHs), perfluorooctane sulfonamides (FOSAs) and perfluorooctane sulfonamidoethanols (FOSEs) and other precursors to perfluoroalkyl acids (PFAA) comes primarily from consumer and industrial products readily available in people’s homes.1–4 FTOHs were the dominant polyfluorinated compounds in indoor air 5 where ~60% of detected per- and polyfluorinated alkyl substances (PFAS) were associated with the particle phase.2 Since most people spend more than 90% of their time indoors,2 indoor air and dust are important uptake pathways for human PFAS exposure6 in addition to the widely recognized exposure sources of diet and water.7–9 Indeed, correlations between elevated indoor air exposure to precursors and increased PFAS serum concentrations have been reported.10,11
The use of passive sampling, which can measure the concentration of freely dissolved or gas-phase trace organic contaminants, has been widely accepted as an effective detection tool.12,13 Single-phase polymers, such as polyethylene (PE) sheets, have been able to detect a wide range of non-polar and moderately polar contaminants in the gas phase or dissolved in water.14,15 In addition, PE sheets are inexpensive, easy to handle, and can be easily transported and deployed.13 Recently, neutral PFAS were successfully measured in outdoor air and water using PE sheets.16 However, the partitioning of neutral PFAS into or onto the PE sheets indoors is not yet fully understood.
To further assess the role that indoor environments play as an exposure source of airborne PFAS in gas-phase and dust, the main objectives of this research were to (i) derive indoor PE-air partitioning coefficients (KPE-air); (ii) compare the volatile PFAS composition in different indoor environments using PE sheets as passive samplers; (iii) evaluate the air-dust partitioning of PFAS in carpeted kindergarten classrooms, and (iv) estimate daily intake (EDI) in children 2-6 years of age.
Materials and Methods
Sampling of neutral PFAS was performed in carpeted kindergarten classrooms, residences, an outdoor gear and apparel store in northern California; university offices, classrooms, laboratories, and a carpet store in southern Rhode Island between 2018 and 2020. A total of 90 PE sheets were deployed in the indoor locations, in addition to eight radiello samplers (Sigma Aldrich) with precleaned XAD-4 as sorbent used for active sampling.
Two types of precleaned PE passive samplers differentiated by thickness (25 μm and 50 μm) were deployed for 14 days (validation study), 21 days (kinetic study), and 28 days (measurements). Active sampling was performed on days 1, 7 and 14 where the radiello samplers were attached to a QuickTake 30 SKC Pump at a constant flow of 5 L min−1 for 240 minutes. All samples were kept in a freezer at −20°C until extraction (for details, see SI).
Instrumental analysis
Samples were analyzed for nine neutral PFAS on an Agilent 7890B gas chromatograph coupled to an Agilent 5977A mass selective detector (MSD) device operating in positive chemical ionization mode using selected ion monitoring (for details, see SI).
Data interpretation
The partitioning constants of neutral PFAS between PE and air (KPE-air) were derived in the validation study as:
| (1) |
Where CPE is the concentration in PE sheets (ng g−1PE ), and Cair is the gas-phase concentration (ng m−3).
Active sampling was used in the KPE-air validation study only. For all other campaigns, Cair was calculated using equation (1). Partitioning within the PE sheets was derived as the ratio of the 25 μm passive sampler (C25) to the 50 μm passive sampler (C50) amounts at equilibrium (for details, see SI).
Daily intake
The total estimated daily intake (EDI) of neutral PFAS via air and dust was calculated from PFAS concentrations measured here, and dust concentrations reported elsewhere17 based on established methods 18,19 (for details, see SI).
QA/QC
Field blanks, matrix spikes, matrix blanks, and field duplicate samples were included with each sample batch. Matrix spikes were prepared by spiking 50 μL of an 80 pg/μL native standard solution and 50 μL of an 80 pg/μL mass labelled standard solution into a clean (unused and never removed from the laboratory) sampler. Method detection limits (MDL) were calculated as the blank average plus three times the standard deviation; however, when a compound was not detected in the blanks, instrumental limits of detection (ILOD) were used. Only values above limits of quantitation (LOQ) were reported (for details, see SI, Table S2). Recoveries of the matrix spikes ranged between 81% (±35) to 111% (±19) for all compounds (for details, see SI, Table S2).
Results and Discussion
PE-air Partitioning Constants
Results from the kinetic study showed that 6:2 FTOH and 8:2 FTOH reached equilibrium after 14 days (see SI Figure S1). Log KPE-air values were approximately 4 -5 for the FTOHs, ~ 5 for 8:2 FTAcr, and increased with molecular weight. Although 10:2 FTAcr, FOSAs and FOSEs were detected by PE sheets, none were detected by active sampling; calculating their equilibrium partitioning constant was not possible (see SI Table S6). There were only minor differences between the 25 μm and 50 μm KPE-air results, indicating good reproducibility of PE sheets as passive samplers.
Mean log KPE-air values from the FTOHs of this study were approximately three log units lower than those reported for outdoors (see SI Table S3)16 where break-through and environmental factors could have affected the partitioning of the compounds. Missing KPE-air values were derived based on a correlation between previously reported16 and currently measured PE-air partitioning constants. Further studies are needed to corroborate the partitioning coefficients of the FOSAs and FOSEs.
PE-air Partitioning ratios
The partitioning ratios of the weight-normalized neutral PFAS between 25 and 50 μm thick PE sheets were ~1 (see SI Figure S2) implying absorption as the mechanism of partitioning. The greater mass of the 50 μm PE sheets for the same size resulted in easier detection and is thus preferable for future studies.
Neutral PFAS Indoor Air Concentrations
Indoor air concentrations were derived from the PEs for neutral PFAS in (1) California Kindergarten classrooms (SI Table S4); (2) offices, classrooms and laboratories at a university, and a nearby carpet store in southern Rhode Island (SI Table S5); and (3) a storage room at an outdoor clothing store in California (SI Table S6), see Figure 1. Neutral PFAS were present at all locations, dominated by FTOHs, in-line with previous results.5,20,21 PFAS profiles and concentrations varied between locations, though, likely driven by the different PFAS-containing products present. These results indicated that PE-sheets can be used to determine differences in PFAS profiles and concentrations in various indoor air settings.
Figure 1. Indoor air concentrations measured at California Kindergarten classrooms and an outdoor clothing store, and university classrooms, offices and laboratories, and a carpet store in southern Rhode Island.
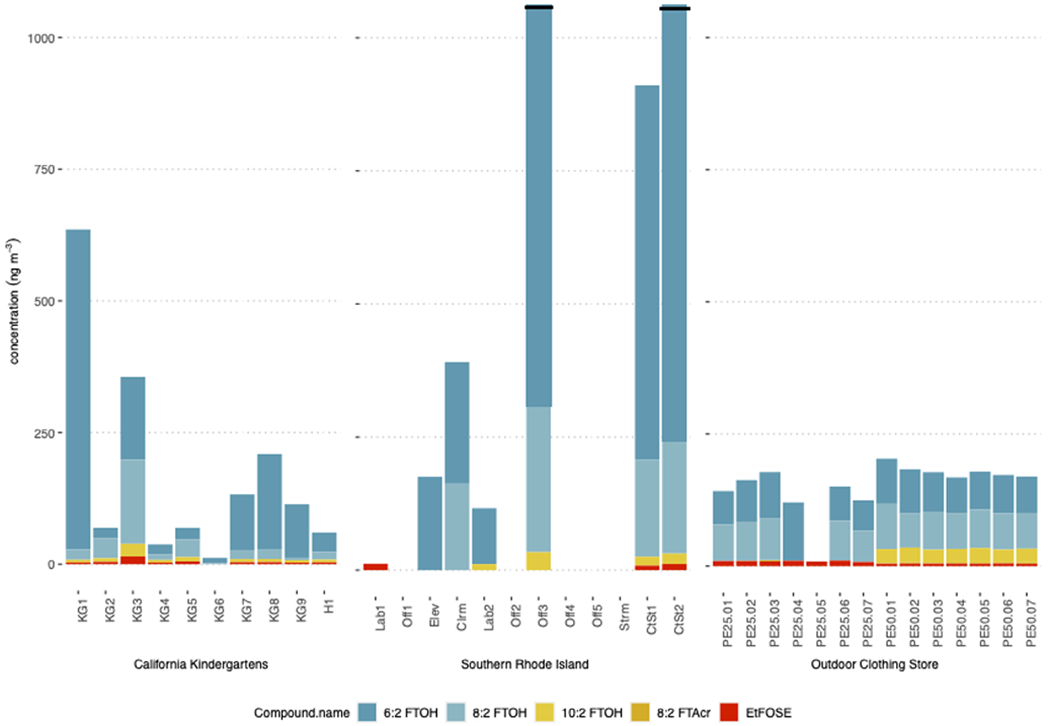
H: home; KG: kindergarten classrooms; Lab: laboratory; Off: office; Elev: elevator; Clrm: classroom; Ctst: carpet store; Strm: storage room. Numbers (i.e. KG7) are indicative of separate/individual samples. Off3 and CtSt2 have concentrations above 1000 ng m−3.
In the California kindergarten classrooms, 6:2 FTOH dominated with concentrations ranging from 10-600 ng m−3 (accounting for 29-96 % of sum of nine PFAS), followed by 8:2 FTOH (2-160 ng m−3, 3-54% of total PFAS) (Figure 1; SI Table S2). In three kindergarten classrooms (KG2, KG3, and KG5), concentrations of 8:2 FTOH exceeded those of 6:2 FTOH. In all kindergarten classrooms, EtFOSE was present at low concentrations, while MeFOSE was below method detection limits (MDLs) (Figure 1; SI Table S2). EtFOSA, 8:2 FTAcr, and 10:2 FTAcr were not detected (SI Table 3).
When detected, 6:2 FTOH (with detection frequency of 83%, and ranging from < MDL – 1900 ng m−3), and 8:2 FTOH (17%, < MDL-270 ng m−3) also dominated total PFAS in the university rooms (Figure 1; SI Table S5). FTOHs were detected only in carpeted rooms and in the analytical laboratory (SI Table S5). The detection of 10:2 FTOH was sporadic, with concentrations up to 33 ng m−3 (Figure 1; SI Table S5). MeFOSA, EtFOSE, and MeFOSE were at or < MDL at all sites, while EtFOSA and FTAcr were rarely above MDLs (SI Table S5).
Volatile PFAS were present in all replicates from the outdoor clothing store (Figure 1). FTOHs were the most abundant and dominant group; consistent with previous studies on the composition of PFAS in various indoor environments.5,20,21 The most abundant compound was 8:2 FTOH, with an average concentration of ~200 ng m−3, followed by 6:2 FTOH and 10:2 FTOH with average concentrations of 70 ng m−3 and 30 ng m−3 respectively (Figure 1). The dominance of 8:2 FTOH is concerning since this and other longer-chain PFAS have been phased out by PFAS producers in the United States, European Union, and Japan22. These results show that these compounds are still being used for textiles, and possibly point to textile imports from other countries where PFAS are poorly regulated.23
Fraser et al. (2011)11 reported concentrations of FTOHs ranging from <MDL to 11 ng m−3 (6:2 FTOH), 0.3 - 70 ng m−3 (8:2 FTOH), and 0.14 - 12 ng m−3 (10:2 FTOH) in multiple office spaces in Boston, Massachusetts, similar to results report here and in other studies.5,21 A study in Ottawa, Canada, in 2005 reported concentrations of MeFOSE, EtFOSE and EtFOSA in indoor air of ~ 7 ng m−3, 2 ng m−3 and 0.1 ng m−3, respectively 2, and even lower in Vancouver, Canada in 2011, at 0.4 ng m−3, 0.06 ng m−3, 0.03 ng m−3, and 0.02 ng m−3 respectively.21 In the present study, although present in many locations, FOSEs rarely exceeded concentrations of 0.001 ng m−3. FOSAs were detected even fewer times. The difference in concentrations of the FOSAs and FOSEs in different locations across North America could reflect geographic differences of indoor sources. Additionally, the difference between older and newer data could point to the phase out of PFOS-based chemicals, including FOSAs and FOSEs since 2002, whereas the use of replacement FTOHs in North America has increased since 2000.21,24,25
Air-dust-carpet partitioning
Concentrations of neutral PFAS in dust and carpet of the same kindergarten classrooms were measured by Wu et al. (2020)17 (see SI Table S7). Strong correlations (RSQ > 0.7, P≤0.05) were observed between different FTOHs in air-dust, and air-carpet (and dust-carpet from Wu et al. (2020)17, see SI Table S8), except for 6:2 FTOH in air-carpet. On the other hand, FOSEs were not strongly correlated in air, dust or carpet.
Distribution of PFAS between indoor air and floor dust were reported to be controlled by partitioning between the gas phase and PFASs sorbed to the organic phases in the dust.26 Our results corroborated that neutral PFAS were present in air and partitioned to dust. Given that the origin of volatile PFAS in air in the (carpet-free) outdoor clothing storage room was likely to be textiles, it is possible that multiple products in the kindergarten classrooms were in fact the source of these compounds that also partitioned into carpet and dust. Previous work demonstrated that FTOHs, FOSAs, and FOSEs degrade in the atmosphere into more stable PFAA.27,28 Significant associations between precursors in air and PFCAs in dust have been reported (e.g., 6:2 FTOH and PFHxA).29 Similarly, significant associations were observed between FOSAs/FOSEs in air and PFOS and PFDS in house dust.28
In contrast to FTOHs, there were no significant correlations between the FOSAs/FOSEs in air, dust and carpet from this study, suggesting that the sources of FOSAs/FOSEs were different and likely not linked to carpets or textiles. A previous study did not find significant correlations between the FOSEs in kindergarten classrooms either, but did however find strong associations in offices30, implying that there were common sources of these sulfonamidoethanols in items associated with office spaces that perhaps were not usually found in kindergarten classrooms. Additionally, as previously mentioned, the production of FOSAs/FOSEs has been largely phased-out of production since 200224, and thus their low concentration or absence is expected31.
Estimated daily intake of volatile PFAS through air and dust
To assess the relevance of volatile and neutral PFAS in indoor air for children aged two to six years old, the estimated daily intake (EDI) was calculated (SI Table S8) for three exposure estimates (low, medium, high, see SI). Biotransformation constants for each compound were used to calculate their contribution to the ∑PFAA intake (SI Table S8).
Total EDI (SI Table S9) was 1.5 ng kg−1 bw day −1 for low exposure, 14 ng kg−1 bw day−1 for intermediate exposure, and 150 ng kg−1 bw day−1 for high exposure. Compounds that were regularly detected in both air and dust were 6:2 FTOH, 8:2 FTOH, and, to a lesser extent, 10:2 FTOH; while MeFOSE and EtFOSE appeared to have significantly larger contributions in dust than air (Figure 2, SI Table S9). Volatile and neutral PFAS measured in air contributed 4.9-62 % to ∑PFAA intake, while ionic PFAS measured in dust contributed 34-95 % (SI Table S9). These results are similar to other studies that found precursors contributing 41–68 % to ∑PFOS uptake via all investigated exposure pathways, 32 and precursors responsible for 90 % to the ∑PFOS intake in air (Figure 2).26,33 Our results imply that air inhalation was a major exposure pathway for FTOHs, while dust ingestion was dominant for FOSEs in children, similar to prior results.30
Figure 2. Percent of volatile and neutral PFAS (top panel) and indirect or biotransformed PFAA (bottom panel) intake via air inhalation (pink) and dust ingestion (blue) for children at ages 2 through 6.
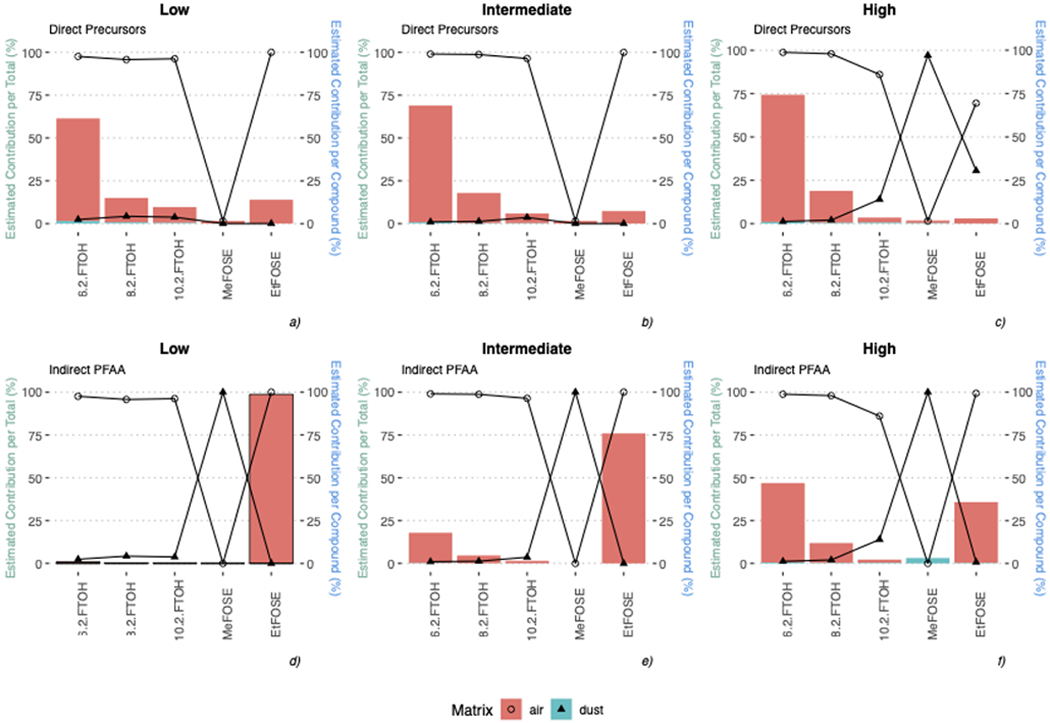
Bars represent the relative contribution of individual precursors to total PFAS (left axis); bars are differentiated by color for both matrices. Lines represent the percent estimated contribution for each compound in air and dust (right axis). MeFOSE was detected at low concentrations in dust and <MDL in air.
Given the potential for precursors to be biotransformed into more stable PFAA, estimations of PFAA indirect exposure were also calculated as 1.2 ng kg−1 bw day−1, 75 ng kg−1 bw day−1, 2800 ng kg−1 bw day−1 for the low, intermediate, and high exposure scenario respectively (SI Table S9). The major contributors to indirect PFAA exposure were 6:2 FTOH and 8:2 FTOH in air, and MeFOSE in dust (Figure 2). This study demonstrated that volatile neutral PFAS, such as FTOHs, are major contributors to exposure in air.
Supplementary Material
Table 1.
Indoor log KPE-air values from the validation study for 25 and 50 um PE sheets.
| Compound | Molecular weight (g mol−1) | Mean log KPE-air 25 (this study) | Mean log KPE-air 50 (this study) |
|---|---|---|---|
| 6:2 FTOH | 364.1 | 4.4 ± 0.1 | 4.3 ± 0.0 |
| 8:2 FTOH | 464.1 | 4.3 ± 0.1 | 4.5 ± 0.0 |
| 10:2 FTOH | 564.1 | 5.0 | 5.0 ± 0.0 |
| 8:2 FTAcr | 518.1 | 4.9 ± 0.4 | 5.0 ± 0.2 |
| 10:2 FTAcr | 618.1 | 5.2* | 5.3* |
| MeFOSA | 527.2 | 5.1* | 5.2* |
| EtFOSA | 513.1 | ND | ND |
| MeFOSE | 571.2 | 5.2* | 5.3* |
| EtFOSE | 557.2 | 5.2* | 5.2* |
ND=not detected,
KPE-air from this study was estimated based on a correlation between those measured here and those reported by Dixon-Anderson and Lohmann, (2018)16.
Estimated KPE-air = 0.44 x KPE-air, measured (Dixon-Anderson and Lohmann, 2018) + 1.30 (RSQPE50=0.67).
Acknowledgements
The authors acknowledge funding from NIEHS (P42ES027706). The analysis was conducted at a Rhode Island NSF EPSCoR research facility, Molecular Characterization Facility, supported in part by the EPSCoR Cooperative Agreement # OIA-1655221. We thank Marta Venier (Indiana U) for comments on a previous version of this manuscript.
Footnotes
Polyethylene (PE) sheets are effective passive samplers for PFAA precursors which are ubiquitous in indoor air and dominate indoor exposure.
Supporting Information
The Supporting Information contains additional details on the analysis, data interpretation and EDI calculation, and is available free of charge.
Conflict of interest
The authors declare no competing financial interests.
References
- (1).Prevedouros K; Cousins IT; Buck R; Korzeniowski SH Critical Review Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol 2006. 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- (2).Shoeib M; Harner T; Wilford BH; Jones KC; Zhu J Perfluorinated Sulfonamides in Indoor and Outdoor Air and Indoor Dust: Occurrence, Partitioning, and Human Exposure. Environ. Sci. Technol 2005. 10.1021/es048340y. [DOI] [PubMed] [Google Scholar]
- (3).Langer V; Dreyer A; Ebinghaus R Polyfluorinated Compounds in Residential and Nonresidential Indoor Air. Environ. Sci. Technol 2010. 10.1021/es102384z. [DOI] [PubMed] [Google Scholar]
- (4).Gremmel C; Fr T; Omel €; Knepper TP Systematic Determination of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Outdoor Jackets. 2016. 10.1016/j.chemosphere.2016.06.043. [DOI] [PubMed] [Google Scholar]
- (5).Schlummer M; Gruber L; Fiedler D; Kizlauskas M; Müller J Detection of Fluorotelomer Alcohols in Indoor Environments and Their Relevance for Human Exposure. Environ. Int 2013, 57–58, 42–49. 10.1016/j.envint.2013.03.010. [DOI] [PubMed] [Google Scholar]
- (6).Ericson Jogsten I; Nadal M; Van Bavel B; Lindström G; Domingo JL Per- and Polyfluorinated Compounds (PFCs) in House Dust and Indoor Air in Catalonia, Spain: Implications for Human Exposure. Environ. Int 2012, 39, 172–180. 10.1016/j.envint.2011.09.004. [DOI] [PubMed] [Google Scholar]
- (7).Sunderland EM; Hu XC; Dassuncao C; Tokranov AK; Wagner CC; Allen JG A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol 2019, 29 (2), 131–147. 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk D; Bignami M; Bodin L; Chipman JK; del Mazo J; Grasl-Kraupp B; Hogstrand C; Hoogenboom L. (Ron); Leblanc J-C; Nebbia CS; Nielsen E; Ntzani E; Petersen A; Sand S; Vleminckx C; Wallace H; Barregård L; Ceccatelli S; Cravedi JP; Halldorsson TI; et al. Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA J. 2020, 18 (9), e06223. 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).ATSDR. Toxicological Profile for Perfluoroalkyls. (Draft for Public Comment); Atlanta, GA, 2018. [Google Scholar]
- (10).Makey CM; Webster TF; Martin JW; Shoeib M; Harner T; Dix-cooper L; Webster GM Airborne Precursors Predict Maternal Serum Perfluoroalkyl Acid Concentrations. Env. Sci Technol 2017, 51, 7697–7675. 10.1021/acs.est.7b00615. [DOI] [PubMed] [Google Scholar]
- (11).Fraser AJ; Webster TF; Watkins DJ; Nelson JW; Stapleton HM; Calafat AM; Kato K; Shoeib M; Vieira VM; McClean MD Polyfluorinated Compounds in Serum Linked to Indoor Air in Office Environments. Env. Sci Technol 2011, No. 46, 1209–1215. 10.1021/es2038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lohmann R Critical Review of Low-Density Polyethylene’s Partitioning and Diffusion Coefficients for Trace Organic Contaminants and Implications for Its Use As a Passive Sampler. 2011. 10.1021/es202702y. [DOI] [PubMed] [Google Scholar]
- (13).Lohmann R; Booij K; Smedes F; Vrana B POPs Workshop, Ten Years after the Signature of the Stockholm Convention. Use of Passive Sampling Devices for Monitoring and Compliance Checking of POP Concentrations in Water. Environ. Sci. Pollut. Res 2012, No. 19, 1885–1895. 10.1007/s11356-012-0748-9. [DOI] [PubMed] [Google Scholar]
- (14).Booij K; Sleiderink HM; Smedes F Calibrating the Uptake Kinetics of Semipermeable Membrane Devices Using Exposure Standards. Environ. Toxicol. Chem 1998, 17 (7), 1236–1245. 10.1897/1551-5028. [DOI] [Google Scholar]
- (15).Adams R; Lohmann R; Fernandez L; MacFarlane J; Gschwend P Polyethylene Devices: Passive Samplers for Measuring Dissolved Hydrophobic Organic Compounds in Aquatic Environments. Environ. Sci. Technol 2007. 10.1021/es0621593. [DOI] [PubMed] [Google Scholar]
- (16).Dixon-Anderson E; Lohmann R Field-Testing Polyethylene Passive Samplers for the Detection of Neutral Polyfluorinated Alkyl Substances in Air and Water. Environ. Toxicol. Chem 2018, 37 (12), 3002–2010. 10.1002/etc.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wu Y; Romanak K; Bruton T; Blum A; Venier M Per-and Polyfluoroalkyl Substances in Paired Dust and Carpets from Childcare Centers. Chemosphere 2020. 10.1016/j.chemosphere.2020.126771. [DOI] [PubMed] [Google Scholar]
- (18).USEPA. Exposure Factors Handbook (EFH); Washington, DC, 2008. https://doi.org/EPA/600/R-06/096F. [Google Scholar]
- (19).Gebbink W; Berger U; Cousins I Estimating Human Exposure to PFOS Isomers and PFCA Homologues: The Relative Importance of Direct and Indirect (Precursor) Exposure. Environ. Int 2015, 74, 160–169. 10.1016/j.envint.2014.10.013. [DOI] [PubMed] [Google Scholar]
- (20).Liu W; Takahashi S; Sakuramachi Y; Harada KH; Koizumi A Polyfluorinated Telomers in Indoor Air of Japanese Houses. 2012. 10.1016/j.chemosphere.2012.09.062. [DOI] [PubMed] [Google Scholar]
- (21).Shoeib M; Harner T; Webster GM; Lee SC Indoor Sources of Poly-and Perfluorinated Compounds (PFCS) in Vancouver, Canada: Implications for Human Exposure. Environ. Sci. Technol 2011, 45, 7999–8005. 10.1021/es103562v. [DOI] [PubMed] [Google Scholar]
- (22).Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; Voogt P. De; Jensen AA; Kannan K; Mabury SA; van Leeuwen SPJ. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag 2011. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Vestergren R; Herzke D; Wang T; Cousins IT Are Imported Consumer Products an Important Diffuse Source of PFASs to the Norwegian Environment? 2015. 10.1016/j.envpol.2014.12.034. [DOI] [PubMed] [Google Scholar]
- (24).Barber JL; Berger U; Chaemfa C; Huber S; Jahnke A; Temme C; Jones KC Analysis of Per- and Polyfluorinated Alkyl Substances in Air Samples from Northwest Europe. J. Environ. Monit 2007, 9 (6), 530–541. 10.1039/b701417a. [DOI] [PubMed] [Google Scholar]
- (25).OECD. RESULTS OF THE 2006 SURVEY ON PRODUCTION AND USE OF PFOS, PFAS, PFOA, PFCA, THEIR RELATED SUBSTANCES AND PRODUCTS/MIXTURES CONTAINING THESE SUBSTANCES; 2006. https://doi.org/ENV/JM/MONO(2006)36. [Google Scholar]
- (26).Winkens K; Giovanoulis G; Koponen J; Vestergren R; Berger U; Karvonen A; Pekkanen J; Kiviranta H; Cousins I Perfluoroalkyl Acids and Their Precursors in Floor Dust of Children’s Bedrooms – Implications for Indoor Exposure. Environ. Int 2018, 119 (June), 493–502. 10.1016/j.envint.2018.06.009. [DOI] [PubMed] [Google Scholar]
- (27).Ellis DA; Martin JW; De Silva AO; Mabury SA; Hurley MD; Sulbaek Andersen MP; Wallington TJ Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environ. Sci. Technol 2004, 38 (12), 3316–3321. 10.1021/es049860w. [DOI] [PubMed] [Google Scholar]
- (28).Haug L; Huber S; Schlabach M; Becher G; Thomsen C Investigation on Per-and Polyfluorinated Compounds in Paired Samples of House Dust and Indoor Air from Norwegian Homes. Environ. Sci. Technol 2011, 45, 7991–7998. 10.1021/es103456h. [DOI] [PubMed] [Google Scholar]
- (29).Huber S; Haug L; Schlabach M Per-and Polyfluorinated Compounds in House Dust and Indoor Air from Northern Norway-A Pilot Study. 2011. 10.1016/j.chemosphere.2011.04.075. [DOI] [PubMed] [Google Scholar]
- (30).Goosey E; Harrad S Perfluoroalkyl Substances in UK Indoor and Outdoor Air: Spatial and Seasonal Variation, and Implications for Human Exposure. Environ. Int 2012, 45 (1), 86–90. 10.1016/j.envint.2012.04.007. [DOI] [PubMed] [Google Scholar]
- (31).Karásková P; Venier M; Melymuk L; Bečanová J; Vojta Š; Prokeš R; Diamond ML; Klánová J Perfluorinated Alkyl Substances (PFASs) in Household Dust in Central Europe and North America. 2016. 10.1016/j.envint.2016.05.031. [DOI] [PubMed] [Google Scholar]
- (32).Vestergren R; Cousins I; Trudel D; Wormuth M; Scheringer M Estimating the Contribution of Precursor Compounds in Consumer Exposure to PFOS and PFOA. Chemosphere 2008, 73, 1617–1624. 10.1016/j.chemosphere.2008.08.011. [DOI] [PubMed] [Google Scholar]
- (33).Winkens K; Koponen J; Schuster J; Shoeib M; Vestergren R; Berger U; Karvonen AM; Pekkanen J; Kiviranta H; Cousins IT Perfluoroalkyl Acids and Their Precursors in Indoor Air Sampled in Children’s Bedrooms. Environ. Pollut 2017, 222, 423–432. 10.1016/J.ENVPOL.2016.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


