Abstract
Objective:
Female patients are more likely to undergo repair of intact and ruptured abdominal aortic aneurysm (AAA) at smaller aortic diameter compared with male patients. By adjusting for inherent anatomic differences between sexes, aortic size index (ASI) and aortic height index (AHI) may provide an additional method for guiding treatment. We therefore analyzed sex-specific criteria for AAA repair using aortic diameter, ASI, and AHI.
Methods:
We identified all patients who underwent AAA repair between 2003 and 2019 in the Vascular Quality Initiative database. The Dubois and Dubois formula was used to calculate body surface area; aortic diameter was divided by body surface area to calculate ASI. Aortic diameter was divided by height to calculate AHI. Cumulative distribution curves were used to plot the proportion of patients who underwent repair of ruptured aneurysm according to aortic diameter, ASI, and AHI. Multivariable logistic regression modeling was used to identify the association of female sex with perioperative mortality and any major postoperative complication.
Results:
We identified 55,647 patients, of whom 12,664 were female (20%). For both intact and rupture repair, female patients were older, less likely to undergo endovascular aneurysm repair, and more likely to have comorbid conditions. Female patients underwent repair at smaller median aortic diameter compared with male patients for intact (5.4 vs 5.5 cm; P < .001) and rupture repair (6.7 vs 7.7 cm; P < .001). However, ASI was higher in female patients for both intact (3.1 vs 2.7 cm/m2; P < .001) and rupture repair (3.8 vs 3.7 cm/m2; P < .001), whereas AHI was higher in female patients for intact repair (3.3 vs 3.1 cm/m; P < .001) but lower for rupture repair (4.1 vs 4.3 cm/m; P < .001). When analyzing the cumulative distribution of rupture repair in male patients, 12% of rupture repairs were performed at an aortic diameter below 5.5 cm. To achieve the same proportion of rupture repair in female patients, the repair diameter was only 4.9 cm. However, when ASI and AHI were used, female and male patients both reached 12% of rupture repair at an ASI of 2.7 cm/m2 and an AHI of 3.0 cm/m.
Conclusions:
Our study provides data to strongly support the sex-specific 5.0-cm aortic diameter threshold suggested for repair in female patients by the Society for Vascular Surgery. The high percentage of patients undergoing rupture repair below 5.5 cm in male patients and 5.0 cm in female patients highlights the need to better identify patients at risk of rupture at smaller aortic diameters.
Keywords: AHI, Aortic aneurysms, Aortic diameter, Aortic height index, Aortic size index, ASI, Diameter, Sex
Abdominal aortic aneurysm (AAA) diameter of 5.5 cm or greater had previously been used as a threshold for repair in both male and female patients. This was based on four randomized controlled trials in which female patients comprised only 3.7% to 17.1% of the entire study cohort.1–4 The United Kingdom Small Aneurysm Trial, which had the largest proportion of female patients (17.1%), demonstrated that female patients were more likely to present with ruptured AAA at a smaller aortic diameter compared with male patients.1 Contemporary studies have also found that female patients have smaller aortic diameter at the time of repair for both intact and ruptured AAA compared with male patients.5–8 Therefore, the Society for Vascular Surgery (SVS) suggests young healthy female patients, with an aortic diameter of 5.0 cm to 5.4 cm may benefit from early repair,9 and the European Society for Vascular Surgery (ESVS) suggests repair should be considered in female patients with an aortic diameter of 5.0 cm.10
This difference in aneurysm diameter by sex at the time of repair may be due to baseline differences in aortic anatomy. Female patients have aortic diameter measurements approximately 2- to 6-mm smaller than male patients along the entire length of the aorta.11–13 Aortic size index (ASI), which indexes aortic diameter to body surface area, and aortic height index (AHI), which indexes aortic diameter to height, may account for differences in body size and provide information on relative as opposed to absolute aortic aneurysm dilation.14–16 In their single-center study, Davies et al identified that an ASI ≥4.25 cm/m2 was predictive of thoracic aortic rupture.14 Recently the same group identified that an AHI ≥3.6 cm/m was also predictive of thoracic aortic rupture.16 These aortic index thresholds for repair are now integral in the management of thoracic aortic aneurysms.17 However, the ideal ASI and AHI criteria for AAA repair remain unclear. Additionally, the SVS and ESVS sex-specific aortic diameter thresholds for repair are suggestions and not recommendations. Both societies cite a lack of strong quality of evidence as a reason for the weaker guidelines (Table I). Therefore, we aimed to analyze sex-specific criteria for AAA repair using aortic diameter, ASI, and AHI.
Table I.
Level of recommendation and supporting quality of evidence cited by the SVS and ESVS for sex-specific aortic diameter thresholds for repair
| SVS | ESVS | |
|---|---|---|
|
| ||
| Level of recommendation | 2 (Weak) Benefits closely balanced with harms and burdens |
IIB Usefulness/efficacy is less well-established by evidence/opinion |
| Quality of evidence | B (Moderate) Evidence from RCTs with important limitations or unusually strong evidence from unbiased observational studies. Further research (if performed) is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
C Consensus of opinion of the experts and/or small studies, retrospective studies, registries. |
ESVS, European Society for Vascular Surgery; RCT, randomized controlled trials; SVS, Society for Vascular Surgery.
METHODS
Data source.
We performed a retrospective cohort study including patients from the SVS Vascular Quality Initiative (VQI). The VQI is a prospectively collected quality improvement registry. With over 550 participating centers, VQI captures over 350 predefined variables including patient characteristics and procedural and anatomical characteristics, as well as in-hospital outcomes and long-term mortality. More information can be found at www.vqi.org. This manuscript adheres to the applicable Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) standards for observational studies.18 The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived the need for patient consent due to the retrospective and de-identified nature of the study.
Patient cohort.
We identified all patients undergoing open and endovascular repair of intact and ruptured AAAs between 2003 and 2019 (n = 64,603). Patients who underwent urgent or emergent repair for reasons other than rupture were excluded from our study. To avoid multiple evaluations of the same patients, we excluded secondary repair procedures when patients had multiple entries in the database (n = 314). When evaluating patients who underwent intact repair, we aimed to include only those who had an elective repair indicated by AAA diameter. Therefore, we excluded intact repairs that were performed on the weekend and were therefore most likely not truly elective (n = 253). Additionally, patients with an isolated iliac aneurysm (n = 730) or those undergoing repair within 24 hours of onset of pain and/or tenderness (n = 5836) were also excluded, as we did not want the repair to be driven by iliac aneurysm disease or symptomatic status. Finally, we excluded patients with essential missing data (missing sex, n = 6; admission status, n = 218; diameter, n = 1028; or height/weight, n = 571).
Definitions and variables.
ASI was defined as aneurysm diameter divided by body surface area (cm/m2); body surface area was calculated using the Dubois and Dubois formula (0.20247 × [height (m)0.725 × weight (kg)0.425]). AHI was defined as aneurysm diameter divided by height (cm/m). Ruptured presentation is captured separately within the VQI database and is defined by computed tomography angiography or operative evidence of rupture. Aortic diameter measurements immediately preceding rupture were not available; therefore, aortic diameter in patients with ruptured aneurysm may not reflect the true measurement right before rupture. Body mass index (BMI) was calculating using the standard weight/height2 (kg/m2) formula. We classified patients with a BMI <18.5 kg/m2 as underweight and ≥30 kg/m2 as obese. The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.8 We defined chronic kidney disease (CKD) as an estimated glomerular filtration rate <30 mL/min/1.73 m2 or currently on dialysis. Major complication was defined as the presence of one of the following: reoperation, postoperative congestive heart failure, stroke, myocardial infarction, reintubation, dialysis requirement, surgically treated intestinal ischemia, surgical site infection, or lower extremity ischemia/emboli.
Statistical analysis.
We stratified our analysis by intact or ruptured AAA repair. Within each group, we used univariate analysis to compare demographics, coexisting conditions, and anatomical and procedural characteristics between female and male patients. Categorical variables were presented as counts and percentages and compared using the χ2 test. Continuous variables were presented as median and interquartile ranges and compared using the Wilcoxon rank-sum test. We then constructed box plots of the aortic diameter, ASI, and AHI for male and female patients at the time of repair. The box spans the interquartile range with the median value represented by the horizontal line within the box. Cumulative distribution curves were used to plot the proportion of male and female patients who underwent repair of ruptured aneurysm according to aortic diameter, ASI, and AHI. We used multivariable logistic regression modeling to assess the independent association between female sex and perioperative mortality as well as any major postoperative complication. We adjusted the models for covariates selected a priori including age, race, current smoker, insulin dependent diabetes mellitus, hypertension, congestive heart failure, chronic obstructive pulmonary disease, CKD, prior AAA repair, coronary artery disease, familial history of AAA, preoperative medication use (aspirin, statin, and beta blocker), AAA diameter, and type of repair (open or endovascular). Subsequently, we replaced AAA diameter with ASI and AHI. We assessed for interactions between female sex and the remaining covariates within each multivariable model. We did not include BMI in the multivariable model as the formula to calculate ASI includes height and weight and the formula to calculate AHI contains height. We did not include BMI in the model using aortic diameter as we wanted all three models to contain the same covariates.
All variables had <5% missing data. All analyses were performed using Stata 15.1 (StataCorp, College Station, Tex).
RESULTS
Aneurysm presentation.
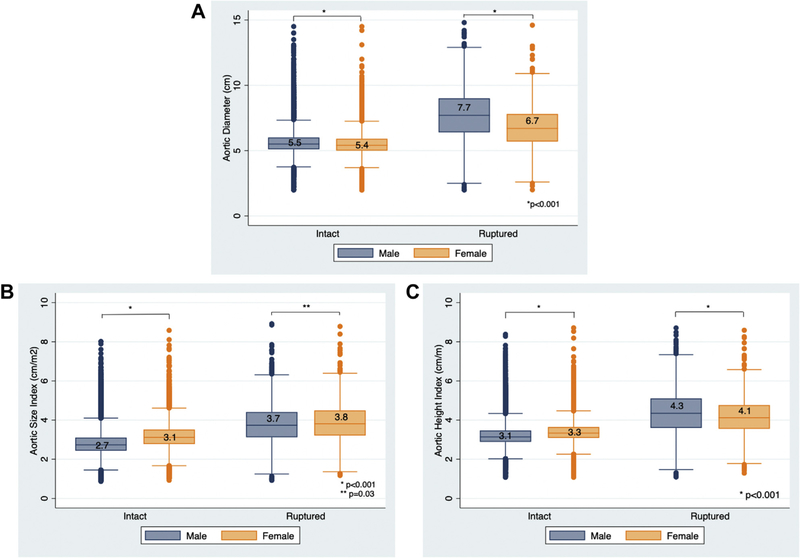
We identified 55,647 patients, of whom 51,136 underwent intact repair and 4511 underwent rupture repair. Female patients represented a larger proportion of rupture repair compared with elective repair (22% vs 20%; P = .002); however, the absolute difference was small. Female patients underwent intact repair at a slightly smaller median aortic diameter compared with male patients (5.4 vs 5.5 cm; P < .001) but larger ASI (3.1 vs 2.7 cm/m2; P < .001) and AHI (3.3 vs 3.1 cm/m; P < .001; Fig 1). Female patients also underwent rupture repair at a smaller aortic diameter (6.7 vs 7.7 cm; P < .001) and AHI (4.1 vs 4.3 cm/m; P < .001), but larger ASI (3.8 vs 3.7 cm/m2; P = .03) compared with male patients (Fig 1). For both intact and rupture repair, female patients were older, less likely to undergo endovascular aneurysm repair (EVAR), and more likely to be Black. Female patients were also more likely to have hypertension, chronic obstructive pulmonary disease, or CKD but were less likely to be on preoperative acetylsalicylic acid/aspirin or statin therapy (Table II).
Fig 1.
Vertical box plots showing the median and interquartile ranges of aortic diameter (A), aortic size index (ASI) (B), and aortic height index (AHI) (C) in male and female patients undergoing intact and rupture repair.
Table II.
Baseline characteristics for female and male patients undergoing AAA repair stratified by intact and rupture repair
| Female | Male | P value | |||
|---|---|---|---|---|---|
|
| |||||
| Intact repair | |||||
| No. patients | 10,118 | 41,018 | |||
| Diameter, cm | 5.4 | 5.0–5.9 | 5.5 | 5.1–6.0 | <.001 |
| ASI, cm/m2 | 3.1 | 2.8–3.5 | 2.7 | 2.4–3.1 | <.001 |
| AHI, cm/m | 3.3 | 3.1–3.6 | 3.1 | 2.9–3.5 | <.001 |
| BSA, m2 | 1.7 | 1.6–1.9 | 2.0 | 1.9–2.2 | <.001 |
| Height, m | 1.6 | 1.6–1.7 | 1.7 | 1.7–1.8 | <.001 |
| EVAR | 7893 | 78 | 34,643 | 85 | <.001 |
| Age, y | 75 | 69–80 | 73 | 67–79 | <.001 |
| Race/ethnicity | <.001 | ||||
| Non-Hispanic white | 8768 | 89 | 36,588 | 92 | |
| Black or African American | 710 | 7.2 | 1570 | 3.9 | |
| Hispanic | 249 | 2.5 | 1094 | 2.7 | |
| Asian | 101 | 1.0 | 535 | 1.3 | |
| Other | 44 | 0.5 | 151 | 0.4 | |
| Underweight | 539 | 5.3 | 728 | 1.8 | <.001 |
| Obese | 2957 | 29 | 12,922 | 32 | <.001 |
| Current smoker | 3665 | 36 | 13,008 | 32 | <.001 |
| Hypertension | 8605 | 85 | 34,170 | 83 | <.001 |
| Insulin-dependent diabetes | 322 | 3.2 | 1474 | 3.6 | .045 |
| Coronary artery disease | 3176 | 31 | 18,204 | 44 | <.001 |
| CHF | <.001 | ||||
| None | 9070 | 90 | 36,196 | 88 | |
| Asymptomatic/mild | 914 | 9.0 | 4141 | 10 | |
| Moderate/severe | 130 | 1.3 | 660 | 1.6 | |
| COPD | 4203 | 42 | 12,920 | 32 | <.001 |
| CKD | 609 | 6.1 | 1462 | 3.6 | <.001 |
| Prior aortic aneurysm repair | 322 | 3.2 | 1428 | 3.5 | .14 |
| Family history of AAA | 1048 | 10 | 3575 | 8.8 | <.001 |
| Preoperative ASA/aspirin use | 6284 | 62 | 27,526 | 67 | <.001 |
| Preoperative beta-blocker use | 5626 | 56 | 23,178 | 57 | .10 |
| Preoperative statin use | 6847 | 68 | 29,704 | 72 | <.001 |
| Rupture repair | |||||
| No. patients | 978 | 3533 | |||
| Diameter, cm | 6.7 | 5.7–7.8 | 7.7 | 6.4–9.0 | <.001 |
| ASI, cm/m2 | 3.8 | 3.2–4.5 | 3.7 | 3.1–4.4 | .030 |
| AHI, cm/m | 4.1 | 3.6–4.8 | 4.3 | 3.6–5.1 | <.001 |
| BSA, m2 | 1.7 | 1.6–1.9 | 2.0 | 1.9–2.2 | <.001 |
| Height, m | 1.6 | 1.5–1.7 | 1.8 | 1.7–1.9 | <.001 |
| EVAR | 562 | 58 | 2097 | 60 | .29 |
| Age, y | 76 | 70–83 | 72 | 65–78 | <.001 |
| Race/ethnicity | .001 | ||||
| Non-Hispanic white | 834 | 88 | 3075 | 90 | |
| Black or African American | 80 | 8.5 | 184 | 5.4 | |
| Hispanic | 23 | 2.4 | 97 | 2.8 | |
| Asian | 6 | 0.6 | 55 | 1.6 | |
| Other | 3 | 0.3 | 7 | 0.2 | |
| Underweight | 73 | 7.5 | 102 | 2.9 | <.001 |
| Obese | 320 | 33 | 1187 | 34 | .61 |
| Current smoker | 409 | 42 | 1619 | 46 | .019 |
| Hypertension | 784 | 80 | 2730 | 78 | .068 |
| Insulin-dependent diabetes | 35 | 3.6 | 117 | 3.3 | .69 |
| Coronary artery disease | 236 | 24 | 1132 | 32 | <.001 |
| CHF | .18 | ||||
| Asymptomatic/mild | 91 | 9.4 | 314 | 9.0 | |
| Moderate/severe | 26 | 2.7 | 62 | 1.8 | |
| COPD | 361 | 37 | 999 | 29 | <.001 |
| CKD | 171 | 18 | 418 | 12 | <.001 |
| Prior aortic aneurysm repair | 47 | 4.8 | 232 | 6.6 | .041 |
| Family history of AAA | 58 | 6.1 | 200 | 5.8 | .78 |
| Preoperative ASA/aspirin use | 356 | 37 | 1412 | 41 | .054 |
| Preoperative beta-blocker use | 388 | 40 | 1345 | 39 | .31 |
| Preoperative statin use | 406 | 42 | 1518 | 44 | .46 |
AAA, Abdominal aortic aneurysm; AHI, aortic height index; ASA, acetylsalicylic acid; ASI, aortic size index; BSA, bodysurface area; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EVAR, endovascular aneurysm repair; Obese, body mass index of 30 or above; Underweight, body mass index below 18.5.
Categorical variables are presented as number (%) and continuous variables as median (interquartile range).
Rupture repair.
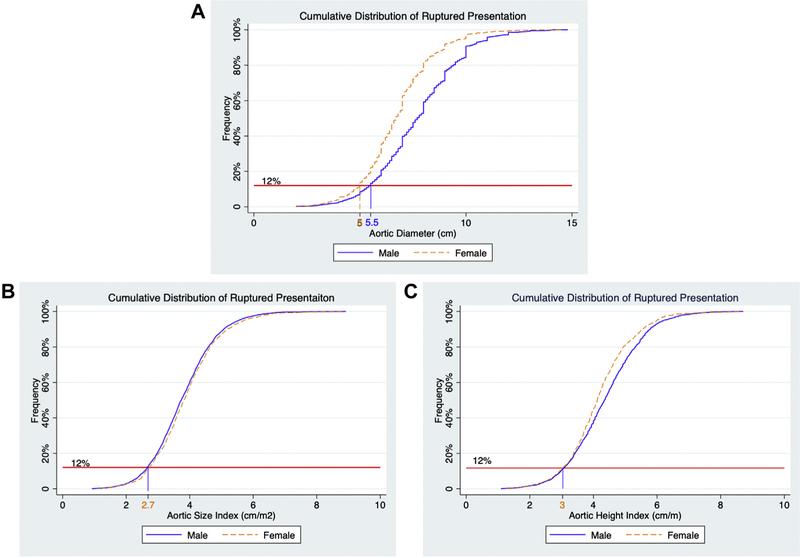
When cumulative distribution of rupture repair was plotted against aortic diameter, 12% of all rupture repairs in male patients were performed at an aortic diameter below 5.5 cm (Fig 2). The same 12% frequency of rupture repair in female patients occurred at an aortic diameter of 4.9 cm. However, 12% of the rupture repairs occurred at similar aortic measurements for female and male patients when using ASI (2.7 vs 2.7 cm/m2) and AHI (3.0 vs 3.0 cm/m).
Fig 2.
Plot of the cumulative distribution function for male and female patients undergoing ruptured abdominal aortic aneurysm (AAA) repair by aortic diameter (A), aortic size index (ASI) (B), and aortic height index (AHI) (C).
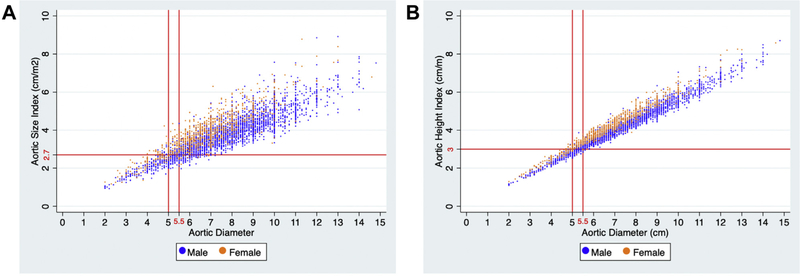
Of female patients undergoing rupture repair (n = 978), 20% had an aneurysm diameter below 5.5 cm, 14% had an aneurysm diameter at or below 5 cm, and 12% had an aneurysm diameter at or below 4.9 cm. Additionally, 12% of female patients undergoing rupture repair had an ASI below 2.7 cm/m2 and an AHI below 3.0 cm/m. Of the female patients undergoing rupture repair below 5.0 cm (n = 134), 31% had an ASI above 2.7 cm/m2, 22% had an AHI above 3.0 cm/m, and 20% had an aortic diameter above 4.9 cm (Fig 3).
Fig 3.
Distribution of male and female patients undergoing ruptured AAA repair stratified by (A) aortic diameter and aortic size index (ASI) (A) or aortic diameter and aortic height index (AHI) (B) at the time of repair.
Of male patients undergoing rupture repair (n = 3533), 12% had an aneurysm diameter below 5.5 cm, and 8.4% had an aneurysm diameter at or below 5 cm. Additionally, 12% of male patients undergoing rupture repair had an ASI below 2.7 cm/m2 or an AHI below 3.0 cm/m. Of the male patients undergoing rupture repair below 5.5 cm (n = 415), 16% had an ASI above 2.7 cm/m2, and 14% had an AHI above 3.0 cm/m (Fig 3).
Perioperative outcomes.
Female patients had higher perioperative mortality after intact EVAR (1.4% vs 0.7%; P < .001), intact open repair (5.1% vs 3.2%; P < .001), ruptured EVAR (26% vs 20%; P < .001), and ruptured open repair (42% vs 31%; P < .001; Table III). Female patients had higher major complication rates after intact EVAR (6.3% vs 3.2%; P < .001) and intact open repair (24% vs 20%; P < .001); this trend was similar when comparing a composite of perioperative mortality and major complication. Following EVAR for ruptured AAA, female patients had similar rates of major complication (34% vs 35%; P = .79) as well as composite perioperative mortality and major complication (46% vs 43%; P = .17). Major complication rates were lower in female patients after ruptured open repair (54% vs 60%; P = .03); however, when composite perioperative mortality and major complication was compared, this difference was mitigated (73% vs 70%; P = .11). Female patients were more likely to be discharged to a skilled nursing facility after intact EVAR (7.7% vs 3.6%; P < .001), intact open repair (26% vs 15%; P < .001), or ruptured EVAR (35% vs 21%; P < .001).
Table III.
Perioperative outcomes for female and male patients undergoing AAA repair stratified by intact and rupture repair
| EVAR |
Open repair |
|||||
|---|---|---|---|---|---|---|
| Female | Male | P value | Female | Male | P value | |
|
| ||||||
| Intact repair | ||||||
| No. patients | 7893 | 34,643 | 2225 | 6375 | ||
| Perioperative mortality | 1.4 | 0.7 | <.001 | 5.1 | 3.2 | <.001 |
| Any major complication | 6.3 | 3.2 | <.001 | 24 | 20 | <.001 |
| Mortality + complication | 6.8 | 3.6 | <.001 | 25 | 20 | <.001 |
| LOS, d | 2 (1–3) | 1 (1–2) | <.001 | 7 (6–9) | 7 (5–9) | <.001 |
| Cardiac complications | 3.9 | 2.6 | <.001 | 17 | 17 | .93 |
| Renal complications | 4.4 | 2.7 | <.001 | 16 | 17 | .1 |
| Respiratory complication | 1.9 | 1.1 | <.001 | 12 | 9.6 | <.001 |
| Access-related complication | 2.9 | 1.8 | <.001 | – | – | – |
| Postoperative stroke | 0.2 | 0.2 | .27 | 1.0 | 0.8 | .33 |
| Intestinal ischemia | 0.7 | 0.3 | <.001 | 4.3 | 3.3 | .044 |
| Discharged to SNF | 7.8 | 3.6 | <.001 | 26 | 15 | <.001 |
| Rupture repair | ||||||
| No. patients | 562 | 2097 | 416 | 1436 | ||
| Perioperative mortality | 26 | 20 | <.001 | 42 | 31 | <.001 |
| Any major complication | 34 | 35 | .80 | 54 | 60 | .03 |
| Mortality + complication | 46 | 43 | .17 | 73 | 69 | .11 |
| LOS, d | 5 (3–10) | 5 (3–10) | .73 | 8.5 (2–17) | 10 (5–19) | .002 |
| Cardiac complications | 20 | 21 | .47 | 34 | 37 | .19 |
| Renal complications | 25 | 26 | .81 | 41 | 41 | .84 |
| Respiratory complication | 20 | 20 | .86 | 33 | 39 | .036 |
| Access-related complication | 4.9 | 4.0 | .36 | – | – | – |
| Postoperative stroke | 2.8 | 2.2 | .45 | 3.3 | 3.6 | .76 |
| Intestinal ischemia | 6.4 | 6.2 | .91 | 18 | 18 | .96 |
| Discharged to SNF | 35 | 21 | <.001 | 32 | 29 | .22 |
AAA, Abdominal aortic aneurysm; EVAR, endovascular aneurysm repair; LOS, length of stay; SNF, skilled nursing facility.
Categorical variables are presented as number (%) and continuous variables as median (interquartile range).
Adjusted outcomes.
After adjustment for demographics, coexisting conditions, type of repair, and aortic diameter, female sex remained associated with higher perioperative mortality after intact repair (odds ratio [OR], 1.5; 95% confidence interval [CI], 1.3–1.8; P < .001) and rupture repair (OR, 1.3; 95% CI, 1.1–1.6; P = .003) (Table IV). When aortic diameter was replaced with ASI in the model, the association between female sex and perioperative mortality remained significant for both intact repair (OR, 1.3; 95% CI, 1.1–1.6; P = .003) and rupture repair (OR, 1.3; 95% CI, 1.1–1.5; P = .01). When aortic diameter was replaced with AHI in the model, the association between female sex and perioperative mortality again remained significant for both intact repair (OR, 1.4; 95% CI, 1.2–1.7; P < .001) and rupture repair (OR, 1.3; 95% CI, 1.1–1.5; P = .006). There was no interaction between female sex and the remaining covariates within each model.
Table IV.
Multivariable adjusted analysis of the effect of female sex on perioperative mortality following intact repair and ruptured repair adjusted for aortic diameter, ASI, or AHI
| Model adjusted for | Odds ratio | 95% CI | P value |
|---|---|---|---|
|
| |||
| Intact repair | |||
| Aortic diameter | 1.5 | 1.3–1.8 | <.001 |
| ASI | 1.3 | 1.1–1.6 | .003 |
| AHI | 1.4 | 1.2–1.7 | <.001 |
| Rupture repair | |||
| Aortic diameter | 1.3 | 1.1–1.6 | .003 |
| ASI | 1.3 | 1.1–1.5 | .010 |
| AHI | 1.3 | 1.1–1.5 | .006 |
ASI, Aortic size index; AHI, Aortic height index; CI, confidence interval.
Models also adjusted for age, race, current smoking status, insulin-dependent diabetes mellitus, hypertension, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, prior abdominal aortic aneurysm repair, coronary artery disease, family history of abdominal aortic aneurysm, preoperative aspirin use, preoperative statin use, preoperative beta-blocker use, and type of repair (endovascular vs open).
When adjusted for demographics, coexisting conditions, type of repair, and aortic diameter, female sex was significantly associated with any major complication after intact repair (OR, 1.5; 95% CI, 1.4–1.7; P < .001) (Table V). When aortic diameter was replaced with ASI in the model, female sex was associated with higher risk of any major complication after intact repair (OR, 1.4; 95% CI, 1.3–1.5; P < .001). Similarly, when aortic diameter was replaced with AHI, female sex was associated with higher risk of any major complication after intact repair (OR, 1.4; 95% CI, 1.3–1.6; P < .001). Again, there was no interaction between female sex and the remaining covariates within each model.
Table V.
Multivariable adjusted analysis of the effect of female sex on any major complication following intact repair and ruptured repair adjusted for aortic diameter, ASI, or AHI
| Model adjusted for | Odds ratio | 95% CI | P value |
|---|---|---|---|
|
| |||
| Intact repair | |||
| Aortic diameter | 1.5 | 1.4–1.7 | <.001 |
| ASI | 1.4 | 1.3–1.5 | <.001 |
| AHI | 1.4 | 1.3–1.6 | <.001 |
| Rupture repair | |||
| Aortic diameter | 0.9 | 0.7–1.0 | .06 |
| ASI | 0.8 | 0.7–0.9 | .016 |
| AHI | 0.8 | 0.7–0.9 | .027 |
AHI, Aortic height index; ASI, aortic size index; CI, confidence interval.
Models also adjusted for age, race, current smoking status, insulin-dependent diabetes mellitus, hypertension, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, prior abdominal aortic aneurysm repair, coronary artery disease, family history of abdominal aortic aneurysm, preoperative aspirin use, preoperative statin use, preoperative beta-blocker use, and type of repair (endovascular vs open).
DISCUSSION
When compared with male patients, female patients had smaller aortic diameter but larger ASI at the time of intact and rupture AAA repair. Female patients had larger AHI at the time of intact AAA repair, but smaller AHI at the time of rupture repair. When comparing the cumulative distribution of rupture repair in male and female patients, the currently recommended criteria for repair in males of 5.5 cm represented an ASI of 2.7 cm/m2, an AHI of 3.0 cm/m, or an aortic diameter of 4.9 cm in female patients. When adjusting for demographics, coexisting conditions, and aortic diameter, female patients had higher odds of perioperative mortality following intact and rupture repair, and higher odds of major complication after intact repair. When aortic diameter was replaced with ASI and AHI, the association of female sex with perioperative mortality following both intact and rupture repair and the association with any major complications after intact repair remained significant.
An ASI of 2.7 cm/m2 and an AHI of 3.0 cm/m were chosen as these measurements represent the point where 12% of male patients and 12% of female patients with ruptured aneurysms were treated. The 12% frequency was chosen to correspond to the proportion of male patients who underwent repair of ruptured aneurysm below the current 5.5 cm aortic diameter threshold for repair. However, as a society, we must determine if it is acceptable to have a threshold of repair, whether it be aortic diameter, ASI, or AHI, below which 12% of the population is at risk for presenting with ruptured aneurysm. Further studies are warranted to identify patients with smaller aneurysms who are at risk for rupture.
It should also be noted that our study population only includes those patients who underwent vascular intervention; therefore, we are not able to capture the true population of patients with small aneurysms. In order to determine the true validity of ASI or AHI over aortic diameter we would need prospective data that identifies all female patients with small aneurysms who are at risk of rupture, not just those with small aneurysms who undergo repair. Until such data are available, we cannot propose the superiority of ASI or AHI over aortic diameter. Therefore, our study primarily supports sex-specific aortic diameter thresholds for repair.
The most recent SVS practice guidelines for AAA management suggest repair in female patients with an AAA between 5.0 cm and 5.4 cm; however, the guidelines note only young, healthy females would derive benefit from repair at this smaller aortic diameter.9 Likewise, the ESVS practice guidelines state aneurysm repair should be considered in female patients with an aortic diameter of 5.0 cm.10 Our study found that 20% of female patients undergoing repair for ruptured aneurysm had an aortic diameter less than 5.5 cm compared with only 12% of males. Furthermore, 14% of female patients underwent rupture repair at an aortic diameter less than 5.0 cm. It should be noted that earlier repair in female patients is not a “recommendation” in either practice guideline; rather, these are suggestions and considerations to be made by the individual surgeon based on patient presentation and health status. Although the guidelines suggest repair in young, healthy female patients, there may be older patients with comorbid conditions who would also benefit from repair. Therefore, operative risk should be calculated for each patient19 and considered together with their estimated life-expectancy when considering eligibility for operative repair.20,21 Our findings suggest a change in practice guidelines to provide a stronger recommendation for sex-specific elective AAA repair threshold for all female patients with an aortic diameter ≥5.0 cm.
Studies across multiple databases including the VQI, National Surgical Quality Improvement Program, and National Inpatient Sample have found that female patients derive less benefit from repair of aortic aneurysms. When compared with males, female patients had increased 30-day mortality,6,22–27 postoperative complications,6–8,23,25,27 type IA endoleak,27,28 and were more likely to be discharged to skilled nursing facilities.7,23,24,29 The worse outcomes observed in female patients in our study and in previously published works cannot be refuted. However, by comparing demographics, patient anatomy, and current practice guidelines, we can begin to understand the causes of these disparate outcomes and identify targeted strategies for improvement. As shown in our study and several others, female patients present with smaller aortic diameter at the time of both intact and rupture6–8,2 Furthermore, female patients are more likely to undergo repair for ruptured aneurysm compared with their male counterparts.
These findings suggest that we may be underdiagnosing aortic aneurysms in female patients and failing to intervene in a timely manner. The effectiveness of screening guidelines is dependent on the prevalence of disease, cost and accuracy of testing, and the expected reduction in morbidity and mortality following intervention. Currently, the United States Preventive Services Task Force and Canadian Task Force on Preventative Care both recommend against AAA screening in females.30,31 The low prevalence of AAA in females and adverse outcomes following repair have been the mainstay for recommending against screening. However, due to increased prevalence of AAA in several high-risk groups, the SVS recommends screening women aged 65 years or older who have a history of smoking or a family history of AAA.9 The ESVS also recommends screening women with a family history of AAA and those with a true peripheral arterial aneurysm.10 The current sex-neutral definition for diagnosis of AAA may also contribute to the low prevalence in female patients. Several population-based studies have shown the aortic diameter in female patients is 2 to 6 mm smaller than males.11–13 When aortic aneurysm diagnosis was defined as the median aortic diameter plus two standard deviations, Wanhainen et al found infrarenal AAA diagnosis should be defined as an aortic diameter greater than 3.0 cm in male patients and 2.7 cm in female patients.11 If sex-specific thresholds for diagnosis were implemented to reflect baseline anatomic differences, the prevalence of AAA in female patients would increase, positively influencing the value of expanding screening guidelines.
Furthermore, female patients are more likely to present with challenging anatomy, including shorter neck length, more angulated neck, and smaller iliac artery diameter.6–8,23,27,28,32 These anatomic differences may further contribute to fewer female patients being offered EVAR and the worse outcomes observed in female patients following endovascular repair. When contemporary low-profile stent graft use was analyzed using the ENGAGE registry, female patients were found to have more challenging anatomy at the time of repair and were more likely to be treated outside the manufacturer’s instructions for use. Despite these anatomic differences, female patients experienced similar perioperative outcomes, long-term survival, freedom from aneurysm-related reinterventions, late rupture, and open conversion compared with male patients.28,32 Device development dedicated to stent grafts with lower profile, widely applicable instructions for use, and conformability better suited for complex anatomy may further help reduce the disparity in outcomes between male and female patients undergoing EVAR.
The vast majority of practice patterns for both open and endovascular aortic aneurysm repair are based on randomized controlled trials in which female patients were only modestly represented.1–4 Female patients have also been underrepresented in pivotal trials for current United States Food and Drug Administration-approved infrarenal devices.33–37 As female patients are less likely to undergo endovascular repair, expanding device development to account for sex-specific anatomy may enhance the range of endovascular repair options. Increasing female representation and treating male and female patients as two separate entities in future research studies, as well as device development research, may expand our understanding of disease pathology and ultimately lead to improved practice patterns in female patients.
This study should be interpreted within the context of its retrospective design. As the VQI is a voluntary quality initiative registry, the participating centers are likely to have a dedicated focus on quality improvement and are more likely to be high-volume centers. Therefore, these outcomes might not be generalizable to the wider population.38 However, as of 2015, AAA repairs in VQI accounted for 24% of all AAA repairs in the United States, and the proportion is increasing over time.38 Device-specific information was blinded for the investigators; therefore, potential device-related confounding could not be accounted for. Furthermore, only patients who have undergone vascular interventions are included in the VQI, introducing a selection bias. We do not have data on patients with small aortic aneurysms who did not undergo repair; therefore, patients with small intact AAAs are not included in our analysis. Patients with ruptured aneurysms that did not reach the hospital to receive medical care are not accounted for in our analysis. As a result, our findings are likely to underrepresent the true mortality rate associated with rupture presentation. Furthermore, without data on this subpopulation we are unable to analyze the effect of aortic diameter, ASI, and AHI for patients that do not undergo repair. The Dubois and Dubois formula has been found to underestimate the true body surface area in obese patients,39 consequently resulting in an overestimation of the ASI.
CONCLUSIONS
Our study provides data to strongly encourage the 5.0-cm aortic diameter threshold suggested for repair in female patients by the SVS and ESVS. Individualized timing for AAA repair should be offered to all patients based on aortic size and operative risk. This study does not include data on patients who were unable to seek timely medical attention; therefore, our study likely underestimates the true severity of ruptured aneurysm. Lastly, the high percentage of male and female patients undergoing repair of ruptured AAA below the current elective repair threshold highlights the need to better identify patients at risk of rupture at smaller aortic diameters.
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective cohort study of prospectively collected data from the Vascular Quality Initiative registry
Key Findings: In our cohort of 55,647 patients who underwent abdominal aortic aneurysm repair, female patients underwent repair at smaller median aortic diameter compared with male patients. To achieve the same proportion of rupture repair as male patients at 5.5 cm (12%), the female repair diameter was only 4.9 cm. However, when aortic size index and aortic height index were used, female and male patients reached 12% of rupture repair at a similar aortic size index of 2.7 cm/m2 and an aortic height index of 3.0 cm/m.
Take Home Message: This study supports sex-specific aortic diameter thresholds for repair of 5.0 cm in female patients and 5.5 cm in male patients.
Acknowledgments
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541 and financial contributions from Harvard University and its affiliated academic health care centers. P.B.P. and K.D. are supported by the Harvard-Longwood Research Training in Vascular Surgery National Institutes of Health T32 Grant 5T32HL00773422. C.L.M. is supported by grant number F32HS027285 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, the National Institutes of Health, or the Agency for Healthcare Research and Quality.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Powell JT, Brown LC, Forbes JF, Fowkes FGR, Greenhalgh RM, Ruckley CV, et al. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg 2007;94:702–8. [DOI] [PubMed] [Google Scholar]
- 2.Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010;51:1081–7. [DOI] [PubMed] [Google Scholar]
- 3.Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E; CAESAR Trial Group. Comparison of Surveillance Versus Aortic Endografting for Small Aneurysm Repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg 2011;41:13–25. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Liitooy FN, Acher CW, et al. Aneurysm Detection and Management Veterans Affairs Cooperative Study Group. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002;346: 1437–44. [DOI] [PubMed] [Google Scholar]
- 5.Lo RC, Lu B, Fokkema MTM, Conrad M, Patel VI, Fillinger M, et al. Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. J Vasc Surg 2014;59:1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deery SE, Soden PA, Zettervall SL, Shean KE, Bodewes TCF, Pothof AB, et al. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg 2017:65: 1006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML; Vascular Study Group of New England. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg 2013:57:1261–8.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matyal R, Shakil O, Hess PE, Lo R, Jainandunsing JS, Mahmood B, et al. Impact of gender and body surface area on outcome after abdominal aortic aneurysm repair. Am J Surg 2015;209:315–23. [DOI] [PubMed] [Google Scholar]
- 9.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee AW, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67:2–77.e2. [DOI] [PubMed] [Google Scholar]
- 10.Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. ; European Society for Vascular Surgery. Management of abdominal aortic aneurysms: clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2011;41:S1–58. [DOI] [PubMed] [Google Scholar]
- 11.Wanhainen A, Themudo R, Ahlström H, Lind L, Johansson L. Thoracic and abdominal aortic dimension in 70-year-old men and women — A population-based whole-body magnetic resonance imaging (MRI) study. J Vasc Surg 2008:47:504–12. [DOI] [PubMed] [Google Scholar]
- 12.Rogers IS, Massaro JM, Truong QA, Mahabade AA, Kriegel MF, Fox CS, et al. Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study). Am J Cardiol 2013;111:1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham MHC, Ballegaard C, de Knegt MC, Sigvardsen PE, Sorgaard MH, Fuchs A, et al. Normal values of aortic dimensions assessed by multidetector computed tomography in the Copenhagen General Population Study. Eur Heart J Cardiovasc Imaging 2019;20:939–48. [DOI] [PubMed] [Google Scholar]
- 14.Davies RR, Gallo A, Coady MA, Tellides G, Botta DDM, Burke B, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169–77. [DOI] [PubMed] [Google Scholar]
- 15.Jones GT, Sandiford P, Hill GB, Williams MJ, Khashram M, Tilyard MW, et al. Correcting for body surface area identifies the true prevalence of abdominal aortic aneurysm in screened women. Eur J Vasc Endovasc Surg 2019;57:221–8. [DOI] [PubMed] [Google Scholar]
- 16.Zafar MA, Chen JF, Wu J, Li Y, Papanikolaou D, Abdelbaky M, et al. Yale Aortic Institute Natural History Investigators. Natural history of descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2021;161:498–511.e1. [DOI] [PubMed] [Google Scholar]
- 17.Coady MA, Ikonomidis JS, Cheung AT, Matsumoto AH, Dake MD, Chaikof EL, et al. American Heart Association Council on Cardiovascular Surgery and Anesthesia and Council on Peripheral Vascular Disease. Surgical management of descending thoracic aortic disease: open and endovascular approaches. Circulation 2010;121: 2780–804. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007;18:800–4. [DOI] [PubMed] [Google Scholar]
- 19.Eslami MH, Rybin D, Doros G, Kalish JA, Farber A. Vascular Study Group of New England. Comparison of a Vascular Study Group of New England risk prediction model with established risk prediction models of in-hospital mortality after elective abdominal aortic aneurysm repair. J Vasc Surg 2015;62:1125–33.e2. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell TFX, Wade JE, Liang P, Li C, Swerdlow NJ, DeMartino RR, et al. Endovascular aneurysm repair in patients over 75 is associated with excellent 5-year survival, which suggests benefit from expanded screening into this cohort. J Vasc Surg 2019;69: 728–37. [DOI] [PubMed] [Google Scholar]
- 21.Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD Jr, Blebea J, et al. ; Veterans Affairs Cooperative Study #417 Investigators. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002;287:2968–72. [DOI] [PubMed] [Google Scholar]
- 22.McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001–2004. J Vasc Surg 2007;45:891–9. [DOI] [PubMed] [Google Scholar]
- 23.Mehta M, Byrne WJ, Robinson H, Roddy SP, Paty PSK, Kreienberg PB, et al. Women derive less benefit from elective endovascular aneurysm repair than men. J Vasc Surg 2012;55:906–13. [DOI] [PubMed] [Google Scholar]
- 24.Egorova NN, Vouyouka AG, McKinsey JF, Faris PL, Kent KC, Moskowitz AJ, et al. Effect of gender on long-term survival after abdominal aortic aneurysm repair based on results from the Medicare national database. J Vasc Surg 2011;54:1–12.e6. [DOI] [PubMed] [Google Scholar]
- 25.de Guerre LEVM Varkevisser RRB, Swerdlow NJ, Liang P, Li C, Dansey K, et al. Sex differences in perioperative outcomes after complex abdominal aortic aneurysm repair. J Vasc Surg 2020;71: 374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abedi NN, Davenport DL, Xenos E, Sorial E, Minion DJ, Endean ED. Gender and 30-day outcome in patients undergoing endovascular aneurysm repair (EVAR): an analysis using the ACS NSQIP dataset. J Vasc Surg 2009;50:486–91. 491.e1–491.e4. [DOI] [PubMed] [Google Scholar]
- 27.Locham S, Shaaban A, Wang L, Bandyk D, Schermerhorn M, Malas MB. Impact of gender on outcomes following abdominal aortic aneurysm repair. Vasc Endovascular Surg 2019;53:636–43. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell TFX, Verhagen HJ, Pratesi G, Tratesi C, Teijink J, Vermassen F, et al. Female sex is associated with comparable 5-year outcomes after contemporary endovascular aneurysm repair despite more challenging anatomy. J Vasc Surg 2020:71:1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boitano LT. lannuzzi JC, Tanious A, Mohebali J. Schwartz SI, Chang DC, et al. Preoperative predictors of discharge destination after endovascular repair of abdominal aortic aneurysms. Ann Vasc Surg 2019;57:109–17. [DOI] [PubMed] [Google Scholar]
- 30.United States Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA 2019;322:2211–8. [DOI] [PubMed] [Google Scholar]
- 31.Canadian Task Force on Preventive Health Care. Abdominal aortic aneurysm. Available at: https://canadiantaskforce.ca/guidelines/published-guidelines/abdominal-aortic-aneurysm/2017. Accessed November 8, 2020.
- 32.Dubois L, Novick TV, Harris JR, DeRose G, Forbes TL. Outcomes after endovascular abdominal aortic aneurysm repair are equivalent between genders despite anatomic differences in women. J Vasc Surg 2013:57382–9.e1. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg RK, Chuter TAM, Sternbergh WC 3rd. Fearnot NE; Zenith Investigators. Zenith AAA endovascular graft: intermediate-term results of the US multicenter trial. J Vasc Surg 2004;39:1209–18. [DOI] [PubMed] [Google Scholar]
- 34.Criado FJ, Fairman RM, Becker GJ; Talent LPS Pivotal Clinical Trial investigators. Talent LPS AAA stent graft: results of a pivotal clinical trial. J Vasc Surg 2003;37:709–15. [DOI] [PubMed] [Google Scholar]
- 35.Singh MJ, Fairman R, Anain P, Jordan WD, Maldonaldo T, Samson R, et al. ; Endurant U.S. Pivotal Trial Investigators. Final results of the Endurant Stent Graft System in the United States regulatory trial. J Vasc Surg 2016;64:55–62. [DOI] [PubMed] [Google Scholar]
- 36.Barleben A, Mathlouthi A, Mehta M, Nolte T, Valdes F, Malas MB; Ovation trial investigators. Long-term outcomes of the Ovation Stent Graft System investigational device exemption trial for endovascular abdominal aortic aneurysm repair. J Vasc Surg 2020;72:1667–73.e1. [DOI] [PubMed] [Google Scholar]
- 37.Malas MB, Hicks CW, Jordan WD, Hodgson KJ, Mills JL, Makaroun MS, et al. ; PYTHAGORAS Investigators. Five-year outcomes of the PYTHAGORAS U.S. clinical trial of the Aorfix endograft for endovascular aneurysm repair in patients with highly angulated aortic necks. J Vasc Surg 2017;65:1598–607. [DOI] [PubMed] [Google Scholar]
- 38.Dansey KD, de Guerre LEVM, Swerdlow NJ, Li C, Lu J, Patel PB, et al. Not all databases are created equal, a comparison of administrative data and quality improvement registries for abdominal aortic aneurysm repair. J Vasc Surg 2021;73:874–88. [DOI] [PubMed] [Google Scholar]
- 39.Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 2006;55:515–24. [DOI] [PubMed] [Google Scholar]