Abstract
Perfluorooctanoic acid (PFOA) is a synthetic fluorosurfactant used in the manufacturing of fluorotelomers. Although PFOA is no longer produced in the United States, it is environmentally persistent and found in imported food packaging, cookware, and textiles. Previous studies have identified developmental toxicity of PFOA, but little is known about the effects of PFOA on the adult ovary. Thus, this study examined the effects of PFOA on hormone levels, ovarian steroidogenic gene expression, and folliculogenesis in mice in vitro and in vivo. For the in vitro studies, antral follicles from adult female mice were cultured with vehicle control or 1, 10, or 100 μg/ml PFOA for 96 h. For the in vivo studies, adult CD-1 female mice were orally dosed with vehicle control or 1, 5, 10, or 20 mg/kg/day PFOA for 10 days. Gene expression of steroidogenic enzymes, levels of sex steroid hormones, and follicle counts were analyzed. In vitro, PFOA (100 μg/ml) significantly decreased follicle growth, estradiol and estrone levels, and gene expression of StaR, Cyp11a1, and Hsd3b1 compared with controls. In vivo, exposure to PFOA significantly decreased progesterone and pregnenolone levels (5 mg/kg), increased testosterone levels (1 mg/kg), and increased gene expression of Cyp19a1 (1 mg/kg) compared with controls. Exposure to PFOA also significantly altered follicle counts by decreasing primordial follicles and increasing preantral and antral follicles (5 and 10 mg/kg) compared with controls. Collectively, these data show that PFOA disrupts adult ovarian function in a nonmonotonic matter and may pose a risk for premature ovarian failure.
Keywords: PFOA, perfluorinated compounds, ovary, endocrine disruption, folliculogenesis
Perfluorooctanoic acid (PFOA) is a synthetic chemical that is part of the perfluoroalkyl family of compounds, in which carbon-hydrogen bonds have been replaced with carbon-fluorine bonds. Perfluoroalkyls repel oil, stains, grease, and water, making them ideal for stain-resistant treatments for textiles and for producing nonstick cookware (Gonsioroski et al., 2020). PFOA commonly enters the body via ingestion of contaminated food or water or dermal contact with PFOA-containing products (Cordner et al., 2019; Franko et al., 2012; Poothong et al., 2012; Vestergren and Cousins, 2009; Xiao et al., 2015). Environmental spills from manufacturing have also led to high drinking water exposures (Frisbee et al., 2009; Vieira et al., 2013). In 2006, the Environmental Protection Agency organized a stewardship program to eliminate PFOA production in the United States by 2015. However, products containing PFOA from other countries can still be imported and reach consumers.
Due to the high stability of carbon-fluorine bonds, perfluoroalkyl compounds are environmentally persistent and bioaccumulative (Post et al., 2012). The half-life of PFOA ranges from 2.3 to 3.8 years in humans and 16–19 days in mice (Lou et al., 2009; Seals et al., 2011). Concentrations of PFOA in human sera typically range from 3 to 5 ng/ml and have been decreasing over time (Kato et al., 2011; Vestergren and Cousins, 2009). However, studies of human populations exposed to contaminated drinking water report average exposures of approximately 10 times higher than the average U.S. exposure, with blood concentrations reaching up to 22 000 ng/ml (Frisbee et al., 2009; Innes et al., 2014). PFOA has been measured in ovarian follicular fluid at levels similar to or slightly less than average serum concentrations (2–5 ng/ml; Heffernan et al., 2018; Kang et al., 2020; Kim et al., 2020; McCoy et al., 2017).
Multiple perfluoroalkyl chemicals, including PFOA, are associated with ovarian toxicity in human and animal studies (reviewed in Ding et al., 2020). In women participating in the National Health and Nutrition Examination Survey in the United States, higher levels of per-and polyfluoroalkyl substances (PFAS) were associated with earlier menopause (Taylor et al., 2014). In a cohort of Chinese women, higher plasma levels of multiple PFAS, including PFOA, were associated with menstrual cycle irregularity (Zhou et al., 2017). Plasma PFOA levels were also associated with primary ovarian insufficiency in another cohort of Chinese women (Zhang et al., 2018), indicating that the ovary is a likely target of PFAS toxicity.
The ovary is responsible for the production and release of female germ cells or oocytes, as well as the production of sex steroid hormones (Hirshfield, 1991). Female mammals are born with a finite number of primordial follicles, which contain an immature oocyte surrounded by a single layer of somatic cells known as granulosa cells. Primordial follicles must grow to primary, preantral, and then antral follicles to be capable of releasing an oocyte for fertilization in a process called folliculogenesis. Furthermore, follicles must grow to the antral stage to be capable of producing large amounts of sex steroid hormones, especially estrogens.
Although numerous studies have identified developmental disruptions, including altered oocyte development, increased apoptosis, and decreased number of primordial follicles following prenatal exposure of PFOA (Ding et al., 2020), few studies have examined models of adult exposure. Disruption of folliculogenesis or steroidogenesis in adulthood can lead to reduced fertility, premature ovarian failure, and hormone-mediated diseases (Craig et al., 2011). Thus, the purpose of this study was to examine the effects of PFOA exposure during adulthood on key ovarian functions, folliculogenesis, and steroidogenesis, using a mouse model. Ovarian physiology is highly conserved in mammalian species, making the rodent an appropriate translatable model for humans (Hirshfield, 1991). Experiments were performed in vitro and in vivo to test the hypothesis that adult exposure to PFOA alters hormone levels and disrupts folliculogenesis in young adult mice.
MATERIALS AND METHODS
Chemicals
PFOA was obtained (Sigma-Aldrich, St Louis, Missouri) and dissolved in 0.5% Tween20 (Sigma-Aldrich). For in vitro studies, follicles were treated with vehicle control (dH2O 0.5% Tween20) or PFOA (1, 10, or 100 μg/ml). For in vivo studies, mice were exposed to vehicle control (dH2O 0.5% Tween20) or PFOA (1, 5, 10, or 20 mg/kg/day). The doses of PFOA were chosen based on relevant human biomonitoring levels of up to 22 µg/ml and doses used in previous toxicology studies (Cordner et al., 2019; Ding et al., 2020). A previous study of CD-1 female mice dosed with 20 mg/kg of PFOA for 17 days measured approximately 175 μg/ml of PFOA in serum immediately following exposure (Lau et al., 2006). Another study on the pharmacokinetics of PFOA in mice predicted serum levels of 50 μg/ml following 10 days of repeated dosing of 5 mg/kg PFOA (Lou et al., 2009), suggesting that the ranges of doses used for our in vitro and in vivo experiments are physiologically comparable to each other.
Animals and in vivo study design
Young adult female CD-1 mice were purchased from Charles River (Wilmington, Massachusetts) at 23 days of age. Mice were housed in the College of Veterinary Medicine Animal Facility at the University of Illinois at Urbana-Champaign and allowed to acclimate to the facility prior to experimentation. Temperature was maintained at 22°C ± 1°C with 12-h light-dark cycles to provide a controlled housing environment and food and water were provided ad libitum. At 30 days of age, mice were orally dosed by pipetting the solution into mouth with either vehicle control (dH2O with 0.5% Tween20) or PFOA (1–20 mg/kg/day) for 10 days. Dosing volumes were determined daily by corresponding mouse weight. Mice were euthanized via carbon dioxide inhalation during diestrus for sample collection within 8 days of the final dose. Cyclicity was assessed daily at the same time via vaginal lavage. Body weight and ovary, uterus, and liver weights were determined following euthanasia. Blood was collected from the inferior vena cava after euthanization, allowed to sit at room temperature for 15 min, and then centrifuged to isolate sera. Sera were frozen at −80°C until analysis of sex steroid hormone levels. Ovaries were collected for follicle counts and gene expression analyses (1 ovary each, randomly assigned). All procedures involving animal care, euthanasia, and tissue collection were approved by the Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign.
Follicle counts
Following exposure to PFOA for 10 days, ovaries were fixed in Dietrich’s fixative, transferred to 70% ethanol, dehydrated, embedded in paraffin wax, and serially sectioned (8 µm) using a microtome. Every 10th serial section was mounted on a glass slide and stained with hematoxylin and eosin. Stage of follicular development was assessed using published criteria (Flaws et al., 1994; Hannon et al., 2014; Pedersen and Peters, 1968). Briefly, primordial follicles contained an oocyte surrounded by a single layer of squamous granulosa cells, primary follicles contained an oocyte surrounded by a single layer of cuboidal granulosa cells, preantral follicles contained an oocyte surrounded by at least 2 layers of cuboidal granulosa cells and theca cells, and antral follicles contained an oocyte surrounded by multiple layers of cuboidal granulosa cells with a fluid-filled antral space and theca cells. All primordial and primary follicles were counted in each section regardless of nuclear material in the oocyte, whereas only preantral and antral follicles with nuclear material in the oocyte were counted to avoid double counting the larger follicle types that can span multiple sections. Follicles transitioning between stages were counted as follicles within the more immature stage of the 2 stages. The total numbers of all follicles and each type of follicle were recorded without knowledge of treatment group.
Antral follicle culture
Adult female CD-1 mice were euthanized at 32–38 days of age. Antral follicles were manually isolated from the ovaries based on relative follicle size (250–400 μm). Interstitial tissue was cleaned using watchmaker forceps. Follicles were pooled from 3 to 6 different mice for each follicle culture, with 10–30 follicles obtained from each mouse. The follicles were individually plated in 96-well tissue culture plates so that each treatment group contained 10–12 follicles. The different treatment groups (dH2O 0.5% Tween20, PFOA at 1, 10, or 100 μg/ml) were supplemented in minimum essential medium α, containing 1% insulin-transferrin-seleium (10 ng/ml insulin, 5.5 µg/ml transferrin, 5 ng/ml sodium selenite, Sigma-Aldrich), 100 U/ml penicillin (Sigma-Aldrich), 100 mg/ml streptomycin (Sigma-Aldrich), 5 IU/ml human recombinant follicle-stimulating hormone (Dr A.F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, California), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, Georgia). Various stock concentrations of PFOA (1.33, 13.33, and 133.33 mg/ml) were prepared, and 0.75 μl was used per 1 ml of supplemented medium. Final working concentrations of PFOA were 1, 10, and 100 μg/ml, respectively. Follicles were cultured for 96 h in an incubator supplying 5% CO2 at 37°C.
Follicle growth analysis
The growth of antral follicles over 96 h was assessed at 24 h timepoints by measuring follicle diameters on perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. The diameters of each follicle were averaged within the treatment group for each 24 h interval, and the average values were divided by the initial average measurement at 0 h of each of the respective treatment groups to calculate the percent change in follicle diameter over time. This percent change in antral follicle diameter over time was used for statistical analysis.
Hormone assays
After 96 h culture, media were collected from each well, pooled according to treatment group, and subjected to enzyme-linked immunosorbent assays (ELISAs) for measurement of the levels of progesterone, testosterone, estradiol, estrone, pregnenolone, and dehydroepiandrosterone (DHEA) which are essential for normal female reproductive function. Levels of progesterone, testosterone, estradiol, pregnenolone, and DHEA were also measured in the sera collected from the in vivo studies. The levels of these hormones were measured using ELISA kits purchased from DRG International, INC (Mountainside, New Jersey) according to manufacturer’s instructions. Samples were run in duplicate. Samples were diluted as needed to match the dynamic range of each ELISA kit and read with Multiskan Ascent software (Thermo Scientific). The detection ranges were 0.0083–16 ng/ml for testosterone, 0–40 ng/ml for progesterone, 10.6–2000 pg/ml for 17β-estradiol, 8.1–2400 pg/ml for estrone, 0.05–25.6 ng/ml for pregnenolone, and 0.07–30 ng/ml for DHEA. The mean values of each sample were used for statistical analysis. If the measurement was lower than the lowest limit of detection, the value was substituted with the lowest limit of detection divided by the square root of 2.
Quantitative real-time polymerase chain reaction
After 96 h culture, antral follicles were pooled according to treatment group. Total RNA was extracted from the follicles using an RNeasy Micro Kit (Qiagen, Inc., Valencia, California). RNA was also extracted for qPCR from whole ovaries collected following in vivo dosing, as described earlier. The concentrations of RNA were measured with a Nanodrop spectrophotometer following manufacturer’s instructions. Subsequently, cDNA was synthesized from 100 ng total RNA per sample using an iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, California). The qPCR reactions were run in duplicate with 1.67 ng cDNA and 7.5 pmol gene-specific primers (Integrated DNA Technologies, Inc. Coralville, Iowa) for a final reaction volume of 10 μl. The expression levels of the genes aromatase (Cyp19a1), 17α-hydroxylase (Cyp17a1), 17β-hydroxysteroid dehydrogenase 1 (Hsd17b1), cytochrome P450 11A1 (Cyp11a1), 3-beta-hydroxysteroid dehydrogenase 1 (Hsd3b1), and steroidogenic acute regulatory protein (Star) were compared among treatment groups (Supplementary Table 1). Beta-actin (Actb) was used as a reference gene for normalization (Craig et al., 2013; Hannon et al., 2014, 2015; Zhou et al., 2015) The Pfaffl method for relative quantification was used to obtain expression data, and the calculated relative fold changes compared with vehicle-treated controls were analyzed (Pfaffl, 2001).
In the in vitro experiments, the average coefficient of variation values across all independent replicates had < 1% variation. In the in vivo experiments, the average coefficient of variation values across all independent replicates had < 1.5% variation.
Statistical analyses
Data were expressed as the mean ± SEM. Data were analyzed by comparing treatment groups to control using IBM SPSS version 26 software (SPSS Inc., Chicago, Illinois). Outliers were removed by the Grubb’s test using GraphPad outlier calculator software (GraphPad Software Inc., La Jolla, California). All data were continuous and assessed for normal distribution by Shapiro-Wilk analysis. If data met assumptions of normal distribution and homogeneity of variance, data were analyzed by 1-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference test or Dunnett’s 2-sided post hoc comparisons. However, if data met assumptions of normal distributions, but not homogeneity of variance, data were analyzed by ANOVA followed by Games-Howell or Dunnett’s T3 post hoc comparisons. If data were not normally distributed or presented as percentages, the independent sample Kruskal-Wallis H followed by Mann-Whitney U non-parametric tests were performed. For all comparisons, statistical significance was determined by p value ≤ .05. If p values were > .05, but < .11, data were considered to exhibit a trend towards significance.
RESULTS
Effects of PFOA on Follicle Growth In Vitro
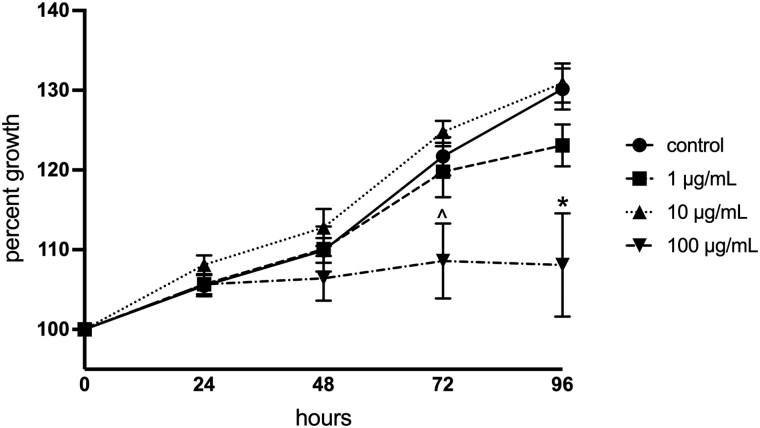
To assess the direct effects of PFOA on follicle growth, isolated antral follicles were cultured in media containing vehicle or various doses of PFOA for 96 h. PFOA at 100 µg/ml moderately inhibited growth at 72 h (p = .062) and dramatically inhibited growth at 96 h compared with vehicle-treated control follicles (p = .012; Figure 1). The 2 lower concentrations of PFOA (1 and 10 µg/ml) did not affect follicle growth at any time points compared with controls (Figure 1).
Figure 1.
Effects of in vitro perfluorooctanoic acid exposure on antral follicle growth in culture. Follicle growth was measured every 24 h for 96 h. Graphs represent means ± SEM from 3 to 7 independent experiments per treatment group. Asterisk (*) indicates significant difference from the control (p ≤ .05) and (^) indicates differences trending towards significance (.05 < p ≤ .1).
Effects of PFOA on Sex Steroid Hormone Levels and Steroidogenic Enzyme Gene Expression In Vitro
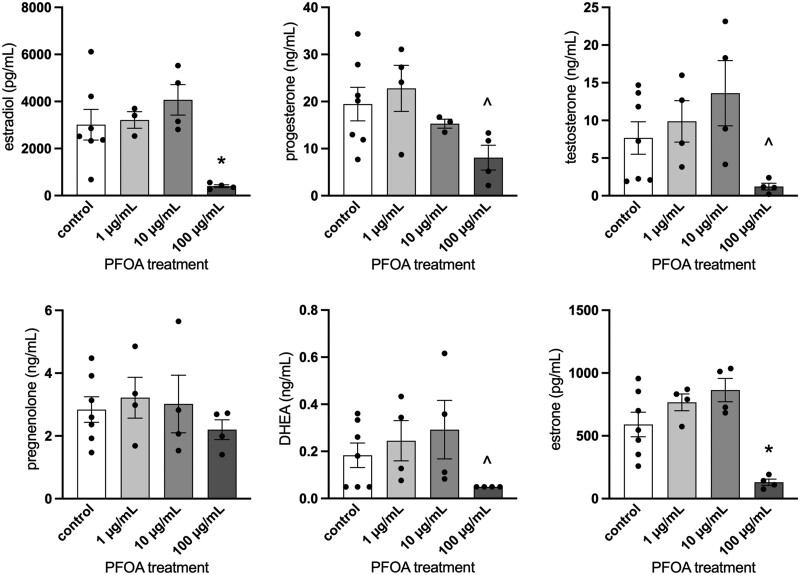
Basal hormone levels of 7 ± 2 ng/ml for testosterone, 19 ± 4 ng/ml for progesterone, 3012 ± 649 pg/ml for 17β-estradiol, 591 ± 98 pg/ml for estrone, 2.8 ± 0.4 ng/ml for pregnenolone, and 0.18 ± 0.05 ng/ml for DHEA were measured for the control groups. Treatment with PFOA at 100 µg/ml statistically significantly decreased estradiol and estrone levels compared with media from vehicle control experiments (p = .03 and .012, respectively; Figure 2). In addition, the levels of testosterone (p = .088), progesterone (p = .107), and DHEA (p = .08) trended towards statistically significant decreases in the 100 µg/ml treatment group compared with vehicle controls (Figure 2). PFOA did not alter levels of pregnenolone (p > .05).
Figure 2.
Effects of in vitro perfluorooctanoic acid exposure on sex steroid hormone level production by antral follicles. Culture media was subjected to enzyme-linked immunosorbent assays. Graphs represent means ± SEM from 3 to 7 independent experiments per treatment group. Asterisk (*) indicates significant difference from the control (p ≤ .05) and (^) indicates differences trending towards significance (.05 < p ≤ .1).
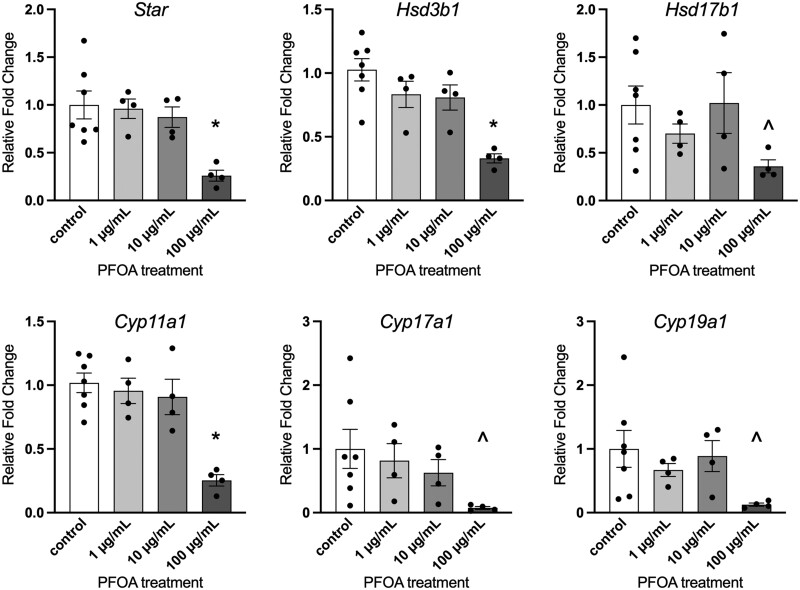
Exposure of PFOA at 100 µg/ml statistically significantly decreased the gene expression of Star (p = .007), Cyp11a1 (p < .001), and Hsd3b1 (p < .001) compared with controls (Figure 3). Additionally, gene expression of Hsd17b1 (p = .067), Cyp19a1 (p = .055), and Cyp17a1 (p = .067) trended towards statistically significant decreases in the 100 µg/ml treatment group compared with controls (Figure 3). Lower concentrations of PFOA did not significantly affect the gene expression of steroidogenic enzymes compared with vehicle-treated control follicles.
Figure 3.
Effects of in vitro perfluorooctanoic acid exposure on steroidogenic enzyme gene expression in antral follicles expressed as fold change compared with vehicle-treated controls. All gene expression is relative to the housekeeping gene, BAct. Graphs represent means ± SEM from 3 to 7 independent experiments per treatment group. Asterisk (*) indicates significant difference from the control (p ≤ .05) and (^) indicates differences trending towards significance (.05 < p ≤ .1).
Effects of PFOA Exposure on Organ Weights In Vivo
PFOA exposure (5, 10, and 20 mg/kg) statistically significantly increased liver weight compared with vehicle-treated control mice (p < .0001 for all, Supplementary Figure 1). When liver weight was normalized to body weight, all 4 doses of PFOA statistically significantly increased liver weight compared with control (p ≤ .05 for all). PFOA exposure did not alter raw (unnormalized) or normalized ovary weight, uteri weight, or body weight at collection compared with control. Initial body weights were statistically indistinguishable between groups and body weight changes over the course of the experiment were not statistically significant between groups (data not shown).
Effects of PFOA Exposure on Follicle Counts and Percentages In Vivo
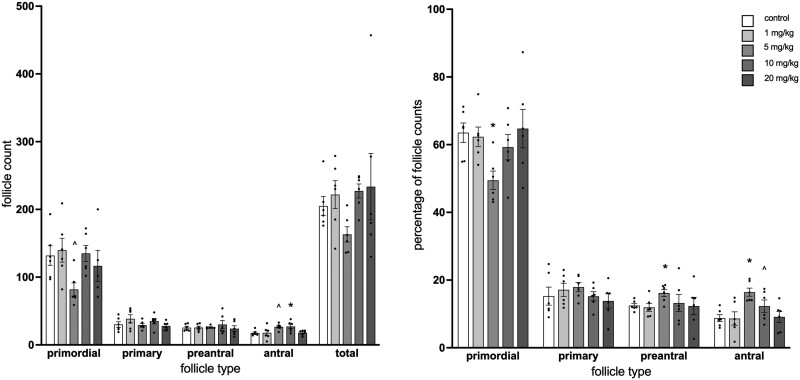
PFOA exposure at 10 mg/kg statistically significantly increased antral follicle counts compared with vehicle-treated control mice (p = .045; Figure 4, left). At 5 mg/kg PFOA, counts of primordial follicles trended towards a statistically significant decrease (p = .10) and counts of antral follicles trended towards a statistically significant increase compared with vehicle-treated controls (p = .067). When follicle counts were analyzed as percentages of the total follicle population in the ovary, primordial follicle counts were statistically significantly decreased at 5 mg/kg PFOA compared with vehicle-treated controls (p = .01) and preantral and antral follicle counts were increased at 5 mg/kg PFOA compared with vehicle-treated controls (p = .02 and .004, respectively; Figure 4, right). At 10 mg/kg, antral follicle percentages trended towards a statistically significant decrease with PFOA exposure compared with vehicle-treated control animals (p = .11).
Figure 4.
Effect of adult exposure to perfluorooctanoic acid on total follicle numbers (left) and percentages of follicle types in mice (right). Ovaries were subjected to histological evaluation of follicle numbers. Graphs represent means ± SEM from 5 to 6 animals per treatment group. Asterisk (*) indicates significant difference from the control (p ≤ .05) and (^) indicates differences trending towards significance (.05 < p ≤ .1).
Effects of PFOA Exposure on Sex Steroid Hormone Levels and Steroidogenic Enzyme Expression In Vivo
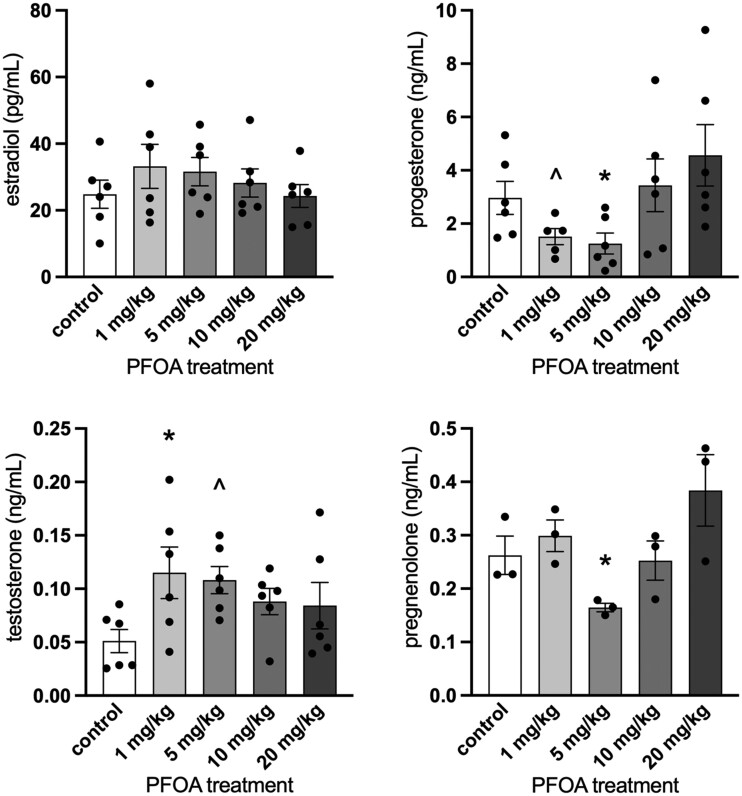
Basal hormone levels of 0.05 ± 0.01 ng/ml for testosterone, 3.0 ± 0.6 ng/ml for progesterone, 25 ± 4 pg/ml for 17β-estradiol, and 0.26 ± 0.04 ng/ml for pregnenolone were measured for the control groups. DHEA values were below the limit of detection and there was not enough serum to measure estrone. PFOA treatment at 1 mg/kg statistically significantly increased sera testosterone levels compared with sera from vehicle-treated controls (p = .048) and marginally increased sera testosterone levels at 5 mg/kg compared with sera from vehicle-treated controls (p = .087; Figure 5). PFOA exposure at 5 mg/kg statistically significantly decreased progesterone (p = .037) and pregnenolone levels (p = .050) compared with vehicle-treated controls and marginally decreased progesterone levels at 1 mg/kg compared with vehicle-treated controls (p = .10; Figure 5). PFOA exposure did not alter levels of estradiol compared with controls (p > .05).
Figure 5.
Effects of perfluorooctanoic acid exposure on serum sex steroid hormone levels in female mice. Sera were subjected to enzyme-linked immunosorbent assays. dehydroepiandrosterone values were below the limit of detection (data not shown). There was not enough serum to measure estrone. Graphs represent means ± SEM from 3 to 6 animals per treatment group. Asterisk (*) indicates significant difference from the control (p ≤ .05) and (^) indicates differences trending towards significance (.05 < p ≤ .10).
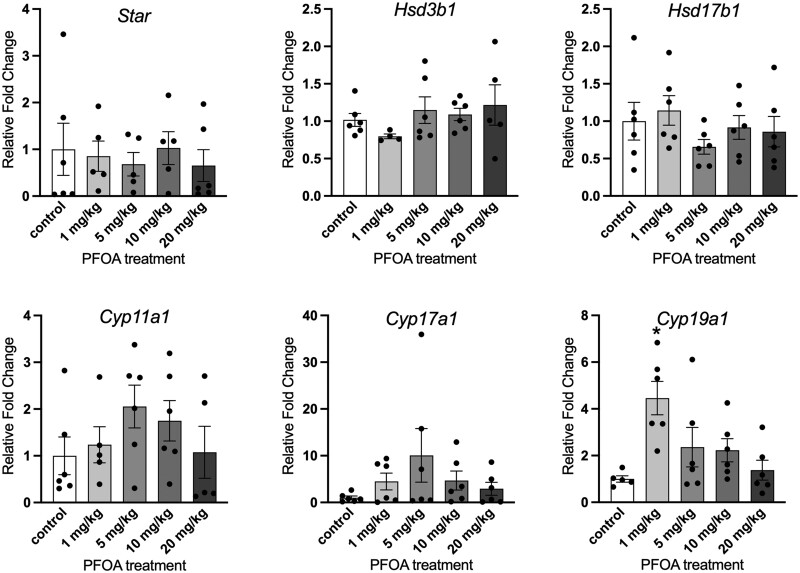
Exposure to PFOA at 1 mg/kg/day statistically significantly increased the gene expression of Cyp19a1 compared with controls (p = .022; Figure 6). Gene expression of Star, Cyp11a1, Cyp17a1, Hsd3b1, and Hsd17b1 in the ovary was not significantly altered compared with controls.
Figure 6.
Effects of adult exposure to perfluorooctanoic acid on ovarian steroidogenic enzyme gene expression changes in female mice expressed as fold change compared with vehicle-treated controls. All gene expression is relative to the housekeeping gene, BAct. Graphs represent means ± SEM from 5 to 6 animals per treatment group. Asterisk (*) indicates significant difference from the control (p ≤ .05).
DISCUSSION
This study was conducted to determine the effects of subacute PFOA exposure on folliculogenesis and steroidogenesis in adult mouse ovaries as a model for the effects of human environmental exposures. Our results indicate that certain levels of PFOA exposure can alter hormone levels, disrupt steroidogenic gene expression, slow follicle growth, and accelerate folliculogenesis. These findings are significant because disruption of normal ovarian function can lead to numerous health conditions, including premature ovarian failure, reduced fertility, and hormone-mediated diseases.
This study was designed to compare in vitro and in vivo analyses at levels of exposure that are relevant to humans and wildlife. The 2 experiments used mice of the same age range for direct comparison. The pharmacokinetics of PFOA in mice, humans, and rats differs noticeably in biological half-life, with mice clearing PFOA from the body in days, compared with years for humans and hours for rats (Lou et al., 2009). Thus, published studies of both internal PFOA levels and estimated exposures were important in the study design. Because humans excrete PFOA at a much slower rate than mice, the results of the current in vivo study may be applicable to humans long after exposure has ceased. Similarly, studies in female rats may underpredict effects in humans because of quick clearance in rats compared with humans. Based on previous pharmacokinetic studies in female CD-1 mice, a 1 mg/kg in vivo dose is expected to lead to blood levels of 10 µg/ml (Lau et al., 2006; Lou et al., 2009). Considering studies showing that the relationship between serum and follicular fluid concentrations in humans is approximately 1:1 (Heffernan et al., 2018; Kang et al., 2020; Kim et al., 2020; McCoy et al., 2017), a rough comparison of the 1 mg/kg in vivo dose to the 10 µg/ml in vitro treatment and 10 mg/kg to 100 µg/ml are reasonable. These doses are relevant to human exposure, representing consumption of contaminated drinking water (Frisbee et al., 2009; Innes et al., 2014).
Our findings of disrupted steroidogenic gene expression and altered hormone levels are consistent with previous studies on PFOA and other perfluoroalkyl compounds in adult and adolescent rodents and cell culture models. Prepubertal female rats exposed to 3 mg/kg of perfluorododecanoic acid for 28 days had altered ovarian gene expression of Star, Cyp11a1, and Hsd17b3 and decreased serum estradiol levels compared with controls (Shi et al., 2009). Twelve-week-old female mice chronically exposed to 0.1 mg/kg perfluorooctanesulfonate had decreased ovarian gene expression of Star and lower levels of estradiol and progesterone compared with vehicle controls (Feng et al., 2015). Cultured porcine theca and granulosa cells exposed to 1.2 µM (0.5 µg/ml) PFOA for 72 h had inhibited secretion of estradiol, progesterone, and androstenedione compared with controls (Chaparro-Ortega et al., 2018). In this study, culture of whole antral follicles with 100 µg/ml PFOA inhibited the production of 5 of the 6 measured hormones and all 6 measured steroidogenic enzymes compared with controls. Interestingly, in vivo PFOA exposure altered serum hormone levels and enzyme gene expression at 1 and 5 mg/kg, with progesterone, pregnenolone, and estradiol being decreased and testosterone and Cyp19a1 expression being increased by PFOA exposure compared with controls. As testosterone is converted to estradiol by Cyp19a1, this suggests that estradiol may be degrading at a faster rate than usual, causing compensatory increases in Cyp19a1 and testosterone. Alternatively, activity of CYP19A1 may be blocked by PFOA action, leading to a buildup of testosterone and increased gene expression. Enzyme activity measurements and time-course experiments should be performed in future experiments to help elucidate the relationship between testosterone, estradiol, and CYP19A1.
Exposure to PFOA-altered follicle counts in the ovaries of exposed animals compared with unexposed animals, indicating disrupted folliculogenesis. Assessing follicles counts as percentages corrects for the size of the ovary and provides information on shifts in the follicle pool. At 5 mg/kg of PFOA, ovaries showed a decrease in the percent of primordial follicles and an increase in the percent of preantral and antral follicles compared with controls, indicating that folliculogenesis may be accelerated in the presence of PFOA. As the primordial follicle pool cannot be renewed, accelerated folliculogenesis can lead to early depletion and premature reproductive senescence. Previous studies of PFOA and related compounds in female rats have not found disrupted follicle counts (Du et al., 2019; Shi et al., 2009), likely due to the quick metabolism of PFOA in rats (Lou et al., 2009), whereas a study of chronic exposure to 0.1 mg/kg of perfluorooctane sulfonate-altered folliculogenesis (Feng et al., 2015). These different outcomes in each rodent model emphasize the need to use species with proper sensitivity and metabolic activity most similar to humans to most accurately study the reproductive toxicity of environmental chemicals (Vandenberg et al., 2013).
This study revealed U- or inverted U-shaped nonmonotonic dose-response curves for many of the assessed endpoints, consistent with previous studies on PFOA (Fenton et al., 2009; Jain and Ducatman, 2019). The 1–10 mg/kg doses of PFOA caused the majority of disruptions in vivo, whereas in vitro effects were restricted to the highest dose of 100 µg/ml. The lack of growth, dark color, and decreased expression of all genes suggests that the follicles exposed to this high dose were dead or dying (atretic). Future experiments using similar high doses should include measurement of cytotoxicity and apoptosis. As previously mentioned, the 1 mg/kg dose can be approximately compared with the 10 µg/ml culture treatment. Although 1 mg/kg treatment disrupted hormone levels and aromatase expression, no effects were observable from the 10 µg/ml culture treatment. Thus, the in vivo experiment may be more sensitive than the in vitro model, emphasizing the importance of whole animal studies. The whole ovaries analyzed from the in vivo studies contain stroma and less developed follicles (primordial, primary, and preantral) that may be more affected by PFOA than the antral follicles. In addition, the relative insensitivity of the in vitro experiments compared with in vivo may indicate that PFOA is not directly targeting the ovary and instead disrupting another part of the hypothalamic-pituitary-gonadal axis. PFOA disruption of the hypothalamus and pituitary could lead to effects on the ovary that would only be observable in vivo. Future studies should examine the hypothalamus and pituitary as targets of PFOA action.
The comparison of in vivo and in vitro experiments is a major strength of this study. Adult exposure is representative of the biological state of the majority of human life. Additionally, this study identified reproductive and endocrine disruptions from PFOA in adulthood, emphasizing the risk of perfluorinated compounds at all ages. However, because mice metabolize PFOA significantly faster than humans, the doses may be representative of lower exposure in humans. Future studies should use lower doses for longer treatment periods and consider other animal models with metabolic half-lives more similar to humans.
CONCLUSIONS
Adult female mice and cultured antral follicles exposed to PFOA had altered steroidogenic gene expression and sex hormone production. In addition, folliculogenesis was accelerated in a nonmonotonic manner, resulting in a depleted pool of primordial follicles that can never be renewed. Overall, these results are consistent with previous studies of the detrimental effects of perfluorinated compounds on the female reproductive system and show that adult exposure to PFOA poses a risk for decreased reproductive function.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the members of the Flaws Lab for assistance.
FUNDING
National Institutes of Health (R01 ES028661 to J.A.F., K99 ES031150 and T32 ES007326 to G.R.W.).
Contributor Information
May Yang, Department of Comparative Bioscience, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA.
Yuna Lee, Department of Comparative Bioscience, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA.
Liying Gao, Department of Comparative Bioscience, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA.
Karen Chiu, Department of Comparative Bioscience, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA; Division of Nutritional Sciences, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA.
Daryl D Meling, Department of Comparative Bioscience, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA.
Jodi A Flaws, Department of Comparative Bioscience, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA; Division of Nutritional Sciences, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA; Institute for Genomic Biology, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA.
Genoa R Warner, Department of Comparative Bioscience, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802, USA.
REFERENCES
- Chaparro-Ortega A., Betancourt M., Rosas P., Vázquez-Cuevas F. G., Chavira R., Bonilla E., Casas E., Ducolomb Y. (2018). Endocrine disruptor effect of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on porcine ovarian cell steroidogenesis. Toxicol. In Vitro 46, 86–93. [DOI] [PubMed] [Google Scholar]
- Cordner A., De La Rosa V. Y., Schaider L. A., Rudel R. A., Richter L., Brown P. (2019). Guideline levels for PFOA and PFOS in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors. J. Expo. Sci. Environ. Epidemiol. 29, 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig Z. R., Hannon P. R., Wang W., Ziv-Gal A., Flaws J. A. (2013). Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol. Reprod. 88, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig Z. R., Wang W., Flaws J. A. (2011). Endocrine-disrupting chemicals in ovarian function: Effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction 142, 633–646. [DOI] [PubMed] [Google Scholar]
- Ding N., Harlow S. D., Randolph J. F., Loch-Caruso R., Park S. K. (2020). Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 26, 724–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Hu J., Huang Z., Yu M., Lu C., Wang X., Wu D. (2019). Neonatal and juvenile exposure to perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS): Advance puberty onset and kisspeptin system disturbance in female rats. Ecotoxicol. Environ. Saf. 167, 412–421. [DOI] [PubMed] [Google Scholar]
- Feng X., Wang X., Cao X., Xia Y., Zhou R., Chen L. (2015). Chronic exposure of female mice to an environmental level of perfluorooctane sulfonate suppresses estrogen synthesis through reduced histone H3K14 acetylation of the StAR promoter leading to deficits in follicular development and ovulation. Toxicol. Sci. 148, 368–379. [DOI] [PubMed] [Google Scholar]
- Fenton S. E., Reiner J. L., Nakayama S. F., Delinsky A. D., Stanko J. P., Hines E. P., White S. S., Lindstrom A. B., Strynar M. J., Petropoulou S. S. E. (2009). Analysis of PFOA in dosed CD-1 mice. Part 2: Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 27, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaws J. A., Doerr J. K., Sipes I. G., Hoyer P. B. (1994). Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod. Toxicol. 8, 509–514. [DOI] [PubMed] [Google Scholar]
- Franko J., Meade B. J., Frasch H. F., Barbero M., Anderson S. E. (2012). Dermal penetration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. J. Toxicol. Environ. Health A 75, 50–62. [DOI] [PubMed] [Google Scholar]
- Frisbee S. J., Brooks A. P., Maher A., Flensborg P., Arnold S., Fletcher T., Steenland K., Shankar A., Knox S. S., Pollard C., et al. (2009). The C8 health project: Design, methods, and participants. Environ. Health Perspect. 117, 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsioroski A., Mourikes V. E., Flaws J. A. (2020). Endocrine disruptors in water and their effects on the reproductive system. Int. J. Mol. Sci. 21, 1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Flaws J. A. (2015). Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol. Reprod. 92, 120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Peretz J., Flaws J. A. (2014). Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice1. Biol. Reprod. 90, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan A. L., Cunningham T. K., Drage D. S., Aylward L. L., Thompson K., Vijayasarathy S., Mueller J. F., Atkin S. L., Sathyapalan T. (2018). Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int. J. Hyg. Environ. Health 221, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Hirshfield A. N. (1991). Development of follicles in the mammalian ovary. Int. Rev. Cytol. 124, 43–101. [DOI] [PubMed] [Google Scholar]
- Innes K. E., Wimsatt J. H., Frisbee S., Ducatman A. M. (2014). Inverse association of colorectal cancer prevalence to serum levels of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in a large Appalachian population. BMC Cancer 14, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. B., Ducatman A. (2019). Perfluoroalkyl substances follow inverted U-shaped distributions across various stages of glomerular function: Implications for future research. Environ. Res. 169, 476–482. [DOI] [PubMed] [Google Scholar]
- Kang Q., Gao F., Zhang X., Wang L., Liu J., Fu M., Zhang S., Wan Y., Shen H., Hu J. (2020). Nontargeted identification of per- and polyfluoroalkyl substances in human follicular fluid and their blood-follicle transfer. Environ. Int. 139, 105686. [DOI] [PubMed] [Google Scholar]
- Kato K., Wong L. Y., Jia L. T., Kuklenyik Z., Calafat A. M. (2011). Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999-2008. Environ. Sci. Technol. 45, 8037–8045. [DOI] [PubMed] [Google Scholar]
- Kim Y. R., White N., Bräunig J., Vijayasarathy S., Mueller J. F., Knox C. L., Harden F. A., Pacella R., Toms L. M. L. (2020). Per- and poly-fluoroalkyl substances (PFASs) in follicular fluid from women experiencing infertility in Australia. Environ. Res. 190, 109963. [DOI] [PubMed] [Google Scholar]
- Lau C., Thibodeaux J. R., Hanson R. G., Narotsky M. G., Rogers J. M., Lindstrom A. B., Strynar M. J. (2006). Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 90, 510–518. [DOI] [PubMed] [Google Scholar]
- Lou I., Wambaugh J. F., Lau C., Hanson R. G., Lindstrom A. B., Strynar M. J., Zehr R. D., Setzer R. W., Barton H. A. (2009). Modeling single and repeated dose pharmacokinetics of PFOA in mice. Toxicol. Sci. 107, 331–341. [DOI] [PubMed] [Google Scholar]
- McCoy J. A., Bangma J. T., Reiner J. L., Bowden J. A., Schnorr J., Slowey M., O'Leary T., Guillette L. J., Parrott B. B. (2017). Associations between perfluorinated alkyl acids in blood and ovarian follicular fluid and ovarian function in women undergoing assisted reproductive treatment. Sci. Total Environ. 605–606, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T., Peters H. (1968). Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 17, 555–557. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poothong S., Boontanon S. K., Boontanon N. (2012). Determination of perfluorooctane sulfonate and perfluorooctanoic acid in food packaging using liquid chromatography coupled with tandem mass spectrometry. J. Hazard. Mater. 205–206, 139–143. [DOI] [PubMed] [Google Scholar]
- Post G. B., Cohn P. D., Cooper K. R. (2012). Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 116, 93–117. [DOI] [PubMed] [Google Scholar]
- Seals R., Bartell S. M., Steenland K. (2011). Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environ. Health Perspect. 119, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Zhang H., Ding L., Feng Y., Xu M., Dai J. (2009). The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod. Toxicol. 27, 352–359. [DOI] [PubMed] [Google Scholar]
- Taylor K. W., Hoffman K., Thayer K. A., Daniels J. L. (2014). Polyfluoroalkyl chemicals and menopause among women 20-65 years of age (NHANES). Environ. Health Perspect. 122, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Colborn T., Hayes T. B., Heindel J. J., Jacobs D. R., Lee D.-H., Myers J. P., Shioda T., Soto A. M., vom Saal F. S., et al. (2013). Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod. Toxicol. 38, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergren R., Cousins I. T. (2009). Tracking the pathways of human exposure to perfluorocarboxylates. Environ. Sci. Technol. 43, 5565–5575. [DOI] [PubMed] [Google Scholar]
- Vieira V. M., Hoffman K., Shin H. M., Weinberg J. M., Webster T. F., Fletcher T. (2013). Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ. Health Perspect. 121, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Simcik M. F., Halbach T. R., Gulliver J. S. (2015). Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a U.S. metropolitan area: Migration and implications for human exposure. Water Res. 72, 64–74. [DOI] [PubMed] [Google Scholar]
- Zhang S., Tan R., Pan R., Xiong J., Tian Y., Wu J., Chen L. (2018). Association of perfluoroalkyl and polyfluoroalkyl substances with premature ovarian insufficiency in Chinese women. J. Clin. Endocrinol. Metab. 103, 2543–2551. [DOI] [PubMed] [Google Scholar]
- Zhou C., Wang W., Peretz J., Flaws J. A. (2015). Bisphenol A exposure inhibits germ cell nest breakdown by reducing apoptosis in cultured neonatal mouse ovaries. Reprod. Toxicol. 57, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Zhang L., Tong C., Fang F., Zhao S., Tian Y., Tao Y., Zhang J.; for the Shanghai Birth Cohort Study. (2017). Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ. Health Perspect. 125, 067012–067016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.