Abstract
Background
Coronavirus disease 19 (COVID-19) may have a severe course in children. Multisystem inflammatory syndrome in children (MIS-C) is the post-COVID complication characterized by an exaggerated inflammation, observed in children. However, data on the underlying pathophysiology are sparse. We therefore aimed to assess the cytokine and chemokine profiles of children with MIS-C and compare these to life-threatening severe SARS-CoV-2 and healthy controls (HCs) to shed light on disease pathophysiology.
Methods
Samples of 31 children with MIS-C, 10 with severe/critical COVID-19 and 11 HCs were included. Cytokine and chemokine profiles were studied and compared in between groups.
Results
Most cytokines and chemokines related to IL-1 family and IFN-γ pathway (including IL-18 and MIG/CXCL9) and IL-17A were significantly higher in the MIS-C group when compared to the severe/critical COVID-19 group and HCs. IP-10/CXCL10 and IL-10 were higher in both MIS-C patients and severe/critical COVID-19 compared to HCs.
Conclusion
Our results suggest that IL-1 and IFN-γ pathways play an important role in the pathophysiology of MIS-C.
Impact
This study defines a pattern of distinctive immune responses in children with MIS-C and in patients with severe/critical COVID-19.
As the COVID-19 pandemic continues, biomarkers to identify MIS-C risk are needed to guide our management that study results may shed light on it.
Introduction
In the early periods of coronavirus disease 19 (COVID-19) pandemics, healthy children were thought to have a milder disease course comprising approximately 1% of hospital admission with favorable outcomes.1,2 However, after the first few months of the pandemic, newly identified entity known as a multisystem inflammatory syndrome in children (MIS-C) has led to change the idea that healthy children are not severely affected by SARS-CoV-2.3–5 MIS-C is suggested to be a post/delayed-infectious event characterized by the classical findings of inflammation, with fever as the main feature and multiorgan dysfunction that frequently affects the gastrointestinal (GI), cardiac, respiratory and neurologic systems.3–6 MIS-C has a distinct clinical presentation compared to severe/critical COVID-19. Children who present with severe COVID-19 in the acute phase of SARS-CoV-2 infection mostly have underlying diseases and respiratory complications; however, most MIS-C patients are healthy and have multisystem involvement and higher inflammatory markers.7–10
There are still many unknowns regarding the pathophysiology of the various clinical presentations of COVID-19. The Kawasaki-like features and resemblance to cytokine storm of childhood rheumatic diseases have led to speculations of an abnormal response by the naïve immune system. Data related to immunologic features explaining severe COVID-19 in children and MIS-C are limited as well as those regarding the pathophysiology of the disease.
We therefore aimed to assess the cytokine and chemokine profiles of children with MIS-C and life-threatening complications of SARS-CoV-2 to shed light on disease pathophysiology that may distinguish these clinical syndromes in children.
Methods
Study design and participants
We enrolled all consecutive pediatric patients hospitalized for MIS-C and severe/critical COVID-19 at Hacettepe University Ihsan Dogramaci Children’s Hospital from April 20, 2021 until August 15, 2021. Pediatric healthy controls (HCs) (n = 11) who were negative for SARS-CoV-2 by reverse transcriptase polymerase chain reaction (RT-PCR) or SARS-CoV-2 serology were recruited.
MIS-C was diagnosed by physicians and all patients met the diagnostic criteria for Centers for Disease Control and Prevention and/or American College of Rheumatology11,12 with documented current or recent SARS-CoV-2 RT-PCR /serology; or documented current/recent household contacts positive for SARS-CoV-2 if testing unavailable at presentation;11,12 or exposure to a suspected/confirmed COVID-19 case within 4 weeks of symptom onset.
The severity of pediatric COVID-19 cases was defined as severe/critical according to the classification of Dong et al.,7 which is based on the clinical characteristics, laboratory results and radiologic imaging. The classifications are described as follows: (a) severe disease, or cases with progressive respiratory disease, dyspnea, and central cyanosis and (b) critically ill, or cases with acute respiratory distress syndrome or respiratory failure, shock and organ dysfunction.7 Macrophage activating syndrome (MAS) was defined based on the 2016 MAS classification.13
As HCs we included 11 children who were both SARS-CoV-2 RT-PCR negative and SARS-CoV-2 seronegative. HCs were pediatric patients without any underlying disease. They had presented to outpatient clinics for routine follow-up or vaccination. They had no history of COVID-19 contact.
Written informed consent was obtained from patients/HCs or their legal caregivers. The study was approved by the Hacettepe University Ethical Committee (2021/09-45).
Data regarding the demographic and clinical characteristics of patients were extracted from electronic records of patients, patients’ charts and laboratory databases of the hospital.
Blood samples were collected from patients and HCs after receiving informed consent. Samples were drawn between day 1 and day 10 of the disease course since patients were admitted to the hospital at different times of the disease. Sampling was performed before the administration of immunomodulatory treatments in all MIS-C patients. Blood samples were collected with serum-separating tubes and centrifuged at 4000 rpm for 10 min at room temperature. Serum samples were stored at –80 °C until further analysis.
Cytokine and chemokine profiling
Serum levels of cytokines and chemokines were studied by the cytometric bead-based multiplex assay panels according to manufacturer’s instructions and analyzed by Novocyte 3005 flow cytometer.
Panel 1 included IL-1β, IFN-α2, IFN-γ, TNF-α, MCP-1 (CCL2), IL-6, IL-8 (CXCL8), IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33 (LEGENDplex Human Inflammation panel 1 (13-plex); catalogue number 740809, Biolegend).
Panel 2 included MCP-1 (CCL2), RANTES (CCL5), IP-10 (CXCL10), Eotaxin (CCL11), TARC (CCL17), MIP-1α (CCL3), MIP-1β (CCL4), MIG (CXCL9), MIP-3α (CCL20), ENA78 (CXCL5), GROα (CXCL1), ITAC (CXCL11) and IL-8 (CXCL8) (LEGENDplex HU Pro-Inflammatory Chemokine panel 1 (13-plex); catalogue number 740985, Biolegend).
Statistics
The statistical analyses and graphical representation of the data were done with SPSS 22 (IBM) and Prism 8 (GraphPad Software). Categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as the mean or median. The Kruskal–Wallis test was performed for all the cytokines, using Bonferroni’s correction for comparisons of all the samples. Pair-wise comparisons using the Mann–Whitney test were only performed when the Kruskal–Wallis test was significant after Bonferroni’s correction. A P value of <0.05 was considered statistically significant.
Receiver-operating characteristic (ROC) curve and area under the ROC curve of the levels of the laboratory parameters were estimated for the patients. After ROC analysis, the best cutoff point was determined by using Youden Index for C-reactive protein level (CRP), IL-17A, IL-18 and MIG/CXCL9. The sensitivity and specificity of these parameters was calculated according to the best cutoff point. Multiple logistic regression analysis was used to identify the risk factors.
Results
Demographics and clinical characteristics
Between April 20, 2021, and August 15, 2021, we enrolled 31 patients with MIS-C, 10 with severe/critical COVID-19 and 11 HCs. The patients’ demographics, underlying diseases, treatments, and interventions are shown in Table 1. Of all, one (3.2%) MIS-C case SARS-CoV-2 PCR positivity on nasopharyngeal swab while 30 MIS-C cases were seropositive for SARS-CoV-2 IgG.
Table 1.
Characteristics of patients.
| Total | MIS-C | Severe/critical COVID-19 | P | |
|---|---|---|---|---|
| No. of patients, n (%) | 41 (100) | 31 (75.6) | 10 (24.4) | |
| Sex F/M, n (%) | 14 (34)/27 (66) | 11 (35.5)/20 (64.5) | 3 (30)/7 (70) | 0.53 |
| Age, years, median (IQR) | 9 (11) | 9 (10) | 4.5 (11) | 0.105 |
| Positive SARS-CoV-2 PCR, n (%) | 11 (26.82) | 1 (3.2) | 10 (100) | <0.001 |
| SARS-CoV-2 IgG positive, n (%) | 30 (70.73) | 30 (96.8) | 0 | |
| Underlying disease, n (%) | 9 (21.95) | 0 | 9 (90) | <0.001 |
| None | 32 (78.04) | 31 (100) | 1 (10) | |
| Chronic pulmonary diseases | 2 (4.9) | 0 | 2 (20) | |
| Hematologic/oncologic/immunologic diseases | 3 (7.3) | 0 | 3 (30) | |
| Neurometabolic/genetic diseases | 3 (7.3) | 0 | 3 (30) | |
| Gastrointestinal disease | 1 (2.4) | 0 | 1 (10) | |
| Clinical presenting features, n (%) | ||||
| Fever | 41 (100) | 31 (100) | 10 (100) | 0.001 |
| Days of fever preceding admission, median (IQR) | – | 5 (3) | 3 (1) | <0.001 |
| Rash | 27 (66) | 27 (87.1) | 0 | 0.018 |
| Conjunctival injection | 30 (73.2) | 30 (96.8) | 0 | 0.061 |
| Edema of the extremities | 26 (63.4) | 26 (84) | 0 | 0.016 |
| Abdominal pain | 32 (78) | 30 (96.8) | 2 (20) | <0.001 |
| Vomiting | 24 (58.5) | 22 (71) | 2 (20) | 0.001 |
| Diarrhea | 16 (39) | 15 (48.4) | 1 (10) | 0.20 |
| Splenomegaly | 1 (2.4) | 1 (3.2) | 0 | |
| Hepatomegaly | 13 (31.7) | 10 (32.3) | 3 (30) | |
| Hepatosplenomegaly | 10 (24.4) | 10 (32.3) | 0 | |
| Respiratory symptoms | 27 (65.8) | 17 (54.8) | 10 (100) | |
| Respiratory distress | 28 (68.3) | 18 (58.1) | 10 (100) | |
| Cough | 17 (41.5) | 7 (22.6) | 10 (100) | |
| Headache | 17 (41.5) | 17 (54.8) | 0 | |
| Lethargy, altered mental status | 26 (63.4) | 24 (77.4) | 2 (20) | |
| Myalgia | 27 (65.9) | 22 (71) | 5 (50) | |
| Cardiac changes, n (%) | ||||
| Left ventricular dysfunction, any | 16 (39.02) | 12 (38.7) | 4 (40) | |
| Pericardial effusion | 14 (34.14) | 12 (38.7) | 2 (20) | |
| Myocarditis | 9 (21.95) | 7 (22.5) | 2 (20) | |
| Radiological findings, n (%) | ||||
| Abnormal chest X-ray | 32 (78) | 22 (70.96) | 10 (100) | 0.076 |
| Abnormal thorax CT | 23 (56) | 16 (51.61) | 7 (70) | 0.48 |
| Abnormal abdominal USG | 16 (39) | 15 (48.4) | 1 (10) | |
| Hospitalization | ||||
| Length of stay at the hospital, days, median (min–max) | 7 (3–29) | 9.5 (4–46) | 0.151 | |
| ICU admission, n (%) | 31 (75.6) | 21 (67.7) | 10 (100) | 0.040 |
| Length of stay in ICU, days, median (IQR) | 2 (3) | 8.5 (16) | 0.055 | |
| Respiratory support, n (%) | ||||
| None | 8 (19.5) | 8 (25.8) | 0 | |
| Oxygen only | 11 (26.8) | 11 (35.5) | 0 | |
| High flow support | 7 (17) | 4 (12.9) | 3 (30) | |
| Non-invasive ventilation | 5 (12.2) | 4 (12.9) | 1 (10) | |
| Invasive mechanical ventilation | 10 (24.4) | 4 (12.9) | 6 (60) | |
| Shock, n (%) | ||||
| Requiring fluid bolus | 29 (70.7) | 23 (74.2) | 6 (60) | 0.44 |
| Requiring inotropes | 19 (46.3) | 15 (48.3) | 4 (40) | 0.46 |
| Immunomodulatory treatmentsa, n (%) | 41 (100) | 31 (100) | 10 (100) | |
| Intravenous immunoglobulin | 34 (82.9) | 31 (100) | 3 (30) | <0.001 |
| IL-1 inhibitor | 27 (65.8) | 26 (83.9) | 1 (10) | <0.001 |
| IL-6 inhibitor | 1 (2.4) | 1 (3.2) | 0 | 1 |
| Corticosteroids (2 mg/kg/day) | 40 (97.5) | 30 (96.7) | 10 (10) | |
| Bolus corticosteroids (30 mg/kg/day) | 7 (17.1) | 7 (22.6) | 0 | |
| Anticoagulantsc | 35 (85.36) | 31 (100) | 4 (40) | <0.001 |
| Acetylsalicylic acid at discharge | 24 (60) | 24 (80) | 0 | |
| Antibiotics, n (%) | 40 (97.5) | 30 (96.7) | 10 (100) | |
| Antivirals, n (%)b | 41 (100) | 31 (100) | 10 (100) | |
| Outcome, n (%) | ||||
| Recovered | 38 (92.7) | 31 (100) | 7 (70) | |
| Mortality | 3 (7.3) | 0 | 3 (30) | |
CT computed tomography, F/M female/male, IL interleukin, ICU intensive care unit, USG ultrasonography.
aIntravenous immunoglobulin, corticosteroids, anakinra or tocilizumab.
bThree patients with MIS-C had remdesivir for 2 days then continued with favipiravir.
cLow molecular weight heparin (Enoxaparin 2 × 0.5 mg/kg/dose).
While 90% of children with severe/critical COVID-19 had underlying diseases, all of the MIS-C patients were healthy children. The severe/critical COVID-19 patients had chronic lung disease (n = 2), hematologic/oncologic (n = 3) and neurometabolic/genetic disorders (n = 3) as underlying diagnoses. Three severe/critical COVID-19 patients had hematologic/oncologic disease and had ongoing chemotherapy. They had received chemotherapeutic agents 13–20 days before the COVID-19 infection and before sampling.
All of the patients in both groups had fever as presenting symptom; however, the duration of fever before admission was longer in MIS-C cases (5 days [IQR, 3]; P = 0.001). All severe/critical COVID-19 patients had respiratory system signs and symptoms at admission. Mucocutaneus, GI and neurologic symptoms were more common in MIS-C patients with statistically significant differences compared with severe/critical COVID-19 patients (P < 0.001, P = 0.005, P < 0.001, respectively). Twenty-one (67.7%) of patients with MIS-C admitted to pediatric intensive care unit for a short period of time (2 days [IQR, 3]) and four (12.9%) of them required invasive mechanical ventilation (IMV) support while six (60%) of patients with severe/critical COVID-19 had IMV support (P = 0.006). Thirty-eight percent of patients with MIS-C had left ventricular dysfunction and 15 (48.3%) patients with MIS-C required cardiovascular support. Forty percent of the patients with severe/critical COVID-19 had left ventricular dysfunction. Two critical COVID-19 patients who had high levels of both B-type natriuretic peptide (BNP) and cardiac troponin I had myocarditis. In this group, two patients had pericardial effusion during the severe disease course.
Laboratory findings
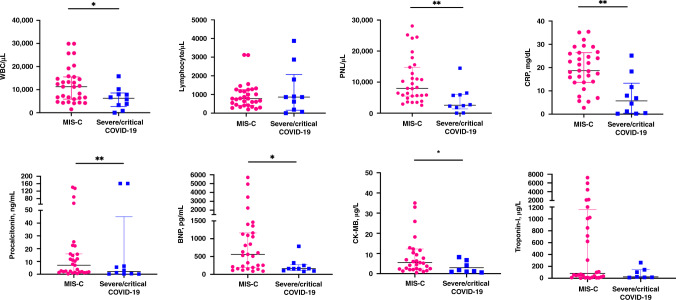
Laboratory tests on admission are summarized in Fig. 1. The children with MIS-C had significantly higher leukocyte counts ([WBC] median, 11,300/µL; min–max, 1500–29,900), neutrophil counts (median, 8000/µL; min–max, 2550–28,100), CRP (median, 18.7 mg/dL; min–max, 2.75) and procalcitonin levels (median, 5.42 ng/mL; IQR, 39) on admission than those with severe/critical COVID-19 (P = 0.031, P = 0.003, P = 0.001, P = 0.008, respectively). Both MIS-C and severe/critical COVID-19 groups had lymphopenia at admission ([lymphocyte count], median [min–max] 780/µL [200–1120]; 860/µL [0–3870], respectively) (P = 0.98). Cardiac biomarkers, such as BNP (median, 417 pg/mL; IQR, 951.75) and creatine kinase myocardial band (median, 5.6 µg/L; IQR 9.53) were significantly higher in MIS-C group (P = 0.044 and P = 0.023, respectively).
Fig. 1. Comparison of laboratory test results on admission.
Statistically significant differences of laboratory values are given. WBC white blood cell, PNL polymorphonuclear lymphocyte, CRP C-reactive protein, BNP B-type natriuretic peptide, CK-MB creatine kinase myocardial band. *P < 0.05; **P < 0.01.
Cytokine and chemokine profiles
To assess whether cytokines and chemokines can distinguish between different clinical phenotypes of COVID-19 in children we measured the serum levels of those biomarkers (Figs. 2 and 3). Median (min–max) levels and their comparisons are given in Table 2. Patients with MIS-C had significantly elevated levels of cytokines, IFN α, IFN γ, IL-6, IL-10, IL-18, IL-23 and IL-33 in comparison to HCs (P = 0.015, P = 0.001, P = 0.001, P < 0.001, P < 0.001, P = 0.015 and P = 0.013, respectively). Severe/critical COVID-19 children had higher levels of IL-10 than children in HC group (P = 0.001). When we compare cytokine levels in between MIS-C and severe/critical COVID-19 children, we found that IL-17A and IL-18 were significantly higher in MIS-C patients (P = 0.004 and P = 0.014, respectively).
Fig. 2. Quantitative circulating levels of selected cytokines.
*P < 0.05; **P < 0.01; ***P < 0.001.
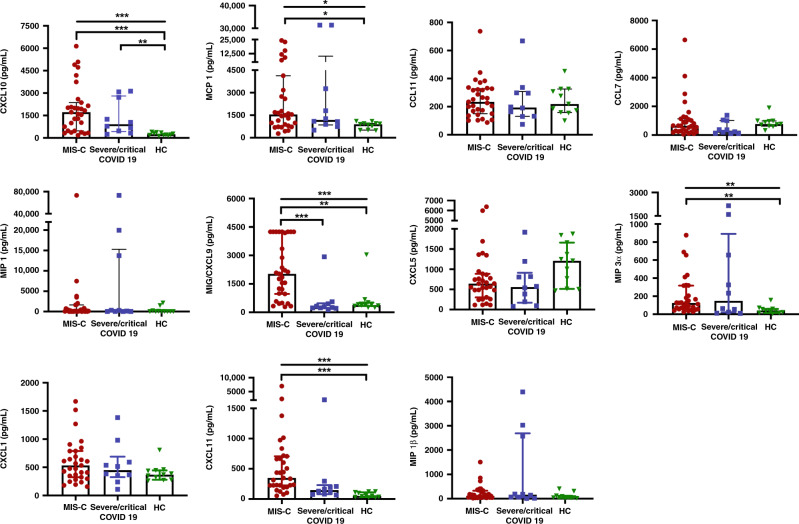
Fig. 3. Quantitative circulating levels of selected chemokines.
*P < 0.05; **P < 0.01; ***P < 0.001.
Table 2.
Cytokine and chemokine levels of MIS-C, severe/critical COVID-19 and healthy controls.
| Cytokine/chemokine levels (pg/mL) | MIS-C | Severe/critical COVID-19 | Healthy controls | P |
|---|---|---|---|---|
| Cytokine levels, median (min–max) | ||||
| IL-1β | 83.61 (0–23,573) | 104.67 (0–20,940) | 49.13 (0–743) | 0.151* |
| IFN-α | 21.67 (0–94.49) | 12.68 (4.68–53.83) | 6.98 (2.43–25.41) | 0.019* |
| 1a | ||||
| 0.015b | ||||
| 0.348c | ||||
| IFN-γ | 45.88 (4.56–1053) | 26.45 (0–188.18) | 8.33 (1.89–39.78) | 0.001* |
| 0.332a | ||||
| 0.001b | ||||
| 0.378c | ||||
| TNF-α | 30.35 (0–1050) | 29.98 (0–5732) | 10.74 (0–200.79) | 0.568* |
| IL-6 | 277.77 (8.38–43459) | 68.6 (18–43,459) | 21.99 (2.92–1297) | 0.002* |
| 1a | ||||
| 0.001b | ||||
| 0.111c | ||||
| IL-10 | 69.48 (13.56–1318) | 112.98 (17.61–1354) | 14.46 (0–40) | <0.001* |
| 1a | ||||
| <0.001b | ||||
| 0.001c | ||||
| IL-12p70 | 7.83 (0–80.61) | 3.85 (0–31.69) | 5.72 (0–18.11) | 0.488* |
| IL-17A | 3.53 (0.22–16.99) | 1.47 (0–8.91) | 0.67 (0–6.16) | 0.002* |
| 0.004a | ||||
| 0.091b | ||||
| 1c | ||||
| IL-18 | 4048 (887.18–27,009) | 1278 (311.28–20,706) | 666.8 (299.3–1779) | <0.001* |
| 0.014a | ||||
| <0.001b | ||||
| 0.411c | ||||
| IL-23 | 37.16 (3.27–297.73) | 16 (2.64–66.58) | 7.02 (0–54.01) | 0.016* |
| 0.521a | ||||
| 0.015b | ||||
| 0.788c | ||||
| IL-33 | 63.37 (0–428.65) | 43.635 (0–98.61) | 16.08 (0–48.08) | 0.014* |
| 0.613a | ||||
| 0.013b | ||||
| 0.613c | ||||
| Chemokine levels, median (min–max) | ||||
| IL-8/CXCL8 | 3413 (8.31–46214) | 1897.5 (77.35–38,804) | 116.79 (0–26,173) | 0.024* |
| 1a | ||||
| 0.02b | ||||
| 0.234 | ||||
| IP-10/CXCL10 | 1722 (179.15–6137) | 912.61 (250.12–3130) | 215.35 (123.96–396.45) | <0.001* |
| 1a | ||||
| <0.001b | ||||
| 0.010c | ||||
| MCP-1/CCL2 | 1548 (279.57–28,045) | 1184 (505.18–31,369) | 893.33 (477.73–1101) | 0.029* |
| 1a | ||||
| 0.032b | ||||
| 0.105c | ||||
| EOTOXIN/CCL11 | 234.26 (87.5–736.88) | 194.05 (74.25–667.74) | 219.07 (100.08–452.04) | 0.849* |
| TARC/CCL17 | 645.35 (54.9–6637) | 297.12 (98.46–1376) | 766.7 (321.7–1897) | 0.173* |
| MIP-1α/CCL3 | 139.2 (0–73,363) | 203.33 (25.63–73,363) | 15.05 (0–2112) | 0.080* |
| MIP-1 β/CCL4 | ||||
| 115.7 (22.35–1507) | 164.56 (9.13–4392) | 44.54 (13.26–406.36) | 0.126 | |
| MIG/CXCL9 | ||||
| 2029 (287.36–4244) | 271.9 (130.62–2926) | 382.81 (249.06–3035) | <0.001* | |
| <0.001a | ||||
| 0.005b | ||||
| 1c | ||||
| ENA78/CXCL5 | ||||
| 643 (112.96–6372) | 557.32 (79.74–1918) | 1209 (467.16–1875) | 0.098 | |
| MIP-3α/CCL20 | ||||
| 125.2 (24.75–876.54) | 148.62 (9.78–2152) | 41.81 (4.87–156.68) | 0.007* | |
| 1a | ||||
| 0.006b | ||||
| GRO-α/CXCL1 | 0.077c | |||
| 532.56 (158.26–2540) | 449.63 (109.96–1381) | 370.46 (260.8–805.93) | 0.262* | |
| ITAC/CXCL11 | ||||
| 348.1 (52.9–6985) | 147.54 (66.46–2110) | 50.3 (24.06–124.47) | <0.001* | |
| 0.081a | ||||
| <0.001b | ||||
| 0.122c |
Statistically significant P values are in bold.
*P value for comparison of three groups.
aP value of comparison in between MIS-C and severe COVID-19.
bP value of comparison in between MIS-C and healthy control.
cP value of comparison in between severe COVID-19 and healthy control.
Median concentrations of chemokines; IL-8/CXCL8, IP-10/CXCL10, MCP-1/CCL2, MIP-3α/CCL20, ITAC/CXCL11 and MIG/CXCL9 were elevated in MIS-C patients and significantly higher than HCs (P = 0.02, P < 0.001, P = 0.032, P = 0.006, <0.001 and 0.005, respectively). Median of IP-10/CXCL10 was also higher in severe/critical COVID-19 group in comparison with HCs (Fig. 3). Patients with MIS-C had significantly higher concentrations of MIG/CXCL9 than did those with sever/critical COVID-19 (2029 pg/mL [min–max,287.36–4244] vs 271.9 pg/mL [min–max, 130.62–2926]; P < 0.001; Fig. 3). Median of IL-1 β was higher in severe/critical COVID-19 group (median [min–max]; 104.67 pg/mL [0–20,940]) than MIS-C group (median [min–max]; 83.61 pg/mL [0–23,573]); however, the difference was not significant (P = 0.82).
The utility of IL-17A, IL-18 and CXCL9 concentrations in differentiating MIS-C from severe/critical COVID-19 were assessed by ROC curve analysis and performed well with area under curve of 0.816 (P = 0.003), 0.819 (P = 0.003) and 0.897 (P < 0.001), respectively (Fig. 4). The optimal MIG/CXCL9 cutoff to differentiate MIS-C from severe/critical COVID-19 was determined to be 561.67 pg/mL with a sensitivity of 80.65% and specificity of 90%. It was found that MIG/CXCL9 (odds ratio [OR], 1.002: 95% confidence interval [CI], 1–1.003; P = 0.018) and CRP (OR, 1.25; 95% CI, 1.04–1.5; P = 0.017) were independent predictors of patients with MIS-C with regression analysis.
Fig. 4. Heatmap and the ROC curve of cytokines and chemokines.
a Heatmap representing the selected cytokines and chemokines. Every column represents a patient. Asterisk (*) identifies the cytokines and chemokines with significant differences between MIS-C and severe/critical COVID-19. Color gradient represents the normalized percentages of cytokine and chemokine levels. MIS-C multisystem inflammatory syndrome in children (n = 31), COVID-19 coronavirus disease 19 (n = 10). b The ROC curve of IL-17A, IL-18 and MIG/CXCL9 concentrations in differentiating MIS-C from severe/critical COVID-19. The utility of IL-17A, IL-18 and MIG/CXCL9 concentrations in differentiating MIS-C from severe/critical COVID-19 were assessed by ROC curve analysis and performed well with area under curve of 0.816 (P = 0.003), 0.819 (P = 0.003) and 0.897 (P < 0.001), respectively.
We classified MIS-C patients into those with MAS and without MAS according to laboratory and clinical features of patients and found that 13 (41.9%) of 31 MIS-C patients satisfied MAS criteria. Patients with MIS-C that satisfied MAS criteria had higher levels of IL-17A and IFN-γ than those who did not fulfill the criteria; however, it was not statistically significant (P = 0.051 and P = 0.056, respectively).
Treatment and outcome
Antiviral treatments without seeking a positive SARS-CoV-2 PCR result were given to all patients in both groups because the pathogenesis of the disease is not clearly understood and the tissue virus load remains unclear. Three patients with MIS-C who presented with multiorgan failure had remdesivir for 2 days and continued with favipiravir later on. While all patients with MIS-C were given IVIg on the day they were hospitalized, 26 (83.9%) of them had IL-1 inhibitor (anakinra) together with IVIg and corticosteroid. We usually started anakinra with the dose of 4 mg/kg/day subcutaneously for MIS-C patients. Interleukin-1 inhibitor (anakinra) dose and treatment duration were tailored depending on patients’ clinical course and acute phase reactants. We individualized therapy for each patient by cautiously balancing effectiveness while assessing risks. Interleukin-1 inhibitor was given to only one patient in the severe/critical COVID-19 group who was with testicular tumor and developed multiorgan failure.
Three patients with severe/critical COVID-19 died: two of them had oncologic malignancy (one with non-Hodgkin lymphoma and one with testicular tumor) and one had neurometabolic disease. We had no mortality in patients with MIS-C.
Discussion
COVID-19 has started a new chapter not just in infectious diseases but in medicine as well. For pediatrics, the associated MIS-C has surely become a challenging and fascinating entity. Studies have tried to address the underlying pathophysiology and why some patients, albeit a small percentage, develop MIS-C or a more severe disease course. We have shown that patients with MIS-C have significantly different cytokine profiles when compared to severe COVID-19 patients and HCs, that complement their clinical and laboratory features.
The differentiation of MIS-C and severe COVID-19 may pose a challenge to the clinician. First of all, the timing of the infection is different between the two conditions. The patients with severe COVID-19 usually have underlying comorbid conditions as was the case of nine of our patients. In severe COVID-19 respiratory problems dominate the clinical picture while in MIS-C the skin features, GI symptoms and neurological symptoms were prominent. If SARS-CoV-2 PCR and antibody tests are not readily available, these features and the existing diagnostic criteria may help differentiate between these two conditions. In the presented paper we also highlight the differences in the cytokine profile between these two conditions.
So far, the relevant data are very limited. Diorio et al.14 studied four cytokines (IFN-γ, IL-10, IL-6 and TNF-α) and one chemokine IL-8/CXCL8 in a small cohort including six MIS-C cases and nine severe COVID-19 pediatric cases. They reported that IL-10 and TNF-α were significantly higher in MIS-C patients.14 In another study, IL-17A and IFN-γ were significantly higher in MIS-C group than the adults with severe COVID-19 and children with acute COVID-19 who did not require mechanical ventilation.15
When we compared the cytokine and chemokine levels, IL-17A, IL-18 and MIG/CXCL9 levels were significantly higher in patients with MIS-C compared to children with severe/critical COVID-19 and all three were independent predictors for the diagnosis of MIS-C. Interleukin 17A is a member of IL-17 family and a proinflammatory cytokine. Interleukin 17A stimulates innate immune cells and epithelial cells and then those cells produce GCSF and IL-8/CXCL8, which causes an increase in the number of neutrophils. It is among the cytokines which increase during the acute phase of MIS-C.16 Consistent with the GI disease of MIS-C, cytokines like IL-17A that enhance mucosal immunity were particularly prominent in terms of both T helper cell function and mucosal chemotaxis.17 Consiglio et al.18 reported that IL-17A was higher in the Kawasaki group than in the MIS-C group; however, children with severe COVID-19 were not studied in this report.
Our results showed that IL-18 and IL-33 were also elevated in MIS-C patients when compared to severe/critical COVID-19. Interleukin 18 and IL-33 are important members of IL-1 superfamily.19 Thus, the increase in these cytokines may be reflecting the IL-1 dependent cytokine storm in these patients. It is tempting to speculate that this may explain the response to anakinra in patients with severe MIS-C. These cytokines may be studied as new therapeutic options as well. Tran et al.20 suggested that IL-1 may play an important role in the pathology of MIS-C and SARS-CoV-2. They reported that especially IL-1 β is higher in MIS-C. In our study, IL-1 β levels were higher in severe COVID-19, but it was not statistically significant. This may reflect the inflammatory nature of severe COVID-19 infection. IL-33 is also being investigated for its role in the pathophysiology of COVID-19.19,21 Interleukin‐33 is normally released by damaged or necrotic barrier cells (endothelial and epithelial cells), acting as an alarmin.22 However, it is not clear whether IL-33 is secreted by activated immune cells or is directly released due to cell death/damage in COVID-19.19 Liang et al.19 commented that IL-33 released from the damaged cell may play an important role in COVID-19 immunopathology.
IL-18 is also known as IFN-γ stimulating factor.23–25 Both proinflammatory cytokines are produced mainly by macrophages. IL-18 together with IL-12 induces IFN-γ production by CD4, CD8 T cells and natural killer cells. This mechanism then acts on macrophage activation.24 This loop contributes to the production of more IFN-γ. This type of inflammation is similar to MAS due to systemic JIA and adult-onset Still’s diseases, mediated by IL-18.26,27 In MIS-C patients, IL-18 and the chemokines in the IFN-γ pathway described below reflect the MAS-like features in these patients. In fact, when these patients were first defined, most pediatricians were guided by the treatment of MAS. Our study has shown that patients with MIS-C had higher levels of MIG/CXCL9, IP-10/CXCL10 and ITAC/CXCL11 than HCs. Monokine-induced gamma interferon/CXCL9 levels of patients with MIS-C are also higher than levels of children with severe/critical COVID-19. High levels of MIG/CXCL9 are important markers of Th1-mediated immune response resulting in the production of IFN-γ.28 Caldarale et al.29 showed that IFN-γ levels were undetectable in both MIS-C and acute COVID-19 children. However, patients with MIS-C had higher levels of IFN-γ related chemokines, MIG/CXCL9 and IP-10/CXCL10 suggesting higher levels of IFN-γ production in children who present with MIS-C. Likewise in a similar manner, patients with MIS-C had higher levels of CXCL9 and CXCL10 in our cohort. IFN-γ and the IFN-γ induced chemokines play an important role in the pathogenesis of MAS.16,30,31 Rodriguez-Smith et al.32 reported that six MIS-C patients who fulfill the criteria of MAS had higher levels of MIG/CXCL9 than patients with MIS-C who did not satisfy MAS criteria. Esteve-Sole et al.33 reported that five patients with MIS-C who had multiorgan involvement had higher levels of IFN-γ, IL-18, GM-CSF, RANTES, IP-10/CXCL10, IL-1α, and SDF-1 that may also be an early sign of MAS. When we divided the patients with MIS-C according to MAS criteria, 41.9% (n = 13) of 31 MIS-C patients had fulfilled the MAS criteria and had higher levels of IL-17A and IFN-γ but this difference was not statistically significant (P = 0.051 and P = 0.056, respectively).
Patients with MIS-C also had increased levels of IL-6 and IL-10 compared to HCs. Increased levels of IL-6, IL-10, MIG/CXCL9 and IL-18 reinforce the idea of interaction between T-cell activation and IFN-γ activation in the pathogenesis of MIS-C.16,29,33–35 In our study, we found that especially the MIG/CXCL9 level was quite high (median 2029 [min–max, 287.36–4244]) in MIS-C patients. A remarkable result in this study is that MIG/CXCL9 and CRP may act as independent biomarkers for MIS-C when regression analysis is applied.
Patients with severe/critical COVID-19 had significantly higher IL-10 and IP-10/CXCL10 levels compared to HCs. Recent reports suggest IP-10 as a marker of disease severity in COVID-19. There are also reports suggesting that IP-10 may play a role both in the progression of COVID-9 and also in the prevention of lung injury.36–38 Lev et al.36 measured serial IP-10 levels in adults with COVID-19 and reported that recurrent fluctuations in abnormally high IP-10 levels were characteristic of patients who subsequently died.
IL-10 is an anti-inflammatory cytokine found elevated in patients with severe COVID-19. It is thought that IL-10 has a dual action in COVID-19; while it acts as an anti-inflammatory in the early stages of the infection, it is speculated that it is one of the biomolecules that act as a trigger for the cytokine storm when the disease becomes severe. In a meta-analysis, it was reported that regardless of age, if IL-6 and IL-10 together can be used as predictors for rapid diagnosis of patients at higher risk of deterioration in COVID-19 disease.39 In our study, even the median IL-6 was slightly higher in the severe/COVID-19 group compared to the HCs; however, the difference was not significant (P = 0.11). IL-6 level was significantly higher in patients with MIS-C compared to HCs (P = 0.001).
Besides the aforementioned cytokines and chemokines, IL-6, IL-10, IL-23, IFN α 2, MCP-1/CCL2 and macrophage inflammatory protein (MIP-3α)/CCL20 were also higher in the MIS-C group compared to HCs. Diorio et al.14 found that TNF α and IL-10 levels were significantly different in MIS-C when compared to severe COVID-19; however, we were not able to confirm this.
When we evaluated all these results, we concluded that the IL-1 and IFN-γ pathways play an important role in the pathophysiology of MIS-C. Although our cohort consisted of severe MIS-C patients, none died. Considering the cytokines and chemokines detected in this study, anakinra used in addition to other immunomodulatory treatments could have had affected this good outcome.
We started IVIg, anakinra and methylprednisolone in the treatment of patients diagnosed with MIS-C. Anakinra neutralizes strong IL-1 response elicited by damaged endothelial cells. Anakinra can indirectly block the production of biologically active IL-18 through inhibiting positive feedback of loop of IL-1β-induced caspase-1 expression.40,41 IVIg and corticosteroids provide more general immunosuppression. However, the exact mechanism of these treatments remains to be determined.
One of the limitations in our study is the relatively small cohort of patients with severe/critical COVID-19. Another limitation is the fact that 90% of the severe/critical COVID-19 group had a comorbid disease and we do not know how the underlying diseases affect the pathogenesis so further investigation is needed. Three severe/critical COVID-19 patients with underlying malignancies had received chemotherapy 13–20 days before the diagnosis of COVID-19. These treatments could have had some impacts on cytokine/chemokine responses. Lastly, serum cytokines and chemokines concentrations were measured only at admission and serial measurements with certain time periods might better clarify their role on disease outcome.
In conclusion, our study defines a pattern of distinctive immune responses in children with MIS-C and in patients with severe/critical COVID-19. As the COVID-19 pandemic continues we need biomarkers that would define the risk for MIS-C to guide our management. In our study IL-17A, IL-18 and MIG/CXCL9 seem to be the major cytokines/chemokines responsible for the pathogenesis of MIS-C and they act as if they are independent predictors of MIS-C.
Author contributions
S.O., A.B.C. and Y.O. conceptualized and designed the study and reviewed and revised the manuscript. S.L.G. conceptualized the study, collected data, carried out the analyses and drafted the initial manuscript. E.S. performed laboratory analysis. P.D.O. collected data, carried out the analyses. J.K. performed statistical analysis. S.K., U.K.A., M.K.C., O.B. and S.G. collected data. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This work was supported by Hacettepe University Research Projects Department [THD‐2021‐19591]. Y.O., S.L.G., P.D.O., A.B.C. and S.O. received the grant.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study complies with the Declaration of Helsinki, that Hacettepe University Ethical Committee has approved the research protocol [2021/09-45] and that informed consent has been obtained from the subjects (or their legally authorized representative).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.She J, Liu L, Liu W. COVID-19 epidemic: disease characteristics in children. J. Med. Virol. 2020;92:747–754. doi: 10.1002/jmv.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones VG, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein LR, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiff DD, Mannion ML, Samuy N, Scalici P, Cron RQ. Distinguishing active pediatric COVID-19 pneumonia from MIS-C. Pediatr. Rheumatol. 2021;19:21. doi: 10.1186/s12969-021-00508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome (MIS-C). https://www.cdc.gov/mis-c/cases/index.html. Accessed June 2021.
- 12.Henderson LA, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. 2020;72:1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravelli A, et al. 2016 Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. (Hoboken, N. J.) 2016;68:566–576. doi: 10.1002/art.39332. [DOI] [PubMed] [Google Scholar]
- 14.Diorio C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Investig. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce CA, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020;12:eabd5487. doi: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter MJ, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 17.Gruber CN, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995.e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consiglio CR, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y, Ge Y, Sun J. IL-33 in COVID-19: friend or foe? Cell. Mol. Immunol. 2021;18:1602–1604. doi: 10.1038/s41423-021-00685-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran VL, Parsons S, Nuibe A. The trilogy of SARS-CoV-2 in pediatrics (Part 2): multisystem inflammatory syndrome in children. J. Pediatr. Pharmacol. Ther. 2021;26:318–338. doi: 10.5863/1551-6776-26.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zizzo G, Cohen PL. Imperfect storm: is interleukin-33 the Achilles heel of COVID-19? Lancet Rheumatol. 2020;2:e779–e790. doi: 10.1016/S2665-9913(20)30340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 23.Yazdi AS, Ghoreschi K. The interleukin-1 family. Adv. Exp. Med. Biol. 2016;941:21–29. doi: 10.1007/978-94-024-0921-5_2. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50(4):778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Migliorini P, Italiani P, Pratesi F, Puxeddu I, Boraschi D. The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmun. Rev. 2020;19:102617. doi: 10.1016/j.autrev.2020.102617. [DOI] [PubMed] [Google Scholar]
- 26.Put K, et al. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: tipping the balance between interleukin-18 and interferon-γ. Rheumatology. 2015;54:1507–1517. doi: 10.1093/rheumatology/keu524. [DOI] [PubMed] [Google Scholar]
- 27.Colafrancesco S, et al. IL-18 serum level in adult onset Still’s disease: a marker of disease activity. Int. J. Inflamm. 2012;2012:156890. doi: 10.1155/2012/156890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kak G, Raza M, Tiwari BK. Interferon-gamma (IFN-γ): exploring its implications in infectious diseases. Biomol. Concepts. 2018;9:64–79. doi: 10.1515/bmc-2018-0007. [DOI] [PubMed] [Google Scholar]
- 29.Caldarale F, et al. Plasmacytoid dendritic cells depletion and elevation of IFN-γ dependent chemokines CXCL9 and CXCL10 in children with multisystem inflammatory syndrome. Front. Immunol. 2021;12:654587. doi: 10.3389/fimmu.2021.654587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bracaglia C, et al. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 2017;76:166–172. doi: 10.1136/annrheumdis-2015-209020. [DOI] [PubMed] [Google Scholar]
- 31.Di Cola I, Ruscitti P, Giacomelli R, Cipriani P. The pathogenic role of interferons in the hyperinflammatory response on adult-onset Still’s disease and macrophage activation syndrome: paving the way towards new therapeutic targets. J. Clin. Med. 2021;10:1164. doi: 10.3390/jcm10061164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Smith JJ, et al. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: a cohort study. Lancet Rheumatol. 2021;3:e574–e584. doi: 10.1016/S2665-9913(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esteve-Sole A, et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J. Clin. Investig. 2021;131:e144554. doi: 10.1172/JCI144554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee PY, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J. Clin. Investig. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farooqi KM, et al. Longitudinal outcomes for multisystem inflammatory syndrome in children. Pediatrics. 2021;148:e2021051155. doi: 10.1542/peds.2021-051155. [DOI] [PubMed] [Google Scholar]
- 36.Lev S, et al. Observational cohort study of IP-10’s potential as a biomarker to aid in inflammation regulation within a clinical decision support protocol for patients with severe COVID-19. PloS One. 2021;16:e0245296. doi: 10.1371/journal.pone.0245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020;146:119–127.e4. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozsurekci Y, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic in Turkey: first report from the Eastern Mediterranean. Clin. Rheumatol. 2021;40:3227–3237. doi: 10.1007/s10067-021-05631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhar SK, K V, Damodar S, Gujar S, Das M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon. 2021;7:e06155. doi: 10.1016/j.heliyon.2021.e06155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenini G, Contassot E, French LE. Potential of IL-1, IL-18 and Inflammasome Inhibition for the Treatment of Inflammatory Skin Diseases. Front. Pharmacol. 2017;8:278. doi: 10.3389/fphar.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jesus AA, Goldbach-Mansky R. IL-1 blockade in autoinflammatory syndromes. Annu. Rev. Med. 2014;65:223–244. doi: 10.1146/annurev-med-061512-150641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.