Abstract
Introduction
Knee osteoarthritis is a leading cause of disability and medical costs. The effect of whole-body vibration in knee osteoarthritis is controversial. The aim of this study was to assess the effects and safety of whole-body vibration on pain, stiffness, physical function, and muscle strength in patients with knee osteoarthritis.
Methods
PubMed, Scopus, Web of Science, Physiotherapy Evidence Database (PEDro) and EMBASE databases were searched (date last accessed 1 April 2021) using the key words “vibration” and “knee osteoarthritis”, to identify all randomized controlled trials related to whole-body vibration and knee osteoarthritis. Outcomes related to pain, stiffness, physical function, muscle strength, adverse events were included. The risk of bias and quality were assessed by the Cochrane Collaboration tool and PEDro scale. A systematic review and meta-analysis were performed. Subgroup analysis was performed for low- and high-frequency interventions.
Results
A total of 14 randomized controlled trials involving 559 patients with knee osteoarthritis met the inclusion criteria. Nine studies were good-quality trials (PEDro score=6–8), and 5 studies were fair-quality trials (PEDro score=4–5). Ten studies were included in the meta-analysis. One study showed negative effects of whole-body vibration on knee osteoarthritis. The duration of whole-body vibration ranged from 4 to 24 weeks. Meta-analysis revealed that whole-body vibration with strengthening exercises has a significant treatment effect on pain score (standardized mean difference (SMD) = 0.46 points, 95% confidence interval (95% CI) = 0.20–0.71, p = 0.0004), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC-function) (SMD = 0.51 points, 95% CI = 0.27–0.75, p < 0.0001), Timed Up and Go (TUG) test (SMD = 0.82 points, 95% CI = 0.46–1.18, p < 0.00001), extensor isokinetic peak torque (SMD = 0.65 points, 95% CI = 0.00–1.29, p = 0.05), peak power (SMD = 0.68 points, 95% CI = 0.26–1.10, p = 0.001), and extensor isometric strength (SMD = 0.44 points, 95% CI = 0.13–0.75, p = 0.006). Both low-frequency (10–30 Hz) and high-frequency (30–40 Hz) whole-body vibration were associated with significant changes in pain, physical function, and knee extensor strength (p < 0.05). WBV was not associated with significant changes in stiffness, balance ability, quality of life, and knee flexor strength. No adverse events were reported.
Conclusion
Meta-analysis showed that low-frequency and high-frequency whole-body vibration had additional positive effects compared with strengthening exercises alone on pain, knee extensor muscle strength, and physical function in individuals with knee OA. Whole-body vibration with strengthening exercises can be incorporated into treatment protocols.
LAY ABSTRACT
Knee osteoarthritis is a leading cause of disability and medical costs. Osteoarthritis leads to pain, stiffness, swelling and loss of function, resulting in poor quality of life. Whole-body vibration is a non-invasive treatment that has been proposed to improve muscle strength and physical performance. This analysis of 14 randomized controlled trials showed that, compared with exercise alone, whole-body vibration with exercise had positive effects on pain, physical function, and knee extensor muscle strength in patients with knee osteoarthritis. Based on these findings, we recommend whole-body vibration used together with strengthening exercises for knee osteoarthritis.
Key words: vibration therapy, knee osteoarthritis, exercise
Knee osteoarthritis (OA) is a leading cause of disability and medical costs (1). OA is one of the most common chronic diseases; it is estimated that there are more than 240 million people worldwide with symptomatic knee OA. Furthermore, radiographic study shows that approximately 30% of people older than 45 years have evidence of knee OA (2). Approximately 43% of the 54 million individuals with OA in the US have limitations in daily activities, with direct medical costs exceeding 100 billion USD (2). Each patient with knee OA has medical costs of more than 15,000 USD over their lifetime (2). The prevalence of knee OA in the UK has increased, with 15% of people aged 85 years or above affected (3). Knee OA currently accounts for 83% of the total burden of OA (3).
Conservative management is the current first-line therapy for knee OA (4), including education, exercises, weight management, and medication. However, current conservative management has major limitations, as common prescribed treatments have poor efficacy, and often do not reach a level of clinical significance (5). Furthermore, there are risks of medication; for example, non-steroidal anti-inflammatory drugs (NSAID) have side-effects of gastrointestinal bleeding and renal failure (4). As a result, additional interventions are warranted, focussing on strengthening of the lower extremity muscles, which improves pain and functioning (2, 6).
Whole-body vibration (WBV) is a cyclic, non-invasive treatment that can improve quadriceps muscle strength and physical performance (7–10). WBV is capable of stimulating muscle spindles, affecting the central mechanism and resulting in the activation of the alpha-motor neurone, followed by vibration tonic reflex, which may explain the positive effects of WBV on knee OA (11). However, current evidence in utilizing WBV on knee OA remains controversial, with conflicting results from various studies. There is also a lack of studies to investigate the therapeutic effects of different parameters of vibration therapy. A meta-analysis of 4 clinical trials has shown that WBV can reduce pain and improve function in patients with knee OA (12). However, other meta-analysis with 5 trials have shown no additional effect of WBV on muscle strength (13), and limited evidence to support its effectiveness. The main reason for the conflicting results is the limited number of trials and sample size. There is also a lack of assessment of longer-term results and adherence of patients. Recently, there has been an increasing number of publications demonstrating WBV as an efficient method for patients with knee OA to relieve pain, strengthen lower limb muscles, and improve quality of life (14–16). In order to address the discrepancies between current publications, and overcome the small sample size in previous studies, an updated meta-analysis is warranted. The aim of this study is to assess the effects and safety of WBV on pain, stiffness, physical function, and muscle strength in patients with knee OA.
METHODS
Data sources
PubMed, Web of Science, Embase, Scopus, and Physiotherapy Evidence Database (PEDro) databases (date last accessed 1 April 2021) were searched with the key words “vibration” and “knee osteoarthritis”. The study was conducted using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 2020.
Search criteria
Inclusion criteria were: (i) randomized controlled trials (RCTs); (ii) investigating effects of WBV; (iii) participants diagnosed with knee OA; (iv) primary outcome measures including pain, stiffness, physical performance, lower limb muscle strength, quality of life, adverse events; and (v) reported in English. Exclusion criteria were: (i) lack of control group; (ii) conference abstracts; (iii) review paper; (iv) protocol paper.
Study selection
Two independent reviewers conducted the selection process for the studies. Each reviewer screened the titles and abstracts. Articles were selected based on inclusion and exclusion criteria. Each article was reviewed, and any disagreement was resolved through discussion and consensus.
Study data extraction
Data from the included studies were extracted as follows: author; year of publication; participants and sample size; demographics; groups; interventions; vibration treatment parameters; duration; outcomes of participants.
Quality assessment of studies
The Cochrane Collaboration tool was used to assess the risk of bias in the domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, and selective reporting (17). Risk of bias in each domain was classified as low risk, high risk, or unclear. The quality of included studies was assessed using the PEDro scale. The scale consists of 11 items to assess the quality of RCTs on internal validity and sufficient statistical information to make it interpretable. Studies scoring ≥ 6 (6/10) were considered “good” quality, 4 – 5 “fair” quality, and < 4 “poor” quality (18).
To proceed to meta-analysis, studies needed to have at least “fair” quality and the control group needed to have exercise as an intervention, as this is currently the recommended conservative management therapy for knee OA. The meta-analysis assessed the additional effect of vibration therapy.
Statistical analysis for meta-analysis
The effects of WBV on outcomes was analysed using the Review Manager (RevMan 5.4, The Cochrane Centre, The Cochrane Collaboration), and forest plots were assembled using a random effects model. The weighted mean difference (WMD) and 95% confidence interval (95% CI) of the outcomes were computed. Heterogeneity was evaluated by Q-value and I2 index, where the I2 index < 25%, < 75%, and ≥ 75% represents low, moderate, and high heterogeneity, respectively. Subgroup analysis was conducted based on different frequencies (high-frequency group 30–40 Hz, and low-frequency group 10–30 Hz). Continuous results were presented by standardized mean difference (SMD) and 95% CI. A p-value < 0.05 was considered statistically significant. The standard deviation (SD) of the change from baseline was calculated as
RESULTS
Literature search
The literature search yielded 610 articles, of which 321 were excluded as duplicate studies. Another 258 studies were excluded as they did not meet the inclusion and exclusion criteria. A total of 31 full-text articles were reviewed for eligibility, 2 studies were removed as only an abstract was available, 1 was not a randomized controlled trial, 11 were excluded as the outcomes were not related to this review, 1 was not published in English, and 2 were protocol papers. A final total of 14 studies was included for qualitative synthesis. Fig. 1 shows the flow chart of the search results.
Fig. 1.
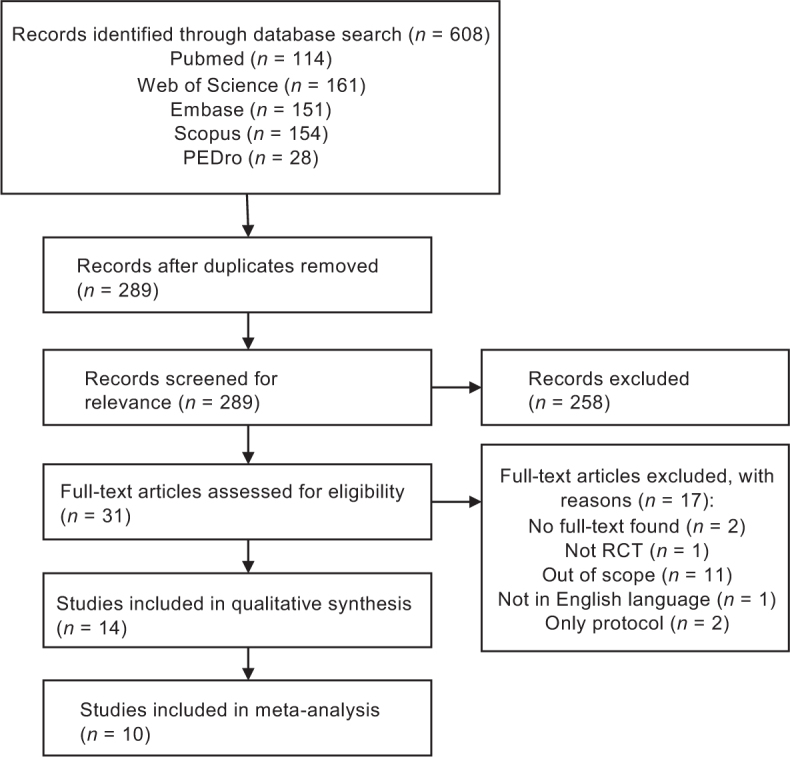
PRISMA flow chart.
Characteristics of included studies
The summary of the included studies for the current systematic review is shown in Table I. The 14 studies were published between 2009 and 2021. A total of 559 individuals were included from all studies, with a mean age range of 51.8–75 years. Sample size ranged from 15 to 99 participants. The studies were conducted in 7 countries, including Brazil (16, 19–22), India (23), China (14, 15, 24, 25), Korea (26), Japan (27), Denmark (28), and Iran (29). In order to assess for knee OA, 8 studies used the American College of Rheumatology guidelines (14, 16, 19, 20, 22, 24, 26, 28), 2 studies used Kellgren and Lawrence classification (27, 29), 3 studies used Lequesne index (15, 23, 25), and 1 study used Ahlbäck classification (21).
Table I.
Characteristics of included studies
| Author (country) Year | Participants n (M/F) | Groups (n) Mean age (SD) years | Vibration parameters | Duration | Outcomes | Key findings |
|---|---|---|---|---|---|---|
| Lai et al (China) (25) 2021 |
Patients diagnosed with KOA 81 (11/70) |
G1 (27): WBV+SE 63.52 (4.98) G2 (27): SE 64.81 (4.04) G3 (27): HE 63.67 (4.84) |
f (Hz): 20 A (mm): 2 a (g): NR | 3 (exposed time: 18–58.5 min) sessions/week ×8 weeks |
Physical function TUG, 6MWT, TDPM Knee strength ISK |
G1 vs G2, sign increase in knee extensor ISK |
| Aggarwal et al (India) (23) 2020 |
Patients diagnosed with KOA 30 (9/21) |
G1 (15): WBV+SE M 59 (5.68) F 57.5 (7.05) G2 (15): SE M 62 (5.88) F 61 (0) |
f (Hz): 25 A (mm): NR a (g): NR | 3 (exposed time: 9–21 min) sessions/week ×4 weeks |
Physical function WOMAC, CST, BBS Pain VAS |
G1 vs G2, Sign increase in VAS and WOMAC |
| Moura-F et al. (Brazil) (21) 2020 | Patients diagnosed with KOA 23 (NR) |
G1 (15): WBV G2 (8): Sham WBV 65 (8) | f (Hz): 5–14 D (mm): 2.5–7.5 a (g): 0.12–2.95 | 2 (exposed time: 18 min) sessions/week ×5 weeks |
Quality of life WHOQOL |
WBV did not contribute to alter the quality of life of participants |
| Moura et al. (Brazil) (22) 2020 | Obese patients diagnosed with KOA 37 (7/30) |
G1 (19): WBV 62.32 (2.52) G2 (18): Sham WBV 68.06 (2.02) |
f (Hz): 5 D (mm): 2.5–7.5 a (g): 0.12–0.37 | 3 bouts, 1 session, total 11 min | Pain VAS Physical function TUG, ATF, Borg scale |
G1 vs G2, sign increase in VAS, TUG and ATF |
| Simão et al (Brazil) (16) 2019 |
Female patients diagnosed with KOA 15 (0/15) |
G1 (7): WBV+ST G1: 75 (6.5) G2 (8): ST G2: 71 (3.3) |
f (Hz):35,40 A (mm): 4 a (g): 2.78–3.26 | 3 (exposed time: 6–16 min) sessions/week ×12 weeks |
Knee strength IQMS |
G1 vs G2, sign increase in IQMS |
| Lai et al. (China) (15) 2019 | Patients diagnosed with KOA 41 (5/36) |
G1 (20): WBV+ST 64.1 (4.95) G2 (21): ST 65 (4.39) |
f (Hz): 20 A (mm): 2 a (g): NR | 3 (exposed time: 18–58.5 min) sessions/week ×8 weeks |
Physical function TUG, 6MWT Knee strength ISK |
G1 vs G2, sign increase in knee extensor ISK |
| Bokaeian et al. (Iran) (29) 2016 | Patients diagnosed with KOA and able to walk 26 (2/24) |
G1 (15): WBV+SE 51.8 (8.3) G2 (11): SE 54.0 (3.9) |
f (Hz): 25–30 A (mm): 2 a (g): NR | 3 (exposed time: 9–31.5 min) sessions/week ×8 weeks |
Knee strength ISK Pain VAS Physical function WOMAC, 2MWT, TUG, 50FWT |
G1 vs G2, sign increase in knee extensor ISK, 2MWT, TUG and 50FWT |
| Wang P et al. (China) (24) 2016 | Patients diagnosed with KOA based on criteria of ACR 39 (16/23) |
G1 (19): WBV+QSE 61.1 (7.1) G2 (20): QSE 61.5 (7.3) |
f (Hz): 35 A (mm): 4–6 a (g): 1.0 | 5 (exposed time: 75 min) sessions/week ×12 weeks |
Physical function TUG, 6MWT, WOMAC, gait analysis Pain VAS |
G1 vs G2, sign increase in VAS, WOMAC, 6MWT, TUG and gait speed |
| Wang et al (China) (14) 2016 |
Patients diagnosed with KOA based on criteria of ACR 99 (28/71) |
G1 (49): WBV+QSE 61.2 (9.6) G2 (50): QSE 61.5 (9.1) |
f (Hz): 35 A (mm): 4–6 a (g): 1.0 | 5 (exposed time: 75 min) sessions/week ×24 weeks |
Quality of life SF-36 Pain VAS Physical function TUG, 6MWT, WOMAC Knee strength ISM | G1 vs G2, sign increase in VAS, SF-36, TUG, 6MWT, WOMAC and knee extensor ISM |
| Tsuji et al (Japan) (27) 2014 |
Postmenopausal women diagnosed with KOA 38 (0/38) |
G1 (29): WBV+HBE 62.1 (5.5) G2 (9): HBE 60.9 (4.6) |
f (Hz): 30,40 A (mm): 2.5 a (g): NR | 3 (exposed time: 54–69 min) sessions/week ×8 weeks |
Knee strength ISM, ISK Pain VAS Physical function JKOM, TUG |
G1 vs G2, sign increase in JKOM and TUG |
| Park et al (Korea) (26) 2013 |
Women diagnosed with KOA 22 (0/22) |
G1 (11): WBV+HBE 62.5 (5.66) G2 (11): HBE 60.0 (6.22) |
f (Hz): 12,14 A (mm): 2.5–5 a (g): NR | 3 (exposed time: 60 min) sessions/week ×8 weeks |
Knee strength ISK, ISM Physical function KWOMAC, LSS, SBCS Pain NRS |
G1 vs G2, sign increase in NRS |
| Simão et al (Brazil) (20) 2012 |
Patients diagnosed with KOA 35 (4/31) |
G1 (12): WBV+ST 75 (7.4) G2 (11): ST 69 (3.7) G3 (12): None 71 (5.3) |
f (Hz): 35,40 A (mm): 4 a (g): 2–2.61 | 3 (exposure time: 6–16 min) sessions/week ×12 weeks |
Physical function WOMAC, BBS, GST, and 6MWT | G1 vs G2, sign increase in WOMAC, BBS, and gait speed |
| Avelar et al (Brazil) (19) 2011 |
Patients diagnosed with KOA 21 (3/18) |
G1 (11): WBV+ST 75 (5) G2 (10): ST 71 (4) |
f (Hz): 35,40 A (mm): 4 a (g): 2.78–3.26 | 3 (exposed time: 6–16 min) sessions/week ×12 weeks |
Physical function BBS, TUG, CST, 6MWT, WOMAC |
G1 vs G2, failed to result in any significant improvement |
| Trans et al. (Denmark) (28) 2009 |
Women diagnosed with KOA 52 (0/52) |
G1 (18): WBV (BB) 58.7 (11) G2 (17): WBV (SP) 61.5 (9.2) G3 (17): None 61.1 (8.5) |
f (Hz): 25,30 A (mm): NR a (g): NR | 2 (exposure time 6–21 min) sessions/week ×8 weeks | Knee strength ISK, ISM Physical function WOMAC, TDPM |
G1 vs G3, sign increase in ISK and ISM; G2 vs G3, sign increase in TDPM |
A: amplitude; a, acceleration; ACR: American College of Rheumatology; ATF: anterior trunk flexion; BB: balance board; BBS: Berg Balance Scale; CG: control group; CST: chair stand test; D: displacement; F: female; f: frequency; G: group; GST: gait speed test; HE: health education; IQMS: isometric quadriceps muscle strength; ISM: isometric muscle strength; ISK: isokinetic muscle strength; JKOM: Japanese Knee Osteoarthritis Measure; KOA: knee osteoarthritis; KWOMAC: Korean Western Ontario McMaster score; LI: Lequesne index; LSS: Lysholm scoring scale; M: male; NR: not reported; NRS: numerical rating scale; QSE: quadriceps strengthening exercise; SBCS: Standing Balance Control Scores; SE: strengthening exercise; SF-36: Medical Outcomes Short Form 36; SP: stable platform; ST: squat training; TDPM: threshold for detective of passive movement; TUG: Timed Up and Go test; VAS: visual analogue scale; WBV: whole-body vibration; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; WHOQOL: World Health Organization Quality-of-Life Scale; 2/6MWT: 2/6-minute walk test; 50FWT: 50-foot walk test.
Vibration therapy regime
Twelve studies used vertical vibration (15, 16, 19–23, 25–29), and 2 studies used multi-directional vibration (14, 24). The frequency of vibration varied from 5 to 40 Hz, with 6 studies using high frequency, at 30–40 Hz (14, 16, 19, 20, 24, 27), and 8 studies using low frequency, at 5–30 Hz (15, 21–23, 25, 26, 28, 29). Ten studies reported the amplitude of vibration, with 4-mm amplitude in 3 studies (16, 19, 20), 4–6-mm in 2 studies (14, 24), 2-mm in 3 studies (15, 25, 29), 2.5–5-mm in 1 study (26), and 2.5-mm in 1 study (27). Two studies documented peak-to-peak displacement instead of amplitude (21, 22). Two studies did not report amplitude or displacement (23, 28). The frequency of vibration therapy ranged from 2 to 5 times per week, except for 1 study, which lasted for only 11 min (22). Regarding duration of treatment, there were 2 studies with vibration treatment longer than 12 weeks (at 24 weeks (14) and 16 weeks (24)). There were 3 studies of 12 weeks’ duration (16, 19, 20), and other studies were of 8 weeks (15, 25–29), 5 weeks (21), and 4 weeks (23) duration. The exposure time to vibration ranged from 6 to 75 min per week.
Control group of studies
Most of the included studies had strengthening exercises as control groups, including squatting exercises (15, 16, 19, 20), strengthening training (23, 25, 29), quadriceps strengthening exercises (14, 24), and home-based exercises (26, 27), whereas the remaining 3 studies used sham WBV (21, 22) and no exercise (28) for the control group.
Adverse events
None of the included studies reported any adverse events with WBV. Only 1 participant in the quadriceps resistance exercise group felt increased knee pain at 2 weeks’ assessment, which was resolved by modifying the exercise technique (14).
Risk of bias
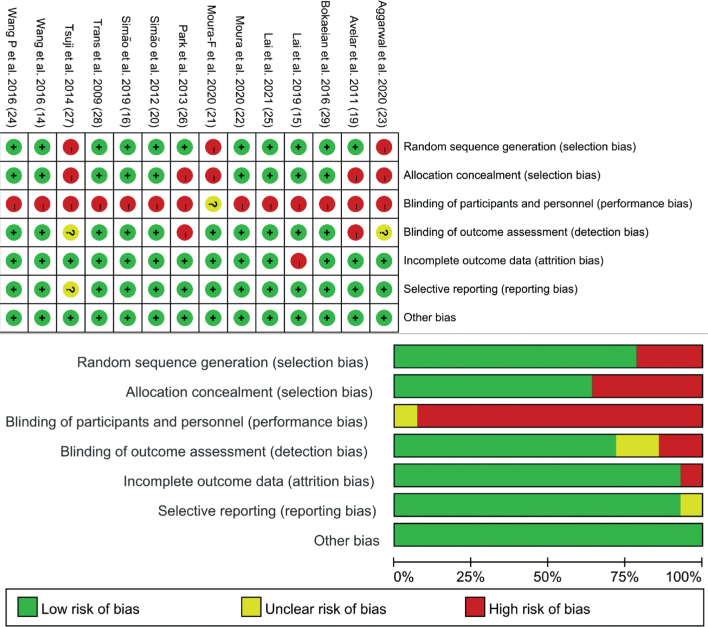
The risk of bias of all studies was assessed (Fig. 2). Eleven studies performed random sequence as low risk of bias (14–16, 19, 20, 22, 24–26, 28, 29), and 3 studies were high risk of bias (21, 23, 27). For allocation concealment, 9 studies were considered low risk of bias (14–16, 20, 22, 24, 25, 28, 29), but 5 studies reported no information on allocation concealment (19, 21, 23, 26, 27). All of the studies lacked blinding of participants and personnel (14–16, 19, 20, 22–24, 26–29), except 1 study that was unclear (21). For the blinding of outcome assessors, 2 studies were high risk of bias (19, 26), 2 were unclear (23, 27), and the remaining studies were low risk of bias (14–16, 20–22, 24, 26, 28, 29). Only 1 study had high risk of bias of incomplete data because of a high drop-out rate (15). Most included studies showed low risk of bias on selective reporting, except 1 which was unclear (27). PEDro was also performed, as summarized in Table II. All studies had a score of 4 or more.
Fig. 2.
Assessment of risk of bias of included studies.
Table II.
Assessment of the methodological quality using the Physiotherapy Evidence Database (PEDro) scale
| Study | Random allocation | Concealed allocation | Baseline comparability | Blind subjects | Blind therapists | Blind assessors | Follow-up | Intention-to-treat analysis | Group comparisons | Point and variability measures | Total scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lai et al. 2021 (25) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Aggarwal et al. 2020 (23) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 4 |
| Moura-F et al. 2020 (21) | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 5 |
| Moura et al. 2020 (22) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Simão et al. 2019 (16) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Lai et al. 2019 (15) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Bokaeian et al. 2016 (29) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Wang P et al. 2016 (24) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Wang et al. 2016 (14) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Tsuji et al. 2014 (27) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 |
| Park et al. 2013 (26) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 4 |
| Simão et al. 2012 (20) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Avelar et al. 2011(19) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Trans et al. 2009 (28) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
Meta-analysis
Four trials were excluded from the meta-analysis. The control group for 3 studies did not include exercise (21, 22, 28), which is a routine treatment for knee OA. One study had missing data (25). Therefore, 10 RCTs were included in the meta-analysis to compare the effectiveness of WBV training together with strengthening exercise, in order to assess the additional effect of WBV. One study did not report any significant results of WBV on knee OA (19). Subgroup analysis was also performed based on different frequencies (high-frequency 30–40 Hz, low-frequency 10–30 Hz).
Pain intensity
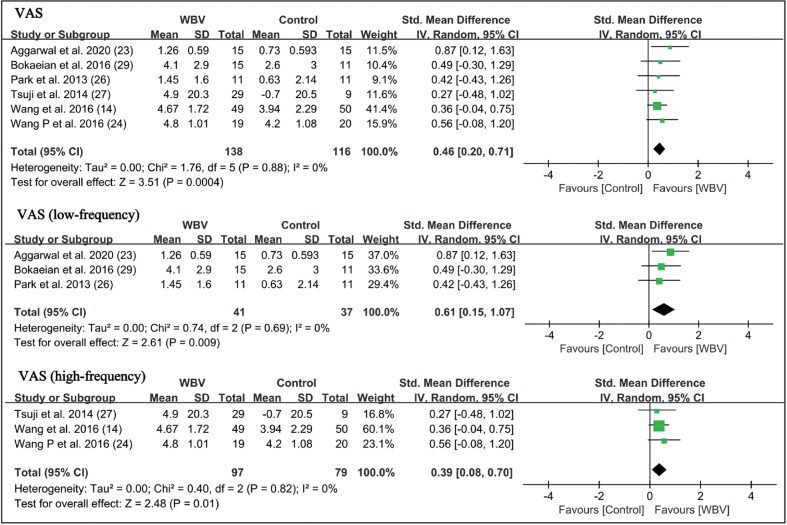
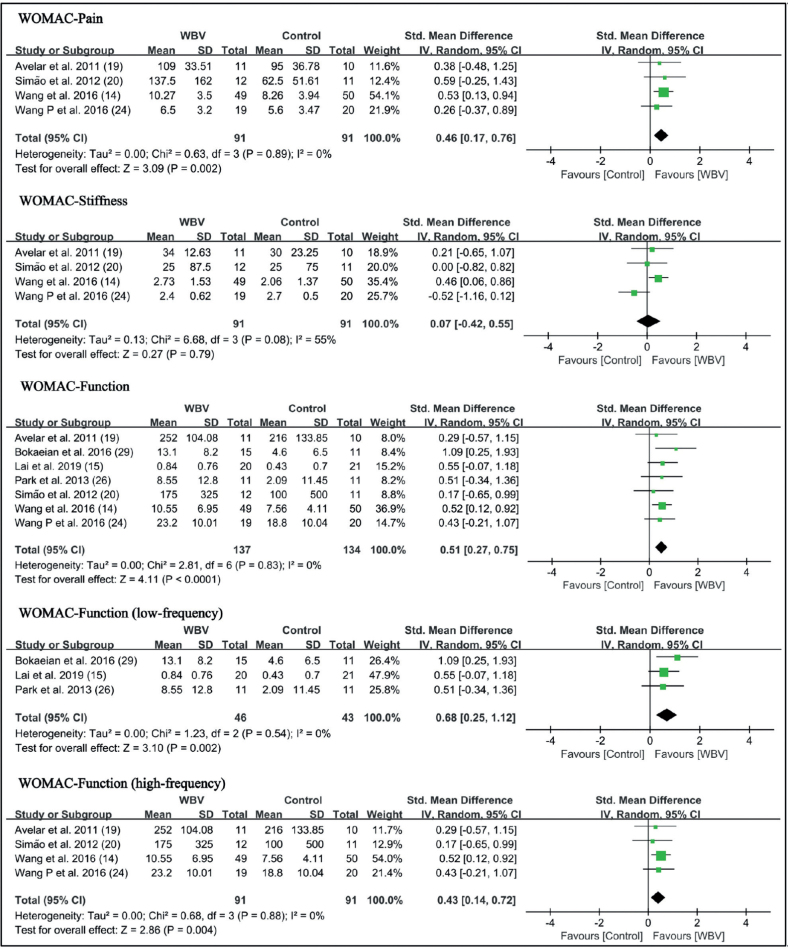
Five studies (14, 23, 24, 27, 29) used a visual analogue scale (VAS) and 1 study (26) used numerical rating scale (NRS) to evaluate pain intensity. The results of these 6 studies showed that WBV significantly reduced pain (SMD = 0.46 points, 95% CI = 0.20 – 0.71, p = 0.0004). Subgroup analysis showed that both low-frequency WBV (SMD = 0.61 points, 95% CI = 0.15 – 1.07, p = 0.009) and high-frequency WBV (SMD = 0.39 points, 95% CI = 0.08–0.70, p = 0.01) significantly reduced pain (Fig. 3). The WOMAC-pain subscale was used in 4 studies, which all used high-frequency vibration. The results also showed a significant reduction in pain intensity (SMD = 0.46 points, 95% CI = 0.17 – 0.76, p = 0.002) (Fig. 4).
Fig. 3.
Forest plot of meta-analysis and subgroup analysis of whole-body vibration (WBV) plus exercise vs exercise alone for pain. SD: standard deviation; VAS: visual analogue scale; 95% CI: 95% confidence interval.
Fig. 4.
Forest plot of meta-analysis and subgroup analysis of whole-body vibration (WBV) plus exercise vs exercise alone for the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). SD: standard deviation; 95% CI: 95% confidence interval.
Stiffness
Four studies (14, 19, 20, 24) with high-frequency vibration as an intervention used the WOMAC-stiffness subscale to assess the effects on knee stiffness. The results showed that WBV did not significantly reduce stiffness (SMD = 0.07 points, 95% CI = −0.42–0.55, p = 0.79) (Fig. 4).
WOMAC-function
Seven studies (14, 15, 19, 20, 24, 26, 29) used the WOMAC-function subscale to evaluate self-reported function. The results showed that WBV significantly improved self-reported function in knee OA (SMD = 0.51 points, 95% CI = 0.27 – 0.75, p < 0.0001). In the subgroup analysis, self-reported function was improved in both the low-frequency group (SMD = 0.68 points, 95% CI = 0.25 – 1.12, p = 0.002) and the high-frequency group (SMD = 0.43 points, 95% CI = 0.14 – 0.72, p = 0.004) (Fig. 4).
Functional performance
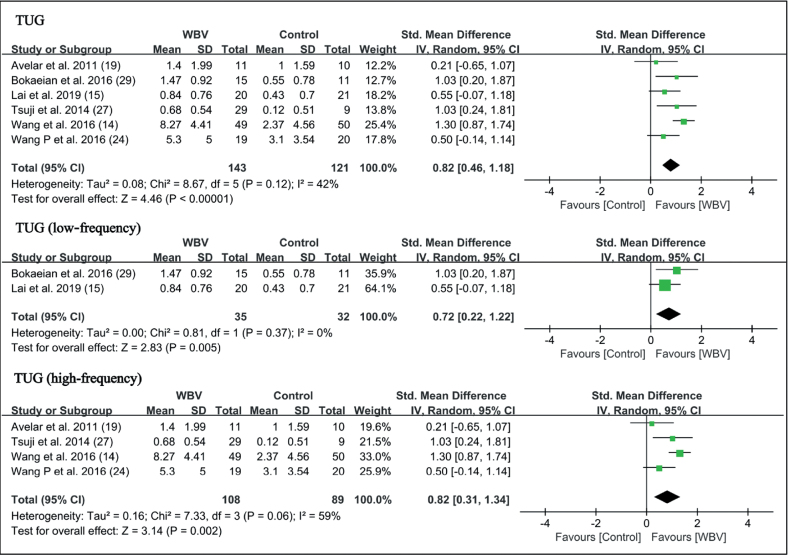
Timed Up and Go (TUG) test. A total of 6 studies reported results of the TUG test (14, 15, 19, 24, 27, 29). Results of meta-analysis showed WBV significantly enhanced the performance of the TUG test in patients with knee OA (SMD = 0.82 points, 95% CI = 0.46 – 1.18, p < 0.00001). In the subgroup analysis, low-frequency WBV significantly improved the performance of the TUG test (SMD = 0.72 points, 95% CI = 0.22 – 1.22, p = 0.005). The high-frequency group also significantly improved in the TUG test (SMD = 0.82 points, 95% CI = 0.31 – 1.34, p = 0.002) (Fig. 5).
Fig. 5.
Forest plot of meta-analysis and subgroup analysis of whole-body vibration (WBV) plus exercise vs exercise alone for Timed Up and Go test (TUG) test. SD: standard deviation; 95% CI: 95% confidence interval.
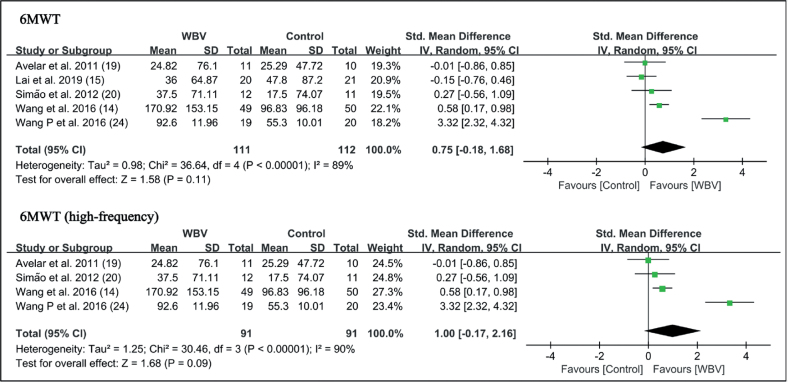
6-minute walk test (6MWT). Five studies reported the results of the 6MWT (14, 15, 19, 20, 24). Meta-analysis showed a trend that WBV was superior to the control group, but did not reach statistical significance (SMD = 0.75 points, 95% CI = –0.18 – 1.68, p = 0.11). In subgroup analysis, 4 studies with high-frequency were analysed, and the results were similar (SMD = 1.00 points, 95% CI = –0.17 – 2.16, p = 0.09) (Fig. 6).
Fig. 6.
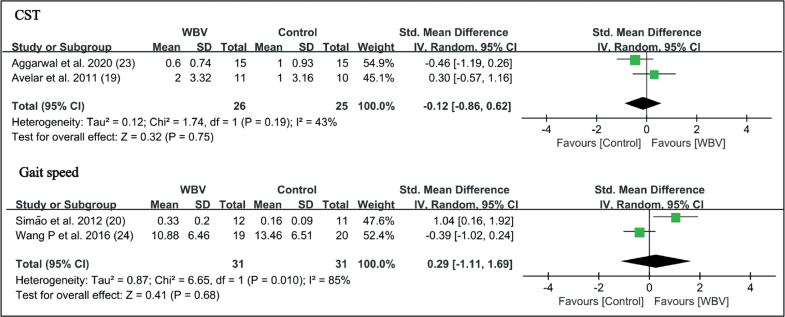
Forest plot of meta-analysis and subgroup analysis of whole-body vibration (WBV) plus exercise vs exercise alone for change in chair stand test (CST) and gait speed. SD: standard deviation; 95% CI: 95% confidence interval.
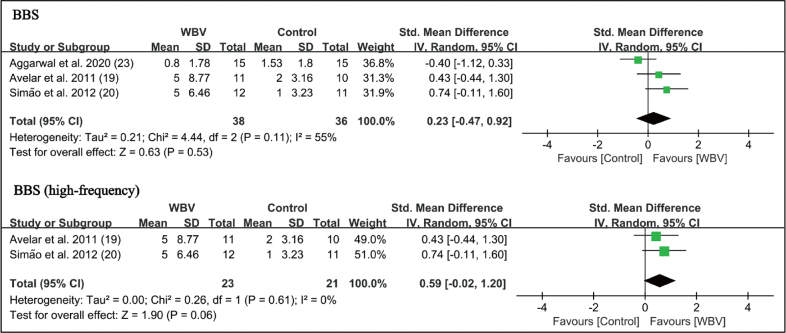
Berg Balance Scale (BBS). Three studies used the BBS to test the ability of balance (19, 20, 23). There was no significant difference between WBV and control groups (SMD = 0.23 points, 95% CI = –0.47 – 0.92, p = 0.53). In the subgroup analysis, high-frequency WBV had marginal significance (SMD=0.59 points, 95% CI = –0.02 – 1.20, p = 0.06). There was insufficient data to perform subgroup analysis in the low-frequency group (Fig. 7).
Fig. 7.
Forest plot of meta-analysis and subgroup analysis of whole-body vibration (WBV) plus exercise vs exercise alone for change in 6-minute walk test (6MWT). SD: standard deviation; 95% CI: 95% confidence interval.
Chair stand test (CST). Two studies used a chair stand test as an outcome measure to assess functional performance (19, 23). WBV with strengthening exercises did not have additional beneficial effects compared with the control group on the CST (SMD = –0.12 points, 95% CI = –0.86 – 0.62, p = 0.75) (Fig. 8).
Fig. 8.
Forest plot of meta-analysis and subgroup analysis of randomized controlled trials (RCTs) of whole-body vibration (WBV) plus exercise vs exercise alone for change in Berg Balance Scale (BBS). SD: standard deviation; 95% CI: 95% confidence interval.
Gait Speed Test. Two studies in the high-frequency group compared the effects of WBV on gait speed (20, 24). In comparison with strengthening exercise, WBV did not significantly increase the gait speed of individuals with knee OA (SMD = 0.29 points, 95% CI = –1.11 – 1.69, p = 0.68) (Fig. 8).
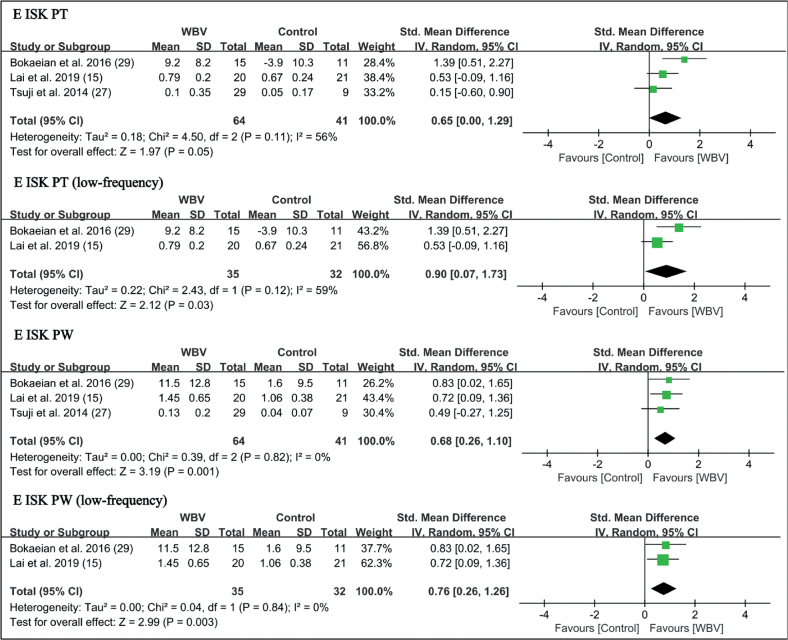
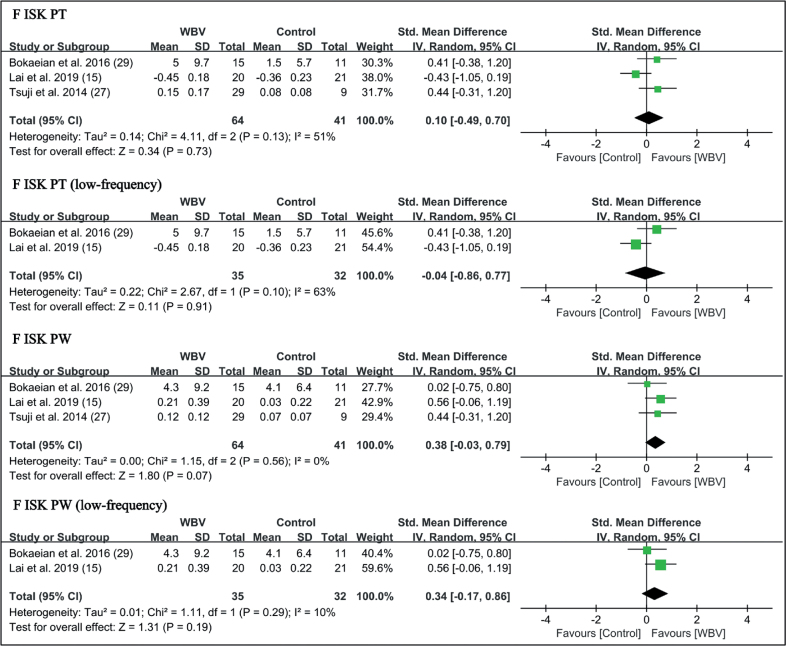
Muscle strength. Six trials reported the outcome measures of muscle strength, involving isokinetic peak torque, peak power at 90°/s, and knee extensor isometric strength (14, 15, 20, 26, 27, 29). WBV significantly improved the extensor isokinetic peak torque (SMD = 0.65 points, 95% CI = 0.00 – 1.29, p = 0.05) and isokinetic peak power (SMD = 0.68 points, 95% CI = 0.26 – 1.10, p = 0.001). For subgroup analysis, low-frequency vibration was beneficial for extensor isokinetic peak torque (SMD = 0.90 points, 95% CI = 0.07 – 1.73, p = 0.03) and isokinetic peak power (SMD = 0.76 points, 95% CI = 0.26 – 1.26, p = 0.003). No significant difference in flexor strength was found (Fig. 9).
Fig. 9.
Forest plot of meta-analysis and subgroup analysis of randomized controlled trials (RCTs) of whole-body vibration (WBV) plus exercise vs exercise alone for change in knee extensor isokinetic peak torque (E ISK PT) and extensor isokinetic peak power (E ISK PW). SD: standard deviation; 95% CI: 95% confidence interval.
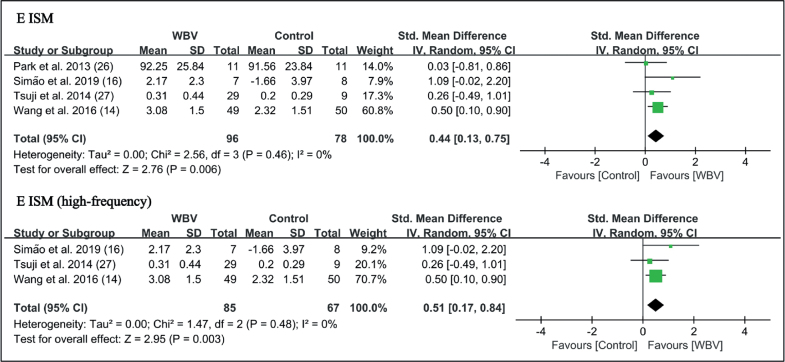
WBV with strengthening exercises showed additional effects on knee extensor isometric muscle strength (SMD = 0.44 points, 95% CI = 0.13–0.75, p = 0.006) (Fig. 11). Subgroup analysis showed high-frequency WBV significantly enhanced knee extensor isometric strength (SMD = 0.51 points, 95% CI = 0.17–0.84, p = 0.003) (Fig. 11). No significant difference in flexor strength was found (Fig. 10).
Fig. 11.
Forest plot of meta-analysis and subgroup analysis of randomized controlled trials (RCTs) of whole-body vibration (WBV) plus exercise vs exercise alone for change in knee flexor isokinetic peak torque (F ISK PT) and flexor isokinetic peak power (F ISK PW). SD: standard deviation; 95% CI: 95% confidence interval.
Fig. 10.
Forest plot of meta-analysis and subgroup analysis of randomized controlled trials (RCTs) of whole-body vibration (WBV) plus exercise vs exercise alone for change in knee extenosr isometric strength (E ISM). SD: standard deviation; 95% CI: 95% confidence interval.
DISCUSSION
The result of this meta-analysis showed that WBV with exercise had additional positive effects on pain, self-reported function, TUG test, and extensor muscle strength in patients with knee OA, compared with a control group performing strengthening exercises alone. More importantly, no adverse outcomes were reported for WBV. However, there was no evidence that WBV with exercise had superior effects on stiffness, 6MWT, balance, CST, and knee flexor strength. Subgroup analysis showed that both low- and high-frequency WBV training significantly reduced pain, improved self-reported function and TUG test. It is known that vibration therapy improves muscle strength (30), although the exact mechanism is unclear. Studies have suggested that WBV modulates neuromuscular adaptations (16), and therefore improves muscle strength. The vibration produced by the oscillating platform is capable of stimulating muscle spindles, resulting in activation of the alpha-motor neurone, followed by vibration tonic reflex (31, 32) and spinal and supraspinal mechanisms, which are possible mechanisms to explain the positive effects of WBV on knee OA (11, 33).
Vibration therapy has been shown to have multiple effects on muscle strength, postural control and balancing ability (34–36). With reduced pain, muscle strength and functional performance, patients with OA can improve (37). Studies have shown that, similar to exercise, WBV can affect the central mechanisms, cortical reorganization and nociceptive activity, and therefore reduce pain and enhance muscle strength. Therefore, several studies have considered vibration therapy as a type of exercise therapy (7, 38, 39). However, the current study found that WBV together with strengthening exercise did not significantly improve 6MWT, BBS, CST, or gait speed test (GST) compared with strengthening exercises alone. The difference may be due to the heterogeneity of included studies and limited sample size. The analysis of CST, GST, and BBS were based on fewer than 100 participants, and the higher I2 statistic of 6MWT suggested higher heterogeneity among the included studies. Therefore, the conclusion regarding functional performance still requires more evidence from larger trials. Stiffness of the knee is not improved with WBV, which may be due to the fact that cartilage wear from OA cannot be reversed. The current study also found that WBV combined with strengthening exercise was more effective for improving knee extensor strength, whereas there was a limited effect on knee flexor strength. The position of participants on the vibratory platform may be responsible for this difference (40). It should be noted that the effects of WBV training on muscle strength are related to the initial length of the muscle. Studies have shown that stretched muscles are more sensitive to exposure to WBV (40, 41). Most participants in the current review had slight flexion of the knees on the vibration platform and therefore the quadriceps were stretched, which explains the increased response to vibration.
Subgroup analysis showed that both high- and low-frequency WBV were effective in improving pain, physical function and knee extensor muscle strength. Studies have shown that the effectiveness of WBV on outcomes may be influenced by various vibration parameters. A previous meta-analysis demonstrated that treatment effects on muscle strength increased linearly with increase in vibration frequency (42). However, these results are controversial, as other studies have demonstrated that high-frequency WBV did not generate significant positive effects in comparison with the control groups with inadequate exposure time (43). Therefore, short-term WBV may not have positive effects on the musculoskeletal system (38). The current meta-analysis showed that frequency may not be the only important parameter, as duration and amplitude probably also play a role. Therefore, additional randomized controlled trials are necessary to compare the effects of different vibration frequencies and amplitudes. Currently, the optimal regime for WBV is unclear.
Previous systematic reviews and meta-analyses (13, 44) had reported that WBV along with exercise did not significantly improve pain control, physical function, and muscle strength, but, in contrast, we found that WBV training produced additional effects on pain, functional performance, and knee extensor muscle strength. This is due to the recent publications that the current meta-analysis also included, which largely increased the sample size. Dong et al. (38) also reported similar results to the current study, and WBV could serve as an effective complementary intervention to alleviate pain in patients with OA. Subgroup analysis for their study was based on duration of treatment. Results had shown that long-term WBV training showed better results for pain control (38) and physical function (12) compared with short-term WBV training. Based on the current findings, we recommend that WBV can be used together with strengthening exercises for knee OA. This study has also clarified current controversy on the effectiveness of WBV on knee OA.
This study has several strengths. To our knowledge, the current systematic review and meta-analysis has the largest sample size to date, with a total of 14 randomized controlled trials for qualitative analysis, and 10 for quantitative analysis. This study provides evidence that WBV has beneficial effects on knee OA, as the results of previous studies are controversial. Furthermore, subgroup analysis was conducted based on vibration frequency, whilst previous reviews were based on treatment duration. This study also has some limitations. Subgroup analyses for muscle strength and function (CST, GST, BBS) were based on limited sample size, and there was a lack of studies comparing the beneficial effects of low-frequency WBV with high-frequency WBV with regards to isokinetic and isometric strength. Furthermore, heterogeneity of data and exercise programmes of included studies may have an effect on results. In addition, the current study did not separately analyse the differences in effects of WBV on male and female participants. Another limitation of this review was that the protocol was not registered.
In conclusion, WBV is a safe and effective training modality for individuals with knee OA. WBV combined with exercise was superior to exercise alone, in improving pain, physical function (TUG test and WOMAC), and knee extensor strength (isokinetic and isometric). WBV training did not produce additional positive effects on stiffness, CST, 6MWT, balance, gait speed, and knee flexor strength compared with a control group with strengthening exercises alone. Both high- and low-frequency WBV had beneficial effects on pain intensity, physical function and knee extensor strength. Further larger-scale studies are necessary to validate the optimal regime for WBV. Furthermore, WBV can be incorporated into conservative management protocols for patients with knee OA.
ACKNOWLEDGEMENTS
This study was supported by the Hong Kong Research Grants Council Early Career Scheme (reference number 24108519).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA 2017; 317: 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 2021; 325: 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spitaels D, Mamouris P, Vaes B, Smeets M, Luyten F, Hermens R, et al. Epidemiology of knee osteoarthritis in general practice: a registry-based study. BMJ Open 2020; 10: e031734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennell KL, Hunter DJ, Hinman RS. Management of osteoarthritis of the knee. BMJ 2012; 345: e4934. [DOI] [PubMed] [Google Scholar]
- 5.Vannabouathong C, Bhandari M, Bedi A, Khanna V, Yung P, Shetty V, et al. Nonoperative Treatments for knee osteoarthritis: an evaluation of treatment characteristics and the intra-articular placebo effect: a systematic review. JBJS Rev 2018; 6: e5. [DOI] [PubMed] [Google Scholar]
- 6.Holla JF, van der Leeden M, Peter WF, Roorda LD, van der Esch M, Lems WF, et al. Proprioception, laxity, muscle strength and activity limitations in early symptomatic knee osteoarthritis: results from the CHECK cohort. J Rehabil Med 2012; 44: 862–868. [DOI] [PubMed] [Google Scholar]
- 7.Pistone EM, Laudani L, Camillieri G, Di Cagno A, Tomassi G, Macaluso A, et al. Effects of early whole-body vibration treatment on knee neuromuscular function and postural control after anterior cruciate ligament reconstruction: a randomized controlled trial. J Rehabil Med 2016; 48: 880–886. [DOI] [PubMed] [Google Scholar]
- 8.Sañudo B, Carrasco L, de Hoyo M, Oliva-Pascual-Vaca Á, Rodríguez-Blanco C. Changes in body balance and functional performance following whole-body vibration training in patients with fibromyalgia syndrome: a randomized controlled trial. J Rehabil Med 2013; 45: 678–684. [DOI] [PubMed] [Google Scholar]
- 9.Ho KK, Lau LC, Chau WW, Poon Q, Chung KY, Wong RM. End-stage knee osteoarthritis with and without sarcopenia and the effect of knee arthroplasty - a prospective cohort study. BMC Geriatr 2021; 21: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow SKH, Ho CY, Wong HW, Chim YN, Wong RMY, Cheung WH. Efficacy of low-magnitude high-frequency vibration (LMHFV) on musculoskeletal health of participants on wheelchair: a study protocol for a single-blinded randomised controlled study. BMJ Open 2020; 10: e038578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaidell LN, Mileva KN, Sumners DP, Bowtell JL. Experimental evidence of the tonic vibration reflex during whole-body vibration of the loaded and unloaded leg. PLoS One 2013; 8: e85247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Yang XT, Yang YH, Yang L, Zhou YJ, Liu C, et al. Effects of whole body vibration on pain, stiffness and physical functions in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil 2015; 29: 939–951. [DOI] [PubMed] [Google Scholar]
- 13.Anwer S, Alghadir A, Zafar H, Al-Eisa E. Effect of whole body vibration training on quadriceps muscle strength in individuals with knee osteoarthritis: a systematic review and meta-analysis. Physiotherapy 2016; 102: 145–151. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Yang L, Liu C, Wei X, Yang X, Zhou Y, et al. Effects of whole body vibration exercise associated with quadriceps resistance exercise on functioning and quality of life in patients with knee osteoarthritis: a randomized controlled trial. Clin Rehabil 2016; 30: 1074–1087. [DOI] [PubMed] [Google Scholar]
- 15.Lai ZQ, Lee S, Hu XY, Wang L. Effect of adding whole-body vibration training to squat training on physical function and muscle strength in individuals with knee osteoarthritis. J Musculoskelet Neuronal Interact 2019; 19: 333–341. [PMC free article] [PubMed] [Google Scholar]
- 16.Simao AP, Mendonca VA, Avelar NCP, da Fonseca SF, Santos JM, de Oliveira ACC, et al. Whole body vibration training on muscle strength and brain-derived neurotrophic factor levels in elderly woman with knee osteoarthritis: a randomized clinical trial study. Front Physiol 2019; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother 2002; 48: 43–49. [DOI] [PubMed] [Google Scholar]
- 19.Avelar NC, Simao AP, Tossige-Gomes R, Neves CD, Rocha-Vieira E, Coimbra CC, et al. . The effect of adding whole-body vibration to squat training on the functional performance and self-report of disease status in elderly patients with knee osteoarthritis: a randomized, controlled clinical study. J Altern Complement Med 2011; 17: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 20.Simao AP, Avelar NC, Tossige-Gomes R, Neves CD, Mendonca VA, Miranda AS, et al. Functional performance and inflammatory cytokines after squat exercises and whole-body vibration in elderly individuals with knee osteoarthritis. Arch Phys Med Rehabil 2012; 93: 1692–1700. [DOI] [PubMed] [Google Scholar]
- 21.Moura-Fernandes MC, Moreira-Marconi E, de Meirelles AG, de Oliveira APF, Silva AR, de Souza LFF, et al. Effect of the combined intervention with passive whole-body vibration and auriculotherapy on the quality of life of individuals with knee osteoarthritis assessed by the whoqol-bref: a multi-arm clinical trial. Appl Sci-Basel 2020; 10: 13. [Google Scholar]
- 22.Moura-Fernandes MC, Moreira-Marconi E, de Meirelles AG, Reis-Silva A, de Souza LFF, da Silva ALP, et al. Acute effects of whole-body vibration exercise on pain level, functionality, and rating of exertion of elderly obese knee osteoarthritis individuals: A randomized study. Applied Sciences (Switzerland) 2020; 10. [Google Scholar]
- 23.Aggarwal A, Paranjape PR, Palekar TJ, Singh G, Rao TS. Effect of whole body vibration on lower body strength and balance in osteoarthritis knee. Int J Physiother 2020; 7: 86–92. [Google Scholar]
- 24.Wang P, Yang L, Li H, Lei Z, Yang X, Liu C, et al. Effects of whole-body vibration training with quadriceps strengthening exercise on functioning and gait parameters in patients with medial compartment knee osteoarthritis: a randomised controlled preliminary study. Physiotherapy 2016; 102: 86–92. [DOI] [PubMed] [Google Scholar]
- 25.Lai Z, Lee S, Chen Y, Wang L. Comparison of whole-body vibration training and quadriceps trength training on physical function and neuromuscular function of individuals with knee osteoarthritis: a randomised clinical trial. J Exerc Sci Fit 2021; 19: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park YG, Kwon BS, Park JW, Cha DY, Nam KY, Sim KB, et al. Therapeutic effect of whole body vibration on chronic knee osteoarthritis. Ann Rehabil Med 2013; 37: 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji T, Yoon J, Aiba T, Kanamori A, Okura T, Tanaka K. Effects of whole-body vibration exercise on muscular strength and power, functional mobility and self-reported knee function in middle-aged and older Japanese women with knee pain. Knee 2014; 21: 1088–1095. [DOI] [PubMed] [Google Scholar]
- 28.Trans T, Aaboe J, Henriksen M, Christensen R, Bliddal H, Lund H. Effect of whole body vibration exercise on muscle strength and proprioception in females with knee osteoarthritis. Knee 2009; 16: 256–261. [DOI] [PubMed] [Google Scholar]
- 29.Bokaeian HR, Bakhtiary AH, Mirmohammadkhani M, Moghimi J. The effect of adding whole body vibration training to strengthening training in the treatment of knee osteoarthritis: a randomized clinical trial. J Bodyw Mov Ther 2016; 20: 334–340. [DOI] [PubMed] [Google Scholar]
- 30.Alghadir AH, Anwer S, Zafar H, Iqbal ZA. Effect of localised vibration on muscle strength in healthy adults: a systematic review. Physiotherapy 2018; 104: 18–24. [DOI] [PubMed] [Google Scholar]
- 31.De Gail P, Lance JW, Neilson PD. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry 1966; 29: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tankisheva E, Jonkers I, Boonen S, Delecluse C, van Lenthe GH, Druyts HL, et al. Transmission of whole-body vibration and its effect on muscle activation. J Strength Cond Res 2013; 27: 2533–2541. [DOI] [PubMed] [Google Scholar]
- 33.Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev 2003; 31: 3–7. [DOI] [PubMed] [Google Scholar]
- 34.Wong RMY, Ho WT, Tang N, Tso CY, Ng WKR, Chow SK, et al. A study protocol for a randomized controlled trial evaluating vibration therapy as an intervention for postural training and fall prevention after distal radius fracture in elderly patients. Trials 2020; 21: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Cui C, Chim YN, Yao H, Shi L, Xu J, et al. Vibration and β-hydroxy-β-methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J Cachexia Sarcopenia Muscle 2020; 11: 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung KS, Li CY, Tse YK, Choy TK, Leung PC, Hung VW, et al. Effects of 18-month low-magnitude high-frequency vibration on fall rate and fracture risks in 710 community elderly--a cluster-randomized controlled trial. Osteoporos Int 2014; 25: 1785–1795. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Ramirez DC, van der Leeden M, van der Esch M, Roorda LD, Verschueren S, van Dieën J, et al. Increased knee muscle strength is associated with decreased activity limitations in established knee osteoarthritis: two-year follow-up study in the Amsterdam osteoarthritis cohort. J Rehabil Med 2015; 47: 647–654. [DOI] [PubMed] [Google Scholar]
- 38.Dong Y, Wang W, Zheng J, Chen S, Qiao J, Wang X. Whole body vibration exercise for chronic musculoskeletal pain: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2019; 100: 2167–2178. [DOI] [PubMed] [Google Scholar]
- 39.Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med 2006; 38: 302–308. [DOI] [PubMed] [Google Scholar]
- 40.Munera M, Bertucci W, Duc S, Chiementin X. Transmission of whole body vibration to the lower body in static and dynamic half-squat exercises. Sports Biomech 2016; 15: 409–428. [DOI] [PubMed] [Google Scholar]
- 41.Bosco C, Colli R, Introini E, Cardinale M, Tsarpela O, Madella A, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol 1999; 19: 183–187. [DOI] [PubMed] [Google Scholar]
- 42.Marín PJ, Rhea MR. Effects of vibration training on muscle power: a meta-analysis. J Strength Cond Res 2010; 24: 871–878. [DOI] [PubMed] [Google Scholar]
- 43.Wei N, Pang MYC, Ng SSM, Ng GYF. Optimal frequency/time combination of whole body vibration training for developing physical performance of people with sarcopenia: a randomized controlled trial. Clin Rehabil 2017; 31: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 44.Zafar H, Alghadir A, Anwer S, Al-Eisa E. Therapeutic effects of whole-body vibration training in knee osteoarthritis: a systematic review and meta-analysis. Arch Phys Med Rehabil 2015; 96: 1525–1532. [DOI] [PubMed] [Google Scholar]