Précis:
Irrigating goniectomy with the TrabEx+ device can lower intraocular pressure (IOP) in patients with glaucoma, as a standalone procedure or combined with cataract surgery.
Purpose:
The aim was to describe the efficacy and safety of irrigating goniectomy performed using the TrabEx+ device, either as a standalone procedure or combined with cataract surgery, in eyes with medically treated open-angle glaucoma.
Methods:
A retrospective case series of eyes treated by a single surgeon at a single UK teaching hospital. Data was collected at follow-up visits at 1 week, 3, 6, 12, 18, and 24 months postoperatively. Primary outcomes included IOP and glaucoma medication reduction after surgery. Proportion of eyes achieving >20% IOP reduction, IOP <21 mm Hg, and no reoperation were classified as surgical success.
Results:
Seventy-three consecutive eyes of 64 patients (mean age 68.4±13.7 y) were enrolled. 62% were treated as combined procedures with cataract surgery. Overall, mean IOP decreased from 31.3±7.3 to 20.9±10.4 mm Hg at the latest follow-up (34% reduction) (P<0.001) at the latest follow-up (16.1±10.3 mo) with mean preoperative medications decreased from 2.9±1.2 to 1.9±1.3 (P<0.001). 73% met the definition of success at latest follow-up. Postoperative complications were recorded including hyphaema (17%), uveitis (3%), hypotony (1%), and persistent vitreous hemorrhage (1%). Eighteen percent required reoperation because of treatment failure.
Conclusion:
TrabEx+ appears to be effective in lowering IOP and medication with or without cataract surgery. However, long-term safety and efficacy will be better understood in a prospective study with longer follow-up.
Key Words: glaucoma, minimally invasive glaucoma surgery, TrabEx, Ab interno trabeculectomy, irrigating goniectomy
Primary open-angle glaucoma and many of the secondary glaucomas are characterized by increased resistance to aqueous humor outflow with a corresponding pressure gradient, primarily across the trabecular meshwork (TM).1–3 Surgical techniques to remove or bypass the TM can thereby lower intraocular pressure (IOP) and reduce the risk of progressive glaucomatous optic neuropathy. A range of ab interno trabeculectomy procedures are available and are part of the minimally invasive glaucoma surgery (MIGS) category.
Ab interno trabeculectomy surgeries use varying methods for removal of TM and for maintaining the anterior chamber (AC). TrabEx+ (MicroSurgical Technology, Redmond, WA) is a serrated trapezoidal dual-bladed single-use device, designed to perform irrigating goniectomy through a corneal microincision, aiming to remove 3 to 6 clock hours of TM. It has irrigation and aspiration (IA) ports built into the handpiece, which attach to standard phacoemulsification machines, giving the benefit of stabilizing the AC, without the need of ocular viscoelastic devices. TrabEx+ is CE marked, allowing use in Europe, but it has not received FDA approval for the indications discussed in this case series.
TrabEx+ bears similarities to the Kahook Dual Blade (KDB) (New World Medical, Rancho Cucamonga, CA) as both remove TM by cutting, though the blade designs differ and TrabEx+ includes IA in the handpiece. There are also similarities with Trabectome (MicroSurgical Technology), as both utilize IA, but Trabectome ablates TM with a bipolar current. These three devices are distinct from other techniques used to incise or rupture TM, rather than removing it. These other techniques include goniotomy, gonioscopy-assisted transluminal trabeculotomy and the Microhook ab interno trabeculotomy (Inami & Co Ltd, Tokyo, Japan).
The purpose of our study was to report efficacy and safety outcomes of the first cohort of patients treated with TrabEx+ in a single glaucoma tertiary referral center.
METHODS
Study Design
This was a retrospective case series of consecutive patients treated by a single surgeon (G.A.) at the Royal Hallamshire NHS teaching hospital in Sheffield, UK between March 9, 2018 and May 1, 2021. The study was approved by the Clinical Effectiveness Unit in our institution and adhered to the tenets of the Declaration of Helsinki.
Patients
All eyes who received TrabEx+ surgery and had a follow-up duration of at least 3 months were included. Patients were selected for this procedure by the clinician according to the following principles: patients medically treated for open-angle glaucoma, patients with uncontrolled IOP >21 mm Hg, open angles on gonioscopy examination, pseudophakic status or having clinically significant cataract for combined surgery, and ability to give consent for the procedure. Patients who, in the judgment of the clinician, had indications for traditional filtration surgery or trans-scleral cyclodiode laser were not offered TrabEx+ surgery. These included evidence of progressive visual field loss, a poor visual prognosis, abnormal angle structures, or neovascular glaucoma.
Surgical Method
Eyes were treated either with the TrabEx+ surgery as a standalone procedure, or with the TrabEx+ surgery combined with cataract surgery. The surgeon had prior experience of angle surgery with Trabectome and iStent (Glaukos, San Clemente, CA). However, this series represents the first series of eyes they treated with the TrabEx+.
The following surgical steps were performed:
The surgery was performed through a 1.8 mm clear corneal incision temporally.
The angle was visualized with a Swan-Jacob gonioprism by turning the patients head away by ∼30 degrees and the operating microscope through 35 degrees.
IOP was lowered to visualize Schlemm’s canal.
3 to 6 clock hours of goniectomy performed under direct visualization with reflux from collector channels observed (Fig. 1).
Corneal sections were hydrated, IOP increased and intracameral cefuroxime given.
FIGURE 1.
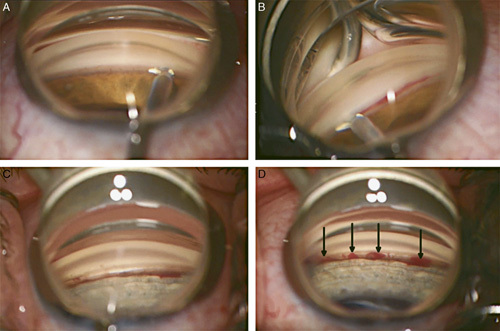
Intraoperative photographs of drainage angle during TrabEx+ surgery. A, Insertion of the device blade into the trabecular meshwork. B, Incision of trabecular meshwork with blood visible in the canal of Schlemm. C, Pale outer wall of the canal of Schlemm visible in treated area. D, Black arrows indicate 4 areas of refluxing heme from collector channels.
If performed as a combined surgery, phacoemulsification and insertion of a 1-piece intraocular lens in the bag was completed either before or after the TrabEx+ procedure according to surgeon’s preference. GA preferred performing phacoemulsification first thereby widening the iridocorneal angle, with improved visualization of TM for the TrabEx+ procedure.
Postoperative Care
All patients received pilocarpine 2% and dexamethasone 0.1% eye drops, both 4 times daily for 4 weeks postoperatively, and levofloxacin 0.5% eye drops 4 times daily for 1 week. Patients continued on their preoperative glaucoma medication regimen, unless they were on oral acetazolamide (which was stopped) or the purpose of surgery was to stop topical treatment because of ocular surface disease. If drops were reduced after treatment, it was by clinician decision, but this was not the treatment goal in the majority of cases.
Statistical Analysis
Data were retrieved from Medisoft (Medisoft Limited, United Kingdom) electronic patient records.
Descriptive data were summarized. Categorical and parametric data were analyzed by χ2 test and the Student t test, respectively. Means were compared within the group with the paired t test and between groups with the 2-sample t test. Kaplan-Meier survival estimate was performed for eyes that failed the success criteria. Data analysis was performed in SPSS (IBM, New York).
Outcome Measures
Outcomes were reported as: mean IOP at 1 week, 3, 6, 12, 18, and 24 months, and latest follow-up, mean change in the number of IOP lowering medications at latest follow-up, and proportion of eyes achieving 30% IOP lowering on the same or fewer IOP lowering agents.
In line with previous studies,4 the success outcome was reported when the following criteria were met: achieving 20% IOP lowering, and IOP of 21 mm Hg or less at latest follow-up, without the need for reoperation, whilst on an equal or fewer number of IOP lowering medications.
To report safety, eyes with intraoperative and postoperative complications, those requiring further glaucoma surgery, and eyes with a loss of 2 or more lines of vision were accounted for.
For eyes that had subsequent glaucoma surgery, their failing IOP were included at the failing timepoint, and in the latest IOP for statistical analysis. IOP measurements at subsequent timepoints after further surgery were excluded.
IOP was measured by the Reichert ocular response analyzer 7CR (Reichert Technologies, New York) using the Goldmann approximation measure. In cases where reliability index for the ocular response analyzer was poor, Goldmann applanation tonometry was used.
RESULTS
A total of 73 eyes of 64 patients (mean age 68.4±13.7 y, range: 31 to 90 y, 59% females) were treated with TrabEx+. 62% and 38% of the eyes were treated as combined procedures with cataract surgery and standalone, respectively (Table 1). The patient ethnicity consisted of predominately white (81%) followed by Afro-Caribbean (6%), Asian (5%), and unknown (8%). The mean latest follow-up was 16.1±10.3 (range: 3 to 38) months. 59% and 41% of the eyes were diagnosed with either primary open-angle glaucoma or ocular hypertension and secondary glaucoma respectively (Table 2). Secondary etiologies include uveitic (57%), steroid induced (23%), pigmentary (13%), and pseudoexfoliative (7%). 23% had follow-up of <6 months at time of data collection, and 10% were lost to follow-up, but were included for real world evaluation.
TABLE 1.
Demographics and Characteristics of Both Groups: TrabEx+ Standalone and Combined TrabEx+ Procedure With Cataract Surgery
| Phaco and TrabEx+(n=45) | TrabEx+ Standalone (n=28) | P | |
|---|---|---|---|
| Age, years | 70.4±11.2 | 65.4±16.8 | 0.2* |
| Sex, female (%) | 22 (49) | 16 (57) | 0.5† |
| Left eye (%) | 22 (49) | 12 (43) | 0.6† |
| Secondary glaucoma (%) | 15 (33) | 15 (54) | 0.09† |
| Latest follow-up, months | 16.0±9.9 | 16.4±11.0 | 0.9* |
| BCVA gain, Snellen lines | 1.2±2.2 | −0.6±2.4 | <0.001‡ |
| Preoperative IOP, mm Hg | 30.4±7.8 | 32.9±6.1 | 0.15* |
| Latest IOP, mm Hg | 18.3±7.1 | 25.0±13.2 | 0.02* |
| Pre op IOP medication | 2.8±1.2 | 3.0±1.1 | 0.5‡ |
| Latest IOP medication | 1.8±1.3 | 2.0±1.4 | 0.5‡ |
Independent Samples t test.
χ2 test.
Mann-Whitney U test.
BCVA indicates best corrected visual acuity; IOP, intraocular pressure.
TABLE 2.
Preoperative and Postoperative IOP and Medications in Primary and Secondary Causes of Glaucoma or Ocular Hypertension Treated With TrabEx+ Procedures With or Without Cataract Surgery
| Primary Glaucoma/OHT (n=43) | Secondary Glaucoma/OHT (n=30) | P | |
|---|---|---|---|
| Preoperative IOP, mm Hg | 28.9±5.9 | 34.9±7.7 | <0.001* |
| Latest IOP, mm Hg | 21.0±9.7 | 20.7±11.5 | 0.9* |
| IOP reduction, mm Hg | −12.0±10.7 | −20.4±13.1 | <0.01* |
| Preoperative IOP medications | 2.6±1.1 | 3.3±1.1 | 0.01† |
| Latest medications | 1.8±1.5 | 2.0±1.0 | 0.6† |
| Medication reduction | −0.8±1.3 | −1.3±1.6 | 0.1† |
| Success (%) | 72 | 73 | 0.9‡ |
Independent samples t test.
Mann-Whitney U test.
χ2 test.
IOP indicates intraocular pressure; OHT, ocular hypertension.
IOP
IOPs were lowered significantly from 31.3±7.3 mm Hg at baseline to 20.9±10.4 mm Hg at the latest follow-up (P<0.001). The mean IOP reduction at each follow-up compared with the preoperative baseline was significant (P<0.001) (Table 3). IOP lowering at 1 week after the surgery, was maintained to latest follow-up (P>0.05).
TABLE 3.
Statistical Difference Was Demonstrated When Comparing Mean IOP Reduction at Each Follow-up Timepoint
| IOP Reduction, mm Hg (mean±SD) | ||||
|---|---|---|---|---|
| Comparing With Preoperative Baseline IOP | TrabEx+ Standalone | P * | Phaco and TrabEx+ | P * |
| 1 wk | −13.4±13.4 | <0.001 | −10.4±14.9 | <0.001 |
| 3 mo | −9.1±13.1 | 0.002 | −13.9±8.6 | <0.001 |
| 6 mo | −13.0±9.0 | <0.001 | −13.4±9.8 | <0.001 |
| 12 mo | −11.7±7.6 | <0.001 | −14.8±10.5 | <0.001 |
| 18 mo | −13.7±8.7 | <0.001 | −13.0±10.8 | <0.001 |
| 24 mo | −12.2±10.3 | 0.012 | −11.7±7.9 | 0.002 |
| Latest | −7.9±13.4 | 0.004 | −12.1±9.8 | <0.001 |
Paired-samples t test.
IOP indicates intraocular pressure.
Figure 2 illustrates the mean IOPs at 1 week, 3, 6, 12, 18, and 24 months for cases divided by combined and standalone treatments. Mean IOP reduction in standalone TrabEx+ and combined procedure with cataract surgery was 7.9±13.4 and 12.1±11.2 mm Hg, respectively (P=0.156). Mean IOP at latest follow-up in the standalone group was 25.0±13.2 versus 18.3±7.1 mm Hg (P<0.05) in the combined procedures. The standalone group had a higher baseline IOP of 32.9±6.1 versus 30.4±7.8 mm Hg.
FIGURE 2.
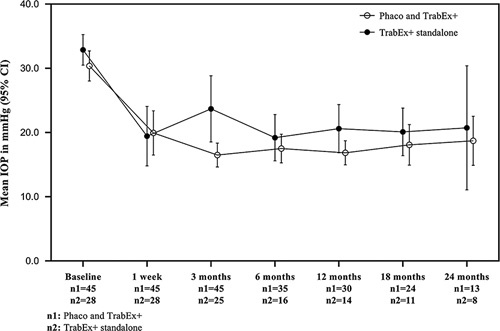
Mean intraocular pressures (IOPs) at 1 week, 3, 6, 12, 18, and 24 months for TrabEx+ standalone and combined TrabEx+ procedure with cataract surgery. CI indicates confidence interval.
Overall, 73% of the eyes achieved an IOP reduction of >20%, IOP <21 mm Hg, and no reoperation or increased medical treatment at latest follow-up. Figure 3 shows a Kaplan-Meier survival estimates graph illustrating the time to failure for the 20 cases which did not meet the above success criteria. The calculation predicts a 54% success rate at 25 months.
FIGURE 3.
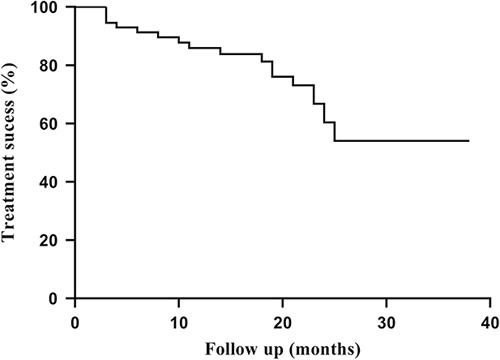
Kaplan-Meier survival estimates graph for treatment “success” at latest follow-up (intraocular pressure <21 mm Hg; intraocular pressure lowering >20%; no reoperation).
Among the 20 eyes that did not meet the above success criteria, 13 had further surgical interventions: 10 had augmented trabeculectomy, 1 Baerveldt tube insertion (Johnson and Johnson Vision, Santa Ana, CA), 1 combined Baerveldt tube insertion with pars plana vitrectomy and AC washout (for persistent hyphaema and vitreous hemorrhage), and 1 cyclodialysis cleft repair for hypotony with IOP of 1 mm Hg and flat AC in the early postoperative period. The mean onset of treatment failure was at 7.8±9.0 months with the range of 1 week to 25 months. One patient had further cyclodiode laser because of uncontrolled IOP of 52 mm Hg and a poor visual prognosis. Four patients had IOP >21 mm Hg (mean IOP 25.2±2.2 mm Hg) at the latest follow-up, but were judged clinically “successful,” as escalation of treatment was not required, but they did not meet the above definition. One patient was treated for fungal endophthalmitis following candidaemia. This occurred 6 months after combined TrabEx+ and cataract surgery, but was diagnosed as endogenous rather than surgical in source. This patient required escalation of medication to lower IOP after the endophthalmitis.
Medications
Mean preoperative IOP lowering medications were 2.9±1.2 (range: 1 to 5), reducing to 1.9±1.3 (range: 0 to 4) postoperatively (P<0.001). Four patients were on oral acetazolamide for longer than 2 weeks before the procedure (2 standalone and 2 combined procedures), which was stopped postoperatively in all cases. Medication numbers were reduced by 1.0±1.5 and 1.0±1.42 in TrabEx+ standalone and combined procedure with cataract surgery respectively (P<0.001 when compared with baseline).
Excluding those who had further glaucoma surgery (n=12), 48% reduced the number of medications and 16% stopped drops altogether and were medication free. 62% and 72% of the eyes achieved IOP reduction of 30% or more and 20% or more on the same or fewer medical agents at the latest follow-up, respectively. 72% and 43% of the eyes achieved an IOP of <21 mm Hg and IOP <16 mm Hg at the latest follow-up, respectively.
Safety Outcomes
Of 73 eyes, there was 1 intraoperative complication in an eye with uveitic glaucoma. Hemorrhage in the iridocorneal angle resulted in inadequate goniectomy, postoperative microscopic hyphaema, and anterior segment inflammation with uncontrolled IOP. This patient required trabeculectomy 1 month after TrabEx+ standalone procedure.
There were 16 postoperative complications (21.9%). Twelve eyes had postoperative hyphaema, of which 4 eyes had an IOP spike >10 mm Hg at first postoperative visit. All resolved rapidly with medical management only and achieved treatment success. One eye with hyphaema needed AC washout, and then pars plana vitrectomy with Baerveldt tube insertion for nonclearing vitreous hemorrhage and treatment failure. The IOP was controlled (21 mm Hg) at 12-month follow-up, with a visual acuity gain of 2 Snellen lines (6/9). There was 1 case with early postoperative hypotony because of cyclodialysis cleft that required urgent surgical repair 1 week after the initial procedure. The hypotony was successfully resolved with IOP increased. Medical management was ongoing with a best corrected visual acuity (BCVA) of 6/36 (loss of 3 Snellen lines) at 3 months follow-up. Postoperative uveitis occurred in 2 eyes (11% of uveitic glaucoma eyes). One case was managed medically with good outcome; the other had treatment failure and required Baerveldt tube, in which, subsequent postoperative IOP showed numeric hypotony of 4mm Hg without clinical manifestations and BCVA was stable at 6/48 (gain of 1 Snellen line). One case of postoperative cystoid macular edema was noted in a combined procedure with cataract surgery who recovered uneventfully with anti-inflammatory topical agents (BCVA of 6/9).
Mean BCVA change was −0.6±2.4 Snellen lines in the standalone group, comparing to a gain of 1.2±2.2 Snellen lines in the group combined with cataract surgery (P<0.001).
Visual acuity was worse in 4 eyes (6%) compared with baseline. One patient has already been described with hypotony because of cyclodialysis cleft. One patient with uveitic glaucoma developed postoperative uveitis, with a loss of 2 Snellen lines at the latest visit, but a controlled IOP (14.7 mm Hg). One patient had lost 4 Snellen lines at the latest follow-up because of concurrent age-related macular degeneration with choroidal neovascular membrane, necessitating treatment with intravitreal anti-VEGF. The choroidal neovascular membrane preceded treatment with TrabEx+. Furthermore, the previously described eye with endogenous endophthalmitis at 6 months postphacoemulsification and TrabEx+ lost 5 Snellen lines to BCVA of 6/60. This was not caused by the procedure.
Secondary Glaucoma Subgroup Analysis
Comparing patients with primary and secondary causes of glaucoma or ocular hypertension, mean IOP lowering was significantly greater in the eyes with secondary glaucoma group (14.2±13.6 vs. 7.9±8.9 mm Hg, P=0.03) (Table 2). However, higher preoperative baseline IOPs were also recorded in this group. A greater number of IOP lowering medications were used in secondary glaucoma group (3.3±1.1 vs. 2.6±1.1, P=0.01) at baseline, but no difference between the 2 groups was observed postoperatively. The success rate between secondary and primary glaucoma groups were similar.
DISCUSSION
Over the last 2 decades, surgical interventions for IOP lowering, collectively termed “MIGS” (minimally invasive glaucoma surgery or microincision glaucoma surgery) has gained popularity in glaucoma practice. These are a heterogenous group of interventions using both new and old technology. The concept of MIGS is to provide the ophthalmic surgeon with surgery that is safer than current filtration surgeries, but more effective than medical or laser treatments. However, there is only low-quality evidence to support their use in practice,5 unlike trabeculectomy and tube shunt surgery. There is ongoing debate on what role these procedures might have in current and future management strategies. Despite this, MIGS continue to attract considerable interest from glaucoma specialists and investment from medical instruments companies.
To our knowledge, this is the first reported cohort with the TrabEx+ device. Its use in ex vivo porcine eyes (reported under a previous branding of Goniotome) demonstrated the intraocular lowering potential of the device.6
Other devices are marketed to perform a similar procedure, sometimes termed ab interno trabeculectomy, goniectomy or goniotomy. The Trabectome ablates TM by applying bipolar current across the tissue. The KDB shares many similarities to the TrabEx+, though without serrated blades, curved foot plate, or mechanism for IA.
Kaplowitz et al4 performed meta-analysis of Trabectome outcomes, analyzing 14 studies at 2 years follow-up, with 5091 cases. These had a lower overall mean baseline IOP for standalone treatment of 26.7 mm Hg and decreased by 10.5 mm Hg on 1.0 fewer medications. This represented a 39% mean reduction. For combined surgery, the baseline IOP was much lower at 21 mm Hg, decreasing by 6.2 mm Hg (a 27% decrease) on 0.8 fewer medications. The 2-year success rate (defined by 20% IOP lowering, IOP <21 mm Hg and no further surgery) was achieved in 66%. Esfandiari et al7 report 5-year outcomes in 96 eyes from a single center with mean IOP lowering of 4.4 mm Hg and a 67.5% success rate by the above criteria. Bendel and Patterson8 report outcomes through to 8 years with maintenance of IOP lowering in 80% by Kaplan-Meier analysis. No randomized controlled trials for Trabectome have been completed.9,10
Our results achieved similar IOP lowering and success rates as these studies. In the meta-analysis by Kaplowitz et al,4 the preoperative IOP was considerably different between cases undergoing standalone and combined surgery (26.7 vs. 21.0 mm Hg), but still lower than our cohort (32.9 vs. 30.4 mm Hg). A linear regression analysis of Trabectome patients demonstrated greater IOP lowering in patients with higher pretreatment IOP.11 Therefore, it is unsurprising to see that our series achieved greater IOP reductions in either group, but fewer cases achieving the <21 mm Hg threshold of success (54% at 25 mo from Kaplan-Meier survival graphs in our case series vs. 66% at 2 years in Kaplowitz et al4).
To date there are no long-term outcomes for the KDB, but case series with 6 or 12 months follow-up report IOP lowering effects of between 2.1 and 10.3 mm Hg.12–21 Again, preoperative IOPs in our series were higher than these studies, which most likely explained greater absolute IOP lowering in our patients treated with TrabEx+ compared with reported series of KDB. However, other factors likely play a role in greater IOP reductions including the etiology of raised IOP, surgical technique used, extent of goniectomy and combination with cataract surgery.
In addition, these KDB studies reported mean medication reduction between 0.6 and 1.7 medications. Incidence of complications were reported between 3.5% and 20%, mainly reporting postoperative hyphaema and IOP spike, and a single case report of cystoid macular edema.22 Our case series also reported postoperative complications including hypotony because of cyclodialysis cleft, postoperative uveitis and persistent hyphaema with vitreous hemorrhage requiring combined vitrectomy and glaucoma tube surgery. Though the majority of cases had significant IOP lowering at first postoperative visit, hyphaema may be accompanied by an IOP spike in a small proportion. Therefore, patients who would not be able to tolerate transient IOP rises may not be good candidates for this procedure.
As with other forms of ab interno trabeculectomy or goniectomy, TrabEx+ spares the conjunctiva. This means the possibility of future bleb forming filtration surgery is not excluded. The dual-bladed device also allows in vivo samples of TM to be excised in a nondestructive manner, which may be of use in research applications.23
Interestingly, our study showed that the IOP reduction in the combined procedure group was greater than standalone TrabEx+ though not statistically significant. The effect of cataract surgery alone on IOP lowering should not be overlooked. It can lower IOP by 1.5 to 3 mm Hg and a greater IOP reduction occurs in eyes with greater baseline preoperative IOP.24,25 In contrast, Parikh et al reported cataract surgery not making a significant contribution to IOP reduction when combined with Trabectome.26
This study added to the growing literature supporting TM procedures in glaucoma management. In many ways it reflects a real world cohort and the learning curve of a single surgeon, reporting all results from their first case. However, significant limitations should be noted, which include a retrospective study design, lack of control group, and no rigorous process for case selection. Causes for lost to follow-up was multifactorial; as our institution was a tertiary referral center for surgical glaucoma management, some patients were followed up locally at their request for convenience. This occurred after early postoperative review (3 to 6 mo) in our center was completed. Subsequent IOPs in this group were difficult to obtain. Also, the study period spanned the COVID-19 pandemic, and many elective ophthalmic services were halted, leading to delayed and missed appointments for some patients.27 Furthermore, it is worth considering cataract surgery as a notable confounding factor because of its effect on IOP.
In conclusion, the early results reported in this study were promising, and demonstrated that irrigating goniectomy with the novel TrabEx+ device in combination with, or without cataract surgery, effectively decreased IOP and glaucoma medications in the postoperative period between 3 months to 2 years. This study added to the surgical safety profile of goniectemy surgery. Long-term effects on IOP and medication from TrabEx+ will be better understood with longer follow-up. A further prospective, preferably randomized study would be required to confirm these preliminary findings and determine long-term safety and efficacy of TrabEx+ both as a standalone procedure and combined with cataract surgery.
Footnotes
D.G. and H.W. are joint first authors and equally contributed to the study.
Disclosure: G.A. receives an educational honorarium from MST. The remaining authors declare no conflict of interest.
Contributor Information
Daniel Gosling, Email: danielgosling@nhs.net.
Haoyu Wang, Email: haoyu.wang2@nhs.net.
Graham Auger, Email: graham.auger1@nhs.net.
REFERENCES
- 1. Carreon T, van der Merwe E, Fellman RL, et al. Aqueous outflow—a continuum from trabecular meshwork to episcleral veins. Prog Retin Eye Res. 2017;57:108–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenquist R, Epstein D, Melamed S, et al. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res. 1989;8:1233–1240. [DOI] [PubMed] [Google Scholar]
- 3. Grant WM. Further studies on facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1958;60:523–533. [DOI] [PubMed] [Google Scholar]
- 4. Kaplowitz K, Bussel II, Honkanen R, et al. Review and meta-analysis of ab-interno trabeculectomy outcomes. Br J Ophthalmol. 2016;100:594–600. [DOI] [PubMed] [Google Scholar]
- 5. Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12:e0183142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang C, Dang Y, Shah P, et al. Intraocular pressure reduction in a pigmentary glaucoma model by Goniotome Ab interno trabeculectomy. PLoS One. 2020;15:e0231360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esfandiari H, Shah P, Torkian P, et al. Five-year clinical outcomes of combined phacoemulsification and trabectome surgery at a single glaucoma center. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bendel RE, Patterson MT. Long-term effectiveness of Trabectome (ab-interno trabeculectomy) surgery. J Curr Glaucoma Pract. 2018;12:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu K, Gazzard G, Bunce C, et al. Ab interno trabecular bypass surgery with Trabectome for open angle glaucoma. Cochrane Database Syst Rev. 2016;8:CD011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ting JLM, Rudnisky CJ, Damji KF. Prospective randomized controlled trial of phaco-trabectome versus phaco-trabeculectomy in patients with open angle glaucoma. Can J Ophthalmol. 2018;53:588–594. [DOI] [PubMed] [Google Scholar]
- 11. Neiweem AE, Bussel II, Schuman JS, et al. Glaucoma surgery calculator: Limited additive effect of phacoemulsification on intraocular pressure in ab interno trabeculectomy. PLoS One. 2016;11:e0153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berdahl JP, Gallardo MJ, ElMallah MK, et al. Six-month outcomes of goniotomy performed with the Kahook Dual blade as a stand-alone glaucoma procedure. Adv Ther. 2018;35:2093–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-month outcomes of goniotomy performed using the Kahook Dual blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther. 2018;35:1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ElMallah MK, Seibold LK, Kahook MY, et al. 12-month retrospective comparison of Kahook dual blade excisional goniotomy with istent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv Ther. 2019;36:2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falkenberry S, Singh IP, Crane CJ, et al. Excisional goniotomy vs trabecular microbypass stent implantation: a prospective randomized clinical trial in eyes with mild to moderate open-angle glaucoma. J Cataract Refract Surg. 2020;46:1165–1171. [DOI] [PubMed] [Google Scholar]
- 16. Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: short-term results. J Cataract Refract Surg. 2017;43:1197–1201. [DOI] [PubMed] [Google Scholar]
- 17. Hirabayashi MT, King JT, An JA. Outcome of phacoemulsification combined with excisional goniotomy using the Kahook Dual blade in severe glaucoma patients at 6 months. Clin Ophthalmol. 2019;13:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kornmann HL, Fellman RL, Feuer WJ, et al. Early results of goniotomy with the Kahook dual blade, a novel device for the treatment of glaucoma. Clin Ophthalmol. 2019;13:2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le C, Kazaryan S, Hubbell M, et al. Surgical outcomes of phacoemulsification followed by istent implantation versus goniotomy with the Kahook dual blade in patients with mild primary open-angle glaucoma with a minimum of 12-month follow-up. J Glaucoma. 2019;28:411–414. [DOI] [PubMed] [Google Scholar]
- 20. Salinas L, Chaudhary A, Berdahl JP, et al. Goniotomy using the kahook dual blade in severe and refractory glaucoma: 6-month outcomes. J Glaucoma. 2018;27:849–855. [DOI] [PubMed] [Google Scholar]
- 21. Wakil SM, Birnbaum F, Vu DM, et al. Efficacy and safety of Kahook Dual blade goniotomy: 18-month results. J Cataract Refract Surg. 2020;46:1165–1171. [DOI] [PubMed] [Google Scholar]
- 22. Arnljots TS, Economou MA. Reversible cystoid macular edema following uneventful microinvasive Kahook dual blade goniotomy in a pseudophakic patient: a case report. J Glaucoma. 2018;27:e128–e130. [DOI] [PubMed] [Google Scholar]
- 23. Ramjiani V, Mudhar HS, Julian T, et al. Sampling trabecular meshwork using TrabEx. BMC Ophthalmol. 2021;21:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mansberger SL, Gordon MO, Jampel H, et al. Ocular Hypertension Treatment Study Group. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology. 2012;119:1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. Am J Ophthalmol. 2013;156:89–94. [DOI] [PubMed] [Google Scholar]
- 26. Parikh HA, Bussel II, Schuman JS, et al. Coarsened exact matching of phaco-trabectome to trabectome in phakic patients: lack of additional pressure reduction from phacoemulsification. PLoS One. 2016;11:e0149384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wickham L, Hay G, Hamilton R, et al. The impact of COVID policies on acute ophthalmology services—experiences from Moorfields Eye Hospital NHS Foundation Trust. Eye. 2020;34:1189–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]


