Abstract
Glutathione S-transferase P (GSTP) is a component of a complex series of pathways that provide cellular redox homeostasis. It is an abundant protein in certain tumors and is over-expressed in cancer drug resistance. It has diverse cellular functions that include, thiolase activities with small electrophilic agents or susceptible cysteine residues on the protein to mediate S-glutathionylation, and chaperone binding with select protein kinases. Preclinical and clinical testing of a nanomolar inhibitor of GSTP, TLK199 (Telintra; Ezatiostat) has indicated a role for the enzyme in hematopoiesis and utility for the drug in the treatment of patients with myelodysplastic syndrome.
Keywords: c-Jun N-terminal kinases, Clinical trials, Cyclophosphamide, Drug resistance, Ezatiostat, Glutathione, Glutathione S-transferase, Glutathionylation, Hematopoiesis, Lung diseases, Myelodysplastic syndromes, Myeloproliferation, Oxidative stress, Telintra
Graphical Abstract
1. Historical Perspectives
In the 1980s and 1990s, there was a great deal of emphasis on understanding those mechanistic aspects of cancer cell drug resistance that characteristically caused failure of chemotherapeutic response and patient relapse. Numerous conferences and books were dedicated to the subject, and while the term multidrug resistance (MDR) was primarily judged to be synonymous with overexpression of the P-glycoprotein (now called ABCB1), there were a few investigators with interests in glutathione pathways and their impact on cancer drug resistance. Since drug combination therapies were de rigueur for clinical treatments, much interest was focused on modulating existing cancer drugs with non-toxic agents that might serve to reverse resistance or, at worst, enhance a therapeutic index. Out of this discipline came the principle that resistance that resulted as a consequence of overexpression of various thiol-based enzyme pathways might be combatted through concomitant inhibition of certain redox-active enzymes. For example, increased levels of intracellular glutathione (GSH) were combatted by treatment with buthionine sulfoximine (BSO) (Griffith and Meister 1979), an inhibitor of γ-glutamylcysteine synthetase (now called glutamate-cysteine ligase), the rate-limiting enzyme in de novo GSH synthesis. Clinical trials with BSO proved to be of limited value (Bailey et al. 1994; O’Dwyer et al. 1992), but were a forerunner of efforts to modulate various GSH pathways linked with drug resistance. The early observation that GST isozymes were overexpressed in drug-resistant cells (Wang and Tew 1985) created an opportunity for a similar modulatory approach. While ethacrynic acid (EA) was first tried, the clinical toxicity associated with its diuretic properties limited its utility (O’Dwyer et al. 1991). Nevertheless, around this time, under the leadership of Dr. Larry Kauvar, Terrapin Technologies of South San Francisco was developing patented technologies that focused on the development of GSH analogs and paralog panels comprising GSH mimics. From this work, TLK199 was identified and became the lead agent that progressed through preclinical studies to eventual clinical testing and is the focus of the remainder of this chapter. Starting life as TER199 (reflecting the original name of Terrapin Technologies), preclinical studies are described in this chapter under the TLK199 moniker. As the drug moved through preclinical development, the name was changed to Telintra and finally Ezatiostat. In each case the essentially chemical entity of the drug is the same, although GLP/GMP manufacturing altered formulation.
2. Why Target GSTP?
When GST isozymes were first described (Boyland and Chasseaud 1969), most early publications detailed their catalytic functions in catalyzing the thioether conjugation of small molecule agents with GSH, usually accommodating a diverse range of electrophilic substrates. For GSTP, these reactions have proved to be quite restricted, and in subsequent decades, more and different biological functions have been ascribed to this GST isozyme family. These additional functions take on direct relevance to understanding why a GSTP inhibitor such as Telintra has translatable utility and has been tested in early stage clinical trials. The fact that GSTP is so highly expressed in certain solid tumors and in cancer cells that have acquired resistance to various anticancer drugs provided the initial rationale for inhibiting GSTP (Tew 1994). In principle, modulating GSTP in these setting could carry therapeutic benefit. Since these studies, the disparate roles of GSTP in cellular growth and stress response pathways have broadened the interest base for this GST isozyme. In particular, its role in regulating stress response pathways of various kinds and its catalytic functions in facilitating the forward reaction of the S-glutathionylation cycle (Townsend et al. 2009) have added to the potential importance of inhibiting such pathways. The following sections detail much of the material.
3. Stress Signaling Pathways
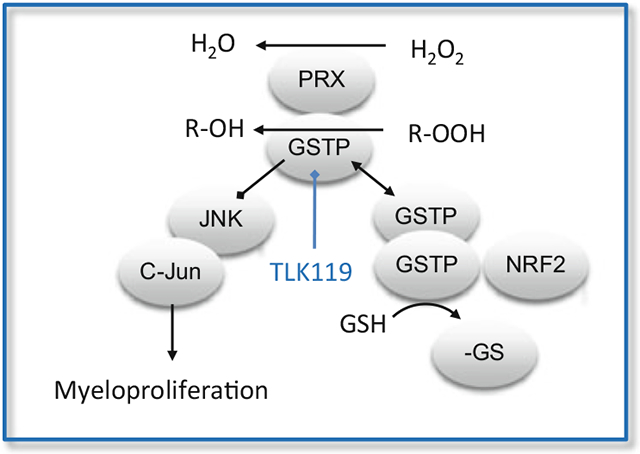
c-Jun N-terminal kinases (JNKs) are a family of stress kinases subject to transient activation in response to a variety of cellular stresses, including reactive oxygen or nitrogen species, heat shock, and perhaps of particular interest in terms of myeloproliferation, growth factors, or inflammatory cytokines (Davis 2000). JNK-mediated phosphorylation of the transcription factors c-Jun and activating transcription factor 2 (ATF2) can effectively facilitate the stress response. Because there would be few advantages in cells existing in a constant stressed condition, by necessity, basal JNK activity is maintained at a low level, and it is at this nexus that GSTP can act as an endogenous negative regulatory switch for the kinase. GSTP has ligand-binding properties that manifest as protein complexes where JNK activity can be regulated through a series of protein:protein interactions. In unstressed cells, low JNK activity is maintained through sequestration of the kinase in a multiprotein complex that includes GSTP-JNK. In this regard, treatment with Telintra has been shown to cause GSTP to dissociate from this complex, accumulating GSTP oligomers, with resultant activation of JNK impacting downstream events as divergent as proliferation or apoptosis (Yin et al. 2000). JNK-dependent stress-induced apoptosis may be suppressed during tumor development, and in this regard, the high levels of GSTP found in many solid tumors, or in drug-resistant cells, may act to sequester JNK in an inactive state, perhaps explaining why elevated GSTP levels can be found even when the selecting drug is not a substrate for GSH conjugation (Tew 1994). Recent studies confirm that for GSTP, binding to other protein partners is quite common. While these events might initially appear promiscuous, they are likely driven by the propensity for GSTP to act as a protein thiolase (see later discussion on S-glutathionylation). However, for the kinase regulatory effects, homology between GSTA and P family members may explain why GSTA1 by a similar mechanism can also suppress JNK signaling caused by inflammatory cytokines or ROS (Romero et al. 2006). Moreover, GSTP also regulates tumor necrosis factor alpha (TNFα) signaling through a protein ligand interaction with tumor necrosis factor receptor-associated factor 2 (TRAF2; Wu et al. 2006).
GSTP inhibits TRAF2-induced activation of both JNK and p38 (but not NFkB) attenuates TRAF2-enhanced apoptosis signal-regulating kinase 1 (ASK1) autophosphorylation and inhibits TRAF2-ASK1-induced apoptosis by suppressing the interaction of these two proteins. When GSTP interacts, its catalytic activity is unaffected, implicating sites distant to those involved in GSH or substrate binding. This would be in agreement with the principle that the interaction occurs in the first place as a conduit to the thiolase activity, i.e., that GSTP interacts as a prelude to S-glutathionylating the adjacent protein. A further example of functional redundancy within the GST family is afforded by the fact that GSTM1 binds to, and inhibits, the activity of ASK1 (Cho et al. 2001). Similar to GSTP:JNK, the interaction of the GSTM1:ASK1 complex is dissociated under stress conditions, leading to GSTM oligomerization and subsequent activation of ASK1 (Dorion et al. 2002). Because ASK1 can activate the JNK and p38 pathways, this disassociation could also serve to activate cytokine- and stress-induced apoptosis (Ichijo et al. 1997). A general conclusion from all these studies is that GST isozymes are not acting in a detoxification fashion, rather they serve to augment intermediary kinase regulation. In this regard, an inhibitor such as Telintra can potentially impact these stress kinase pathways through interference with this regulation.
4. GSTP and S-Glutathionylation
In previous reviews, we have drawn parallels between the processes of phosphorylation and S-glutathionylation (Ye et al. 2017). Each exact critical regulatory control functions on target proteins susceptible to these post-translational modifications. However, S-glutathionylation of either kinases or phosphatases (7) can be critical in maintaining the cyclical nature of phosphorylation/dephosphorylation and demonstrates the layered nature of how sulfur-based post-translational modifications may actually supersede those of phosphorus. S-glutathionylation generally occurs on cysteines in basic environments within the protein (e.g., vicinal to Arg, His, or Lys residues). GSTP can lower the pKa of the cysteine thiol of GSH, producing a nucleophilic thiolate anion (Graminski et al. 1989). Under the right conditions, cysteines on the surfaces of proteins may undergo spontaneous S-glutathionylation (Ghezzi 2005); nevertheless GSTP can influence the rate and extent of the process in a catalytic manner. In this regard, Telintra has been shown to interfere with S-glutathionylation, with subsequent influence on the structure and function of a variety of target proteins (McMillan et al. 2016; Jones et al. 2016).
Relative to the proteome, the number of S-glutathionylated proteins is not proportionally large, and those can be categorized into functional clusters. These include enzymes with catalytically important cysteines especially those involved with protein folding/stability, nitric oxide regulation, and redox homeostasis; cytoskeletal; signaling – particularly kinases and phosphatases; transcription factors; ras proteins; heat shock proteins; ion channels, calcium homeostasis; and energy metabolism and glycolysis. Under stress conditions, the half-life of S-glutathionylation approximates 4 h (Townsend et al. 2009), although this value is contingent upon both stress-induced conditions and cell type. However, relevant to the utility of Telintra, there are instances (as described in this chapter) where interference with S-glutathionylation can have a plausible therapeutic effect. In this regard, the enhanced myeloproliferative phenotype of the GSTP knockout mouse (Gate et al. 2004), together with the other indications where pharmacological inhibition of GSTP influences bone marrow proliferation and migration (Zhang et al. 2014), dictates that S-glutathionylation is an important factor in regulating myeloproliferation in the bone marrow. In this regard, the next two sections detail how preclinical studies with Telintra have created opportunities for its use in either myeloproliferative or lung diseases.
5. Modulation of Drug Resistance
Elevated levels of GSTs, especially GSTP1-1, are often associated with an increased resistance of tumors to a variety of anticancer drugs (Tew 1994; Tew et al. 1997; Townsend and Tew 2003). Potentiation of the cytotoxicity by GST inhibitors, e.g., ethacrynic acid, has been observed both in vitro and in vivo (O’Dwyer et al. 1991; Petrini et al. 1993; Tew et al. 1988). EA inhibits GSTs by binding to the H-site (substrate-binding site) of the isozyme, as well as by depleting its cofactor, GSH, via covalent binding (Michael addition), with Ki (μM) of 4.6–6.0, 0.3–1.9, and 3.3–4.8 for GSTA1-1, GSTM1-1, and GSTP1-1, respectively (Ploemen et al. 1993). EA was shown to potentiate the toxicity of chlorambucil in several cancer cell lines (Tew et al. 1988) and increase the sensitivity of melphalan in xenograft models in SCID mice. The therapeutic value of EA as a chemosensitizer has also been reported in patients (O’Dwyer et al. 1991; Petrini et al. 1993). However, EA is not GST isozyme specific (Ploemen et al. 1993) and causes extreme diuresis (O’Dwyer et al. 1991), a side effect that proved to be an important dose-limiting toxicity, making it less suitable for clinical modulation. Partly as a consequence, further attempts at selective inhibition of GST isozymes focused on synthesis of a number of GSH (γ-Glu-Cys-Gly) analogs (Flatgaard et al. 1993; Lyttle et al. 1994). The GSH analogs were designed and synthesized based on the observations that the γ-glutamyl residue of GSH was absolutely critical for binding (Adang et al. 1990), whereas substituting the C-terminal glycine of GSH and functionalizing the sulfur of the cysteine residue of GSH with different alkyl and aryl groups only affected the potency and selectivity of the GSH analogs as GST inhibitors (Adang et al. 1990; Askelof et al. 1975). Among those GSH analogs, TLK117 (γ-glutamyl-S-(benzyl)cysteinyl-R(–)-phenylglycine), which contains substituents at both the glycine α-carbon and cysteine thiol group, was produced as a specific GSTP1-1 inhibitor (Ki = 0.4 μM). Its binding affinity to the G-site of GSTP1-1 is greater than that of GSH, and its selectivity for GSTP1-1 is over 50-fold higher compared with GSTA1-1, GSTM1-1, and GSTM2-2 (Flatgaard et al. 1993; Lyttle et al. 1994). The high-resolution (2.0 Å) crystal structure of GSTP1-1 in complex with TLK117 provides an explanation as to why this compound inhibits the pi-class GST much better than the other GST classes. The phenyl moiety of TLK117 is stacked against the benzyl moiety and interacts with Phe8 and Trp38 in a lipophilic region of GSTP1-1. However, in the case of GSTA1-1, the phenyl substitution would clash with Phe220 and Phe222, while in the case of GSTM1-1, it would clash with Trp7, Met34, and Arg42 (Oakley et al. 1997). TLK117 was designed for efficient inhibition of the most abundant allelic variant GSTP1*A (Ile105, Ala114), but it also competitively inhibits GSTP1*B (Val105, Ala114) with similar potency (Johansson et al. 2000). The inhibitory effects of TLK117 on GSTP1*C (Val105, Val114) and GSTP1*D (Ile105, Ala114) have not been determined. Such considerations are quite relevant since there is evidence that of the four allelic variants of GST, the wild type GSTP*1A has the highest catalytic efficiency for the forward S-glutathionylation reaction (Manevich et al. 2013), and there is significant evidence that racial differences in expression of the polymorphic variants exist (Zhang et al. 2019a).
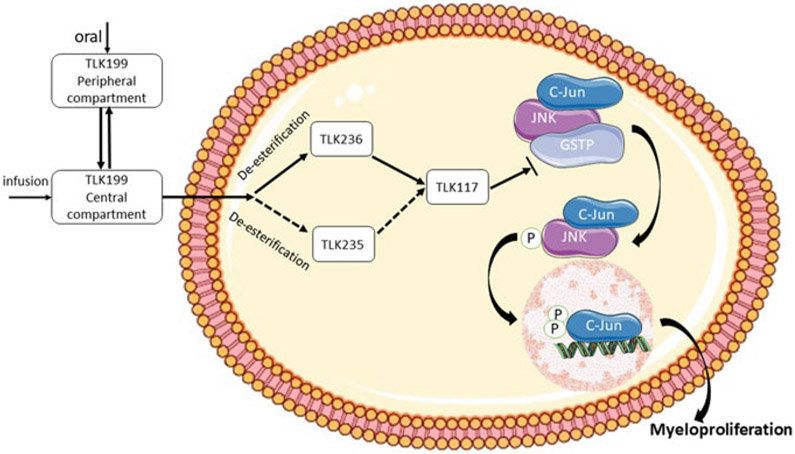
However, since TLK117 has two free carboxyl groups, the resulting charge was expected to inhibit cell uptake of the compound. Indeed, when tested in tumor cells which express GSTP1-1 as the predominant GST isozyme, TLK117 had neither toxicity nor potentiation. However, the diethyl ester form, TLK199 (γ-glutamyl-S-(benzyl)cysteinyl-R(–)-phenylglycine diethyl ester) did. The IC50 values were >200 μM for TLK117 compared with 22 μM for TLK199 in HT29 human colon adenocarcinoma cells line that express high levels of GSTP1-1 (Morgan et al. 1996). Similar IC50 values (26–28 μM) for TLK199 were obtained with other human colon adenocarcinoma cell lines, e.g., SW620, LoVo, and Caco2 (Beaumont et al. 1998). TLK199 is easily taken up by the cells, rapidly converted to phenylglycine monoethyl ester TLK236, and then gradually converted to TLK117. TLK199 undergoes deesterification to glutamyl monoethyl ester TLK235 as well, but this metabolite is only produced in very limited quantities (Figs. 1 and 2) (Morgan et al. 1996). The absorption, distribution, metabolism, and elimination properties of TLK199 were characterized in rat and dog. The primary metabolites are TLK236 and TLK117. Unchanged TLK199 was not detected in the blood, although the metabolites TLK117 and TLK236 were, indicating that the systemic clearance of the parent compound was both rapid and extensive (Raza et al. 2009a). TLK199 has a half-life in rodents of <1 min and in monkeys of ~15 min (Kauvar et al. 1998).
Fig. 1.
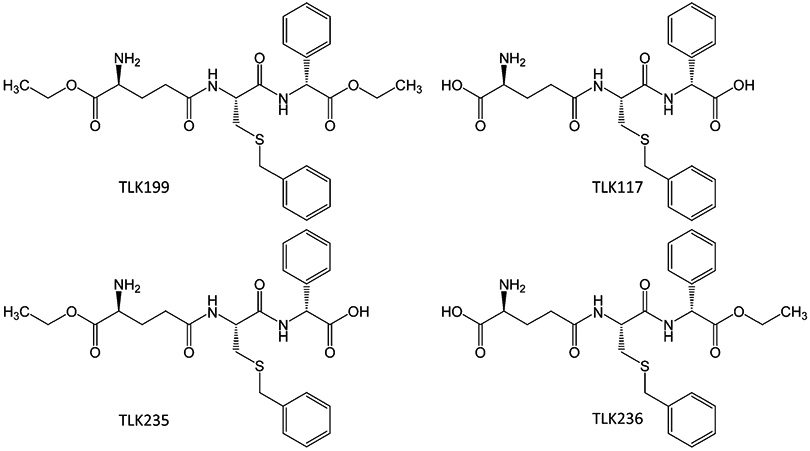
Structures of Telintra (TLK199) and its metabolites
Fig. 2.
Schematic of the influence of TLK199 on myeloproliferation
Since GSTP1-1 is frequently overexpressed in tumors and correlates with the development of drug resistance, combinations of TLK199 with several chemotherapeutic agents has been tested to determine whether TLK199 would act as a chemosensitizer. In human cancer cell lines overexpressing GSTP1-1, e.g., HT29 colon adenocarcinoma, HT4-1 (HT29 subclone), SKOV3 ovarian carcinoma, and SK VLB (vinblastine-resistant variant of SKOV3), TLK199 (12.5 or 25 μM) was found to potentiate the toxicity of chlorambucil and doxorubicin by up to 2.5-fold (Morgan et al. 1996). In a separate study, the GSTP1-1 antisense cDNA construct was shown to sensitize the human colon cancer cells to doxorubicin about as well as TLK199 (Ban et al. 1996). Furthermore, increased sensitivity of melphalan (5 mg/kg), measured by human colon tumor growth in SCID mice, was achieved by TLK199 (60 mg/kg). No tumor growth inhibition was observed with TLK199 as a single agent (Morgan et al. 1996). As a chemosensitizer, TLK199’s activity seemed easy to understand. TLK199 inhibits GSTP1-1, thus interferes with cellular phase II detoxification, leaving a cell susceptible to chemotherapeutic agents such as chlorambucil or melphalan. However, it was not immediately clear why TLK199 potentiated such a wide variety of drugs, including doxorubicin, which is not a specific GSTP1-1 substrate. We have posited at least one possible explanation in that TLK199 interfered with the GSTP1-1 and JNK interaction, leading to JNK activation (Bailey et al. 1994) and subsequent promotion of cancer cell apoptosis (Adler et al. 1999). In addition to GSTP1-1 inhibition, TLK199 has been implicated as an effective inhibitor (O’Brien et al. 1999) of multidrug resistance-associated protein 1 (MRP1 coded by the ABCC1 gene). MRP1 is an ATP-binding cassette transporter protein that plays an active role in multidrug resistance by its ability to efflux a vast array of anticancer drugs to sub-lethal levels (Cole et al. 1992). Using MRP1-transfected NIH3T3 mouse fibroblast cells with little detectable GSTP1-1 levels, TLK199 significantly inhibited the ATP-dependent transport, enhanced the accumulation, and subsequently reversed the resistance of numerous anticancer reagents, e.g., vincristine, etoposide, doxorubicin, daunorubicin, and mitoxantrone (O’Brien et al. 1999). Moreover, information now available indicates that a variety of genes, e.g., dihydrodiol dehydrogenase and γ-glutamylcysteine synthetase (rate-limiting enzyme in glutathione biosynthesis), are induced by TLK199 in cultured tumor cells (O’Dwyer et al. 1995). Modulation of expression of some of these genes might occur through the drug-induced perturbation of redox sensitive transcription factors such as Nrf2 and contribute further to the pharmacological actions of TLK199.
To elucidate how tumor cells may acquire resistance to TLK199, resistant cell lines were established. A human promyelocytic leukemia cell line (HL-60), which expresses GSTP1-1 as the predominant isozyme, was made tenfold resistant to TLK199. Both mRNA and protein levels of MRP1 were significantly increased by approximately 52- and 10-fold in the resistant cell line (HL60/TLK199). In addition, the HL60/TLK199 cells exhibited a drug resistance profile commensurate with a MRP1 overexpressing phenotype, with resistance to vinca alkaloids, epipodophyllotoxins, and anthracyclines (O’Brien et al. 1999). Further analysis of these cells revealed that TLK199 resistance was also associated with increased kinase activities of JNK1 (with threefold increase of basal expression levels) and ERK1/ERK2 (without modification of basal protein levels) (Ruscoe et al. 2001). The increased ERK1/ERK2 activities were suggested to protect the HL60/TLK199 cells against UV-induced apoptosis (Ruscoe et al. 2001) and PMA (phorbol 12-myristate 13-acetate)-induced cell growth arrest during monocyte/macrophage cytodifferentiation (Gate et al. 2003).
6. Hematopoiesis
Although TLK199 acted as a moderately effective chemosensitizer, the potentiation of therapeutic index was generally modest, a reflection of the state of the field at that time (Kauvar et al. 1998). Therefore, its effect on sensitizing normal cells to cytotoxins was examined, particularly in the case of the bone marrow where dose-limiting toxicity can sometimes predominate. Surprisingly, TLK199 showed a striking hematopoietic stimulatory effect.
In normal Gstp1/p2+/+ mice, treatment with TLK199 (75 mg/kg i.p.) caused a twofold increase of circulating white blood cells, whereas no increase in white blood cell count was observed in Gstp1/p2−/− mice (Ruscoe et al. 2001). In addition, TLK199 administration caused significant increases in neutrophil levels in rodents and dogs (Hamilton and Batist 2005). Moreover, using a granulocyte/macrophage colony forming unit (CFU-GM) assay, direct effects of TLK199 on mouse bone marrow progenitor cell proliferation were found by in vivo (75 mg/kg i.p.) or in vitro (10 μM) treatments, and in each case, TLK199 induced a proliferative response, approximately twofold above vehicle control. Similar effects were also found in human bone marrow progenitor cells with TLK199 (1–10 μM) treatment. Furthermore, increased mobilization of the GM progenitors from mouse bone marrow to the spleen and peripheral blood was observed following treatment with TLK199. In contrast, the bone marrow from Gstp1/p2−/− mice did not respond to TLK199 (Kauvar et al. 1998; Ruscoe et al. 2001). These data are consistent with the results that Gstp1/p2−/− mice had higher basal levels of white blood cells compared with Gstp1/p2+/+ mice, and cytokines (IL-3, GM-CSF, G-CSF, SCF, TPO, and Flt3L) were more effective at stimulating hematopoietic cell proliferation in Gstp1/p2−/− than in Gstp1/p2+/+ mice (Gate et al. 2004; Zhang et al. 2014; Ruscoe et al. 2001). In addition, Gstp1/p2−/− mouse embryo fibroblast (MEF) cells doubled faster than Gstp1/p2+/+ cells (26.2 versus 33.6 h) (Ruscoe et al. 2001).
Taken together, such evidence suggests that the presence, as well as the subsequent inhibition of GSTP1-1, is critical for the proliferative effects of TLK199. However, the mechanism underlying TLK199’s hematopoietic stimulatory effects is not fully understood. These effects might be explainable by the ability of TLK199 to disrupt the GSTP1-1 and JNK interaction (Adler et al. 1999), resulting in the activation of the JNK pathway that regulates proliferation, differentiation, and survival of hematopoietic cells (Geest and Coffer 2009) (Fig. 2). Indeed, treatment of TLK199 led to twofold increase in basal JNK activity in Gstp1/p2+/+ cells (Adler et al. 1999), and the JNK inhibitor SP600125 completely inhibited the myelostimulant effects of TLK199 (Gate et al. 2004). Consistently, Gstp1/p2−/− cells exhibited higher basal levels of JNK activity (Adler et al. 1999), and SP600125 abrogated the differential myeloproliferation between Gstp1/p2−/− and Gstp1/p2−/− cells (Gate et al. 2004). Sustained activation of STAT proteins has been associated with increased proliferation of Gstp1/p2−/− bone marrow and mast cells (Gate et al. 2004). GSTP1-1 has been shown to be a negative regulator of STAT3. It binds to STAT3 and protects cells against EGF and angiotensin II-induced proliferation and migration through inhibition of STAT3 phosphorylation (Chen et al. 2014; Kou et al. 2013). However, whether TLK199 could interrupt the interaction between GSTP1-1 and STAT and in this way regulate myeloproliferation needs further investigation. Moreover, GSTP1-1 has the potential to mediate the S-glutathionylation of a number of proteins that may be involved in myeloproliferative events (Townsend et al. 2009), and this may provide a framework for explaining the myelostimulatory effects of TLK199.
The impact of TLK199 on normal animals and human bone marrow certainly provides opportunities for clinical application; however, the drug’s effects on myelosuppressed subjects may also prove to be clinically relevant. Therefore, several preclinical studies have been performed in which TLK-199 was administered following chemotherapy. Data on the use of TLK199 in rodents demonstrated that TLK199 accelerated the recovery of circulating neutrophil levels following 5-fluorouracil treatment. Mice treated with TLK199 in addition to cisplatin or 5-fluorouracil demonstrated 60 or 100% of normal CFU-GM, respectively, compared with <10% observed when the cytotoxins were administered alone. In murine experiments, comparable results with TLK199 were also obtained following carboplatin and cyclophosphamide treatment. Overall, preclinical studies have demonstrated that (1) TLK199 reduces the severity of the cell count nadir in some animals; (2) cell count recovery to normal levels is accelerated by at least the same margin as that provided by G-CSF; and (3) the effects observed apply to both neutrophils and platelets, an advantage over G-CSF, which generally increases neutrophil numbers only (Kauvar et al. 1998; Hamilton and Batist 2005). TLK199 was non-toxic when parenterally administered daily for 7 days to both rats and dogs at doses up to 480 mg/m2 and 800 mg/m2, respectively (Hamilton and Batist 2005). No significant toxicities were observed in rats and dogs following daily oral administration of TLK199 for 14 days at doses up to 1,000 mg/kg and 20 mg/kg, respectively (Raza et al. 2009a). The collective preclinical results have been translated into phase I and phase II clinical trials in patients with myelodysplastic syndromes (MDS).
7. Use in Myelodysplastic Syndrome
Myelodysplastic syndromes represent a diverse group of bone marrow stem cell disorders predominantly affecting older individuals, with a median age at diagnosis of 65–70 years. The syndromes are characterized by ineffective hematopoiesis leading to cytopenia and in a third of patients, by progression to acute myeloid leukemia (AML) (Ades et al. 2014). The treatment options available are largely based on the patient’s age and their prognosis as determined by the International Prognostic Scoring System (IPSS) (Greenberg et al. 1997, 2012). For patients in the IPSS low/intermediate [Int]-1 risk categories, the goal of the treatment is to improve infective hematopoiesis while providing the appropriate supportive care, including RBC and platelet transfusions, use of hematopoietic growth factors, antibiotics, and use of iron chelation therapy as appropriate. In higher-risk patients, the goal is to extend survival and delay transformation to AML. Currently, there are three FDA-approved drugs: the hypomethylating agents (HMAs) azacitidine and decitabine beneficial for higher-risk MDS patients and lenalidomide specific for lower-risk transfusion-dependent patients with del(5q) cytogenetic abnormalities. These agents, in addition to supportive care, immunosuppressive therapies, and allogeneic stem cell transplantation (allo-SCT), constitute the most commonly used therapeutic interventions (Zeidan et al. 2013). Overall, outside of a curative intent allo-SCT, the rest of the treatment modalities are palliative (Mahadevan and Sutton 2015). Even for those patients who proceed to allo-SCT, significant treatment-related mortality and morbidity and high relapse rates compromise long-term disease-free survival (Luger et al. 2012). There remains a clear need for new treatment options.
In this regard, TLK199, Telintra in its FDA-approved formulation, ezatiostat hydrochloride, was employed to treat MDS patients with low to intermediate risk. The drug company utilized the preclinical results and claimed that the promotion of proliferation and differentiation in normal hematopoietic cells and apoptosis of malignant cells was a sound rationale for phase I/II trials. Such a molecular mechanism was further supported by MDS patient pretreatment genomic data. Pathway analysis of the response profiles revealed that the genes comprising the JNK pathway, which is known to be activated by TLK199, are underexpressed in patients who were responders and overexpressed in patients who were non-responders to TLK199, suggesting that both the biology of the disease and the molecular mechanisms of action of the drug are positively correlated (Galili et al. 2012). There have been several clinical trials with MDS patients showing the safety and ezatiostat alone and in combination with lenalidomide (Table 1). In these trials, efficacy was based on the International Working Group (IWG) 2000 or 2006 criteria for hematologic improvement (HI) in the erythroid (HI-E), platelet (HI-P), or neutrophil (HI-N) lineages (Cheson et al. 2000, 2006). Adverse events (AEs) were graded by the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE) version 3.0.
Table 1.
Summary of clinical trials with ezatiostat
| Phase | Formulation | Dose | Patient | Response rate | Ref. |
|---|---|---|---|---|---|
| I | Tablet, orally | 200, 400, 1,000, 1,400, 200, 2,400, 3,000, 4,000, 5,000, or 6,000 mg divided into two oral doses twice daily on days 1–7 of a 21-day treatment cycle | All WHO classification types of MDS with IPSS low to intermediate-2 risk | IWG 2000: HI-E: 6/29 (21%) HI-N: 4/19 (21%) HI-P: 7/21 (33%) Unilineage: 3/14 (21%) Bilineage: 3/25 (12%) IWG 2006: RBC transfusion reduction: 14/23 (61%) RBC transfusion independence: 8/23 (35%) |
Raza et al. (2009a) |
| I | Tablet, orally | 2000 mg divided into two oral doses twice daily in combination with lenalidomide 10 mg oral dose once daily on days 1–21 of a 28-day treatment cycle | All WHO classification types of MDS with IPSS low to intermediate-1 risk | IWG 2006: HI-E: 4/10 (40%) HI-N: 1/3 (33%) HI-P: 3/5 (60%) Bilineage: 5/11 (45%) Trilineage: 1/3 (33%) RBC transfusion independence: 3/7 (43%) |
Raza et al. (2012b) |
| 2,500 mg divided into two oral doses twice daily in combination with lenalidomide 10 mg oral dose once daily on days 1–21 of a 28-day treatment cycle | IWG 2006: HI-E: 1/4 (25%) HI-N: 0/2 (0%) HI-P: 0/2 (0%) Bilineage: 0/5 (0%) Trilineage: 0/1 (0%) RBC transfusion independence: 0/2 (0%) |
||||
| I | Liposomes, intravenous injection | 50, 100, 200, 400, and 600 mg/m2 daily at a constant rate infusion over 60 min on days 1–5 of a 14-day treatment cycle | All FAB classification types of MDS with IPSS low to high risk | IWG 2000: HI-E: 9/38 (24%) HI-N: 11/26 (42%) HI-P: 12/24 (50%) Unilineage: 7/14 (50%) Bilineage: 1/13 (8%) Trilineage: 4/16 (25%) RBC transfusion reduction: 5/31 (16%) |
Raza et al. (2009b) |
| IIa | 600 mg/m2 daily on days 1–5 or days 1–3 of a 21-day treatment cycle | ||||
| II | Tablet, orally | 3,000 mg divided into two oral doses twice daily on days 1–14 of a 21-day treatment cycle | All WHO classification types of MDS with IPSS low to intermediate-1 risk | IWG 2006: HI-E: 7/29 (24%) HI-N: 1/10 (10%) HI-P: 1/14 (7%) Bilineage: 1/9 (11%) Trilineage: 1/6 (17%) RBC transfusion reduction: 6/20 (30%) RBC transfusion independence: 1/20 (5%) |
Raza et al. (2012a) |
| 2000 mg divided into two oral doses twice daily on days 1–21 of a 28-day treatment cycle | IWG 2006: HI-E: 6/32 (19%) HI-N: 3/11 (27%) HI-P: 0/0 (0%) Bilineage: 3/11 (27%) Trilineage: 0/0 (0%) RBC transfusion reduction: 5/18 (28%) RBC transfusion independence: 3/18 (17%) |
FAB French-American-British, IPSS International Prognostic Scoring System, IWG International Working Group, MDS myelodysplastic syndromes, RBC red blood cells, WHO World Health Organization
The first-in-human phase I-IIa study of the intravenous (IV) formulation of ezatiostat was designed on the basis of safety demonstrated in multidose toxicity studies and efficacy reported in animal models. Fifty-four MDS patients were enrolled. Phase I patients received liposomal ezatiostat at five dose levels (50, 100, 200, 400, and 600 mg/m2) intravenously on days 1–5 of a 14-day cycle until MDS progression or unacceptable toxicity. In phase IIa, ezatiostat was administered on two dose schedules (DSs): 600 mg/m2 IV on days 1–5 or days 1–3 of a 21-day treatment cycle. The most common AEs were grades 1 or 2 and non-hematologic, including chills, back pain, flushing, nausea, bone pain, fatigue, extremity pain, dyspnea, and diarrhea related to acute infusion hypersensitivity reactions. Pharmacokinetic parameters were estimated and derived for TLK199, TLK236, and TLK117. The ezatiostat elimination half-life was 0.20 h, an AUC/dose of 0.008 h/L, and a distribution half-life of 0.03 h. The active metabolite TLK236 had a half-life of 2.65 h, with an AUC/dose of 0.341 h/L, and TLK117 had a half-life of 0.24–0.60 h with an AUC/dose of 0.0116 h/L. Overall, trilineage responses were observed in 25% patients with trilineage cytopenia. HI-E, HI-N, and HI-P were observed in 24%, 42%, and 50% patients, respectively. These responses were accompanied by improvement in clinical symptoms and independence or reduction in red blood cell (RBC) and platelet transfusion requirements (Raza et al. 2009b).
Based on the promising clinical results obtained from the intravenous formulation of ezatiostat, a phase I study with an oral formulation (ezatiostat tablets) was initiated. Forty-five patients with low to Int-2 risk MDS were enrolled and received ten dose levels (200, 400, 1,000, 1,400, 2000, 2,400, 3,000, 4,000, 5,000, and 6,000 mg) of ezatiostat tablets divided into two oral doses twice daily on days 1–7 of a 21-day cycle. No dose-limiting toxicities were observed. The most common treatment-related AEs were non-hematologic and mild or moderate in grade (1 or 2), including nausea, diarrhea, vomiting, abdominal pain, constipation, anorexia, and dyspepsia. Levels of the major metabolite TLK236 increased proportionate to ezatiostat dosage. Eleven of the seventeen HI responses were observed at doses of 4,000–6,000 mg/day, reflecting a dose response. HI responses occurred in all lineages including three bilineage and one complete cytogenetic response. Decreased numbers of RBC and platelet transfusions and in some cases transfusion independence were attained (Raza et al. 2009a). These findings supported the further development of extended dose schedules of ezatiostat tablets in MDS.
Subsequently, a phase II study was conducted to evaluate two extended dose schedules of oral ezatiostat in 89 heavily pretreated patients with low to Int-1 risk MDS. In DS1, patients received 3,000 mg of ezatiostat tablets divided into two oral doses twice daily for 14 days of a 21-day cycle, and in DS2, patients received 2000 mg of ezatiostat tablets divided into two oral doses twice daily for 21 days of a 28-day cycle. Most common ezatiostat-related AEs were grade 1 and 2 gastrointestinal, including nausea, diarrhea, and vomiting. Overall, 29% of the RBC transfusion-dependent patients had transfusion reduction, with 11% achieving transfusion independence. The median duration of HI-E response was 34 weeks. Multilineage responses were observed. There was one cytogenetic complete response in a del (5q) MDS patient. An important trend was the effect of prior therapy on response. A 40% HI-E rate was observed in patients who had prior lenalidomide and were HMA naive, with 45% patients achieving significant RBC transfusion reduction and 27% achieving transfusion independence. In contrast, a 28% HI-E rate was observed in patients who were both lenalidomide and HMA naive, with 50% patients achieving clinically significant RBC transfusion reductions. The higher responses of ezatiostat in the subsets of patients previously treated with lenalidomide suggested a potential role for combining the two drugs. In addition, DS2 was selected for further ezatiostat studies due to its longer median duration of HI-E response (46 weeks), better tolerability of the lower daily dose and the greater convenience for patients of dosing with two tablets twice a day (Raza et al. 2012a).
Therefore, a phase I study was conducted to determine the safety and efficacy of ezatiostat in combination with lenalidomide. Nineteen patients with non-del(5q) MDS received one of two doses of ezatiostat (2000 mg or 2,500 mg/day) in combination with 10 mg of lenalidomide on days 1–21 of a 28-day cycle. No unexpected toxicities occurred, and the incidence and severity of AEs were consistent with those expected for each drug alone. All multilineage responses were observed in the 2000/10 mg doses, recommended for future studies. In the 2000/10 mg dose group, 4 of 10, 1 of 3, and 3 of 5 evaluable patients experienced an HI-E, HI-N, and HI-P response, respectively. Bilineage responses, HI-E/HI-P, HI-E/HI-N, and HI-N and HI-P, occurred in 3 of 5, 1 of 3, and 1 of 3 patients, respectively. One of three patients with pancytopenia experienced a complete trilineage response. In addition, three of seven RBC transfusion-dependent patients became RBC transfusion independent, including one patient for whom prior lenalidomide monotherapy was ineffective (Raza et al. 2012b).
Additionally, there are two case reports of MDS patients who responded unexpected well to ezatiostat (Quddus et al. 2010; Lyons et al. 2011). Both patients participated in the phase II study comparing two DSs of ezatiostat tablets for low to intermediate-1 risk MDS. The first patient was a 77-year-old male who relapsed after a short course of lenalidomide, with the disappearance of the del(5q) but the appearance of a new clonal abnormality t(2; 3) upon relapse. The patient discontinued lenalidomide and was randomized to receive ezatiostat tablets at 3000 mg/day for 14 days of a 21-day cycle. Five days into his second treatment cycle, he was withdrawn from the study due to intolerable side effects. However, striking improvement in all three blood counts had been observed since the initiation of the study and continued to remain high a year post-therapy, suggesting a role of ezatiostat in the treatment of patients who are resistant to lenalidomide (Quddus et al. 2010). Another patient was a 64-year-old female who suffered from longstanding idiopathic chronic neutropenia (ICN) with frequent episodes of sepsis, and had an inadequate response to G-CSF. She was randomized to receive ezatiostat tablets at 2000 mg/day for 21 days of a 28-day cycle. She responded by the end of the first cycle of treatment with stabilization of her absolute neutrophil count (ANC), clearing of fever and healing of areas of infection. Following eight cycles of treatment, she had continued to show remarkable improvement of ANC, suggesting a potential role of ezatiostat in the treatment of patients with ICN who are not responsive to G-CSF (Lyons et al. 2011).
Overall, the available clinical data have shown favorable tolerability and hematopoietic-promoting activity profiles for ezatiostat in MDS patients and indicated that the drug was worthy of further evaluation in randomized phase II and phase III trials. Missing from all of these clinical efforts was any type of “precision medicine” approach to patient selection. Given the time period that these drugs emerged and the subsequent trial design, there were no efforts to strategize patient selection on the basis of GST polymorphisms and no trial components to use possible biomarkers to assess drug efficacy. Given the variable catalytic efficiencies for GSTP variants (Manevich et al. 2013) and the recent indications of the utility of S-glutathionylated serum biomarkers in predicting response to electrophilic stress in patients (Zhang et al. 2019b), the absence of any pharmacogenetics approach to trial design may have restricted the chances for positive outcomes.
8. Lung Diseases
One of us (Y.J.) has specialized in studying lung pathologies such as idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD). The former is characterized by excessive collagen production and fibrogenesis and the latter by airway wall thickening and/or emphysema. Obviously, the lung is exposed to high oxygen concentrations, and aberrant GSH homeostasis has also been implicated in the presentation of each of these two disease states. As a consequence, various evidence have been generated to identify that GSTP-mediating S-glutathionylation is involved in these pathologies and that, by extension, Telintra may have relevance in their management.
As a first example, pro-inflammatory signaling cascades frequently begin with stimulation of the transcription factor nuclear factor kappa B (NfκB). To this end, S-glutathionylation has been shown to regulate the activity of inhibitory kappa B kinase beta (IKKβ), and GSTP also interacts with the adaptor protein TRAF2, a known regulator of NfκB (Jones et al. 2016). In mouse lung alveolar epithelial cells, a constitutive association between GSTP and IκBα was reported in unstimulated cells, rapidly lost when treated with LPS, but at a time that preceded IκBα degradation. In principle, this GSTP/IκBα interaction could prevent the phosphorylation and ubiquitination of IκBα, thereby preventing NFκB activation. LPS-induced nuclear contents of RelA and RelA phosphorylation were increased in cells following siRNA-mediated GSTP knockdown. In this regard, both GSTP knockdown and TLK117 (active metabolite see Fig. 1) treatments mediated GSTP inhibition and enhanced LPS-induced NF-κB luciferase activity and cytokine production, suggesting a potential regulatory function of GSTP in preventing IκBα phosphorylation and/or degradation. Cysteine189 of IκBα is the site of S-glutathionylation causing a decrease in phosphorylation by IKK that can attenuate ubiquitination (Kil et al. 2008), limiting its degradation and subsequent activation of NF-κB (Seidel et al. 2011). There are reasons to believe that the protein interaction(s) between GSTP and IκBα may be stabilized in some manner by the process of S-glutathionylating the target cysteine residue. In context, these events may then control activation and/or assembly of the IKK signalsome. Since GSTP does not affect IKKβ S-glutathionylation until 6 h after LPS exposure (a time at which NF-κB transcriptional activity is beginning to subside (Jones et al. 2016)), S-glutathionylation of IKK proteins may represent a mechanism whereby GSTP can attenuate NF-κB. This possible model predicts that in the absence of stimulus, GSTP prevents degradation of endogenous IκBα and that GSTP-mediated S-glutathionylation shuts down IKK activity providing a versatile mechanism for regulation of NFκB by GSTP. Overall, in light of the reported relevance of GSTP polymorphisms in allergic asthma (McCunney 2005), pharmacological manipulation of GSTP by drugs like Telintra may prove, in the future, to be a useful therapeutic approach to regulate pro-inflammatory signaling in these types of lung diseases.
Lung tissue remodeling in chronic obstructive pulmonary disease (COPD) is characterized by airway wall thickening and/or emphysema. Surfactant protein C (SPC)-TNF-α mice showed remodeling in alveolar and airway walls similar to those observed in patients with COPD. Epithelial cells are able to undergo a phenotypic shift, gaining mesenchymal properties, a process in which JNK signaling is involved. Consequently, TNF-α induces JNK-dependent epithelial plasticity, contributing to lung matrix remodeling. A pharmacological inhibitor of JNK attenuated this phenotypic shift, indicating the role of JNK signaling in this process. Activation of JNK signaling was also present in the lungs of SPC-TNF-α mice and patients with COPD. Together, these studies provide evidence for the involvement of the TNF-alpha-JNK axis in extracellular matrix remodeling. In light of the known connections between JNK and GSTP, this may also indicate a role for Telintra in impacting JNK activity, particularly since the drug is known to interfere with the interactions between the two proteins.
IPF is a debilitating disease characterized by the development of excess fibrous tissue that causes thickening of alveolar walls and diminished lung function (Lomas et al. 2012). It is the most common subtype of interstitial lung disease, impacting >120,000 Americans with 40,000 deaths each year (Blackwell et al. 2014; Raghu et al. 2006). Apoptosis in lung epithelial cells is a critical determinant for the extent of disease progression, since increased loss of these cells promotes fibroblast activation and remodeling. Changes in glutathione and GST expression patterns have been reported in IPF patients (Anathy et al. 2012), and this provided an opportunity to consider a therapeutic intervention strategy with Telintra (McMillan et al. 2016). GSTP mediates lung fibrogenesis in part through FAS S-glutathionylation, a critical event in epithelial cell apoptosis. GSTP expression (as well as the FAS-GSTP interaction) is increased in the lungs of IPF patients, mostly within type II epithelial cells. Bleomycin- and AdTGFβ-induced increases in collagen content, α-SMA, FAS S-glutathionylation, and total protein S-glutathionylation were strongly attenuated in GSTP knockout mice (McMillan et al. 2016). Oropharyngeal administration of TLK117, at a time when fibrosis was already apparent, attenuated bleomycin- and AdTGFβ-induced remodeling, α-SMA, caspase activation, FAS S-glutathionylation, and total protein S-glutathionylation. GSTP is an important driver of protein S-glutathionylation and lung fibrosis, and GSTP inhibition via inhalation of the Telintra active moiety may prove to be a novel therapeutic strategy for the future management of IPF.
9. Future Perspectives
In general terms the clinical success of drugs designed to target redox homeostasis have had limited success. There are many potential reasons for this, highlighted perhaps by the necessary redundancy inherent in maintaining cellular redox homeostasis (Zhang et al. 2018) and the evolutionary importance of oxidative regulation of a variety of transcription factors that control critical cell function (Hayes et al. 2020). Although clinical trials in MDS indicate that the drug has activity, the present absence of a corporate sponsor and supply of available GMP drug suggests that instigation of further clinical studies may be limited by these exigencies. When Telik, Inc. was reverse merged into privately held MabVax Therapeutics Inc. in May 2014, Telintra development was deemphasized, and this year, this company filed for bankruptcy. Patent coverage of the drug has expired, perhaps contributing to the reduction of corporate interest, but should remove limitations in further academic developments. In moving forward, the fact that the time-consuming components of formulation and initial IND application have already been accomplished does provide opportunities for more rapid clinical development. It should be noted though that positive preclinical results in lung disorders made use of a nasopharangeal administration route. Moreover, there are emerging examples of where S-glutathionylated proteins are critical in regulating important pathways. Since inhibition of GSTP limits this post-translational modification, there may prove to be a role for the drug in this area. We and others have discussed previously the importance of sulfur amino acid homeostasis to the bone marrow environment (Gate et al. 2004). Indeed, many leukemias, including CLL, rely upon cystine transporters from the surrounding marrow stromal environment to provide sufficient cystine as a precursor of cysteine (Zhang et al. 2012). Since both qualitative and quantitative aspects of protein S-glutathionylation will depend upon a balanced supply of GSH and GSTP, drugs like Telintra may hold promise in delineating the physiological importance of these pathways in both normal and malignant marrow tissues.
Contributor Information
Jie Zhang, Department of Cell and Molecular Pharmacology and Experimental Therapeutics, Medical University of South Carolina, Charleston, SC, USA.
Zhi-Wei Ye, Department of Cell and Molecular Pharmacology and Experimental Therapeutics, Medical University of South Carolina, Charleston, SC, USA.
Yvonne Janssen-Heininger, Department of Pathology and Laboratory Medicine, The University of Vermont, Burlington, VT, USA.
Danyelle M. Townsend, Department of Pharmaceutical and Biomedical Sciences, Medical University of South Carolina, Charleston, SC, USA
Kenneth D. Tew, Department of Cell and Molecular Pharmacology and Experimental Therapeutics, Medical University of South Carolina, Charleston, SC, USA
References
- Adang AE et al. (1990) The glutathione-binding site in glutathione S-transferases. Investigation of the cysteinyl, glycyl and gamma-glutamyl domains. Biochem J 269(1):47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades L, Itzykson R, Fenaux P (2014) Myelodysplastic syndromes. Lancet 383(9936):2239–2252 [DOI] [PubMed] [Google Scholar]
- Adler V et al. (1999) Regulation of JNK signaling by GSTp. EMBO J 18(5):1321–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anathy V et al. (2012) Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxid Redox Signal 16(6):496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askelof P et al. (1975) Purification and characterization of two glutathione S-aryltransferase activities from rat liver. Biochem J 147(3):513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HH et al. (1994) Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: an attempt at modulation of glutathione. J Clin Oncol 12(1):194–205 [DOI] [PubMed] [Google Scholar]
- Ban N et al. (1996) Transfection of glutathione S-transferase (GST)-pi antisense complementary DNA increases the sensitivity of a colon cancer cell line to adriamycin, cisplatin, melphalan, and etoposide. Cancer Res 56(15):3577–3582 [PubMed] [Google Scholar]
- Beaumont PO et al. (1998) Role of glutathione S-transferases in the resistance of human colon cancer cell lines to doxorubicin. Cancer Res 58(5):947–955 [PubMed] [Google Scholar]
- Blackwell TS et al. (2014) Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med 189(2):214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland E, Chasseaud LF (1969) Glutathione S-aralkyltransferase. Biochem J 115(5):985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D et al. (2014) GSTpi protects against angiotensin II-induced proliferation and migration of vascular smooth muscle cells by preventing signal transducer and activator of transcription 3 activation. Biochim Biophys Acta 1843(2):454–463 [DOI] [PubMed] [Google Scholar]
- Cheson BD et al. (2000) Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 96(12):3671–3674 [PubMed] [Google Scholar]
- Cheson BD et al. (2006) Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108(2):419–425 [DOI] [PubMed] [Google Scholar]
- Cho SG et al. (2001) Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem 276(16):12749–12755 [DOI] [PubMed] [Google Scholar]
- Cole SP et al. (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258(5088):1650–1654 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103(2):239–252 [DOI] [PubMed] [Google Scholar]
- Dorion S, Lambert H, Landry J (2002) Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Ask1. J Biol Chem 277 (34):30792–30797 [DOI] [PubMed] [Google Scholar]
- Flatgaard JE, Bauer KE, Kauvar LM (1993) Isozyme specificity of novel glutathione-S-transferase inhibitors. Cancer Chemother Pharmacol 33(1):63–70 [DOI] [PubMed] [Google Scholar]
- Galili N et al. (2012) Prediction of response to therapy with ezatiostat in lower risk myelodysplastic syndrome. J Hematol Oncol 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate L, Lunk A, Tew KD (2003) Resistance to phorbol 12-myristate 13-acetate-induced cell growth arrest in an HL60 cell line chronically exposed to a glutathione S-transferase pi inhibitor. Biochem Pharmacol 65(10):1611–1622 [DOI] [PubMed] [Google Scholar]
- Gate L et al. (2004) Increased myeloproliferation in glutathione S-transferase pi-deficient mice is associated with a deregulation of JNK and Janus kinase/STAT pathways. J Biol Chem 279 (10):8608–8616 [DOI] [PubMed] [Google Scholar]
- Geest CR, Coffer PJ (2009) MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol 86(2):237–250 [DOI] [PubMed] [Google Scholar]
- Ghezzi P (2005) Regulation of protein function by glutathionylation. Free Radic Res 39(6):573–580 [DOI] [PubMed] [Google Scholar]
- Graminski GF, Kubo Y, Armstrong RN (1989) Spectroscopic and kinetic evidence for the thiolate anion of glutathione at the active site of glutathione S-transferase. Biochemistry 28(8):3562–3568 [DOI] [PubMed] [Google Scholar]
- Greenberg P et al. (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89(6):2079–2088 [PubMed] [Google Scholar]
- Greenberg PL et al. (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120(12):2454–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254(16):7558–7560 [PubMed] [Google Scholar]
- Hamilton D, Batist G (2005) TLK-199 (Telik). IDrugs 8(8):662–669 [PubMed] [Google Scholar]
- Hayes JD et al. (2020) Oxidative stress in cancer. Cancer Cell. 10.1016/jxcell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo H et al. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275(5296):90–94 [DOI] [PubMed] [Google Scholar]
- Johansson AS, Ridderstrom M, Mannervik B (2000) The human glutathione transferase P1-1 specific inhibitor TER 117 designed for overcoming cytostatic-drug resistance is also a strong inhibitor of glyoxalase I. Mol Pharmacol 57(3):619–624 [DOI] [PubMed] [Google Scholar]
- Jones JT et al. (2016) Glutathione S-transferase pi modulates NF-kappaB activation and pro-inflammatory responses in lung epithelial cells. Redox Biol 8:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauvar LM et al. (1998) Glutathione based approaches to improving cancer treatment. Chem Biol Interact 111–112:225–238 [DOI] [PubMed] [Google Scholar]
- Kil IS, Kim SY, Park JW (2008) Glutathionylation regulates IkappaB. Biochem Biophys Res Commun 373(1):169–173 [DOI] [PubMed] [Google Scholar]
- Kou X et al. (2013) GSTP1 negatively regulates Stat3 activation in epidermal growth factor signaling. Oncol Lett 5(3):1053–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas NJ et al. (2012) Idiopathic pulmonary fibrosis: immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers. Int J Clin Exp Pathol 5(1):58–71 [PMC free article] [PubMed] [Google Scholar]
- Luger SM et al. (2012) Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant 47(2):203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RM et al. (2011) Oral ezatiostat HCl (Telintra(R), TLK199) and idiopathic chronic neutropenia (ICN): a case report of complete response of a patient with G-CSF resistant ICN following treatment with ezatiostat, a glutathione S-transferase P1-1 (GSTP1-1) inhibitor. J Hematol Oncol 4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttle MH et al. (1994) Isozyme-specific glutathione-S-transferase inhibitors: design and synthesis. J Med Chem 37(1):189–194 [DOI] [PubMed] [Google Scholar]
- Mahadevan D, Sutton GR (2015) Ezatiostat hydrochloride for the treatment of myelodysplastic syndromes. Expert Opin Investig Drugs 24(5):725–733 [DOI] [PubMed] [Google Scholar]
- Manevich Y et al. (2013) Allelic variants of glutathione S-transferase P1-1 differentially mediate the peroxidase function of peroxiredoxin VI and alter membrane lipid peroxidation. Free Radic Biol Med 54:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCunney RJ (2005) Asthma, genes, and air pollution. J Occup Environ Med 47(12):1285–1291 [DOI] [PubMed] [Google Scholar]
- McMillan DH et al. (2016) Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase pi. JCI Insight 1(8):e85717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AS et al. (1996) Isozyme-specific glutathione S-transferase inhibitors potentiate drug sensitivity in cultured human tumor cell lines. Cancer Chemother Pharmacol 37(4):363–370 [DOI] [PubMed] [Google Scholar]
- O’Dwyer PJ et al. (1992) Depletion of glutathione in normal and malignant human cells in vivo by buthionine sulfoximine: clinical and biochemical results. J Natl Cancer Inst 84(4):264–267 [DOI] [PubMed] [Google Scholar]
- Oakley AJ et al. (1997) The structures of human glutathione transferase P1-1 in complex with glutathione and various inhibitors at high resolution. J Mol Biol 274(1):84–100 [DOI] [PubMed] [Google Scholar]
- O’Brien ML et al. (1999) Glutathione peptidomimetic drug modulator of multidrug resistance-associated protein. J Pharmacol Exp Ther 291(3):1348–1355 [PubMed] [Google Scholar]
- O’Dwyer PJ et al. (1991) Phase I study of thiotepa in combination with the glutathione transferase inhibitor ethacrynic acid. Cancer Res 51(22):6059–6065 [PubMed] [Google Scholar]
- O’Dwyer PJ et al. (1995) Modulation of glutathione and related enzymes in reversal of resistance to anticancer drugs. Hematol Oncol Clin North Am 9(2):383–396 [PubMed] [Google Scholar]
- Petrini M et al. (1993) Reversing of chlorambucil resistance by ethacrynic acid in a B-CLL patient. Br J Haematol 85(2):409–410 [DOI] [PubMed] [Google Scholar]
- Ploemen JH et al. (1993) Ethacrynic acid and its glutathione conjugate as inhibitors of glutathione S-transferases. Xenobiotica 23(8):913–923 [DOI] [PubMed] [Google Scholar]
- Quddus F et al. (2010) Oral Ezatiostat HCl (TLK199) and Myelodysplastic syndrome: a case report of sustained hematologic response following an abbreviated exposure. J Hematol Oncol 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G et al. (2006) Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174(7):810–816 [DOI] [PubMed] [Google Scholar]
- Raza A et al. (2009a) Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood 113(26):6533–6540 [DOI] [PubMed] [Google Scholar]
- Raza A et al. (2009b) Phase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra, TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndrome. J Hematol Oncol 2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A et al. (2012a) A phase 2 randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Cancer 118 (8):2138–2147 [DOI] [PubMed] [Google Scholar]
- Raza A et al. (2012b) Phase 1 dose-ranging study of ezatiostat hydrochloride in combination with lenalidomide in patients with non-deletion (5q) low to intermediate-1 risk myelodysplastic syndrome (MDS). J Hematol Oncol 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L et al. (2006) Human GSTA1-1 reduces c-Jun N-terminal kinase signalling and apoptosis in Caco-2 cells. Biochem J 400(1):135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscoe JE et al. (2001) Pharmacologic or genetic manipulation of glutathione S-transferase P1–1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther 298(1):339–345 [PubMed] [Google Scholar]
- Seidel P et al. (2011) IkappaBalpha glutathionylation and reduced histone H3 phosphorylation inhibit eotaxin and RANTES. Eur Respir J 38(6):1444–1452 [DOI] [PubMed] [Google Scholar]
- Tew KD (1994) Glutathione-associated enzymes in anticancer drug resistance. Cancer Res 54 (16):4313–4320 [PubMed] [Google Scholar]
- Tew KD, Bomber AM, Hoffman SJ (1988) Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res 48(13):3622–3625 [PubMed] [Google Scholar]
- Tew KD, Dutta S, Schultz M (1997) Inhibitors of glutathione S-transferases as therapeutic agents. Adv Drug Deliv Rev 26(2–3):91–104 [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD (2003) The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22(47):7369–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM et al. (2009) Novel role for glutathione S-transferase pi. Regulator of protein S-glutathionylation following oxidative and nitrosative stress. J Biol Chem 284(1):436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Tew KD (1985) Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep 69(6):677–682 [PubMed] [Google Scholar]
- Wu Y et al. (2006) Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene 25(42):5787–5800 [DOI] [PubMed] [Google Scholar]
- Ye ZW et al. (2017) Glutathione S-transferase P-mediated protein S-glutathionylation of resident endoplasmic reticulum proteins influences sensitivity to drug-induced unfolded protein response. Antioxid Redox Signal 26(6):247–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z et al. (2000) Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res 60(15):4053–4057 [PubMed] [Google Scholar]
- Zeidan AM, Linhares Y, Gore SD (2013) Current therapy of myelodysplastic syndromes. Blood Rev 27(5):243–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W et al. (2012) Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol 14(3):276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J et al. (2014) Glutathione S-transferase P influences redox and migration pathways in bone marrow. PLoS One 9(9):e107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J et al. (2018) An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation. Free Radic Biol Med 120:204–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J et al. (2019a) Racial disparities, cancer and response to oxidative stress. Adv Cancer Res 144:343–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L et al. (2019b) S-glutathionylated serine proteinase inhibitors as biomarkers for radiation exposure in prostate cancer patients. Sci Rep 9(1):13792. [DOI] [PMC free article] [PubMed] [Google Scholar]