Abstract
Background:
Sacubitril/valsartan, the first agent to be approved in a new class of drugs called angiotensin receptor neprilysin inhibitors (ARNIs), has been shown to reduce cardiovascular mortality and morbidity compared to enalapril in outpatient subjects with chronic heart failure (HF) and reduced left ventricular ejection fraction (HFrEF). However, there is little real-world evidence about the efficacy of ARNIs in elderly hypertensive patients with HFrEF and comorbidities.
Methods:
In this prospective open-label study, 108 subjects, 54 of them (mean age 78.6 ± 8.2 years, 75.0 % male), with HFrEF (29.8 ± 4.3 %) and New York Heart Association (NYHA) class II-III symptoms were assigned to receive ARNIs twice daily, according to the recommended dosage of 24/26, 49/51, 97/103 mg. Patients were gender- and age-matched with a control arm of patients with HFrEF receiving the optimal standard therapy for HF. The clinic blood pressure (BP), N-terminal pro-B-type natriuretic peptide (NT-proBNP), estimated glomerular filtration rate (eGFR), blood glucose and glycated hemoglobin (HbA1c), uric acid (UA), left ventricular ejection fraction (LVEF) and NYHA class were evaluated at a mean follow-up of 12 months. During the follow-up, the clinical outcomes, including mortality and re-hospitalization for HF, were collected.
Results:
NYHA class significantly improved in the ARNI arm compared to the control (24.9 vs. 6.4 %, shifting from class III to II, and 55.4 vs. 25.2 %, from class II to I, p < 0.05 for all). A significant improvement in LVEF and eGFR levels was found in the ARNI arm compared to controls (42.4 vs. 34.2 %, 73.8 vs. 61.2 mL/min, respectively; p < 0.001 for all). NT-proBNP, clinic systolic and diastolic BP, blood glucose, HbA1c and UA values were reduced in both treatment arms, but they were lower in the ARNI arm compared controls (3107 vs. 4552 pg/mL, 112.2 vs. 120.4 and 68.8 vs. 75.6 mmHg, 108.4 vs. 112.6 mg/dL, 5.4 vs. 5.9 % and 5.9 vs. 6.4 mg/dL, respectively, p < 0.05). Mortality and re-hospitalization for HF was lower in the ARNI arm than controls (20.1 vs. 33.6 % and 27.7 vs. 46.3 % respectively; p < 0.05 for all). Gender differences were not found in either arm. No patients refused to continue the study, and no side effects to the ARNI treatment were observed.
Conclusions:
In elderly patients with HFrEF and comorbidities, ARNI treatment seems effective and safe. The improvement in LVEF and cardiac remodeling, BP, eGFR, serum glucose, UA and HbA1c could be the mechanisms by which ARNIs play their beneficial role on clinical outcomes. However, these results need to be confirmed in studies involving a greater number of subjects, and with a longer follow-up.
Keywords: Ejection fraction, Chronic heart failure, Hypertension, Internal medicine, Mortality, Sacubitril/valsartan
1. Introduction
Heart failure (HF) is the main cause of hospitalization in Internal Medicine units, and HF patients have a 50 % reduction in 5-year survival, similar to that of patients with cancer [1]. The prevalence of HF increases with age, and doubles with each decade, both due to the progressive aging of the population [2] and to the improvement in the treatment of the main risk factors for HF [1].
In the European general population, including Italy and North America, although HF it is considered a typical condition of advanced age [3,4], in other countries HF is diagnosed in younger subjects [5]. This is one of the reasons why the average age of the subjects enrolled in HF clinical trials is generally lower than patients with HF managed in an outpatient or Internal Medicine setting [5]. Furthermore, in order to have the chance to obtain significant results in terms of efficacy and safety, controlled interventional clinical studies on HF enrolled frequently younger subjects and with fewer comorbidities than those treated in real-world settings [6]. In fact, patients hospitalized for HF in Internal Medicine units or managed at an outpatient level, as well as being very old, commonly have multiple comorbidities, take a polytherapy and are fragile, so much so that they are only partially representative of the patients enrolled in most HF clinical trials [7,8]. For these reasons, in the absence of strong recommendations from the main guidelines based mostly on subjects under the age of 70, “internistic” HF patients often receive sub-optimal medical treatment [9]. Fortunately, the standard optimal medical treatment of HF with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-II receptor blockers (ARBs) (in case of ACEI intolerance), beta-blockers and mineralocorticoid receptor antagonists (MRA) reduces the risk of mortality and hospitalization due to HF by up to 37 % [10]. However, data from the ARNO study [11], the largest Italian registry that collects information on 41,413 H F patients from administrative databases (for the majority, patients managed in Internal Medicine), indicate that HF mortality remains high. The analysis of these data also reveals a significant under-prescribing of drugs for HF, where only 66 %, 49 %, and 42 % of patients underwent treatment with ACE inhibitors/ARBs, beta-blockers and MRA, respectively [11]. Fortunately, the clinician has the task of adapting therapeutic strategies considering the availability of new treatments for HF. In this context, sacubitril/valsartan is the progenitor of the inhibitors of the AT1 receptor of angiotensin-II and neprilysin (ARNIs, angiotensin receptor neprilysin inhibitors) and, thanks to the results of the “Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure” (PARADIGM-HF) trial [12], ARNIs were approved by the US Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) as a new pharmacological class for the treatment of HF. The results of PARADIGM-HF have shown in the short term that treatment with ARNI allowed the reduction of total and cardiovascular mortality and the number of hospitalizations for HF by 20 % and this trial was suspended early for an excess of benefit, at a follow-up of 27 months. In the PARADIGM-HF study, the elderly population was well represented; 23 % of the 8442 outpatients with HF with reduced ejection fraction (HFrEF < 40 %) randomized to ARNI or enalapril treatment were 75 years or older [12].
Although treatment with ARNI has shown promising results, it is still unclear whether it is applicable to the Internal Medicine setting, consisting mostly of frail, elderly patients with multiple comorbidities and requiring a complex pharmacological and non-pharmacological therapy for HF [9].
The aim of this prospective study, conducted in hypertensive, elderly and comorbid patients managed in an internistic setting, was to evaluate whether treatment with ARNI compared to the standard drug therapy for HF was able to improve the symptoms of HF with reduced EF and have a positive impact on mortality and re-hospitalization due to HF.
2. Materials and methods
Three months after discharge from an Internal Medicine unit, 54 hypertensive subjects, with an average age of 78.6 ± 8.2 years (75 % male), with HF with reduced left ventricular EF (LVEF) and in NYHA class II-III, were consecutively enrolled in an HF outpatient clinic, from April 2017 to April 2019. The diagnosis of HF was obtained by analyzing the hospital discharge sheets (HDSs) and taking into account the following codes, based on the 9th revision of the international statistical classification of diseases (ICD-9) and related health problems: 398.91, 420.1, 402.11, 420.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1. The study protocol was approved by the local Ethics Committee and conducted according to the “Guide to Good Clinical Practice” established by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). All procedures were conducted, taking into consideration the ethical standards of the Commission responsible for human experimentation and in compliance with the Helsinki Declaration of 1964, revised in 2013. All subjects were informed of the study protocol and expressed their informed consent to participate in the same.
2.1. Data collection
At the screening visit, blood pressure (BP) was measured 3 times in both clinostatism and orthostatism, with a mercury sphygmomanometer, at 10-minute intervals. The average of the last two measurements was approximated by default to minimize the “white coat” effect, and at the same time heart rate (HR) was also measured. Arterial hypertension was defined as a systolic BP value > 140 mmHg and/or diastolic BP > 90 mmHg or any BP during antihypertensive treatment. Body Mass Index (BMI) was calculated as the ratio of weight (in kilograms) to height (in meters) squared.
The N-terminal pro-B-type natriuretic peptide (NT-proBNP) was analyzed with the electrochemiluminescence immunoassay on human plasma, according to the Elecsys pro-BNP II STAT system [13]. The values of total cholesterol (TC), triglycerides (TG), cholesterol associated with high-density lipoproteins (HDL-C), glycemia, glycated hemoglobin (HbA1c). The cholesterol values (in mg/dL) associated with low-density lipoproteins (LDL-C) were calculated with Friedewald’s formula [14].
The subjects, based on their smoking habit (or absence thereof), were classified into non-smokers and smokers (> 1 cigarette/day). Patients were considered diabetic if they had repeated blood glucose values (in mg/dL) ≥ 126, or glycated hemoglobin (HbA1c) values > 6.5 %, or if they were treated with hypoglycemic drugs, regardless of their blood sugar values. Serum creatinine values (SCr, in mg/dL) were measured by means of an enzymatic method, using an auto-analyzer (Hitachi Modular P, Roche diagnostic, USA). Estimated glomerular filtration rate (eGFR, in mL/min) was obtained from the serum creatinine value in both genders, using the Cockroft-Gault formula:
Chronic kidney disease (CKD) was diagnosed based on eGFR values < 60 mL/min. Patients who, upon spirometry, showed a non-reversible ratio between forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) < 0.7, or who were taking bronchodilator drugs, were considered to be suffering from chronic obstructive pulmonary disease (COPD). Finally, the clinical history of myocardial infarction (confirmed by relevant medical documentation), angina pectoris confirmed by medical documentation or by treatment with anti-anginal drugs, and the presence of positive cycle ergometer or positive myocardial scintigraphy tests, classified the subjects as officially suffering from ischemic heart disease (IHD).
2.2. Study plan
As recommended by the 2016 European guidelines on HF management, ARNI treatment [9] was started in patients with HF with reduced EF who remained symptomatic despite optimal treatment at 3 months with ACEI, or ARB (in case of intolerance to ACEI), beta-blocker and MRA. The inclusion criteria for participating in the study were as follows: adult patients with LVEF < 40 %, NT-pro BNP ≥ 900 pg/mL, eGFR > 30 mL/min, serum potassium < 5.2 mmol/L, systolic and/or diastolic BP ≥ 110/70 mmHg. The exclusion criteria were a history of hypersensitivity or intolerance to ACEI or ARB, history of angioedema, symptomatic hypotension and/or systolic BP < 100 mmHg, serum potassium ≥ 5.3 mmol/L, presence of neoplasms or liver failure, dementia or inability to cooperate.
2.3. Active ARNI treatment
Upon the initial visit, ARNI therapy was administered in patients presenting with clinical symptoms of HF despite standard optimal medical therapy (OMT). ARNI therapy was administered in addition to OMT twice a day at different dosages of sacubitril/valsartan, 24–26 mg, 49–51 mg or 97–103 mg, respectively (Fig. 1), after the suspension of the treatment with ACEI or ARB. In detail, for patients taking ACEI therapy, ARNI treatment was administered at least 36 h after stopping the ACEI, while in patients taking ARBs, ARNI treatment was administered 24 h after stopping the ARB. The choice of the initial dose of ARNI was based on baseline systolic BP, eGFR, and potassium values.
Fig. 1.
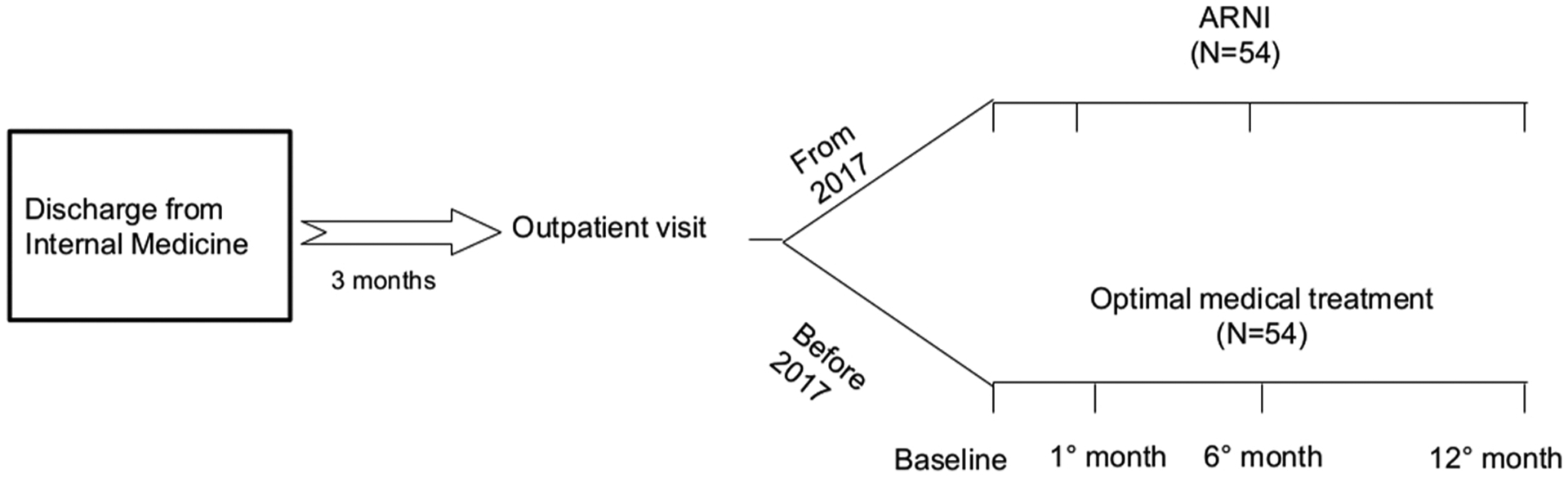
Study plan of patient follow-up after hospital discharge for the ARNI (active treatment) group and the OMT (control) group. ARNI angiotensin receptor neprilysin inhibitor; OMT standard optimal medical treatment.
2.4. Control arm
Patients treated with ARNI were compared with an arm of the same size (n = 54) and matched by age and gender, selected from our database for HF before the marketing of ARNIs and who were taking a standard OMT for HF with reduced EF, characterized by ACEI or ARB, beta-blocker, and MRA. The posology adopted for the drugs included in OMT and the type of ACEI or ARB and beta-blockers used is shown in Table 2.
Table 2.
Type and posology of drugs included in the optimal medical therapy (OMT).
| Type of drugs | Prevalence (%) | Maximum tolerated dose/daily (mg) |
|---|---|---|
| ACEIs | ||
| Enalapril | 75 | 20 |
| Ramipril | 20 | 10 |
| Perindopril | 5 | 10 |
| ARBs | ||
| Valsartan | 70 | 160 |
| Candesartan | 20 | 16 |
| Losartan | 10 | 100 |
| Beta-blockers | ||
| Metoprolol | 30 | 200 |
| Carvedilol | 30 | 25 |
| Atenolol | 20 | 100 |
| Bisoprolol | 20 | 10 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers.
The mean duration of follow-up in the 2 treatment arms was 12 months. To evaluate the effectiveness and the titration of the ARNI and OMT treatment, the NYHA class and the values of systolic BP, serum potassium and creatinine were analyzed upon the initial visit, at Month 1 and 6, and at the end of the follow-up, while NT-proBNP and left ventricle EF and cardiac remodeling parameters were assessed upon the initial visit and at the end of follow-up in both arms (Fig. 1). Data on clinical outcomes of mortality and re-hospitalization due to HF were collected through the analysis of the HDSs or by contacting the General Practitioner, when the patient did not report at follow-up.
2.5. Echocardiographic study of the systo-diastolic function
Echocardiographic data were analyzed by two observers who were not aware of the patient’s arm, and consequently, of the current treatment. M-mode and two-dimensional echocardiography was performed with the IE33 system tool (Philips Medical System, Bothell, WA, United States), in accordance with the recommendations of the American Society of Echocardiography [15]. The thicknesses of the septum and the posterior wall, the size of the left ventricle (LV) in end-diastole and the mass of the LV were measured. LV mass was calculated using the Devereux formula [16] and then indexed for body surface area (LVM). LV hypertrophy was defined as an indexed LVM ≥ 115 g/m2 for males and ≥ 95 g/m2 for females. Left atrium volumes were obtained by the disc method [17] and, where possible, the estimate of systolic BP in the pulmonary artery was obtained by tricuspidal regurgitation jet [18]. All information obtained from Doppler imaging was gained during normal breathing. From the apical vision in 4 chambers, the sample volume was positioned at the mitral valve level, and 5–10 cardiac cycles were recorded to obtain the pulsed Doppler. From the mitral inflow rate, the early (E = early) and late (A = late) peak filling rate, the deceleration time of the E wave velocity and the atrial filling fraction were obtained [19]. As regards the pulmonary veins, the systolic peak velocity (S), the antegrade diastolic peak velocity (D), and the S/D ratio were calculated; the Doppler imaging program was set to the pulsed wave mode. The filters were set to exclude high-frequency signals and the Nyquist limit was adjusted to a velocity range of 15–20 cm/s. Gains were minimized, to allow for a clear tissue signal, with minimal background noise. The pulsed wave Doppler imaging was performed in apical vision, to acquire the mitral annular speeds [20], and the sample volume was positioned at ± 1 cm inside the sites of septal and lateral insertion of the mitral flaps, and adjusted as needed (usually 5–10 mm) to cover the longitudinal excursions of the mitral ring in systole and diastole. The main end-points of the echocardiographic study were the changes in EF, end-systolic volume, end-diastolic volume and left ventricular mass index (LVMI) between the treatment groups.
2.6. Statistical analysis
Continuous variables were analyzed and expressed as mean ± standard deviation and compared by means of the analysis of variance (ANOVA). For the comparison between the categorical variables, Pearson’s chi-squared test was used. The analysis of variance for repeated measures was used to compare the differences in the values of NT-proBNP, systolic BP, eGFR, and potassium at baseline and at follow-up. To obtain an 80 % probability of finding a difference of at least 1000 pg/mL of the NT-proBNP concentration between the two study arms, a potency test was performed. Considering a significance level of 5%, the sample size had to be at least 40 subjects. Statistical analyses were obtained using the SPPS package version 18.0 of Windows (SPSS, Chicago, IL, USA). The null hypothesis was always rejected for values of p < 0.05.
3. Results
The general characteristics of the patients, distributed by treatment arm (OMT and ARNI) are summarized in Table 1. In the OMT group, the proportion of patients taking ACEIs or ARBs and beta-blockers is shown in Table 2. Specifically, among patients on ACEIs, 75 % took enalapril, 20 % ramipril, and 5 % perindopril. Among those on ARBs, 70 % took valsartan, 20 % candesartan and 10 % losartan. For beta-blockers, the proportion was 30 % each for metoprolol and carvedilol and 20 % each for atenolol and bisoprolol. The maximum tolerated dose of the different drugs used in OMT is also shown in Table 2. At 1 and 6 months of follow-up, there were no significant changes in the levels of ambulatory BP, creatinine, and potassium (data not shown) between the two arms.
Table 1.
General characteristics of patients upon initial assessment, by treatment arms.
| Items | All (N = 108) | OMT (N = 54) | ARNI (N = 54) | P |
|---|---|---|---|---|
| Age (years) | 78.6 ± 8.2 | 78.6 ± 8.2 | 78.6 ± 8.2 | 1.000 |
| Male gender (%) | 75 | 75 | 75 | 1.000 |
| Body Mass Index (g/m2) | 27.1 ± 3.6 | 26.8 ± 2.7 | 27.3 ± 4.3 | 0.655 |
| Creatinine (mg/dL) | 1.23 ± 0.45 | 1.24 ± 0.45 | 1.23 ± 0.44 | 0.906 |
| eGFR (mL/min/1.73 m2) | 62.5 ± 23.4 | 63.8 ± 22.3 | 61.2 ± 24.6 | 0.571 |
| SBP (mmHg) | 125.0 ± 10.3 | 126.2 ± 8.9 | 123.8 ± 11.5 | 0.384 |
| DBP (mmHg) | 75.6 ± 10.1 | 75.2 ± 9.6 | 74.0 ± 10.6 | 0.638 |
| HR (bpm) | 66.7 ± 8.9 | 68.5 ± 9.1 | 65.1 ± 8.5 | 0.156 |
| Glycemia (mg/dL) | 115.1 ± 31.3 | 112.7 ± 28.5 | 115.4 ± 34.1 | 0.583 |
| HbA1c (%) | 5.7 | 5.6 | 5.8 | 0.169 |
| Uricemia (mg/dL) | 6.3 ± 0.59 | 6.4 ± 0.58 | 6.2 ± 0.62 | 0.169 |
| Potassium (mmol/L) | 4.0 ± 0.5 | 4.1 ± 0.4 | 4.0 ± 0.5 | 0.645 |
| Sodium (mg/dL) | 139.1 ± 6.8 | 141.2 ± 7.7 | 137.2 ± 5.3 | 0.025 |
| Hemoglobin (g/L) | 12.8 ± 1.4 | 13.0 ± 1.6 | 12.6 ± 1.7 | 0.319 |
| Cardiac parameters | ||||
| LVMI (kg/m2) | 152.2 ± 10.8 | 151.9 ± 9.5 | 152.4 ± 12.1 | 0.884 |
| EF (%) | 30.1 ± 5.1 | 29.8 ± 4.3 | 30.4 ± 5.4 | 0.462 |
| History of HF (years) | 13.4 ± 2.2 | 12.9 ± 2.5 | 13.8 ± 1.7 | 0.091 |
| NT-proBNP (pg/mL) | 5437.4 ± 3450.5 | 5363.9 ± 3562.8 | 5503.1 ± 3402.5 | 0.878 |
| NYHA Class (%) | ||||
| II | 55.4 | 57.1 | 53.6 | 0.072 |
| III | 44.6 | 42.9 | 46.4 | 0.064 |
| CRT (%) | 14.3 | 10.7 | 17.9 | 0.583 |
| ICD (%) | 21.4 | 21.4 | 21.4 | 0.627 |
| Concomitant treatment | ||||
| ACEIs (%) | 87.1 | 57.1 | 71.4 | 0.310 |
| ARBs (%) | 12.6 | 14.8 | 11.1 | 0.120 |
| Diuretics (%) | 92.5 | 94.4 | 90.7 | 0.642 |
| Beta-blockers (%) | 71.4 | 67.9 | 75.0 | 0.384 |
| Mineralocorticoid antagonists (%) | 55.4 | 53.6 | 57.1 | 0.071 |
| Digitalis (%) | 32.1 | 28.6 | 35.7 | 0.388 |
| Concomitant conditions | ||||
| Ischemic heart disease (%) | 72.2 | 70.3 | 74.1 | 0.202 |
| CKD (%) | 58.9 | 57.1 | 60.7 | 0.074 |
| Atrial fibrillation (%) | 35.7 | 32.1 | 39.3 | 0.390 |
| COPD (%) | 42.0 | 32.5 | 48.1 | 0.226 |
| Diabetes mellitus (%) | 35.7 | 32.1 | 39.3 | 0.390 |
| History of stroke (%) | 19.6 | 21.4 | 17.9 | 0.500 |
Values are percent of patients or mean ± standard deviation (SD).
OMT, optimal medical therapy; ARNI, angiotensin receptor-neprilysin inhibitor; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, glomerular filtration rate; LVMI, left ventricle mass index; LVEF, left ventricle ejection fraction; LV, left ventricle; EF, ejection fraction; HF, heart failure; NYHA, New York Heart Association; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid antagonist; CHD, coronary heart disease; CKD, chronic kidney disease; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease.
At the final follow-up (Fig. 2), NYHA class improved significantly in the arm treated with ARNI compared to the control, where 24.9 % vs. 6.4 % of the patients switched from class III to class II and 55.4 % vs. 25.2 % from class II to class I, respectively (p < 0.05). The LVEF and eGFR values increased significantly (p < 0.001) in the ARNI arm compared to the control arm, 42.4 % vs. 34.2 %; 73.8 vs. 61.2 mL/min, respectively (Fig. 3A and B). On the contrary, the values of NT-proBNP (Fig. 3C), systolic and diastolic BP, HbA1c, blood sugar, and uricemia decreased in both arms, but more significantly in the arm treated with ARNI compared to the control (3107 vs. 4552 pg/mL, 112.2 vs. 120.4 and 68.8 vs. 75.6 mmHg, 108.4 vs. 112.6 mg/dL, 5.4 vs. 5.9 % and 5.9 vs. 6.4 mg/dL respectively; p < 0.05). Furthermore, the LVMI and cardiac remodeling parameters improved significantly only in the arm treated with ARNI (Table 3). At the final follow-up, 38.5 % of patients in the ARNI treatment arm were taking the 24–26 mg dose of sacubitril/valsartan, 33 % the 49–51 mg dose and 28.5 % the 97–103 mg dose. There was a favorable trend towards a reduction in the use of loop diuretics in the ARNI arm compared to the OMT arm (74.2 % vs. 86.4 %, NS) which, however, was not significant.
Fig. 2.
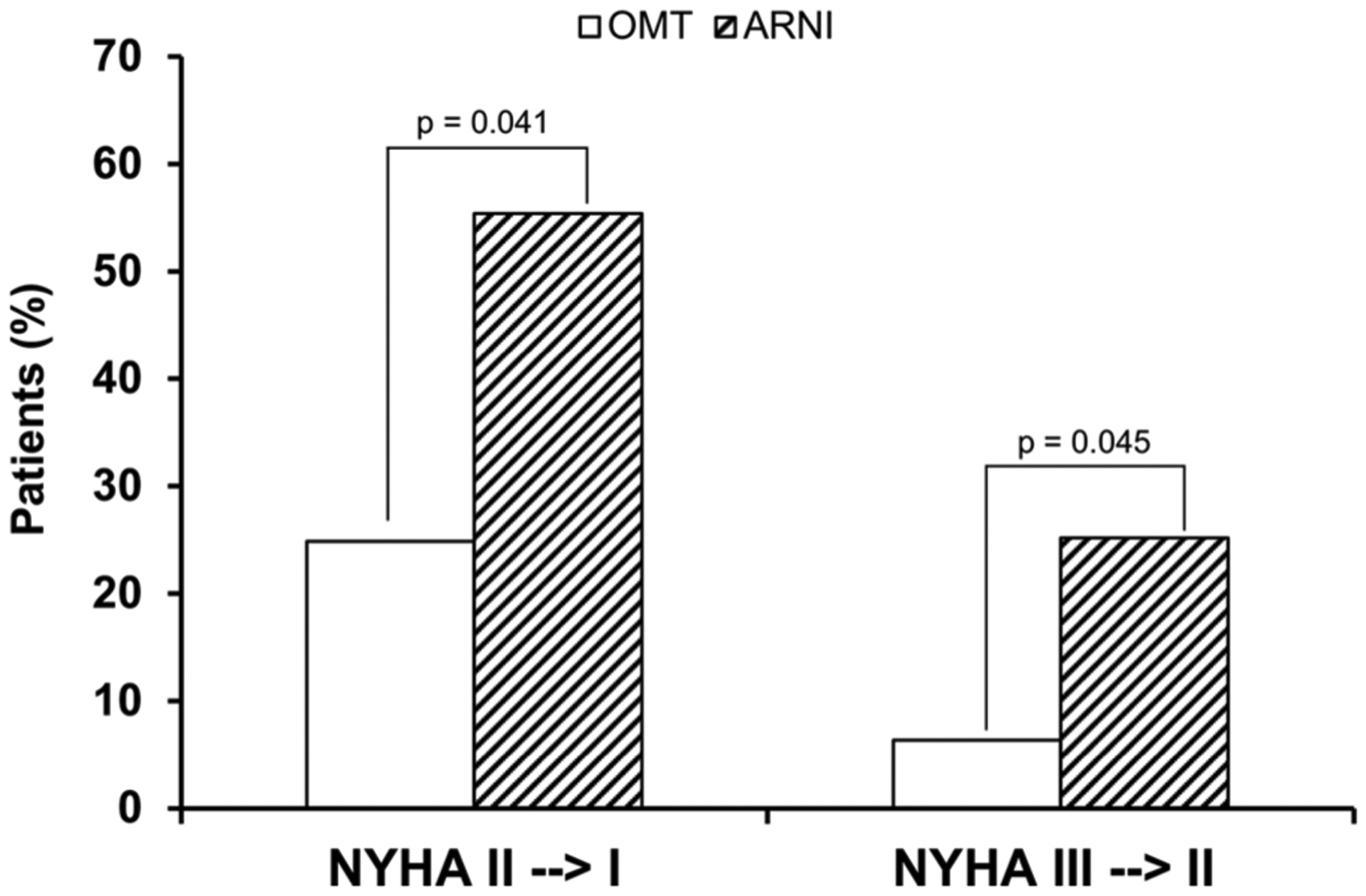
Change in New York Heart Association (NYHA) class between baseline and the final follow-up. ARNI angiotensin receptor neprilysin inhibitor; OMT standard optimal medical treatment.
Fig. 3.
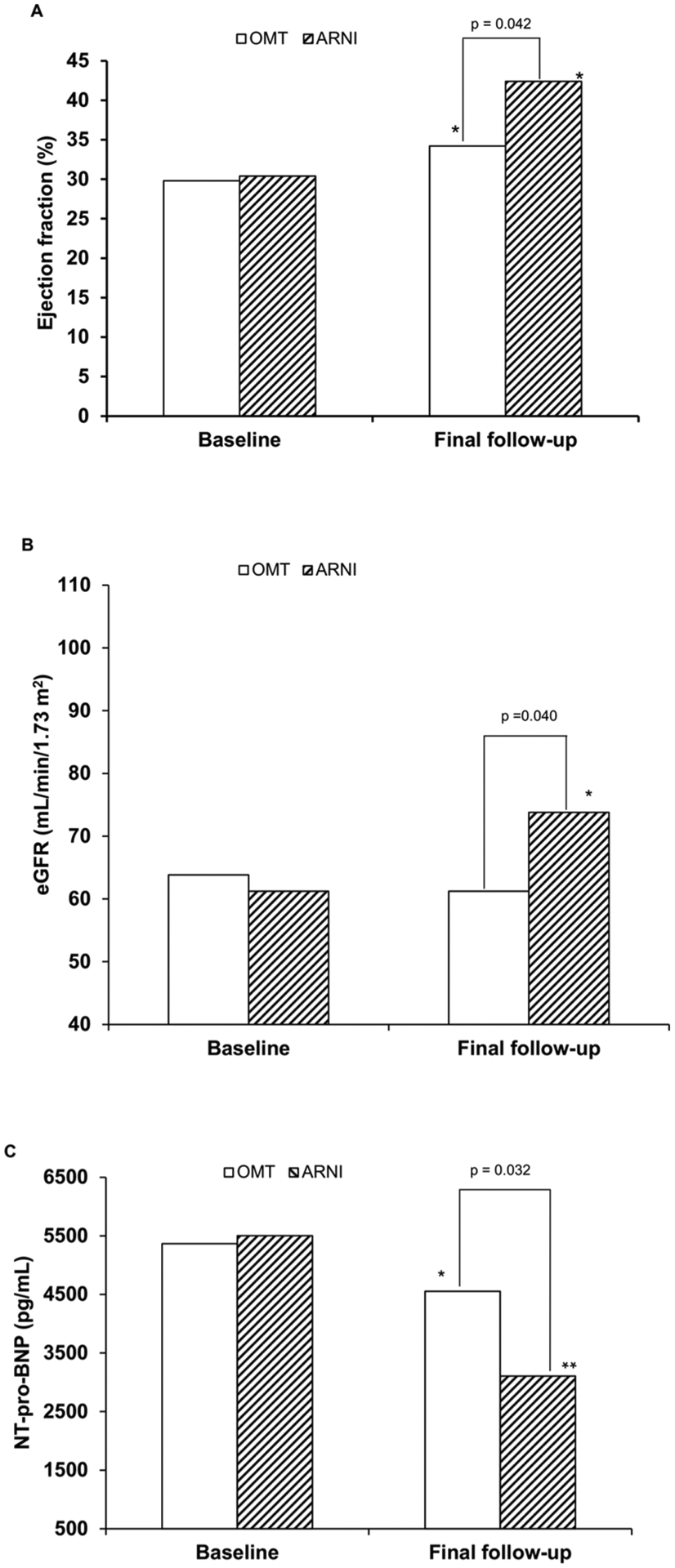
Change from baseline to final follow-up in A) left ventricular ejection fraction (EF), B) estimated glomerular filtration rate (eGFR), and C) N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations. (*p < 0.05, ** p < 0.001 vs. baseline), ARNI angiotensin receptor neprilysin inhibitor; OMT standard optimal medical treatment.
Table 3.
Hemodynamic, metabolic, renal, and cardiac remodeling parameters upon the initial evaluation and at the end of the follow-up after treatment with ARNI.
| Items | Baseline (N = 54) | Follow-up (N = 42) | Mean difference of values ± SD | P |
|---|---|---|---|---|
| SBP (mmHg) | 123.8 | 117.0 | −6.9 ± 11.6 | 0.001 |
| DBP (mmHg) | 73.9 | 69.5 | −4.1 ± 10.9 | 0.04 |
| HR (bpm) | 65.1 | 62.0 | −2.57 ± 7.21 | 0.04 |
| Glycemia (mg/dL) | 115.3 | 101.5 | −15.9 ± 21.3 | 0.001 |
| HbA1c (%) | 5.9 | 5.4 | −0.36 ± 0.31 | 0.04 |
| eGFR (ml/min/1.73 m2) | 64.8 | 70.1 | 8.46 ± 7.9 | 0.04 |
| NT-pro BNP (pg/mL) | 5503.8 | 3107.1 | −2740.2 ± 1760.1 | 0.001 |
| Uricemia (mg/dL) | 6.21 | 5.92 | −0.31 ± 0.23 | 0.04 |
| EF (%) | 29.8 | 42.1 | 11.7 ± 6.1 | 0.001 |
| End-systolic volume (mL) | 123.12 | 83.1 | −39.8 ± 25.1 | 0.014 |
| End-diastolic volume (mL) | 173.3 | 144.2 | −29.3 ± 19.7 | 0.002 |
| LVMI (g/m2) | 152.4 | 147.8 | −4.68 ± 2.19 | 0.001 |
ARNI, angiotensin receptor-neprilysin inhibitor; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; HR, heart rate; eGFR, glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SD, standard deviation; EF, ejection fraction; LVMI, left ventricular mass index.
Mortality and re-hospitalization due to HF (Fig. 4) were lower in the arm treated with ARNI compared to the control (20.1 vs. 33.6 % and 27.7 vs. 46.3 %, respectively; p < 0.05). No patient left the study voluntarily or because of the onset of adverse events.
Fig. 4.
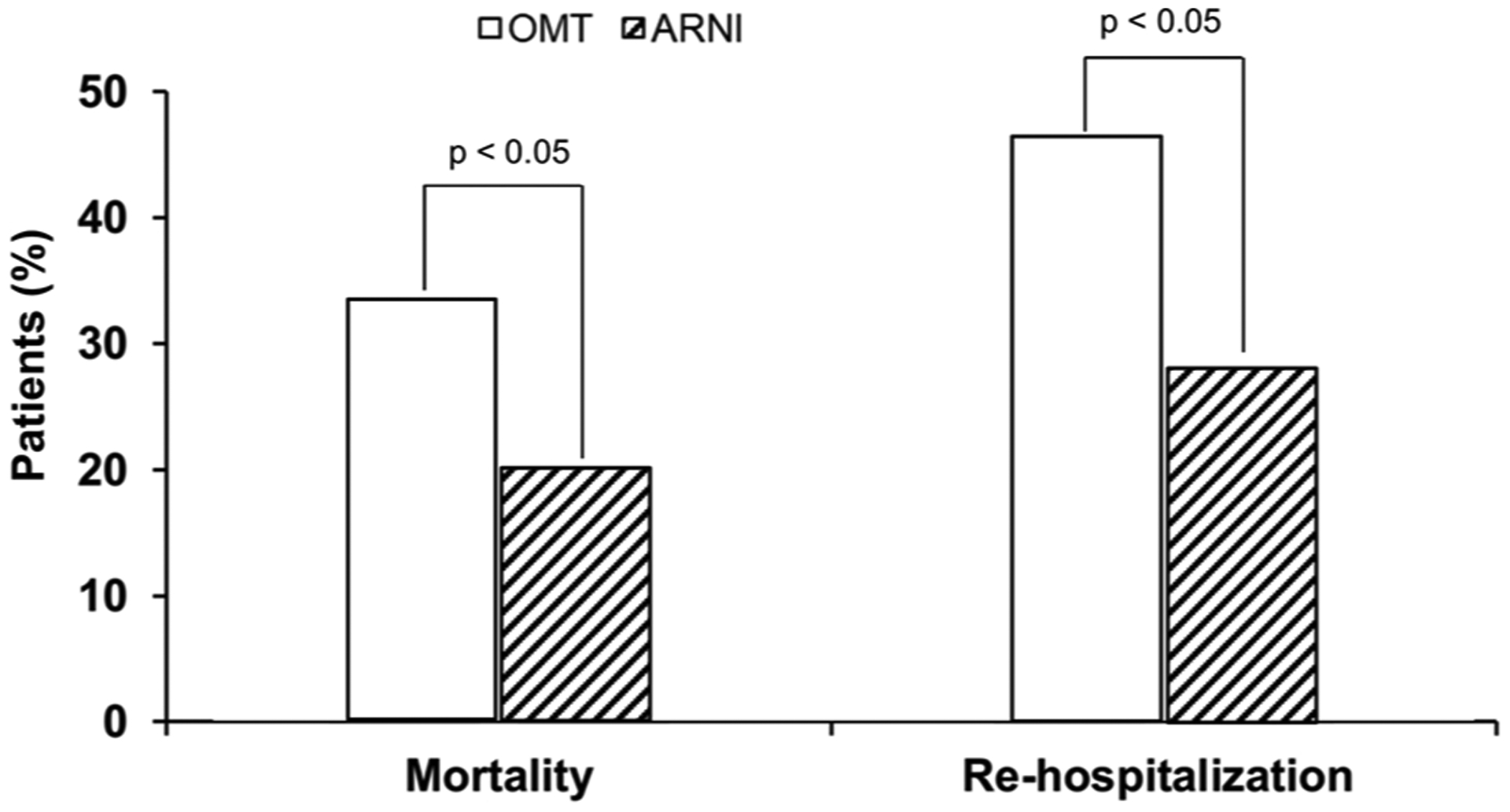
Rates of mortality and re-hospitalization for heart failure in patients treated with angiotensin receptor neprilysin inhibitor (ARNI) or standard optimal medical treatment (OMT).
4. Discussion
Over 12 years ago, the results of Comorbidities and Outcome in patients with chronic heart Failure: a study in Internal Medicine units (CONFINE) study, an observational epidemiological study conducted in Italy in Internal Medicine patients [7], had shown a high prevalence of HF patients over the age of seventy years (80 %) having multiple comorbidities. The frequent association of other disorders in HF patients is not a new observation and has also emerged in a large American study that recruited subjects with an average age of 76, who in 39 % of cases suffered from 5 conditions [21].
In our study, conducted in elderly subjects with HF with reduced EF and with comorbidities, treatment with ARNI compared to standard optimal therapy was shown to be safe and effective in improving the symptoms of HF and in reducing its rate of mortality and re-hospitalization due to HF. These effects would appear to be attributable both to the action of ARNI on cardiovascular hemodynamics for improvement of cardiac function (EF and cardiac remodeling) and BP and to the modulation of the renal filtrate and some metabolic parameters, such as blood glucose (and HbA1c) and uricemia, which are described below.
4.1. ARNI and cardiovascular hemodynamics
Although the physiological mechanisms of ARNI are well known, their effects on the remodeling of the left ventricle (LV) and EF have been poorly studied, especially in elderly patients with multiple comorbidities [9]. In arterial hypertension, the remodeling of the LV is the main mechanism by which progression from sub-clinical cardiac injury secondary to arterial hypertension leads to the signs and symptoms of HF [22]. As a result, the actions aimed at improving the left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), LVEF, and left ventricular hypertrophy (LVH) are the main goals of medical treatment, since these parameters are closely related to the clinical outcomes and survival of HF patients.
In our study, ARNI therapy at 12 months resulted in an improvement in LVEDV, LVESV, EF, and LVH, as also demonstrated by Almufleh and colleagues [23]. However, in the latter observational study, the absence of a control arm precluded a comparison between an arm treated with ARNI and an arm treated with OMT for HF. As regards the latter aspect, current guidelines [9] emphasize the role of OMT for HF, but at the same time recommend the role of adherence and persistence to treatment.
However, in the real-world setting, although OMT is the basis of the management of HF, there is much evidence to show that many HF patients do not receive OMT, or that the therapy is sub-optimal [11,24]. There are numerous reasons that can explain the failure of OMT in chronic HF, partly related to the patient, such as advanced age, comorbidities and polytherapy (which, as is known, affect compliance with therapy), to the doctor (such as inertia or lack of knowledge of the guidelines) and finally to the difficulty in using medical care due to a lack of resources of the health systems [25].
In this study, in accordance with current HF guidelines [9], patients with OMT achieved an improvement in EF, thus confirming that the treatment with ACEI or ARB, beta-blocker, and MRA is effective and safe in changing the clinical history of patients with chronic HF. However, as already demonstrated in clinical studies and animal models [26,27], the benefits observed in our study on EF and cardiac remodeling are mainly attributable to the treatment with ARNI. In addition to the action on the remodeling of the LV, it is well known that the OMT is able to interact with the physio-pathological mechanisms that determine the onset of LVH, a known predictor of cardiovascular morbidity and mortality, regardless of BP and the classic cardiovascular risk factors [9]. The prevalence of LVH gradually increases with age, and it is now established that the therapy with antihypertensive drugs, which are also the cornerstone of the treatment of chronic HF, reduces LVMI in hypertensive patients compared to placebo [28]. The regression of LVMI, in particular thanks to the treatment with renin-angiotensin-aldosterone system (RAAS) blockers, is significantly more effective than other antihypertensive drugs, such as calcium channel blockers or beta-blockers. Among the RAAS blockers, the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial [29], which included 7800 high-risk hypertensive subjects, showed that valsartan treatment resulted in less morbidity and mortality due to HF compared to the control arm taking amlodipine. These data confirmed the previous results of the Valsartan in Heart Failure (Val-HeFT) trial, where there was a 13.2 % reduction of the combined end-points in the 5010 H F patients in the valsartan arm compared to the arm treated with placebo [30].
In the light of this evidence, it can be deduced that the reduction of LVH observed in this study is also due to a significant reduction in the values of the BP, in particular for systolic BP, as observed in the arm treated with sacubitril/valsartan compared to the arm receiving OMT. In fact, in addition to the known action of valsartan on the BP reduction observed in the above studies, the inhibition of neprilysin with sacubitril increases the plasma concentrations of the atrial natriuretic peptide and the cerebral natriuretic peptide, which have the effect in lowering BP mainly through a volume reduction [31] and natriuresis [32]. Furthermore, the action of sacubitril (as discussed below) causes an increase in the glomerular filtration rate and the renal blood flow, with inhibition of the release of renin and aldosterone, which is associated with a reduction in the activity of the ortho-sympathetic system and an anti-hypertrophic and anti-fibrotic action. These mechanisms explain the greater BP reduction observed in the arm being treated with the sacubitril/valsartan combination, compared to the anti-hypertensive therapy with a single RAAS inhibitor present in the control arm receiving OMT (ACEI or ARB).
On the other hand, the magnitude of the modulation/regression of the LVH of the OMT over time has not yet been clearly defined and it seems that it can take months or years to observe significant effects on cardiac remodeling. However, it is commonly held that it takes a few months to observe a significant reduction in LV mass during an antihypertensive treatment, which is compatible with the results of our study [33]. In fact, in agreement with Thurmann and colleagues [34], the antihypertensive treatment with valsartan for 6–8 months produces a significant regression of LVH, which in turn is associated with a reduction in hospitalization for HF [30].
4.2. ARNI and kidney function
ARNI treatment also appears to improve renal function, due to the combined effect of the RAAS blockade, the increase in atrial natriuretic peptides due to the inhibition of neprilysin and the reduction of the diuretic therapy [35]. In detail, valsartan, as a RAAS AT1 receptor antagonist, blocks the effects of angiotensin II, especially in the glomerular efferent arterioles, producing a vasodilator effect, reducing glomerular filtration pressure and decreasing the extent of intra-glomerular filtration [36]. In addition, animal models have also shown how atrial natriuretic peptides act directly on the kidney by dilating and constricting the afferent and efferent arterioles, respectively, causing an increase in intra-glomerular capillary pressure and increasing the glomerular filtration rate [37]. In healthy subjects, in fact, the infusion of atrial natriuretic peptides determines an increase in glomerular filtrate [38] and the inhibition of neprilysin increases plasma levels not only of atrial natriuretic peptides but also of bradykinin and adrenomedullin [39]. The latter are modulating substances of renal hemodynamics which, at the level of the glomerulus, determine an increase in natriuresis, with a consequent reduction of the circulating plasma flow which, by reduction of the renal flow, leads to a reduction in perfusion and glomerular filtration [40]. In addition, different nephron-protective effects of bradykinin have also been described, including the inhibition of renal inflammation, apoptosis and glomerulosclerosis [41]. Consequently, ARNI treatment, by blocking the action of angiotensin II and increasing the biological action of the atrial natriuretic peptides, leads to an improvement in renal function which is superior to treatment with RAAS antagonists alone, particularly in diabetic subjects [42]. Although the benefit of ARNI treatment on renal function was more evident in subjects < 65 years old, Spannella and colleagues [43] recently observed that it was preserved in older subjects too.
Finally, ARNI treatment has been associated with a reduction in the use of loop diuretics, suggesting that such therapy may decrease the need for diuretics in patients with chronic HF with reduced EF [44]. In our study, after titration to the tolerated target dose of ARNI, while observing a trend favorable to the reduction in the use of loop diuretics, patients showed an improvement in the hydro-electrolyte balance without a significant reduction in the diuretic. In hypertensive patients with chronic HF, especially the elderly and those with CKD, the reduction or discontinuation of the diuretic therapy should be carefully evaluated, since there is a risk of creating a “rebound” effect, with retention of sodium and water, and the consequent occurrence of peripheral edema [45].
On the other hand, when long-term trials on the treatment of hypertension were examined to evaluate the effects of diuretics on the development of HF, diuretics were found to reduce the risk of HF by 52 % [46]. In particular, treatment with diuretics compared with calcium channel blockers or ACEI from the Antihypertensive in the Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) showed that chlorthalidone was superior to amlodipine in preventing HF progression and that the arm treated with amlodipine had a 38 % higher risk of developing HF at 6 years of follow-up [47]. The results of our study confirmed the beneficial clinical role of the use of diuretics (particularly loop diuretics) in the management of HF, the need for which is probably reduced by the ARNI therapy [44].
4.3. ARNI and glucose metabolism
Emerging data support a beneficial effect of ARNI treatment on glycemic control [42]. In detail, in a post-hoc analysis of the PARADIGM-HF study [48], in 3778 patients with a known diagnosis of diabetes or with a HbA1c > 6.5 %, ARNI treatment was associated with persistently lower values of HbA1c than in the control arm. In addition, the initiation of insulin therapy was 29 % less frequent in patients treated with ARNI than in those treated with enalapril, and there was lower use of oral antidiabetic agents in the ARNI arm.
The potential mechanisms through which sacubitril/valsartan could lead to an improvement in glycemic control are manifold. Natriuretic peptides, which are increased by the inhibition of neprilysin by sacubitril, could play a crucial role in insulin metabolism [49]. Serum glucose concentrations have also been shown to decrease after natriuretic peptide infusion and higher concentrations of NT-proBNP are associated with a significantly reduced risk of diabetes, after adjustment for the classic risk factors and the fasting blood glucose levels [50]. Furthermore, glucagon-like peptide-1 (GLP-1), a neuropeptide of the in-cretin family, a powerful hypoglycemic hormone with a very short circulating half-life, is partially degraded by neprilysin [51]. In animal models deficient in neprilysin, the improvement of glycemic control has been associated with high active concentrations of GLP-1, reduced activity of plasma dipeptidyl peptidase-4 and an improvement in the function of beta-cells, suggesting beneficial metabolic effects with the inhibition of neprilysin [52]. Furthermore, since angiotensin II promotes insulin resistance, the inhibition of RAAS with valsartan helps to modulate glycemic control [53]. As a consequence, it is commonly believed that the improvement in glucose metabolism by RAAS inhibition alone is likely to be very modest, and that it is mainly due to the effects of sacubitril on glucose homeostasis [54]. These biomolecular mechanisms could in part explain the reduction in blood glucose and glycated hemoglobin observed in our study in patients treated with ARNI.
4.4. ARNI and uric acid
Uric acid (UA) is the final product of purine metabolism, and hyperuricemia reflects the balance between the dietary intake of purines, the synthesis of UA by xanthine oxidase, and the renal excretion of UA [55]. In this respect, diuretic therapy, often used in HF treatment, is also associated with hyperuricemia, probably because diuretics alter the excretion of UA [55]. Indeed, hyperuricemia potentially reflects oxidative stress as a consequence of the activity of xanthine oxidase, as UA itself can have harmful effects, due to increased expression of cytokines, induction of inflammation, impaired endothelial function and activation of the RAAS [56]. These mechanisms in part explain why hyperuricemia is associated with worse clinical outcomes in patients with acute and chronic HF [57].
Whether UA represents an independent predictor of outcomes in HF is still under debate, since the renal function and the use of diuretics increase its plasma concentration [55,57]. However, in a sub-analysis of the PARADIGM-HF study, ARNI treatment at 12 months significantly reduced UA values by 0.24 mg/dL, and improved the outcomes, regardless of UA concentration [58]. This correlation between UA and HF prognosis is therefore still uncertain as hyperuricemia has been shown to be a marker of reduced excretion of UA, due to renal failure or to a higher diuretic dose [59]. On the other hand, hyperuricemia seems to increase oxidative stress (via xanthine oxidase activity) and endothelial dysfunction, which play a harmful role in HF [60]. Whether the reduction in UA levels observed in the ARNI therapy contributed or not to the reduction in morbidity and mortality observed in the PARADIGM-HF study remains to be confirmed in controlled clinical trials. However, the possibility that the reduction in UA levels may have a role in HF cannot be ruled out, as the sacubitril/valsartan mechanism on UA is still unknown. In this respect, losartan has a well-known uricosuric action [61], not observed for valsartan. On the other hand, it would seem that inhibition of neprilysin may cause a small increase in urinary excretion of UA [58].
4.5. ARNI, indicators of efficacy, comorbidity, and outcomes
As widely demonstrated, since atrial natriuretic peptides are degraded by neprilysin, treatment with ARNI increases the concentration of the atrial natriuretic peptide, the type-B natriuretic peptide (BNP) and the type-C natriuretic peptide [62]. On the contrary, neprilysin has no effect on the degradation of NT-proBNP and therefore the NT-proBNP levels are not affected by the inhibition of neprilysin. In agreement with the PARADIGM-HF study, in our study the NT-proBNP levels decreased significantly during treatment with ARNI. In detail, a NT-proBNP level lower than 1000 pg/mL was associated with an improvement NYHA functional class. However, unlike the PARADIGM study, where 70 % of the enrolled subjects were in NYHA class I or II [12], in our experience, 54 % of the subjects were in NYHA class II, therefore with a worse clinical picture of cardiac HF upon the initial visit. The improvement in the NYHA class observed in our study encourages the use of sacubitril/valsartan even in elderly people over the age of 78 and with multiple comorbidities, since NYHA functional classes, as recommended by international guidelines, are recognized as indicators able to influence the therapeutic decisions on HF management. In particular, comorbidities can worsen the management and prognosis of HF [63], in particular by promoting the patient’s functional decline, which in turn negatively affects compliance and long-term adherence to HF drug therapy. In our study, the positive effects of ARNIs on BP and blood glucose reflect in part the improvement of the two comorbidities most frequently associated with HF, i.e., arterial hypertension and diabetes, conditions which in turn, if not adequately controlled, lead to a worsening of outcomes.
In agreement with PARADIGM-HF [64], ARNIs had a positive impact on clinical outcomes in our study performed in elderly subjects. However, unlike PARADIGM-HF, where HF mortality was assessed with a composite end-point, together with cardiovascular mortality, only HF mortality was assessed in our study. The same applies to re-hospitalization due to HF, which was assessed separately and not in combination with cardiovascular mortality, as occurred in PARADIGM-HF. In detail, patients of our study who took ARNIs presented a significantly reduced HF mortality compared to those who received OMT. The data on the reduction in HF mortality observed in our study with ARNIs versus OMT are encouraging but still remain high, as one in five patients died due to HF at 12 months. This is in part also due to the advanced age of our patients, which was about 15 years greater than that of the patients enrolled in the PARADIGM-HF study, and to the greater prevalence of comorbidities present in our study (i.e. hypertension, CKD and COPD, the latter not included in the PARADIGM-HF trial), which have a negative impact on the management of HF.
Finally, the results on the hospitalizations due to HF were also very interesting, since fewer than one patient out of three on ARNI treatment was re-admitted for HF. Conversely, re-hospitalization due to HF in the arm treated with OMT was still very high (46.3 %). These data are quite in line with that observed in clinical and pivotal studies involving HF subjects of advanced age and with comorbidities [7,11]. In general, the reduction of re-hospitalizations, which determine an unsustainable burden both for the quality of life of the patients and for the overall management costs, is one of the main goals of the national health system [65].
Studies on the re-hospitalization phenomenon show that the problem is global and increasing, not only for healthcare units, as well as for the political choices of the States [66]. Data on models aimed at reducing the re-hospitalization of patients with HF is not yet able to provide conclusive indications, and the interpretation of the results must be prudent [67]. The problem of re-hospitalization does not only concern HF therapy, but it includes multifactorial approaches between the hospital, at the time of discharge, and the local management [68].
In this perspective, the implementation of diagnostic and therapeutic care pathways for the management of HF (namely PDTA), also requested by the Veneto Region, can offer intervention models targeted at the needs of the individual HF patient with comorbidities, in order to offer more intensive treatment and to reduce the risk of re-hospitalization. Indeed, as required by the Italian National Outcome Plan [69], namely Piano Nazionale Esiti (PNE), the evaluations of the HF management are based on data from health information systems, and consequently, the validity of the results depends heavily on the quality of the data collected. The auditing activity that could emerge from a diagnostic and therapeutic care pathway for HF (ongoing in our ULSS5 Polesana Health Unit) could be useful to reveal errors (for example in the coding of the clinical information used for the calculation of the PNE), to provide more reliable data on the real management of HF in Internal Medicine. However, these concepts do not reduce the role of ARNIs versus OMT in lowering the rate of re-hospitalizations for HF, but identify them as a new tool for the management of HF after hospital discharge.
This study has some limitations. The administration of the treatment in an open-label setting and the small size of the sample arm and the control, which was not treated simultaneously with the active arm (due to evident ethical problems), constitute the main limitations of the study. However, the uniformity of the two arms matched by age and gender has reduced the possible initial bias.
5. Conclusions
In conclusion, ARNI treatment appears to be safe and effective in reducing HF mortality and re-hospitalization in elderly HF subjects with reduced EF ejection and multiple comorbidities. These effects would appear to be attributable both to the action of the ARNIs on cardiac hemodynamics and BP and to the modulation of renal function and some metabolic parameters. However, these results must be confirmed in studies involving a greater number of subjects, and with a longer follow-up.
Acknowledgements
We thank Ray Hill, an independent medical writer, who provided English-language editing and journal styling prior to submission on behalf of Springer Healthcare Communications. This assistance was funded by Novartis Farma SpA.
Funding
The study was supported by an unrestricted grant from Novartis Farma SpA.
Footnotes
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
References
- [1].Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, Kadam UT, Kwok CS, Clark AB, Murchie P, Buchan I, Hannaford PC, Myint PK, Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland, Eur. J. Heart Fail 19 (9) (2017) 1095–1104. [DOI] [PubMed] [Google Scholar]
- [2].Stewart S, MacIntyre K, Capewell S, McMurray JJ, Heart failure and the aging population: an increasing burden in the 21st century? Heart 89 (1) (2003) 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mazza A, Tikhonoff V, Casiglia E, Pessina AC, Predictors of congestive heart failure mortality in elderly people from the general population, Int. Heart J 46 (3) (2005) 419–431. [DOI] [PubMed] [Google Scholar]
- [4].Casiglia E, Tikhonoff V, Pizziol A, Onesto C, Ginocchio G, Mazza A, Pessina AC, Should digoxin be proscribed in elderly subjects in sinus rhythm free from heart failure? A population-based study, Heart J. 39 (5) (1998) 639–651. [DOI] [PubMed] [Google Scholar]
- [5].Callender T, Woodward M, Roth G, Farzadfar F, Lemarie JC, Gicquel S, Atherton J, Rahimzadeh S, Ghaziani M, Shaikh M, Bennett D, Patel A, Lam CS, Sliwa K, Barretto A, Siswanto BB, Diaz A, Herpin D, Krum H, Eliasz T, Forbes A, Kiszely A, Khosla R, Petrinic T, Praveen D, Shrivastava R, Xin D, MacMahon S, McMurray J, Rahimi K, Heart failure care in low- and middle-income countries: a systematic review and meta-analysis, PLoS Med. 11 (8) (2014) e1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors A, Nielsen OW, Zannad F, Tavazzi L, Heart failure association of the ESC, EURObservational research programme: the heart failure pilot survey (ESC-HF pilot), Eur. J. Heart Fail 12 (10) (2010) 1076–1084. [DOI] [PubMed] [Google Scholar]
- [7].Biagi P, Gussoni G, Iori I, Nardi R, Mathieu G, Mazzone A, Panuccio D, Scanelli G, Cicatello C, Rinollo C, Muriago M, Galasso D, Bonizzoni E, Vescovo G, CONFINE Study Group, Clinical profile and predictors of in-hospital outcome in patients with heart failure: the FADOI “CONFINE” Study, Int. J. Cardiol 152 (1) (2011) 88–94. [DOI] [PubMed] [Google Scholar]
- [8].Aimo A, Barison A, Mammini C, Emdin M, The Barthel Index in elderly acute heart failure patients. Frailty matters, Int. J. Cardiol 254 (2018) 240–241. [DOI] [PubMed] [Google Scholar]
- [9].Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group, 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC, Eur. Heart J 37 (27) (2016) 2129–2200. [DOI] [PubMed] [Google Scholar]
- [10].Zaman S, Zaman SS, Scholtes T, Shun-Shin MJ, Plymen CM, Francis DP, Cole GD, The mortality risk of deferring optimal medical therapy in heart failure: a systematic comparison against norms for surgical consent and patient information leaflets, Eur. J. Heart Fail 19 (11) (2017) 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maggioni AP, Orso F, Calabria S, Rossi E, Cinconze E, Baldasseroni S, Martini N, Arno Observatory, The real-world evidence of heart failure: findings from 41 413 patients of the ARNO database, Eur. J. Heart Fail 18 (4) (2016) 402–410. [DOI] [PubMed] [Google Scholar]
- [12].McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators Committees, Angiotensin-neprilysin inhibition versus enalapril in heart failure, N. Engl. J. Med 371 (11) (2014) 993–1004. [DOI] [PubMed] [Google Scholar]
- [13].Chien TI, Chen HH, Kao JT, Comparison of Abbott AxSYM and Roche Elecsys 2010 for measurement of BNP and NT-proBNP, Clin. Chim. Acta 369 (1) (2006) 95–99. [DOI] [PubMed] [Google Scholar]
- [14].Knopfholz J, Disserol CC, Pierin AJ, Schirr FL, Streisky L, Takito LL, Massucheto Ledesma P, Faria-Neto JR, Olandoski M, da Cunha CL, Bandeira AM, Validation of the Friedewald formula in patients with metabolic syndrome, Cholesterol (2014) (2014) 261878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sahn DJ, DeMaria A, Kisslo J, Weyman A, Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements, Circulation 58 (6) (1978) 1072–1083. [DOI] [PubMed] [Google Scholar]
- [16].Devereux RB, Reichek N, Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method, Circulation 55 (4) (1977) 613–618. [DOI] [PubMed] [Google Scholar]
- [17].Kircher B, Abbott JA, Pau S, Gould RG, Himelman RB, Higgins CB, Lipton MJ, Schiller NB, Left atrial volume determination by biplane two-dimensional echocardiography: validation by cine computed tomography, Am. Heart J 121 (3 Pt 1) (1991) 864–871. [DOI] [PubMed] [Google Scholar]
- [18].Yock PG, Popp RL, Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation, Circulation 70 (4) (1984) 657–662. [DOI] [PubMed] [Google Scholar]
- [19].Mulvagh S, Quiñones MA, Kleiman NS, Cheirif J, Zoghbi WA, Estimation of left ventricular end-diastolic pressure from Doppler transmitral flow velocity in cardiac patients independent of systolic performance, J. Am. Coll. Cardiol 20 (1) (1992) 112–119. [DOI] [PubMed] [Google Scholar]
- [20].Rossvoll O, Hatle LK, Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: relation to left ventricular diastolic pressures, J. Am. Coll. Cardiol 21 (7) (1993) 1687–1696. [DOI] [PubMed] [Google Scholar]
- [21].Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW, Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure, J. Am. Coll. Cardiol 42 (7) (2003) 1226–1233. [DOI] [PubMed] [Google Scholar]
- [22].Cuspidi C, Sala C, Casati A, Bombelli M, Grassi G, Mancia G, Clinical and prognostic value of hypertensive cardiac damage in the PAMELA Study, Hypertens. Res 40 (4) (2017) 329–335. [DOI] [PubMed] [Google Scholar]
- [23].Almufleh A, Marbach J, Chih S, Stadnick E, Davies R, Liu P, Mielniczuk L, Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients, Am. J. Cardiovasc. Dis 7 (6) (2017) 108–113. [PMC free article] [PubMed] [Google Scholar]
- [24].Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM, Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials, J. Am. Heart Assoc 5 (6) (2016) e002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gjesing A, Schou M, Torp-Pedersen C, Kober L, Gustafsson F, Hildebrandt P, Videbaek L, Wiggers H, Demant M, Charlot M, Gislason GH, Patient adherence to evidence-based pharmacotherapy in systolic heart failure and the transition of follow-up from specialized heart failure outpatient clinics to primary care, Eur. J. Heart Fail 15 (6) (2013) 671–678. [DOI] [PubMed] [Google Scholar]
- [26].Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K, LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice, Eur. J. Heart Fail 18 (4) (2016) 386–393. [DOI] [PubMed] [Google Scholar]
- [27].Prenner SB, Shah SJ, Yancy CW, Role of angiotensin receptor-neprilysin inhibition in heart failure, Curr. Atheroscler. Rep 18 (8) (2016) 48. [DOI] [PubMed] [Google Scholar]
- [28].Simpson HJ, Gandy SJ, Houston JG, Rajendra NS, Davies JI, Struthers AD, Left ventricular hypertrophy: reduction of blood pressure already in the normal range further regresses left ventricular mass, Heart 96 (2) (2010) 148–152. [DOI] [PubMed] [Google Scholar]
- [29].Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A, VALUE Trial Group, Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial, Lancet 363 (9426) (2004) 2022–2031. [DOI] [PubMed] [Google Scholar]
- [30].Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators, A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure, N. Engl. J. Med 345 (23) (2001) 1667–1675. [DOI] [PubMed] [Google Scholar]
- [31].Wong PC, Guo J, Zhang A, The renal and cardiovascular effects of natriuretic peptides, Adv. Physiol. Educ 41 (2) (2017) 179–185. [DOI] [PubMed] [Google Scholar]
- [32].Sagnella GA, Saggar-Malik AK, Buckley MG, Markandu ND, Eastwood JB, MacGregor GA, Association between atrial natriuretic peptide and cyclic GMP in hypertension and in chronic renal failure, Clin. Chim. Acta 275 (1) (1998) 9–18. [DOI] [PubMed] [Google Scholar]
- [33].Cuspidi C, Tadic M, Grassi G, Mancia G, Treatment of hypertension: the ESH/ESC guidelines recommendations, Pharmacol. Res 128 (2018) 315–321. [DOI] [PubMed] [Google Scholar]
- [34].Thürmann PA, Kenedi P, Schmidt A, Harder S, Rietbrock N, Influence of the angiotensin II antagonist valsartan on left ventricular hypertrophy in patients with essential hypertension, Circulation 98 (19) (1998) 2037–2042. [DOI] [PubMed] [Google Scholar]
- [35].Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, Zile MR, Packer M, Desai AS, Solomon SD, McMurray JJV, Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure, JACC Heart Fail. 6 (6) (2018) 489–498. [DOI] [PubMed] [Google Scholar]
- [36].Gervasini G, Robles NR, Potential beneficial effects of sacubitril-valsartan in renal disease: a new field for a new drug, Expert Opin. Investig. Drugs 26 (5) (2017) 651–659. [DOI] [PubMed] [Google Scholar]
- [37].Ohishi K, Hishida A, Honda N, Direct vasodilatory action of atrial natriuretic factor on canine glomerular afferent arterioles, Am. J. Physiol 255 (3 Pt 2) (1988) F415–20. [DOI] [PubMed] [Google Scholar]
- [38].Pham I, Sediame S, Maistre G, Roudot-Thoraval F, Chabrier PE, Carayon A, Adnot S, Renal and vascular effects of C-type and atrial natriuretic peptides in humans, Am. J. Physiol 273 (4) (1997) R1457–64. [DOI] [PubMed] [Google Scholar]
- [39].Kaplan AP, Ghebrehiwet B, The plasma bradykinin-forming pathways and its interrelationships with complement, Mol. Immunol 47 (13) (2010) 2161–2169. [DOI] [PubMed] [Google Scholar]
- [40].Mejia R, Sands JM, Stephenson JL, Knepper MA, Renal actions of atrial natriuretic factor: a mathematical modeling study, Am. J. Physiol 257 (6 Pt 2) (1989) F1146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tabrizchi R, Dual ACE and neutral endopeptidase inhibitors: novel therapy for patients with cardiovascular disorders, Drugs 63 (20) (2003) 2185–2202. [DOI] [PubMed] [Google Scholar]
- [42].Packer M, Claggett B, Lefkowitz MP, McMurray JJV, Rouleau JL, Solomon SD, Zile MR, Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial, Lancet Diabetes Endocrinol. 6 (7) (2018) 547–554. [DOI] [PubMed] [Google Scholar]
- [43].Spannella F, Marini M, Giulietti F, Rosettani G, Francioni M, Perna GP, Sarzani R, Renal effects of Sacubitril/Valsartan in heart failure with reduced ejection fraction: a real life 1-year follow-up study, Intern. Emerg. Med 14 (8) (2019) 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJ, Solomon SD, PROSPECTIVE COMPARISON of ARNI with ACEI to DETERMINE iMPACT on GLOBAL MORTALITY MORBIDITY in HEART FAILURE Investigators, Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial, Eur. J. Heart Fail 18 (10) (2016) 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Missouris CG, MacGregor GA, Rebound sodium and water retention occurs when diuretic treatment is stopped, BMJ 316 (7131) (1998) 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moser M, Hebert PR, Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials, J. Am. Coll. Cardiol 27 (5) (1996) 1214–1218. [DOI] [PubMed] [Google Scholar]
- [47].ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group, Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to prevent Heart Attack Trial (ALLHAT-LLT), JAMA 288 (23) (2002) 2998–3007. [DOI] [PubMed] [Google Scholar]
- [48].Seferovic JP, Claggett B, Seidelmann SB, Seely EW, Packer M, Zile MR, Rouleau JL, Swedberg K, Lefkowitz M, Shi VC, Desai AS, McMurray JJV, Solomon SD, Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial, Lancet Diabetes Endocrinol. 5 (5) (2017) 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Santhekadur PK, Kumar DP, Seneshaw M, Mirshahi F, Sanyal AJ, The multi-faceted role of natriuretic peptides in metabolic syndrome, Biomed. Pharmacother 92 (2017) 826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Heinisch BB, Vila G, Resl M, Riedl M, Dieplinger B, Mueller T, Luger A, Pacini G, Clodi M, B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: a randomised placebo-controlled cross-over study in healthy men, Diabetologia 55 (5) (2012) 1400–1405. [DOI] [PubMed] [Google Scholar]
- [51].Packer M, Augmentation of glucagon-like peptide-1 receptor signalling by neprilysin inhibition: potential implications for patients with heart failure, Eur. J. Heart Fail 20 (6) (2018) 973–977. [DOI] [PubMed] [Google Scholar]
- [52].Willard JR, Barrow BM, Zraika S, Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels, Diabetologia 60 (4) (2017) 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Luther JM, Brown NJ, The renin-angiotensin-aldosterone system and glucose homeostasis, Trends Pharmacol. Sci 32 (12) (2011) 734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Davidson EP, Coppey LJ, Shevalye H, Obrosov A, Yorek MA, Vascular and neural complications in type 2 diabetic rats: improvement by sacubitril/valsartan greater than valsartan alone, Diabetes 67 (8) (2018) 1616–1626. [DOI] [PubMed] [Google Scholar]
- [55].Ueno S, Hamada T, Taniguchi S, Ohtani N, Miyazaki S, Mizuta E, Ohtahara A, Ogino K, Yoshida A, Kuwabara M, Yoshida K, Ninomiya H, Kotake H, Taufiq F, Yamamoto K, Hisatome I, Effect of antihypertensive drugs on uric acid metabolism in patients with hypertension: cross-sectional cohort study, Drug Res. (Stuttg.) 66 (12) (2016) 628–632. [DOI] [PubMed] [Google Scholar]
- [56].Spiga R, Marini MA, Mancuso E, Di Fatta C, Fuoco A, Perticone F, Andreozzi F, Mannino GC, Sesti G, Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the nf-kappab signaling pathway in HepG2 cells, Arterioscler. Thromb. Vasc. Biol 37 (6) (2017) 1241–1249. [DOI] [PubMed] [Google Scholar]
- [57].Volterrani M, Iellamo F, Sposato B, Romeo F, Uric acid lowering therapy in cardiovascular diseases, Int. J. Cardiol 213 (2016) 20–22. [DOI] [PubMed] [Google Scholar]
- [58].Mogensen UM, Køber L, Jhund PS, Desai AS, Senni M, Kristensen SL, Dukat A, Chen CH, Ramires F, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Solomon SD, Swedberg K, Packer M, McMurray JJV, PARADIGM-HF Investigators Committees, Sacubitril/valsartan reduces serum uric acid concentration, an independent predictor of adverse outcomes in PARADIGM-HF, Eur. J. Heart Fail 20 (3) (2018) 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sudharshana Murthy KA, Ashoka HG, Aparna AN, Evaluation and comparison of biomarkers in heart failure, Indian Heart J. 68 (Suppl 1) (2016) S22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cai W, Duan XM, Liu Y, Yu J, Tang YL, Liu ZL, Jiang S, Zhang CP, Liu JY, Xu JX, Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway, Biomed Res. Int 2017 (2017) 4391920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Matsumura K, Arima H, Tominaga M, Ohtsubo T, Sasaguri T, Fujii K, Fukuhara M, Uezono K, Morinaga Y, Ohta Y, Otonari T, Kawasaki J, Kato I, Tsuchihashi T, COMFORT Investigators, Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study, Clin. Exp. Hypertens 37 (3) (2015) 192–196. [DOI] [PubMed] [Google Scholar]
- [62].Myhre PL, Vaduganathan M, Claggett B, Packer M, Desai AS, Rouleau JL, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD, B-type natriuretic peptide during treatment with Sacubitril/Valsartan: the PARADIGM-HF trial, J. Am. Coll. Cardiol 73 (11) (2019) 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Manemann SM, Chamberlain AM, Boyd CM, Gerber Y, Dunlay SM, Weston SA, Jiang R, Roger VL, Multimorbidity in heart failure: effect on outcomes, J. Am. Geriatr. Soc 64 (7) (2016) 1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jhund PS, Fu M, Bayram E, Chen CH, Negrusz-Kawecka M, Rosenthal A, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray JJ, Packer M, PARADIGM-HF Investigators Committees, Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF, Eur. Heart J 36 (38) (2015) 2576–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Perrone V, Sangiorgi D, Degli Esposti L, Modugno G, Dambrosio G, Diaferia G, Delvecchio B, Dell’Orco ML, Masullo M, Ancona D, Deluca G, Campanile V, Narracci O, Nica M, Colombo D, Buda S, Heart failure in Apulia Region - Italy (Local Health Unit Barletta-Andria-Trani): analysis of the therapeutic pathways, healthcare resource consumption and related costs Italian, Recenti Prog. Med 110 (1) (2019) 23–32. [DOI] [PubMed] [Google Scholar]
- [66].Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO, Rehospitalization for heart failure: problems and perspectives, J. Am. Coll. Cardiol 61 (4) (2013) 391–403. [DOI] [PubMed] [Google Scholar]
- [67].Golas SB, Shibahara T, Agboola S, Otaki H, Sato J, Nakae T, Hisamitsu T, Kojima G, Felsted J, Kakarmath S, Kvedar J, Jethwani K, A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data, BMC Med. Inform. Decis. Mak 18 (1) (2018) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sevilla-Cazes J, Ahmad FS, Bowles KH, Jaskowiak A, Gallagher T, Goldberg LR, Kangovi S, Alexander M, Riegel B, Barg FK, Kimmel SE, Heart failure home management challenges and reasons for readmission: a qualitative study to understand the patient’s perspective, J. Gen. Intern. Med 33 (10) (2018) 1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Barbato A, Meggiolaro A, Rossi L, Fioravanti C, Palermita F, La Torre G, Tuscan chronic care model: a preliminary analysis Italian, Ig. Sanita Pubbl 71 (5) (2015) 499–513. [PubMed] [Google Scholar]


