Abstract
A novel l-nucleoside analog of deoxycytidine, 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-fluorocytidine (β-l-Fd4C), was recently shown to strongly inhibit hepatitis B virus (HBV) replication in the 2.2.15 cell line. Therefore, its antiviral activity was evaluated in the duck HBV (DHBV) infection model. Using a cell-free system for the expression of the DHBV polymerase, β-l-Fd4C-TP exhibited a concentration-dependent inhibition of dCTP incorporation into viral minus-strand DNA with a 50% inhibitory concentration of 0.2 μM which was lower than that of other tested deoxycytidine analogs, i.e., lamivudine-TP, ddC-TP, and β-l-FddC-TP. Further analysis showed that β-l-Fd4C-TP is likely to be a competitive inhibitor of dCTP incorporation and to cause premature DNA chain termination. In primary duck hepatocyte cultures infected in vitro, β-l-Fd4C administration exhibited a long-lasting inhibitory effect on viral DNA synthesis but could not clear viral covalently closed circular DNA (CCC DNA). Results of short-term antiviral treatment in experimentally infected ducklings showed that β-l-Fd4C exhibited the most potent antiviral effect, followed by β-l-FddC, lamivudine, and ddC. Longer administration of β-l-Fd4C induced a sustained suppression of viremia (>95% of controls) and of viral DNA synthesis within the liver. However, the persistence of trace amounts of viral CCC DNA detected only by PCR was associated with a recurrence of viral replication after drug withdrawal. In parallel, β-l-Fd4C treatment suppressed viral antigen expression within the liver and decreased intrahepatic inflammation and was not associated with any sign of toxicity. Our data, therefore, demonstrate that in the duck model of HBV infection, β-l-Fd4C is a potent inhibitor of DHBV reverse transcriptase activity in vitro and suppresses viral replication in the liver in vivo.
Chronic hepatitis B virus (HBV) infection remains a major public health problem worldwide, with three hundred million chronic carriers of the virus, and its serious clinical consequences include liver cirrhosis and hepatocellular carcinoma (19). Unfortunately, alpha interferon therapy induces a sustained antiviral response in only 20 to 30% of the patients (12). The development of new nucleoside analogs, such as β-l(−)-2′,3′-dideoxy-3′-thiacytidine [l(−)SddC or 3TC or lamivudine] that exhibit a potent inhibitory effect on HBV reverse transcriptase activity and viral replication in vitro (2, 6, 31), has opened new avenues in the antiviral therapy of chronic hepatitis B. Results of phase II and phase III clinical trials have shown that administration of lamivudine results in a dramatic suppression of viral replication which is accompanied by an improvement in liver histology (16, 27, 44). However, because of the relatively low rate of anti-HBe seroconversion and of the special features of the viral kinetics, long-term therapy with a nucleoside analog is required to eradicate viral infection (16, 28). Indeed, chronic HBV infection is characterized by a high rate of virus production, by the absence of a cytopathogenic effect (and therefore a long half-life of infected hepatocytes), and by the persistence of viral genomes as a covalently closed circular DNA (CCC DNA) in the nucleus of infected cells (9, 26, 28, 36, 40). Because of the spontaneous error rate of the viral reverse transcriptase, prolonged administration of a single nucleoside analog in chronically infected patients may select for the replication of resistant viral strains. The rate of selection of resistant mutants is 23% after 1 year of lamivudine treatment and increases to 38% at the end of the second year of therapy (16). The same observation has been made with long-term treatment with famciclovir, another inhibitor of HBV polymerase, and it was demonstrated that the resistant viruses harbor mutations in conserved domains of the viral reverse transcriptase (29, 44).
In order to design new strategies that combine several antiviral agents with different mechanisms of action to prevent the emergence of resistant strains, the development of new inhibitors of HBV replication is required (44). In the search for new potent antiviral agents, 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-fluorocytidine (β-l-Fd4C) was found to exhibit a potent antiviral activity against human immunodeficiency virus and HBV replication in tissue culture (7, 23). β-l-Fd4C was found to be at least 10 times more potent (50% inhibitory concentration [IC50] at 1 nM) than lamivudine (IC50 at 15 nM) on HBV DNA synthesis in the hepatoma cell line HepG2 2.2.15, and its triphosphate derivative specifically inhibited the virion associated HBV DNA polymerase activity (41). Detailed analysis of the intracellular metabolism of β-l-Fd4C revealed that the degree of phosphorylation and retention time of the triphosphate metabolites were higher than for lamivudine which may explain, at least in part, both the more potent and sustained antiviral effects of β-l-Fd4C observed after drug withdrawal in tissue culture. β-l-Fd4C was found to be slightly more cytotoxic (IC50, 20 μM) than lamivudine (IC50, 50 μM), but β-l-Fd4C had no inhibitory activity against mitochondrial DNA synthesis at concentrations up to 10 μM (41). Furthermore, β-l-Fd4C-triphosphate was found to be a poor substrate for polymerase gamma, a very poor substrate for polymerase alpha, and not a substrate for polymerase epsilon (15). Given its antiviral activity and its pharmacodynamic properties, β-l-Fd4C should be considered for development as an anti-HBV agent. We have therefore characterized its antiviral activity in the duck hepatitis B virus (DHBV) infection model in comparison with lamivudine and other deoxycytidine analogs. This model provides relevant tools to study the mechanism of action of new antiviral compounds on the viral polymerase expressed in vitro, in primary hepatocyte cultures and in vivo in experimentally infected animals (1, 4, 8, 20, 25, 42). In the studies reported herein, we give evidence that β-l-Fd4C suppresses DHBV reverse transcription and inhibits the initiation of infection as well as viral antigen expression in hepatocytes.
MATERIALS AND METHODS
Drugs.
β-l-Fd4C and its triphosphate form (β-l-Fd4C-TP) were synthesized in the Department of Pharmocology and the Comprehensive Cancer Center, Yale University School of Medicine, New Haven, Conn., as described by Lin et al. (23) and Kukhanova et al. (15), respectively. 2′,3′-Dideoxy-β-l-5-fluorocytidine (β-l-FddC) and its triphosphate form (β-l-FddC-TP) were also designed and synthesized in the same laboratory as described by Lin et al. (21, 22). 2′,3′-Dideoxycytidine (ddC) and its triphosphate form (ddC-TP) were purchased from Sigma, Saint Quentin Fallavier, France. l(−)SddC (also called 3TC or lamivudine) was provided by VION pharmaceuticals and its triphosphate form (3TC-TP) was a generous gift from J. Kitson (Glaxo Research, Greenford, United Kingdom).
An in vitro assay for the expression of enzymatically active DHBV reverse transcriptase and the study of the inhibitory effect of nucleoside analog triphosphates.
The enzymatically active DHBV polymerase polypeptide was synthesized from the plasmid pHP, which contains the viral polymerase gene under the control of the SP6 promoter, and the RNA template of reverse transcription, as previously described (39, 43). The DHBV polymerase gene was transcribed and translated in a coupled transcription-translation rabbit reticulocyte lysate system (TNT SP6 Coupled Reticulocyte Lysate System; Promega, Charbonnières, France), according to the manufacturer's instructions. The reverse transcription assay was performed as previously described (42). After translation, the viral polymerase was incubated for 30 min at 30°C in a mixture containing 50 mM Tris-HCl (pH 7.5), 15 mM NaCl, 10 mM MgCl2, dATP, dGTP, dTTP (100 μM each), and 0.165 μM [α-32P]dCTP (3,000 Ci/mmol). The inhibition of [α-32P]dCMP incorporation in DHBV minus-strand DNA was performed with the addition of various polymerase inhibitors (β-l-Fd4C-TP, 3TC-TP, β-l-FddC-TP, and ddC-TP) at the concentrations indicated below. Radiolabelled viral DNA covalently attached to polymerase was subjected to electrophoresis through 0.1% sodium dodecyl sulfate (SDS)–10% polyacrylamide gels as already published (39, 43), and the dried gels were exposed to X-ray film. A quantitative dot assay was performed after spotting 2 μl of the radiolabelled reverse transcription assay mixture onto a DE-81 filter (Whatman), followed by three washes in 2× SSC (300 mM NaCl and 30 mM sodium citrate) for 20 min each and two washes in 95% ethanol for 15 min each, and then the radioactivity incorporated in viral minus-strand DNA was measured in a Beckman LS 6000SC scintillation counter (4, 42). To determine whether β-l-Fd4C-TP acts as a competitive inhibitor of [α-32P]dCTP incorporation into the nascent viral DNA, the reverse transcription assay described above was performed with [α-32P]dCTP at a final concentration of 0.165 or 0.825 μM together with β-l-Fd4C-TP at increasing concentrations as indicated in the results section.
To gain more insight into the mechanism of action of β-l-Fd4C-TP, we asked whether this compound acts as a DNA chain terminator. With this aim, we used a mutated pHP plasmid in which the TTAC sequence in the bulge of epsilon had been replaced by site-directed mutagenesis by a TGAC sequence. With this construct, the priming of reverse transcription leads to the synthesis of the short DNA primer whose sequence is modified from GTAA (wild type) to GTCA (mutant). The viral polymerase expressed in the reticulocyte lysate system was then incubated with dGTP, dTTP, and dCTP at 0.165 μM each, with or without β-l-Fd4C-TP or 3TC-TP, and with [α-32P]dATP (0.165 μM). Then the kinetics of incorporation of [α-32P]dAMP in the viral primer in the presence of varying concentrations of dCTP and β-l-Fd4C-TP were determined.
Primary duck hepatocyte cultures.
Primary hepatocyte cultures were prepared from 4-week-old Pekin ducks (Anas domesticus). The procedures of liver perfusion and hepatocyte isolation and culture conditions were described previously by Tuttleman et al. (37). Hepatocytes were seeded on six-well plates at a density of 5 × 105 cells per well, and the serum-free growth medium was changed daily. Infection of primary hepatocyte cultures was performed, 1 day postplating, with a DHBV-positive serum (4 × 108 DNA genome equivalents per well). To determine the effect of antiviral administration on viral DNA synthesis, the addition of drugs to the culture medium at the indicated concentrations was carried out from day 7 to day 13 postseeding. To determine the capacity of antiviral therapy to inhibit the initial steps of viral infection including CCC DNA formation and its amplification, the cultures were inoculated with an infectious serum at day 2 postplating, and drugs were added to the culture medium from day 1 to day 4 postseeding.
Cellular toxicity was analyzed daily by light microscope examination and measurement of the lactic acid level in cell supernatants (Lactate PAP; bioMérieux, Marcy l'Etoile, France). Furthermore, cellular viability was assessed by cellular uptake of neutral red dye (Sigma). Briefly, hepatocytes were seeded in 12-well tissue culture plates and were cultured in medium containing increasing concentrations of β-l-Fd4C from day 2 to day 8, with daily change of medium. Four wells were analyzed for concentration at the end of treatment. Cell viability was estimated according to a protocol already described (11), and the IC50 was defined as the drug concentration required to reduce cell viability by 50%.
Experimental inoculation of ducklings.
Ducklings were maintained under normal daylight, fed with standard commercial diet and water ad libitum, in accordance with the guidelines for animal care at the facilities of the National Veterinary School of Lyon, Marcy l'Etoile, France. Three day-old Pekin ducklings were inoculated intravenously with a DHBV-positive serum containing 1.5 × 107 viral genome equivalents, following a protocol previously described (1, 17, 42). Ducklings received deoxynucleoside analogs by intraperitoneal administration, starting 3 days postinoculation (therapeutic regimen) for a duration of 5 days or 4 weeks, according to the protocols described in the results section. Viremia, animal weight, and lactic acid levels were monitored throughout the study period.
Analysis of viral DNA.
DHBV DNA from the serum of experimentally infected ducklings and from hepatocyte culture supernatants was detected by a specific dot blot hybridization assay at different time points as indicated. Fifty microliters of serum or 800 μl of culture supernatant were spotted directly on nitrocellulose filters (Sartorius, Göttingen, France) using the Hybri-Dot Manifold apparatus (Life Technologies, Cergy Pontoise, France). After denaturation (0.2 M NaOH, 1 M NaCl), neutralization (0.5 M Tris-HCl [pH 8] with 1 M NaCl followed by 2× SSC), and fixation (80°C for 2 h), filters were hybridized with a full-length, α-32P-labelled DHBV genomic DNA probe. Filters were autoradiographed, and spots were counted in a scintillation counter (42). The limit of detection of serum viral DNA by this assay is 100 pg/ml.
Intrahepatic viral DNA from experimentally inoculated ducklings was extracted at different time points as indicated according to a procedure described in detail elsewhere (13). Liver samples were snap-frozen in liquid nitrogen, stored at −80°C, and then analyzed for viral DNAs. One hundred milligrams of liver was homogenized in a solution containing 10 mM Tris-HCl (pH 7.5) and 10 mM EDTA and was divided in two parts, one for isolation of total viral DNA (after proteinase K digestion, phenol-chloroform extraction followed by ethanol precipitation) and the other for isolation of non-protein-bound viral CCC DNA (after SDS-KCl precipitation of protein-bound DNA, phenol-chloroform extraction of the supernatant followed by ethanol precipitation). To normalize cellular DNA concentration in each well, DNA concentration was determined using both spectrophotometric analysis and optical density analysis after electrophoresis through agarose gels. Five micrograms of total DNA or CCC DNA preparation was then subjected to electrophoresis through 1.5% agarose gels and were transferred by blotting to nylon membranes (Hybond N+; Amersham, Courtaboeuf, France), and viral DNAs were detected by hybridization with α-32P-labelled probe representing the complete viral genome.
PCR detection of DHBV DNA was performed on viral CCC DNA preparations with a specific primer pair as described elsewhere (14). Primer P1 (5′-GCG CTT TCC AAG ATA CTG GAG CCC AA-3′) at nucleotide positions 1426 to 1451 was used with primer P3 (5′-CCC TGT GTA GTC TGC CAG AAG TCT TC-3′) at nucleotide positions 2843 to 2818 to amplify the gap region of the viral genome which is completely double stranded only in CCC DNA. After 30 amplification cycles (1 min, 94°C; 3 min, 72°C), PCR products were separated through 1.5% agarose gels and analyzed by Southern blotting as previously described.
To analyze the viral DNA in primary duck hepatocytes, cells were rinsed with phosphate-buffered saline and stored at −80°C for DNA isolation. Viral CCC DNA (non-protein-bound DNA) and replicative intermediate DNAs (protein-bound DNA) were isolated as described by Summers et al. (34). CCC DNA preparations as well as an equivalent volume of the corresponding replicative intermediate DNA preparations were analyzed by electrophoresis through 1.5% agarose gels, were transferred by blotting to nylon membranes (Hybond N+; Amersham), and were hybridized with α-32P-labelled full-length DHBV genomic DNA probe.
Analysis of liver histology in experimentally infected ducklings.
Formalin-fixed liver tissue sections embedded in paraffin, sectioned at 3-μm thickness, and stained with hematoxylin, eosin, and safran were examined by light microscopy. The level of hepatocyte necrosis (acidophilic bodies), portal tract and intralobular inflammation, steatosis, and ductular proliferation were assessed semiquantitatively, under code.
Immunostaining of liver sections for DHBV pre-S antigen.
Five-micrometer deparaffinized liver tissue sections were incubated overnight with an anti-DHBV pre-S murine monoclonal antibody (MAb), MAb 900, previously characterized by Chassot et al. (3). This step was followed by incubation with biotinylated goat anti-mouse immunoglobulin G (Dako, Trappes, France). The antigen-antibody complex was then revealed with streptavidin-horseradish peroxidase (Dako). The visualization was performed by using DAB chromogen substrate according to the manufacturer's instructions (Dako) (35). All specimens were evaluated blind.
RESULTS
β-l-Fd4C-TP exhibits a potent inhibitory effect on the DHBV reverse transcriptase.
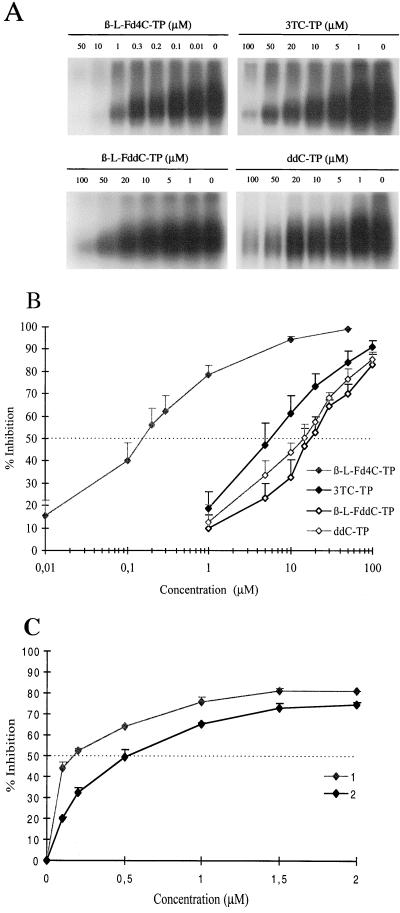
Using a cell-free system for the expression of an enzymatically active DHBV polymerase, we analyzed the inhibitory effect of the triphosphate form of β-l-Fd4C on the DHBV polymerase activity, in comparison with other deoxycytidine analog triphosphates, ddC-TP, β-l-FddC-TP, and 3TC-TP. The level of inhibition of DHBV minus-strand DNA synthesis obtained with these compounds was assessed by quantifying the incorporation of [α-32P]dCMP into the nascent viral DNA. The results showed that the incorporation of radiolabelled dCMP in viral minus-strand DNA was reproducibly inhibited in a dose-dependent manner by the addition of increasing concentrations of deoxycytidine analog triphosphates (Fig. 1A). For a substrate concentration of 0.165 μM, the IC50s for dCMP incorporation in viral minus-strand DNA were in decreasing order: 19.4 ± 5.3 μM for β-l-FddC-TP, 15.2 ± 5.9 μM for ddC-TP, 6.3 ± 2.2 μM for 3TC-TP, and 0.2 μM ± 0.064 μM for β-l-Fd4C-TP (means of five experiments) (Fig. 1B). More than 80% inhibition of viral DNA synthesis was obtained at 100 μM with all these molecules. However, β-l-Fd4C-TP exhibited the most potent inhibitory activity with an IC75 of only 0.96 μM ± 0.6 μM and an IC90 of 8.15 μM ± 1.65 μM (means of 13 experiments performed).
FIG. 1.
β-l-Fd4C-TP is a potent inhibitor of viral minus-strand DNA synthesis by the DHBV reverse transcriptase and acts as a competitive inhibitor of dCTP. The in vitro-expressed DHBV polymerase was incubated in presence of [α-32P]dCTP (0.165 μM) and the cold deoxynucleotides together with dCTP analog triphosphates (β-l-Fd4C-TP, ddC-TP, β-l-FddC-TP, and 3TC-TP) at the indicated concentrations, as described in Materials and Methods. Viral nascent minus-strand DNA covalently linked to the viral polymerase was analyzed through 0.1% SDS–10% polyacrylamide gels, and representative results of one experiment are depicted in panel A. Viral nascent minus-strand DNA was analyzed quantitatively by a dot blot assay, and the results of the inhibition of [α-32P]dCMP incorporation in viral DNA (mean of five experiments) are plotted on the graph in panel B (logarithmic scale). Competitive inhibition of the incorporation of dCTP by β-l-Fd4C-TP was performed as described in Materials and Methods. Results plotted in panel C for 0.165 μM of [α-32P]dCTP (plot 1) and 0.825 μM of [α-32P]dCTP (plot 2). Standard deviations are indicated.
To determine whether β-l-Fd4C-TP acts as a competitive inhibitor of dCTP incorporation in DHBV minus-strand DNA, the same reaction was performed with increasing concentrations of the substrate [α-32P]dCTP which was used at 0.165 μM and at 0.825 μM. Increasing the concentration of [α-32P]dCTP by fivefold shifted the IC50 from 0.17 μM to 0.50 μM, suggesting a competitive inhibitory effect of β-l-Fd4C-TP on dCMP incorporation in viral DNA (Fig. 1C).
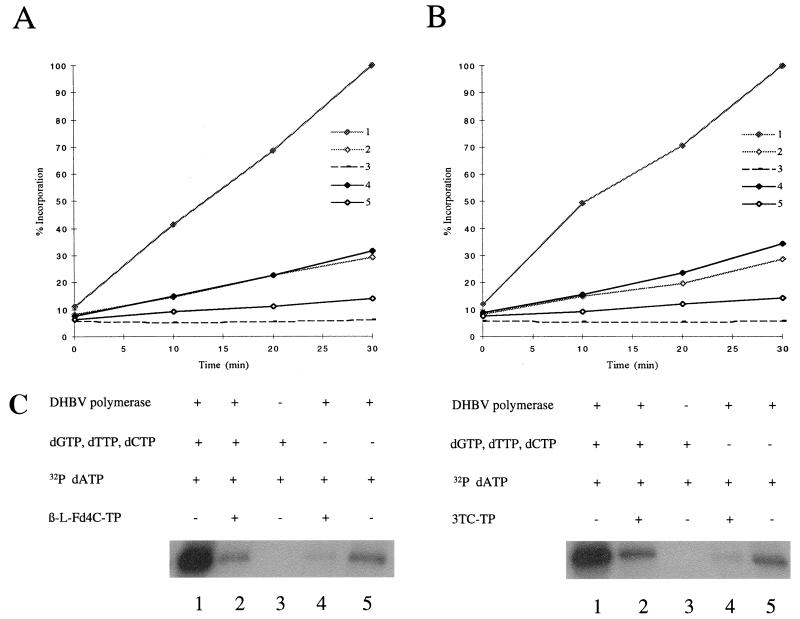
We then asked whether β-l-Fd4C-TP acts as a terminator of viral DNA chain elongation. A mutated pHP plasmid allowing the synthesis, by the priming of reverse transcription, of a short DNA primer whose sequence is modified from GTAA (wild type) to GTCA (mutant) was used. The viral polymerase was incubated with dGTP, dTTP, and dCTP at 0.165 μM each with or without β-l-Fd4C-TP (10 μM) or 3TC-TP (100 μM) and with [α-32P]dATP (0.165 μM). In the control experiment, the results showed the incorporation of [α-32P]dAMP in the viral DNA primer and an arrest in viral DNA synthesis after the priming reaction, as previously described by Wang and Seeger (38, 39) (Fig. 2). When no deoxynucleoside triphosphates (dNTPs) were added, a discrete incorporation of [α-32P]dAMP was still detectable, suggesting that priming of reverse transcription occured with a pool of endogeneous dNTPs in the reticulocyte lysate. When β-l-Fd4C-TP (10 μM) (Fig. 2A) or 3TC-TP (100 μM) (Fig. 2B) were added to the reaction, a dramatic inhibition of [α-32P]dAMP incorporation to the level of background was observed, suggesting that these compounds not only were capable of competing dCTP incorporation in the viral primer but also inhibited the incorporation of the next nucleotide, dATP. Altogether, these results suggested that β-l-Fd4C-TP may act as a viral DNA chain terminator.
FIG. 2.
β-l-Fd4C-TP acts as a viral DNA chain terminator. A mutated pHP plasmid encoding the viral polymerase with the natural TTAC sequence in the bulge of epsilon replaced by site-directed mutagenesis by a TGAC sequence was used. With this construct, the priming of reverse transcription leads to the synthesis of a short DNA primer whose sequence is modified from GTAA (wild type) to GTCA (mutant). The viral polymerase expressed in the reticulocyte lysate system was incubated with or without cold deoxynucleotides (dGTP, dTTP, and dCTP at 0.165 μM each), in the presence or absence of β-l-Fd4C-TP (10 μM) or 3TC-TP (100 μM), and with [α-32P]dATP (0.165 μM). Kinetics of incorporation of [α-32P]dAMP in the viral primer covalently attached to the viral polymerase were analyzed after electrophoresis through 0.1% SDS–10% polyacrylamide gels and autoradiography (panel C) as well as by a dot blot assay as described in Materials and Methods. Results of kinetics were plotted on the graphs: panel A, β-l-Fd4C-TP experiment; panel B, 3TC-TP experiment. Plot 1, viral polymerase incubated with cold dNTPs and [α-32P]dATP; plot 2, viral polymerase incubated with cold dNTPs, [α-32P]dATP, and a reverse transcriptase inhibitor; plot 3, no polymerase with cold dNTPs and [α-32P]dATP; plot 4, viral polymerase incubated with [α-32P]dATP and a reverse transcriptase inhibitor, but without cold dNTPs; plot 5, viral polymerase incubated with [α-32P]dATP, but without cold dNTPs or reverse transcriptase inhibitor. The level of [α-32P]dAMP incorporation without drug (plot 1), after an incubation of 30 min, was arbitrarily chosen as 100%.
Inhibition of DHBV DNA synthesis and CCC DNA formation in primary duck hepatocytes by β-l-Fd4C.
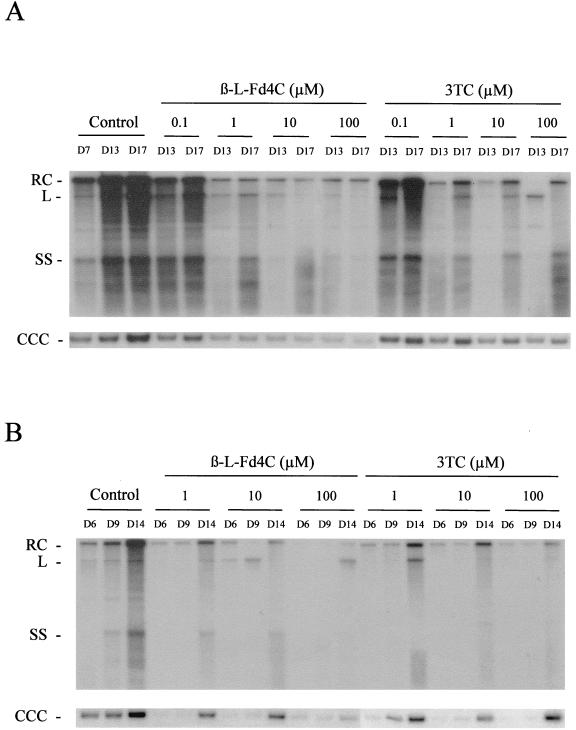
The inhibitory activity of β-l-Fd4C on DHBV replication was then analyzed in experimentally infected primary duck hepatocyte cultures. In the first set of experiments, we investigated the inhibitory effect of β-l-Fd4C on DHBV DNA synthesis. Different concentrations of β-l-Fd4C or 3TC were studied in parallel. Six days after virus inoculation of the hepatocyte cultures, drugs were added to the culture medium for 6 consecutive days with a daily medium change. The supernatant of each culture well was collected daily for virion DNA analysis, and intracellular viral DNA was analyzed prior to therapy, at the end of treatment, and 4 days posttreatment. Dot blot analysis of viral DNA in the culture supernatants indicated that virion release by hepatocytes in the culture medium was reproducibly inhibited by β-l-Fd4C in a concentration-dependent fashion (data not shown). The antiviral effect of β-l-Fd4C seemed to last up to 4 days after cessation of treatment. Southern blot analysis of the intracellular viral DNAs showed a more pronounced inhibition of the synthesis of viral replicative intermediates (relaxed circular (RC) DNA, single strand (SS) DNA, and linear (L) DNA) and viral CCC DNA by β-l-Fd4C compared to 3TC (Fig. 3A). However, viral CCC DNA was still detected at the end of the treatment, indicating the absence of viral clearance from infected hepatocytes. No particular sign of cellular toxicity was detected by the daily light microscope examination and by the incorporation of neutral red (IC50 > 100 μM). Quantification of lactic acid in cell culture supernatants showed no significant increase, and examination of cellular DNA on agarose gels did not show any DNA ladder (5).
FIG. 3.
β-l-Fd4C inhibits viral DNA synthesis in primary duck hepatocyte cultures. (A) Primary duck hepatocyte cultures were inoculated with an infectious serum on day 1 postseeding, and intracellular viral DNA was analyzed after Southern blotting and specific hybridization at the indicated time points during cell culture. β-l-Fd4C and 3TC were added 6 days postinoculation (day 7), at the indicated concentrations, for 6 consecutive days with a daily medium change in the curative protocol. In this experiment, viral DNA was analyzed at the end of treatment (day 13) and 4 days posttreatment (day 17). (B) In the preventive treatment, cultures were inoculated with an infectious serum 2 days postplating. Drugs (β-l-Fd4C and 3TC) were added 1 day prior to the inoculation and were maintained at the indicated concentrations for 4 days postinoculation. In this experiment, viral DNA was analyzed at the end of treatment (day 6), and 3 and 8 days posttreatment (days 9 and 14, respectively). The viral replicative intermediates are indicated.
In the second set of experiments, we examined whether β-l-Fd4C could inhibit the initial steps of viral infection including CCC DNA formation and its amplification in experimentally infected primary hepatocytes. To answer this question, primary hepatocytes were treated by β-l-Fd4C or 3TC 1 day before the infection, the day of inoculation, and for the next 4 days. Southern blot analysis of the viral replicative intermediates and CCC DNA was performed at the end of treatment and at 3 and 8 days posttreatment (Fig. 3B). The results showed that both drugs inhibited the synthesis of viral DNA replicative intermediates and the synthesis of CCC DNA, resulting in a delay in the initiation of viral replication in tissue culture (Fig. 3B). Eight days after 3TC withdrawal, CCC DNA synthesis as well as replicative intermediates became detectable. Administration of β-l-Fd4C at 10 or 100 μM was associated with the more pronounced suppression of the synthesis of all forms of viral DNA, and this inhibitory effect was sustained even 8 days posttreatment. Interestingly, in cells treated with β-l-Fd4C, only faint bands of RC DNA and CCC DNA could be detected, suggesting that β-l-Fd4C had blocked the viral replication cycle after the transformation of viral RC DNA in CCC DNA form (Fig. 3B). These results were confirmed by the analysis of virion DNA release in cell culture supernatants (data not shown). This delay in the initiation of DHBV infection suggested that β-l-Fd4C or its active metabolite had a long half-life in primary duck hepatocytes.
Short-term administration of β-l-Fd4C transiently inhibits viral replication in vivo in experimentally infected ducklings.
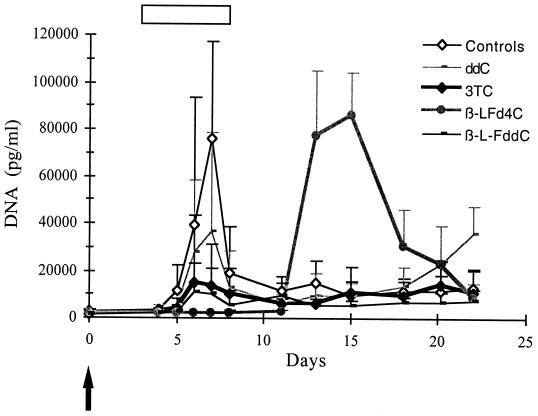
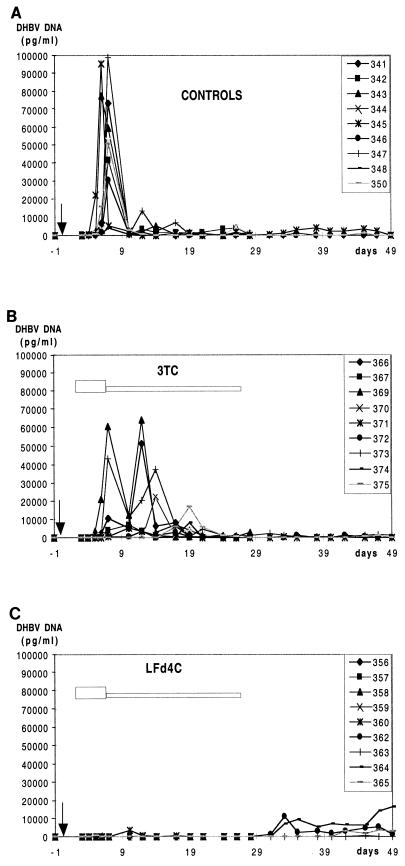
The previous results, indicating a strong inhibitory effect on the DHBV reverse transcriptase activity and a potent antiviral effect in primary hepatocyte cultures, led us to evaluate the therapeutic potential of β-l-Fd4C in vivo. Experimentally infected ducklings underwent short-term administration of β-l-Fd4C in comparison with 3TC, β-l-FddC, or ddC at different doses. One hundred twenty-five animals were included in these experiments for the dose-finding study. Three days after inoculation of virus, drugs were administered intraperitoneally at 0.2 mg/kg/day (five animals in each treatment group), 2 mg/kg/day (five animals in each treatment group), 5 mg/kg/day (six animals in each treatment group), 20 mg/kg/day (five animals in the β-l-Fd4C treatment group), and 25 mg/kg/day (eight animals in each treatment group) for 5 days. Twenty-four animals did not receive any treatment and served as controls. Analysis of viral replication showed that β-l-Fd4C was the most potent antiviral compound at each tested dosage. Results of the administration of the antivirals at 25 mg/kg/day are presented (Fig. 4). Eight animals were included in each treatment group. Four animals in each group were sacrificed at the end of treatment for analysis of intrahepatic viral DNA, pre-S protein expression, and liver histology. The other animals were observed for 2 additional weeks. β-l-Fd4C was the most active drug since the inhibition of the peak of viremia reached 52% for ddC, 82% for 3TC, 87% for β-l-FddC, and 97% for β-l-Fd4C (Fig. 4). Southern blot analysis of intrahepatic viral DNA at the end of the therapy demonstrated that β-l-Fd4C exhibited a very potent inhibitory effect on viral DNA synthesis since the DHBV replicative intermediates and CCC DNA were almost completely suppressed. The inhibition of viral DNA synthesis within the liver was only moderate in animals treated with 3TC, β-l-FddC, or ddC (data not shown). Immunostaining of liver sections for viral pre-S proteins showed that 3% to 50% of the liver cells expressed these viral proteins in the β-l-Fd4C group, while >95% of cells were positive for pre-S proteins in the control and 3TC-treated group (Fig. 7). However, after drug withdrawal, a relapse of viral replication occured in all β-l-Fd4C-treated animals, as indicated by the delayed onset of viremia (Fig. 4) and by the detection of viral DNA synthesis by Southern blot analysis of liver DNA (data not shown). At this dose, no significant variation of lactate levels or in animal weight was observed in β-l-Fd4C-treated animals as compared with control animals. Histological examination showed no sign of liver toxicity at the end of β-l-Fd4C administration. However, microvesicular steatosis was observed in all animals treated with ddC at the end of follow-up (data not shown).
FIG. 4.
Short-term administration of β-l-Fd4C transiently inhibits viral replication in experimentally infected ducklings. Animals were inoculated with an infectious serum at 3 days of age, and antiviral treatment was started 3 days postinoculation. Animals received intraperitoneal injection of either β-l-Fd4C or 3TC or β-l-FddC or ddC at a dose of 25 mg/kg/day for 5 days. Eight animals were included in each of the treatment groups as well as in a control group. Viremia was quantitatively analyzed by dot blot hybridization. The mean serum viral DNA levels in each group of animals is plotted on the graph. The arrow indicates the time of virus inoculation. The white bar indicates the antiviral treatment period. Standard deviations are indicated by the error bars.
FIG. 7.
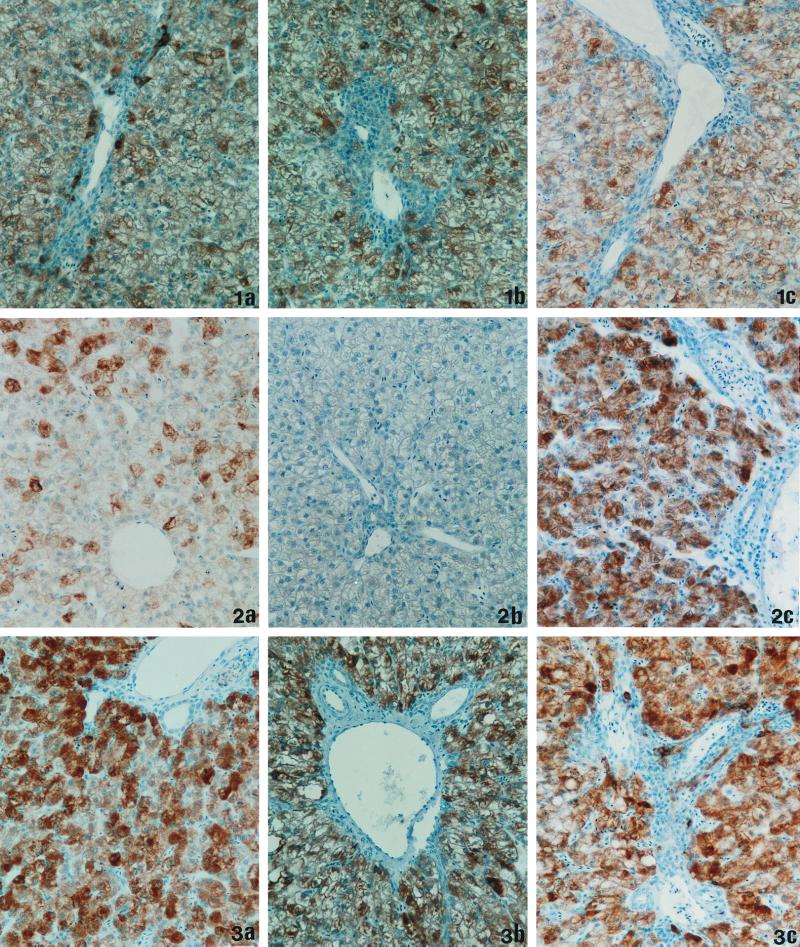
Inhibition of viral spread in the liver of infected animals by a 4-week β-l-Fd4C treatment regimen. Liver sections were analyzed for DHBV pre-S antigen by immunostaining with an anti-pre-S monoclonal antibody (magnification, ×660). Representative results obtained in the different groups of animals are depicted. (1a) Liver section of control animal at day 5 of the protocol (duck number 130), >95% positive cells. (1b) Liver section of control animal at day 28 of the protocol (duck number 341), >95% positive cells. (1c) Liver section of control animal at day 49 of the protocol (duck number 347), >95% positive cells. (2a) Liver section at day 5 of β-l-Fd4C treatment (duck number 118), <30% positive cells. (2b) Liver section at day 28 of β-l-Fd4C therapy (duck number 356), <1% positive cells. (2c) Liver section at end of follow-up (day 49), 21 days post-β-l-Fd4C treatment (duck number 365), >95% positive cells. (3a) Liver section at day 5 of 3TC treatment (duck number 111), >95% positive cells. (3b) Liver section at day 28 of 3TC therapy (duck number 366), >95% positive cells. (3c) Liver section at end of follow-up (day 49), 21 days post-3TC treatment (duck number 373), >95% positive cells. Differences in staining intensities from animal to animal were related to the degree of liver steatosis.
A 4-week administration of β-l-Fd4C suppresses viral DNA synthesis and viral protein expression in vivo in infected ducklings.
Then we investigated whether a more prolonged administration of β-l-Fd4C beginning early after the inoculation of an infectious serum would be successful in eradicating or controlling viral infection. Animals received β-l-Fd4C or 3TC by intraperitoneal administration at day 3 postinoculation as an induction therapy with β-l-Fd4C or 3TC at a dose of 25 mg/kg/day for 5 consecutive days (day 3 to day 7 postinoculation), followed by a maintenance therapy with β-l-Fd4C or 3TC at a dose of 25 mg/kg three times weekly for 3 additional weeks. Nine animals received β-l-Fd4C, nine received 3TC, and nine served as controls. Four animals in the control and the 3TC groups and five animals in the β-l-Fd4C group were sacrificed at the end of the maintenance therapy to study liver histology and intrahepatic viral DNA and viral protein expression in the liver. The other animals were observed for 3 additional weeks and were then also sacrificed for the same studies. Viremia was analyzed throughout the study. During the induction phase of therapy, the inhibition of the peak of viremia reached 80% for 3TC and 97% for β-l-Fd4C by comparison with that observed in control animals, confirming the results obtained in the short-term experiment (Fig. 5). During the maintenance phase of therapy, suppression of viremia was sustained in all β-l-Fd4C-treated animals. In 3TC-treated animals, viremia was not completely suppressed and declined progressively, leading to a broader peak of viremia (16 days) as compared to the control group (5 days). Then a low-level viremia was detected as also observed in the control group.
FIG. 5.
A 4-week course of β-l-Fd4C suppresses viremia but does not clear viral infection in infected ducks. Animals were inoculated with an infectious serum at 3 days of age, and antiviral treatment was started 3 days postinoculation. Animals received intraperitoneal injection of either β-l-Fd4C or 3TC at a dose of 25 mg/kg/day for 5 days, followed by a maintenance therapy of 25 mg/kg three times weekly for 3 additional weeks. Nine animals were included in each of the treatment groups as well as in a control group. Four animals in the 3TC and control groups and five animals in the β-L-Fd4C group were sacrificed at the end of treatment, and the other animals were observed after cessation of therapy. Viremia was quantitatively analyzed by dot blot hybridization. Results of serum viral DNA levels in individual animals are plotted on the graph. The arrow indicates the time of virus inoculation. The white bar indicates the antiviral treatment period.
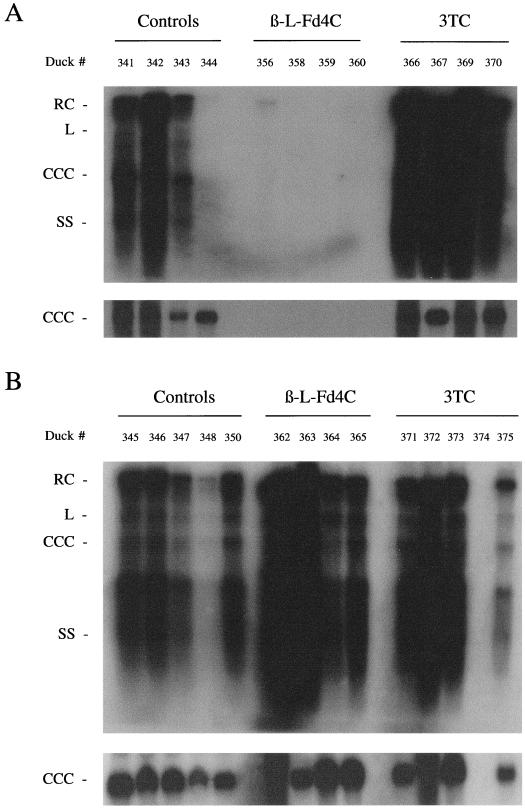
Analysis of intrahepatic viral DNA by Southern blot was performed at the end of therapy. The results showed a dramatic suppression of all viral DNA replicative intermediates and CCC DNA in all β-l-Fd4C-treated animals (Fig. 6A). In contrast, intrahepatic synthesis of viral DNA in 3TC-treated animals was not decreased compared to that of controls. Furthermore, to examine the effect of β-l-Fd4C on viral spread, we studied at the end of treatment the number of hepatocytes expressing viral pre-S envelope proteins by immunostaining of liver sections using a monoclonal anti-pre-S antibody. 3TC administration had no effect on the number of hepatocytes expressing the pre-S proteins, which was estimated to be >95% and similar to the number observed in the control animals (Fig. 7). In contrast, in β-l-Fd4C-treated animals, a careful examination of liver slides failed to detect a single hepatocyte or biliary cell expressing the viral pre-S proteins. Therefore, these results indicated that a 4-week administration of β-l-Fd4C in acutely infected ducklings not only suppresses viral DNA synthesis but also inhibits viral antigen expression in the liver.
FIG. 6.
β-l-Fd4C therapy suppresses viral DNA synthesis in the liver of infected animals but is not sufficient to eradicate viral infection. We extracted total viral DNA, including the replicative intermediates as well as viral CCC DNA, from the livers of all animals that received a 4-week course of β-l-Fd4C, as well as from animals in the lamivudine and control groups and analyzed the samples by Southern blotting and specific hybridization. Panel A shows the end of treatment analysis and panel B shows the analysis 3 weeks after cessation of therapy. The liver sample of duck number 357 was not available for DHBV DNA analysis. In animal number 374, the absence of detectable DHBV DNA may be due to spontaneous viral clearance or due to postmortem analysis (accidental death).
Suppression of viral replication by a 4-week course of β-l-Fd4C is not sufficient to clear viral infection in vivo in acutely infected ducklings.
In the 3TC group, a low-level viremia was detected by dot blot hybridization after drug withdrawal and was comparable with that observed in the control group (range of DHBV DNA levels, <100 to 2,361 pg/ml). After cessation of β-l-Fd4C therapy, three out of four animals exhibited a relapse of viremia on day 5 (two animals) and on day 14 (one animal) posttreatment. One animal did not show viremia during the 3-week follow-up period. In these animals, the level of DHBV DNA in serum ranged from <100 to 16,272 pg/ml, suggesting that prolonged administration of β-l-Fd4C had delayed the occurrence of the onset of viremia (Fig. 5).
Southern blot analysis of intrahepatic viral DNA performed 3 weeks after drug withdrawal showed the presence of all viral DNA replicative intermediates as well as CCC DNA, indicating that in the β-l-Fd4C group, viral DNA replication had been initiated in all previously treated animals (Fig. 6B). The occurrence of viral DNA replication in the β-l-Fd4C group was associated with viral spread as determined by the number of infected cells expressing the viral pre-S proteins, which was >95% and similar in all 3 groups of animals (Fig. 7). These data suggest that a 4-week administration of β-l-Fd4C in acutely infected animals suppressed viral replication but is not able to clear viral infection, even when therapy is started early after the virus inoculation.
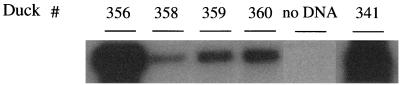
These results also suggest that during β-l-Fd4C therapy, persistence of trace amounts of virus as recalcitrant genomes (CCC DNA) within a number of infected cells may have explained this phenomenon. To answer this question, a PCR assay specific for CCC DNA detection was performed on protein-free liver DNA extracted from β-l-Fd4C-treated animals to determine whether viral CCC DNA was still present in the liver at the end of therapy. The results showed the absence of detectable CCC DNA by ethidium bromide staining of PCR products electrophoresed through agarose gels in this group of animals. Southern blot hybridization of the PCR products allowed to detect trace amounts of CCC DNA in the liver at the end of β-l-Fd4C treatment (Fig. 8), suggesting that the recalcitrant viral CCC DNA had not been cleared from infected hepatocytes by β-l-Fd4C administration in vivo.
FIG. 8.
Persistence of low levels of viral CCC DNA in the liver despite a dramatic suppression of viral DNA synthesis during β-l-Fd4C therapy. At the end of the 4-week treatment with β-l-Fd4C, CCC DNA in the liver was analyzed after amplification by a specific PCR assay followed by hybridization of 25% of the PCR products with a full-length DHBV genome probe. CCC DNA was amplified with primers P1 and P3 (see Materials and Methods). The result of DHBV DNA detection by the same PCR assay in the liver of a control animal is also shown (duck number 341; 1% of PCR products were loaded on the gel). The results demonstrate viral persistence at the end of β-l-Fd4C treatment in ducks numbers 356, 358, 359, and 360.
A 4-week course of β-l-Fd4C in experimentally infected ducklings is not associated with liver cell damage.
The potential side effects of β-l-Fd4C were monitored throughout the in vivo studies. Animal weight and lactic acid levels were carefully evaluated during the therapy and after the cessation of treatment. The results showed a similar evolution of these markers in the three groups of animals (controls, 3TC, and β-l-Fd4C), suggesting that a 4-week administration of β-l-Fd4C was not associated with a significant clinical toxicity. Moreover, analysis of liver histology performed at the end of the maintenance therapy and at the end of follow-up revealed the absence of liver toxicity of β-l-Fd4C at the studied time points (Table 1). Indeed, by comparison with the control group, there was no difference in terms of cellular necrosis or micro- or macrovesicular steatosis. Interestingly, at the end of β-l-Fd4C therapy, analysis of liver histology showed a decrease in the inflammatory infiltrates in portal tracts compared to the controls and 3TC-treated animals, which could be related to the suppression of viral antigen expression within the liver. At the end of follow-up, when viral replication occured in β-l-Fd4C-treated animals, inflammatory infiltrates were comparable in the β-l-Fd4C group and in the 3TC and control groups.
TABLE 1.
Analysis of liver histology in experimentally infected ducklings undergoing β-l-Fd4C therapy
| Test period | Treatment protocol | Duck no. | Cells presenting:
|
|||
|---|---|---|---|---|---|---|
| Hepatocyte necrosis | Portal inflammation | Microsteatosisa (%) | Macrosteatosis (%) | |||
| End of treatment | Control | 341 | − | +++ | − | − |
| 342 | − | +++ | 5 | 5 | ||
| 343 | ± | +++ | 10 | 40 | ||
| 344 | ± | +++ | 30 | 40 | ||
| β-l-Fd4C | 356 | − | + | − | − | |
| 357 | − | + | − | − | ||
| 358 | ± | ++ | − | 30 | ||
| 359 | ± | + | − | − | ||
| 360 | − | + | − | − | ||
| 3TC | 366 | − | ++ | − | <5 | |
| 367 | ± | +++ | − | <5 | ||
| 369 | − | ++ | − | − | ||
| 370 | − | +++ | − | − | ||
| 3-Wk posttreatment | Control | 345 | − | +++ | − | − |
| 346 | − | +++ | − | − | ||
| 347 | − | +++ | − | − | ||
| 348 | − | +++ | − | − | ||
| 350 | ± | + | − | − | ||
| β-l-Fd4C | 362 | − | +++ | − | − | |
| 363 | − | +++ | − | − | ||
| 364 | − | + | − | − | ||
| 365 | − | ++ | − | − | ||
| 3TC | 371 | − | +++ | − | − | |
| 372 | − | +++ | − | − | ||
| 373 | − | +++ | 60 | 20 | ||
| 374b | − | +++ | 100 | − | ||
| 375 | − | + | − | − | ||
Percentages indicate the proportion of cells presenting a given lesion (micro- or macrosteatosis). For portal inflammation and cell necrosis, − indicates a negative result, ± indicates a rare event, and + indicates a minimal degree, ++ indicates a mild degree, and +++ indicates a moderate degree of the given histological lesion.
Postmortem analysis of liver histology was carried out for duck number 374.
DISCUSSION
In this work, the anti-HBV activity of β-l-Fd4C was evaluated in the DHBV infection model. Using an in vitro assay for the expression of an enzymatically active DHBV reverse transcriptase, we could demonstrate that the triphosphate form of β-l-Fd4C is an inhibitor of viral minus-strand DNA synthesis. It was shown to be 30 times more potent than 3TC triphosphate, while ddC triphosphate and β-l-FddC triphosphate were much less active inhibitors. The IC50 of β-l-Fd4C triphosphate on nucleotide incorporation in viral minus-strand DNA is one of the lowest that has been observed so far using this assay (1, 4, 30, 33, 42). Furthermore, we gained new information on its mechanism of action and showed that β-l-Fd4C triphosphate is likely to be a competitive inhibitor of dCTP incorporation in nascent viral minus-strand DNA and that β-l-Fd4C triphosphate and 3TC triphosphate may terminate viral DNA chain synthesis, as was also shown with 3TC triphosphate in a nucleocapsid-based polymerase assay (31). Further experiments using radiolabelled compounds are warranted to demonstrate whether β-l-Fd4C-triphosphate is indeed incorporated in viral minus-strand DNA. Altogether, these results indicate that β-l-Fd4C-triphosphate is one of the most potent inhibitors of hepadnavirus reverse transcriptase and are consistent with the dramatic antiviral effect of β-l-Fd4C observed in the 2.2.15 cell line (41).
In primary duck hepatocyte cultures that were infected in vitro, β-l-Fd4C administration induced a dose-dependent inhibition of viral DNA synthesis accompanied with a significant decrease in viral DNA replicative intermediates. However, the persistence of the recalcitrant viral CCC DNA was demonstrated at the end of therapy, indicating that short-term treatment is not able to eradicate the viral genome from infected cells. The same phenomenon was also observed with other nucleoside analogs (1, 9, 26, 32, 41, 42). Interestingly, we observed that the antiviral effect was maintained 4 days posttreatment, which suggests that β-l-Fd4C and/or its triphosphate derivative has a long half-life in infected hepatocytes. This is consistent with the observation made in HepG2 cells showing that the apparent half-life of β-l-Fd4C triphosphate was 20 h, compared with 4 h in the case of lamivudine triphosphate (41). This long-lasting antiviral effect may, therefore, be helpful in designing protocols of maintenance therapy with a spacing of dose in vivo. Moreover, when the drugs were administered prior to the inoculation of virus, neither β-l-Fd4C nor 3TC could prevent the initiation of viral infection, suggesting their lack of effect on the initial formation of CCC DNA. As it was described with carbocyclic 2′-deoxyguanosine (9), another potent inhibitor of the hepadnavirus reverse transcriptase (4, 9), our data strongly emphasize that prevention of CCC DNA formation will be a difficult task to achieve with nucleoside analogs.
The antiviral activity of β-l-Fd4C was also studied in vivo in experimentally infected ducklings, in comparison with other cytidine analogs (3TC, β-l-FddC, and ddC). Analysis of viral replication showed that, at each dose, β-l-Fd4C was the most potent antiviral, followed by β-l-FddC, 3TC, and ddC, respectively (Fig. 4). While no signs of toxicity were observed with β-l-Fd4C, the delayed appearance of microvesicular steatosis was observed in the liver of ddC-treated animals as already described (1). The dose of 25 mg/kg/day was found to induce the best antiviral effect and was therefore selected for the evaluation of longer administration in animals. Interestingly, the greater the antiviral effect during therapy, the higher was the rebound of viral replication after drug withdrawal, as previously observed with β-l-FddC (42). This may suggest that the suppression of viral replication in acutely infected animals is not associated with a strong specific antiviral response, leading to a delayed peak of viral replication after drug withdrawal whose intensity is comparable to control animals. We then asked whether therapy with β-l-Fd4C, when started as soon as 3 days postinoculation and maintained for 4 weeks, would be able to eradicate viral infection. Monitoring of viremia showed that β-l-Fd4C administration dramatically suppressed viral replication, while lamivudine treatment was less potent (Fig. 5). Sequence analysis of the viral polymerase gene during the peak of viremia showed the absence of mutations in 3TC-treated birds (data not shown), suggesting that the less-potent antiviral effect may have been due to a lesser transformation in its triphosphate derivative and to a less potent inhibitory activity on the viral reverse transcriptase. Specific studies of the in vivo metabolism of 3TC and β-l-Fd4C are required to identify the differences in deoxycytidine analog metabolism in duck, woodchuck, and humans. The strong antiviral effect of β-l-Fd4C was associated with a profound suppression of viral DNA synthesis and CCC DNA in the liver of infected animals. However, a specific PCR assay detected the persistence of trace amounts of viral CCC DNA, which was sufficient to initiate viral replication when β-l-Fd4C administration was stopped. β-l-Fd4C treatment was also capable of reducing intrahepatic viral protein expression, which may reflect an inhibition of viral spread within the liver (Fig. 7). Another hypothesis that remains to be proven is that a significant number of cells were infected but harbored a very-low copy number of CCC DNA, resulting in the observed lack of viral antigen expression. In contrast, 3TC administration had little effect on the number of infected cells expressing viral antigens, as was recently shown in the woodchuck model of woodchuck hepatitis virus infection (24). However, within 3 weeks of β-l-Fd4C withdrawal, the initiation of viral replication was associated with viral spread in almost all hepatocytes (Fig. 7). These results therefore suggest that the clearance of infected cells during antiviral therapy with a potent reverse transcriptase inhibitor is a long process which requires the maintenance of antiviral pressure for prolonged durations to control viral infection, as has been described with carbocyclic 2′-deoxyguanosine and penciclovir in the duck model (8, 20) and with deoxycytidine or deoxyguanosine analogs in the woodchuck model (10, 24). Noteworthy is the degree to which liver inflammation was decreased during β-l-Fd4C therapy, which may be beneficial in patients with chronic hepatitis, as was recently reported in clinical trials with lamivudine (16). During the 4-week administration protocol, no sign of toxicity was observed, but careful studies, including examination of hepatocyte mitochondria by electron microscopy, are required in several animal models (18).
In conclusion, the results of our study have shown that β-l-Fd4C is a potent inhibitor of the DHBV reverse transcriptase, inhibits DHBV DNA synthesis in hepatocyte cultures, and suppresses both viral DNA replication and viral antigen expression in the liver of infected animals in vivo, without significant signs of toxicity. However, even when administered shortly after experimental inoculation, β-l-Fd4C treatment was not sufficient to eradicate DHBV infection. In view of its development as a potential anti-HBV agent in humans, evaluation of long-term administration of β-l-Fd4C in the woodchuck mammalian model (25) is warranted to characterize its antiviral activity and capacity to eradicate viral infection as well as its toxic effect.
ACKNOWLEDGMENTS
We thank C. Borel and O. Schorr for their help in tissue culture experiments, S. Aguesse-Germon for providing DHBV polymerase mutants, and L. Cova for the generous gift of MAb 900.
This work was supported by grants from the INSERM, the French Association for Research against Cancer, and the French League Against Cancer. Franck Le Guerhier was a recipient of a fellowship from the French League Against Cancer.
REFERENCES
- 1.Aguesse-Germon S, Liu S-H, Chevallier M, Pichoud C, Jamard C, Borel C, Chu C K, Trépo C, Cheng Y-C, Zoulim F. Inhibitory effect of 2′-fluoro-5-methyl-β-l-arabinofuranosyl-uracyl on duck hepatitis B virus replication. Antimicrob Agents Chemother. 1998;42:369–376. doi: 10.1128/aac.42.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang C-N, Doong S-L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C-H, Cheng Y-C. Deoxycytidine deaminase-resistant stereoisomer is the active form of (±) 2′,3′ dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. [PubMed] [Google Scholar]
- 3.Chassot S, Lambert V, Kay A, Godinot C, Roux B, Trépo C, Cova L. Fine mapping of neutralization epitopes on duck hepatitis B virus (DHBV) pre-S protein using monoclonal antibodies and overlapping peptides. Virology. 1993;192:217–223. doi: 10.1006/viro.1993.1024. [DOI] [PubMed] [Google Scholar]
- 4.Dannaoui E, Trépo C, Zoulim F. Inhibitory effect of penciclovir-triphosphate on duck hepatitis B virus reverse transcription. Antivir Chem Chemother. 1997;8:38–46. [Google Scholar]
- 5.Diaz-Guerra M, Rivas C, Esteban M. Activation of the IFN-inducible enzyme RNase L causes apoptosis of animal cells. Virology. 1997;236:354–363. doi: 10.1006/viro.1997.8719. [DOI] [PubMed] [Google Scholar]
- 6.Doong S-L, Tsai C-H, Schinazi R F, Liotta D C, Cheng Y-C. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutschman G E, Bridges E G, Liu S-H, Gullen E, Guo X, Kukhanova M, Cheng Y-C. Metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine and its activity in combination with clinically approved anti-human immunodeficiency virus β-d(+) nucleoside analogs in vitro. Antimicrob Agents Chemother. 1998;42:1799–1804. doi: 10.1128/aac.42.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fourel I, Cullen J M, Saputelli J, Aldrich C E, Schaffer P, Averett D R, Pugh J, Mason W S. Evidence that hepatocyte turnover is required for rapid clearance of duck hepatitis B virus during antiviral therapy of chronically infected ducks. J Virol. 1994;68:8321–8330. doi: 10.1128/jvi.68.12.8321-8330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fourel I, Saputelli J, Schaffer P, Mason W S. The carbocyclic analog of 2′-deoxyguanosine induces a prolonged inhibition of duck hepatitis B virus DNA synthesis in primary hepatocyte cultures and in the liver. J Virol. 1994;68:1059–1065. doi: 10.1128/jvi.68.2.1059-1065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genovesi E V, Lamb L, Medina I, Taylor D, Seifer M, Innaimo S, Colonno R J, Standring D N, Clark J M. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother. 1998;42:3209–3217. doi: 10.1128/aac.42.12.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hantz O, Borel C, Trabaud C, Zoulim F, Dessolin J, Camplo M, Vlieghe P, Bouygues M, Trépo C, Kraus J L. Selective inhibition of the duck hepatitis B virus by a new class of tetraazamacrocycles. Antimicrob Agents Chemother. 1997;41:2579–2581. doi: 10.1128/aac.41.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoofnagle J H, Di Bisceglie A M. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 13.Jilbert A R, Wu T T, England J M, De La P, Hall M, Carp N Z, O'Connell A P, Mason W S. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992;66:1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kock J, Schlicht H G. Analysis of the earliest steps of hepadnavirus replication: genome repair after infectious entry into hepatocytes does not depend on viral polymerase activity. J Virol. 1993;67:4867–4874. doi: 10.1128/jvi.67.8.4867-4874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kukhanova M, Li X, Chen S-H, King I, Doyle T, Prusoff W, Cheng Y-C. Interaction of β-l-2′,3′-dideoxy-2′,3′-didehydro-5-fluoro-CTP with human immunodeficiency virus-1 reverse transcriptase and human DNA polymerases: implications for human immunodeficiency virus drug design. Mol Pharmacol. 1998;53:801–807. [PubMed] [Google Scholar]
- 16.Lai C L, Chine R W, Leung N W Y, Chang T T, Guan R, Tai D I, Ng K Y, Wu P C, Dent J C, Barber J, Stephenson S L, Gray F. A one year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 17.Lambert V, Chassot S, Kay A, Trépo C, Cova L. In vivo neutralization of duck hepatitis B virus by antibodies specific to the N-terminal portion of pre-S protein. Virology. 1991;185:446–450. doi: 10.1016/0042-6822(91)90796-e. [DOI] [PubMed] [Google Scholar]
- 18.Lee W. Drug-induced hepatotoxicity. N Eng J Med. 1995;333:1118–1127. doi: 10.1056/NEJM199510263331706. [DOI] [PubMed] [Google Scholar]
- 19.Lee W M. Hepatitis B virus infection. N Eng J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 20.Lin E, Luscombe C, Colledge D, Wang Y Y, Locarnini S. Long-term therapy with the guanine nucleoside analog penciclovir controls chronic duck hepatitis B virus infection in vivo. Antimicrob Agents Chemother. 1998;42:2132–2137. doi: 10.1128/aac.42.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin T-S, Luo M-Z, Liu M-C, Pai S-B, Dutschman G-E, Cheng Y-C. Antiviral activity of 2′,3′-dideoxy-β-l-5-Fluorocytidine (β-L-F-ddC) and 2′,3′-dideoxy-β-l-cytidine (β-L-ddC) against hepatitis B virus and human immunodeficiency virus type 1 in vitro. Biochem Pharmacol. 1994;47:171–174. doi: 10.1016/0006-2952(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 22.Lin T-S, Luo M-Z, Liu M-C, Pai S B, Dutschman G E, Cheng Y-C. Synthesis and biological evaluation of 2′,3′-dideoxy-l-pyrimidine nucleosides as potential antiviral agents against human immunodeficiency virus (HIV) and hepatitis B virus (HBV) J Med Chem. 1994;37:798–803. doi: 10.1021/jm00032a013. [DOI] [PubMed] [Google Scholar]
- 23.Lin T-S, Luo M-Z, Liu M-C, Zhu Y-L, Gullen E, Dutschmann G E, Cheng Y-C. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-β-l-cytidine (β-l-d4C) and 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-fluorocytidine (β-l-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent human immunodeficiency virus (HIV) in vitro. J Med Chem. 1996;39:1757–1759. doi: 10.1021/jm950836q. [DOI] [PubMed] [Google Scholar]
- 24.Mason W S, Cullen J, Moraleda G, Saputelli J, Aldrich C E, Miller D S, Tennant B, Frick L, Averett D, Condreay L D, Jilbert A R. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- 25.Mason W S, Taylor J M. Experimental systems for the study of hepadnavirus and hepatitis delta virus infections. Hepatology. 1989;9:635–645. doi: 10.1002/hep.1840090420. [DOI] [PubMed] [Google Scholar]
- 26.Moraleda G, Saputelli J, Aldrich C E, Averett D, Condreay L, Mason W S. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9399. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nevens F, Main J, Honkoop P, Tyrrell D L, Barber J, Sullivan M T, Fevery J, De Man R, Thomas H C. Lamivudine therapy for chronic hepatitis B: a six month randomized dose-ranging study. Gastroenterology. 1997;113:1258–1263. doi: 10.1053/gast.1997.v113.pm9322520. [DOI] [PubMed] [Google Scholar]
- 28.Nowak M, Bonhoeffer S, Hill A, Boehme R, Thomas H, McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci USA. 1996;93:4398–4402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichoud C, Seignères B, Wang Z, Trepo C, Zoulim F. Transient selection of a hepatitis B virus polymerase gene mutant associated with a decreased replication capacity and famciclovir resistance. Hepatology. 1999;29:230–237. doi: 10.1002/hep.510290119. [DOI] [PubMed] [Google Scholar]
- 30.Seifer M, Hamatake R K, Colonno R J, Standring D N. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob Agents Chemother. 1998;42:3200–3208. doi: 10.1128/aac.42.12.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severini A, Liu X-Y, Wilson J, Tyrell D. Mechanism of inhibition of duck hepatitis B virus polymerase by (−)-β-L-2′,3′-dideoxy-3′-thyacytidine. Antimicrob Agents Chemother. 1995;39:1430–1435. doi: 10.1128/aac.39.7.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw T, Amor P, Civitico G, Boyd M, Locarnini S. In vitro antiviral activity of penciclovir, a novel purine nucleoside, against duck hepatitis B virus. Antimicrob Agents Chemother. 1994;38:719–723. doi: 10.1128/aac.38.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staschke K, Colacino J. Priming of duck hepatitis B virus reverse transcription in vitro: premature termination of primer DNA induced by the 5′-triphosphate of fialuridine. J Virol. 1994;68:8265–8269. doi: 10.1128/jvi.68.12.8265-8269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summers J, Smith P M, Horwich A L. Hepadnaviral envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trigueiro de Araujo M S, Guerret S, Gérard F, Chossegros P, Chevallier M, Grimaud J A. Quantitative studies on liver fibrosis and alpha-smooth muscle actin expression in heroin abusers. Cell Mol Biol. 1997;43:589–596. [PubMed] [Google Scholar]
- 36.Tuttleman J, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 37.Tuttleman J S, Pugh J C, Summers J W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986;58:17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G H, Seeger C. A novel mechanism for reverse transcription in hepatitis B viruses. J Virol. 1993;67:6507–6512. doi: 10.1128/jvi.67.11.6507-6512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G H, Seeger C. The reverse transcriptase of hepatitis B virus acts a protein primer for viral DNA synthesis. Cell. 1992;71:663–670. doi: 10.1016/0092-8674(92)90599-8. [DOI] [PubMed] [Google Scholar]
- 40.Wu T-T, Coates L, Aldrich C E, Summers J, Mason W S. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y-L, Dutschman G E, Liu S-H, Bridges E G, Cheng Y-C. Anti-hepatitis B virus activity and metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine. Antimicrob Agents Chemother. 1998;42:1805–1810. doi: 10.1128/aac.42.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoulim F, Dannaoui E, Borel C, Hantz O, Lin T-S, Liu S-H, Trépo C, Cheng Y-C. 2′,3′-dideoxy-β-l-5-fluorocytidine inhibits duck hepatitis B virus reverse transcription and suppresses viral DNA synthesis in hepatocytes, both in vitro and in vivo. Antimicrob Agents Chemother. 1996;40:448–453. doi: 10.1128/aac.40.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zoulim F, Seeger C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J Virol. 1994;68:6–13. doi: 10.1128/jvi.68.1.6-13.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zoulim F, Trépo C. Drug therapy for chronic hepatitis B: antiviral efficacy and influence of hepatitis B virus polymerase mutations on the outcome of therapy. J Hepatol. 1998;29:151–168. doi: 10.1016/s0168-8278(98)80191-8. [DOI] [PubMed] [Google Scholar]