Abstract
Background:
Considering the diverse socio-economic and demographic factors in a vast country like India, it is important to study the long-term trends of hepatitis A (HAV) and hepatitis E (HEV) viruses. This study describes their seroprevalence and long-term trends in a tertiary care center of North India.
Methods:
The present retrospective observational study was conducted over a period of 8 years (January 2011–December 2018). Serological testing was done for detecting IgM antibodies against HAV and HEV using enzyme-linked immunosorbent assay.
Results:
A total of 5319 samples were received during the study period, of which 903 (16.9%) were reactive for anti-HAV IgM antibodies and 795 (14.9%) were reactive for anti-HEV IgM antibodies. Majority of the cases occurred from June to October while HEV cases had a constant presence during the later years. Among HAV group, 534 (59.1%) were children, 336 (37.2%) were adults, and 33 (3.7%) were pregnant females. In HEV group, 632 (79.5%) were adults, 114 (14.3%) were pregnant females, whereas only 49 (6.2%) were children. Among those who were co-infected (n = 87), 48 (55.2%) were adults, 22 (25.3%) were pregnant females, and the rest 17 (19.5%) were children.
Conclusions:
The shift in seroprevalence toward adults, along with an increasing trend of the number of cases reporting to the hospital, warrants active surveillance of HAV. Similarly, screening protocols for HEV should be set up as part of the antenatal management for early detection of the cases among pregnant females.
Keywords: Enteric hepatitis, hepatitis A, hepatitis E, seroprevalence, viral hepatitis
Introduction
Hepatitis A (HAV) and hepatitis E (HEV) viruses are important public health problems in developing countries such as India.[1] Both these viruses are predominantly enterically transmitted through feco-oral route and cause a spectrum of infection ranging from asymptomatic infection, usually in children, to acute viral hepatitis (AVH) of varying severity in adults.[2]
Hepatitis A is a vaccine-preventable disease but the vaccine has not been deployed in India[3] as more than 80% of children by the age of 10 years develop antibodies as a result of natural infection[4] and since the disease is often clinically insignificant in this age group, the use of vaccine is not justified and is still a subject of debate. It is pertinent to note that more than half of the world’s population practicing defecation in the open is residing in India.[5] However, there is a noticeable shift in the disease spectrum from children to adults as a result of improvement in the socio-economic conditions.[6] To add to this, there has been an emphasis on the promotion of increased sanitary infrastructure by India under the Swachh Bharat (Clean India) mission since 2014.[7]
HEV on the other hand is known to cause infection in adult population as compared to children,[8] with a greater predilection to cause outbreaks in the community as compared to HAV.[9] It is also documented to cause severe disease in pregnant females leading to increased mortality and pregnancy-related complications.[10] There is evidence in the literature regarding this virus still being a public health menace in industrialized countries as well.[11,12]
There has been no case-based surveillance for these viral diseases in India and the mode of surveillance till date has been outbreak-oriented where the weekly numbers are analyzed for predefined threshold by the Integrated Disease Surveillance Project, depending upon the geographical area.[4] The National Viral Hepatitis Control Program (NVHCP), launched in July 2018, intends to address the public health problem caused by these viruses and it aims to substantially reduce the risk, morbidity, and mortality associated with HAV and HEV by 2030.[13]
There are limited long-term studies from India regarding the extent of the disease burden of these two viruses. Considering the diverse socio-economic and demographic factors in a vast country like India, coupled with recent improvements in the sanitation infrastructure under clean India Mission 2014, it is important to study the long-term trends of HAV and HEV infections. Hereby, we present our hospital-based study on seroprevalence of hepatitis A and hepatitis E in patients attending a tertiary care center in North India over a period of 8 years (2011–2018) with the following objectives: (i) to determine the seroprevalence of HAV and HEV in sera of the suspected enteric hepatitis patients attending a tertiary care hospital, (ii) to study the yearly and month-wise trends of the positive cases, and (iii) to study the seroprevalence in special risk categories, namely, children, adults, and pregnant females.
Materials and Methods
The present study was a retrospective observational study conducted from January 2011 to December 2018 at a tertiary care center of North India. All patients presenting to the hospital with features of AVH whose samples were received for serological testing against hepatitis A and hepatitis E in the Microbiology laboratory of the institute during the study period were included in the study. Repeat samples were excluded from the study so that each patient is represented by a single sample only.
For microbiological confirmation, serological testing was done using enzyme-linked immunosorbent assay (ELISA)-based anti-HAV IgM (DiaPro Diagnostic Bioprobes, Italy) and anti-HEV IgM (DiaPro Diagnostic Bioprobes, Italy) antibody detection tests. Five milliliters of blood sample was collected in blood collection vials without additives under strict aseptic precautions and the serum was separated by standard methods. If not possible to put up immediately, the sera were stored at −20°C till testing (usually up to 1–3 days). Sera exhibiting hemolysis, lipemia, and turbidity were rejected. ELISA testing was performed as per manufacturer instructions supplied as package inserts/kit literature along with the ELISA kits. Positive and negative controls supplied with the kits were run for test validation as per instructions and internal quality control was also set up by testing known positive samples every time ELISA testing was done on patient samples.
Data were collected from the laboratory and hospital records for the study. Patients were categorized into three groups for analysis: (i) pregnant females, (ii) pediatric, and (iii) adult patients. The categorization into pediatric and adult was as per the previous description[14] and was based on the hospital protocol for patient enrolment in the pediatrics (≤12 years) or medicine (>12 years) specialties.
Results
A total of 5319 patient samples were included in the study, of which 903 (16.9%) were reactive for anti-HAV IgM antibodies and 795 (14.9%) were reactive for anti-HEV IgM antibodies. Samples of 87 (1.6%) patients among these were reactive for both anti-HAV and anti-HEV IgM antibodies indicating a co-infection of HAV and HEV in these patients.
The year-wise distribution of the cases showed an overall increasing trend of cases of both HAV and HEV during the study period [Figure 1]. HAV caused more infections throughout the study period except in 2016 and 2017 where HEV became the dominant etiological agent. The month-wise trend of both HAV and HEV showed two patterns during the study period [Figure 2]. During the first half of the study period (2011–2014), the number of cases peaked during the monsoon season (June–October) and persisted till around early winters/December [Figure 2]. The numbers of HAV cases were more than the number of HEV cases for more than 75% of this duration. The bars in Figure 2 show the relative difference between the number of cases between HAV and HEV, with black bars indicating higher number of HAV cases and white bars indicating higher number of HEV cases. During the second half of the study period (2015–2018), the numbers of HEV cases were more than HAV for just more than 50% of this duration. HEV also had a more consistent presence from October 2015 till May 2018 as shown in Figure 2.
Figure 1.

Yearly trends of HAV and HEV cases
Figure 2.
Month-wise trends of HAV and HEV cases
The gender-wise distribution in the patients was assessed and the number of males (n = 924) was slightly higher as compared to females (n = 788). HAV was more commonly associated with infection in males (n = 522; 57.8%) whereas HEV caused infection in slightly higher number of females (n = 401; 50.4%) as compared to males. Among the co-infected patients, 39 were males and 48 were females. None of these differences were statistically significant.
Based on the seropositivity, the patients were divided into two groups, that is, HAV and HEV, and the seropositivity within these groups was assessed in three special categories, namely, children, adults, and pregnant females. Among HAV group, 534 (59.1%) were children, 336 (37.2%) were adults, and 33 (3.7%) were pregnant females. The proportion of cases in adults, however, increased from 15.6% to 58.3% during this period [Figure 3]. In HEV group, we observed that 632 (79.5%) were adults, 114 (14.3%) were pregnant females whereas only 49 (6.2%) were children [Figure 4]. The proportion of pregnant females was more or less at similar levels except for a spike in 2014 (32.5%) as shown in Figure 4. Among those who were co-infected (n = 87), 48 (55.2%) were adults, 22 (25.3%) were pregnant females, and the rest 17 (19.5%) were children.
Figure 3.

HAV in special risk groups
Figure 4.
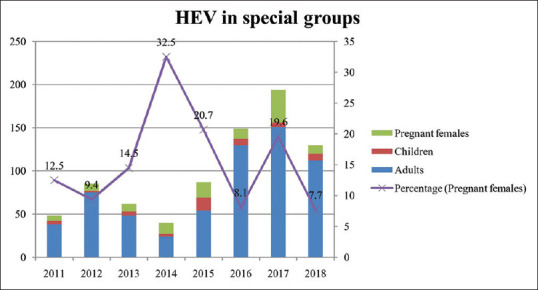
HEV in special risk groups
We also noted clustering of cases among patients admitted in the intensive care unit (ICU) and labor rooms on four occasions. Within the HEV group, three patients were found positive from ICU in October 2013, six patients from ICU in April 2015, whereas eight positive patients were reported from labor room in August 2016. Three patients were positive for HAV from the ICU in May 2018. A total of four outbreaks were reported from geographical localities around the institute in Chandigarh (India) during this period, two each caused by HAV and HEV.
Discussion
The present study was conducted over a period of 8 years and highlights the changing trend of infections caused by HAV and HEV in a hospital-based setting. There was an overall rise in the number of cases of hepatitis A as well as hepatitis E. In a study by Murhekar et al.,[4] the data from Virus Research and Diagnostic Laboratory (VRDL) network across India from 2014 to 2017 had a HAV: HEV ratio of 1.19 which is consistent with our overall ratio of 1.14 during the study period. However, it should be noted that the ratio of cases of HAV and HEV was unstable throughout our study period with a wide fluctuation ranging from 0.61 to 2.53.
The numbers of HAV cases were more than HEV for most of the study period which is in contrast to previous studies from India where HEV was found to be the dominant agent.[14,15] Joon et al.[16] reported higher prevalence of HAV (19.31%) as compared to HEV (10.54%) from Karnataka (India). The data from VRDL network laboratory also show higher prevalence of HAV in northern India while the western, central, and eastern part of India have higher prevalence of HEV.[4]
With regard to HAV, it is also worthwhile to mention that 40.9% cases in our study were >12 years old. This puts a high proportion of patients at risk of developing jaundice as per the model described by Aggarwal et al.[17] The rising proportion of adults among hepatitis A patients in this study may have a bearing in the community as well. This is important to investigate further in view of clean India mission 2014 and NVHCP 2018 of India. A seroprevalence of more than 80% in children less than 10 years of age has been described in the literature from India.[4] In the last two decades, there is a noticeable shift in the seroprevalence with a gradual decrease in seropositivity among children less than 10 years of age.[18] In a review of available literature, Agrawal et al.[19] noted that the shift in seroprevalence towards adolescents and adults was significant in both urban areas as well as populations with high or middle socioeconomic status as compared to rural and lower socioeconomic status, respectively. Arankalle et al.[20] on the other hand observed that this shift was evident only among higher socio-economic group from urban areas while those belonging to lower socio-economic group from urban areas as well as residents of all socio-economic groups in rural households demonstrated similar, high seroprevalence among children.
This increases the predisposition for outbreaks of viral hepatitis in the susceptible population, leading to higher incidence of clinical disease and its related complications including death. A seasonal peak was also observed in our study around the monsoon season, that is, June to October which is consistent with other studies from India.[4,19,21] The understanding of the fact that there is a seasonal peak of cases in these months can help contain the incidence by a coordinated effort by the public health department before and at the time of monsoons every year. The primary and secondary health-care system can be an important part of this strategy as they are often the first point of contact with the cases in the local community.
Pregnant females constituted 14.3% of the HEV cases which is consistent with data from a previous study from India.[22] HEV is known to cause pregnancy-related complications in up to 30% of infected females[23] that includes abortion, preterm labor, still births, low birth weight, fulminant hepatitis, and maternal mortality.[9] Among HAV–HEV co-infected patients, 25% were pregnant females that suggest that pregnant females are susceptible to severe manifestations of these infections. This is backed by the presence of clustering of HEV cases in ICU and obstetrics wards in our study, thus indicating the need for their screening during routine antenatal follow-up.
Both HAV and HEV have a single serotype each but have three and eight genotypes, respectively.[11,24,25,26,27,28] Among HEV, genotype (gt) 1, gt2 (and gt4 to some extent) are predominant in developing countries while gt3 and gt4 are seen in western countries where sanitation and drinking water supply are much superior.[11,24] Both gt3 and gt4 are considered to be transmitted by zoonotic route, pigs being the chief reservoir for gt3 and cattle for gt4.[11] The resurgence of HEV in the developed countries is said to be a result of these differences in genotype that have a zoonotic potential coupled with imported cases from the developing countries as a result of travel-related activities.[11,25]
HEV also differs from HAV in the context of its survival in the environment and its structure. It is found as a non-enveloped virus in the environment but is a quasi-enveloped virus in the body that helps it evade the immune system and cause disease.[29,30] In the present study, only 6.2% of the HEV cases were children below 12 years. Previous studies have also made similar observations.[4,16,21,23] It is postulated that these differences in genotype and structure are responsible for higher prevalence of HEV in adults as compared to HAV in the same geographical region[31] despite both transmitted via feco-oral route. Another possible reason could be the fact that more HEV infections in children could be asymptomatic leading to lower numbers in hospital-based studies.[16,21]
The present study has a few limitations. First, this was a hospital-based study, and since asymptomatic individuals (usually children) are less likely to know their illness and seek medical assistance, the seroprevalence in the community may comprise a fraction of children/pediatric population that did not report to the hospital. Second, we could not do molecular or genotype study owing to financial constraints and the fact that the study was a retrospective study. Lastly, serological diagnosis of HEV is challenging as the assays demonstrate a wide variation in performance characteristics such as sensitivity as well as specificity owing to factors such as high titers of IgG interfering with IgM detection assay[26] or lower antibody levels in immunocompromised as compared to immunocompetent individuals.[27]
Key Points and Highlights
The present study adds long-term data regarding the changes and trends in the seroprevalence of these enterically transmitted viruses in cases of AVH at a teaching hospital from North India.
The incidence of enteric hepatitis showed a rising trend in the present hospital-based study.
Hepatitis E became the dominant virus for major part of the second half of the study, which is in contrast to previous data that showed hepatitis A virus to be the dominant virus in the northern part of India.
The seroprevalence in the adults showed a rising trend in this study which is a cause of concern. Continuous surveillance should aim at ascertaining the role of vaccine in the community.
Conclusions
Both hepatitis A and hepatitis E are endemic in this part of the country and demonstrate a seasonal peak around the monsoon season. A better coordinated public health effort around this time can help contain the seasonal cases to some extent. The shift in HAV seroprevalence toward adults, along with an increasing trend of the number of cases reporting to the hospital, warrants the active community-based surveillance to assess the incidence of HAV in adults in this region. A long-term, continuous serosurveillance for presence of this virus is important to ascertain the utility of the vaccine for its prevention. In view of the consistent presence of HEV in pregnant females, screening protocols should be set up for all pregnant females as part of the antenatal management for early detection of the cases.
Ethical considerations
This retrospective study using available laboratory data was conducted as per ethical guidelines for biomedical research on human subjects as given by the Central Ethics Committee on Human Research (CECHR) of Indian Council of Medical Research (ICMR), New Delhi, in 2017 and as given in the “Declaration of Helsinki” 1975 revision of 2013.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kalita D, Paul M, Deka S, Badoni G, Gupta P. Simultaneous infection of hepatitis A and hepatitis E viruses amongst acute viral hepatitis patients:A hospital-based study from Uttarakhand. J Family Med Prim Care. 2020;9:6130–4. doi: 10.4103/jfmpc.jfmpc_1373_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurav YK, Retheesh Babu G, Vinu KP, Lole KS. Suspected spread of hepatitis A virus from a restaurant among adults in rural area of the Kerala state, India. Epidemiol Infect. 2019;147:e210. doi: 10.1017/S0950268819000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batra Y, Bhatkal B, Ojha B, Kaur K, Saraya A, Panda SK, et al. Vaccination against hepatitis A virus may not be required for schoolchildren in northern India:Results of a seroepidemiological survey. Bull World Health Organ. 2002;80:728–31. [PMC free article] [PubMed] [Google Scholar]
- 4.Murhekar MV, Ashok M, Kanagasabai K, Joshua V, Ravi M, Sabarinathan R, et al. Epidemiology of hepatitis A and hepatitis E based on laboratory surveillance data-India, 2014-2017. Am J Trop Med Hyg. 2018;99:1058–61. doi: 10.4269/ajtmh.18-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar M, Sarin SK. Viral hepatitis eradication in India by 2080- gaps, challenges &targets. Indian J Med Res. 2014;140:1–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Arankalle VA, Sarada Devi KL, Lole KS, Shenoy KT, Verma V, Haneephabi M. Molecular characterization of hepatitis A virus from a large outbreak from Kerala, India. Indian J Med Res. 2006;123:760–9. [PubMed] [Google Scholar]
- 7.Dandabathula G, Bhardwaj P, Burra M, Rao PVVP, Rao SS. Impact assessment of India's Swachh Bharat Mission-Clean India Campaign on acute diarrheal disease outbreaks:Yes, there is a positive change. J Family Med Prim Care. 2019;8:1202–8. doi: 10.4103/jfmpc.jfmpc_144_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murhekar MV, Sehgal SC, Murhekar KM, Padbhidri SP, Chitambar SD, Arankalle VA. Changing scenario of hepatitis A virus and hepatitis E virus exposure among the primitive tribes of Andaman and Nicobar Islands, India over the 10-year period 1989-99. J Viral Hepat. 2002;9:315–21. doi: 10.1046/j.1365-2893.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaur M, Sidhu SK, Singh K, Devi P, Kaur M, Singh NJ. Hepatitis E virus:A leading cause of waterborne viral hepatitis in Northwest Districts of Punjab, India. J Lab Physicians. 2017;9:121–4. doi: 10.4103/0974-2727.199636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A, Ali IA, Ghazal H, Fazili J, Nusrat S. Mystery of hepatitis E virus:Recent advances in its diagnosis and management. Int J Hepatol. 2015;2015:31. doi: 10.1155/2015/872431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton HR, Seghatchian J. Hepatitis E virus:Emerging from the shadows in developed countries. Transfus Apher Sci. 2016;55:271–4. doi: 10.1016/j.transci.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Fenaux H, Chassaing M, Berger S, Gantzer C, Bertrand I, Schvoerer E. Transmission of hepatitis E virus by water:An issue still pending in industrialized countries. Water Res. 2019;151:144–57. doi: 10.1016/j.watres.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Reddy DC. Elimination of viral hepatitis:Evolution and India's response. Indian J Public Health. 2019;63:275–6. doi: 10.4103/ijph.IJPH_581_19. [DOI] [PubMed] [Google Scholar]
- 14.Radhakrishnan S, Raghuraman S, Abraham P, Kurian G, Chandy G, Sridharan G. Prevalence of enterically transmitted hepatitis viruses in patients attending a tertiary--care hospital in south India. Indian J Pathol Microbiol. 2000;43:433–6. [PubMed] [Google Scholar]
- 15.Netra S, Bithu R, Maheshwari RK. Epidemiological study of hepatitis A virus and hepatitis E virus infection in patients presenting with acute viral hepatitis. Int J Curr Microbiol App Sci. 2018;7:899–904. [Google Scholar]
- 16.Joon A, Rao P, Shenoy SM, Baliga S. Prevalence of hepatitis A virus (HAV) and hepatitis E virus (HEV) in the patients presenting with acute viral hepatitis. Indian J Med Microbiol. 2015;33(Suppl):102–5. doi: 10.4103/0255-0857.150908. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal R, Goel A. Hepatitis A:Epidemiology in resource-poor countries. Curr Opin Infect Dis. 2015;28:488–96. doi: 10.1097/QCO.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 18.Arankalle V, Mitra M, Bhave S, Ghosh A, Balasubramanian S, Chatterjee S, et al. Changing epidemiology of hepatitis A virus in Indian children. Vaccine Development Therapy. 2014;4:7–13. [Google Scholar]
- 19.Agrawal A, Singh S, Kolhapure S, Hoet B, Arankalle V, Mitra M. Increasing burden of hepatitis A in adolescents and adults and the need for long-term protection:A review from the Indian subcontinent. Infect Dis Ther. 2019;8:483–97. doi: 10.1007/s40121-019-00270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arankalle VA, Chadha MS, Chitambar SD, Walimbe AM, Chobe LP, Gandhe SS. Changing epidemiology of hepatitis A and hepatitis E in urban and rural India (1982-98) J Viral Hepat. 2001;8:293–303. doi: 10.1046/j.1365-2893.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 21.Sarangi G, Dash M, Mahapatra D, Paty BP, Mohanty DP, Chayani N. Fecal–oraltransmitted hepatitis A and E prevalence in Eastern India:A 3 year retrospective study. J Med Soc. 2019;33:86–90. [Google Scholar]
- 22.Chandra NS, Sharma A, Rai RR, Malhotra B. Contribution of hepatitis E virus in acute sporadic hepatitis in north western India. Indian J Med Res. 2012;136:477–82. [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Ratho RK, Chawla YK, Chakraborti A. Virological investigation of a hepatitis E epidemic in North India. Singapore Med J. 2006;47:769–73. [PubMed] [Google Scholar]
- 24.Shalimar, Kedia S, Gunjan D, Sonika U, Mahapatra SJ, Nayak B, et al. Acute liver failure due to hepatitis E virus infection is associated with better survival than other etiologies in Indian patients. Dig Dis Sci. 2017;62:1058–66. doi: 10.1007/s10620-017-4461-x. [DOI] [PubMed] [Google Scholar]
- 25.Hofmeister MG, Foster MA, Teshale EH. Epidemiology and transmission of hepatitis A virus and hepatitis E virus infections in the United States. Cold Spring Harb Perspect Med. 2019;9:a033431. doi: 10.1101/cshperspect.a033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta E, Agarwala P. Hepatitis E virus infection:An old virus with a new story! Indian J Med Microbiol. 2018;36:317–23. doi: 10.4103/ijmm.IJMM_18_149. [DOI] [PubMed] [Google Scholar]
- 27.Al-Sadeq DW, Majdalawieh AF, Mesleh AG, Abdalla OM, Nasrallah GK. Laboratory challenges in the diagnosis of hepatitis E virus. J Med Microbiol. 2018;67:466–80. doi: 10.1099/jmm.0.000706. [DOI] [PubMed] [Google Scholar]
- 28.Prakash S, Shukla S, Shukla R, Bhagat A, Srivastava SS, Jain A. Molecular characterization of hepatitis A virus circulating in Uttar Pradesh, India:A hospital-based study. Indian J Med Res. 2020;151:375–9. doi: 10.4103/ijmr.IJMR_429_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker CM. Adaptive immune responses in hepatitis A virus and hepatitis E virus infections. Cold Spring Harb Perspect Med. 2019;9:a033472. doi: 10.1101/cshperspect.a033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nan Y, Wu C, Zhao Q, Sun Y, Zhang YJ, Zhou EM. Vaccine development against zoonotic hepatitis E Virus:Open questions and remaining challenges. Front Microbiol. 2018;9:266. doi: 10.3389/fmicb.2018.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren X, Wu P, Wang L, Geng M, Zeng L, Zhang J, et al. Changing epidemiology of hepatitis A and hepatitis E viruses in China, 1990-2014. Emerg Infect Dis. 2017;23:276–9. doi: 10.3201/eid2302.161095. [DOI] [PMC free article] [PubMed] [Google Scholar]



