Abstract
Objective:
The aim of this study is to determine the aetiology and characteristics of pulmonary cavities that developed in patients recovering from COVID-19 infection.
Materials and Methods:
Between 1st May 2021 and 30st June 2021, we found 9 post COVID-19 patients who developed lung cavities on chest radiograph or CT during the follow-up period. These patients underwent routine blood examination, sputum examination and bronchoscopy to identify the aetiologies for the lung cavities.
Results:
The duration from the onset of COVID-19 symptoms to the detection of lung cavities ranged from 18 to 82 days. Out of 7 patients, 4 had recovered from severe COVID-19 disease, 2 from moderate and 1 from mild disease. After the diagnostic workup, 5 patients were found to have COVID-19 associated pulmonary aspergillosis (CAPA), 1 patient with mucormycosis and 1 patient with mycobacterium infection. Two patients with CAPA also had bacterial infection; sputum culture from both these patients grew Klebsiella pneumonia.
Conclusion:
Lung cavities can develop in patients recovering from COVID-19 pneumonia and fungal infection is the most common cause for such cavities.
Keywords: COVID-19 associated pulmonary aspergillosis, COVID-19 pneumonia, fungal pneumonia, pulmonary cavity
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19 disease, was first time detected in humans from city Wuhan, China, in December 2019.[1] This is the third coronavirus emerged in the last 20 years. In last two decades, after the SARS-CoV outbreak in 2002 and the Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in 2012, this 2019-novel coronavirus is the third coronavirus to emerge in the human population which challenge the health care system.[2]
To date, several studies and a meta-analysis have described the typical computerised tomography (CT) findings of COVID-19 pneumonia. The typical abnormalities seen on CT of the chest in patients with COVID-19 lung disease have been well described.[3,4,5,6] Almost all studies have described the ground glass opacities as the most common radiological finding. The other findings included consolidations with air bronchograms, linear opacities, crazy-paving pattern and interlobular septal thickening.[7,8] There was no description of formation of cavities in any of these studies. Pulmonary cavitation, lymphadenopathy and pleural effusion are considered rare in COVID-19 pneumonia.[9] There were some patients who end up in OPD with persistent COVID-19 symptom or new onset symptoms with pulmonary cavitation in chest X-ray or CT scan chest. Pulmonary cavity occurs due to wide variety of pathological processes in lung associated with both infective and noninfective condition. This report describes the clinical, imaging and microbiological findings in 7 patients with COVID-19 pneumonia who developed lung cavities during follow-up period.
Materials and Methods
This retrospective study was performed during 1 May and 30 June, 2021 at All India Institute of Medical Sciences, Patna (Bihar). In our outpatient care facility for post COVID-19 patients, 9 patients were found to have lung cavities, which developed during the recovery phase of the disease. Of 9 patients, 7 patients had documented normal chest radiograph before the onset of the COVID-19. The diagnosis of COVID-19 in all patients were made by positive RT-PCR test on nasopharyngeal swab. A detailed history was obtained from all patients. All patients underwent routine blood examination including complete blood count, sputum examination and bronchoscopy to identify aetiologies for the lung cavities. Bronchoalveolar lavage fluid were analyzed for Gram stain, culture, sensitivity, fungal KOH stain, fungal culture, AFB culture and GeneXpert analysis. The clinical characteristics of all patients are summarised in Table 1. The clinical history of these patients are as follows.
Table 1.
Case summary of the 7 post COVID-19 patients with cavitation
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Age | 26 | 59 | 55 | 44 | 67 | 45 | 56 |
| Sex | F | F | M | M | M | F | M |
| Covid severity | Severe | Severe | Severe | Mild | Severe | Mild | Moderate |
| Date of RTPCR positive | 28/04/2021 | 21/04/2021 | 26/04/2021 | 15/05/2021 | 24/04/21 | 21/04/2021 | 15/05/2021 |
| ICU | yes | yes | yes | No | No | No | No |
| Mechanical ventilation | No | No | No | No | No | No | No |
| Comorbidity | Diabetes, | Diabetes | - | Diabetes, CKD | - | Diabetes | |
| Treatment received | Tocilizumab IV Methyl prednisolone |
IV Methyl Prednisolone Ticilizumab |
IV Methylpred | Doxycycline, Azithromycin, Ivermectin Zinc Methyl pred tab |
Methyl prednisolone | Methyl pred tab, Doxycycline, Azithromycin, Ivermectin | Oxygen Dexamethasone Antibiotic Heparin |
| Post covid presenting Symptom |
Fever Cough |
Fever | Weakness, Dyspnea, cough | Hemoptysis Dyspnea |
Cough | Chest pain Dry cough |
Productive cough, Fever |
| Presentation after SARS cov-2 positivity (day) | 36 | 43 | 38 | 18 | 50 | 82 | 70 |
| Cavity | Single, thick wall, left lower lobe | Single Right Lower lobe | Single. Right lower lobe | Left lower lobe | Single, Left upper lobe | Single Left lower lobe | Bilateral, lower lobe |
| Hemoglobin (gm/dl) | 11.6 | 10.2 | 9.8 | 12.6 | 8.1 | 10.8 | 10.4 |
| TLC | 11460 | 13700 | 12300 | 7000 | 10.28 | 9800 | 8.40 |
| Neutrophil (%) | 71 | 83 | 79 | 72 | 76.2 | 67 | 77 |
| Lymphocyte (%) | 21 | 11 | 13 | 21 | 8.8 | 23 | 18.3 |
| SERUM GALACTOMAN | Not done | Positive (0.59) | Not done | 0.58 | 1.47 | No done | Not done |
| BAL GALCTOMAN | Positive (1.909) | Positive (1.909) | Positive (1.226) | Positive (3.2) | Positive (1.50) | Positive (2.8) | Not done |
| BAL Fungal stain | Negative | Negative | Negative | Negative | Aseptate hyphae | Septate hyphae | Not done |
| BAL fungal culture | Negative | Negative | Negative | Negative | Awaited | Aspergillus | Not done |
| BAL bacterial culture | Neg | Negative (PCR for Klebsiella positive) | Klebsiella pneumoniae | Negative | Negative | Negative | Not done |
| BAL fluid-GeneXpert for MTB | Negative | Negative | Negative | Negative | Negative | Negative | Detected (Sputum) |
| Outcome | Discharged | Discharged | Discharged | Discharged | Discharged | Discharged | Discharged |
BAL; Bronchoalveolar lavage M; Male F; Female CKD; Chronic Kidney disease
Case 1
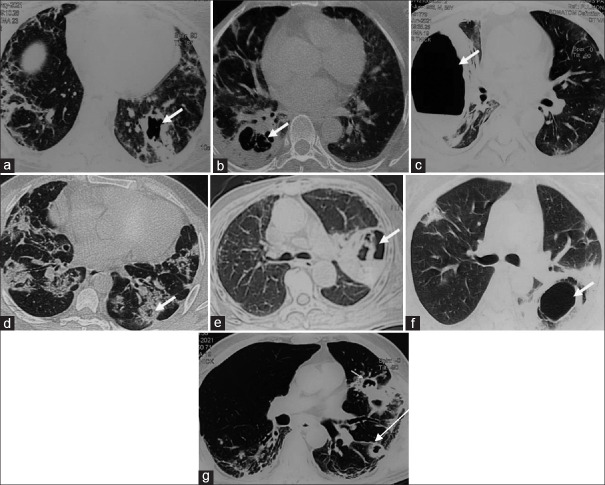
A 26-year-old girl developed with sudden onset fever and was diagnosed for COVID-19. After 5 days, she developed dyspnoea at rest and O2 saturation fell to 82% at room air; she was admitted in Intensive care unit (ICU) with Pulse 112/min and BP 112/76 mmHg. Her respiratory rate at that time was 34/min and O2 saturation was 56% at room air. She was started with O2 inhalation and put on noninvasive ventilatory support. During hospitalisation, she received injection ceftriaxone, methyl prednisolone and injectionremdesivir. She was discharged after 1 month of hospitalisation. Then, she again presented with fever, dry cough and weakness. The patient underwent CT examination on the same day, which showed thick-walled cavity in left lower lobe along with the parenchymal changes typical of COVID-19 pneumonia [Figure 1a]. Notably, she had chest CT one month ago at which time CT showed only bilateral diffuse ground glass opacities without cavitation. Bronchoscopy was performed and BAL fluid sent for bacterial culture and sensitivity, Fungal KOH stain, culture and Galactomannan. The BAL galactomannan test was found positive for aspergillus. Therefore, patient was diagnosed as COVID-19 associated pulmonary aspergillosis (CAPA) and started on tab Voriconazole 200 mg twice a day. She became asymptomatic after 2 weeks and follow-up radiograph showed disappearance of lung cavities
Figure 1.
(a-g). Axial high-resolution CT of 7 consecutive patients showing cavitary lung lesions indicated by arrows. Note that all lesions occurred in the setting of COVID-19 pneumonia which is evident by subpleural reticulations and fibrotic bands in the surrounding lung parenchyma in all cases
Case 2
A 59-year-old women was admitted to our hospital with severe COVID-19. She was a known diabetic for 10 years. Her blood glucose level (random) was 212 with oxygen saturation of 88% at room air. She was treated in ICU with maximum O2 requirement up to 12 litres with Non reservoir mask. She received medication like parenteral antibiotic, steroid, Remdesivir and also 2 doses of Tocilizumab. High resolution computerized tomography (HRCT) chest showed diffuse ground glass opacity (GGO) predominantly middle and lower lobe [Figure 1b]. She was treated for a period of 21 days including 6 days of ICU. After 9 days of discharge, patient developed fever, for which she consulted to us. On examination, vital are within normal limit. Repeat HRCT showed thick-walled cavity in Right Lower Lobe with GGO and fibrosis [Figure 1b]. Serum galactomannan and BAL galactomannan both was found positive. The diagnosis of CAPA was made and started on parenteral Voriconazole. She was asymptomatic after 1 week and discharged on Tab Voriconazole and asked for review after 4 weeks.
Case 3
A 55-year-old male had recovered from severe COVID-19. He presented with generalised weakness, dyspnea on exertion (mMRC grade 3) and dry cough. Patient denied any history of fever or blood in sputum. The chest CT showed thick-walled cavity with fluid level in right lower lobe [Figure 1c]. Bronchoscopy was performed and BAL fluid sent for bacterial, fungal and mycobacterial and galactomannan test. Bacterial culture grew Klebsiella and the BAL galactomannan was found positive for Aspergillus. On susceptibility test results, the Klebsiella species were found sensitive to imipenem, amikacin and ciprofloxacin. Accordingly, the patient received antibiotics. In addition, he also received oral voriconazole considering possibility of invasive pulmonary aspergillosis.
Case 4
A 44-year-old women had just recovered from mild COVID-19. She again presented with cough with blood-tinged sputum and breathlessness on exertion. She was non-smoker and had no comorbidity. Her vitals were normal and maintained oxygen saturation of 96% at room air. Her chest CT revealed multiple cavities in bilateral lower lobe [Figure 1d]. Video bronchoscopy was performed and BAL fluid was found negative for bacterial, fungal and acid-fast bacilli. BAL Fluid was also sent for Genexpert which did not detect Mycobacterium tuberculosis. Serum and Bal Galctomann both were strongly positive and diagnosis of CAPA was made. The Patient was discharged on oral voriconazole 200 mg twice a day.
Case 5
A 67-year-old man had recovered from severe COVID-19. He was a known diabetic and was suffering from chronic kidney disease. His chief complaint at the time of presentation included worsening dry cough from last 10 days. His vitals were normal with oxygen saturation of 95% at room air. The chest CT showed thick-walled cavity in left upper lobe [Figure 1e]. Bronchoscopy was performed and the BAL fluid was sent for various tests including bacteriological and fungal culture, bacteriological fungal stain aseptate hyphae and culture grown mucor species. The BAL galactomannan and serum galactomannan both were found positive for aspergillus. The diagnosis of covid associated pulmonary mucormycosis was made. Liposomal amphotericin was started. After 7 days of amphotericin, it was not available and discharged on tab Posaconazole.
Case 6
A 45-year-old women was presented with left sided chest pain. She had recovered from mild COVID-19 infections 15 days ago. Her vitals were normal and she maintained oxygen saturation of 97% at room air. The chest CT showed thick-walled cavity in left lower lobe [Figure 1f]. BAL fluid obtained by bronchoscopy revealed septate hyphae and culture reported grown aspergillus. BAL galactomannan was found positive.The Diagnosis of CAPA was made and discharged on tab voriconazole.
Case 7
A 56-year-old male, diabetic from last 2 years, presented with productive cough with whitish expectoration and low-grade fever from last 10 days. He had moderate COVID-19 for which he was admitted in a private hospital 2 months ago. During the COVID-19 illness, he had received supplemental oxygen, parenteral steroid and antibiotics. On examination, the patient was afebrile and he denied for haemoptysis, anorexia and weight loss. His vitals are with in normal limit. His chest CT showed multiple small cavities in both lungs, predominantly in bilateral upper lobes [Figure 1g]. His sputum examination revealed acid fast bacilli which was rifampicin sensitive on GeneXpert test. Accordingly, the patient received anti-tubercular treatment and became asymptomatic after 3 months.
Result
A total of 9 patients were presented in the given period but only 7 patients undergo detailed workup including bronchoscopy included in study. Characteristics of all patients are summarised in Table 1. In our case series, 5 patients suspected of aspergillus infection, 1 patient suspected for mucor and 1 patient with mycobacterium tuberculosis infection. Two patients had also bacterial infection and Klebsiella pneumoniae grown as bacterial pathogen in both of them.
Discussion
Pulmonary cavity, defined as an air-filled space formed within an area of consolidation, mass or nodule, results from liquefication of the necrotic portion of the lesion and the discharge of this necrotic material via bronchial airway. Although, both infective (mycobacterial, bacterial and fungal) and noninfective disease (neoplastic, autoimmune or infarct) can cause cavity formation within lung, lung cavities resulting from a viral pneumonia is rare. The occurrence of lung cavities has rarely been reported even in patients with severe SARS-CoV and MERS-CoV.[10,11,12,13,14] The exact mechanism of cavitation in COVID-19 pneumonia is unknown. It may be related to diffuse alveolar damage, intra-alveolar haemorrhage and necrosis of parenchymal cells based on prior autopsy reports.[15] It is unclear whether the secondary bacterial infection or invasive fungal infection contribute to the development of the cavities, or cavity is formed due to SARS cov-2 itself. This case series found aspergillus as most common cause of cavitation. In one patient, it was due to mucormycosis and in another patient, mycobacterium tuberculosis caused the cavity formation. Two patients had coinfection with both Klebsiella pneumonia. The diagnosis of associated pulmonary aspergillosis (CAPA) was made on basis of typical CT appearance and BAL galactomannan test.[16] Although pulmonary tuberculosis was found in only one patient in this series, a recent case series has reported 6 patients of COVID-19 with pulmonary tuberculosis.[17] Out of 7 patients, 4 patients had recovered from severe COVID-19, 1 had moderate and 1 had mild infection. Four patients were known diabetic, but no comorbidities were found for the other 3 patients. All the patients received methyl prednisolone injectable or tablet, while 2 patients received injection tocilizumab. Although recent trial showed survival benefit with steroid[18] and tocilizumab,[19,20] it also suppresses the immune system by impairing innate immunity and person susceptible for opportunistic infection which could be a cause of pulmonary cavitation. Pulmonary cavitation frequently treated as pulmonary tuberculosis by primary care physician considering endemic nature of disease. But fungal aetiology is found as more common cause than mycobacterium in patient recovered from COVID-19 infection. All the patients recovered from COVID-19 presented with pulmonary cavitation should be evaluated by bronchoscopy to get early diagnosis and treatment.
Details of mechanism and pathogenesis of cavity formation is needed
Pulmonary cavitation in COVID-19 pneumonia may be multifactorial, with contributing factors could be bacterial, mycobacterial, fungal coinfection, the immunosuppressive effects of glucocorticoids and tocilizumab, thromboembolic pathway leading to infarct lading to cavitation[21] or SARS-CoV-2 itself through inflammatory phase can be the cause of cavitation.[22]
Conclusion
Pulmonary cavitation in patients who recover from severe COVID-19 infection is not uncommon. Secondary infection, particularly fungal, can cause development of cavities in setting of COVID-19 pneumonia. A detailed, and early workup specially with bronchoscopy required to clinch the underlying aetiology. Regular follow-up is therefore essential in high-risk patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, et al. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–11. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China —Key questions for impact assessment. N Engl J Med. 2020;382:692–4. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 3.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–7. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong W, Agarwal PP. Chest imaging appearance of COVID-19 infection. Radiol Cardiothorac Imaging. 2020;2:e200028. doi: 10.1148/ryct.2020200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Zeng X, Liu B, Yu Y. COVID-19 infection presenting with CT halo sign. Radiol Cardiothorac Imaging. 2020;2:e200026. doi: 10.1148/ryct.2020200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19):A systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, Wang L, Ben S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J Med Virol. 2021;93:241–9. doi: 10.1002/jmv.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. Ct imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–7. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, Zhang N, Li Y, Xu X. A systematic review of chest imaging findings in COVID-19. Quant Imaging Med Surg. 2020;10:1058–79. doi: 10.21037/qims-20-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong KT, Antonio GE, Hui DSC, Lee N, Yuen EHY, Wu A, et al. Severe acute respiratory syndrome:Radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228:401–6. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- 11.Das KM, Lee EY, Langer RD, Larsson SG. Middle east respiratory syndrome coronavirus:What does a radiologist need to know? AJRAm J Roentgenol. 2016;206:1193–201. doi: 10.2214/AJR.15.15363. [DOI] [PubMed] [Google Scholar]
- 12.Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle east respiratory syndrome coronavirus (MERS-CoV) infection:Chest CT findings. AJR Am J Roentgenol. 2014;203:782–7. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Chen W, Zhou J, Sun C, Lei Y. Large pulmonary cavity in COVID-19 cured patient case report. Ann Palliat Med. 2020;9:5–452. doi: 10.21037/apm-20-452. [DOI] [PubMed] [Google Scholar]
- 14.Selvaraj V, Dapaah-Afriyie K. Lung cavitation due to COVID-19 pneumonia. BMJ Case Rep. 2020;13:e237245. doi: 10.1136/bcr-2020-237245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. European Confederation of Medical Mycology;International Society for Human Animal Mycology;Asia Fungal Working Group;INFOCUS LATAM/ISHAM Working Group;ISHAM Pan Africa Mycology Working Group;European Society for Clinical Microbiology;Infectious Diseases Fungal Infection Study Group;ESCMID Study Group for Infections in Critically Ill Patients;Interregional Association of Clinical Microbiology and Antimicrobial Chemotherapy;Medical Mycology Society of Nigeria;Medical Mycology Society of China Medicine Education Association;Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology;Association of Medical Microbiology;Infectious Disease Canada Defining and managing COVID-19-associated pulmonary aspergillosis:The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–62. [Google Scholar]
- 17.Yousaf Z, Khan AA, Chaudhary HA, Mushtaq K, Parengal J, Aboukamar M, et al. Cavitary pulmonary tuberculosis with COVID-19 coinfection. IDCases. 2020;22:e00973. doi: 10.1016/j.idcr.2020.e00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure:A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19:A retrospective cohort study. Lancet Rheumatol. 2020;2:e474–84. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruse JM, Zickler D, Lüdemann WM, Piper SK, Gotthardt I, Ihlow J, et al. Evidence for a thromboembolic pathogenesis of lung cavitations in severely ill COVID-19 patients. Sci Rep. 2021;11:16039. doi: 10.1038/s41598-021-95694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Gupta RK, Layek A, Bala M, Akhlesh Pulmonary cavitation post COVID-19 pneumonia:A case report. Ann Clin Med Res. 2021;2:1035. [Google Scholar]