Abstract
Background
Otitis media (OM) is one of the most common infections in young children, arising from bacterial and/or viral infection of the middle ear. Globally, Streptococcus pneumoniae and non-typeable Haemophilus influenzae (NTHi) are the predominant bacterial otopathogens. Importantly, common upper respiratory viruses are increasingly recognized contributors to the polymicrobial pathogenesis of OM. This study aimed to identify predominant bacteria and viruses in the nasopharynx, adenoids and middle ears of peri-urban/urban South-East Queensland Australian children, with and without clinical history of chronic otitis media with effusion (COME) and/or recurrent acute otitis media (RAOM).
Methods
Sixty children, 43 diagnosed with OM and 17 controls with no clinical history of OM from peri-urban/urban South-East Queensland community were recruited to the study. Respiratory tract bacterial and viral presence were examined within nasopharyngeal swabs (NPS), middle ear effusions (MEE) and adenoids, using real-time polymerase chain reaction (RT-PCR) and bacterial culture.
Results
At least one otopathogen present was observed in all adenoid samples, 86.1% and 82.4% of NPS for children with and without OM, respectively, and 47.1% of the MEE from the children with OM. NTHi was the most commonly detected bacteria in both the OM and control cohorts within the adenoids (90.0% vs 93.8%), nasopharynx (67.4% vs 58.8%) respectively, and in the MEE (OM cohort 25.9%). Viruses were detected in all adenoid samples, 67.4% vs 47.1% of the NPS from the OM and control cohorts, respectively, and 37% of the MEE. Rhinovirus was the predominant virus identified in the adenoids (85.0% vs 68.8%) and nasopharynx (37.2% vs 41.2%) from the OM and control cohorts, respectively, and the MEE (19.8%).
Conclusions
NTHi and rhinovirus are predominant otopathogens within the upper respiratory tract of children with and without OM from peri-urban and urban South-East Queensland, Australia. The presence of bacterial otopathogens within the middle ear is more predictive of concurrent URT infection than was observed for viruses, and the high otopathogen carriage within adenoid tissues confirms the complex polymicrobial environment in children, regardless of OM history.
Keywords: otitis media, etiology, Haemophilus influenzae, Streptococcus pneumoniae, Moraxella (Branhamella) catarrhalis, otitis media with effusion (OME), recurrent acute otitis media (RAOM)
1 Introduction
Otitis media (OM) is one of the most common infections in young children and is associated with otopathogenic bacteria and/or viruses within the upper respiratory tract (Rovers et al., 2004; Nokso-Koivisto et al., 2015; Phillips et al., 2020; Thornton et al., 2020). Globally, Streptococcus pneumoniae, non-typeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis are the three main bacterial otopathogens of OM. S. pneumoniae is identified as the predominant bacterial otopathogen in the middle ear of children experiencing acute otitis media (AOM), while NTHi is more frequently detected in the middle ear of children with recurrent acute otitis media (RAOM) and/or chronic otitis media with effusion (COME) (Ngo et al., 2016). These bacteria are commensal flora in the upper respiratory tract (URT), including nasopharynx and adenoids, which are considered potential reservoirs for both bacterial and viral otopathogens causing middle ear infection (Pettigrew et al., 2012; Stol et al., 2013; Fago-Olsen et al., 2019).
Viral infection of the upper respiratory tract can contribute to OM development, through direct causation of AOM (Chonmaitree and Heikkinen, 1997; Heikkinen and Chonmaitree, 2003; Nokso-Koivisto et al., 2015; Chonmaitree et al., 2016; Schilder et al., 2016; Thornton et al., 2020) and/or initiation of inflammation prolonging middle ear effusion (MEE) (Chonmaitree and Heikkinen, 1997; Heikkinen and Chonmaitree, 2003; Nokso-Koivisto et al., 2015). A range of respiratory viruses, including adenovirus (ADV), rhinovirus (HRV) and respiratory syncytial virus (RSV), have been detected in the middle ear, nasopharynx and adenoids of children with AOM (Heikkinen et al., 1999; Chonmaitree, 2000; Heikkinen and Chonmaitree, 2003; Ishibashi et al., 2003; Monobe et al., 2003; Nokso-Koivisto et al., 2004; Ruohola et al., 2006; Bulut et al., 2007; Drago et al., 2008; Binks et al., 2011; Wiertsema et al., 2011a; Ruohola et al., 2013; Marom et al., 2019; Sawada et al., 2019). Globally, the predominant viruses identified within the middle ear, vary more widely, with RSV reported as the most frequently detected virus in the MEE of children with AOM in the United States (Heikkinen et al., 1999; Chonmaitree, 2000; Marom et al., 2019) and Asian countries (Ishibashi et al., 2003; Monobe et al., 2003; Bulut et al., 2007; Sawada et al., 2019). HRV is identified most often within the MEE of children in Australia (Wiertsema et al., 2011a) and European countries (Heikkinen and Chonmaitree, 2003; Nokso-Koivisto et al., 2004; Ruohola et al., 2006) whilst enterovirus is most frequently identified in Brazil (Buzatto et al., 2017).
Overall, otopathogen detection frequencies appear to relate to geographical location and should be actively considered. For example, in Australia, a majority of studies of OM otopathogen identification have examined rural and/or remote Australian Aboriginal children, who experience significant increased risk of severe OM. These studies include children with severe OM, chronic suppurative otitis media (CSOM) with tympanic perforation and ear discharge (Gibney et al., 2005; Leach and Morris, 2007; Smith-Vaughan et al., 2013). These studies were undertaken in the Northern Territory, a tropical region incorporating many rural and remote communities. Two studies identified bacterial and viral otopathogens within the MEE and nasopharynx of urban children with RAOM (Wiertsema et al., 2011a; Wiertsema et al., 2011b) and were undertaken in the temperate region of Western Australia. The current study aimed to identify predominant bacterial and viral carriage within the nasopharynx, adenoids and middle ears of the upper respiratory tract in peri-urban/urban children undergoing ventilation tube insertion for COME and/or RAOM in South-East Queensland, a subtropical region of Australia. The frequency of bacterial and viral co-infection and their distribution throughout the upper respiratory tracts and middle ear of children with OM were compared to the otopathogen distribution in the upper respiratory tracts of children undergoing adenoidectomy, who had no significant clinical history of OM.
2 Methods
2.1 Recruitment and Study Cohorts
Children aged 1 to 8 years old who were undergoing ventilation tube insertion (VTI) +/- adenoidectomy for the treatment of COME and/or RAOM were recruited to the OM cohort between December 2008 and December 2010 at Royal Children’s Hospital, Brisbane and January and November 2015 at Pindara Private Hospital, Gold Coast, Queensland, Australia. The clinical history of these children included: the presence of MEE for >3months or recurrent acute OM infection of either 3 episodes within 6 months or 4 or more episodes within 12 months. The cohort without OM history, recruited children of similar age undergoing adenoidectomy as treatment for adenoidal hypertrophy (AH) and/or obstructive sleep apnoea (OSA). These participants had no significant history of OM. All children recruited to this study were fully vaccinated with pneumococcal conjugate vaccines in accordance with the Australian National Immunisation Program schedule [Australian Technical Advisory Group on Immunisation (ATAGI), 2018]. Children with diagnosed immunological abnormality either intrinsic or pharmacological; anatomical or physiological defect; respiratory tract infection; purulent middle ear effusion and any malformations were excluded. All children were examined and clinically well on the day of sample collection and surgery.
This study was approved by the Children’s Health Services District Ethics Committee (2008/063 and HREC/14/QRCH/33), Greenslopes Hospital Human Research Ethics Committee (14/18) and the Griffith University Human Research Ethics Committee (MSC/05/08/HREC and MSC/19/13/HREC). Prior, informed consent was provided by each child’s parent or guardian.
A series of samples were collected from each child by the surgeon, after anesthesia induction but prior to insertion of VTI or adenoid removal. The samples collected for the OM cohort included NPS, adenoid swab/tissue and MEE, the latter were not collected from participants without OM history. All samples were placed on ice and transferred to the laboratory for processing for RT-PCR and bacterial culture within 4 hours of collection.
2.1.1 Nasopharyngeal Swabs
Nasopharyngeal swabs were collected by trans-nasal insertion of a sterile flexible cotton-wool swab (Copan, Brescia, Italy) reaching the nasopharyngeal space. Swabs were stored in sterile Skim-Milk-Tryptone-Glucose-Glycerol-Broth and placed on ice until processing.
2.1.2 Middle Ear Samples
Prior to MEE collection, outer ear canals were rinsed with sterile physiological saline. An anterior-inferior myringotomy incision was made and MEE from each ear was collected separately using individual sterile Argyle Specimen Traps (Covidien, Dublin, Ireland) connected to a suction system, washed through with 2ml of saline. The MEE was immediately placed on ice until processed. MEE samples were collected from both left and right ears for each OM patient.
2.1.3 Adenoid Tissues
Adenoid tissue was removed using a curette and placed immediately in sterile Hanks buffered saline solution (Invitrogen, Australia) and kept on ice until transferred and processed for bacterial culture. Part of each adenoidal tissue sample was homogenized and placed in sterile Skim-Milk-Tryptone-Glucose-Glycerol-Broth for storage and RT-PCR.
2.2 Bacterial Culture
Following collection, MEE, NPS and adenoid samples were transported on ice to the hospital pathology laboratory and analyzed using standard pathology laboratory culture protocols (Mahon et al., 2014). All predominant bacterial colonies were recorded after 24-72h incubation and visual identification of colonies for S. pneumoniae, NTHi and M. catarrhalis were confirmed by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (Shimadzu, Australia). Serotypes for S. pneumoniae were initially identified using a Pneumotest kit (Statens Serum Institut – (SSI), Denmark) by Quellung reaction, in accordance with manufacturer instructions. Further identification of serotypes was performed using latex agglutination (Satzke et al., 2015) with latex reagents prepared using antisera from SSI Diagnostica (Ortika et al., 2013) by the Pneumococcal Research Group at the Murdoch Children’s Research Institute (Victoria, Australia).
2.3 Bacterial and Viral PCR
Bacterial DNA was extracted from 300µl of MEE, NPS and adenoid samples, as previously described (Smith-Vaughan et al., 2006). RT-PCR detection of S. pneumoniae, NTHi and M. catarrhalis utilized specific primers for each bacterial target as follows: S. pneumoniae (autolysin gene – lytA) (Marsh et al., 2012), NTHi (haemophilus protein D gene – hpd and L-fucose permease gene – FucP) (Binks et al., 2012; Price et al., 2015) and M. catarrhalis (outer membrane protein gene – copB) (Marsh et al., 2012), respectively.
Viral otopathogen detection used total nucleic acid extracted using Qiagen x-tractor gene from a 200µl sample spiked with 104 copies of Equine Herpes virus, before RT-PCR was performed to detect eight viral pathogens. The viruses included influenza A virus (IAV), influenza B virus (IBV), parainfluenza virus (PIV, including types 1, 2, 3), Human Adenovirus (ADV), Human metapneumovirus (hMPV), Human Respiratory Syncytial Virus (RSV), Human Rhinovirus (HRV) and WU polyomavirus (WU) by Rotorgene instruments, Qiagen Australia, as described previously (Rockett et al., 2013).
2.4 Statistical Analyses
Demographic data including participant age and frequency of OM episodes were compared using independent samples t-tests. Sex, URT presence of bacteria/viruses, patterns of co-colonizing or infecting species were compared between OM and control cohorts using Pearson Chi-square analyses with comparison of detection frequencies between RT-PCR and culture results performed using McNemar’s tests. For all statistical analyses, a p-value <0.05 was considered significant and all data analyses were performed using SPSS for Windows, Version 23 (IBM).
3 Results
3.1 Demographics and Sample Collection
A total of 60 children, aged 1-8 years were recruited to the OM group (mean = 3.7 years +/- 1.9 SD, n=43) and the control group without a history of OM (mean = 4.5 years +/- 1.5 SD, n=17). There were no significant differences in age or sex between the groups, as shown in Table 1 . The number of episodes of OM differed significantly (p<0.001) between the OM group (Mean = 5.6 +/- 3.2 SD) and control group (Mean = 0.9 +/- 0.9 SD). Overall, the otopathogens identified in all sample types collected from each group are illustrated in Supplemental Figure 1 . The otopathogens identified in the MEE samples collected and analysed for each group are shown in Table 2 . Notably within the OM group, a MEE sample was collected from both left and right ears, however one MEE sample was not collected due to pre-existing tympanic perforation. Adenoid samples were only available for 20 children due to OM treatment decisions not including adenoidectomy. Viral RT-PCR results for two children (4 MEE samples) were excluded due to the presence of inhibitors, thus only 81 MEE samples (or 40 paired + 1 single MEE samples) from 43 recruited children were used for viral detection. In addition, one adenoid sample was not successfully collected from a control group participant (n=16) ( Table 1 ).
Table 1.
Demographic data and samples collected from peri-urban and urban children in South-East Queensland undergoing ventilation tube insertion for otitis media (OM) or adenoidectomy (Control) in the absence of a clinical history of OM.
| OM | Control | |
|---|---|---|
| Number | 43 | 17 |
| Mean age in year (range) | 3.7 (1-8) | 4.5 (3–8) |
| Male (%) | 23 (53.5%) | 12 (70.6%) |
| Mean episodes of AOM within last 12 months (range) | 5.6 (4-11) | 0.9 (0-2) |
| Middle ear effusion (MEE) | 85 | – |
| Nasopharyngeal swabs (NPS) | 43 | 17 |
| Adenoid samples | 20 | 16 |
Table 2.
Bacterial otopathogens and viruses identified by RT-PCR in the middle ears of peri-urban and urban children in South-East Queensland undergoing ventilation tube insertion for otitis media (OM).
| OM(n=85 ears) | |
|---|---|
| Bacterial otopathogens (n=85 ears) | 40 (47.1%) |
| S. pneumoniae | 15 (17.6%) |
| H. influenzae | 22 (25.9%) |
| M. catarrhalis | 17 (20.0%) |
| Viruses (n=81 ears) | 30 (37.0%) |
| Adenovirus | 3 (3.7%) |
| Human metapneumovirus | 3 (3.7%) |
| Influenza B virus | 1 (1.2%) |
| Respiratory syncytial virus | 6 (7.4%) |
| Rhinovirus | 16 (19.8%) |
| WU polyomavirus | 5 (6.2%) |
| No pathogen | 30 (37.0%) |
| Bacterial otopathogens alone | 21 (25.9%) |
| Viruses alone | 15 (18.5%) |
| Bacterial otopathogens and viruses | 15 (18.5%) |
Number and percentage (between brackets) of samples in which bacteria were detected. Viral detections are from 81 MEE samples due to presence of inhibitors in 4 samples and one sample not collected due to pre-existing tympanic membrane perforation.
3.2 Bacterial Otopathogens and Viruses Present in the Middle Ear
Within the middle ear effusate (MEE), bacterial otopathogen identification was significantly higher using RT-PCR compared to bacterial culture for each of the 3 predominant bacteria (P<0.001 for each bacterium). Bacterial otopathogens were identified in 47.1% of all MEE samples (n=85) ( Table 2 ) compared to 5.9% (n=5) from bacterial culture ( Supplemental Table 1 ), thus only RT-PCR data are reported further. NTHi, identified using RT-PCR, was the most common bacterium detected in the 85 MEE samples (25.9%), followed by M. catarrhalis (20.0%) and S. pneumoniae (17.6%) ( Table 2 ). Overall, at least one of the three predominant bacteria were identified within either one or both ears of 26 of the 42 participants with paired MEE samples. Comparison of the left and right ear samples from the same child (n=26) showed that different bacteria were identified in each ear (76.9%, n=20 children) whilst only 6 children had the same bacteria present in each ear ( Supplemental Table 2 ).
At least one of the viruses tested was found in 37.0% of MEE samples (n=81), with HRV (19.8%), RSV (7.4%) and WU (6.2%) detected most frequently ( Table 2 ). Overall, 22 paired left and right MEE samples had virus present in one or both ears (55.0%, n=40 pairs), with 15 of these pairs having a different virus in each ear (68.2%) compared to 8 pairs showing the same virus in both the left and right ears (31.8%) ( Supplemental Table 3 ).
Together these data demonstrated that 63.0% (51/81 ears) contained at least one bacteria or virus, with the remaining 30 ears (37.0%) being negative for either the three predominant bacteria causal for OM, or the selected panel of 8 common respiratory tract viruses. Bacterial detection alone (virus negative) or concurrent detection of bacteria and virus was observed in 25.9% of MEE samples, with viral detection alone observed in 18.5% of the MEE samples. With respect to concurrent bacterial and viral presence within the MEE, ADV detection is likely correlated with the presence of NTHi (r=0.321, p=0.001, Supplemental Table 4 ).
3.3 Bacterial Otopathogens and Viruses Present in the Nasopharynx
Bacterial identification within the nasopharynx of children with and without a clinical history of OM by RT-PCR demonstrated that at least one of the 3 predominant otopathogenic bacteria were present in 86.0% and 82.4% of NPS samples respectively. Bacteria were more commonly identified using RT-PCR than using bacterial culture (55.8% and 70.6% for OM and control cohorts respectively) thus only RT-PCR data is reported further. NTHi was identified most frequently in the NPS of children with OM (67.4%, 29/43) and the control cohort (58.8%, 10/17). Neither RT-PCR or bacterial culture data demonstrated any statistically significant difference in otopathogen identification between the OM and control groups ( Table 3 and Supplemental Table 5 ).
Table 3.
Otopathogens and viruses identified by RT-PCR in the nasopharynx and adenoids of peri-urban and urban children in South-East Queensland undergoing ventilation tube insertion for otitis media (OM) or adenoidectomy in the absence of a clinical history of OM (Control).
| Nasopharynx | OM (n=43) | Control (n=17) | P |
|---|---|---|---|
| Otopathogens | 37 (86.1%) | 14 (82.4%) | 0.718 |
| S. pneumoniae | 20 (46.5%) | 7 (41.2%) | 0.708 |
| H. influenzae | 29 (67.4%) | 10 (58.8%) | 0.528 |
| M. catarrhalis | 23 (53.5%) | 8 (47.1%) | 0.653 |
| Viruses | 29 (67.4%) | 8 (47.1%) | 0.143 |
| Adenovirus | 5 (11.6%) | 0 (0.0%) | 0.142 |
| Human metapneumovirus | 3 (7.0%) | 0 (0.0%) | 0.264 |
| Influenza A virus | 1 (2.3%) | 0 (0.0%) | 0.526 |
| Respiratory syncytial virus | 2 (4.7%) | 0 (0.0%) | 0.366 |
| Rhinovirus | 16 (37.2%) | 7 (41.2%) | 0.776 |
| Parainfluenza virus | 5 (11.6%) | 1 (5.9%) | 0.666 |
| WU polyomavirus | 8 (18.6%) | 0 (0.0%) | 0.056 |
| No otopathogen | 2 (4.7%) | 1 (5.9%) | 0.841 |
| Bacteria alone | 11 (25.6%) | 8 (47.1%) | 0.114 |
| Viruses alone | 4 (9.3%) | 2 (11.8%) | 0.772 |
| Bacteria and viruses | 26 (60.5%) | 6 (35.3%) | 0.091 |
| Adenoids | OM (n=20) | Control (n=16) | P |
| Otopathogens | 20 (100%) | 16 (100%) | |
| S. pneumoniae | 13 (65.0%) | 12 (75.0%) | 0.517 |
| H. influenzae | 18 (90.0%) | 15 (93.8%) | 0.686 |
| M. catarrhalis | 13 (65.0%) | 11 (68.8%) | 0.813 |
| Viruses | 20 (100%) | 16 (100%) | |
| Adenovirus | 13 (65.0%) | 7 (43.8%) | 0.202 |
| Rhinovirus | 17 (85.0%) | 11 (68.8%) | 0.244 |
| Parainfluenza virus | 12 (60.0%) | 10 (62.5%) | 0.875 |
| WU polyomavirus | 11 (55.%) | 5 (31.3%) | 0.154 |
| No pathogen | 0 (0.0%) | 0 (0.0%) | – |
| Bacteria alone | 0 (0.0%) | 0 (0.0%) | – |
| Viruses alone | 0 (0.0%) | 0 (0.0%) | – |
| Bacteria and viruses | 20 (100.0%) | 16 (100.0%) | – |
Number and percentage (between brackets) of samples in which bacteria were detected. P value was analyzed by Pearson Chi-square analyses.
Viral identification showed that 67.4% and 47.1% of NPS samples contained one of the 8 viruses investigated in the OM and control groups, respectively. HRV was the most commonly detected virus in NPS samples from both cohorts, with ADV, hMPV, RSV, IAV and WU viruses detected at lower frequencies within the OM cohort only ( Table 3 ).
Further examination of the frequency of co-detection of bacteria and viruses was assessed in NPS from both cohorts. Overall, the frequencies where no pathogen, and virus alone were detected within NPS were low, compared to bacteria alone, however there was no significant difference between children with and without OM (4.7% vs 5.9%, 9.3% vs 11.8% and 25.6% vs 47.1%, respectively) ( Table 3 ). The frequency of co-detection of bacteria and viruses in the OM group appeared higher but did not differ significantly from the control group (60.5% vs 35.3%, p=0.091) ( Table 3 ).
3.4 Bacterial Otopathogens and Viruses Present in the Adenoids
Adenoid sample collections were not collected from every recruited participant due to clinical treatment decisions however adenoid tissues were obtained from both the OM (n=20) and control cohorts (n=16) ( Table 1 ). RT-PCR analyses confirmed concurrent bacterial and viral otopathogens within the adenoids of all children, regardless of OM history. NTHi was most frequently detected in both the OM (90.0%, n=18/20) and control cohorts (93.8%, n=15/16). There were no significant differences in bacterial identifications between OM and control cohorts ( Table 3 and Supplemental Table 5 ).
All adenoid samples, from both OM and control cohorts were positive for at least one of the 8 viruses tested. HRV was the predominant virus identified within OM (85.0%, n=17/20) and control group (68.8%, n=11/17) adenoids. ADV, PIV and WU virus were detected in adenoids from both cohorts but did not differ in frequency between the OM and control groups ( Table 3 ).
3.5 Concurrent Bacterial Otopathogens and Viruses Present in the Same Child
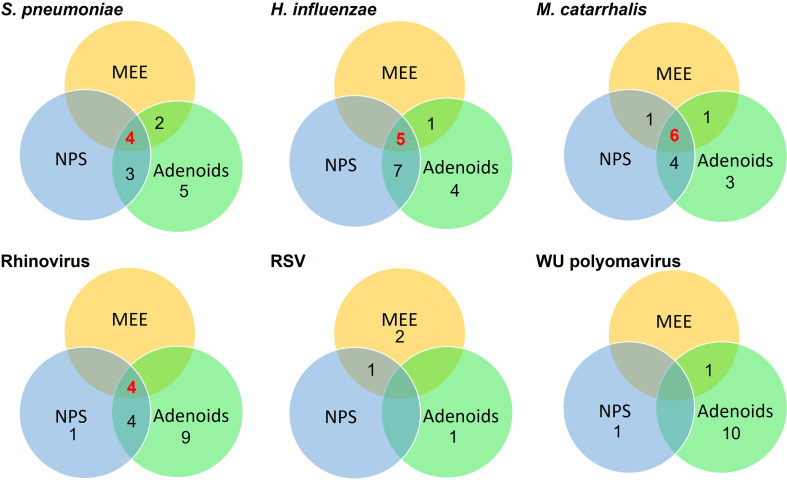
Concurrent bacterial and viral detection of the three predominant bacteria, NTHi, S. pneumoniae and M. catarrhalis and the three most frequently detected viruses, HRV, WU polyoma virus and RSV, were examined within the upper respiratory tract of children with a history of OM, undergoing VTI for OM. For each child, the otopathogens identified within the MEE, NPS and adenoids were mapped and their concurrent presence in multiple upper respiratory tract locations within the same child are presented in Figure 1 . These data show that when any of the 3 predominant bacteria are present within the middle ear, it is highly indicative of the same bacteria being present within the NPS and adenoids concurrently. Interestingly, only the presence of HRV within the MEE may indicate concurrent presence within the nasopharynx and adenoids, with RSV not identified as frequently in other areas of the upper respiratory tract ( Figure 1 ).
Figure 1.
Distribution of concurrent bacterial and viral detection of predominant otopathogenic microbes within the middle ear effusate (MEE), nasopharyngeal swab (NPS) and adenoid samples of the same peri-urban/urban children in South-East Queensland who were undergoing ventilation tube insertion for otitis media (OM). Each circle represents the sample locations within each child and their intersections indicate the number of children with the same microbe identified within two or three of the sample locations.
3.6 Pneumococcal Serotypes
There were 31 pneumococcal isolates serotyped from samples collected in this study. Fourteen (45.2%) were serogroup 23 (serotype 23A was 12.9%, 23B was 32.3%), eight were serotype 11A (25.8%), four were serotype 35B (12.9%), three were serotype 16F (9.7%) and there were single isolates of serotypes 19A and 21 (3.2% each respectively).
4 Discussion
This study identified the predominant bacterial and viral carriage of OM in young peri-urban and urban children of South-East Queensland, Australia undergoing VTI for OM. Otopathogen presence was determined using RT-PCR in MEE, NPS and adenoid samples from the same child, who were clinically well at collection. The frequency of detection of the three predominant bacteria considered causal for OM within the URT, S. pneumoniae, NTHi and M. catarrhalis, did not differ significantly regardless of the child’s clinical history of OM. Rhinovirus was most frequently detected with the MEE of children with OM, with RSV and WU polyomavirus also observed, albeit at slightly, but not significantly lower rates of detection. Overall, regardless of the children’s history for OM, otopathogen detection was highest in the adenoids, then comparatively less in the nasopharynx and for children with OM, the middle ear. Despite significantly different geographical location and ethnicity demographics, these findings are consistent with the findings of a study of Australian Aboriginal children from Central Australia. Australian Aboriginal children are reported to experience significantly increased risk of OM and this report identified Streptococcus sp., H. influenzae and M. catarrhalis as common operational taxonomic units (OTUs) found in the adenoids, nasopharynx, and middle ear in these children undergoing treatment for OM (Jervis-Bardy et al., 2015). Similarly, more recent studies in Australia, Finland and Switzerland also found that these OTUs were abundant in the MEE of children with OM (Chan et al., 2016; Sillanpää et al., 2017; Brugger et al., 2019).
Interestingly, a larger study (n=143) conducted in Western Australia reported that children undergoing VTI surgery for RAOM at a younger age than our cohorts, had significantly higher rates of nasopharyngeal colonization with NTHi and S. pneumoniae compared to healthy controls (Wiertsema et al., 2011b). These younger children (mean age=1.7 years) were more severely affected by RAOM than observed for the older (mean age=3.7 years) children being treated for RAOM/COME in the current study. These findings perhaps reflect different OM cohorts with the more clinically recognizable, severe and repeated AOM in children undergoing VTI at an earlier age. It is recognized that for most children, OM frequency typically reduces with age, in association with facial growth (Bluestone, 1996; Swarts et al., 2013) but particularly with progressive maturation of their immune system (Wiertsema and Leach, 2009). The smaller cohort sizes in this study and older children recruited may help to explain the lack of significance between the frequencies of otopathogen detection observed between OM affected children and control children, in conjunction with physical and immunological development.
Consistent with previous publications, this study also demonstrated that RT-PCR was more sensitive than traditional culture techniques to the detection of bacterial otopathogens (Ngo et al., 2016). This is due to the organism's fastidious growth requirements and the presence of viable organisms within a biofilm, which reduce the sensitivity of detection using culture (Thornton et al., 2013; Niedzielski et al., 2021). In the current study, which was undertaken when the children were well, it is not possible to differentiate whether the magnitude of the difference between RT-PCR and culture results was technical or due to a lower bacterial load at the time of sampling. Technically, RT-PCR may detect unviable bacterial fragments, inflating detection frequencies, although previous research confirms that live bacteria present in biofilms, identified using fluorescence in situ hybridisation (FISH) are not always culturable (Thornton et al., 2013).
Co-detection of different bacteria in different locations of the URT in this study has not only confirmed the role of the nasopharynx and adenoids as potential sources of NTHi and M. catarrhalis but has also demonstrated that S. pneumoniae detection in the middle ear of children with OM is also linked to colonization of the nasopharynx and/or adenoids of the same child. In all cases where S. pneumoniae was isolated from the MEE, it was also isolated from either the NPS or adenoids, suggesting that S. pneumoniae in the middle ear has originated from the nasopharynx and/or adenoids, although further investigation, including typing studies are needed. Similarly, NTHi and M. catarrhalis may also have originated from the nasopharynx and/or adenoids in the current study, but not all NTHi or M. catarrhalis identifications within the same child were concurrently identified in the MEE, nasopharynx and/or adenoids. A report by Stol et al. (2013), has shown a genetic match between S. pneumoniae found in the middle ear and nasopharynx of children with RAOM/COME. Importantly, these children were undergoing VTI, similar to the current study, and were also not experiencing current active OM infection at the time of sample collection. Furthermore, only 80% of NTHi isolates from the middle ear and nasopharynx were a genetic match (Stol et al., 2013). Together, the current findings and those by Stol, suggest that NTHi or M. catarrhalis in the middle ears of children with OM may persist and are not necessarily seeded from those bacteria in the nasopharynx and/or adenoids.
Throughout this study, NTHi was the predominant otopathogen detected in the URT, particularly in the nasopharynx and adenoids of children without a clinical history of OM, in addition to within the middle ear of children with RAOM/COME. A limitation of this study and any other studies using a control group comprised of children undergoing surgery for adenotonsillar hypertrophy/disease, is that this pathogenic process may also impact the high prevalence of NTHi in the control group. Despite this limitation, the current results are consistent with reports from Western Australia and New Zealand (Wiertsema et al., 2011b; Mills et al., 2015; Seppanen et al., 2020) and reflect the regional prevalence of NTHi as the most common otopathogen in the middle ear and nasopharynx of children with RAOM/COME reported in our global systematic review (Ngo et al., 2016).
Importantly in this study, the predominant bacterial otopathogen, NTHi was progressively increasingly resistant to β-lactam antibiotics over the study period, rising from 14.3% (2009-2010) to 67.9% (2015) ( Supplemental Table 6 ). In addition, multidrug resistance was not observed for NTHi within the 2009-2010 isolates but was present within the 2015 cohort (12/28 isolates data not shown). These findings highlight the importance of continuous surveillance for antimicrobial resistance to inform antibiotic therapy. Monitoring of serotype variants and their frequency in children of different ages undergoing a range of national immunization programs (NIP) from around the world would assist in evaluation of the potential impact of existing NIP vaccinations on OM prevalence. For example, recently, a 10-valent pneumococcal NTHi protein D conjugate vaccine was reported to reduce the frequency of middle ear infection caused by NTHi in Australian Indigenous communities (Leach et al., 2015). Development of an efficacious vaccine for NTHi has potential benefits for reduction in OM prevalence in both urban children and Indigenous Australian children, the latter children are known to experience significantly increased risk of severe OM development at a young age (Leach et al., 1994; Boswell and Nienhuys, 1996).
Limitations of the current study, in addition to those mentioned previously, include the selection of the comparative control group. Children undergoing adenoidectomy+/- tonsillectomy do not reflect a “healthy” cohort but are a sample of convenience, undergoing ear, nose and throat surgery and anesthesia, permitting collection of comparative clinical samples. Children with a clinically recognizable history of OM were excluded from control group recruitment and this is reflected in fewer OM episodes. The slightly but not significantly higher number of boys in the control group reflects recruitment is consistent with previous studies (Schupper et al., 2018) showing a higher incidence of adenoidectomy for boys. Similarly, the age range distribution between the control and OM groups is reflective of the diagnostic and clinical pathways, which tend to result in surgical resection of adenoids at a later age than for VTI due to RAOM or COME.
In the current study, despite the small sample size, S. pneumoniae and M. catarrhalis were detected at similar or lower frequencies compared to NTHi in the different regions of the URT in children with and without RAOM/COME. These results are consistent with the results of a systematic review of previous reports from the Pacific region, including Australia and New Zealand (Ngo et al., 2016).
Viral detections within the MEE of the present study occurred within 37.0% of MEE samples. The presence of these viruses, within the middle ears of OM prone children, in the absence of AOM symptomology provides support to the potential role of viruses in COME pathogenesis. Persistence of unresolving infection and presence of otopathogens in the middle ear may contribute to RAOM pathogenesis through dysregulated innate immune responses including inflammation and accumulation of middle ear effusate reflective of COME (Massa et al., 2015). Viral presence within the middle ear of OM-prone children contrasts to the sterile middle ear reported in children and adults without OM (Westerberg et al., 2009). Improved detection of viral otopathogens using RT-PCR may continue to better inform our understanding of the role of viruses within OM pathogenesis, particularly COME (Massa et al., 2015).
In this study, HRV was the predominant virus detected in all URT locations. The predominance of this virus is consistent with previous reports from Finland, the Netherlands and Western Australia, where viral detection within the middle ear was reported using PCR methods (Pitkäranta et al., 1998; Nokso-Koivisto et al., 2004; Ruohola et al., 2006; Wiertsema et al., 2011a; Stol et al., 2013). In contrast, RSV was the most common virus identified (via PCR) in the MEE of children with OM from Japan and Turkey (Monobe et al., 2003; Bulut et al., 2007). Similar studies from the US also confirmed RSV as the predominant virus within the middle ear, however these studies did not use PCR based detection methods (Heikkinen et al., 1999; Patel et al., 2007). Identification of the predominant virus within the MEE of children experiencing OM clearly varies with the region and recruitment criteria for participants in each study, including age and clinical symptomology. Furthermore, viral incidence may vary at different times within a year or between years as evidenced by a recent report from the US. The study reported significantly increased frequency of RSV detection in the URT of children with OM in the peak season of RSV, compared to the shoulder seasons of RSV (Makari et al., 2015). Therefore, the timing of patient recruitment may impact the study population detection frequencies of the otopathogen, if it occurred during the low-activity RSV seasons rather than the peak period (Chonmaitree, 2006). Each virus may present differently over the year, for example, HRV infections occur year round (Winther et al., 2006), which may increase the opportunity for detection and reporting. Regardless of which virus is predominant, an effective vaccine against that virus would benefit children with OM, who are positive for the virus, unfortunately, for the two most commonly detected viruses, HRV and RSV, vaccine development has been difficult (Campbell et al., 2015; Glanville and Johnston, 2015).
The frequency of viral detection varied by location within the URT, with the frequency of detecting at least one virus present in the NPS of children with OM tended to be higher than observed in control, although not significantly. This trend is consistent with a previous study in which children with RAOM had significantly higher frequency of viral detection in the nasopharynx compared to their control counterparts (Wiertsema et al., 2011a). In contrast, all adenoid tissues from children with or without OM in the current study had at least one virus present and this finding is consistent with previous studies whereby 90-100% adenoid samples from children with adenoidal hypertrophy, RAOM, or with OME had viruses detected (Herberhold et al., 2009; Sato et al., 2009; Szalmás et al., 2013). Interestingly, the current study showed that children with OM had higher frequencies of ADV and WU detection in both the nasopharynx and adenoids compared to children without OM. These findings are consistent with previous studies in Australia conducted on non-Indigenous children with/without OM (Wiertsema et al., 2011a) and Indigenous Australian Aboriginal children with/without the disease (Binks et al., 2011). In addition, those viruses were also detected in the middle ear of children with OM in the current study and further research is needed to investigate the potential role of these viruses in OM pathogenesis.
Finally, bacterial-viral co-infection or colonization in the URT may increase the incidence of OM development in children. Indeed, the current study showed that children with OM tended to show higher frequency of bacterial-viral detection in the nasopharynx compared to children without OM although the difference was not statistically significant (60.5% vs 35.3%). However, the role of co-occurrence of specific microbes in potentially increasing the risk of OM pathogenesis was demonstrated in the present study by the co-detection and correlation of ADV and NTHi. Further exploration of co-infection and its impact on OM pathogenesis has been provided through animal models, with elegant research using the chinchilla often considered the most representative of the human condition. Using the chinchilla model, Suzuki and Bakaletz (1994) demonstrated that intranasal inoculation with both ADV type 1 and NTHi resulted in development of more severe OM than was observed using either agent as a single pathogen (Suzuki and Bakaletz, 1994; Miyamoto and Bakaletz, 1997). In addition, intranasal inoculation of chinchillas using ADV may increase the transduction of NTHi from the nasopharynx to the middle ear and induce NTHi OM (Miyamoto and Bakaletz, 1997). Murine viral pre-infection models that utilize polymicrobial bacterial pathogens including S. pneumoniae and M. catarrhalis have demonstrated increased infection frequency and severity (Krishnamurthy et al., 2009), whilst bacterial co-infection models using NTHi and M. catarrhalis have also shown enhanced bacterial persistence and antimicrobial resistance (Armbruster et al., 2010). Interestingly, a study by Short et al. (2011) demonstrated that infection with influenza virus enabled development and persistence of pneumococcal OM via neutrophil extracellular trap (NET) induction in the mouse (Short et al., 2011). Persistent effusion, bacteria and NETs have been reported in children with RAOM (Thornton et al., 2013) and together, viral-bacterial co-infection play significant roles in the development and persistence of OM.
Overall, the current study identified NTHi and HRV as the predominant otopathogens within the URT of peri-urban and urban children, with and without COME/RAOM from South-East Queensland Australia. The presence of multiple otopathogens, both viral and bacterial within the middle ear and the upper respiratory tracts of clinically well children undergoing VTI surgery for COME/RAOM confirm the complexity of vaccine development to reduce the risk and impact of this frequent childhood disease. For the future, improved routine surveillance of otopathogens present in the URT of children experiencing OM, including affected children of differing ages from across the world, will better reflect the impact of existing vaccines and the support the development of new vaccines for OM prevention.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Children’s Health Services District Ethics Committee (2008/063 and HREC/14/QRCH/33), Greenslopes Hospital Human Research Ethics Committee (14/18) and the Griffith University Human Research Ethics Committee (MSC/05/08/HREC and MSC/19/13/HREC), in accordance with the National Statement on Ethical Conduct in Human Research 2007. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
HM, RT and AC designed the study. HM, CP and BM developed and planned surgical collection protocols. BM, CP, HM, RT and MN designed recruitment and collection protocols and HM, BM and CP oversaw patient recruitment and sample collections. CN, TS, RT and QPID, MCRI and GCUH Pathology consortia processed samples. CN, HM, RT, TS, AC analysed data. CN and HM drafted the manuscript and all authors contributed to the article and approved the submitted manuscript.
Funding
This research received funding support from the Royal Children’s Hospital Foundation grant (10258), Griffith Health Institute, Gold Coast Hospital Foundation Collaborative grant (40837 03/12/2729) and Financial Markets for Children Foundation grant (2008-216). CN received a Vietnam International Education Development-Griffith University scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to all children and their parents who participated in and supported this research. The expertise of Dr’s E. Perry, A. Chang, R. Barr, E. Hodge, S.B. Lambert, the former Royal Children’s Hospital, (Brisbane) theatre team, Dr R. Rockett and Mr N. O’Neill, Queensland Paediatric Infectious Diseases (QPID) Research Group is gratefully acknowledged. The expertise and assistance of Dr E. Dunne and Dr B. Ortika, Murdoch Children's Research Institute (MRCI) Pneumococcal Research Group is gratefully valued. The collaboration and expertise of Dr D. Maguire, the Pindara Private Hospital theatre team, J. Tier, J. McGrath, G. Davies, M. Apps and colleagues, in addition to Pathology Queensland, Gold Coast University Hospital group, specifically, Dr P. Derrington, D. Thorley, S. Fentiman, M. Mitov and M. Alkhdaidi are acknowledged and appreciated.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.775535/full#supplementary-material
References
- Armbruster C. E., Hong W., Pang B., Weimer K. E., Juneau R. A., Turner J., et al. (2010). Indirect Pathogenicity of Haemophilus Influenzae and Moraxella Catarrhalis in Polymicrobial Otitis Media Occurs via Interspecies Quorum Signaling. mBio 1, e00102–10. doi: 10.1128/mBio.00102-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Technical Advisory Group on Immunisation (ATAGI) (2018). Australian Immunisation Handbook (Canberra: Australian Government Department of Health; ). [Google Scholar]
- Binks M., Cheng A., Smith-Vaughan H., Sloots T., Nissen M., Whiley D., et al. (2011). Viral-Bacterial Co-Infection in Australian Indigenous Children With Acute Otitis Media. BMC Infect. Dis. 11, 1–8. doi: 10.1186/1471-2334-11-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binks M., Temple B., Kirkham L., Wiertsema S., Dunne E., Richmond P., et al. (2012). Molecular Surveillance of True Nontypeable Haemophilus Influenzae: An Evaluation of PCR Screening Assays. PloS One 7, e34083. doi: 10.1371/journal.pone.0034083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone C. (1996). Pathogenesis of Otitis Media: Role of Eustachian Tube. Pediatr. Infect. Dis. J. 15, 281–291. doi: 10.1097/00006454-199604000-00002 [DOI] [PubMed] [Google Scholar]
- Boswell J., Nienhuys T. (1996). Patterns of Persistent Otitis Media in the First Year of Life in Aboriginal and Non-Aboriginal Infants. Ann. Otol. Rhinol. Laryngol. 105, 893–900. doi: 10.1177/000348949610501110 [DOI] [PubMed] [Google Scholar]
- Brugger S. D., Kraemer J. G., Qi W., Bomar L., Oppliger A., Hilty M. (2019). Age-Dependent Dissimilarity of the Nasopharyngeal and Middle Ear Microbiota in Children With Acute Otitis Media. Front. Genet. 10, 555. doi: 10.3389/fgene.2019.00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut Y., Güven M., Otlu B., Yenişehirli G., Aladağ I., Eyibilen A., et al. (2007). Acute Otitis Media and Respiratory Viruses. Eur. J. Pediatr. 166, 223–228. doi: 10.1007/s00431-006-0233-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzatto G. P., Tamashiro E., Proenca-Modena J. L., Saturno T. H., Prates M. C., Gagliardi T. B., et al. (2017). The Pathogens Profile in Children With Otitis Media With Effusion and Adenoid Hypertrophy. PloS One 12, e0171049. doi: 10.1371/journal.pone.0171049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell H., Bont L., Nair H. (2015). Respiratory Syncytial Virus (RSV) Disease - New Data Needed to Guide Future Policy. J. Glob. Health 5, 20101. doi: 10.7189/jogh.05.020101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. L., Wabnitz D., Bardy J. J., Bassiouni A., Wormald P. J., Vreugde S., et al. (2016). The Microbiome of Otitis Media With Effusion. Laryngoscope 126, 2844–2851. doi: 10.1002/lary.26128 [DOI] [PubMed] [Google Scholar]
- Chonmaitree T. (2000). Viral and Bacterial Interaction in Acute Otitis Media. Pediatr. Infect. Dis. J. 19, S24–S30. doi: 10.1097/00006454-200005001-00005 [DOI] [PubMed] [Google Scholar]
- Chonmaitree T. (2006). Acute Otitis Media Is Not a Pure Bacterial Disease. Clin. Infect. Dis. 43, 1423–1425. doi: 10.1086/509329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonmaitree T., Heikkinen T. (1997). Role of Viruses in Middle-Ear Disease. Ann. N. Y. Acad. Sci. 830, 143–157. doi: 10.1111/j.1749-6632.1997.tb51886.x [DOI] [PubMed] [Google Scholar]
- Chonmaitree T., Trujillo R., Jennings K., Alvarez-Fernandez P., Patel J. A., Loeffelholz M. J., et al. (2016). Acute Otitis Media and Other Complications of Viral Respiratory Infection. Pediatrics 137 (4), e20153555. doi: 10.1542/peds.2015-3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago L., Esposito S., De Vecchi E., Marchisio P., Blasi F., Baggi E., et al. (2008). Detection of Respiratory Viruses and Atypical Bacteria in Children's Tonsils and Adenoids. J. Clin. Microbiol. 46, 369–370. doi: 10.1128/jcm.01819-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fago-Olsen H., Dines L. M., Sorensen C. H., Jensen A. (2019). The Adenoids But Not the Palatine Tonsils Serve as a Reservoir for Bacteria Associated With Secretory Otitis Media in Small Children. mSystems 4, e00169–18. doi: 10.1128/mSystems.00169-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney K. B., Morris P. S., Carapetis J. R., Skull S. A., Smith-Vaughan H. C., Stubbs E., et al. (2005). The Clinical Course of Acute Otitis Media in High-Risk Australian Aboriginal Children: A Longitudinal Study. BMC Pediatr. 5, 16. doi: 10.1186/1471-2431-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N., Johnston S. (2015). Challenges in Developing a Cross-Serotype Rhinovirus Vaccine. Curr. Opin. Virol. 11, 83–88. doi: 10.1016/j.coviro.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Heikkinen T., Chonmaitree T. (2003). Importance of Respiratory Viruses in Acute Otitis Media. Clin. Microbiol. Rev. 16, 230–241. doi: 10.1128/CMR.16.2.230-241.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T., Thint M., Chonmaitree T. (1999). Prevalence of Various Respiratory Viruses in the Middle Ear During Acute Otitis Media. N. Engl. J. Med. 340, 260–264. doi: 10.1056/NEJM199901283400402 [DOI] [PubMed] [Google Scholar]
- Herberhold S., Eis-Hübinger A., Panning M. (2009). Frequent Detection of Respiratory Viruses by Real-Time PCR in Adenoid Samples From Asymptomatic Children. J. Clin. Microbiol. 47, 2682–2683. doi: 10.1128/JCM.00899-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Monobe H., Nomura Y., Shinogami M., Yano J. (2003). Multiplex Nested Reverse Transcription-Polymerase Chain Reaction for Respiratory Viruses in Acute Otitis Media. Ann. Otol. Rhinol. Laryngol. 112, 252–257. doi: 10.1177/000348940311200311 [DOI] [PubMed] [Google Scholar]
- Jervis-Bardy J., Rogers G., Morris P., Smith-Vaughan H., Nosworthy E., Leong L., et al. (2015). The Microbiome of Otitis Media With Effusion in Indigenous Australian Children. Int. J. Pediatr. Otorhinolaryngol. 79, 1548–1555. doi: 10.1016/j.ijporl.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy A., Mcgrath J., Cripps A. W., Kyd J. M. (2009). The Incidence of Streptococcus Pneumoniae Otitis Media Is Affected by the Polymicrobial Environment Particularly Moraxella Catarrhalis in a Mouse Nasal Colonisation Model. Microbes Infect. 11, 545–553. doi: 10.1016/j.micinf.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Leach A., Boswell J., Asche V., Nienhuys T., Mathews J. (1994). Bacterial Colonization of the Nasopharynx Predicts Very Early Onset and Persistence of Otitis Media in Australian Aboriginal Infants. Pediatr. Infect. Dis. J. 13, 983–989. doi: 10.1097/00006454-199411000-00009 [DOI] [PubMed] [Google Scholar]
- Leach A. J., Morris P. S. (2007). The Burden and Outcome of Respiratory Tract Infection in Australian and Aboriginal Children. Pediatr. Infect. Dis. J. 26, S4–S7. doi: 10.1097/INF.0b013e318154b238 [DOI] [PubMed] [Google Scholar]
- Leach A., Wigger C., Hare K., Hampton V., Beissbarth J., Andrews R., et al. (2015). Reduced Middle Ear Infection With non-Typeable Haemophilus Influenzae, But Not Streptococcus Pneumoniae, After Transition to 10-Valent Pneumococcal Non-Typeable H. Influenzae Protein D Conjugate Vaccine. BMC Pediatr. 15, 1–13. doi: 10.1186/s12887-015-0483-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon C., Lehman D. C., George M. (2014). Textbook of Diagnostic Microbiology (USA: Saunders; ). [Google Scholar]
- Makari D., Staat M., Henrickson K., Wu X., Ambrose C. (2015). The Underrecognized Burden of Respiratory Syncytial Virus Among Infants Presenting to US Emergency Departments. Clin. Pediatr. (Phila) 54, 594–597. doi: 10.1177/0009922814546040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom T., Fellner A., Hirschfeld Z. E., Lazarovitch T., Gavriel H., Muallem-Kalmovich L., et al. (2019). The Yield of Respiratory Viruses Detection Testing Is Age-Dependent in Children With Acute Otitis Media. Ther. Adv. Infect. Dis. 6, 2049936119871127. doi: 10.1177/2049936119871127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. L., Binks M. J., Beissbarth J., Christensen P., Morris P. S., Leach A. J., et al. (2012). Quantitative PCR of Ear Discharge From Indigenous Australian Children With Acute Otitis Media With Perforation Supports a Role for Alloiococcus Otitidis as a Secondary Pathogen. BMC Ear Nose Throat Disord. 12, 1–10. doi: 10.1186/1472-6815-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa H., Lim D., Kurono Y., Cripps A. (2015). “Middle Ear and Eustachian Tube Mucosal Immunology,” in Mucosal Immunology, 4th ed. Eds. Jiri M., Warren S., Michael W., Hilde C., Bart N., Brian L. (Waltham, MA: Elsevier Inc; ), 1923–1942. doi: 10.1016/B978-0-12-415847-4.00101-4 [DOI] [Google Scholar]
- Mills N., Best E., Murdoch D., Souter M., Neeff M., Anderson T., et al. (2015). What Is Behind the Ear Drum? The Microbiology of Otitis Media and the Nasopharyngeal Flora in Children in the Era of Pneumococcal Vaccination. J. Paediatr. Child Health 51, 300–306. doi: 10.1111/jpc.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N., Bakaletz L. (1997). Kinetics of the Ascension of NTHi From the Nasopharynx to the Middle Ear Coincident With Adenovirus-Induced Compromise in the Chinchilla. Microb. Pathog. 23, 119–126. doi: 10.1006/mpat.1997.0140 [DOI] [PubMed] [Google Scholar]
- Monobe H., Ishibashi T., Nomura Y., Shinogami M., Yano J. (2003). Role of Respiratory Viruses in Children With Acute Otitis Media. Int. J. Pediatr. Otorhinolaryngol. 67, 801–806. doi: 10.1016/s0165-5876(03)00124-1 [DOI] [PubMed] [Google Scholar]
- Niedzielski A., Chmielik L. P., Stankiewicz T. (2021). The Formation of Biofilm and Bacteriology in Otitis Media with Effusion in Children: A Prospective Cross-Sectional Study. Int. J. Res. Public Health 18 (7), 3555. doi: 10.3390/ijerph18073555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo C., Massa H., Thornton R., Cripps A. (2016). Predominant Bacteria Detected From the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review. PloS One 11, e0150949. doi: 10.1371/journal.pone.0150949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokso-Koivisto J., Marom T., Chonmaitree T. (2015). Importance of Viruses in Acute Otitis Media. Curr. Opin. Pediatr. 27, 110–115. doi: 10.1097/MOP.0000000000000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokso-Koivisto J., Räty R., Blomqvist S., Kleemola M., Syrjänen R., Pitkäranta A., et al. (2004). Presence of Specific Viruses in the Middle Ear Fluids and Respiratory Secretions of Young Children With Acute Otitis Media. J. Med. Virol. 72, 241–248. doi: 10.1002/jmv.10581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortika B. D., Habib M., Dunne E. M., Porter B. D., Satzke C. (2013). Production of Latex Agglutination Reagents for Pneumococcal Serotyping. BMC Res. Notes 6 (1), 49–49. doi: 10.1186/1756-0500-6-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J. A., Nguyen D. T., Revai K., Chonmaitree T. (2007). Role of Respiratory Syncytial Virus in Acute Otitis Media: Implications for Vaccine Development. Vaccine 25, 1683–1689. doi: 10.1016/j.vaccine.2006.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew M. M., Laufer A. S., Gent J. F., Kong Y., Fennie K. P., Metlay J. P. (2012). Upper Respiratory Tract Microbial Communities, Acute Otitis Media Pathogens, and Antibiotic Use in Healthy and Sick Children. Appl. Environ. Microbiol. 78, 6262–6270. doi: 10.1128/aem.01051-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M., Finelli L., Saiman L., Wang C., Choi Y., Patel J. (2020). Respiratory Syncytial Virus-Associated Acute Otitis Media in Infants and Children. J. Pediatr. Infect. Dis. Soc. 9, 544–550. doi: 10.1093/jpids/piaa094 [DOI] [PubMed] [Google Scholar]
- Pitkäranta A., Jero J., Arruda E., Virolainen A., Hayden F. (1998). Polymerase Chain Reaction-Based Detection of Rhinovirus, Respiratory Syncytial Virus, and Coronavirus in Otitis Media With Effusion. J. Pediatr. 133, 390–394. doi: 10.1016/s0022-3476(98)70276-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E., Sarovich D., Nosworthy E., Beissbarth J., Marsh R., Pickering J., et al. (2015). Haemophilus Influenzae: Using Comparative Genomics to Accurately Identify a Highly Recombinogenic Human Pathogen. BMC Genomics 16, 1-10. doi: 10.1186/s12864-015-1857-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett R., Sloots T., Bowes S., O'neill N., Ye S., Robson J., et al. (2013). Detection of Novel Polyomaviruses, TSPyV, HPyV6, HPyV7, HPyV9 and MWPyV in Feces, Urine, Blood, Respiratory Swabs and Cerebrospinal Fluid. PloS One 8, e62764. doi: 10.1371/journal.pone.0062764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovers M., Schilder A., Zielhuis G., Rosenfeld R. (2004). Otitis Media. Lancet 363, 465–473. doi: 10.1016/S0140-6736(04)15495-0 [DOI] [PubMed] [Google Scholar]
- Ruohola A., Meurman O., Nikkari S., Skottman T., Salmi A., Waris M., et al. (2006). Microbiology of Acute Otitis Media in Children With Tympanostomy Tubes: Prevalences of Bacteria and Viruses. Clin. Infect. Dis. 43, 1417–1422. doi: 10.1086/509332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruohola A., Pettigrew M., Lindholm L., Jalava J., Räisänen K., Vainionpää R., et al. (2013). Bacterial and Viral Interactions Within the Nasopharynx Contribute to the Risk of Acute Otitis Media. J. Infect. 66, 247–254. doi: 10.1016/j.jinf.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Li H., Ikizler M., Werkhaven J., Williams J., Chappell J., et al. (2009). Detection of Viruses in Human Adenoid Tissues by Use of Multiplex PCR. J. Clin. Microbiol. 47, 771–773. doi: 10.1128/JCM.02331-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satzke C., Dunne E. M., Porter B. D., Klugman K. P., Mulholland E. K. (2015). The PneuCarriage Project: A Multi-Centre Comparative Study to Identify the Best Serotyping Methods for Examining Pneumococcal Carriage in Vaccine Evaluation Studies. PloS Med. 12 (11), 1–30. doi: 10.1371/journal.pmed.1001903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S., Okutani F., Kobayashi T. (2019). Comprehensive Detection of Respiratory Bacterial and Viral Pathogens in the Middle Ear Fluid and Nasopharynx of Pediatric Patients With Acute Otitis Media. Pediatr. Infect. Dis. J. 38, 1199–1203. doi: 10.1097/inf.0000000000002486 [DOI] [PubMed] [Google Scholar]
- Schilder A. G. M., Chonmaitree T., Cripps A. W., Rosenfeld R. M., Casselbrant M. L., Haggard M. P., et al. (2016). Otitis Media. Nat. Rev. Dis. Primers 2, 16063–16063. doi: 10.1038/nrdp.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupper A. J., Nation J., Pransky S. (2018). Adenoidectomy in Children: What Is the Evidence and What Is Its Role? Curr. Otorhinolaryngol. Rep. 6 (1), 64–73. doi: 10.1007/s40136-018-0190-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppanen E. J., Thornton R. B., North H. J., Corscadden K. J., Wiertsema S. P., Vijayasekaran S., et al. (2020). Bacterial Reservoirs in the Middle Ear of Otitis-Prone Children Are Associated With Repeat Ventilation Tube Insertion. Pediatr. Infect. Dis. J. 39, 91–96. doi: 10.1097/inf.0000000000002541 [DOI] [PubMed] [Google Scholar]
- Short K., Diavatopoulos D., Thornton R., Pedersen J., Strugnell R., Wise A., et al. (2011). Influenza Virus Induces Bacterial and Nonbacterial Otitis Media. J. Infect. Dis. 204, 1857–1865. doi: 10.1093/infdis/jir618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpää S., Kramna L., Oikarinen S., Sipilä M., Rautiainen M., Aittoniemi J., et al. (2017). Next-Generation Sequencing Combined With Specific PCR Assays To Determine the Bacterial 16s rRNA Gene Profiles of Middle Ear Fluid Collected From Children With Acute Otitis Media. mSphere 2, e00006-00017. doi: 10.1128/mSphere.00006-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vaughan H. C., Binks M. J., Marsh R. L., Kaestli M., Ward L., Hare K. M., et al. (2013). Dominance of Haemophilus Influenzae in Ear Discharge From Indigenous Australian Children With Acute Otitis Media With Tympanic Membrane Perforation. BMC Ear Nose Throat Disord. 13 (12), 1–9. doi: 10.1186/1472-6815-13-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vaughan H., Byun R., Nadkarni M., Jacques N., Hunter N., Halpin S., et al. (2006). Measuring Nasal Bacterial Load and Its Association With Otitis Media. BMC Ear Nose Throat Disord. 6. doi: 10.1186/1472-6815-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stol K., Verhaegh S. J., Graamans K., Engel J. A., Sturm P. D., Melchers W. J., et al. (2013). Microbial Profiling Does Not Differentiate Between Childhood Recurrent Acute Otitis Media and Chronic Otitis Media With Effusion. Int. J. Pediatr. Otorhinolaryngol. 77, 488–493. doi: 10.1016/j.ijporl.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Bakaletz L. (1994). Synergistic Effect of Adenovirus Type 1 and Nontypeable Haemophilus Influenzae in a Chinchilla Model of Experimental Otitis Media. Infect. Immun. 62, 1710–1718. doi: 10.1128/iai.62.5.1710-1718.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts J., Alper C., Luntz M., Bluestone C., Doyle W., Ghadiali S., et al. (2013). Panel 2: Eustachian Tube, Middle Ear, and Mastoid–Anatomy, Physiology, Pathophysiology, and Pathogenesis. Otolaryngol. Head Neck Surg. 148, E26–E36. doi: 10.1177/0194599812472631 [DOI] [PubMed] [Google Scholar]
- Szalmás A., Papp Z., Csomor P., Kónya J., Sziklai I., Szekanecz Z., et al. (2013). Microbiological Profile of Adenoid Hypertrophy Correlates to Clinical Diagnosis in Children. BioMed. Res. Int. 2013, 1–10. doi: 10.1155/2013/629607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton R. B., Hakansson A., Hood D. W., Nokso-Koivisto J., Preciado D., Riesbeck K., et al. (2020). Panel 7 - Pathogenesis of Otitis Media - A Review of the Literature Between 2015 and 2019. Int. J. Pediatr. Otorhinolaryngol. 130 Suppl 1, 109838. doi: 10.1016/j.ijporl.2019.109838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton R. B., Wiertsema S. P., Kirkham L. S., Rigby P. J., Vijayasekaran S., Coates H. L., et al. (2013). Neutrophil Extracellular Traps and Bacterial Biofilms in Middle Ear Effusion of Children With Recurrent Acute Otitis Media–a Potential Treatment Target. PloS One 8, e53837. doi: 10.1371/journal.pone.0053837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg B., Kozak F., Thomas E., Blondel-Hill E., Brunstein J., Patrick D. (2009). Is the Healthy Middle Ear a Normally Sterile Site? Otol. Neurotol. 30, 174–177. doi: 10.1097/MAO.0b013e31819225a0 [DOI] [PubMed] [Google Scholar]
- Wiertsema S., Chidlow G., Kirkham L., Corscadden K., Mowe E., Vijayasekaran S., et al. (2011. a). High Detection Rates of Nucleic Acids of a Wide Range of Respiratory Viruses in the Nasopharynx and the Middle Ear of Children With a History of Recurrent Acute Otitis Media. J. Med. Virol. 83, 2008–2017. doi: 10.1002/jmv.22221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertsema S. P., Kirkham L. S., Corscadden K. J., Mowe E. N., Bowman J. M., Jacoby P., et al. (2011. b). Predominance of Nontypeable Haemophilus Influenzae in Children With Otitis Media Following Introduction of a 3 + 0 Pneumococcal Conjugate Vaccine Schedule. Vaccine 29, 5163–5170. doi: 10.1016/j.vaccine.2011.05.035 [DOI] [PubMed] [Google Scholar]
- Wiertsema S., Leach A. (2009). Theories of Otitis Media Pathogenesis, With a Focus on Indigenous Children. Med. J. Aust. 191, S50–S54. doi: 10.5694/j.1326-5377.2009.tb02927.x [DOI] [PubMed] [Google Scholar]
- Winther B., Hayden F., Hendley J. (2006). Picornavirus Infections in Children Diagnosed by RT-PCR During Longitudinal Surveillance With Weekly Sampling: Association With Symptomatic Illness and Effect of Season. J. Med. Virol. 78, 644–650. doi: 10.1002/jmv.20588 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.