Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a rare but serious complication of infection with SARS-CoV-2. A possible involvement of pathogenetically relevant autoantibodies has been discussed. Recently, neutralising autoantibodies against inflammatory receptor antagonists progranulin and interleukin-1 receptor antagonist (IL-1Ra) were found in adult patients with critical COVID-19. The aim of this study was to investigate the role of such autoantibodies in MIS-C.
Methods
In this multicentre, retrospective, cohort study, plasma and serum samples were collected from patients (0–18 years) with MIS-C (as per WHO criteria) treated at five clinical centres in Germany and Spain. As controls, we included plasma or serum samples from children with Kawasaki disease, children with inactive systemic juvenile idiopathic arthritis, and children with suspected growth retardation (non-inflammatory control) across four clinical centres in Germany and Spain (all aged ≤18 years). Serum samples from the CoKiBa trial were used as two further control groups, from healthy children (negative for SARS-CoV-2 antibodies) and children with previous mild or asymptomatic COVID-19 (aged ≤17 years). MIS-C and control samples were analysed for autoantibodies against IL-1Ra and progranulin, and for IL-1Ra concentrations, by ELISA. Biochemical analysis of plasma IL-1Ra was performed with native Western blots and isoelectric focusing. Functional activity of the autoantibodies was examined by an in vitro IL-1β-signalling reporter assay.
Findings
Serum and plasma samples were collected between March 6, 2011, and June 2, 2021. Autoantibodies against IL-1Ra could be detected in 13 (62%) of 21 patients with MIS-C (11 girls and ten boys), but not in children with Kawasaki disease (n=24; nine girls and 15 boys), asymptomatic or mild COVID-19 (n=146; 72 girls and 74 boys), inactive systemic juvenile idiopathic arthritis (n=10; five girls and five boys), suspected growth retardation (n=33; 13 girls and 20 boys), or in healthy controls (n=462; 230 girls and 232 boys). Anti-IL-1Ra antibodies in patients with MIS-C belonged exclusively to the IgG1 subclass, except in one patient who had additional IL-1Ra-specific IgM antibodies. Autoantibodies against progranulin were only detected in one (5%) patient with MIS-C. In patients with MIS-C who were positive for anti-IL-1Ra antibodies, free plasma IL-1Ra concentrations were reduced, and immune-complexes of IL-1Ra were detected. Notably, an additional, hyperphosphorylated, transiently occurring atypical isoform of IL-1Ra was observed in all patients with MIS-C who were positive for anti-IL-1Ra antibodies. Anti-IL-1Ra antibodies impaired IL-1Ra function in reporter cell assays, resulting in amplified IL-1β signalling.
Interpretation
Anti-IL-1Ra autoantibodies were observed in a high proportion of patients with MIS-C and were specific to these patients. Generation of these autoantibodies might be triggered by an atypical, hyperphosphorylated isoform of IL-1Ra. These autoantibodies impair IL-1Ra bioactivity and might thus contribute to increased IL-1β-signalling in MIS-C.
Funding
NanoBioMed fund of the University of Saarland, José Carreras Center for Immuno and Gene Therapy, Dr Rolf M Schwiete Stiftung, Staatskanzlei Saarland, German Heart Foundation, Charity of the Blue Sisters, Bavarian Ministry of Health, the Center for Interdisciplinary Clinical Research at University Hospital Münster, EU Horizon 2020.
Introduction
The course of SARS-CoV-2 infection is typically mild or asymptomatic in children.1, 2, 3 Multisystem inflammatory syndrome in children (MIS-C; also known as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2) is a rare but serious complication that usually occurs after SARS-CoV-2 infection following a latency period (approximately 2–6 weeks).4, 5, 6, 7, 8, 9 All affected children present with persistent fever while other clinical features might vary. Acute abdominal pain, diarrhoea or vomiting, muscle pain, headache, and fatigue have been reported.6 Initial descriptions have highlighted overlapping features with those observed in Kawasaki disease, including bilateral conjunctival injection (hyperaemia) and exanthema, swollen hands and feet, and so-called strawberry tongue, as well as cardiovascular features such as myocardial dysfunction, arterial hypotension, myocarditis, pericarditis, and involvement of the valves and coronary arteries (dilatation or aneurysma) or systemic shock.4, 5 Laboratory data have revealed excessive inflammation with highly elevated serum concentrations of C-reactive protein, procalcitonin, and ferritin, and elevated troponin and N-terminal pro-B-type natriuretic peptide reflecting cardiac involvement.6 Furthermore, hyponatraemia, markers of coagulopathy (elevated D-dimers and prolonged prothrombin time and partial thromboplastin time) and haematological abnormalities (anaemia, lymphocytopenia, and thrombocytopenia or thrombocytosis) have been reported.10 Most affected children need intensive care due to multiorgan failure and shock.7, 8, 9, 11, 12, 13
Research in context.
Evidence before this study
Before starting our analyses, we searched PubMed (May 1, 2021) using the search terms “MIS-C”, “PIMS”, “COVID-19”, and “autoantibodies” OR “anti-IL-1Ra” OR “anti- progranulin” for articles with these terms appearing in publication titles and abstracts of English language articles. Multisystem inflammatory syndrome in children (MIS-C; also called paediatric inflammatory multisystem syndrome paediatric inflammatory temporally associated with SARS-CoV-2) has emerged as a rare but serious complication after infection with SARS-CoV-2 in children and adolescents. Published work has already described different autoantibody responses in COVID-19 and MIS-C and a possible pathogenetic involvement has been discussed, particularly in the context of type I interferon-targeting autoantibodies. Recently, neutralising autoantibodies against anti-inflammatory receptor antagonists progranulin and interleukin-1 receptor antagonist (IL-1Ra) were identified in adult patients with critical COVID-19. Neutralising antibodies against IL-1Ra have also been reported in IgG4-associated disease. However, we found no published studies that have investigated the occurrence of antibodies against IL-1Ra or progranulin in MIS-C.
Added value of this study
Our retrospective multicentre cohort study identified IL-1Ra-specific autoantibodies in a high proportion of patients with MIS-C, but not in healthy or disease controls. Generation of the anti-IL-1Ra antibodies might be triggered by an atypical, hyperphosphorylated isoform of IL-1Ra, which we observed in all autoantibody-positive patients with MIS-C. IL-1Ra-specific antibodies were also associated with decreased IL-1Ra plasma concentration and impaired IL-1Ra bioactivity. In contrast to critical COVID-19 in adults, in MIS-C, antibodies against IL-1Ra belonged predominantly to the IgG1 subclass and antibodies against progranulin were infrequent.
Implications of all the available evidence
Functional neutralising autoantibodies directed against IL-1Ra can contribute to excessive IL-1 signalling in MIS-C.
Despite numerous published studies since its initial description, understanding of MIS-C pathogenesis remains limited. The formation of pathogenetically relevant autoantibodies has been suggested to contribute to a hyperinflammatory state.14, 15 The autoantibody hypothesis is supported by, firstly, the observation of a latency period between SARS-CoV-2 infection and MIS-C onset, during which priming and activation of adaptive immune mechanisms might occur; and, secondly, the favourable clinical response of patients to intravenous immunoglobulins (IVIGs) and glucocorticoids. Furthermore, the American College of Rheumatology recommends treatment with a high dose of the recombinant interleukin-1 receptor antagonist (IL-1Ra) anakinra for IVIG-refractory patients with MIS-C.16, 17, 18
Recently, in adult patients with critical COVID-19, we identified neutralising autoantibodies against progranulin,19 an anti-inflammatory ligand of the pro-inflammatory receptors tumour necrosis factor receptor 1 (TNFR1), TNFR2, and DR3 (also known as TNF receptor superfamily members 1A, 1B, and 25),20, 21, 22, 23 that acts as direct receptor antagonist of TNFα and TL1A (also known as TNF ligand superfamily member 15).22, 23 In these adult patients we also detected autoantibodies against IL-1Ra,19 a ligand of the pro-inflammatory IL-1 receptor and antagonist of IL-1α and IL-1β binding and signalling. 24, 25, 26, 27, 28 The aim of this study was to investigate the role of such autoantibodies in MIS-C.
Methods
Study design and participants
In this multicentre, retrospective, cohort study, serum and plasma samples were collected from patients (≤18 years) with MIS-C treated at five clinical centres in Germany and Spain. Blood samples of patients with MIS-C were drawn in the Department of Pediatric Cardiology (Saarland University Hospital, Homburg, Germany), the Department of Pediatrics (Klinikum Saarbrücken, Saarbrücken, Germany), the Department of Pediatric Rheumatology and Immunology (University Children's Hospital Münster, Münster, Germany), the Department of Pediatrics (Klinikum Kempten, Kempten, Germany), and the Department of Pediatrics (Hospital Sant Joan de Déu, Universitat de Barcelona, Barcelona, Spain). All patients with MIS-C fulfilled the WHO criteria.12 As a control group, we collected plasma samples from patients with Kawasaki disease at the Hospital Sant Joan de Déu (Universitat de Barcelona) and at the Department of Paediatric Cardiology (Ludwig Maximilians University, Munich, Germany). Furthermore, we included plasma samples from children with inactive systemic juvenile idiopathic arthritis at the Department of Pediatric Rheumatology and Immunology (University Children's Hospital Münster). As a non-inflammatory control group, serum samples were collected from children with suspected growth retardation at the Department of Pediatrics (Saarland University, Homburg, Germany). We also included serum samples negative for SARS-CoV-2 antibodies from healthy paediatric control patients, referred to as healthy controls herein, and serum samples from children with a history of asymptomatic or mild COVID-19, from the CoKiBa study.29 Healthy controls were age and sex-matched with the MIS-C group (multiple matched cases for each MIS-C group). Samples in the CoKiBa study were obtained 1–3 months after the peak of the first pandemic wave (spring 2020) in Bavaria. Most control groups were aged 0–18 years apart from groups from the CoKiBa trial (aged ≤17 years). Plasma samples of adult patients (>18 years) with critical COVID-19 were obtained from the Department of Internal Medicine V, Saarland University Medical School (Homburg, Germany).
This study was approved by the Ethics Committee of the Saarland Medical Association (reference number 41/21) and conducted according to the Declaration of Helsinki. All parents or guardians and adult patients signed written informed consent.
Procedures
Baseline data including demographic characteristics, clinical manifestations, laboratory parameters, and current drug therapies at the time of blood sampling were collected by study staff with the exception of the CoKiBa study samples. In the CoKiBa trial, data were collected by questionnaire at the time of blood sampling. We were not able to obtain follow-up samples systematically but had access to longitudinal samples for two patients in the MIS-C group.
Product details of all kits and antibodies used are listed in the appendix (p 38). ELISA for autoantibodies was performed as described previously.30 In brief, the antigens were obtained with use of the coding sequences of the GRN gene encoding progranulin and isoform 1 precursor of IL1RN, and were recombinantly expressed with a C-terminal FLAG tag in HEK293 cells under the control of a cytomegalovirus promoter (pSFI).31 Total cell extracts were prepared and bound to Nunc MaxiSorp plates (eBioscience, Frankfurt, Germany) precoated with murine anti-FLAG monoclonal antibody at a dilution of 1:2500 (volume/volume; Sigma-Aldrich, Munich, Germany) at 4°C overnight. After blocking with 1·5% (weight/volume) gelatin in Tris-buffered saline (TBS) for 1 h at room temperature and washing steps with TBS with Triton X-100, the individual plasma samples were diluted 1:100. ELISA was performed according to standard manufacturer protocols with the antibodies: biotinylated goat antihuman heavy and light chain IgG at a dilution of 1:2500 (Dianova, Hamburg, Germany); subclass-specific sheep antihuman IgG1, IgG2, IgG3, and IgG4 (Binding Site Group, Birmingham, UK) at a dilution of 1:5000; goat antihuman IgM (Dianova) at a dilution of 1:2500; or goat antihuman IgA (Dianova) at a dilution of 1:2500. Following this step, corresponding biotinylated secondary antibodies were used for immunoassays performed to detect IgG subclasses and IgM. Peroxidase-labelled streptavidin (Roche Applied Science, Indianapolis, IN, USA) was used at a dilution of 1:50 000. As a cutoff for positivity, the average of the optical density (OD) of the negative samples plus three SDs was applied. To determine the epitope region of the anti-IL-1Ra antibodies, we expressed fragments of full-length IL-1Ra (amino acids 59–75, 98–116, 125–143, and 161–177), and, as a control antigen, full-length IL-36Ra, with a C-terminal FLAG tag in HEK293 cells under the control of a cytomegalovirus promoter (pSFI).31 As a control for possible bystander humoral immune responses, IgG antibodies directed against Clostridium tetani tetanus toxin (Argio Biolaboratories, Hsinchu City, Taiwan) were analysed by ELISA.
Isoelectric focusing and western blotting (including native western blotting with non-reducing sample pretreatment and gradient gels without SDS) were performed. Blocking was done overnight at 4°C in 10% non-fat dry milk (weight/volume). Plasma samples were analysed for IL-1Ra isoforms. Plasma from anti-IL-1Ra antibody-positive patients was treated with alkaline phosphatase as previously described with FastAP Thermosensitive Alkaline Phosphatase (Fermentas, Darmstadt, Germany).32
IL-1Ra plasma concentrations were measured with an IL1RA Human ELISA Kit (Thermo Fisher Scientific, Waltham, MA USA) according to the manufacturer's instructions. The accuracy of IL-1Ra plasma concentrations measured by ELISA has been validated previously by a dilution series with recombinant human IL-1Ra in the presence of recombinant anti-IL-1Ra antibodies or anti-IL-1Ra antibodies purified from patient plasma.19 Progranulin plasma concentrations were determined with a Progranulin (human) ELISA Kit (AdipoGen, Incheon, South Korea) according to the manufacturer's instructions.
For an IL-1β signalling reporter assay, HEK-Blue IL-1β Cells (Invivogen, San Diego, CA, USA) were used, which react specifically to IL-1β and IL-1α by induction of nuclear factor-κB and activator protein 1, leading to expression of secreted embryonic alkaline phosphatase reporter. Recombinant IL-1Ra at 40 ng/mL (Biozol, Eching, Germany) alone or with either rabbit antihuman IL-1Ra antibody at 5 μg/mL (antibodies-online, Aachen, Germany), recombinant anti-SLP2 antibody at 5 μg/mL (Abcam, Cambridge, UK), diluted plasma (1:20) from a patient with acute MIS-C and high-titred (1:800) anti-IL-1Ra antibodies, or diluted plasma (1:20) from the same patient 7 months after onset of MIS-C but without detectable anti-IL-1Ra antibodies, were preincubated for 2 h at room temperature. Subsequently, these compounds were added with either 2 ng/mL IL-1β (Biozol) or 2 ng/mL TNF (Biozol) in 100 μl Dulbecco's Modified Eagle Medium to HEK-Blue IL-1β reporter cells (2 × 104 cells per well) and incubated overnight at 37°C. Thereafter, 180 μL of each supernatant was collected, 20 μl QUANTI-Blue (Invivogen) was added, and secreted embryonic alkaline phosphatase activity was measured at an OD of 655 nm. Experiments were performed in triplicate. Diluted plasma (1:20) from adult patients with critical COVID-19 with and without anti-IL-1Ra antibodies served as controls.
Statistical analysis
Differences in proportions of Anti-IL-1Ra autoantibody positivity between the MIS-C and control groups were compared by Fisher's exact test, not corrected for multiple testing. Associations between two categorical variables were tested with two-tailed Fisher's exact test. IL-1Ra plasma concentrations measured by ELISA were analysed for normality distribution with Shapiro–Wilk and Kolmogorov–Smirnov tests. Mean IL-1Ra plasma concentration in autoantibody-positive patients with MIS-C was compared with mean concentration in seronegative patients with MIS-C, and with mean values in the control groups, via Brown–Forsythe and Welch ANOVA and Dunnett's T3 multiple comparisons test. All analyses were repeated excluding two MIS-C cases that had received IVIGs before the blood sampling.
A p value less than 0·05 was regarded to indicate statistical significance. GraphPad Prism (version 9.3.0) was used for analysis.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Serum and plasma samples analysed in this study were collected between March 6, 2011, and June 2, 2021. We analysed 21 patients (11 girls and ten boys) with MIS-C, with a median age of 7·0 years (range 1·5–17·0 years), and a mean weight of 31·6 kg (SD 19·2; range 9·8–71·8; table , appendix pp 1–2). All patients with MIS-C were seropositive or PCR positive (or both seropositive and PCR positive) for SARS-CoV-2, with the exception of one patient who had only reported contact with SARS-CoV-2. The control groups comprised 146 children with asymptomatic or mild COVID-19 (72 girls and 74 boys, median age 8 years [range 0–16]), 24 children with Kawasaki disease (nine girls and 15 boys; median age 3·0 years [0·1–7·5]), ten children with systemic juvenile idiopathic arthritis in remission (five girls and five boys; median age 15·5 [9·0–18·0]), 33 non-inflammatory patients with suspected growth retardation (13 girls and 20 boys; median age 10·6 years [3·0–15·3]), and 462 healthy controls (230 girls and 232 boys; median age 8 years [0–16 years]). All samples from patients with MIS-C, with the exception of two patients, and all patients with Kawasaki disease were collected before administration of IVIGs. Further baseline characteristics of the MIS-C and control groups are presented in the table.
Table.
Study population
| Healthy controls (n=462) | Non-inflammatory controls* (n=33) | MIS-C (n=21) | Kawasaki disease (n=24) | Inactive systemic juvenile idiopathic arthritis (n=10) | Asymptomatic or mild COVID-19 (n=146) | ||
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age, years | 8 (0–16) | 10·6 (3·0–15·3) | 7·0 (1·5–17·0) | 3·0 (0·1–7·5) | 15·5 (9·0–18·0) | 8 (0–16) | |
| Sex | |||||||
| Female | 230 (50%) | 13 (39%) | 11 (52%) | 9 (38%) | 5 (50%) | 72 (49%) | |
| Male | 232 (50%) | 20 (61%) | 10 (48%) | 15 (63%) | 5 (50%) | 74 (51%) | |
| Clinical manifestations | |||||||
| Fever >3 days | 163 (35%)† | 0 | 21 (100%) | 24 (100%) | 0 | 57 (39%)† | |
| Rash | 23 (5%) | 0 | 14 (67%) | 17 (71%) | 0 | 3 (2%) | |
| Bilateral conjunctivitis | 20 (4%)‡ | 0 | 15 (71%) | 21 (88%) | 0 | 5 (3%)‡ | |
| Gastrointestinal symptoms | 108 (23%)§ | 0 | 18 (86%) | 4 (17%) | 0 | 29 (20%)§ | |
| Cardiac involvement | NA | 0 | 13 (62%) | 24 (100%) | 0 | NA | |
| Coronary artery aneurysms | NA | 0 | ND | 4 (17%) | 0 | NA | |
| Arthritis | NA | 0 | 0 | 2 (8%) | 1 (10%) | NA | |
| Laboratory parameters | |||||||
| C-reactive protein, mg/L | ND | ND | 214 (74–440) | 95 (3–382) | <0·5 (not detectable–13) | ND | |
| Ferritin, ng/mL | ND | ND | 565 (67–40 006) | ND | 35 (24–226) | ND | |
| Natrium, mmol/L | ND | ND | 133 (124–141) | 135 (126–138) | 140 (138–142) | ND | |
| Leukocyte count, ×109 cells/L | ND | ND | 17·8 (10·3–30·6) | 15·9 (7·9–37·3) | 6·4 (4·4–32·5) | ND | |
| Neutrophils, % | ND | ND | NA | 82 (53–86) | 55 (36–85) | ND | |
| Platelet count, ×103 cells/mL | ND | ND | 151 (78–1248) | 547 (135–974) | 296 (214–339) | ND | |
| SARS-CoV-2, serology positive | 0 | ND¶ | 19/20 (95%) | ND¶ | ND‖ | 146 (100%) | |
| SARS-CoV-2, PCR positive** | ND | ND‖ | 4/20 (20%) | ND‖ | ND‖ | ND | |
| Treatment | |||||||
| IVIG†† | 0 | 0 | 19 (90%) | 24 (100%) | 0 | 0 | |
| Steroids | 0 | 0 | 20 (95%) | 1 (4%) | 3 (30%) | 0 | |
| Anakinra | 0 | 0 | 15 (71%) | 21 (88%) | 5 (50%) | 0 | |
| Ventilation | 0 | 0 | 9 (43%) | 0 | 0 | 0 | |
Data are median (range), n (%), or n/N (%) where N is patients with available data. Body weight is reported for the MIS-C group in the appendix (pp 1-2); weight data were not collected or unavailable for all other groups. MIS-C=multisystem inflammatory syndrome in children. NA=not available. ND=not determined. IVIG=intravenous immunoglobulin.
Children with suspected growth retardation.
Parent report of fever >38·5°C.
Parent report of conjunctivitis.
Parent report of diarrhoea or nausea.
Collected before 2019.
Collected before 2020.
PCR at admission to hospital.
The analysed serum or plasma study samples were all collected from IVIG-naive patients (except two MIS-C cases); patients were subsequently treated during the disease course as medically indicated.
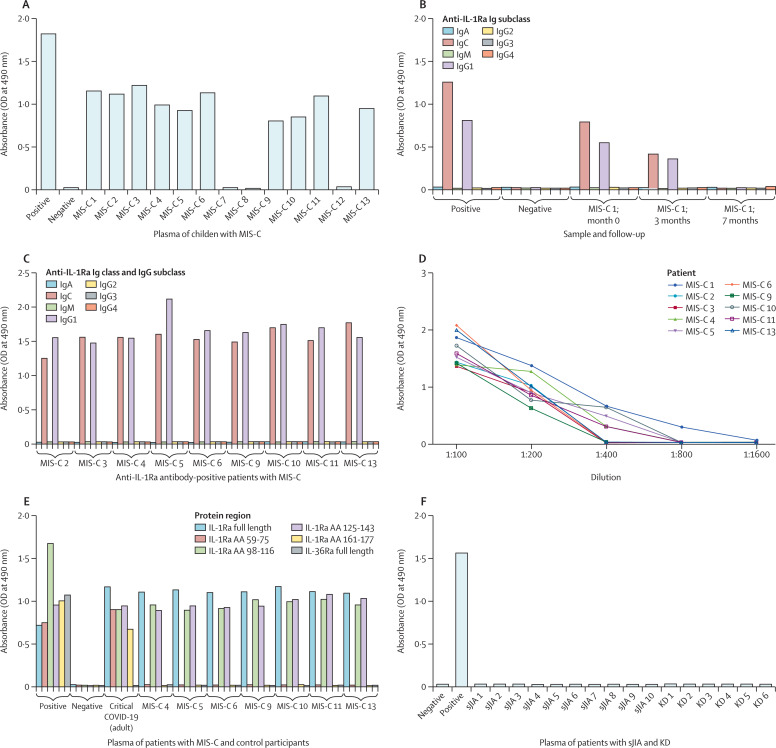
Anti-IL-1Ra antibodies were detected in 13 (62%) of 21 patients with MIS-C, with titres ranging between 1:200 to 1:800 (figure 1A and 1D , appendix pp 3–5, 19–20). All anti-IL-1Ra antibodies in patients with MIS-C belonged exclusively to the IgG1 subclass, with the exception of one patient, who additionally had anti-IL-1Ra antibodies of the IgM subclass (figure 1C, appendix p 19). Anti-IL-1Ra antibodies bound to a region spanning from amino acid 98 to 143 (figure 1E). Autoantibodies against progranulin were only detected in one (5%) patient with MIS-C at a titre of 1:200 (appendix p 5).
Figure 1.
ELISA of IL-1Ra antibodies in patients with MIS-C and control participants.
(A) ELISA data for anti-IL-1Ra antibodies in plasma from an exemplary 13 patients with MIS-C. Data for the other eight patients with MIS-C are shown in the appendix (pp 5, 19). Anti-IL-1Ra antibody subclasses in follow-up samples (initial presentation and 3-month and 7-month follow-up) of a single patient (B) and in samples collected at presentation of acute inflammation (C). (D) Titres of anti-IL-1Ra antibodies in patients with MIS-C at presentation. (E) Epitope mapping of anti-IL-1Ra antibodies in MIS-C. (F) ELISA of anti-IL-1Ra antibodies in plasma of intravenous immunoglobulin-naive patients with Kawasaki disease at presentation (n=6) and serum samples obtained from patients with systemic juvenile idiopathic arthritis (in remission; n=10). Positive control was anti-FLAG antibody; negative control was healthy paediatric control plasma. MIS-C=multisystem inflammatory syndrome in children. IL-1Ra=interleukin-1 receptor antagonist. OD=optical density. IL-36Ra=interleukin-36 receptor antagonist. AA=amino acid. sJIA=systemic juvenile idiopathic arthritis. KD=Kawasaki disease.
Anti-IL-1Ra antibodies were not detectable in children with asymptomatic or mild COVID-19 (vs MIS-C group, Fisher's exact test p<0·0001; appendix pp 23–26), Kawasaki disease (p<0·0001; figure 1F, appendix p 22), or inactive systemic juvenile idiopathic arthritis (p=0·0013; figure 1F). Anti-IL-1Ra antibodies were also not detectable in the non-inflammatory group (p<0·0001; appendix p 20) or healthy control group (p<0·0001, appendix pp 23–26). Progranulin autoantibodies were undetectable in all control groups (appendix pp 6–18, 20–22, 27–30).
Clostridium tetanus toxin IgG antibody concentrations in patients MIS-C did not differ from the concentrations measured in patients with Kawasaki disease or healthy controls (appendix p 31).
IL-1Ra typically produces two bands on isoelectric focusing.19 Isoelectric focusing of total protein from plasma of one patient with MIS-C with several follow-up samples revealed the presence of an additional, more negatively charged third band of IL-1Ra (appendix p 33) at the time of initial presentation. Pretreatment with alkaline phosphatase before isoelectric focusing resulted in disappearance of protein bands resembling both the normally occurring second IL-1Ra isoform as well as the atypical additional third, indicating a hyperphosphorylation. In the disease course of this patient, hyperphosphorylated IL-1Ra was no longer detectable at 3 or 7 months after initial MIS-C onset (appendix p 33).
Hyperphosphorylated IL-1Ra was observed in all 13 MIS-C patients with anti-IL-1Ra antibodies but not in the patients with MIS-C who were autoantibody negative (appendix pp 33–34), healthy controls (appendix p 35), or control patients with Kawasaki disease (appendix p 36), inactive systemic juvenile idiopathic arthritis, or non-inflammatory conditions (n=49 negative samples) analysed by isoelectric focusing, resulting in a significant association (Fisher's exact test, two-tailed p<0·0001), which was maintained when excluding the two patients with MIS-C who had received IVIGs before blood sampling (p<0·0001).
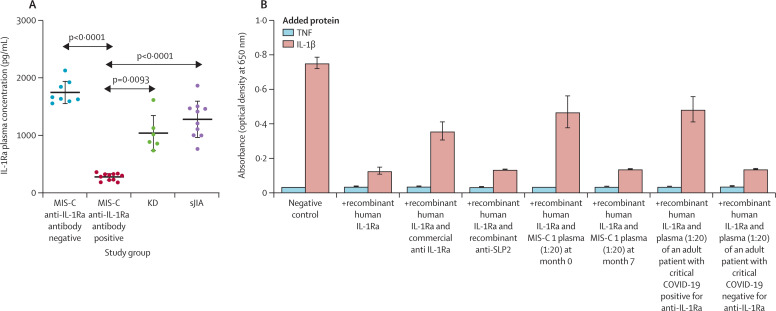
In MIS-C patients positive for anti-IL-1Ra antibody, we observed that free IL-1Ra plasma concentrations were significantly decreased (figure 2A , appendix 3–5) at presentation of acute inflammation (n=13, mean 279·4 pg/mL [SD 58·2]; or excluding the two MIS-C patients who received IVIGs before blood sampling, n=11, mean 275·0 pg/mL [62·6]), compared with MIS-C patients negative for anti-IL-1Ra antibody (n=8; mean 1746·0 pg/mL [200·4], p<0·0001). A significant decrease was also observed compared with patients with Kawasaki disease (n=6 with available data; mean 1038·0 pg/mL [SD 312·8], p=0·0094; or versus 11 MIS-C cases, p=0·0093) or patients with inactive systemic juvenile idiopathic arthritis (n=10; mean 1278·0 [323·1], p<0·0001; figure 2A).
Figure 2.
Neutralising and functional effect of anti-IL-1Ra antibodies in MIS-C
(A) Free IL-1Ra plasma concentrations as measured by ELISA in patients with MIS-C (n=21), Kawasaki disease (n=6) and systemic juvenile idiopathic arthritis (n=10). Horizontal lines represent the mean and SD. Data were analysed by Brown–Forsythe and Welch ANOVA with Dunnett's T3 multiple comparisons. (B) IL-1β-signalling reporter assay on selected MIS-C plasma compared with an adult critical COVID-19 plasma sample (both 1:20 dilution) as well as commercially available anti-IL-1Ra antibody or control (anti-SLP2) antibody. The absorbance of secreted embryonic alkaline phosphatase, as a marker for IL-1β pathway activation in HEK IL-1β reporter cells, was detected at 650 nm. Error bars show mean (SD). MIS-C=multisystem inflammatory syndrome in children. IL-1Ra=interleukin-1 receptor antagonist. KD=Kawasaki disease. sJIA=systemic juvenile idiopathic arthritis. TNF=tumour necrosis factor. IL-1β=interleukin-1β.
Similar to the absolute concentrations of free IL-1Ra as measured by ELISA, native western blots of total plasma protein also revealed weakened protein bands resembling IL-1Ra in all patients with MIS-C who were positive for anti-IL-1Ra antibodies. However, all autoantibody-positive patients had an additional protein band representing IgG-bound IL-1Ra (appendix pp 33–34). In the only patient (MIS-C-21) presenting with IgM antibodies in addition to IgG1, we observed an additional protein band representing IgM-bound IL-1Ra.
We further analysed the two MIS-C patients with longitudinal samples available. In one patient (MIS-C-1), free IL-1Ra plasma concentration was strongly reduced at the time of MIS-C presentation (172·0 pg/mL) compared with values at 3 months (1297·1 pg/mL) and 7 months (847·5 pg/mL). Native western blot of total plasma protein at initial presentation and the 3-month follow-up produced a weakened free IL-1Ra signal and revealed the presence of IgG-bound IL-1Ra, which was absent at 7 months when the patient was seronegative for anti-IL-1Ra antibodies (appendix p 32). In the other patient (MIS-C-6), the initial plasma sample was positive for anti-IL-1Ra antibodies, which coincided with hyperphosphorylated IL-1Ra (appendix p 33) and reduced free IL-1Ra plasma concentration (323·5 pg/mL). At a 5-week follow-up visit, anti-IL-1Ra antibodies and hyperphosphorylated IL-1Ra were no longer detectable (data not shown), whereas IL-1Ra plasma concentration had increased to 1642·0 pg/mL.
To investigate a possible functional effect of the observed anti-IL-1Ra antibodies, we performed an in vitro IL-1β signalling reporter assay. Addition of plasma from a patient with MIS-C who was positive for anti-IL-1Ra antibodies significantly weakened the antagonism by recombinant IL-1Ra, resulting in a stronger stimulatory effect of IL-1β (figure 2B). We observed similar impairment of IL-1Ra bioactivity when adding commercially available antihuman IL-1Ra antibody or plasma obtained from an adult patient with critical COVID-19 who had tested positive for IL-1Ra antibodies. By contrast, IL-1Ra function was not affected when adding plasma from the MIS-C patient when the sample was obtained at the 7-month follow-up and negative for anti-IL-1Ra antibodies (figure 2B). IL-1Ra function was also not affected when adding plasma from an adult patient with critical COVID-19 who had tested negative for anti-IL1-Ra antibodies.
Discussion
In this study we report on neutralising autoantibodies against the anti-inflammatory molecule IL-1Ra as an exclusive phenomenon in patients with MIS-C, when compared with Kawasaki disease and other inflammatory and non-inflammatory paediatric conditions, including asymptomatic and mild courses of SARS-CoV-2 infection. Although we did not observe anti-IL-1Ra antibodies in any of the control conditions enrolled in this study, our findings are in accordance with those in our preprint paper on adults with critical COVID-19,19 in whom anti-IL-1Ra antibodies were detected in a high proportion of patients (about 50%).
In contrast to MIS-C, in adult patients with critical COVID-19 we observed autoantibodies against progranulin in about 40% of patients.19 However, we only detected anti-progranulin antibodies in one of 21 patients with MIS-C in the present study. Compared with critical COVID-19 in adults, anti-IL-1Ra antibodies were found in a higher proportion in patients with MIS-C (13 [62%] of 21). Notably, the plasma concentrations of free IL-1Ra were significantly reduced in all patients with MIS-C who had anti-IL-1Ra antibodies, compared with patients with Kawasaki disease or systemic juvenile idiopathic arthritis, which coincided with detection of immune-complexed IL-1Ra. Anti-IL-1Ra antibodies in patients with MIS-C belonged exclusively to the IgG1 subclass, apart from one patient who had additional antibodies of the IgM class, which is in contrast to adults with critical COVID-19, in whom anti-IL-1Ra antibodies of the IgM class and several IgG subclasses were universally detectable in all investigated patients.19 These findings might suggest that a class switch of the anti-IL-1Ra antibodies from IgM to IgG might have already occurred within the latency period between infection and manifestation of hyperinflammation, or that the children might have had a clinically inapparent previous autoimmune response against IL-1Ra and consequently already established respective immunological memory. IL-1Ra-specific autoantibodies were also recently reported in IgG4-associated diseases,33 which might support the idea of IL-1Ra-specific immunity outside of a SARS-CoV-2 infection context. Furthermore, our epitope mapping data indicated that anti-IL-1Ra antibodies in patients with MIS-C recognise a more confined epitope (amino acids 98–143) compared with respective antibodies in adult COVID-19,19 which might argue for at least a partly distinct immunological mechanism and origin of this immune response.
However, in both critical COVID-19 and MIS-C, we observed a hyperphosphorylated isoform of the IL-1Ra antigen, which coincided with the presence of anti-IL-1Ra antibodies. In patient follow-up samples, we observed the disappearance of hyperphosphorylated IL-1Ra, which preceded the disappearance of anti-IL-1Ra antibodies. Conversely, data from longitudinal samples of adult patients with severe or critical COVID-19 has shown that hyperphosphorylation of IL-1Ra preceded the formation of respective autoantibodies. Collectively, data from both COVID-19 and MIS-C suggest that atypical post-translational modifications are associated with SARS-CoV-2-infection itself or the resulting inflammatory environment,19 and that these modifications are likely to be immunogenic.
Collectively, the findings of our study need to be discussed in view of three main limitations. First, we do not have a detailed molecular understanding of the circumstances and mechanism of the IL-1Ra hyperphosphorylation, and we cannot delineate particular HLA associations with the reported autoantibody phenotypes, even though specific HLA haplotypes have been reported to associate with the general immunopathology in patients with MIS-C and COVID-19.34, 35, 36 Importantly, however, the coinciding autoantibodies proved to be functional and impaired IL-1Ra bioactivity, and this might thus offer an additional explanation for the hyperinflammatory phenotype in patients with MIS-C. Second, due to the small number of predominantly White patients with MIS-C included in our study, we did not observe differences in the clinical presentation or severity of MIS-C between patients with or without autoantibodies against IL-1Ra. Further studies with larger cohorts and mixed ethnicity are necessary to address this question. Third, based on our data we cannot provide specific treatment recommendations for autoantibody-positive patients. IVIG treatment has recently been suggested to selectively deplete IL-1-producing neutrophils in both Kawasaki disease and MIS-C.37 In case of non-response to IVIG, this effect could support application of selective IL-1 targeting therapies,17, 18, 38 which might also help to override temporarily imbalanced IL-1 signalling due to IL-1Ra-neutralising autoantibodies in patients with MIS-C. However, we cannot make predictions about the in vivo concentrations of anakinra that would potentially be required to override anti-IL-1Ra immunity in MIS-C or whether use of the anti-IL-1β monoclonal antibody canakinumab might offer an adequate alternative treatment particularly in this scenario.
In summary, autoantibodies against IL-1Ra together with a hyperphosphorylated isoform of IL-1Ra were observed in a high proportion of children with MIS-C. Although the small number of patients with MIS-C enrolled in this study might pose a limitation, the high numbers of controls (both inflammatory and non-inflammatory) highlight our findings as unique rather than an epiphenomenon. Our data suggest that anti-IL-1Ra autoantibodies are pathogenetically relevant and potentially contribute to hyperinflammation in patients with MIS-C.
Data sharing
All data required to evaluate the conclusions in the paper are present in the manuscript or its appendix. Further information on the study protocol or de-identified datasets generated and analysed within this publication are available from the corresponding author on reasonable request.
Declaration of interests
LT has received research grants from Wilhelm Sander-Stiftung, BioNanoMed, and the Homburger Forschungsförderung programme of the University of Saarland; travel grants for meeting attendance from AbbVie, Janssen, and EUSA-Pharm; and has participated in advisory boards for Takeda, AstraZeneca, Merck, and EUSA Pharma. LT and K-DP were listed among inventors of a patent on progranulin antibodies as marker for autoimmune diseases filed by University of Saarland, which expired in 2017. CK has received consulting fees from Novartis and Swedish Orphan Biovitrum (SOBI) and receives research support from Novartis. MBö has received speakers honoraria from or participated in advisory boards for Abbott, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Cyotkinetics, Medtronic, Novartis, Servier, and Vifor. HW has received honoraria (lecture fees) from Novartis and Takeda, and travel support from Octapharma and CSL-Behring. DF has received speaker fees or honoraria from Chugai-Roche, Novartis, and SOBI, and research support from Novartis, Pfizer, and SOBI. JA has received grants and travel grants for meeting attendance from SOBI and Novartis, and participated in advisory boards for SOBI and Novartis. BT has received honoraria for lectures from Nutricia Milupa and is a private shareholder of BioNTech. MK has received research support for the CoKiBa trial from Roche, who provided the diagnostic antibodies at a time when they were not yet commercially available. In addition, MK has received honoraria for lectures from Pädnetz Bayern, Ärztlicher Kreisverband, and Bayerischer Berufsverband der Kinder- und Jugendärzte, and participated in the advisory board for COVID-19 in children of the Bavarian Ministry of Health. SLB has participated in advisory boards and received honoraria for lectures from Shinogi and Pfizer. RB has received grants and personal fees from AstraZeneca, Boehringer Ingelheim, and Novartis; personal fees from GlaxoSmithKline, Grifols, and CSL Behring; grants from the German Ministry of Education and Research, Competence Network on Asthma and COPD, Sander Stiftung, Schwiete Stiftung, Krebshilfe, and Mukosviszidose; and has a leadership role at the Alpha-1-Center in Bad Lippspringe, Germany. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the patients and their parents, who supported this research project. We also want to thank all physicians, nurses, and other staff not mentioned here, who cared for the patients. We thank Susanne Brandstetter for compiling the symptoms of the control groups obtained from the CoKiBa trial. This work was supported by a young investigator NanoBioMed fund of the University of Saarland (to LT); by funding from the Dr Rolf M Schwiete Stiftung and the Staatskanzlei of the Federal State of Saarland (to SS); and by funding from the German Heart Foundation (to AJ). CK and DF were supported by the Center for Interdisciplinary Clinical Research at University Hospital Muenster (grant number Fo2/018/20) and the EU's Horizon 2020 research and innovation programme (grant agreement number 779295; via the ImmunAID consortium). The CoKiBa trial was funded by the Charity of the Blue Sisters and the project Post-COVID Kids Bavaria of the Bavarian Ministry of Health.
Acknowledgments
Contributors
LT, BT, JP, NF, ER, and CK planned the study, reviewed and verified the data, and wrote the manuscript. NF, ER, M-CH, and LT performed experiments. KR, YF, K-DP, IAK, SLB, PK, SG, KH, TR, SM, SS, AJ, MBe, MBö, HA-K, CK, EB, AK, DF, and MK reviewed the data and manuscript. SM, SL, SS, M-CD, RB, MF, HJ, HS, TR, HA-K, HW, SG, PK, KM, AJ, JA, and RMP-R provided patient and control samples and clinical data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 4.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Sarmiento J, De Souza D, Jabornisky R, Gonzalez GA, Arias López MDP, Palacio G. Paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): a narrative review and the viewpoint of the Latin American Society of Pediatric Intensive Care (SLACIP) Sepsis Committee. BMJ Paediatr Open. 2021;5 doi: 10.1136/bmjpo-2020-000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Smith JJ, Verweyen EL, Clay GM, et al. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: a cohort study. Lancet Rheumatol. 2021;3:e574–e584. doi: 10.1016/S2665-9913(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harwood R, Allin B, Jones CE. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. 2021;5:133–141. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Scientific brief. May 15, 2020. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 13.Esteve-Sole A, Anton J, Pino-Ramirez RM, et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J Clin Invest. 2021;131 doi: 10.1172/JCI144554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber CN, Patel RS, Trachtman R, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982. doi: 10.1016/j.cell.2020.09.034. 95.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikén M, Hallén B, Kullenberg T, Koskinen LO. Development and effect of antibodies to anakinra during treatment of severe CAPS: sub-analysis of a long-term safety and efficacy study. Clin Rheumatol. 2018;37:3381–3386. doi: 10.1007/s10067-018-4196-x. [DOI] [PubMed] [Google Scholar]
- 17.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. 2020;72:1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. 2021;73:e13–e29. doi: 10.1002/art.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurner L, Fadle N, Bewarder M, et al. Autoantibodies against progranulin and IL-1 receptor antagonist due to immunogenic posttranslational isoforms contribute to hyperinflammation in critically ill COVID-19. bioRxiv. 2021 doi: 10.1101/2021.04.23.441188. published online Oct 20. (preprint). [DOI] [Google Scholar]
- 20.Kessenbrock K, Fröhlich L, Sixt M, et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest. 2008;118:2438–2447. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Nathan C, Jin W, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 22.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Li XX, Gao W, Liu W, Liu DS. Progranulin-derived atsttrin directly binds to TNFRSF25 (DR3) and inhibits TNF-like ligand 1A (TL1A) activity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinarello CA, Rosenwasser LJ, Wolff SM. Demonstration of a circulating suppressor factor of thymocyte proliferation during endotoxin fever in humans. J Immunol. 1981;127:2517–2519. [PubMed] [Google Scholar]
- 25.Arend WP, Joslin FG, Massoni RJ. Stimulation of production of interleukin-1 and an interleukin-1 inhibitor in human monocytes. Rheumatology. 1985;24(suppl 1):175–178. [Google Scholar]
- 26.Arend WP. Interleukin-1 receptor antagonist: discovery, structure and properties. Prog Growth Factor Res. 1990;2:193–205. doi: 10.1016/0955-2235(90)90018-f. [DOI] [PubMed] [Google Scholar]
- 27.Arend WP. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991;88:1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 29.Laub O, Leipold G, Toncheva AA, et al. Symptoms, SARS-CoV-2 antibodies, and neutralization capacity in a cross sectional-population of German children. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.678937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurner L, Preuss KD, Fadle N, et al. Progranulin antibodies in autoimmune diseases. J Autoimmun. 2013;42:29–38. doi: 10.1016/j.jaut.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Bornkamm GW, Berens C, Kuklik-Roos C, et al. Stringent doxycycline-dependent control of gene activities using an episomal one-vector system. Nucleic Acids Res. 2005;33:e137. doi: 10.1093/nar/gni137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grass S, Preuss KD, Ahlgrimm M, et al. Association of a dominantly inherited hyperphosphorylated paraprotein target with sporadic and familial multiple myeloma and monoclonal gammopathy of undetermined significance: a case-control study. Lancet Oncol. 2009;10:950–956. doi: 10.1016/S1470-2045(09)70234-7. [DOI] [PubMed] [Google Scholar]
- 33.Jarrell JA, Baker MC, Perugino CA, et al. Neutralizing anti-IL-1 receptor antagonist autoantibodies induce inflammatory and fibrotic mediators in IgG4-related disease. J Allergy Clin Immunol. 2022;149:358–368. doi: 10.1016/j.jaci.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porritt RA, Paschold L, Rivas MN, et al. HLA class I-associated expansion of TRBV11–2 T cells in multisystem inflammatory syndrome in children. J Clin Invest. 2021;131 doi: 10.1172/JCI146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreews M, Le Gouge K, Khaldi-Plassart S, et al. Polyclonal expansion of TCR Vbeta 21·3+ CD4+ and CD8+ T cells is a hallmark of multisystem inflammatory syndrome in children. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abh1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner J, 3rd, Suwalski P, Holtgrewe M, et al. Increased risk of severe clinical course of COVID-19 in carriers of HLA-C*04:01. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu YP, Shamie I, Lee JC, et al. Immune response to intravenous immunoglobulin in patients with Kawasaki disease and MIS-C. J Clin Invest. 2021;131 doi: 10.1172/JCI147076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koné-Paut I, Tellier S, Belot A, et al. Phase II open label study of anakinra in intravenous immunoglobulin-resistant Kawasaki disease. Arthritis Rheumatol. 2021;73:151–161. doi: 10.1002/art.41481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data required to evaluate the conclusions in the paper are present in the manuscript or its appendix. Further information on the study protocol or de-identified datasets generated and analysed within this publication are available from the corresponding author on reasonable request.