Many immunosuppressed patients with rheumatic and musculoskeletal disease have a poor antibody response to two-dose SARS-COV-2 mRNA vaccination,1, 2 prompting widescale authorisation of a third vaccine dose for these patients. High antibody concentrations are required to overcome immune evasion by variants of concern in immunocompetent patients,3 and although a third dose augments the immune response against SARS-CoV-2 in some immunosuppressed patients,4, 5 it is uncertain whether this response is sufficient for protection. Thus, identifying patients with rheumatic and musculoskeletal disease with poor response following a third dose is important in the selection of appropriate candidates for further medical interventions such as additional vaccine doses or prophylactic therapies. Herein, we describe the antibody response and factors associated with poor antibody response following a third vaccine dose in immunosuppressed patients with rheumatic and musculoskeletal disease.
Patients with rheumatic and musculoskeletal disease (aged ≥18 years) in the USA on immunosuppression without previous known COVID-19 who completed three-dose SARS-CoV-2 vaccination (two-dose mRNA series followed by single mRNA or adenoviral vector dose) were recruited via a social media campaign and provided informed consent electronically. This study was approved by the Johns Hopkins Institutional Review Board (IRB00248540). Clinical characteristics were collected via participant report. Serial antibody responses were assessed using the semi-quantitative Roche Elecsys (Rotreuz, Switzerland) anti-SARS-CoV-2 S enzyme immunoassay, which measures total antibody to the SARS-CoV-2 S-receptor binding domain (RBD; range 0·4 with upper limit >2500 U/mL), and is recognised as a consistent correlate of neutralising antibody.6 Poor antibody response was defined as anti-RBD titre less than 500 U/mL on the basis of predicted correlates of protective plasma neutralising capacity in COVID-19 vaccine trials.7, 8 Participant demographics and clinical characteristics were stratified by antibody response (appendix pp 1–2). Poisson regression with robust standard error was done to evaluate factors identified a priori to be associated with poor antibody response (age, third dose vaccine type, immunosuppression, and number of immunosuppressive therapies).
We evaluated serial anti-RBD titres in 511 participants (appendix p 1). 471 (92%) were women, 38 (7%) were men, and the median age was 50 years (IQR 41–60). The most common diagnosis was inflammatory arthritis (210 [41%] of 511). Participants completed standard vaccination with BNT162b2 (271 [53%] of 511) or mRNA-1273 (240 [47%]). At a median of 159 days (IQR 92–185) after participants' second dose, anti-RBD titres were negative (anti-RBD <0·8 U/mL) in 57 (11%) of 511 participants, and the median anti-RBD titre was 238 U/mL (IQR 47·9–839·6).
For their third vaccine dose, participants either received BNT162b2 (266 [52%] of 511), mRNA-1273 (240 [47%]), or Ad.26.COV2.S (5 [1%]), with most (485 [95%]) receiving a homologous third-dose vaccination. Repeat anti-RBD testing was done at a median of 30 days (IQR 28–34) after dose three. 233 (46%) of 511 participants reported holding immunosuppression peri-D3. Methotrexate was the most commonly held medication; 82 (62%) of 133 prescribed methotrexate withheld immunosuppression for a median of 1 (IQR1–2) doses in the peri-D3 period (appendix p 3). An increased antibody titre was seen in most participants (470 [92%] of 511) following the third dose.
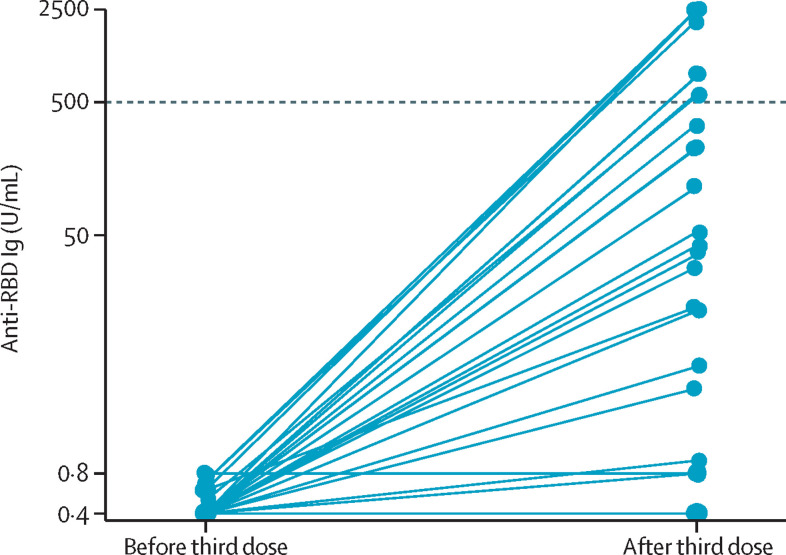
Of those participants who were negative before the third dose, 23 (40%) of 57 showed de novo humoral response, of whom 16 (70%) of 23 were on regimens containing rituximab or mycophenolate mofetil, and 34 (60%) remained negative following the third dose (figure ). The proportion of participants with titres of at least 2500 U/mL following a third dose was similar irrespective of homologous or heterologous third-dose vaccination (379 [78%] of 485 vs 17 [65%] of 26, p=0·1). Participants on immunosuppressant regimens containing rituximab were 10 times more likely to have a poor response following a third dose (adjusted incident rate ratio [aIRR] 10·00 [95% CI 6·61–15·13]; p<0·0010), and those on mycophenolate were twice as likely to have a poor response (aIRR 2·01 [95% CI 1·25–3·23]; p=0·0040; appendix p 3). 28 (7%) of 386 participants reported disease flare requiring treatment from a physician within 1 month of vaccination; appendix pp 1–2); no patient reported the need for intravenous therapy or hospital admission for treatment of flare.
Figure.
Anti-SARS-CoV-2 RBD antibody titres before and after a third dose in patients negative for anti-RBD antibodies after the second vaccine dose
Ig=immunoglobin. RBD=receptor binding domain.
Limitations of this study include that neutralisation capacity was not measured directly and there was not a healthy control group as a comparator. Antibody response is durable over 6 months, but titres might wane over time,8 which might have affected titres before the third dose. We did not assess B-cell or T-cell responses. A larger sample size is required to determine differential immunogenicity of homologous versus heterologous vaccine schedules, as well as determination of optimal perivaccination modulation of immunosuppression. Disease flares were based on patient report. Although administration of a third dose of SARS-CoV-2 vaccine has been associated with a significantly lower rate of COVID-19 infection in immunocompetent persons compared to two-dose vaccination,9, 10 associations between vaccine doses and antibody titres and clinical outcomes in immunosuppressed patients is required.
In summary, we observed an augmented humoral response in the majority (92%) of patients with rheumatic and musculoskeletal disease following a third dose of SARS-CoV-2 vaccination, highlighting the benefit of a three-dose vaccination schedule for these patients. We also identified a subset of patients, namely those on regimens containing either mycophenolate or CD20-depleting therapy, in which antibody responses remained suboptimal after a third dose. These patients are ideal candidates for prophylactic therapies or might require additional vaccine doses to confer increased protection against COVID-19 infection. The rapidly evolving SARS-CoV-2 requires continued development and refinement of medical countermeasures such as antibody testing, vaccine schedule, and prophylactic therapies to enhance the protection of these vulnerable patients.
DLS reports consulting and speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallincroft, Thermo Fisher Scientific, Regeneron, and AstraZeneca. LS-C reports consultant fees from Janssen, Boehringer-Ingelheim, Mallinckroft, Serono, Roivant, Octapharm, Allogene, and ArgenX. All other authors declare no competing interests. CMC and TP-YC contributed equally. CMC, TP-YC, JLA, WAW, DLS, and JJP agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors made substantial contributions to the conception or design of the work; made substantial contributions to the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; and finally approved the version to be published. DLS and JJP were co-senior authors. This work was made possible by the generous support of the Ben-Dov and Trokhan Patterson families. This work was supported by grant number F32DK124941 (Boyarsky), T32DK007713 (Alejo) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), K24AI144954 (Segev), U01AI138897 and K23AI157893 (Werbel) from National Institute of Allergy and Infectious Diseases (NIAID), K23AR073927 (Paik) from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. We would like to acknowledge the contributions of Dr Brian J Boyarsky, Jake A Ruddy, and Dr Jacqueline M Garonzik-Wang.
Supplementary Material
References
- 1.Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connolly CM, Boyarsky BJ, Ruddy JA, et al. Absence of humoral response after two-dose SARS-CoV-2 messenger rna vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann Intern Med. 2021;174:1332–1334. doi: 10.7326/M21-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaba AH, Zhu X, Liang T, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2021 doi: 10.1111/ajt.16933. published online Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly CM, Teles M, Frey S, et al. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis. 2021;81:291–293. doi: 10.1136/annrheumdis-2021-221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lusvarghi S, Pollett SD, Sabari NN, et al. SARS-CoV-2 omicron neutralization by therapeutic antibodies, convalescent sera, and post-mRNA vaccine booster. BioRxiv. 2021 doi: 10.1101/2021.12.22.473880. published online Dec 28, 2021. (preprint). [DOI] [Google Scholar]
- 6.Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59:e03149–e03150. doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey S, Chiang TP, Connolly CM, et al. Antibody durability 6 months after two doses of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal disease. Lancet Rheumatol. 2022;4:e241–e243. doi: 10.1016/S2665-9913(21)00417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer A, Angel Y, Marudi O, et al. Association of a third dose of BNT162b2 vaccine with incidence of SARS-CoV-2 infection among health care workers in Israel. JAMA. 2022;327:341–349. doi: 10.1001/jama.2021.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.