Abstract
Background
COVID-19 is associated with acute respiratory distress and cytokine release syndrome. The Janus kinase (JAK)1/JAK2 inhibitor ruxolitinib reduces inflammatory cytokine concentrations in disorders characterised by cytokine dysregulation, including graft-versus-host disease, myelofibrosis, and secondary hemophagocytic lymphohistiocytosis. We assessed whether treatment with the JAK1/JAK2 inhibitor ruxolitinib would be beneficial in patients with COVID-19 admitted to hospital.
Methods
RUXCOVID was an international, randomised, double-blind, phase 3 trial of ruxolitinib plus standard of care versus placebo plus standard of care in patients with COVID-19. Patients who were hospitalised but not on mechanical ventilation or in the intensive care unit [ICU] were randomly assigned (2:1) to oral ruxolitinib 5 mg twice per day or placebo for 14 days (14 additional days were allowed if no improvement). The primary endpoint was a composite of death, respiratory failure (invasive ventilation), or ICU care by day 29, analysed by logistic regression including region, treatment, baseline clinical status, age, and sex as covariates. This trial is registered with ClinicalTrials.gov, NCT04362137.
Findings
Between May 4 and Sept 19, 2020, 432 patients were randomly assigned to ruxolitinib (n=287) or placebo (n=145) plus standard of care; the mean age was 56·5 years (SD 13·3), 197 (46%) were female, and 235 (54%) were male. The primary objective was not met: the composite endpoint occurred in 34 (12%) of 284 ruxolitinib-treated patients versus 17 (12%) of 144 placebo-treated patients (odds ratio 0·91, 95% CI 0·48–1·73; p=0·77). By day 29, nine (3%) of 286 ruxolitinib-treated patients had died compared with three (2%) of 145 placebo-treated patients; 22 (8%) of 286 ruxolitinib-treated patients had received invasive ventilation compared with ten (7%) of 145 placebo-treated patients; and 30 (11%) of 284 ruxolitinib-treated patients had received ICU care compared with 17 (12%) of 144 placebo-treated patients. In an exploratory analysis, median time to recovery was 1 day faster with ruxolitinib versus placebo (8 days vs 9 days; hazard ratio 1·10, 95% CI 0·89–1·36). Adverse events included headache (23 [8%] of 281 on ruxolitinib vs 11 [8%] of 143 on placebo) and diarrhoea (21 [7%] vs 12 [8%]).
Interpretation
Ruxolitinib 5 mg twice per day showed no benefit in the overall study population. A larger sample is required to determine the clinical importance of trends for increased efficacy in patient subgroups.
Funding
Novartis and Incyte.
Introduction
COVID-19 was declared a global pandemic on March 11, 2020, by WHO.1 As of March 1, 2022, more than 437 million cases of COVID-19 had been reported, with 5·96 million deaths worldwide.2 Although most people with COVID-19 develop mild or uncomplicated illness, many have some form of respiratory involvement.3, 4, 5 Approximately 20% of people develop severe disease resulting in pneumonia, hospitalisation, and oxygen support; 5% require admission to the intensive care unit (ICU) and invasive mechanical ventilation.3, 4, 5
On infection, the virus activates the innate and adaptive immune systems, resulting in the release of pro-inflammatory cytokines in an attempt to eliminate the virus.6 As the disease progresses, the innate immune system response contributes to oxidative injury and alveolar membrane damage, resulting in hypoxia.6 Hypoxemia is further exacerbated by pulmonary microthromboses and macrothromboses.7, 8
Severe disease can be complicated by acute respiratory distress syndrome (the primary cause of death in 70% of COVID-19 fatalities), sepsis and septic shock, or multiorgan failure, or a combination of these, which have all been linked to the host inflammatory response.9, 10, 11, 12 The marked increase in immune cells and pro-inflammatory chemokines and cytokines, including interleukin (IL)-1, IL-6, IL-8, and tumour necrosis factor (TNF), drives lung injury and the activation of additional pro-inflammatory pathways via the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, resulting in further lung inflammation, lung lesions, respiratory dysfunction and failure, and in some cases, death.8, 10
Research in context.
Evidence before this study
We searched PubMed for articles published between Jan 1 and May 1, 2020, that evaluated treatments for COVID-19 using the search terms "(COVID-19 OR SARS-COV-2) AND (therap* OR treatment OR drug)". The search focused on clinical studies, clinical trials, meta-analyses, and systematic reviews. Before the start of this study (May, 2020), evidence regarding pharmacotherapy in severe COVID-19 was scarce. There were indications that severe disease and death might be related to hyperinflammation, with similarities to cytokine release syndrome. Studies suggested that agents that block inflammatory pathways, including the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, should be evaluated as treatment for severe COVID-19. Because ruxolitinib, a potent and selective inhibitor of JAK1 and JAK2, had previously shown efficacy in controlling inflammatory cytokine dysregulation in other disorders, such as graft-versus-host disease, myelofibrosis, and secondary hemophagocytic lymphohistiocytosis, it was considered a candidate for the treatment of severe COVID-19 disease.
During the conduct of this trial, the North American ACTT-2 study of baricitinib (a JAK1/JAK2 inhibitor) combined with remdesivir in COVID-19 found that addition of the JAK inhibitor to antiviral treatment reduced time to recovery, in particular in hospitalised patients requiring high-flow oxygen support. A trial of tofacitinib (a JAK1/JAK3 inhibitor) in Brazil found that tofacitinib plus standard care reduced mortality or respiratory failure in a population consisting mainly of patients with severe COVID-19. However, in another study in patients with mild-to-moderate COVID-19, tofacitinib treatment showed a reduction in inflammatory markers, but no clinical benefits. Trials of tocilizumab in COVID-19 also found that patients with more severe disease were more likely to benefit from immunosuppressive therapy, and in the large RECOVERY trial (n=4116), tocilizumab improved outcomes in patients with hypoxia and substantial inflammation.
Added value of this study
To our knowledge, this was the first randomised, controlled, phase 3 study to evaluate ruxolitinib plus standard of care (according to local practice) for patients with COVID-19. This study showed that, in a broad, international population of patients admitted to hospital for COVID-19 who were not on invasive mechanical ventilation or in the intensive care unit, a low dose of ruxolitinib (5 mg twice per day) plus standard-of-care treatment did not significantly improve outcomes or slow progression of severe disease in an international population compared with placebo plus standard-of-care treatment. Hence our data suggest that JAK1/JAK2 inhibitor therapy in COVID-19 needs to be considered with caution.
Implications of all the available evidence
RUXCOVID was compatible with previous studies of JAK inhibitors in COVID-19: although the proportion of patients on high-flow oxygen was small (22 [5%] of 432), a trend toward greater efficacy of ruxolitinib versus placebo was noted in this subgroup. No benefit was observed in patients with no or low-flow oxygen requirements. Substantial inflammation was not an inclusion criterion. Taken together, studies suggest that JAK inhibitor treatment might improve outcomes in patients with COVID-19, especially in a certain subset; however, our findings do not support the use of ruxolitinib as a treatment option for patients with mild-to-moderate COVID-19 who are admitted to hospital. Much is still being learned about COVID-19, and a need exists to identify patient subsets that would benefit from specific treatments. In novel global health crises, robust a priori sample-size estimates are not always possible. Therefore, we suggest that adaptive designs, including futility analyses and sample-size reestimation, be built into future studies conducted under these conditions. When all the evidence is viewed in context, JAK1/JAK2 inhibitor therapy in general is not effective in all subsets of hospitalised patient populations. More research is needed to better understand what inhibitors are beneficial in which subpopulations.
Many patients with severe respiratory disease due to COVID-19 have features consistent with cytokine release syndrome (CRS),13 also referred to as cytokine storm, which is related to increased activation of the JAK/STAT pathway.11 Unlike the systemic CRS that can be caused by chimeric antigen receptor T-cell therapy, CRS-like cytokine storm in COVID-19 predominantly occurs within the lungs.12 Predictive criteria for cytokine storm risk in COVID-19 were recently proposed, with potential to enable a tailored preventive approach by identifying patients at high risk.14 In the early stages of the development of treatment strategies for severe COVID-19 disease, it was suggested that host-directed therapies, including JAK inhibition and other immunotherapies, might be of benefit to patients with cytokine storm.15, 16, 17
Ruxolitinib is a potent and selective inhibitor of JAK1 and JAK2, approved for the treatment of myelofibrosis, polycythemia vera, and steroid-refractory acute graft-versus-host disease (USA only).18 Ruxolitinib reduces concentrations of inflammatory cytokines in disorders in which cytokine dysregulation plays a role, including graft-versus-host disease19 and hemophagocytic lymphohistiocytosis.20, 21, 22
The activity of ruxolitinib in CRS-related diseases warranted investigation of its use in patients with severe COVID-19 with clear symptoms and a positive test for SARS-CoV-2 without progression to intubation or need for ICU care. Furthermore, independent investigator-initiated studies revealed potential clinical benefit from the addition of ruxolitinib to best available therapy.23, 24 Here we report the primary analysis of RUXCOVID (NCT04362137), a global phase 3 study evaluating ruxolitinib plus standard of care versus placebo plus standard of care in hospitalised patients with COVID-19 not requiring invasive ventilation.
Methods
Study design
RUXCOVID was a randomised, double-blind, placebo-controlled, multicentre, phase 3 study (appendix p 13) evaluating the efficacy and safety of ruxolitinib plus standard of care versus placebo plus standard of care in patients with COVID-19.25 The study was conducted in 61 centres across 12 countries (Russia, USA, Brazil, Spain, Argentina, Peru, Turkey, Mexico, UK, Colombia, France, and Germany; appendix p 5). The study was approved by the institutional review board or central ethics committee at each participating institution and conducted in accordance with the Declaration of Helsinki.
Patients
Patients were required to be aged 12 years or older and hospitalised for confirmed COVID-19 (by PCR test or another rapid test from the respiratory tract). Additionally, patients had to meet at least one of: pulmonary infiltrates (chest x-ray or chest CT scan); respiratory frequency of at least 30 breaths per min; requiring supplementary oxygen; oxygen saturation of 94% or less on room air; or arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) of less than 300 mm Hg (40 kPa). Patients were excluded due to any of the following conditions: uncontrolled infection besides COVID-19; currently intubated or intubated between screening and randomisation; in ICU at time of randomisation; on antirejection, immunosuppressant, or immunomodulatory drugs (ie, tocilizumab, ruxolitinib, canakinumab, sarilumab, or anakinra); unable to ingest tablets at randomisation; pregnant or nursing. Full inclusion and exclusion criteria can be found in the appendix (p 3). As per other studies early in the pandemic,26, 27 inclusion was based on clinical criteria rather than hyperinflammation or cytokine storm because, at the time of study initiation, there were no clear cytokine-related criteria associated with COVID-19 that could have reliably been used. Eligible participants were only included in the study after informed consent was obtained, as approved by each institutional review board or independent ethics committee.
Randomisation and masking
Patients were randomly assigned (2:1) to receive oral ruxolitinib or oral matching-image placebo. Block randomisation, with a block size of 3, was used to decrease the risk of imbalance. Randomisation was stratified by geographical region (North America, western Europe, eastern Europe, Latin America, and other). Randomisation was done by interactive response technology. The investigator contacted the interactive response technology system, which assigned a randomisation number to each participant, linking them to their unique medication number. Medication numbers were automatically assigned to medication packs. Study treatments were identical in packaging, appearance, taste, and odour.
Participants, investigator staff, persons performing the assessments, and the clinical trial team remained masked throughout the trial. Unmasking occurred in the case of participant emergencies and at the conclusion of the study.
Procedures
Patients received oral ruxolitinib (Novartis Pharma AG, Stein, Switzerland) 5 mg twice per day or oral placebo twice per day, for 14 days. An additional 14 days of study drug was allowed if, in the opinion of the investigator, the patient's clinical signs and symptoms were not improving or worsened, and the potential benefit outweighed the risk.
Ruxolitinib 5 mg twice per day is the approved starting dose in the USA for treatment of steroid-refractory acute graft-versus-host disease with demonstrated anti-inflammatory effect.28 It is also the starting dose recommended for patients with myelofibrosis with a platelet count of 50 × 109 per L to less than 100 × 109 per L.18 Therefore, ruxolitinib 5 mg twice per day was included in this study.
Study treatment was given in combination with standard-of-care therapy according to the investigator's clinical judgment, with appropriate monitoring of potential drug–drug interactions. Permitted concomitant therapies included antivirals (including remdesivir), corticosteroids (including dexamethasone), heparin, anticoagulants, antiemetics, calcineurin inhibitors, azole fungal prophylaxis, broad-spectrum antibiotics, narcotics, and sedatives. Prohibited medications were other JAK inhibitors, aspirin (>150 mg/day), and fluconazole (>200 mg/day).
Dose reductions or interruptions were allowed in the case of drug toxicities (appendix p 2). If the patient became intubated during the study, an aqueous suspension of the study medication could be delivered via nasogastric tube. Hospitalised patients were assessed daily up to day 29 (end of study) for vital signs, oxygen saturation (SpO2), fraction of inspired oxygen (FiO2), consciousness, haematology (every other day), clinical chemistry (every other day), in-hospital outcomes, and biomarkers (day 7). Patients who were discharged during the study period were subsequently assessed daily up to day 29, via telephone, for clinical status, ventilatory status, adverse events, and previous or concomitant nondrug therapies. On the date of discharge, patients on oxygen by nasal cannula (≤2 L/min) were assessed for SpO2 on room air, based on investigator medical judgement. On days 15 and 29, discharged patients had all assessments performed in clinic.
Outcomes
The primary endpoint was a composite of death, respiratory failure (requiring invasive mechanical ventilation), or ICU care, by day 29.
Secondary efficacy endpoints included mortality rate by day 29, respiratory failure by day 29, ICU care by day 29 (post hoc), duration of hospitalisation, changes in clinical status, and changes in the National Early Warning Score 2 (NEWS2; appendix p 14). Changes in clinical status were measured using the COVID-19-specific 9-point (0–8) ordinal scale proposed by the WHO in February, 2020 (appendix pp 2, 6). Assessments included the proportion of patients with improved or deteriorated clinical status scores at day 29; time to 1 or more points of improvement from baseline; and mean change in the score from baseline at days 15 and 29. Changes in NEWS2 included time to discharge or NEWS2 score of 2 or less for 24 h, whichever came first; and change from baseline in NEWS2 score.
Exploratory efficacy endpoints included time to recovery (a post-hoc measure to allow comparison with the ACTT-2 study), independence from non-invasive ventilation, and oxygen therapy; duration of ICU stay, supplementary oxygen, and invasive mechanical ventilation; and ratio to baseline in concentrations of exploratory biomarkers, including C-reactive protein (CRP), ferritin, and D-dimer. Biomarker samples were analysed in central (for post-hoc measures of TNF, interferon [IFN]-γ, IL-10, IL-2RA, IL-6, IL-8) and local (ferritin, CRP, procalcitonin, IL-6 [if available], D-dimer) laboratories.
Treatment-emergent adverse events were defined as those occurring, or increasing in severity, between the first dose of study medication and the last study visit and were assessed and graded according to the Common Terminology Criteria for Adverse Events (version 5.0). The safety population included all patients who received at least one dose of study medication.
Statistical analysis
The study was designed to have at least 80% power to detect an absolute difference of 15% between the treatment groups in the proportion of patients meeting the primary endpoint (based on multiple sample size calculations assuming the rate of the primary outcome in the control group to be in the range of 30–80%)—the required sample size was 402 patients.
The primary endpoint was analysed by a logistic regression model with treatment group, region, baseline WHO (0–8) clinical status (≤3 or ≥4), age, and sex as covariates. The estimated odds ratio (OR; <1 favours ruxolitinib), p values, and 95% CIs were calculated. Retrieved dropout data after study treatment discontinuation were collected. If retrieved dropout data were available up to day 29, those were used for analysis. If no retrieved dropout data were collected after study treatment discontinuation, the retrieved dropout data were not complete to day 29, or patients withdrew from the study before day 29, then the patient was considered to meet the primary endpoint, unless they were in one of the following scenarios: there was no occurrence of death, mechanical ventilation, or ICU care in all the available data and patients were discharged from the hospital; or, the last available data (either on treatment or off treatment) were from day 15 or later and there was no occurrence of death, mechanical ventilation, or ICU care in all the available data.
A post-hoc analysis of the primary endpoint examined subgroups defined by baseline demographic and disease state parameters. The subgroup analyses were explored using the same logistic regression model as described for the primary analysis with the additional term of subgroup factor (if not already included in the model) and the interaction term of subgroup and treatment. No adjustment was made for multiplicity. Secondary and exploratory endpoints were similarly analysed without adjustments for multiplicity. Time to discharge or recovery was analysed using a proportional hazards model for competing risk analysis, which included treatment, region, age, sex, baseline WHO (0–8) clinical status, and the interaction term of baseline WHO (0-8) clinical status and treatment as covariates. Patients who were not discharged and did not die were censored at their last assessment date. Median (95% CI) times to discharge or recovery were estimated by the Kaplan-Meier method stratified by baseline clinical status, with dead patients being censored at the maximum follow-up time in the study. For both time to discharge and time to recovery, hazard ratio (HR) and 95% CIs were calculated. An HR of more than 1 favours ruxolitinib. This study was registered with ClinicalTrials.gov, NCT04362137.
Role of the funding source
The study was funded and designed by Novartis and Incyte. Data were analysed and interpreted by the funder in collaboration with all the authors; the funder was unaware of the treatment group assignments until database lock.
Results
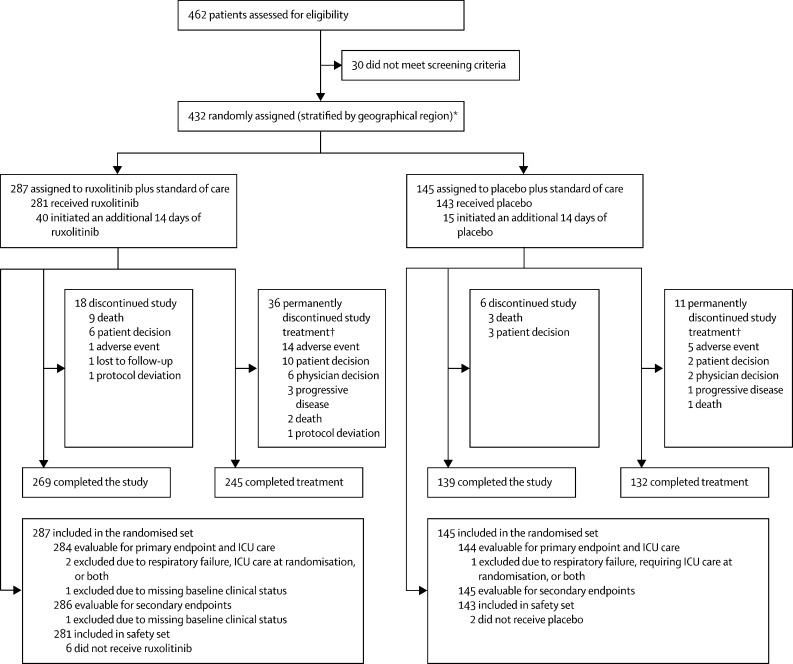
Between May 4 and Sept 19, 2020, 432 patients were randomly assigned (2:1) to receive ruxolitinib (n=287) plus standard of care or placebo (n=145) plus standard of care (randomised analysis set; figure 1 ). The greatest proportion of the 432 patients were from Russia (171 [40%]), followed by the USA (48 [11%]), Brazil (41 [9%]), and Spain (39 [9%]; appendix p 5 for all study sites). Patients who developed respiratory failure or required ICU care, or both, at randomisation (ruxolitinib n=2 and placebo n=1) were excluded from the primary efficacy analyses. The safety set comprised 424 patients who received at least one dose of study drug (ruxolitinib n=281 and placebo n=143).
Figure 1.
Trial profile
ICU=intensive care unit. *Eight patients were randomly assigned but did not receive treatment due to consent withdrawal (n=4), patient decision (n=3), and misrandomisation (n=1). †Includes patients who completed first course of 14-day treatment, but discontinued from second course of 14-day treatment.
Baseline demographics and disease characteristics were balanced between the two treatment groups (table 1 ). Mean patient age was 56·5 years (SD 13·3); 122 (28%) of 432 were aged 65 years or older and none were younger than 18 years (range 20–90). Most patients were White (351 [81%]), and nearly half (201 [47%] of 426) had a body-mass index (BMI) of more than 30 kg/m2. The median time between the onset of COVID-19 symptoms and randomisation was 11 days (IQR 8–14). Most patients had mild disease (WHO [0–8] clinical status of 3 [hospitalised, no oxygen support], 141 [33%] patients; WHO [0–8] clinical status of 4 [low-flow oxygen support], 268 [62%]); only 22 [5%] patients had severe disease (WHO [0–8] clinical status of 5 [non-invasive ventilation or high-flow oxygen support]). Most patients had pneumonia (428 [99%]). At baseline, 249 (58%) patients were receiving steroids and 28 (6%) were receiving remdesivir. Rates of concomitant therapy use at baseline by region (eg, antithrombotics, systemic steroids, remdesivir) are shown in the appendix (p 7). Concomitant therapy used at any time during the study is shown in the appendix (p 8).
Table 1.
Baseline patient demographics and disease characteristics
| Ruxolitinib (n=287) | Placebo (n=145) | Total (N=432) | ||
|---|---|---|---|---|
| Age, years | 56·4 (13·7; 22–90) | 56·9 (12·5; 20–84) | 56·5 (13·3; 20–90) | |
| Age category, ≥65 years | 83 (29%) | 39 (27%) | 122 (28%) | |
| Sex | ||||
| Female | 125 (44%) | 72 (50%) | 197 (46%) | |
| Male | 162 (56%) | 73 (50%) | 235 (54%) | |
| Race | ||||
| White | 242 (84%) | 109 (75%) | 351 (81%) | |
| American Indian or Alaska Native | 26 (9%) | 13 (9%) | 39 (9%) | |
| Black or African American | 6 (2%) | 9 (6%) | 15 (3%) | |
| Asian | 5 (2%) | 5 (3%) | 10 (2%) | |
| Multiple | 3 (1%) | 2 (1%) | 5 (1%) | |
| Unknown | 5 (2%) | 7 (5%) | 12 (3%) | |
| Ethnicity | ||||
| Hispanic or Latino | 93 (32%) | 39 (27%) | 132 (31%) | |
| Not Hispanic or Latino | 184 (64%) | 93 (64%) | 277 (64%) | |
| Not reported | 2 (1%) | 6 (4%) | 8 (2%) | |
| Unknown | 8 (3%) | 7 (5%) | 15 (3%) | |
| Weight, kg | 85·2 (18·8) | 87·2 (18·7) | 85·9 (18·8) | |
| n | 283 | 145 | 428 | |
| Body-mass index, kg/m2 | ||||
| n | 282 | 144 | 426 | |
| Mean (SD) | 29·9 (5·6) | 31·0 (6·5) | 30·3 (5·9) | |
| >30 kg/m2 | 129 (46%) | 72 (50%) | 201 (47%) | |
| Country | ||||
| Russia | 114 (40%) | 57 (39%) | 171 (40%) | |
| USA | 32 (11%) | 16 (11%) | 48 (11%) | |
| Brazil | 28 (10%) | 13 (9%) | 41 (9%) | |
| Spain | 29 (10%) | 10 (7%) | 39 (9%) | |
| Argentina | 16 (6%) | 11 (8%) | 27 (6%) | |
| Peru | 15 (5%) | 10 (7%) | 25 (6%) | |
| Turkey | 13 (5%) | 7 (5%) | 20 (5%) | |
| Mexico | 14 (5%) | 4 (3%) | 18 (4%) | |
| UK | 10 (3%) | 4 (3%) | 14 (3%) | |
| Colombia | 7 (2%) | 3 (2%) | 10 (2%) | |
| France | 4 (1%) | 6 (4%) | 10 (2%) | |
| Germany | 5 (2%) | 4 (3%) | 9 (2%) | |
| Time between onset of symptoms and randomisation, days | 11·0 (8·0–14·0) | 11·0 (8·0–13·5) | 11·0 (8·0–14·0) | |
| Time between diagnosis and randomisation, days | 5·0 (3·0–8·0) | 5·0 (3·0–7·0) | 5·0 (3·0–8·0) | |
| WHO (0–8) clinical status | ||||
| 3, hospitalised with mild disease (no oxygen therapy [defined as SpO2 ≥94% on room air]) | 94 (33%) | 47 (32%) | 141 (33%) | |
| 4, hospitalised with mild disease (oxygen by mask or nasal prongs) | 175 (61%) | 93 (64%) | 268 (62%) | |
| 5, hospitalised with severe disease (noninvasive ventilation or high-flow oxygen) | 17 (6%) | 5 (3%) | 22 (5%) | |
| Missing baseline clinical status | 1 (<1%) | 0 | 1 (<1%) | |
| Pneumonia | 284 (99%) | 144 (99%) | 428 (99%) | |
| Steroid use | 170 (59%) | 79 (54%) | 249 (58%) | |
| Remdesivir use | 21 (7%) | 7 (5%) | 28 (6%) | |
Data are mean (SD; range); n (%); mean (SD); n; or median (IQR). SpO2=oxygen saturation. WHO (0–8)=COVID-19-specific 9-point ordinal scale for clinical status proposed by WHO (appendix p 6).
The study failed to meet the primary objective (table 2 ). The composite endpoint of death, respiratory failure requiring invasive mechanical ventilation, or ICU care by day 29 occurred in 34 (12%) of 284 patients in the ruxolitinib group versus 17 (12%) of 144 patients in the placebo group (OR 0·91, 95% CI 0·48–1·73; p=0·77).
Table 2.
Primary, selected secondary, and exploratory efficacy outcomes
| Ruxolitinib (n=287) | Placebo (n=145) | Comparison (95% CI) | ||
|---|---|---|---|---|
| Primary endpoint | ||||
| Composite endpoint of death, respiratory failure requiring mechanical ventilation, or ICU care by day 29* | 34/284 (12%) | 17/144 (12%) | OR 0·91 (0·48–1·73); p=0·77 | |
| Secondary endpoints | ||||
| Mortality rate by day 29 | 9/286 (3%) | 3/145 (2%) | OR 1·21 (0·35–5·11) | |
| Respiratory failure by day 29* | 22/286 (8%) | 10/145 (7%) | OR 0·99 (0·45–2·21) | |
| ICU care by day 29*† | 30/284 (11%) | 17/144 (12%) | OR 0·81 (0·42–1·55) | |
| Change in WHO (0–8) clinical status at day 29‡ | ||||
| ≥1-point improvement | 261/286 (91%) | 136/145 (94%) | OR 0·79 (0·35–1·79) | |
| ≥2-point improvement | 252/286 (88%) | 129/145 (89%) | OR 1·00 (0·52–1·92) | |
| ≥1-point deterioration | 14/286 (5%) | 5/145 (3%) | OR 1·18 (0·40–3·49) | |
| Death by baseline clinical status by day 29†§ | ||||
| WHO (0–8) clinical status of 3 | 2/94 (2%) | 1/47 (2%) | OR 0·80 (0·10–9·53) | |
| WHO (0–8) clinical status of 4 | 7/175 (4%) | 2/93 (2%) | OR 1·35 (0·32–7·89) | |
| Duration of hospitalisation, days¶ | 9·0 (8·0–10·0) | 9·0 (8·0–12·0) | HR 1·04 (0·84–1·28) | |
| Time to hospital discharge or NEWS2 of ≤2 maintained for 24 h, days¶ | 4·0 (3·0–4·0) | 4·0 (3·0–5·0) | HR 1·02 (0·84–1·23) | |
| Exploratory endpoints | ||||
| Time to recovery (no longer infected, or ambulatory with no or minimal limitations), days†¶ | 8·0 (8·0–9·0) | 9·0 (7·0–11·0) | HR 1·10 (0·89–1·36) | |
| Time to independence from non-invasive ventilation, days | 19·0 (11·5–25·0) | 12·0 (9·0–22·0) | NA‖ | |
| Time to independence from supplementary oxygen, days | 5·5 (3·0–10·5) | 6·0 (3·0–10·0) | NA‖ | |
| Duration of ICU care, days | 9·0 (7·0–13·0) | 9·0 (4·0–21·0) | NA‖ | |
| Duration of supplementary oxygen, days | 5·0 (2·0–10·0) | 6·0 (3·0–10·0) | NA‖ | |
| Duration of invasive mechanical ventilation, days | 7·5 (5·0–16·0) | 12·0 (5·0–28·0) | NA‖ | |
Data are n/total number of patients included in the analysis (M [not model based]; [%]), median (95% CI), or median (IQR), unless otherwise specified. ORs are based on logistic regression models incorporating treatment group, region, baseline WHO (0–8) clinical status (≤3, ≥4), age, and sex as covariates. An OR of less than 1 means an event was less likely in the ruxolitinib group (which favoured ruxolitinib for all except the positive outcome events assessing ≥1-point or ≥2-point improvements in WHO (0–8) clinical status, in which an OR >1 favoured ruxolitinib). An HR of more than 1, representing higher instantaneous rates of discharge or recovery, favoured ruxolitinib. HR=hazard ratio. ICU=intensive care unit. NA=not analysed. NEWS2=National Early Warning Score 2. OR=odds ratio. WHO (0–8)=COVID-19-specific 9-point ordinal scale for clinical status proposed by WHO (appendix p 6).
Patients who developed respiratory failure or required ICU care, or both at randomisation are excluded from the analysis.
Post hoc.
Patients with missing data at day 29 were treated as non-responders.
There were no deaths in the ruxolitinib and placebo groups in patients with a baseline WHO (0–8) clinical status of 5.
Patients who did not have the event and did not die were censored at their last assessment date. Median is estimated by Kaplan-Meier method, with deaths being censored at the maximum follow-up time in the study.
Only summary statistics were conducted for these exploratory outcomes; all were evaluated on subsets of patients defined by post-baseline events, which could be confounded with treatment effect.
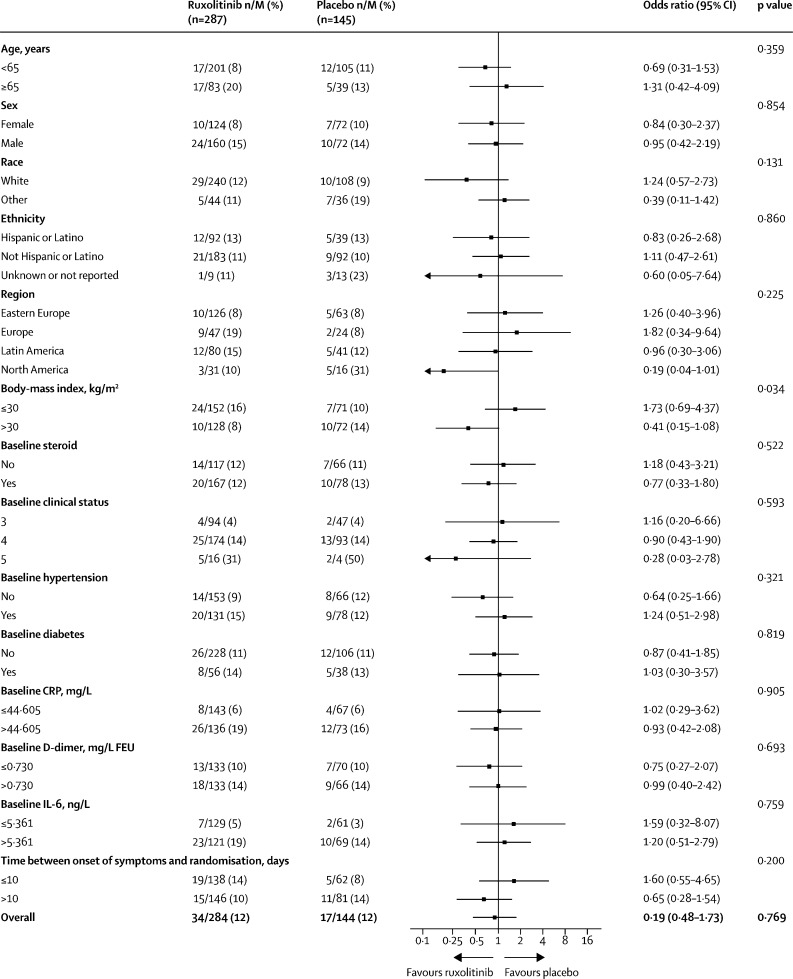
The subgroup analysis for the primary outcome (figure 2 ) revealed that for most subgroups, the proportions of patients who met the primary endpoint were similar between ruxolitinib and placebo. The strongest interaction between subgroup and primary endpoint in ruxolitinib versus placebo was for BMI (>30 kg/m2 vs ≤30 kg/m2; unadjusted p=0·034). Patients with a BMI of more than 30 kg/m2 had a better response with ruxolitinib versus placebo (OR 0·41, 95% CI 0·15–1·08); however, since the analysis was not adjusted for multiplicity, it should be interpreted with caution. Patients in North America had a better response with ruxolitinib versus placebo than patients in other regions (OR 0·19; 95% CI 0·04–1·01); however, this was driven by a high proportion of patients meeting the primary endpoint in the small placebo subgroup (five [31%] of 16). When assessed by baseline WHO (0–8) clinical status (3, 4, and 5), findings suggested that patients with a higher clinical status score (ie, more-severe disease) had a better response to ruxolitinib than those with a lower baseline clinical status (status 3 OR 1·16 [95% CI 0·20–6·66]; status 4 OR 0·90 [0·43–1·90]; status 5 OR 0·28 [0·03–2·78]). However, the sample size for patients with the most severe clinical status (score of 5) at baseline was small (n=20). Lower ORs were observed (which favoured ruxolitinib over placebo, with wide and overlapping CIs) in patients who were aged younger than 65 years (OR 0·69, 95% CI 0·31–1·53); used corticosteroids at baseline (OR 0·77, 0·33–1·80); had no hypertension at baseline (OR 0·64, 0·25–1·66); and had more than 10 days between onset of symptoms and randomisation (OR 0·65, 0·28–1·54). No subgroup analysis was done by remdesivir use because the proportion of patients receiving remdesivir was small (28 [6%] of 432 with baseline use and 49 [12%] of 424 with use at any time). An additional post-hoc analysis examined the effect of steroid treatment at any time during the study on the proportion of patients meeting the primary endpoint: among patients with any steroid use, 28 (14%) of 203 in the ruxolitinib group and 13 (13%) of 100 in the placebo group met the primary endpoint; among patients with no steroid use, seven (9%) of 82 in the ruxolitinib group and four (9%) of 44 in the placebo group met the primary endpoint. Note that these steroid use subgroups were defined partly by post-randomisation variables, and the subgroup memberships were influenced by treatments the patients received during the study. Thus, we cannot attribute any observed effect (or lack thereof) in this subgroup analysis to the investigational treatment since it could be due to differences in patient population.
Figure 2.
Primary endpoint (death, respiratory failure, or ICU care by day 29) according to subgroup analysis
ICU=intensive care unit. M=total number of patients included in the analysis. CRP=C-reactive protein. FEU=fibrinogen equivalent units.
The proportions of patients meeting the individual components of the primary endpoint were similar between the treatment groups (table 2). Change in WHO (0–8) clinical status over time was similar across treatment groups (appendix p 15) as were the median times to discharge and NEWS2 value of 2 or less maintained for 24 h (table 2). The median time to recovery was numerically shorter in the ruxolitinib group versus placebo group (8 days [95% CI 8–9] vs 9 days [7–11]; HR 1·10, 95% CI 0·89–1·36]; table 2). The difference in median time to recovery between ruxolitinib and placebo groups was numerically larger in patients with higher baseline WHO (0–8) clinical status scores (WHO [0–8] clinical status 3, 9 days vs 7 days; WHO [0–8] clinical status 4, 8 days vs 10 days; WHO [0–8] clinical status 5, 11 days vs 15 days; appendix p 9). Additional secondary endpoints are reported in the appendix (p 10).
The effect of treatment on inflammatory biomarkers (CRP, ferritin, D-dimer, procalcitonin, TNF, IFN-γ, IL-10, IL-2RA, IL-6, and IL-8) was also assessed. Over the 29 days of study, decreases were observed in the median concentrations of CRP (42·4 mg/L [IQR 16·6–93·2] to 3·1 mg/L [1·3–7·2] with ruxolitinib vs 45·0 mg/L [16·7–81·6] to 2·6 mg/L [1·0–5·7] with placebo), ferritin (628 μg/L [301–1276] to 254 μg/L [113–513] with ruxolitinib and 462 μg/L [264·5–999·5] to 200 μg/L [91–464] with placebo), and D-dimer (0·735 mg/L FEU [0·400–1·335] to 0·540 mg/L FEU [0·300–1·000] with ruxolitinib and 0·700 mg/L FEU [0·440–1·260] to 0·520 mg/L FEU [0·320–1·020] with placebo). Concentrations of IFN-γ, IL-10, IL-2RA (marker of T-cell activation), and IL-6 decreased (ie, improved) over time while IL-8, procalcitonin, and TNF concentrations did not. However, no appreciable difference in biomarker levels was observed between ruxolitinib and placebo groups.
Overall, 266 (63%) of 424 patients had an adverse event (appendix p 11). The most common treatment-emergent adverse events in the ruxolitinib versus placebo groups were headache (23 [8%] of 281 vs 11 [8%] of 143) and diarrhoea (21 [7%] of 281 vs 12 [8%] of 143; table 3 ). No meaningful differences in rates of adverse events were observed between treatment groups (any adverse events 173 [62%] of 281 with ruxolitinib vs 93 (65%) of 143 with placebo; and grade 3 or more adverse events, 35 (12%) of 281 with ruxolitinib vs 23 (16%) of 143 with placebo). Rates of infection and cytopenia, which were adverse events of special interest, were similar between the ruxolitinib and placebo treatment groups: infection (excluding tuberculosis), 24 (9%) of 281 versus 13 (9%) of 143; leukopenia, seven (2%) of 281 versus five [3%] of 143; anaemia, six (2%) of 281 versus one (1%) of 143; thrombocytopenia, three (1%) of 281 versus two (1%) of 143, respectively.
Table 3.
Frequent treatment-emergent adverse events (≥2% in any treatment group) by preferred term
| Ruxolitinib (n=281)* | Placebo (n=143) | ||
|---|---|---|---|
| Number of patients with ≥1 adverse event† | 173 (62%) | 93 (65%) | |
| Headache | 23 (8%) | 11 (8%) | |
| Diarrhoea | 21 (7%) | 12 (8%) | |
| Alanine aminotransferase increased | 17 (6%) | 6 (4%) | |
| COVID-19‡ | 12 (4%) | 3 (2%) | |
| Cough | 12 (4%) | 3 (2%) | |
| Fatigue | 10 (4%) | 2 (1%) | |
| Constipation | 9 (3%) | 7 (5%) | |
| Hypokalaemia | 8 (3%) | 7 (5%) | |
| Transaminases increased | 7 (2%) | 3 (2%) | |
| Anxiety | 6 (2%) | 1 (1%) | |
| Asthenia | 6 (2%) | 0 (0%) | |
| Hyperkalaemia | 6 (2%) | 6 (4%) | |
| Nausea | 6 (2%) | 11 (8%) | |
| Neutropenia | 6 (2%) | 4 (3%) | |
| Pyrexia | 6 (2%) | 2 (1%) | |
| Thrombocytosis | 6 (2%) | 3 (2%) | |
| Aspartate aminotransferase increased | 5 (2%) | 3 (2%) | |
| Hypoxia | 5 (2%) | 5 (3%) | |
| Abdominal pain | 4 (1%) | 4 (3%) | |
| Dyspnoea | 4 (1%) | 3 (2%) | |
| Hyperglycaemia | 4 (1%) | 5 (3%) | |
| Hypertension | 4 (1%) | 3 (2%) | |
| Hypoproteinaemia | 4 (1%) | 3 (2%) | |
| Leukocytosis | 4 (1%) | 4 (3%) | |
| Insomnia | 3 (1%) | 4 (3%) | |
| Urinary tract infection | 3 (1%) | 5 (3%) | |
| Dizziness | 2 (1%) | 4 (3%) | |
| Hyponatremia | 1 (<1%) | 3 (2%) | |
A patient with multiple adverse events within a preferred term is counted only once for that preferred term.
Preferred terms are presented in descending order of frequency in the RUX group.
A patient with multiple adverse events is counted only once.
COVID-19 relates to adverse events of worsening disease.
There were 46 (11%) of 424 patients who had serious adverse events (appendix p 12), with 45 (11%) of 424 patients having a serious adverse event with a grade 3 or more (31 [11%] of 281 in the ruxolitinib group, and 14 [10%] of 143 in the placebo group). 12 patients died during the study (nine [3%] of 281 patients in the ruxolitinib group and three [2%] of 143 patients in the placebo group); no deaths were considered related to treatment.
Discussion
RUXCOVID was a randomised, phase 3 study evaluating the safety and efficacy of ruxolitinib plus standard of care compared with placebo plus standard of care in patients with COVID-19. The study did not meet its primary objective, and ruxolitinib was not associated with clinically meaningful improvements versus placebo in the secondary or exploratory endpoints. Overall, clinical status and inflammatory biomarker levels improved over time and were similar in both treatment groups. Ruxolitinib was well tolerated, and rates of treatment-emergent adverse events and serious adverse events were comparable between groups. Findings suggested that patients with more severe disease and those with a high BMI had a better response to ruxolitinib.
It should be noted that the study was designed at a very early stage of the COVID-19 pandemic. The standard of care evolved rapidly, and current knowledge might have led to differences in the timing of dosing and in the inclusion and exclusion criteria for choosing patients most likely to benefit from immunomodulatory treatment of the COVID-19 cytokine storm.
The results from our study differ from those reported in the ACTT-2 study of baricitinib (a JAK1/JAK2 inhibitor) plus remdesivir.26 Several possible factors could account for these differences. Ruxolitinib and baricitinib inhibit JAK1 and JAK2 with similar potency,17 but potential differences in how downstream proteins such as STAT3 are impacted, especially in the presence of an antiviral drug, cannot be discounted. Levels of phosphorylated STAT3—which has an immunomodulatory role—were significantly greater in various immune cell types isolated from patients with COVID-19-related pneumonia and decreased after treatment with baricitinib in ACTT-2.26 Although ruxolitinib can also inhibit STAT3 phosphorylation,29 this was not specifically examined in the present study and could contribute to the observed differences. Study designs were different: the RUXCOVID study had a composite primary endpoint that included mortality, respiratory failure, and ICU care, whereas ACTT-2 had a primary endpoint of time to recovery. Differences in regions might also have affected outcomes. Most patients (953 [92%] of 1033) in the ACTT-2 study were treated in North America compared with only 11% (48/432) in the present study (in which the small North American subgroup appeared to do better with ruxolitinib). The variability in clinical settings, standards of care, and outcomes (such as variation in how patients are triaged to ICUs, with ICU care being a component of the composite endpoint) was probably higher in our study, and thus the sensitivity to detect a clinical effect of ruxolitinib might have been lower, even with the inclusion of region in the logistic regression model.
More patients had severe COVID-19 (eg, more patients requiring high-flow oxygen or non-invasive mechanical ventilation) in ACTT-2 than in the RUXCOVID study. In the present study, median time to recovery for ruxolitinib was 11 days versus 15 days with placebo (HR 1·51, 95% CI 0·44–5·19) in patients with more severe disease (WHO [0–8] score 5; non-invasive ventilation or high-flow oxygen); median time to recovery was lower for patients treated with baricitinib plus remdesivir than placebo plus remdesivir in this subgroup of the ACTT-2 study (10 days vs 18 days; rate ratio for recovery 1·51 [95% CI 1·10–2·08]). Median time to recovery was 8 days with ruxolitinib vs 9 days with placebo in the overall study (HR 1·10, 95% CI 0·89–1·36), similar to what was seen in the ACTT-2 study (7 days with baricitinib plus remdesivir vs 8 days with placebo plus remdesivir; rate ratio for recovery 1·16, 95% CI 1·01–1·32).
Findings from tocilizumab studies also suggested that patients with more severe disease are the most likely to benefit from treatment with immunomodulatory agents.30, 31 It is possible that patients with COVID-19 require treatment with an immunomodulatory agent in combination with antiviral medication. In ACTT-2, all patients received remdesivir in combination with baricitinib; however, in RUXCOVID, only 49 (12%) of 424 patients received remdesivir at any time during the study (appendix p 8). Additionally, baricitinib has been found to prevent viral entry through inhibition of numb-associated kinases (NAKs),32 which suggests that the mechanism of action in COVID-19 might be different from that of ruxolitinib, which does not substantially inhibit NAKs at tolerated doses.17
A further difference between the ACTT-2 study and RUXCOVID was the dose: the dose of ruxolitinib used in RUXCOVID (5 mg twice per day) was at the low end of the dosing range (5–25 mg twice per day), while that of baricitinib in ACTT-2 (4 mg per day) was at the high end of the dosing range (1–4 mg daily).18, 26 Ruxolitinib at a dose of 5 mg twice per day showed benefit in a phase 2 study of ruxolitinib in COVID-1924 and demonstrated efficacy in graft-versus-host disease.28 A further motivation for using this dose for the present study was to minimise the risk of cytopenia and infection (ultimately, these adverse events were not more prevalent in the ruxolitinib group in this study). Nevertheless, higher initial doses (≥10 mg twice per day) are routinely used in treating myelofibrosis and graft-versus-host disease,18 with a pronounced decrease in inflammatory cytokines observed.29
Although the design of our study was scientifically sound (a randomised, double-blind, placebo-controlled study), some limitations and weaknesses could not have been predicted at the time of the study design. The overall therapeutic landscape and standard of care changed substantially during the study, and this change might have impacted the proportion of patients meeting the primary endpoint in the control group. Remdesivir became the standard of care in the USA, but not in all countries where our study took place. More recent studies, like ACTT-2, demonstrated the benefit of combination therapy in this setting. Therefore, although the large geographical diversity of our study was a potential strength, making more generalisable conclusions possible, it might be a limitation due to geographical variation in standard of care.
Although no other studies have used ICU care as an outcome measure, the possibility of exceeding local ICU capacities was of urgent concern at the time of the study design. However, the timing and use of ICU care varied according to medical practice among centres, which might have impacted our results both by reducing the numbers of eligible patients on high-flow oxygen, who may or may not have been in ICU care, and by introducing variation in the determination of the primary endpoint. Given the inevitable uncertainty in designing studies in a global health crisis caused by a novel disease, futility analysis or sample-size re-estimation, or both, could be considered, especially when no earlier-phase trials have been done and it is not clear whether a drug will demonstrate clinical benefit or what the treatment effect could be.
Additionally, at the inception of the study, it was not known which patient groups might benefit the most from treatment. In RUXCOVID, patients were not screened for cytokine storm, and it was assumed that this was the major mechanism of pulmonary hyperinflammation. Patients with COVID-associated hyperinflammation have since been defined as those with a CRP of more than 150 mg/L or a ferritin level of more than 1500 μg/L, or both.33 In RUXCOVID, fewer than one-quarter of the patients met this criterion (based on the IQRs of CRP and ferritin). Recent studies suggest that patients with more-severe disease, including people with signs of hyperinflammation, might be the ones who benefit most from treatment with immunomodulatory agents. The DEVENT study evaluated ruxolitinib 5 mg twice per day and 15 mg twice per day versus placebo in patients with COVID-19 who required mechanical ventilation.25 The DEVENT study did not meet its primary endpoint: mortality up to day 29 in the two treatment groups versus placebo was 55% versus 74% (OR 0·42, 95% CI 0·171–1·023; p=0·028) in the 5-mg group and 52% versus 70% (OR 0·46, 95% CI 0·201–1·028; p=0·029) in the 15-mg group.34 These findings should be considered in the design of future studies. Moreover, there is a need to identify the subset of patients who would benefit the most from specific treatments, including treatment with immunomodulatory agents. Finally, because robust a priori sample-size estimates are unlikely to be possible in novel global health crises, we suggest that adaptive designs, including futility analyses and sample-size re-estimation, be built into future studies conducted under these conditions.
Data sharing
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com
Declaration of interests
IG, AG, ALB, AR, JS-O, FT, RT, and SS have no competing interests. MKH reports personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, United Therapeutics, UpToDate, Altesa Biopharma, Medscape, and Integrity. MKH has received either in kind research support or funds paid to the institution from the National Institutes of Health, Novartis, Sunovion, Nuvaira, Sanofi, AstraZeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association. MKH has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to their institution. MKH has received stock options from Meissa Vaccines and Altesa Biopharma. MA has participated in clinical studies funded by AbbVie, AstraZeneca, EMS, Eurofarma, GSK, Humanigen, Janssen, Novartis, Sanofi Genzyme, Angion Biomedica Corporation, and Beigene; has received honoraria from Aché, AstraZeneca, Chiesi, Eurofarma, IPI ASAC Brasil, and Sanofi; has received meeting or travel support from AstraZeneca, GSK, Novartis, and Sanofi Genzyme; and has participated in data safety monitor boards or advisory boards, or both, for Sanofi Genzyme, Chiesi, AstraZeneca, Abbott, and Zambom. JHF has received research funding from Novartis; grants from Boehringer Ingelheim and CSL-Behring; consulting fees from Novartis, Boehringer Ingelheim, and CSL-Behring; honoraria from AstraZeneca, Boehringer Ingelheim, CSL-Behring, GSK, MSD, and Novartis; meeting or travel support from AstraZeneca, Boehringer Ingelheim, CSL-Behring, and Novartis; and materials from CSL-Behring; and has participated in data safety monitor boards or advisory boards, or both, for Boehringer Ingelheim and AstraZeneca. LB, DSB, JMF, BK, WC, TL, and ML are employees and stockholders of Novartis. XS was an employee of Novartis during the conduct of the study. PL is an employee of Incyte.
Acknowledgments
Acknowledgments
We thank the patients and their families, investigators, and study site staff for their dedicated efforts contributing to the successful completion of this study, despite the challenges faced during the COVID-19 pandemic. This study was funded by Novartis and Incyte. The first draft of the manuscript was prepared by medical writers funded by Novartis, with guidance from the authors. The authors thank Amos Race and Karen Chinchilla (ArticulateScience, Hamilton, NJ, USA) for providing medical writing support, which was funded by Novartis, in accordance with Good Publication Practice guidelines.
Acknowledgments
Contributors
All authors contributed to the design of the study, or to the collection, analysis, or interpretation of study data, or to both. MKH and WC had full access to the raw data and verified it. All authors contributed to the drafting and revising of the manuscript and have approved the final version of the manuscript for publication. All authors are accountable for the content and integrity of the manuscript.
Supplementary Material
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) 2020. https://coronavirus.jhu.edu/map.html
- 3.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 5.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goker Bagca B, Biray Avci C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020;54:51–62. doi: 10.1016/j.cytogfr.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khadke S, Ahmed N, Ahmed N, et al. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol J. 2020;17:154. doi: 10.1186/s12985-020-01415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Liu X, Wu S, et al. Definition and risks of cytokine release syndrome in 11 critically ill COVID-19 patients with pneumonia: analysis of disease characteristics. J Infect Dis. 2020;222:1444–1451. doi: 10.1093/infdis/jiaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caricchio R, Gallucci M, Dass C, et al. Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis. 2021;80:88–95. doi: 10.1136/annrheumdis-2020-218323. [DOI] [PubMed] [Google Scholar]
- 15.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395:e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakafi (ruxolitinib) Incyte; Wilmington, DE: 2020. Prescribing information. [Google Scholar]
- 19.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29:2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 2019;6:e630–e637. doi: 10.1016/S2352-3026(19)30156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldsmith SR, Saif Ur Rehman S, Shirai CL, Vij K, DiPersio JF. Resolution of secondary hemophagocytic lymphohistiocytosis after treatment with the JAK1/2 inhibitor ruxolitinib. Blood Adv. 2019;3:4131–4135. doi: 10.1182/bloodadvances.2019000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keenan C, Nichols KE, Albeituni S. Use of the JAK inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistiocytosis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.614704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34:1805–1815. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137–146. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langmuir P, Yeleswaram S, Smith P, Knorr B, Squier P. Design of clinical trials evaluating ruxolitinib, a JAK1/JAK2 inhibitor, for treatment of COVID-19-associated cytokine storm. Del J Public Health. 2020;6:50–54. doi: 10.32481/djph.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135:1739–1749. doi: 10.1182/blood.2020004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stebbing J, Sánchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manson JJ, Crooks C, Naja M, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2:e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Incyte Press Release. March 18, 2021. https://www.businesswire.com/news/home/20210318005781/en/Incyte-Announces-Results-from-the-Phase-3-DEVENT-Study-Evaluating-Ruxolitinib-Jakafi%C2%AE-as-a-Treatment-for-Patients-with-COVID-19-Associated-Acute-Respiratory-Distress-Syndrome-ARDS-on-Mechanical-Ventilation
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com