Abstract
Objective.
Autoantibodies against proteins encoded by human endogenous retrovirus K (HERV-K) have been reported in patients with rheumatoid arthritis (RA), but their relevance, if any, has remained unresolved. We revisited this question and tested if such autoantibodies may react with citrullinated epitopes on the envelope (Env) protein of HERV-K.
Methods.
Immunoblotting and ELISAs were conducted with unmodified Env protein and with Env citrullinated by protein arginine deiminase 4 (PAD4). Sera from 100 patients with RA, plasma from 32 patients with juvenile idiopathic arthritis (JIA), and healthy adult and pediatric controls were included. Antibody reactivity was evaluated for correlations with clinical and laboratory variables of the patients.
Results.
We replicated and expanded upon published data suggesting that patients with RA or JIA have autoantibodies against HERV-K Env, some with high titers. Anti-HERV-K antibodies correlated with cigarette smoking and with circulating myeloperoxidase-DNA complexes indicative of nonapoptotic neutrophil cell death. Further, most of the patients with RA, but not those with JIA, had autoantibodies that reacted more strongly with Env that was citrullinated by PAD4. These anticitrullinated Env autoantibodies correlated with seropositivity and tended to be higher in patients with erosive disease.
Conclusion.
Our data suggest that anti-HERV-K immunity is elevated in RA and JIA and may have a connection with pathogenic protein citrullination in RA.
Key Indexing Terms: citrullination, endogenous retrovirus K, envelope, juvenile idiopathic arthritis, rheumatoid arthritis
A well-documented, but at the time, puzzling, discovery in the 1990s was that serum immunoglobulins from patients with rheumatoid arthritis (RA) or other autoimmune diseases1,2,3,4 often reacted with HIV proteins (e.g., p24 of the HIV capsid), even if these patients had never encountered the virus. Such HIV-reactive antibodies were found in exceedingly few healthy subjects, but reportedly in up to 60% of patients with RA. A likely answer to this conundrum was provided by the subsequent discovery5 that members of a family of HIV-related endogenous retroviruses in the human genome, particularly human endogenous retrovirus K (HERV-K),6 are transcriptionally activated in some patients with RA.7,8 This raised the possibility that HIV-reactive antibodies in patients are, in fact, antibodies against HERV-K proteins that have a sufficient degree of sequence homology with HIV proteins. Indeed, 2 papers9,10 reported that 19% of patients with RA have antibodies against an epitope in the HERV-K envelope (Env) protein (amino acids 19–37) and HERV-K gag, respectively.
A DNA copy of the RNA genome of HERV-K first entered our ancestral early hominid genome 32–44 million years ago11 and represents the only HERV family that has continued to infect our germline until as recently as 150,000 years ago,12 resulting in over 120 HERV-K provirus loci, some of which show insertional polymorphisms (i.e., only some people have them13,14,15) as well as polymorphic deletions.16 The most recent human insertions of the HERV-K subfamily termed HML-2 (human mouse mammary tumor virus–like 2; e.g., HERV-K113 on chromosome 19p12b17), are also intact enough to produce virions,18 albeit with poor infectivity. Another seemingly intact and young HERV-K provirus is located at Xq21.33 in approximately 2% of people, most of whom are of African ancestry.14 These youngest loci are transcriptionally silent in healthy individuals, but can be activated under certain circumstances, such as during very early embryonic development,19 in malignancies of the breast20 and prostate,21 and in HIV-infected individuals.22,23,24,25,26 Increased levels of HERV-K transcripts have also been detected in RA blood and synovial tissue.8,27
We sought to test the notion that reactivated HERV-K might contribute to the pathogenesis of RA by making 2 tentative assumptions: first, that reactivation of the youngest and most intact HERV-K locus would be more likely to cause immune pathology than expression of older and more “domesticated” loci with frame-shifts, point-mutations, and stop codons; and second, that “parasitic” genomic elements like HERV-K proviruses that are suppressed by DNA methylation and other epigenetic mechanisms are likely silent during the development of T and B cell antigen receptor repertoires in early life, resulting in weak immunological tolerance against the proteins that they encode. If so, aberrant expression of these proteins later in life would likely provoke both cellular and humoral immunity.28 We report that patients with RA indeed have autoantibodies that react well with the Env protein, and particularly well with citrullinated Env.
METHODS
Proteins and antibodies.
The extracellular portion of the HERV-K108 Env (Figure 1A) was purchased from Abcam (ab238358). cDNA encoding the surface (SU) and transmembrane (TM) portions of the Env protein of HERV-K_Xq21.33 (Figure 1A), both with an N-terminal 6xHis tag, were designed with codons optimized for prokaryotic expression and synthesized by Twist Biosciences in the pET28 expression plasmid. The proteins were expressed in transformed Escherichia coli and purified by Ni-NTA-agarose (Olympic Protein Technologies LLC). The SU protein was expressed and purified on a larger scale.
Figure 1.
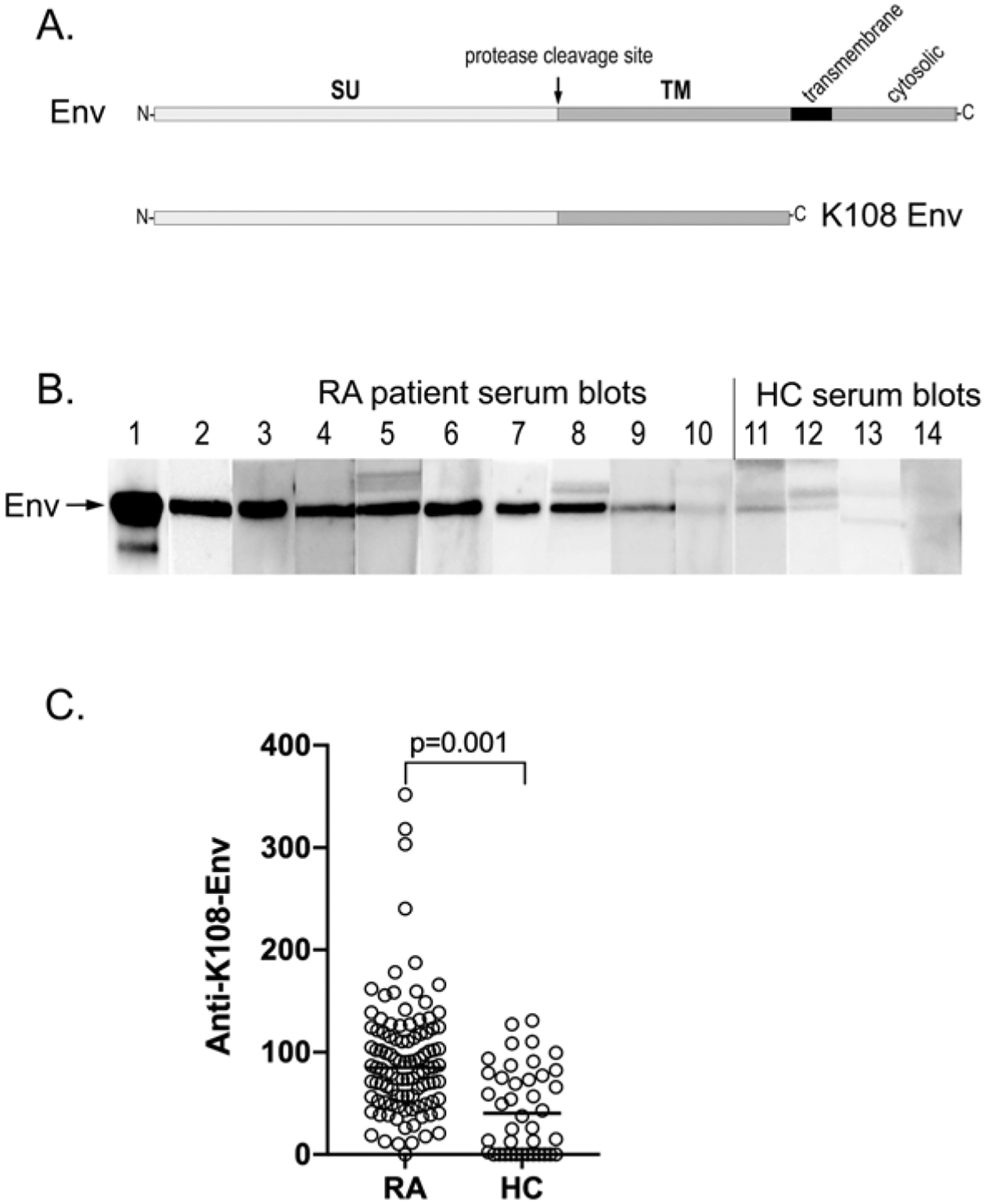
RA patient autoantibodies recognize HERV-K envelope protein. (A) Schematic representation of the HERV-K Env protein and the used extracellular portion of Env from HERV-K108. (B) Representative strip blots of HERV-K108 Env protein with 1:100 dilutions of sera from 10 RA patients and 4 healthy volunteers. (C) Quantitation of IgG autoantibodies against HERV-K108 Env (FL-Env) by ELISA in patients with RA (n = 100) and healthy volunteers (n = 40). The horizontal line in the graphs represents the median of each data set. Statistical significance was determined by the Mann-Whitney U test. Env: envelope; HC: healthy controls; HERV-K: human endogenous retrovirus K; RA: rheumatoid arthritis; SU: surface; TM: transmembrane.
Human subjects.
Sera from patients with RA (n = 100) and healthy controls (HCs; n = 40) were obtained from the University of Washington Rheumatology Biorepository and kept at −20 °C until use. All patients met American College of Rheumatology criteria for RA. The patient characteristics and clinical and serological variables of this cohort were described previously.29 The average age was 52.6 ± 14.0 years (females 52.1 ± 14.3 yrs, n = 61; males 54.6 ± 13.4 yrs, n = 18; n = 21 not recorded). Twelve of the patients had high disease activity (Clinical Disease Activity Index [CDAI] > 22), 15 were classified as moderate (CDAI > 10 to < 22), and 10 had mild disease (CDAI > 2.8–10). Fifty-nine patients were positive for anticitrullinated protein antibodies (ACPA). Seropositivity was defined as positivity for both ACPA and rheumatoid factor (RF). Healthy individuals (n = 40) of similar average age were used as controls (data not shown). Approval for this study was obtained from the University of Washington Institutional Review Board (STUDY00006196) and informed written consent was obtained from all participants according to the Declaration of Helsinki.
Plasma from a cohort of patients with juvenile idiopathic arthritis (JIA; n = 32) were from Seattle Children’s Hospital. The average age of this cohort was 13.9 ± 2.2 years. Twenty-four patients had active disease with an average of 3.3 involved joints, 13 had oligoarticular JIA, and 19 had polyarticular JIA; 2 patients in the latter category were ACPA-positive. Healthy children (n = 18) with a similar average age (12.3 ± 4.4 yrs) were used as controls. The study was approved by the Seattle Children’s Research Hospital Human Subjects Committee (PIROSTUDY14045). Informed written consent was obtained from the parents or guardians of all participants according to the Declaration of Helsinki.
Immunoblotting.
HERV-K108 Env protein, 100 ng per lane, was resolved by sodium dodecyl sulfate (SDS) gel electrophoresis, transferred to nitro-cellulose membranes, and cut into 12–15 strips. The membrane strips were immunoblotted with 1:100 diluted serum from patients or HCs and developed by horseradish peroxidase (HRP)-conjugated antihuman IgG and enhanced chemiluminescence.
ELISA.
Purified HERV-K108 Env or HERV-K_Xq21.33 Env-SU protein was adsorbed onto 96-well polystyrene plates at 50 ng/well in 0.1 M carbonate (pH 9.6) buffer overnight, washed in phosphate-buffered saline with Tween, and blocked in 2% bovine serum albumin (BSA) in phosphate-buffered saline for 2 hours. Control wells without Env-SU were also included for each patient. Patient, or HC, serum was added at 0.03% in blocking buffer for overnight incubation at 4 °C, washed extensively, and then incubated with 1:2000 dilution HRP-conjugated antihuman IgG. The reaction was then washed and developed with 3,3´,5,5´-tetramethylbenzidine (BioLegend), with the color reaction terminated with 2N sulfuric acid, and the absorbance measured at 450 nm using a plate reader (Synergy, BioTek). Background values of BSA-blocked wells without Env-SU were subtracted from values obtained in the presence of immobilized Env-SU. A dilution series from a specifically selected and highly reactive RA patient was included on every ELISA plate and used to normalize all data points. Measurements were made in triplicate, which were within 3.3 % (average 1.8%) of each other. Independent repeat ELISA data points were at most 19.4% different and generally much less.
Competition ELISAs.
To compare the autoantibodies against Env-SU reported here with the autoantibodies recognizing a dominant epitope peptide (VWVPGPTDDRCPAKPEEEG) from Env reported by Mameli et al,9 we performed 2 types of competition ELISAs. First, we coated wells with 50 ng of Env-SU, as described above, but added 1 μM of the peptide to half the replicates of the patient sera 30 minutes before adding them to the ELISA plates. Second, we coated wells with the peptide and added 50 ng Env-SU to half the serum samples 30 minutes before adding them to the plates.
ELISA for citrullinated HERV-K Env.
Autoantibodies reactive with citrullinated HERV-K_Xq21.33 Env-SU protein were measured by immobilizing Env on ELISA plates as above, washing, and blocking the plates with 1.5 μg/mL of poly(Glu, Lys, Tyr) 6:3:1, which lacks arginine residues and therefore cannot be citrullinated. We have previously shown that this protein can block nonspecific binding as well as BSA.29 Recombinant protein arginine deiminase 4 (PAD4), 250 ng, in 100 μl Tris-buffered saline, pH 7.7, 5 mM CaCl2, and 1 mM dithiothreitol, was added and incubated at 37 °C for 1 hour. After extensive washing, the plates were incubated with patient sera, washed, and developed as described above. Data were normalized using the same selected patient with RA as in the ELISA for anti-Env. Controls included wells without PAD4 but treated the same way, in addition to wells without Env-SU with or without PAD4 treatment. Wells without Env-SU but treated with PAD4 gave as low a background as untreated wells, indicating that residual PAD4 recognized by anti-PAD4 autoantibodies did not contribute to our results. As positive controls, fibrinogen and histone H3 were included in separate wells on the same ELISA plates. Reactivity against these proteins was low before and high after treatment with PAD4 (data not shown). RA patient-derived monoclonal ACPA 1325:04C03 was used to demonstrate that Env was citrullinated in our ELISAs.30
ELISA for circulating DNA-neutrophil myeloperoxidase complexes.
Myeloperoxidase (MPO)-DNA complexes (including neutrophil extracellular traps) were quantitated as before.31,32 Briefly, microtiter plates were coated with a mouse monoclonal anti-MPO antibody (4 μg/mL, Bio-Rad, clone 4A4), and then blocked with BSA. Plasma or serum (10%) was added and incubated overnight at 4 °C. Anti-dsDNA-HRP (diluted 1:100, Roche Diagnostics) was added for 2 hours. The reaction was developed, and absorbance was measured at 450 nm. Data were normalized using a standard curve with 1 U/mL equaling neutrophil extracellular traps released by 10,000 neutrophils.
Statistics.
For nonpaired sample sets with non-Gaussian distribution, Mann-Whitney U test and Spearman correlation test were used, as applicable. For paired sample sets, Wilcoxon matched-pairs signed-rank test was used. In some analyses, logistic regression analysis was used for dichotomized variables. Mean + 2 SD of the HCs was used as a cut-off for positivity. GraphPad Prism and IBM SPSS were used for the analyses. All analyses were considered statistically significant at P < 0.05.
RESULTS
Recognition of HERV-K Env by RA patient autoantibodies.
The Env gene of HERV-K108 encodes an 80-kDa type I transmembrane protein with a long extracellular N-terminal portion, a transmembrane α helix, and a short cytoplasmic domain (Figure 1A). We first used a commercially available Env protein from HERV-K108 consisting of its first 543 amino acid residues (the entire extracellular portion) to determine if RA patient serum contained IgG autoantibodies reactive with this protein. The protein was resolved on SDS gels and immunoblotted with 1:100 dilutions of sera from 73 patients with RA, which revealed that 47 patients indeed had such autoantibodies, some very strongly reactive (n = 16) and some modestly reactive (n = 31; Figure 1B). In contrast, most healthy volunteers had no such antibodies or, at most, weak reactivity (Figure 1B, lanes 11–14).
The purified HERV-K108 Env protein was also used to optimize ELISA conditions to allow for a better quantitation of anti-Env autoantibodies, which were found to be higher in patients with RA (n = 100) than in HCs (n = 40), albeit with a considerable overlap (Figure 1C). The difference was statistically significant by the Mann-Whitney U test (P = 0.001). Using the mean + 2 SD (128 U/mL) as a cut-off, 18% of the patients with RA were positive.
HERV-K proviruses are classified based on the presence (type 1) or absence (type 2) of a 292-bp deletion, which affects primary transcript splicing resulting in a different N-terminus of the respective Env proteins (Figure 2A). During the assembly of HERV-K virions, the Env protein undergoes a proteolytic cleavage step, resulting in 2 parts, termed SU and TM, which remain associated by a disulfide bond. To determine which of the 2 proteins patient antibodies recognize, we expressed the sequence of the SU protein shared by both type 1 and type 2 Env proteins (calculated molecular weight 42 kDa) and the 20-kDa extracellular portion of the TM protein (Figure 2B) with 6xHis tags in E. coli and immunoblotted bacterial lysates with patient serum, which revealed a strong reactivity with the SU protein (Figure 2C, lower panel), but none with the TM protein (Figure 2D, lower panel). Based on this result, we focused on the SU protein and purified milligrams of it by Ni-NTA-agarose affinity chromatography. The purified Env-SU protein was strongly detected by RA serum (Figure 2E).
Figure 2.
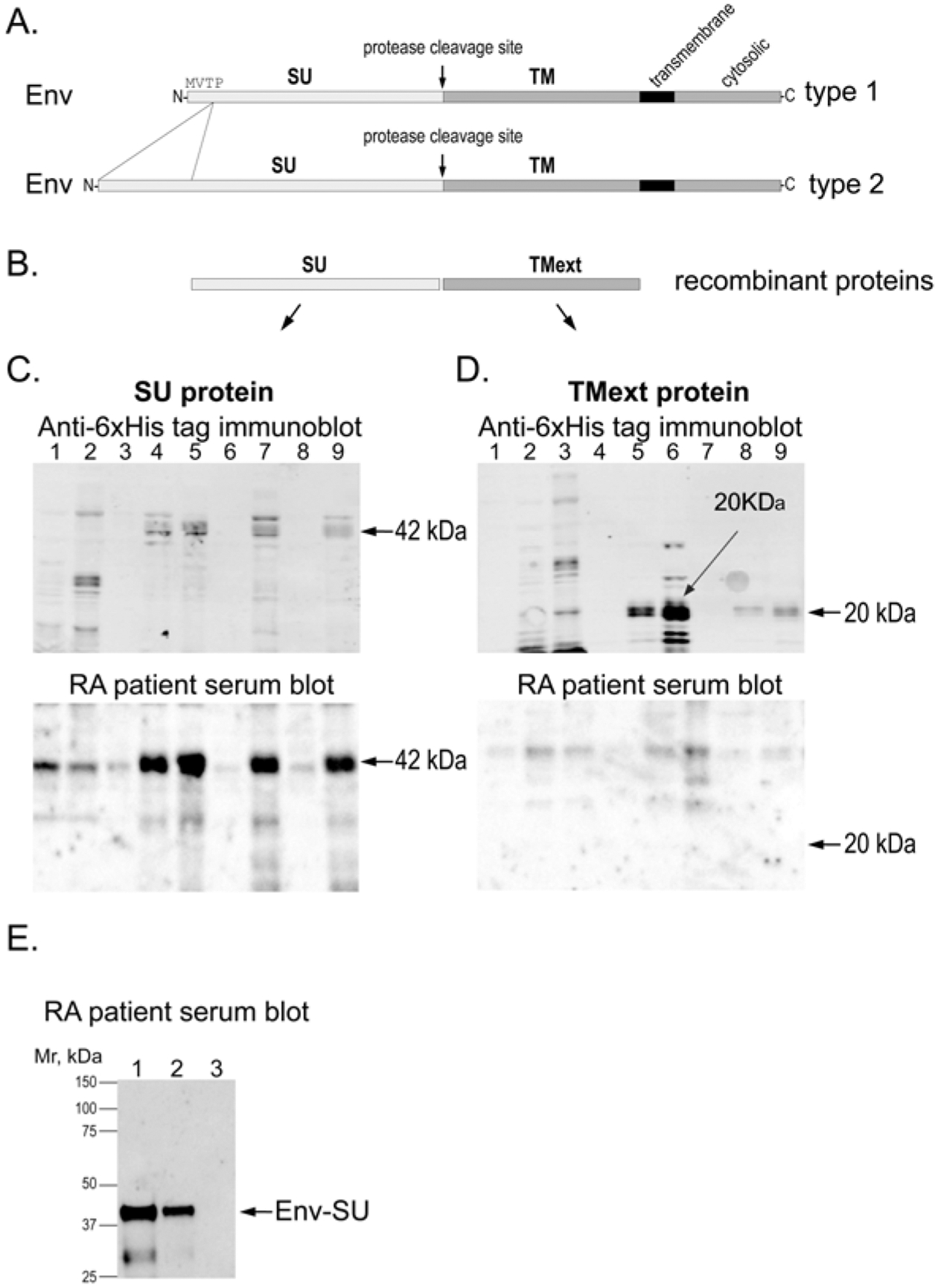
RA patient autoantibodies recognize the SU portion of HERV-K Env. (A) Schematic representation of type 1 and type 2 Env proteins. (B) The used recombinant SU and extracellular portion of TM (TMext) proteins from HERV-K_Xq21.33. (C) E. coli lysates expressing the SU portion of HERV-K Env protein immunoblotted with anti-6xHis tag antibody (upper panel) or RA patient serum (lower panel). (D) E. coli lysates expressing the extracellular region of the TM portion of HERV-K Env immunoblotted with anti-6xHis tag antibody (upper panel) or RA patient serum (lower panel). (E) Immunoblot of 1 μg (lane 1), 500 ng (lane 2), or 0 ng (lane 3) of purified HERV-K Env-SU with RA patient serum. The weaker lower band in lane 1 is a fragment of SU. E. coli: Escherichia coli; Env: envelope; HERV-K: human endogenous retrovirus K; RA: rheumatoid arthritis; SU: surface; TM: transmembrane.
Anti-Env-SU autoantibodies in adult patients with RA and in children with JIA.
ELISAs with the Env-SU protein showed that patients with RA had elevated IgG antibodies against this part of the Env protein (P = 0.0018; Figure 3A), with a similar pattern as was seen with the whole extracellular part of Env (Figure 1). Although the normalized values cannot be directly compared, the overall pattern appeared a bit lower, perhaps due to differences in accessibility of some epitopes between less denatured plastic-bound and fully denatured nitrocellulose-bound proteins. Another possibility is that the 5 amino acid residues that differed between the commercial HERV-K108 and our SU construct based on HERV-K_Xq21.33 resulted in some differences in autoantibody reactivity. There may also have been a small number of patients with autoantibodies principally against the TM portion of Env. Indeed, a minority of patients had autoantibodies that recognized Env-TM as assessed by immunoblots (data not shown).
Figure 3.
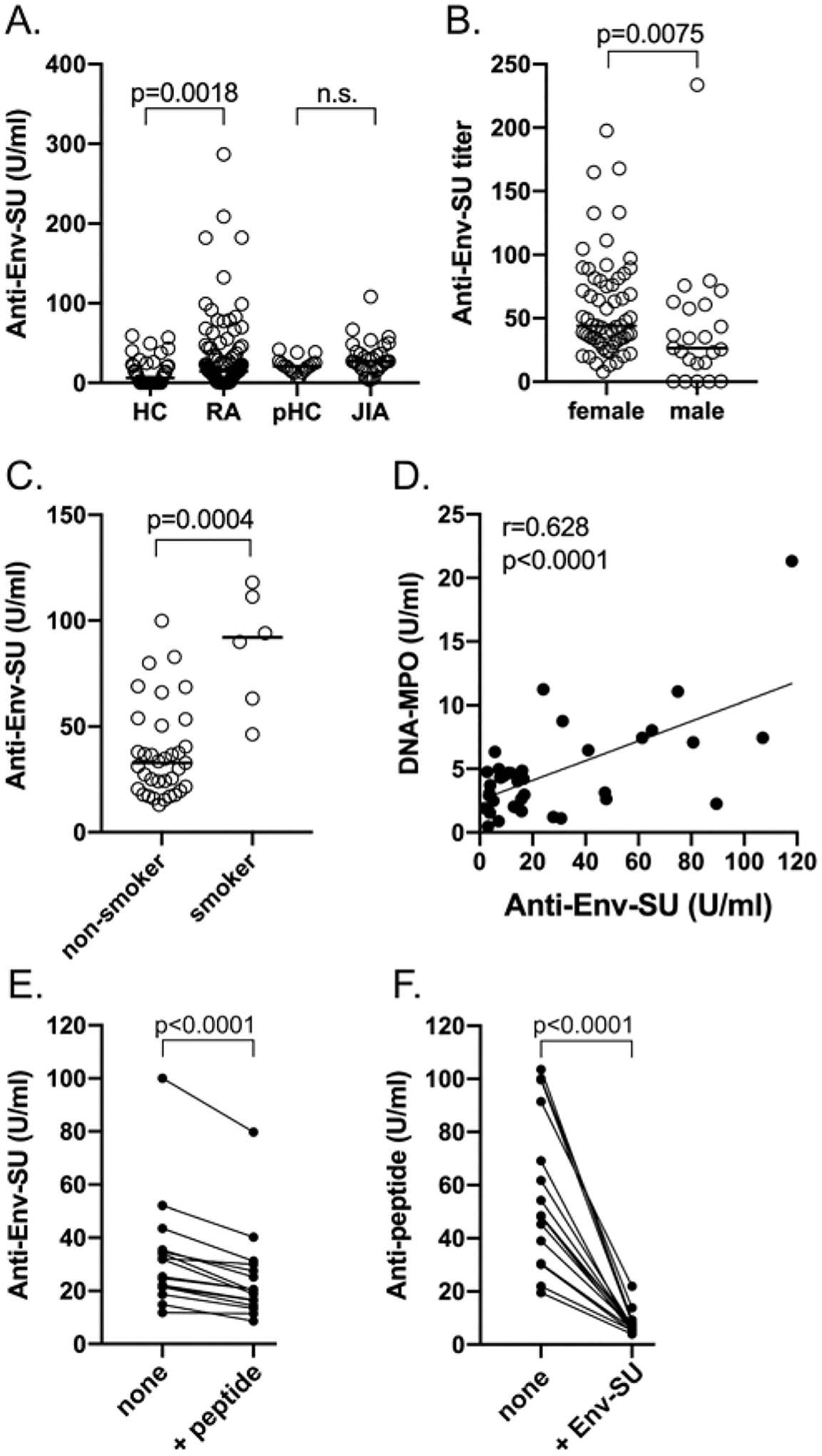
ELISA for RA patient autoantibodies against the SU portion of HERV-K Env. (A) Quantitation of IgG autoantibodies against Env-SU by ELISA in HCs, adult patients with RA (n = 53), pediatric HCs (n = 18), and JIA patients (n = 32). (B) Distribution of anti-Env-SU autoantibodies in RA patients (n = 84) by sex (males n = 23, females n = 61). (C) Current smokers (n = 6) have higher anti-Env-SU autoantibodies than RA patients who are not currently smokers (n = 32). (D) Correlation between anti-Env-SU autoantibodies and circulating MPO-DNA complexes indicative of recent nonapoptotic neutrophil death (n = 38). (E) Anti-Env-SU reactivity in the absence or presence of 1 μM of the Env peptide VWVPGPTDDRCPAKPEEEG (n = 16). (F) IgG antibodies reactive with the same peptide in the absence or presence of 50 ng of Env-SU (n = 16). Statistical significance was determined by the Mann-Whitney U test and Spearman correlation test (D), and the Wilcoxon matched-pairs signed-rank test ([E] and [F]). Env: envelope; HC: healthy controls; HERV-K: human endogenous retrovirus K; JIA: juvenile idiopathic arthritis; MPO: myeloperoxidase; pHC: pediatric HCs; RA: rheumatoid arthritis; SU: surface; TM: transmembrane.
A cohort of 32 patients diagnosed with JIA was also assessed for anti-Env-SU autoantibodies (Figure 2A). The patients with JIA had marginally elevated anti-Env reactivity, which was not statistically different from the pediatric HCs. With the mean + 2 SD of the pediatric controls as a cut-off, only 6 of the patients with JIA were positive. Together, these data indicate that a subset of patients with RA or JIA have IgG autoantibodies that can recognize denatured, nonglycosylated HERV-K Env-SU.
Anti-Env correlate with patient sex, ACPA status, smoking, and in vivo neutrophil cell death.
Anti-Env reactivity was higher in female than in male patients with RA (P = 0.0075; Figure 3B); in active cigarette smokers (P = 0.0004; Figure 3C); in patients with higher amounts of serum MPO-DNA complexes (P < 0.0001; Figure 3D), a sign of ongoing death of neutrophils by extrusion of extracellular traps; and in ACPA-positive patients, albeit not statistically significant (data not shown). There were also trends toward association with CDAI, presence of RF, and erythrocyte sedimentation rate, but these did not reach statistical significance.
Comparison with previously reported anti-Env autoantibodies.
Mameli and et al9 reported that 19% of patients with RA have IgG autoantibodies that recognize a synthetic peptide representing a computationally predicted epitope in the SU portion of the HERV-K Env protein. We used this peptide in competition experiments and found that it was able to reduce reactivity of RA serum IgG autoantibodies with Env-SU by 0–20% in a subset of 16 patients (Figure 3E). Conversely, antibodies reactive with the immobilized peptide were competed out by Env-SU (which contains this sequence with only 1 amino acid difference) to 80% in 1 and over 90% in the other patients (Figure 3F). These results support the notion that some anti-Env autoantibodies indeed recognize this epitope, but they represent a minority of the anti-Env autoantibody repertoire.
Patients with RA have higher autoantibody reactivity against citrullinated HERV-K Env.
Since RA autoantibodies often recognize citrullinated epitopes, we tested whether citrullinated Env-SU would be differentially recognized by RA patient autoantibodies (Figure 4). To this end, we immobilized Env-SU on the ELISA plates, blocked all remaining protein-binding capacity with a protein that cannot be citrullinated, the 20–50 kDa poly(Glu, Lys, Tyr) 6:3:1, and treated the plates with or without PAD4 for 60 minutes, followed by extensive washing. To directly demonstrate that immobilized Env was indeed citrullinated by soluble PAD4, we used a broadly reactive RA patient monoclonal ACPA,30 which recognized Env in ELISA wells treated with PAD4, but not untreated Env, and not blocked wells without Env treated with PAD4 (Figure 4F). The subsequent ELISA revealed that patient IgG autoantibodies reacted considerably better with citrullinated Env-SU than with untreated Env-SU in most patients (Figure 4A). Interestingly, serum from healthy donors also contained IgG that reacted better with citrullinated Env-SU than with untreated Env-SU, but the titers were mostly much lower (Figure 4B). There were 2 patterns among the patients: those with clearly higher anticitrullinated Env reactivity compared to their anti-Env-SU reactivity, suggesting that most of their autoantibodies only recognized citrullinated epitopes; and those with similar reactivity against unmodified and citrullinated Env-SU (Figures 4A,C), suggesting that the antibodies mostly recognized epitopes that were not citrullinated. Stratifying the patients by seropositivity vs seronegativity revealed that the titers were increased in both subgroups, but that the difference became statistically significant (P = 0.03) only in the citrullinated Env-SU group (Figure 4D).
Figure 4.
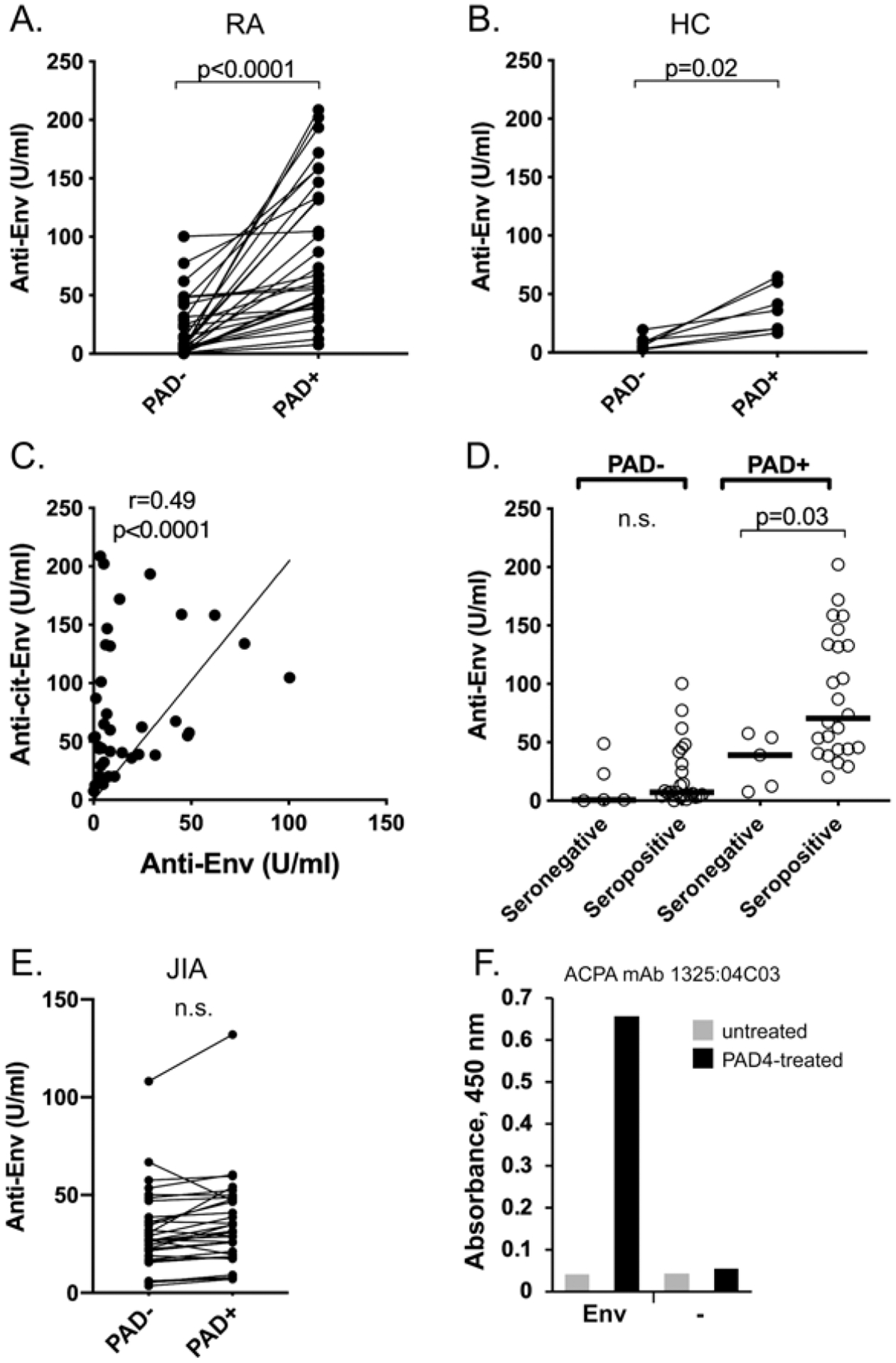
Many patients with RA have higher titers of autoantibodies recognizing citrullinated Env protein compared to unmodified Env. (A) Titers in patients with RA (n = 40) against HERV-K Env treated with buffer alone (PAD–) or with PAD4 for 1 hour at 37 °C (PAD+). Each individual patient is connected with a line between the 2 data points. (B) Titers in healthy subjects (n = 7) against HERV-K Env treated with buffer alone (PAD–) or with PAD4 for 1 hour at 37 °C (PAD+). Each individual subject is connected with a line between the 2 data points. (C) Relation between autoantibodies against unmodified (X-axis) and citrullinated (Y-axis) Env in each patient with RA. (D) Segregation by seronegativity (n = 5) vs seropositivity (n = 24) of autoantibodies against unmodified (PAD–) or citrullinated (PAD+) Env. The horizontal line in the graphs represents the median of each dataset. (E) Titers in patients with JIA (n = 32) against Env treated with buffer alone (PAD–) or with PAD4 (PAD+). (F) ELISA with detection by the patient-derived monoclonal ACPA 1325:04C03 of wells coated with Env, or only blocked, and treated with PAD4 (black bars) or citrullination buffer alone (gray bars). Statistical significance was calculated with the Wilcoxon matched-pairs signed-rank test ([A] and [B]), Spearman correlation test (C), and Mann-Whitney U test (D). ACPA: anticitrullinated protein antibodies; Anti-cit-Env: anticitrullinated Env; Env: envelope; HC: healthy controls; HERV-K: human endogenous retrovirus K; JIA: juvenile idiopathic arthritis; PAD: protein arginine deiminase; RA: rheumatoid arthritis; SU: surface; TM: transmembrane.
In contrast to the adult patients with RA, only 2 children with JIA showed any increase in reactivity against Env when it was citrullinated (Figure 4E). In most of them, the reactivity was essentially unchanged and in 2, it was decreased. As in the adults, the low reactivity in healthy children also did not change significantly upon citrullination of Env (data not shown).
As positive controls to ensure that PAD4 was catalytically active, we carried out ELISAs in which wells were coated with histone H3 (Figure 4F) or fibrinogen (data not shown) instead of Env. Serum IgG from 4 ACPA-positive RA patients recognized these well-known autoantibody targets particularly well after treatment with PAD4.
Clinical correlations in the full RA cohort.
Based on the data described above, we decided to run ELISAs on the entire RA cohort for which clinical variables, laboratory measures, and treatment history exists.29 As shown in Figure 5A, the difference between healthy subjects and patients with RA was statistically significant (P < 0.0001 by the Mann-Whitney U test). Using the mean + 2 SD of the HC dataset (66.7 U/mL) as a cut-off, 55% of the patients with RA were positive. Further, the difference between ACPA-positive and -negative patients was significant (P = 0.0007; Figure 5B), as was the differences between RF-positive and -negative patients (P = 0.03; Figure 5C). Figure 5D shows a weak correlation between anticitrullinated Env titers and ACPA titers (cyclic citrullinated peptide test) reported in patients’ medical records, suggesting that anticitrullinated Env reactivity does not solely represent cross-reacting nonselective ACPA. Last, anticitrullinated Env reactivity was also somewhat higher in patients with joint erosions than in those without, but this did not quite reach statistical significance (Figure 5E).
Figure 5.
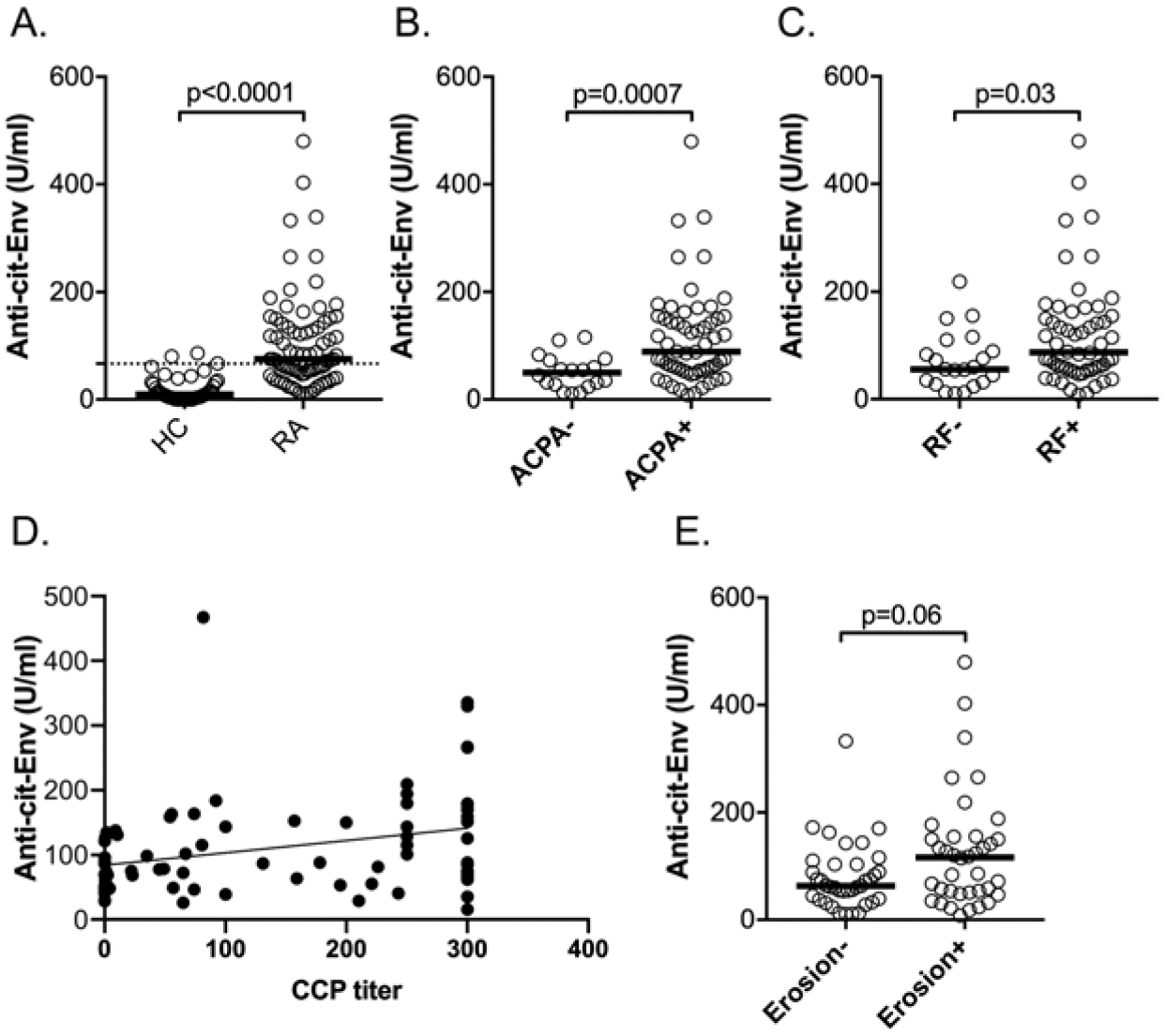
Autoantibodies against citrullinated Env in the full RA cohort. (A) Titers of autoantibodies against citrullinated Env in HC (n = 40) and the full cohort of patients with RA (n = 100). The mean + 2 SD of the HC data set is indicated by a dotted line. (B) Segregation of anticitrullinated Env titers by ACPA status (positive: n = 58; negative: n = 18). (C) Correlation of anticitrullinated Env titers with RF status (positive: n = 56; negative n = 21). (D) Correlation between anticitrullinated Env titers (Y-axis) and ACPA titer reported in patient records. (E) Anticitrullinated Env antibody titers in patients with (n = 38) or without (n = 37) radiographic erosions. Note that the difference does not reach statistical significance. The horizontal lines in the graphs represent the median. Statistical significance was calculated using the Mann-Whitney U test. ACPA: anticitrullinated protein antibodies; Anti-cit-Env: anticitrullinated Env; CCP: cyclic citrullinated peptide; Env: envelope; HC: healthy control; HERV-K: human endogenous retrovirus K; PAD: protein arginine deiminase; RA: rheumatoid arthritis; RF: rheumatoid factor; SU: surface; TM: transmembrane.
DISCUSSION
The molecular mechanisms that underpin the pathogenesis of RA remain incompletely understood. The presence of ACPA in 70–80% of patients33 is a unique feature of RA34,35 and commonly used in its diagnosis. A pathogenic role of autoimmunity against citrullinated epitopes is also suggested by the genetic association of RA with polymorphisms in the genes for 2 of the citrullinating enzymes, PAD2 and PAD4.36,37,38,39 However, it still remains unclear how and when these 2 enzymes cross the line between physiological citrullination and the quantitatively or qualitatively abnormal modification of self-proteins to create immune-reactive epitopes that drive an autoimmune response.40,41,42
Our finding that many patients with RA have IgG autoantibodies that recognize the Env protein of HERV-K suggests that one or several of the HERV-K loci that encode this protein are, or recently were, transcriptionally active and translated into immunogenic protein in the antibody-positive patients. In this context, it is interesting to note that children with JIA had only marginally elevated anti-Env reactivity compared to adult patients with RA. However, nearly all but 2 of the patients with JIA were ACPA-negative and none of them had higher reactivity against citrullinated Env. These findings suggest that anti-HERV-K immunity may be an early feature of autoimmune arthritis, whereas anticitrullinated Env autoantibodies are more closely associated with ACPA-positive RA.
Clearly, humoral and cellular autoimmunity against HERV-K proteins, as it occurs in HIV-infected individuals and in patients with breast cancer,26,43,44 is not sufficient by itself to cause RA. At a minimum, other events and factors must participate. For example, the right MHC alleles may be needed for such autoimmunity to result in ACPA-positive RA. Many other predisposing genetic variants (e.g., of PTPN22)45 may also need to be present for anti-HERV-K immunity to tip the balance toward RA pathogenesis. The magnitude of HERV-K expression, its duration and tissue location(s), and the cell surface exposure of HERV-K proteins likely affect the nature of this immunity. Arguably, autoimmunity against HERV-K expressed in a breast cancer cell, or in an HIV-infected T lymphocyte, is likely to be beneficial to the patient. In contrast, autoimmunity against HERV-K–derived proteins in other tissues—for example, the synovium—are more likely to be detrimental.
In our study, autoantibodies that specifically recognize citrullinated Env correlated more clearly with ACPA and seropositivity and showed a trend toward correlation with more aggressive diseases. However, ACPA titers did not correlate closely with anticitrullinated Env autoantibodies and some patients with the latter were ACPA-negative, suggesting that anticitrullinated Env may not simply be broadly reactive ACPA, although we cannot rule out this possibility. A comparison of known citrullination sites with the sequence surrounding the 21 arginine residues in Env-SU only revealed 1 instance of arginine-glycine (Arg-Gly) and 1 of Gly-Arg, which are seen in some other citrullinated proteins. While the latter would be predicted to be recognized by RA patient-derived monoclonal ACPA 1325:04C03,30 future work will determine if this Arg residue is indeed citrullinated. At present, it also remains unclear if and how Env-TM may become citrullinated in patients with RA, but a plausible mechanism may involve the surface exposure of catalytically active PAD4 and secretion of active PAD2 by human neutrophils.46 Another viral protein, Epstein-Barr virus nuclear antigen 1, is also known to be citrullinated and then recognized by ACPA in patients with RA.47
An intriguing feature of HERV-K as a potential player in RA, which is a female-dominated disease with a 4:1 female–male ratio, is the strong effect of estrogen and progesterone on its expression, particularly in breast cancer cells.48,49 Indeed, we observed somewhat higher anti-Env reactivity in female patients with RA compared to males (Figure 3B). The correlation between anti-Env autoantibodies and smoking (Fig 3D) could also reflect the increased expression of HERV-K by cigarette smoking.50,51 Smoking is also a well-recognized risk factor for RA.
The presence of autoantibodies against citrullinated Env may implicate another potential mechanism by which autoimmunity against citrullinated Env may promote RA development, namely molecular mimicry. In this “modified molecular mimicry” case, citrullinated epitopes in Env may have sufficient sequence similarity to citrullinated epitopes in other proteins for anticitrullinated Env autoantibodies to cross-react with these self-proteins. This possibility should be explored.
We also note that autoantibodies against HERV-K Env, either unmodified or citrullinated, could serve as a biomarker for more aggressive disease. However, demonstrating their utility in this respect will require testing in larger, ideally longitudinal, studies. We also believe that a deeper understanding of the molecular events that lead to these autoantibodies and their possible involvement in initiating, perpetuating, or shaping RA will be required to uncover their true value as biomarkers in clinical practice.
In this paper we extend prior knowledge of autoantibodies in patients with arthritis against the Env protein of the HERV-K (HML-2) endogenous retrovirus family in several important ways: by using proteins encompassing most of the Env protein, rather than selected peptides, and both immunoblots and ELISA, we found that a portion of patients with RA are positive, but that no more than 20% of the anti-Env autoantibodies recognize the previously reported epitope. Even more importantly, we find that many patients with RA have higher titers of autoantibodies for Env when it is first citrullinated. Together, these findings raise the possibility that expression of endogenous retroviral proteins provoke a citrullination-dependent immune response that may be important in RA pathogenesis.
ACKNOWLEDGMENT
We thank Caroline Grönwall and Vivianne Malmström for the patient-derived monoclonal ACPA. We also thank the patients who participated in this study.
This work was supported by National Institutes of Health (R01 AR074939, R21 AR075134, and R21 AR077266 to TM and T32 AR007108 to KCU), and by Lupus Research Alliance (grant number 519414 to CL).
Footnotes
TM has received consulting and advisory board fees from Glysantis, Kiniksa, Cugene, QiLu, and Miro Bio. MAB is an employee of Allogene Therapeutics, and AS is an employee of Janssen Research and Development. The remaining authors declare no conflicts of interest relevant to this article.
REFERENCES
- 1.Talal N, Dauphinee MJ, Dang H, Alexander SS, Hart DJ, Garry RF. Detection of serum antibodies to retroviral proteins in patients with primary Sjögren’s syndrome (autoimmune exocrinopathy). Arthritis Rheum 1990;33:774–81. [DOI] [PubMed] [Google Scholar]
- 2.Fraziano M, Montesano C, Lombardi VR, et al. Epitope specificity of anti-HIV antibodies in human and murine autoimmune diseases. AIDS Res Hum Retroviruses 1996;12:491–6. [DOI] [PubMed] [Google Scholar]
- 3.Dang H, Dauphinee MJ, Talal N, et al. Serum antibody to retroviral gag proteins in systemic sclerosis. Arthritis Rheum 1991;34:1336–7. [DOI] [PubMed] [Google Scholar]
- 4.Talal N, Garry RF, Schur PH, et al. A conserved idiotype and antibodies to retroviral proteins in systemic lupus erythematosus. J Clin Invest 1990;85:1866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson PN, Lever AM, Smith S, et al. Molecular investigations implicate human endogenous retroviruses as mediators of anti-retroviral antibodies in autoimmune rheumatic disease. Immunol Invest 1999;28:277–89. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Montojo M, Doucet-O’Hare T, Henderson L, Nath A. Human endogenous retrovirus-K (HML-2): a comprehensive review. Crit Rev Microbiol 2018;44:715–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freimanis G, Hooley P, Ejtehadi HD, et al. A role for human endogenous retrovirus-K (HML-2) in rheumatoid arthritis: investigating mechanisms of pathogenesis. Clin Exp Immunol 2010;160:340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynier F, Verjat T, Turrel F, et al. Increase in human endogenous retrovirus HERV-K (HML-2) viral load in active rheumatoid arthritis. Scand J Immunol 2009;70:295–9. [DOI] [PubMed] [Google Scholar]
- 9.Mameli G, Erre GL, Caggiu E, et al. Identification of a HERV-K Env surface peptide highly recognized in rheumatoid arthritis (RA) patients: a cross-sectional case-control study. Clin Exp Immunol 2017;189:127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson PN, Roden D, Nevill A, et al. Rheumatoid arthritis is associated with IgG antibodies to human endogenous retrovirus gag matrix: a potential pathogenic mechanism of disease? J Rheumatol 2014;41:1952–60. [DOI] [PubMed] [Google Scholar]
- 11.Hohn O, Hanke K, Bannert N. HERV-K(HML-2), the best preserved family of HERVs: endogenization, expression, and implications in health and disease. Front Oncol 2013;3:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jha AR, Nixon DF, Rosenberg MG, et al. Human endogenous retrovirus K106 (HERV-K106) was infectious after the emergence of anatomically modern humans. PLoS One 2011;6:e20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahyo T, Yamada H, Tao H, Kurabe N, Sugimura H. Insertionally polymorphic sites of human endogenous retrovirus-K (HML-2) with long target site duplications. BMC Genomics 2017;18:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wildschutte JH, Williams ZH, Montesion M, Subramanian RP, Kidd JM, Coffin JM. Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc Natl Acad Sci U S A 2016;113:E2326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belshaw R, Dawson AL, Woolven-Allen J, Redding J, Burt A, Tristem M. Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family HERV-K(HML2): implications for present-day activity. J Virol 2005;79:12507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenz J HERV-K HML-2 diversity among humans. Proc Natl Acad Sci U S A 2016;113:4240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beimforde N, Hanke K, Ammar I, Kurth R, Bannert N. Molecular cloning and functional characterization of the human endogenous retrovirus K113. Virology 2008;371:216–25. [DOI] [PubMed] [Google Scholar]
- 18.Boller K, Schonfeld K, Lischer S, et al. Human endogenous retrovirus HERV-K113 is capable of producing intact viral particles. J Gen Virol 2008;89:567–72. [DOI] [PubMed] [Google Scholar]
- 19.Grow EJ, Flynn RA, Chavez SL, et al. Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature 2015;522:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montesion M, Bhardwaj N, Williams ZH, Kuperwasser C, Coffin JM. Mechanisms of HERV-K (HML-2) transcription during human mammary epithelial cell transformation. J Virol 2018;92:e01258–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goering W, Ribarska T, Schulz WA. Selective changes of retroelement expression in human prostate cancer. Carcinogenesis 2011;32:1484–92. [DOI] [PubMed] [Google Scholar]
- 22.Contreras-Galindo R, Kaplan MH, Markovitz DM, Lorenzo E, Yamamura Y. Detection of HERV-K(HML-2) viral RNA in plasma of HIV type 1-infected individuals. AIDS Res Hum Retroviruses 2006;22:979–84. [DOI] [PubMed] [Google Scholar]
- 23.Contreras-Galindo R, González M, Almodovar-Camacho S, González-Ramírez S, Lorenzo E, Yamamura Y. A new real-time-RT-PCR for quantitation of human endogenous retroviruses type K (HERV-K) RNA load in plasma samples: increased HERV-K RNA titers in HIV-1 patients with HAART non-suppressive regimens. J Virol Methods 2006;136:51–7. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Hernandez MJ, Swanson MD, Contreras-Galindo R, et al. Expression of human endogenous retrovirus type K (HML-2) is activated by the Tat protein of HIV-1. J Virol 2012;86:7790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhardwaj N, Maldarelli F, Mellors J, Coffin JM. HIV-1 infection leads to increased transcription of human endogenous retrovirus HERV-K (HML-2) proviruses in vivo but not to increased virion production. J Virol 2014;88:11108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Mulder M, SenGupta D, Deeks SG, et al. Anti-HERV-K (HML-2) capsid antibody responses in HIV elite controllers. Retrovirology 2017;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlhardt S, Seifert M, Schneider J, Ojak A, Zang KD, Mehraein Y. Human endogenous retrovirus HERV-K(HML-2) Rec expression and transcriptional activities in normal and rheumatoid arthritis synovia. J Rheumatol 2006;33:16–23. [PubMed] [Google Scholar]
- 28.Michaud HA, de Mulder M, SenGupta D, et al. Trans-activation, post-transcriptional maturation, and induction of antibodies to HERV-K (HML-2) envelope transmembrane protein in HIV-1 infection. Retrovirology 2014;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hefton A, Liang SY, Ni K, et al. Autoantibodies against citrullinated serum albumin in patients with rheumatoid arthritis. J Transl Autoimmun 2019;2:100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steen J, Forsstrom B, Sahlstrom P, et al. Recognition of amino acid motifs, rather than specific proteins, by human plasma cell-derived monoclonal antibodies to posttranslationally modified proteins in rheumatoid arthritis. Arthritis Rheumatol 2019;71:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bach M, Moon J, Moore R, Pan T, Nelson JL, Lood C. A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol 2020;72:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duvvuri B, Pachman LM, Morgan G, et al. Neutrophil extracellular traps in tissue and periphery in juvenile dermatomyositis. Arthritis Rheumatol 2020;72:448–58. [DOI] [PubMed] [Google Scholar]
- 33.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 1998;101:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada R Peptidylarginine deiminase type 4, anticitrullinated peptide antibodies, and rheumatoid arthritis. Autoimmun Rev 2005;4:201–6. [DOI] [PubMed] [Google Scholar]
- 35.van Jaarsveld CH, ter Borg EJ, Jacobs JW, et al. The prognostic value of the antiperinuclear factor, anti-citrullinated peptide antibodies and rheumatoid factor in early rheumatoid arthritis. Clin Exp Rheumatol 1999;17:689–97. [PubMed] [Google Scholar]
- 36.Yamada R, Suzuki A, Chang X, Yamamoto K. Peptidylarginine deiminase type 4: identification of a rheumatoid arthritis-susceptible gene. Trends Mol Med 2003;9:503–8. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, Yamada R, Chang X, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet 2003; 34:395–402. [DOI] [PubMed] [Google Scholar]
- 38.Hua J, Huang W. Peptidylarginine deiminase 4 -104C/T polymorphism and risk of rheumatoid arthritis: a pooled analysis based on different populations. PLoS One 2018;13:e0193674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Too CL, Murad S, Dhaliwal JS, et al. Polymorphisms in peptidylarginine deiminase associate with rheumatoid arthritis in diverse Asian populations: evidence from MyEIRA study and meta-analysis. Arthritis Res Ther 2012;14:R250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 2003;25:1106–18. [DOI] [PubMed] [Google Scholar]
- 41.Jones JE, Causey CP, Knuckley B, Slack-Noyes JL, Thompson PR. Protein arginine deiminase 4 (PAD4): current understanding and future therapeutic potential. Curr Opin Drug Discov Devel 2009;12:616–27. [PMC free article] [PubMed] [Google Scholar]
- 42.Raijmakers R, van Beers JJ, El-Azzouny M, et al. Elevated levels of fibrinogen-derived endogenous citrullinated peptides in synovial fluid of rheumatoid arthritis patients. Arthritis Res Ther 2012;14:R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones RB, Leal FE, Hasenkrug AM, et al. Human endogenous retrovirus K(HML-2) Gag and Env specific T-cell responses are not detected in HTLV-I-infected subjects using standard peptide screening methods. J Negat Results Biomed 2013;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones RB, Garrison KE, Mujib S, et al. HERV-K-specific T cells eliminate diverse HIV-1/2 and SIV primary isolates. J Clin Invest 2012;122:4473–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mustelin T, Bottini N, Stanford SM. The contribution of PTPN22 to rheumatological disease. Arthritis Rheumatol 2019;71:486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Chen B, Mittereder N, et al. Spontaneous secretion of the citrullination enzyme PAD2 and cell surface exposure of PAD4 by neutrophils. Front Immunol 2017;8:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pratesi F, Tommasi C, Anzilotti C, Chimenti D, Migliorini P. Deiminated Epstein-Barr virus nuclear antigen 1 is a target of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum 2006;54:733–41. [DOI] [PubMed] [Google Scholar]
- 48.Ono M, Kawakami M, Ushikubo H. Stimulation of expression of the human endogenous retrovirus genome by female steroid hormones in human breast cancer cell line T47D. J Virol 1987;61:2059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montesion M, Williams ZH, Subramanian RP, Kuperwasser C, Coffin JM. Promoter expression of HERV-K (HML-2) provirus-derived sequences is related to LTR sequence variation and polymorphic transcription factor binding sites. Retrovirology 2018;15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabriel U, Steidler A, Trojan L, Michel MS, Seifarth W, Fabarius A. Smoking increases transcription of human endogenous retroviruses in a newly established in vitro cell model and in normal urothelium. AIDS Res Hum Retroviruses 2010;26:883–8. [DOI] [PubMed] [Google Scholar]
- 51.Wallace TA, Downey RF, Seufert CJ, et al. Elevated HERV-K MRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis 2014;35:2074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


