Abstract
The effects of conventional amphotericin B (AmB) dissolved in sodium deoxycholate on microsomal cytochrome P-450 concentrations and propafenone metabolism to 5-hydroxy-propafenone and N-desalkyl-propafenone were compared with those of liposomal AMB (Li-AMB) in rats. AmB (3 mg/kg/day, intravenously [i.v.]) given for 4 days caused a significant decrease in the concentration of hepatic microsomal cytochrome P-450 (0.43 ± 0.06 nmol/mg versus 0.62 ± 0.05 nmol/mg for the control [P < 0.05]). Following the application of Li-AMB (15 mg/kg/day, i.v.), hepatic microsomal cytochrome P-450 concentrations were unchanged at 0.64 ± 0.08 nmol/mg. AmB decreased ex vivo propafenone metabolism to 5-hydroxy-propafenone and N-desalkyl-propafenone significantly. Sodium deoxycholate (the vehicle of AmB) by itself induced a significant decline of 5-hydroxy-propafenone and N-desalkyl-propafenone production, while microsomal cytochrome P-450 concentrations remained unchanged. In contrast, Li-AMB did not change the levels of production of 5-hydroxy-propafenone or of N-desalkyl-propafenone at either substrate concentration tested (50 μmol and 200 μmol). Microsomal AmB concentrations were significantly higher following Li-AMB application (21.1 ± 6.2 μg/g versus 3.7 ± 1.4 μg/g for AmB [P < 0.05]). We conclude that Li-AMB, in contrast to AmB, decreases neither hepatic microsomal cytochrome P-450 nor hepatic propafenone metabolism in rats ex vivo. Sodium deoxycholate alone decreases propafenone metabolism in a similar way to AmB, suggesting that it participates in AmB-induced disturbance of hepatic metabolic function.
The polyene macrolide amphotericin B is the most effective antibiotic agent used for the treatment of systemic fungal infections in humans (13). However, its clinical use is limited due to its pronounced side effects such as chills, fever, nausea, and organ damage (especially the impairment of kidney function). Amphotericin B has a high affinity for biological membranes, resulting in binding to sterols, which is most likely responsible for its excellent antifungal properties. On the other hand, this activity may potentially alter cellular membrane functions, resulting in organ dysfunction. The distribution of amphotericin B differs widely between organs, and the highest amphotericin B concentrations are reached in the liver (2) with the potential to alter hepatic cellular integrity (12). In perfused rat livers, it has been demonstrated that amphotericin B reduces bile flow and decreases bile acid secretion (6). Studies performed with human liver microsomes suggest that amphotericin alters hepatic metabolic function and results in a decrease of antipyrine clearance (7). However, the effect of amphotericin B on hepatic metabolic function is not known in detail. To overcome the pronounced side effects of conventional amphotericin B, novel lipid-containing formulations of amphotericin B with fewer side effects have been developed. These formulations include the small unilamellar vesicle liposomal amphotericin B known as AmBisome, which has been shown to be both safe and effective against systemic fungal infections (14). The influence of liposomal amphotericin B drug preparations on hepatic metabolic function are not well known.
The present investigations were performed to compare the effects of conventional amphotericin B and liposomal amphotericin B (AmBisome) on hepatic cytochrome P-450 concentrations with special respect to the metabolism of propafenone, which is metabolized by hepatic cytochrome P-450 isoenzymes (5). For this purpose, rats were treated with either conventional amphotericin B (dissolved in deoxycholate) or liposomal amphotericin, and hepatic microsomal propafenone metabolism was studied ex vivo.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (Institut für Versuchstierzucht, Hannover, Germany) weighing 200 to 310 g were held in individual cages on a standard diet (Altromin; Lage, Germany) and received water ad libitum.
For the in vivo experiments, rats were anesthetized with pentobarbitone (0.048 mg/kg), and indwelling silastic catheters were inserted into the jugular vein 12 h before the start of the treatment period. The animals were randomized and divided into four groups. Each group consisted of 10 animals. Group I received 5% glucose (0.6 ml/kg/day, intravenously [i.v.]) alone. Group II received conventional amphotericin B (3 mg/kg/day, i.v.). Conventional amphotericin B was used as in a solution containing 50 mg of amphotericin B and 41 mg of sodium deoxycholate as a detergent per vial (Bristol-Myers Squibb, Munich, Germany). Group III received the vehicle sodium deoxycholate (2.46 mg/kg/day, i.v.) alone. Group IV received liposomal amphotericin B (15 mg/kg/day, i.v.) (AmBisome; Nexstar, Munich, Germany).
Treatments were performed for 4 days, and administration was done twice daily. Twelve hours after the last drug application, the animals were exsanguinated under pentobarbital anesthesia. Blood samples of 5 ml were taken, and the livers were perfused with ice cold physiological saline solution for 60 min. Livers were frozen in liquid nitrogen and were stored at −80°C until further use.
Ex vivo measurements.
Liver microsomes were prepared as described in detail earlier (7), according to the method of De Duve et al. (3). Microsomal protein concentrations were measured by using a commercial protein assay kit (Bio-Rad, Munich, Germany). The hepatic microsomal cytochrome P-450 concentrations were determined according to the method of Estabrook and Werringloer (4) by using a split-beam spectrophotometer with an extinction coefficient of 91 mM−1 · cm−1 (16).
Microsomal propafenone metabolism was determined by using a 0.1 M phosphate buffer containing NADPH (0.5 mM) and supplemented with either 0.05 or 0.2 mM propafenone. Two milliliters of this solution was incubated for 30 min at 37°C in a metabolic shaker. Reactions were started by the addition of 50 μg of microsomes per sample and were terminated by adding 50 μl of 60% perchloric acid.
Analytical procedures.
Samples were drawn from each incubation, and the propafenone metabolites 5-hydroxy-propafenone and N-desalkyl-propafenone were measured by high-performance liquid chromatography (9).
Individual hepatic amphotericin B concentrations were determined by using a plate diffusion bioassay (8). From each sample, 100 μl of microsomal suspension was used. In this assay, Candida albicans served as the test organism. After incubation of the samples for 20 h at 37°C, the diameters of the zones of inhibited growth were measured. The coefficient of correlation (r) between this assay and the high-performance liquid chromatography is 0.88 (8).
Statistical analysis.
Unless otherwise stated, all results were expressed as mean values ± standard deviations. For data analyses, the Kruskal-Wallis test was performed. To resolve differences, the Mann-Whitney U test was used. A P value of <0.05 was considered to reflect statistically significant differences.
RESULTS
Hepatic proteins.
Treatment of rats for 4 days with conventional amphotericin B (3 mg/kg/day, i.v.) resulted in a significant decrease in liver weight and total amount of hepatic microsomal cytochrome P-450 in comparison to all other groups (Table 1). Conventional amphotericin B did not influence hepatic microsomal protein, whereas its vehicle, sodium deoxycholate, induced a significant increase in hepatic microsomal protein compared to the controls and to animals treated with liposomal amphotericin B (Table 1). Application of liposomal amphotericin B (15 mg/kg/day) influenced neither liver weight nor total amount of microsomal cytochrome P-450 or hepatic microsomal protein in comparison to controls (Table 1). The hepatic microsomal cytochrome P-450 concentrations decreased significantly following treatment with conventional amphotericin B to 0.43 ± 0.06 nmol/mg (controls, 0.62 ± 0.05 nmol/mg [P < 0.05]), whereas its vehicle, sodium deoxycholate, had no influence on cytochrome P-450 concentrations (0.60 ± 0.03 nmol/mg). Liposomal amphotericin B did not significantly change cytochrome P-450 concentrations (0.64 ± 0.08 nmol/mg).
TABLE 1.
Comparison of 4-day treatments of rats with agents used in this study
| Treatmenta | Liver weight (g) | Amt of hepatic microsomal protein (mg/ml) | Total amt of hepatic microsomal cytochrome P-450 (nmol) |
|---|---|---|---|
| Control | 11.0 ± 0.9 | 12.9 ± 1.7 | 6.9 ± 1.0 |
| NaDo | 11.9 ± 1.2 | 21.5 ± 3.4b | 7.2 ± 1.0 |
| AmB | 9.2 ± 1.1b | 11.1 ± 2.1 | 3.9 ± 0.8b |
| Li-AMB | 10.8 ± 1.1 | 12.8 ± 1.3 | 6.9 ± 0.9 |
NaDo, sodium deoxycholate; AmB, conventional amphotericin B; Li-AMB, liposomal amphotericin B.
P < 0.05 compared to all other groups.
Hepatic microsomal amphotericin B drug concentrations.
Administration of liposomal amphotericin B resulted in a significantly higher microsomal concentration of amphotericin B than administration of conventional amphotericin B (21.1 ± 6.2 versus 3.7 ± 1.4 μg/mg [P < 0.05]).
Hepatic microsomal propafenone metabolism.
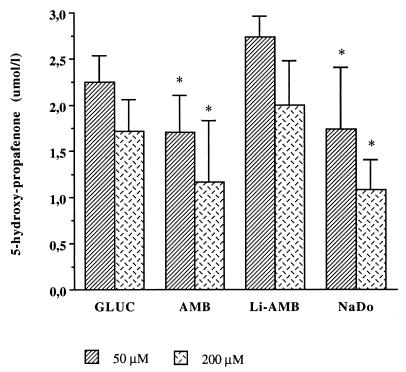
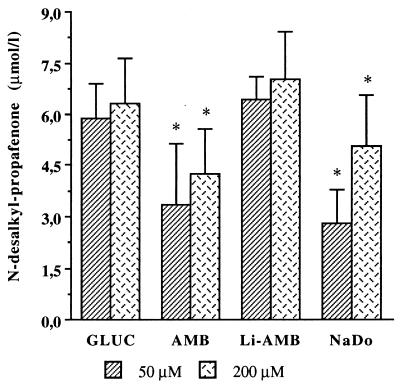
Propafenone metabolism to 5-hydroxy-propafenone and N-desalkyl-propafenone was significantly decreased in microsomes prepared from rats having received conventional amphotericin B or sodium deoxycholate alone. Following treatment with conventional amphotericin B, production of 5-hydroxy-propafenone decreased significantly to 1.7 ± 0.41 μmol/liter with 50 μmol of propafenone and to 1.17 ± 0.66 μmol/liter with 200 μmol of propafenone, respectively (Fig. 1) (controls were 2.25 ± 0.29 μmol/liter [50 μmol of propafenone] and 1.72 ± 0.34 μmol/liter [200 μmol of propafenone]). Treatment with conventional amphotericin B also resulted in a significant decrease of hepatic propafenone metabolism to N-desalkyl propafenone: 3.34 ± 1.86 μmol/liter (with 50 μmol of propafenone) (controls, 5.92 ± 0.98 μmol/liter) and 4.24 ± 1.34 μmol/liter (with 200 μmol of propafenone) (controls, 6.27 ± 1.34 μmol/liter) (Fig. 2). A similarly significant decrease of propafenone metabolism was observed after treatment with sodium deoxycholate alone. In rats treated with liposomal amphotericin B, hepatic microsomal metabolism of propafenone to 5-hydroxy-propafenone or N-desalkyl-propafenone was not significantly changed at either propafenone concentration tested (Fig. 1 and 2).
FIG. 1.
Ex vivo metabolism of propafenone to 5-hydroxy-propafenone by hepatic microsomes prepared from rats following i.v. treatment for 4 days with conventional amphotericin B (3 mg/kg/day) (AMB) or liposomal amphotericin B (15 mg/kg/day) (Li-AMB). NaDo, treatment with sodium deoxycholate; GLUC, treatment with glucose. ∗, P < 0.05 compared to all other groups.
FIG. 2.
Ex vivo metabolism of propafenone to N-desalkyl-propafenone by hepatic microsomes prepared from rats following i.v. treatment for 4 days with conventional amphotericin B (3 mg/kg/day) (AMB) or liposomal amphotericin B (15 mg/kg/day) (Li-AMB). NaDo, treatment with sodium deoxycholate; GLUC, treatment with glucose. ∗, P < 0.05 compared to all other groups.
DISCUSSION
The results of this study show that conventional amphotericin B (dissolved in sodium deoxycholate) and its vehicle, sodium deoxycholate, decrease the metabolism of propafenone by rat liver microsomes. Furthermore, the results show that the daily administration of amphotericin B to rats reduces the concentration of the hepatic microsomal cytochrome P-450. In contrast, treatment of rats with liposomal amphotericin B (AmBisome) neither inhibits the metabolism of propafenone nor decreases the concentration of hepatic microsomal cytochrome P-450.
This study provides, for the first time, evidence that the novel liposomal amphotericin B preparation AmBisome has fewer untoward effects on hepatic cytochrome P-450 and on propafenone metabolism in rat hepatic microsomes than conventional amphotericin B-deoxycholate. These effects seem not to be dependent on the actual amphotericin B tissue concentrations, since liposomal amphotericin B administration resulted in higher microsomal amphotericin B concentrations than did administration of conventional amphotericin B. A decrease in the concentration of the hepatic cytochrome P-450 may either be caused by reduced synthesis or increased catabolism. In rabbits, neither conventional amphotericin B nor liposomal amphotericin B given for 28 days induced any significant elevation of transaminases (11). We cannot exclude enhanced degradation of cytochrome P-450 isoforms by amphotericin B. However, we consider this possibility unlikely, since it has been shown in rabbits that macrolide antibiotics inhibit the degradation of cytochrome P-450, leading to elevated enzyme concentrations (17).
Amphotericin B has a high affinity for biological membranes and low cytochrome P-450 concentrations following drug administration, suggesting selective inhibition of cytochrome P-450, possibly resulting from xenobiotic interaction. It therefore seems possible that amphotericin B or its vehicle, sodium deoxycholate, impairs certain monooxygenases located on the endoplasmic reticulum. This assumption is confirmed by the results of our earlier study, suggesting selective inhibition of the microsomal cytochrome P-450 but not of the microsomal glucose-6-phosphatase (7).
Conventional amphotericin B is dissolved in the secondary bile acid sodium deoxycholate (ratio, 1.0 to 0.82), and the results show that sodium deoxycholate by itself decreases the metabolism of propafenone. Microsomal amphotericin B concentrations following application of liposomal amphotericin B were higher than microsomal amphotericin B concentrations in rats after treatment with conventional amphotericin B, which probably reflects a higher uptake by the reticuloendothelial system (11). In contrast to conventional amphotericin B, liposomal amphotericin B neither influenced microsomal cytochrome P-450 concentrations nor inhibited propafenone metabolism to 5-hydroxy-propafenone or N-desalkyl propafenone. Therefore, it might be assumed that the vehicle of conventional amphotericin B causes impairment of the hepatic microsomal propafenone metabolism observed following treatment with amphotericin B dissolved in deoxycholate. There is evidence that the hepatic cytochrome P-450db1 catalyzes the biotransformation of propafenone (10). From this study, we cannot conclude that the inhibitory effects of amphotericin B and sodium deoxycholate are limited to the cytochrome P-450db1.
Although severe hepatotoxicity is a rare side effect of conventional amphotericin B treatment in humans (1, 15), from the present data it is obvious that conventional amphotericin B may affect metabolic liver function in rats. Thus, a careful drug monitoring system seems advisable, especially for patients concomitantly receiving other drugs which undergo hepatic metabolism. Furthermore, the data suggest that, even in the presence of a high amphotericin B tissue concentration following application of liposomal amphotericin B, the hepatic metabolic function is not substantially altered.
ACKNOWLEDGMENT
We appreciate the excellent technical assistance of T. Kock.
REFERENCES
- 1.Abajo F J, Carcas A J. Amphotericin hepatotoxicity. Br Med J. 1986;293:1243. [Google Scholar]
- 2.Christiansen K J, Bernard E M, Gold J W M, Armstrong D. Distribution and activity of amphotericin B in humans. J Infect Dis. 1985;152:1037–1043. doi: 10.1093/infdis/152.5.1037. [DOI] [PubMed] [Google Scholar]
- 3.De Duve C, Pressman B C, Gianetto R, Wattiaux R, Appelmans F. Tissue fractionating studies. 6. Intracellular distribution patterns of enzymes in rat liver tissues. Biochem J. 1955;60:604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estabrook R W, Werringloer J. The measurement of different spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- 5.Funck Brentano C H, Kroemer H J, Lee J T, Roden D M. Propafenone. N Engl J Med. 1990;322:518–525. doi: 10.1056/NEJM199002223220806. [DOI] [PubMed] [Google Scholar]
- 6.Geata G B, Utili R, Adilnolfi L E, Tripodi M F, Esposito V. Effects of amphotericin B on the excretory function and the colloid clearance capacity of the perfused rat liver. J Hepatol. 1989;8:344–350. doi: 10.1016/0168-8278(89)90033-0. [DOI] [PubMed] [Google Scholar]
- 7.Heidemann H T, Holzlöhner U, Heydemann J, Freitag T, Inselmann G. Effect of amphotericin B on the hepatic cytochrome P-450, glucose-6-phosphatase and lipid peroxidation in the rat. J Hepatol. 1992;14:300–304. doi: 10.1016/0168-8278(92)90174-n. [DOI] [PubMed] [Google Scholar]
- 8.Hulsewede J W, Dermoumi H. Comparison of high performance liquid chromatography and bioassay of amphotericin B in serum. Mycoses. 1994;37:17–21. doi: 10.1111/j.1439-0507.1994.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer H K, Botsch S, Heinkele G, Schick M. In vitro assessment of various cytochromes P450 and glucuronosyltransferases using the antiarrhythmic propafenone as a probe drug. Methods Enzymol. 1996;272:99–105. doi: 10.1016/s0076-6879(96)72012-8. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer H K, Mikus G, Kronbach T, Meyer U A, Eichelbaum M. In vitro characterisation of the human cytochrome P-450 involved in polymorphic oxidation of propafenone. Clin Pharmacol Ther. 1989;45:28–33. doi: 10.1038/clpt.1989.5. [DOI] [PubMed] [Google Scholar]
- 11.Lee J W, Amantea M A, Francis P A, Navarro E E, Bacher J, Pizzo P A, Walsh T J. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (Ambisome) in rabbits. Antimicrob Agents Chemother. 1994;38:713–718. doi: 10.1128/aac.38.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massa T, Sinha D P, Frantz J D, Filipek M E, Weglein R C, Steinberg S A, McGrath J T, Murphy B F, Szot R J, Black H E, Schartz E. Subcronic toxicity studies of N-d-ornithyl amphotericin B methyl ester in dogs and rats. Fundam Appl Toxicol. 1984;5:737–753. doi: 10.1016/0272-0590(85)90198-8. [DOI] [PubMed] [Google Scholar]
- 13.Medoff G, Kobayashi G S. Strategies in the treatment of fungal infections. N Engl J Med. 1983;302:145–155. doi: 10.1056/NEJM198001173020304. [DOI] [PubMed] [Google Scholar]
- 14.Meunier F, Prentice H G, Ringden O. Liposomal amphotericin B (Ambisome) safety data from a phase II/III clinical trial. J Microb Chemother. 1991;28(Suppl. B):83–91. doi: 10.1093/jac/28.suppl_b.83. [DOI] [PubMed] [Google Scholar]
- 15.Miller M A. Reversible hepatotoxicity related to amphotericin B. Can Med Assoc J. 1984;131:1245–1247. [PMC free article] [PubMed] [Google Scholar]
- 16.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification and properties. J Biol Chem. 1964;239:2370–2380. [PubMed] [Google Scholar]
- 17.Watkins P B, Wrighton S A, Schuetz E G, Maurel P, Guzelian P S. Macrolide antibiotics inhibit the degradation of the glucocorticoid responsive cytochrome P-450p in rat hepatocytes in vivo and in primary monolayer culture. J Biol Chem. 1986;261:6264–6271. [PubMed] [Google Scholar]