OBJECTIVES:
Circulating nucleic acids, alone and in complex with histones as nucleosomes, have been proposed to link systemic inflammation and coagulation after trauma to acute kidney injury (AKI). We sought to determine the association of circulating nucleic acids measured at multiple time points after trauma with AKI risk.
DESIGN:
We conducted a prospective cohort study of trauma patients, collecting plasma on presentation and at 6, 12, 24, and 48 hours, defining AKI over the first 6 days by Kidney Disease Improving Global Outcomes serum creatinine and dialysis criteria. We determined kinetics of plasma mitochondrial DNA (mtDNA), nuclear DNA (nDNA), and nucleosome levels across time points and associations with AKI using multivariable linear mixed-effects models, adjusted for injury characteristics and blood transfusions. We evaluated the association of presentation nucleic acid damage-associated molecular patterns (DAMP) concentrations with subsequent AKI, adjusting for injury severity using multivariable logistic regression.
SETTING:
Academic level I trauma center.
PATIENTS:
Trauma patients (n = 55) requiring intensive care for greater than or equal to 24 hours after presentation.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
AKI developed in 17 patients (31%), a median of 12.0 hours (interquartile range, 6.2–24.1 hr) after presentation. mtDNA demonstrated a time-varying association with AKI (p = 0.022, interaction with time point), with differences by AKI status not emerging until 24 hours (β = 0.97 [95% CI, 0.03–1.90] log copies/uL; p = 0.043). Patients who developed AKI had higher nDNA across all time points (overall β = 1.41 log copies/uL [0.86–1.95 log copies/uL]; p < 0.001), and presentation levels were significantly associated with subsequent AKI (odds ratio [OR], 2.55 [1.36–4.78] per log copy/uL; p = 0.003). Patients with AKI had higher nucleosome levels at presentation (β = 0.32 [0.00–0.63] arbitrary unit; p = 0.048), a difference that was more pronounced at 24 hours (β = 0.41 [0.06–0.76]; p = 0.021) and 48 hours (β = 0.71 [0.35–1.08]; p < 0.001) (p = 0.075, interaction with time point).
CONCLUSIONS:
Plasma nucleic acid DAMPs have distinct kinetics and associations with AKI in critically ill trauma patients. nDNA at presentation predicts subsequent AKI and may be amenable to targeted therapies in this population.
Keywords: acute kidney injury, damage-associated molecular pattern, mitochondrial DNA, nuclear DNA, nucleosomes, trauma
The development of acute kidney injury (AKI) following major trauma portends a nearly four-fold increase in mortality and remains a leading contributor to late posttrauma death and disability (1–4). Mechanisms underlying AKI in trauma patients, however, remain incompletely understood (5). This uncertainty hampers efforts to develop effective preventive and therapeutic strategies for AKI and, given the burden of AKI-associated morbidity, constitutes a major priority for improving outcomes in critically ill trauma patients (6).
Damage-associated molecular patterns (DAMPs) are endogenous intracellular molecules with proinflammatory and procoagulant capabilities that may contribute to AKI when released into circulation by stressed or dying cells following trauma (7). Cell-free mitochondrial DNA (mtDNA), nuclear DNA (nDNA), and nucleosomes (DNA in complex with histones) are nucleic acid–associated DAMPs that cause endothelial activation and tissue injury in organs remote from the initial insult in preclinical models of ischemia and trauma (8–14). In murine models, mtDNA caused AKI via toll-like receptor 9 (TLR9) and the cyclic GMP-AMP Synthase-Stimulator of Interferon Genes (cGAS-STING) pathway, which was successfully ameliorated by specific inhibition of each pathway by TLR9 knockout, small molecule inhibition, nucleic acid scavenging (15–18), and STING small interfering RNA knockdown and small molecule inhibition (12, 19, 20), respectively. Cell-free DNA stabilizes thromboses and forms the scaffold of neutrophil extracellular traps (NETs) contributing to immunothrombosis, processes observed in ischemia-induced AKI models (21–23). NET formation initiated by heme-activated platelets mediated rhabdomyolysis-induced AKI, a common complication following trauma, and was mitigated with NET-targeted therapies such as DNase and peptidyl-arginine deiminase inhibitors (23, 24). Histones released after ischemia-reperfusion injury or directly injected intravenously caused AKI in murine models, which was suppressed by antihistone IgG (23, 25). Thus, relevant animal models of trauma and ischemia-reperfusion injury demonstrate specific nucleic acid DAMP-induced AKI. Furthermore, targeted therapeutics that have been trialed or used in humans ameliorated AKI in these models, suggesting the potential utility of targeting this pathway in critically ill trauma patients (17, 23, 25–29).
Despite this preclinical evidence, a few studies have examined the association of circulating nucleic acid DAMPs with AKI in critically ill patients. Two studies showed elevated levels of either mtDNA or total cell-free DNA with AKI after cardiac surgery (27, 30), and we found that plasma mtDNA 48 hours after ICU admission was associated with AKI in sepsis patients (31). Studies of cell-free DNA in trauma patients have focused on late outcomes such as infections (32, 33) and prolonged critical illness (34). These studies lacked serial early blood sampling to identify changes in circulating mtDNA and nDNA levels over the first 48 hours postinjury, during which nearly three-quarters of AKI cases are manifest (35).The association of nucleosomes with AKI is not described in any critical illness population (32–34, 36). Whether concentrations of these three nucleic acid DAMPs follow similar postinjury patterns is an additional area of uncertainty (27, 30, 37, 38). Although nucleic acid DAMPs are released simultaneously during necrotic cell death, mtDNA and nucleosomes are also released by live cells undergoing stress, suggesting their kinetics may diverge from those of nDNA. The distinct mechanisms of release and clearance for each nucleic acid DAMP may have implications for associated targeted therapies (39–45). Clarifying postinjury nucleic acid DAMP kinetics and associations with AKI are thus key steps in moving toward such therapies with the potential to prevent or ameliorate AKI after trauma (15, 46–49).
We, therefore, undertook a prospective study to determine the associations of plasma nDNA, mtDNA, and nucleosome concentrations, measured serially over the first 48 hours after trauma, with AKI, and to define the early kinetics of these DAMPs. We hypothesized that similarities in mechanisms of mtDNA and nucleosome release would lead to a time-varying, progressively stronger association with AKI, whereas nDNA would exhibit a constant association with AKI over the first 48 hours following trauma.
MATERIALS AND METHODS
Study Design and Enrollment
We conducted a prospective cohort study of critically ill trauma patients admitted to the emergency department (ED) at our level I trauma center. On-site study personnel responded to all alerts to the ED trauma bay to assess patients for eligibility (50). Patients determined by the clinical team within the first 30 minutes to require emergent surgery or admission to the ICU were enrolled. Exclusion criteria included age less than 18, pregnancy, police custody, transfer to floor or death within 12 hours of presentation, and isolated severe head injury (Abbreviated Injury Score [AIS] head ≥ 3 and AIS all other regions ≤1). The Institutional Review Board of the University of Pennsylvania approved the study with a waiver of timely informed consent given minimal risk (protocol 826515). Study personnel collected blood samples at presentation to the trauma bay and approximately 6, 12, 24, and 48 hours after presentation.
DAMP Measurement
DNA was extracted from plasma using the Qiagen DNeasy Blood and Tissue Kit (Hilden, Germany). We quantified plasma mtDNA and nDNA in log copies/uL using polymerase chain reaction (PCR) for the mitochondrial ND1 gene and the nuclear COXIV gene (Life Technologies, Carlsbad, CA) and nucleosomes in duplicate using the Cell Death Detection enzyme-linked immunosorbent assay (Roche, Basel, Switzerland), reported in arbitrary units (Aus; detailed in Supplemental Methods, http://links.lww.com/CCX/A952).
Defining AKI
The primary outcome was the development of AKI defined and staged by Kidney Disease Improving Global Outcomes (KDIGO) creatinine and kidney replacement therapy criteria (KRT) (51). To mimic clinical practice, we used serum creatinine at presentation as the baseline value unless a preinjury value within the prior year was available. We determined incident AKI based on: 1) creatinine increase of 0.3 mg/dL over a 48-hr period, 2) creatinine increase to 150% of baseline, or 3) need for acute KRT, phenotyped through the first 6 days in order to focus on the acute physiologic effects of trauma. We then applied KDIGO staging criteria using creatinine and KRT data. Due to the small size of the study, we combined KDIGO stages 2 and 3 in analyses.
Statistical Analysis
We constructed multivariable linear mixed-effects models leveraging repeated measures to test whether DAMP concentrations through 48 hours differed by AKI status, clustered on individual patients. We adjusted these models for potential confounders identified a priori based on variables described or hypothesized to be associated with AKI or circulating DAMP levels: injury severity score (ISS), presence of shock in the ED (mean arterial blood pressure < 60 mm Hg or use of vasopressor medications), injury mechanism, and time-varying total units of transfused packed red blood cells (PRBCs) (31, 52). We did not include transfusion of fresh frozen plasma or platelets, as the 1:1:1 resuscitation strategy for trauma patients resulted in high collinearity between blood product receipts (Supplemental Data, http://links.lww.com/CCX/A952). A timeby-AKI interaction term was used to determine whether the DAMP-AKI association was time-varying. If no interaction was detected (likelihood ratio test p > 0.1), the overall difference of DAMP level between the AKI and non-AKI group across all time points is presented; otherwise, time point-specific differences are presented. We used multivariable logistic regression models to determine the associations of presentation DAMP levels with subsequent AKI and estimated standardized risk of AKI across a range of DAMP levels, adjusted for ISS, using postestimation marginal analysis. Discrimination and calibration were assessed using area under the receiver operator curve (AUC) and the Hosmer-Lemeshow goodness-of-fit test, respectively. In sensitivity analyses, we adjusted these logistic regression models for shock, transfusion, and injury mechanism, separately. We used ordered logistic regression to assess the association of presentation nDNA with AKI stage. Differences between DAMP levels at presentation and later time points, overall and stratified by AKI status, were assessed using linear mixed-effects models. Differences in plasma DAMP concentrations at presentation and 6 hours between patients who underwent operation pre-ICU transfer and those who did not were assessed using the Wilcoxon rank-sum test. We used Stata/IC Version 15 (StataCorp LLC, College Station, TX) for all analyses and considered a p value of less than 0.05 significant.
RESULTS
Cohort Characteristics
We prospectively enrolled 55 critically ill trauma patients with a median age of 34 (interquartile range [IQR], 25–51) and a male predominance (78%) (Table 1). Shock was present in 19 (35%), 55% had blunt injury mechanism, and median ISS was 20 (IQR, 13–29). AKI developed in 17 patients (30.9%) at a median of 12.0 hours (IQR, 6.2–24.1 hr) after presentation (Supplemental Fig. 1, Code as: http://links.lww.com/CCX/A966; legend, http://links.lww.com/CCX/A952). Twelve patients developed stage 1 AKI, no patients developed stage 2 AKI, and five developed stage 3, four of whom (23.5% of AKI cases) required acute hemodialysis. One patient died on day 22 of hospitalization. There was high adherence to protocol with minimal sample missingness (Supplemental Tables 3–5, http://links.lww.com/CCX/A953).
TABLE 1.
Patient Characteristics
| Patient Characteristics | Total, n = 55 | No AKI, n = 38 | AKI, n = 17 |
|---|---|---|---|
| Demographics | |||
| Age | 34 (25–51) | 35 (26–52) | 31 (24–48) |
| Sex | |||
| Female | 12 (22%) | 10 (26%) | 2 (12%) |
| Male | 43 (78%) | 28 (74%) | 15 (88%) |
| Body mass index | 28.7 (16.2–48.2) | 28.8 (16.2–48.2) | 28.5 (21.3–39.5) |
| Race | |||
| Asian | 1 (2%) | 0 (0%) | 1 (6%) |
| Black | 37 (67%) | 25 (66%) | 12 (71%) |
| White | 13 (24%) | 12 (32%) | 1 (6%) |
| Unknown | 4 (7%) | 1 (3%) | 3 (18%) |
| Ethnicity | |||
| Not Hispanic/Latino | 55 (100%) | 38 (100%) | 17 (100%) |
| Medical history | |||
| Chronic kidney disease | 1 (2%) | 1 (3%) | 0 (0%) |
| Diabetes mellitus | 3 (5%) | 2 (5%) | 1 (6%) |
| Congestive heart failure | 2 (4%) | 2 (5%) | 0 (0%) |
| Hypertension | 13 (20%) | 11 (29%) | 2 (12%) |
| Illness characteristics | |||
| Mechanism of trauma | |||
| Blunt | 30 (55%) | 24 (63%) | 6 (35%) |
| Penetrating | 25 (45%) | 14 (37%) | 11 (65%) |
| Injury Severity Score | 20 (13–29) | 18.5 (13–25) | 22 (16–34) |
| Peak creatine kinase (24 hr)a | 3,573 (1,521–4,962) | 2,063 (461–3,531) | 4,962 (3,573–12,699) |
| Peak lactate (24 hr) | 3.7 (2.4–6.4) | 2.9 (2.2–4.9) | 6.4 (5.1–13.1) |
| Packed red blood cell transfusion | |||
| % patients transfused (48 hr)b | 65 | 53 | 94 |
| Median units transfused (6 hr)c | 2.5 (1.5–8) | 2 (1–3) | 9.5 (3–13) |
| Intubated in ED | 25 (45.5%) | 14 (36.8%) | 11 (64.7%) |
| Shock in ED | 19 (34.5%) | 9 (24%) | 10 (59%) |
| Operation prior to ICU | 36 (65%) | 21 (55%) | 15 (88%) |
| 30-d mortality | 1 (2%) | 0 (0%) | 1(6%) |
AKI = acute kidney injury, ED = emergency department.
aCreatine kinase values were measured in 16 of 55 patients.
bIncludes all patients transfused within 48 hr.
cExcludes patients who did not receive any packed red blood cell transfusion within 48 hr.
Data are presented as median (interquartile range) for continuous measures and n (%) for categorical measures. No patients received transfusion prior to presentation.
Plasma DAMP Levels and AKI Risk
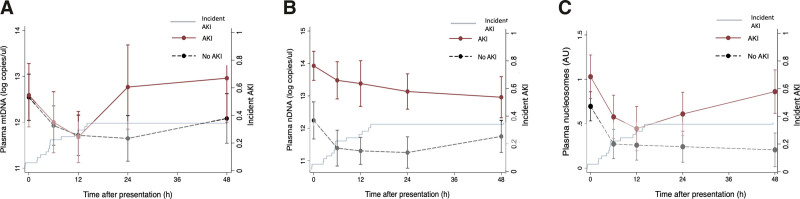
In adjusted mixed-effects models, mtDNA concentration demonstrated a time-varying association with AKI (Fig. 1A; p = 0.019 for interaction of AKI with time point), with similar levels in AKI and non-AKI patients through a 12-hour nadir, followed by an increase at 24 hours in those with AKI (β = 0.97 log copies/uL [95% CI, 0.03–1.90 log copies/uL]; p = 0.043 vs no AKI). AKI patients had higher plasma nDNA across all time points (overall β = 1.41 log copies/uL [0.86–1.95 log copies/uL]; p < 0.001; Fig. 1B), without significant interaction by time point (p = 0.311). Patients with AKI had higher presentation nucleosome levels (β = 0.32 AU [0.00–0.63 AU]; p = 0.048; Fig. 1C), a difference that was more pronounced at 24 hours (β = 0.41 [0.06–0.76]; p = 0.021) and 48 hours (β = 0.71 [0.35–1.08]; p < 0.001), p = 0.075, interaction with time point.
Figure 1.
At serial time points over the first 48 hr after trauma, plasma mitochondrial DNA (mtDNA) and nucleosomes demonstrated time-varying associations with acute kidney injury (AKI), while nuclear DNA (nDNA) was associated with AKI independent of time. Association of plasma mtDNA (A), nDNA (B), and nucleosome (C) levels over 48 hr with AKI. Linear mixed-effects models, clustered on individual patients and adjusted for Injury Severity Score, injury mechanism, packed red blood cell transfusion, and shock in the emergency department, show associations of each damage-associated molecular pattern with AKI. Kaplan-Meier curve of incident AKI over the referent period is noted on each panel. A, mtDNA concentration differences by AKI status varied significantly over time (interaction p = 0.019), with little early difference (β at presentation: –0.06 log copies/uL [–0.91 to 0.80 log copies/uL], p = 0.900; 6 hr: 0.03 [–0.87 to 0.93], p = 0.949; and 12 hr: 0.11 [–0.81 to 1.03], p = 0.810) and subsequent higher levels in AKI patients (β at 24 hr: 0.97 [0.03–1.90], p = 0.043; 48 hr: 0.79 [–0.17 to 1.75], p = 0.107). B, The association of nDNA concentration with AKI showed no significant difference over time (interaction p = 0.311), with nDNA levels consistently higher in those with AKI (overall β = 1.08 log copies/uL [0.55–1.61 log copies/uL], p < 0.001). C, Nucleosome levels did show some difference in association with AKI by time point (interaction p = 0.075), marginally higher at early time points (β at presentation: 0.32 [0.00–0.63] arbitrary unit (AU), p = 0.048; 6 hr: 0.34 [0.01–0.67], p = 0.045; and 12 hr: 0.22 (–0.12 to 0.57] AU, p = 0.205) but more markedly different at 24 and 48 hr (0.41 [0.06–0.76] AU, p = 0.021 and 0.71 [0.35–1.08], p < 0.001, respectively).
Association of DAMP Levels at Presentation With Subsequent AKI
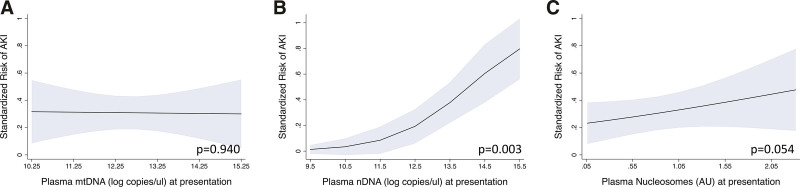
In logistic regression models adjusted for ISS, only presentation nDNA was significantly associated with subsequent AKI (odds ratio [OR], 2.55 (95% CI, 1.36–4.78) per log copy/uL; p = 0.003; Fig. 2B). Standardized risk of AKI increased from less than 5% for patients with presentation nDNA concentrations in the 5th percentile to greater than 75% for patients in the 95th percentile. Excluding AKI cases occurring within 6 hours of presentation did not substantially affect this association (2.35 [1.21–4.58] per log copy/uL; p = 0.012). Presentation nDNA remained associated with AKI in sensitivity analyses adjusting for shock (OR, 2.40; 95% CI, 1.31–4.40 per log copy/uL; p = 0.005), PRBC transfusion through 6 hours (OR, 2.16 [95% CI, 1.12–4.18]; p = 0.022), and blunt versus penetrating mechanism of injury (3.93; 95% CI, 1.73–8.93; p = 0.001). Presentation nDNA was also associated with AKI stage (OR, 2.26 [95% CI, 1.35–3.77] per log copy/uL per AKI stage category [none, stage 1, and stage 2/3]). Although increasing standardized risk of subsequent AKI was noted with higher presentation nucleosome concentration (Fig. 2C), this association was not statistically significant (OR, 1.81; 95% CI, 0.99–3.31 per log copy/uL; p = 0.054). There was no association of presentation mtDNA with AKI (OR, 0.98; 95% CI, 0.65–1.48 per log copy/uL; p = 0.940; Fig. 2A).
Figure 2.
Standardized risks of subsequent acute kidney injury (AKI) from 5th to 95th percentiles of presentation nucleic acid damage-associated molecular pattern concentrations. Risks were calculated using postestimation marginal analysis of logistic regression models adjusted for injury severity score. Presentation mitochondrial DNA (mtDNA) (A) was not associated with subsequent AKI (odds ratio [OR], 0.98 log copies/uL [95% CI, 0.65–1.48 log copies/uL], p = 0.94). Presentation nuclear DNA (nDNA) (B) was significantly associated with subsequent AKI (OR, 2.55 log copies/uL [1.36–4.78 log copies/uL], p = 0.003). Although risk of AKI increased with increasing presentation nucleosome levels (C), odds ratio confidence limits included the null (OR, 1.81 [0.99–3.31] arbitrary unit [AU], p = 0.054).
Presentation nDNA predicted subsequent AKI well, with an AUC of 0.80 (95% CI, 0.69–0.92; Supplemental Fig. 2, http://links.lww.com/CCX/A966; legend, http://links.lww.com/CCX/A952). Combining presentation nDNA with relevant clinical variables improved their predictive ability for AKI from an AUC of 0.59 for ISS (95% CI, 0.42–0.76) to 0.81 for ISS + presentation nDNA (95% CI, 0.69–0.92), from 0.68 for shock in the ED (95% CI, 0.54–0.81) to 0.85 for shock + presentation nDNA (0.75–0.96), from 0.83 for PRBC transfusion by 6 hours (95% CI, 0.70–0.96) to 0.91 for PRBC + presentation nDNA (95% CI, 0.82–0.99), and from 0.64 for mechanism of injury (95% CI, 0.50–0.78) to 0.88 for mechanism + presentation nDNA (95% CI, 0.79–0.97).
Kinetics of Plasma DAMPs
Plasma nDNA, mtDNA, and nucleosome concentrations all declined over the first 12 hours after presentation (Fig. 1 and Table 2). mtDNA concentrations at presentation, and 24 and 48 hours were higher referent to the apparent nadir at 12 hours, a pattern more pronounced in AKI patients compared with non-AKI patients (Table 2). Overall, nDNA levels plateaued after 12 hours, though the pattern in AKI patients was more of slow decline from presentation through 48 hours (Table 2). Nucleosome kinetics mirrored those of mtDNA, demonstrating a 12-hour nadir (Table 2). In AKI patients only, the initial decline was followed by a significant increase by 48 hours (Table 2). Surgical intervention impacted only plasma nDNA concentrations at subsequent time points. In the 42 patients who underwent surgery prior to ICU transfer versus those who did not, nDNA concentrations were not significantly different at presentation (p = 0.24) but were increased in operative versus nonoperative patients at 6 hours (p = 0.03). Plasma mtDNA and nucleosome concentrations did not differ at presentation or at 6 hours between surgical and nonsurgical patients.
TABLE 2.
Kinetics of Circulating Nucleic Acids Over 48 hr After Trauma
| Time Point | Overall (n = 55) | AKI (n = 17) | No AKI (n = 38) |
|---|---|---|---|
| Mitochondrial DNA (log copies/uL) | |||
| Presentation | 0.81 (0.49–1.14), p < 0.001 | 0.78 (0.14–1.41), p = 0.016 | 0.83 (0.47–0.1.19), p < 0.001 |
| 6 hr | 0.23 (–0.10 to 0.56), p = 0.166 | 0.19 (–0.44 to 0.82), p = 0.558 | 0.25 (–0.11 to 0.61), p = 0.167 |
| 12 hr | Reference | ||
| 24 hr | 0.41 (0.07–0.74), p = 0.017 | 0.95 (0.32–1.59), p = 0.003 | 0.12 (–0.26 to 0.50), p = 0.531 |
| 48 hr | 0.75 (0.40–1.10), p < 0.001 | 1.15 (0.51–1.78), p < 0.001 | 0.50 (0.10–0.91), p < 0.001 |
| Nuclear DNA (log copies/uL) | |||
| Presentation | Reference | ||
| 6 hr | –0.72 (–1.06 to –0.38), p < 0.001 | –0.45 (–0.96 to 0.07), p = 0.090 | –0.85 (–1.28 to –0.42), p < 0.001 |
| 12 hr | –0.89 (–1.24 to –0.55), p < 0.001 | –0.55 (–1.08 to –0.03), p = 0.039 | –1.04 (–1.48 to –0.60), p < 0.001 |
| 24 hr | –0.97 (–1.32 to –0.62), p < 0.001 | –0.79 (–1.31 to –0.27), p = 0.003 | –1.05 (–1.51 to –0.61), p < 0.001 |
| 48 hr | –0.86 (–1.22 to –0.49), p < 0.001 | –0.97 (–1.49 to –0.045), p < 0.001 | –0.76 (–1.24 to –0.28), p = 0.002 |
| Nucleosomes (arbitrary unit) | |||
| Presentation | 0.48 (0.33–0.64), p < 0.001 | 0.58 (0.32–0.84), p < 0.001 | 0.44 (0.25–0.62), p < 0.001 |
| 6 hr | 0.05 (–0.11 to 0.20), p = 0.540 | 0.13 (–0.14 to 0.39), p = 0.347 | 0.01 (–0.18 to 0.19), p = 0.935 |
| 12 hr | Reference | ||
| 24 hr | 0.04 (–0.12 to 0.21), p = 0.587 | 0.16 (–0.10 to 0.42), p = 0.236 | –0.10 (–0.21 to 0.18), p = 0.885 |
| 48 hr | 0.13 (–0.04 to 0.29), p = 0.140 | 0.41 (0.15–0.68), p = 0.002 | –0.06 (0.26–0.15), p = 0.594 |
AKI = acute kidney injury.
DISCUSSION
We defined the relationship of early serial plasma concentrations of three nucleic acid DAMPs with the development of AKI in critically ill trauma patients. Circulating nDNA, mtDNA, and nucleosomes cause kidney injury in preclinical models (48, 53–55), and therapeutics targeting these DAMPs in human populations are under active study (17, 19, 20, 23, 24, 26, 28, 54, 56). Our study addresses key knowledge gaps in understanding how these targeted therapies might apply to severely injured patients, in whom pathophysiologic processes rapidly evolve. nDNA levels from presentation through 48 hours were strongly associated with AKI independent of injury severity and other confounders. Associations of mtDNA and nucleosome concentrations with AKI showed greater time variability, with differences between AKI and non-AKI patients most notable at 24 and 48 hours after presentation. Our study adds substantial knowledge to the understanding of early posttrauma kinetics of circulating nucleic acid DAMPs, being the first to characterize how these three interrelated but distinct DAMPs change over time and relate to AKI. In addition to corroborating preclinical data implicating nucleic acid DAMPs in the pathophysiology of acute organ dysfunction, our findings suggest that circulating nDNA might be leveraged for early AKI risk stratification.
The use of AKI as an outcome is an important advantage of our study and is solidly grounded in preclinical evidence, which demonstrates a pathogenic role for nucleic acids and their receptors in sepsis- and hemorrhage-induced AKI models but until now has not been validated in clinical trauma cohorts (17, 19, 23, 26, 28, 54). nDNA exhibited the strongest association with AKI at presentation through 48 hours of ICU course. Although oxidized nDNA is recognized by the pattern recognition receptors cGAS-STING and NLRP3, the immune response elicited is weaker than that provoked by mtDNA since the latter contains hypomethylated CpG motifs homologous to bacterial DNA (14, 57). nDNA does, however, contribute to immunothrombosis and NETs, both implicated in acute organ dysfunction syndromes including AKI and acute respiratory distress syndrome (ARDS), and may underlie the strong association we observed (58). Not surprisingly, since nucleosomes comprise circulating nDNA complexed to histones, their concentrations were correlated with nDNA at all time points and associated with AKI over the first 48 hours, although less robustly than nDNA. This may corroborate preclinical data, suggesting that nDNA in complex with histones is less injurious than free nDNA by shielding the negatively charged nucleic acids from inducing coagulation (57). The association of mtDNA with AKI became evident only in plasma drawn at 24–48 hours. Eppensteiner et al (59) demonstrated that trauma patient plasma caused TLR9 activation in an ex vivo model, a finding likely mediated by mtDNA given the specificity of hypomethylated DNA and mtDNA as ligands for TLR9. Trauma patient plasma activated TLR9 most robustly when sampled from later time points and from patients with multiple organ failure, consistent with the timing of mtDNA elevation and association with AKI we report. These findings may validate experimental observations that stressed cells actively release mtDNA and nucleosomes, representing a second wave of DAMP release in a subset of trauma patients (36, 39, 40, 60). In light of the appearance of this association after many AKI cases were clinically evident, elevations of mtDNA and nucleosomes may reflect active release by injured cells, including in the kidneys, as a consequence rather than a cause of AKI (25, 42, 57, 61), limiting conclusions about whether this relationship is causal and the direction of causality. Nonetheless, it remains possible that mtDNA and nucleosomes ejected by stressed or dying cells in the first 24 hours after trauma might constitute a “second hit” to the kidneys that could precipitate more severe or prolonged AKI.
Our study provides critical data regarding the relative strength and timing of each nucleic acid DAMP’s association with AKI, a finding with potential clinical utility to guide AKI prediction. The clear association of presentation nDNA, prior to any transfusions or operative interventions, with subsequent AKI suggests its potential value to inform AKI risk. Although nDNA correlated with ISS at all time points, presentation nDNA outperformed ISS, presence of shock, and injury mechanism, and was equivalent to transfusion, for AKI prediction. Models combining presentation nDNA with each of these clinical variables better predicted AKI compared with each variable alone, suggesting that its measurement may provide unique prognostic information. Should presentation nDNA reproducibly predict increased AKI risk, it may be useful to prognostically enrich trials for patients more likely to develop AKI. Indeed, PCR-based nucleic acid quantification has become indispensable during the COVID-19 pandemic, driving significant advances in its availability, cost, and speed. Beyond COVID, SARS-CoV-2, viral respiratory detection panels and GeneXpert for Mycobacterium tuberculosis already utilize rapid PCR testing to inform diagnosis and clinical decision-making. These gains in testing capability could be applied to quantification of endogenous nucleic acids to allow for their incorporation into clinical trials and ultimately guide therapeutic decision-making.
Our findings may also inform pharmacologic targeting of nucleic acid DAMPs, the subject of increasing clinical interest. Multiple clinical trials of DNase and other nucleic acid-directed therapies are currently underway, including nebulized recombinant human DNAse to prevent or treat ARDS in trauma patients and COVID-19 pneumonia (Inhaled Dornase Alpha to Reduce Respiratory Failure After Severe Trauma [NCT03368092], Efficacy and Safety of aerosolized Dornase Alfa Administration in Patients With COVID19 Induced ARDS [NCT04355364], I-SPY COVID-19 [NCT04488081], NCT04402944, and NCT04445285), another critical illness in which nucleic acids and NETs contribute to pathophysiology of organ dysfunction. Dornase alfa has been given intravenously safely in trials of lupus nephritis treatment (53) and ischemic stroke (NCT04785066). Mesenchymal stem cells secreting DNase and nucleic acid-scavenging nanospheres are also under study to prevent multiple organ dysfunction after trauma and ischemic injury (15, 46). Nucleic acid quantification may be useful to predictively enrich similar trials by identifying patients more likely to benefit from nucleic acid DAMP-directed treatments. This highlights the importance of understanding the patterns of nucleic acid DAMP release and clearance in critically ill patients.
Circulating levels of all three DAMPs fell from presentation through 12 hours, but in patients with AKI, both mtDNA and nucleosomes rose significantly over the subsequent 36 hours. The differential kinetics of nucleic acid DAMPs may underlie contradictory associations with clinical outcomes observed in prior trauma cohort investigations. Khubutia Sh et al (32) demonstrated that mtDNA, but not nDNA, was associated with mortality in 25 severe polytrauma patients, though samples were only obtained from ~50% of patients at each time point. McIlroy et al (36) found that plasma mtDNA exceeded nDNA concentrations immediately pre- and postoperation for orthopedic injuries, although interventions were done a median of 48 hours after presentation to the trauma bay and, thus, did not address the early posttrauma period when most AKI occurs. Other studies showed elevation in nDNA but not mtDNA before, at, and several days after ED presentation (33) as well as correlation of nDNA but not mtDNA with mortality and chronic critical illness (34). Both studies included only two time points in the first 48 hours after presentation, and the former was further limited by ~40% sample missingness at the second time point. Our study adds the most detailed accounting of early posttrauma nDNA and mtDNA kinetics to date, novel measurement of circulating nucleosomes, an analytic approach to account for our limited sample missingness, and a greater understanding of how nucleic acid DAMP kinetics relate to a specific organ dysfunction syndrome—AKI—known to be precipitated by these mediators in animal models.
Our study has notable strengths. First, we focused on AKI as study of nucleic acid DAMPs and AKI in trauma patients has been extremely limited, despite ample preclinical evidence suggesting that the kidneys may be highly susceptible to nucleic acid-mediated injury (12, 16, 17, 19, 23, 26, 28, 54). Posttrauma AKI occurs more frequently than death, the most commonly studied outcome, and is imprecisely captured by organ dysfunction scores, lending our study increased power to detect the observed associations. Second, we prospectively collected samples at multiple standardized early time points after major trauma, providing novel insight into nucleic acid DAMP kinetics during a period of complex, rapidly evolving physiology in which incipient AKI is common. Specifically, our study design permitted observations about the relationship of mtDNA, nDNA, and nucleosome concentrations both to each other and to AKI that have not previously been reported; prior studies have utilized at most two time points within the first 48 hours after trauma. Third, sample missingness was limited, and we accounted for missingness in statistical analyses in order to accurately assess early DAMP changes over time. Prior studies of mtDNA and nDNA in trauma largely report time point data based solely on available samples—despite substantial missingness—such that apparent changes over time may simply have been due to differences in the patients included at each time point. Limitations include the study’s single-center design and relatively small sample size, although multiple standardized sampling time points and low subject dropout partially mitigate this weakness. The study design does not enable us to determine whether elevations in nucleic acid DAMPs are due to sustained generation through active or passive release or to decreased clearance, as previously proposed (43). It is possible that plasma DAMP levels may be attributed to decreased renal elimination in AKI patients, although the available data suggest this is a minor component of nucleic acid clearance (62–64). Finally, determining causality of any DAMP in human AKI pathogenesis is not possible from an observational study. Larger follow-up studies may better answer questions about such AKI mechanisms associated with nucleic acid DAMPs, as well as subgroups such as AKI persistence, acute kidney disease, and ultimate development of chronic kidney disease. Our findings could, however, indicate which DAMPs to investigate and when to assay them for future studies of AKI in trauma patients and other critical illness populations.
CONCLUSION
We found distinct time-based associations of circulating mtDNA, nDNA, and nucleosomes, measured serially over the first 48 hours following presentation, with AKI in critically ill trauma patients. Our novel finding that nDNA levels in particular were strongly associated with AKI from presentation through 48 hours may have implications for understanding AKI pathophysiology, prediction, and investigational therapies. Further, we described for the first time in any ICU population the kinetics and relationship of these three related nucleic acid DAMPs during incipient critical illness and may corroborate experimental evidence of active release of mtDNA and nucleosomes in response to stress. Knowledge about which nucleic acid DAMPs are most associated with AKI and when they become elevated will be critical to designing trials of existing nucleic acid DAMP-targeted therapies, opening novel mechanistic pathways for prevention and treatment of organ dysfunction in trauma. Given the compelling preclinical evidence that nucleic acid DAMPs cause AKI, our results, if validated, may support further enquiry into the use of existing targeted therapies for treatment and prevention of posttrauma AKI.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by 2T32HL007775-21 and 1KL2TR002374-01 (to Dr. Faust), K23-HL125723 (to Dr. Reilly), and Penn Acute Research Collaboration Pilot Award (to Dr. Shashaty).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Hutchings L, Watkinson P, Young JD, et al. : Defining multiple organ failure after major trauma: A comparison of the Denver, Sequential Organ Failure Assessment, and Marshall scoring systems. J Trauma Acute Care Surg 2017; 82:534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrois A, Soyer B, Gauss T, et al. ; Traumabase® Group: Prevalence and risk factors for acute kidney injury among trauma patients: A multicenter cohort study. Crit Care 2018; 22:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Wessem KJP, Leenen LPH: Incidence of acute respiratory distress syndrome and associated mortality in a polytrauma population. Trauma Surg Acute Care Open 2018; 3:e000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauaia A, Moore EE, Johnson JL, et al. : Temporal trends of postinjury multiple-organ failure: Still resource intensive, morbid, and lethal. J Trauma Acute Care Surg 2014; 76:582–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Søvik S, Isachsen MS, Nordhuus KM, et al. : Acute kidney injury in trauma patients admitted to the ICU: A systematic review and meta-analysis. Intensive Care Med 2019; 45:407–419 [DOI] [PubMed] [Google Scholar]
- 6.Perkins ZB, Haines RW, Prowle JR: Trauma-associated acute kidney injury. Curr Opin Crit Care 2019; 25:565–572 [DOI] [PubMed] [Google Scholar]
- 7.Burmeister DM, Gómez BI, Dubick MA: Molecular mechanisms of trauma-induced acute kidney injury: Inflammatory and metabolic insights from animal models. Biochim Biophys Acta Mol Basis Dis 2017; 1863:2661–2671 [DOI] [PubMed] [Google Scholar]
- 8.Li J, Ma Z, Tang ZL, et al. : CpG DNA-mediated immune response in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 2004; 287:L552–L558 [DOI] [PubMed] [Google Scholar]
- 9.El Kebir D, József L, Pan W, et al. : Bacterial DNA activates endothelial cells and promotes neutrophil adherence through TLR9 signaling. J Immunol 2009; 182:4386–4394 [DOI] [PubMed] [Google Scholar]
- 10.Alekseeva AY, Kameneva LV, Kostyuk SV, et al. : Multiple ways of cfDNA reception and following ROS production in endothelial cells. Adv Exp Med Biol 2016; 924:127–131 [DOI] [PubMed] [Google Scholar]
- 11.Knuefermann P, Baumgarten G, Koch A, et al. : CpG oligonucleotide activates toll-like receptor 9 and causes lung inflammation in vivo. Respir Res 2007; 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hägele H, Allam R, Pawar RD, et al. : Double-stranded DNA activates glomerular endothelial cells and enhances albumin permeability via a toll-like receptor-independent cytosolic DNA recognition pathway. Am J Pathol 2009; 175:1896–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada K, Crother TR, Karlin J, et al. : Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012; 36:401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magna M, Pisetsky DS: The alarmin properties of DNA and DNA-associated nuclear proteins. Clin Ther 2016; 38:1029–1041 [DOI] [PubMed] [Google Scholar]
- 15.Aswani A, Manson J, Itagaki K, et al. : Scavenging circulating mitochondrial DNA as a potential therapeutic option for multiple organ dysfunction in trauma hemorrhage. Front Immunol 2018; 9:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao W, Xia H, Liang Y, et al. : Toll-like receptor 9 can be activated by endogenous mitochondrial DNA to induce podocyte apoptosis. Sci Rep 2016; 6:22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SJ, Li H, Kim M, et al. : Kidney proximal tubular TLR9 exacerbates ischemic acute kidney injury. J Immunol 2018; 201:1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han SJ, Williams RM, D’Agati V, et al. : Selective nanoparticle-mediated targeting of renal tubular toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int 2020; 98:76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maekawa H, Inoue T, Ouchi H, et al. : Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep 2019; 29:1261–1273.e6 [DOI] [PubMed] [Google Scholar]
- 20.Gong W, Lu L, Zhou Y, et al. : The novel STING antagonist H151 ameliorates cisplatin-induced acute kidney injury and mitochondrial dysfunction. Am J Physiol Renal Physiol 2021; 320:F608–F616 [DOI] [PubMed] [Google Scholar]
- 21.Oklu R, Albadawi H, Watkins MT, et al. : Detection of extracellular genomic DNA scaffold in human thrombus: Implications for the use of deoxyribonuclease enzymes in thrombolysis. J Vasc Interv Radiol 2012; 23:712–718 [DOI] [PubMed] [Google Scholar]
- 22.Skendros P, Mitsios A, Chrysanthopoulou A, et al. : Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest 2020; 130:6151–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakazawa D, Kumar SV, Marschner J, et al. : Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol 2017; 28:1753–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okubo K, Kurosawa M, Kamiya M, et al. : Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat Med 2018; 24:232–238 [DOI] [PubMed] [Google Scholar]
- 25.Kawai C, Kotani H, Miyao M, et al. : Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol 2016; 186:829–843 [DOI] [PubMed] [Google Scholar]
- 26.Tsuji N, Tsuji T, Ohashi N, et al. : Role of mitochondrial DNA in septic AKI via toll-like receptor 9. J Am Soc Nephrol 2016; 27:2009–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merkle J, Daka A, Deppe AC, et al. : High levels of cell-free DNA accurately predict late acute kidney injury in patients after cardiac surgery. PLoS One 2019; 14:e0218548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allam R, Scherbaum CR, Darisipudi MN, et al. : Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 2012; 23:1375–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Relja B, Mörs K, Marzi I: Danger signals in trauma. Eur J Trauma Emerg Surg 2018; 44:301–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Likhvantsev VV, Landoni G, Grebenchikov OA, et al. : Nuclear DNA as predictor of acute kidney injury in patients undergoing coronary artery bypass graft: A pilot study. J Cardiothorac Vasc Anesth 2017; 31:2080–2085 [DOI] [PubMed] [Google Scholar]
- 31.Faust HE, Reilly JP, Anderson BJ, et al. : Plasma mitochondrial DNA levels are associated with ARDS in trauma and sepsis patients. Chest 2020; 157:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khubutia Sh M, Shabanov AK, Skulachev VP, et al. : Mitochondrial and nuclear DNA in patients with severe polytrauma. General Reanimatology 2013; 9:24 [Google Scholar]
- 33.Timmermans K, Kox M, Vaneker M, et al. : Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med 2016; 42:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stortz JA, Hawkins RB, Holden DC, et al. : Cell-free nuclear, but not mitochondrial, DNA concentrations correlate with the early host inflammatory response after severe trauma. Sci Rep 2019; 9:13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shashaty MG, Meyer NJ, Localio AR, et al. : African American race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. J Crit Care 2012; 27:496–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIlroy DJ, Bigland M, White AE, et al. : Cell necrosis-independent sustained mitochondrial and nuclear DNA release following trauma surgery. J Trauma Acute Care Surg 2015; 78:282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Relja B, Land WG: Damage-associated molecular patterns in trauma. Eur J Trauma Emerg Surg 2020; 46:751–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paunel-Görgülü A, Wacker M, El Aita M, et al. : cfDNA correlates with endothelial damage after cardiac surgery with prolonged cardiopulmonary bypass and amplifies NETosis in an intracellular TLR9-independent manner. Sci Rep 2017; 7:17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIlroy DJ, Jarnicki AG, Au GG, et al. : Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care 2014; 29:1133.e1–1133.e5 [DOI] [PubMed] [Google Scholar]
- 40.Ingelsson B, Söderberg D, Strid T, et al. : Lymphocytes eject interferogenic mitochondrial DNA webs in response to CpG and non-CpG oligodeoxynucleotides of class C. Proc Natl Acad Sci U S A 2018; 115:E478–E487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon S, Park SJ, Han JH, et al. : Caspase-dependent cell death-associated release of nucleosome and damage-associated molecular patterns. Cell Death Dis 2014; 5:e1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silk E, Zhao H, Weng H, et al. : The role of extracellular histone in organ injury. Cell Death Dis 2017; 8:e2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hazeldine J, Dinsdale RJ, Naumann DN, et al. : Traumatic injury is associated with reduced deoxyribonuclease activity and dysregulation of the actin scavenging system. Burns Trauma 2021; 9:tkab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotz MJ, Qing D, Shashaty MGS, et al. : Red blood cells homeostatically bind mitochondrial DNA through TLR9 to maintain quiescence and to prevent lung injury. Am J Respir Crit Care Med 2018; 197:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin M, Leffler J, Smoląg KI, et al. : Factor H uptake regulates intracellular C3 activation during apoptosis and decreases the inflammatory potential of nucleosomes. Cell Death Differ 2016; 23:903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen HH, Yuan H, Cho H, et al. : Theranostic nucleic acid binding nanoprobe exerts anti-inflammatory and cytoprotective effects in ischemic injury. Theranostics 2017; 7:814–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan X, Li B, Kuang M, et al. : Synthetic human TLR9-LRR11 peptide attenuates TLR9 signaling by binding to and thus decreasing internalization of CpG oligodeoxynucleotides. Int J Mol Sci 2016; 17:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han SJ, Williams RM, D’Agati V, et al. : Selective nanoparticle-mediated targeting of renal tubular toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int 2020; 98:76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shrestha B, Ito T, Kakuuchi M, et al. : Recombinant thrombomodulin suppresses histone-induced neutrophil extracellular trap formation. Front Immunol 2019; 10:2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker SP, O’Neill B, Haddon W, et al. : The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma 1974; 14:187–196 [PubMed] [Google Scholar]
- 51.Singbartl K, Kellum JA: AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int 2012; 81:819–825 [DOI] [PubMed] [Google Scholar]
- 52.Reilly JP, Meyer NJ, Shashaty MGS, et al. : ABO blood type A is associated with increased risk of ARDS in Whites following both major trauma and severe sepsis. Chest 2014; 145:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis JC, Jr, Manzi S, Yarboro C, et al. : Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 1999; 8:68–76 [DOI] [PubMed] [Google Scholar]
- 54.Peer V, Abu Hamad R, Berman S, et al. : Renoprotective effects of DNAse-I treatment in a rat model of ischemia/reperfusion-induced acute kidney injury. Am J Nephrol 2016; 43:195–205 [DOI] [PubMed] [Google Scholar]
- 55.McIlroy DJ, Minahan K, Keely S, et al. : Reduced deoxyribonuclease enzyme activity in response to high postinjury mitochondrial DNA concentration provides a therapeutic target for systemic inflammatory response syndrome. J Trauma Acute Care Surg 2018; 85:354–358 [DOI] [PubMed] [Google Scholar]
- 56.Gao M, Wan X, Ma M, et al. : Kidney injury induced by elevated histones in community-acquired pneumonia. Mol Cell Biochem 2020; 471:155–163 [DOI] [PubMed] [Google Scholar]
- 57.Noubouossie DF, Whelihan MF, Yu YB, et al. : In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood 2017; 129:1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Longstaff C, Varjú I, Sótonyi P, et al. : Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem 2013; 288:6946–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eppensteiner J, Kwun J, Scheuermann U, et al. : Damage- and pathogen-associated molecular patterns play differential roles in late mortality after critical illness. JCI Insight 2019; 4:127925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.West AP, Shadel GS: Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol 2017; 17:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Zhang X, Pelayo R, et al. : Extracellular histones are major mediators of death in sepsis. Nat Med 2009; 15:1318–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korabecna M, Opatrna S, Wirth J, et al. : Cell-free plasma DNA during peritoneal dialysis and hemodialysis and in patients with chronic kidney disease. Ann N Y Acad Sci 2008; 1137:296–301 [DOI] [PubMed] [Google Scholar]
- 63.Gauthier VJ, Tyler LN, Mannik M: Blood clearance kinetics and liver uptake of mononucleosomes in mice. J Immunol 1996; 156:1151–1156 [PubMed] [Google Scholar]
- 64.Lam LKM, Murphy S, Kokkinaki D, et al. : DNA binding to TLR9 expressed by red blood cells promotes innate immune activation and anemia. Sci Transl Med 2021; 13:eabj1008. [DOI] [PMC free article] [PubMed] [Google Scholar]